The Synthesis of a New Glycoluryl–Melamine–Formaldehyde Polymer under the Action of HEDP and the Investigation of the Content of Methylol Groups and Free Formaldehyde
Abstract
1. Introduction
2. Materials and Methods
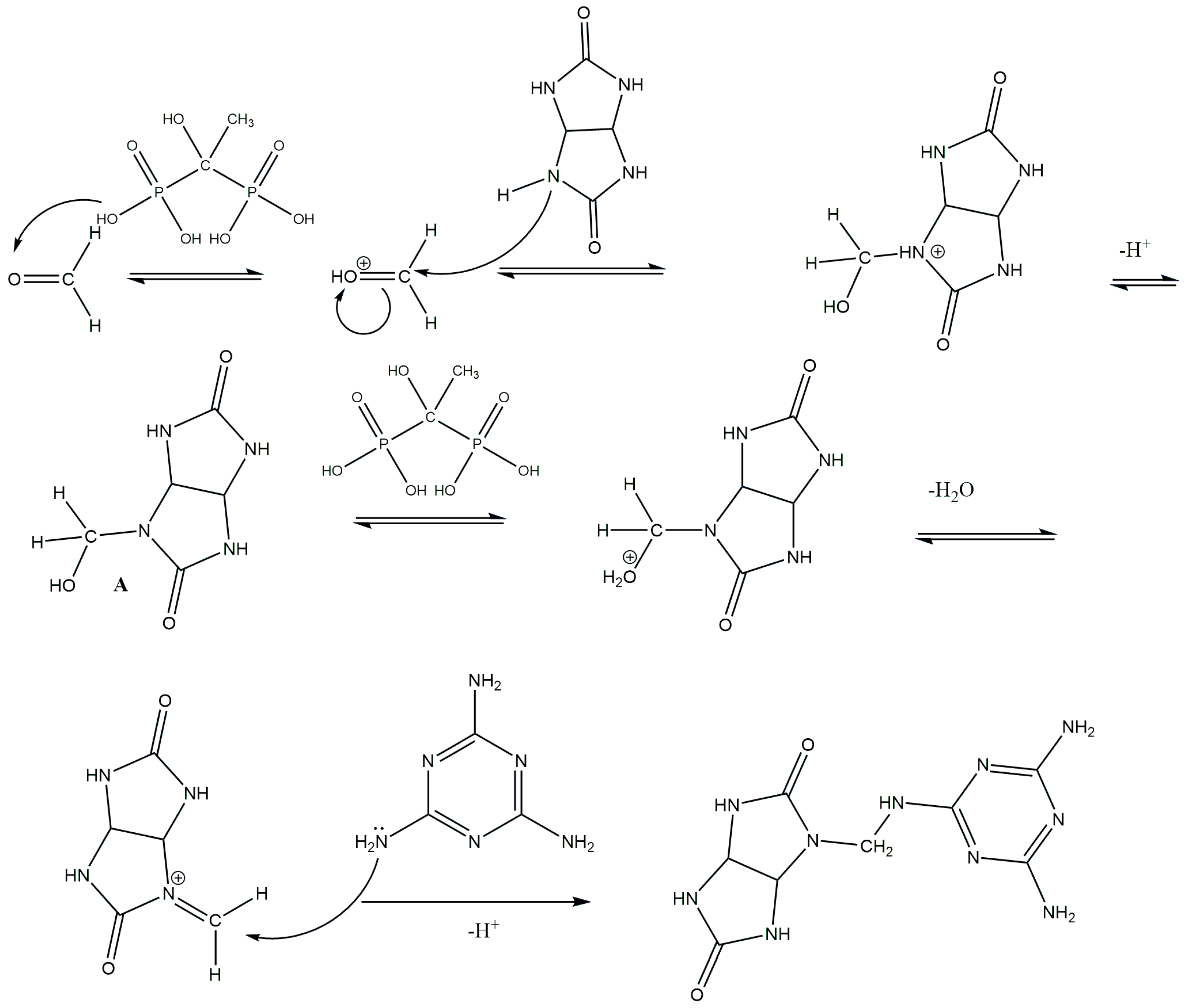
2.1. Synthesis of Glycoluril–Melamine–Formaldehyde Resin (GUMEFA)
2.1.1. Synthesis of the Glycoluril–Melamine Complex (GU-ME)
2.1.2. Synthesis of GUMEFA via Plasticization with Hydrochloric and HEDP Acids
- Method 1: by adding HEDP solution (1 mL of HEDP solution with a concentration of 0.5 g per 1 mL of water), with a plasticization time from 20 h to 24 h [25];
- Method 2: by adding crystalline HEDP acid (0.5 g of HEDP acid), with a plasticization time from 5 to 10 min [25];
- Method 3: an experiment conducted without using a plasticizer, serving as a control; with the plasticization period lasting three days.
- Method 4: by adding hydrochloric acid solution (1 mL of 8% diluted solution), with the time for complete plasticization being from 20 h to a day;
- Method 5: by adding concentrated hydrochloric acid (0.2 mL of 36% concentrated acid), with a plasticization time from 5 to 10 min.
2.2. Physicochemical Research Methods
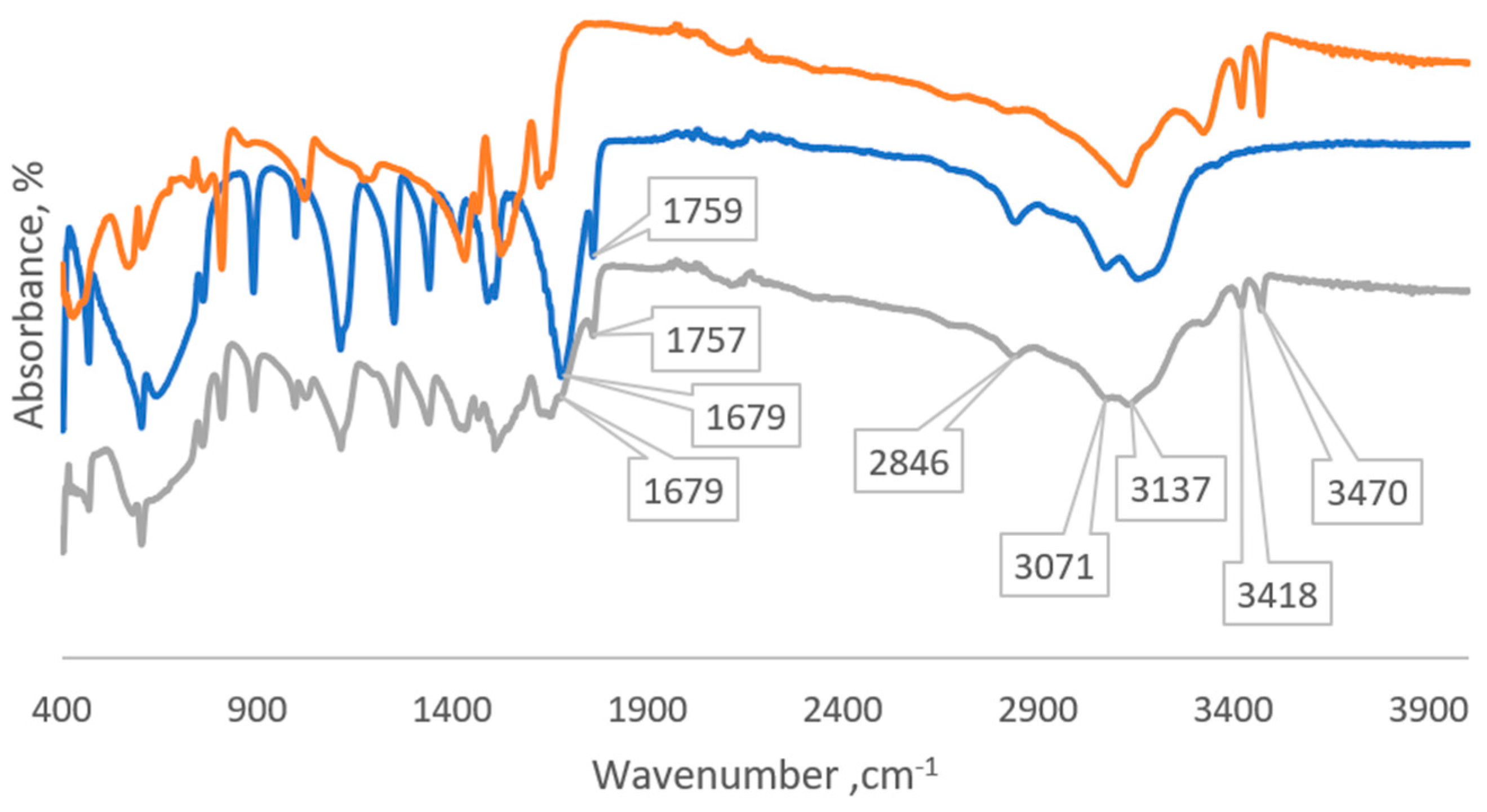
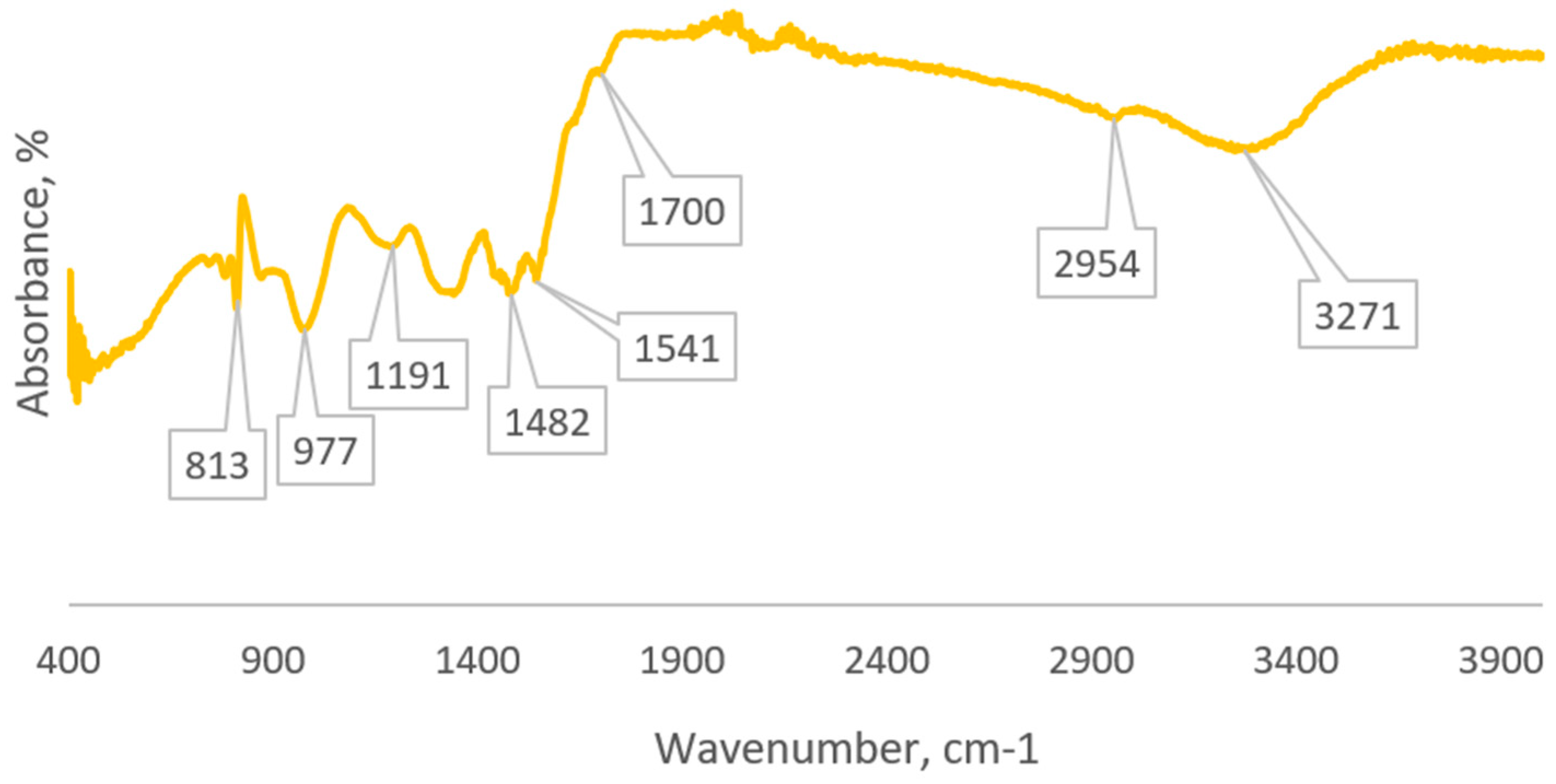
2.2.1. IR Spectroscopy
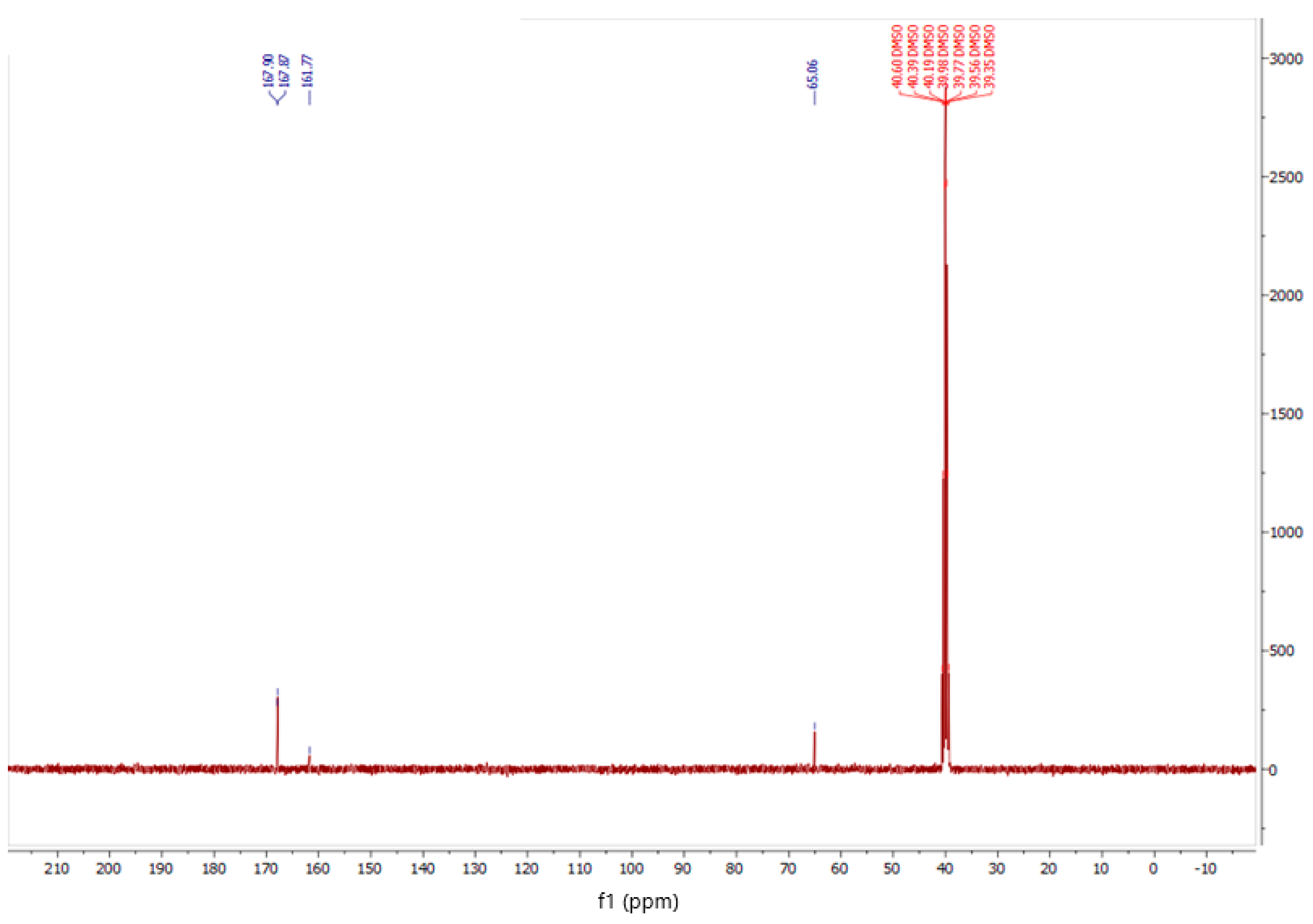
2.2.2. NMR Spectroscopy
2.2.3. Melting Temperature
2.2.4. Gel Permeation Chromatography
2.2.5. Determination of Methylol Group Contents
2.2.6. Determination of Formaldehyde Content
- Optical density: from 3.000 to 0.000;
- Directional transmittance: from 0.0 to 100.0%.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hollande, L.; Marcolino, I.D.; Balaguer, P.; Domenek, S.; Gross, R.A.; Allais, F. Preparation of Renewable Epoxy-Amine Resins With Tunable Thermo-Mechanical Properties, Wettability and Degradation Abilities From Lignocellulose- and Plant Oils-Derived Components. Front. Chem. 2019, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Solt, P.; Konnerth, J.; Gindl-Altmutter, W.; Kantner, W.; Moser, J.; Mitter, R.; van Herwijnen, H.W.G. Technological Performance of Formaldehyde-Free Adhesive Alternatives for Particleboard Industry. Int. J. Adhes. 2019, 94, 99–131. [Google Scholar] [CrossRef]
- Réh, R.; Igaz, R.; Krišťák, Ľ.; Ružiak, I.; Gajtanska, M.; Božíková, M.; Kučerka, M. Functionality of Beech Bark in Adhesive Mixtures Used in Plywood and Its Effect on the Stability Associated with Material Systems. Materials 2019, 12, 1298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Song, S.; Wu, G.; Pu, J. Modified Nanocrystalline Cellulose from Two Kinds of Modifiers Used for Improving Formaldehyde Emission and Bonding Strength of Urea-Formaldehyde Resin Adhesive. BioRes 2011, 6, 4430–4438. [Google Scholar] [CrossRef]
- Kawalerczyk, J.; Siuda, J.; Mirski, R.; Dziurka, D. Hemp Flour as a Formaldehyde Scavenger for Melamine-Urea-Formaldehyde Adhesive in Plywood Production. BioRes 2020, 15, 4052–4064. [Google Scholar] [CrossRef]
- Brown, D.; Sherdron, G.; Kern, W. A Practical Guide to the Synthesis and Study of Polymer Properties; Chemistry: Moscow, Russia, 1976. [Google Scholar]
- Malkov, V.S.; Perminova, D.A.; Knyazeva, S.L. Method of Producing Urea Formaldehyde Resin. RU 2541522C1, 20 February 2015. [Google Scholar]
- No, B.Y.; Kim, M.G. Syntheses and Properties of Low-Level Melamine-Modified Urea–Melamine–Formaldehyde Resins. J. Appl. Polym. Sci. 2004, 93, 2559–2569. [Google Scholar] [CrossRef]
- Antunes, A.; Paiva, N.; Ferra, J.; Martins, J.; Carvalho, L.; Barros-Timmons, A.; Magalhães, F.D. Highly Flexible Glycol-Urea-Formaldehyde Resins. Eur. Polym. J. 2018, 105, 167–176. [Google Scholar] [CrossRef]
- Perminova, D.A.; Malkov, V.S.; Guschin, V.; Eisenreich, N. Influence of Glyoxal on Curing of Urea-Formaldehyde Resins. Int. J. Adhes. Adhes. 2019, 92, 1–6. [Google Scholar] [CrossRef]
- Yan, S.; Wang, Y. 4(0,0-Diethyl Phosphoryl)Glycoluril Flame Retardant Composition and Application Method Thereof. CN104151795A, 19 November 2014. [Google Scholar]
- Liu, Y.; Li, S.; Chen, Y.; Li, M.; Chen, Z.; Hu, T.; Shi, L.; Pudukudy, M.; Shan, S.; Zhi, Y. Urea/Amide-Functionalized Melamine-Based Organic Polymers as Efficient Heterogeneous Catalysts for CO2 Cycloaddition. Chem. Eng. J. 2023, 474, 145918. [Google Scholar] [CrossRef]
- Lan, P.; Yang, R.; Mao, H.; Cui, J.; Brosse, N. Production of Melamine Formaldehyde Resins Used in Impregnation by Incorporation of Ethylene Glycol and Caprolactam with High Flexibility, Storage Stability, and Low Formaldehyde Content. BioResources 2019, 14, 9916–9927. [Google Scholar] [CrossRef]
- Dai, F. 10—Understanding Residual Stresses in Thick Polymer Composite Laminates. In Residual Stresses in Composite Materials (Second Edition); Shokrieh, M.M., Ed.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Cambridge, UK, 2021; pp. 313–349. [Google Scholar] [CrossRef]
- Chrobak, J.; Iłowska, J.; Chrobok, A. Formaldehyde-Free Resins for the Wood-Based Panel Industry: Alternatives to Formaldehyde and Novel Hardeners. Molecules 2022, 27, 4862. [Google Scholar] [CrossRef] [PubMed]
- Roffael, E. Emission of Formaldehyde from Particle Boards; Ecology: Moscow, Russia, 1991. [Google Scholar]
- Mohammadi, Z.; Rahsepar, M. The Use of Green Bistorta Officinalis Extract for Effective Inhibition of Corrosion and Scale Formation Problems in Cooling Water System. J. Alloy. Compd. 2019, 770, 669–678. [Google Scholar] [CrossRef]
- Pansuriya, A.M.; Savant, M.M.; Bhuva, C.V.; Singh, J.; Naliapara, Y.T. One-pot Synthesis of 5-Carboxanilide-Dihydropyrimidinones Using Etidronic Acid. Arkivoc 2009, 7, 79–85. [Google Scholar] [CrossRef]
- Savant, M.M.; Pansuriya, A.M.; Bhuva, C.V.; Kapuriya, N.P.; Naliapara, Y.T. Etidronic Acid: A New and Efficient Catalyst for the Synthesis of Novel 5-Nitro-3,4-Dihydropyrimidin-2(1H)-Ones. Catal. Lett. 2009, 132, 281–284. [Google Scholar] [CrossRef]
- Panshina, S.Y.; Bakibaev, A.A.; Gusliakov, A.N.; Malkov, V.S. Synthesis of Cucurbit[6]uril Using 1-Hydroxyethylidene-1,1-Diphosphonic Acid as a “Green Catalyst”. Bull. Univ. Karaganda-Chem. 2022, 4, 5–13. [Google Scholar] [CrossRef]
- Panshina, S.Y.; Ponomarenko, O.V.; Bakibaev, A.A.; Sidelnikov, V.S.; Kurgachev, D.A.; Malkov, V.S.; Khlebnikov, A.I.; Tashenov, A.K. A Study of Products of Tetrakis(Hydroxymethyl)Glycoluril Dehydroxymethylation in Aqueous Solutions. Russ. Chem. Bull 2021, 70, 140–147. [Google Scholar] [CrossRef]
- Bakibaev, A.A.; Uhov, A.; Malkov, V.S.; Yu. Panshina, S. Synthesis of Glycolurils and Hydantoins by Reaction of Urea and 1, 2-Dicarbonyl Compounds Using Etidronic Acid as a “Green Catalyst”. J. Heterocycl. Chem. 2020, 57, 4262–4270. [Google Scholar] [CrossRef]
- Panshina, S.Y.; Ponomarenko, O.V.; Bakibaev, A.A.; Malkov, V.S. New Synthesis of 2,4,6,8-Tetramethyl-2,4,6,8-Tetraazabicyclo[3.3.0]Octane-3,7-Dione Using Etidronic Acid as a “Green” Catalyst. Russ. J. Org. Chem. 2020, 56, 2067–2073. [Google Scholar] [CrossRef]
- Rottmaier, L.; Merten, R. Glycoluril Salts and a Process for the Preparation Thereof. US4433144A, 21 February 1984. Available online: https://patents.google.com/patent/US4433144A/en (accessed on 22 July 2024).
- Bakibaev, A.A.; Ukhov, A.E.; Guslyakov, A.N.; Gubankov, A.A.; Malkov, V.S.; Knyazev, A.S. Polymer Based on Glycoluril and Melamine and Method for its Production. RU 2822105C1, 1 July 2024. [Google Scholar]
- Parunov, I.V. Aminoplastic Resin. RU 2696859C1, 7 August 2019. [Google Scholar]
- Kalinina, L.S. Analysis of Condensation Polymers; Chemistry: Moscow, Russia, 1984. [Google Scholar]
- ISO 16000-3:2022; Part 3: Determination of Formaldehyde and Other Carbonyl Compounds in Indoor and Test Chamber Air-Active Sampling Method. ISO: Geneva, Switzerland, 2023.
- Kabieva, S.K.; Zhumanazarova, G.M.; Kanasheva, N.; Bakibaev, A.A.; Panshina, S.Y.; Malkov, V.S.; Mamaeva, E.A.; Knyazev, A.S. Methods of Synthesizing Glycoluril-Based Macrocyclic Compounds as Precursors for Polymeric Compounds. J. Saudi Chem. Soc. 2023, 27, 101768. [Google Scholar] [CrossRef]
- Duliban, J.; Galina, H.; Lubczak, J. 1H NMR Study of (Hydroxymethyl)Melamine Rearrangement in DMSO Solutions. Appl. Spectrosc. 1996, 50, 528–532. [Google Scholar] [CrossRef]
- Li, X.; Miao, L.; Yang, W.; Zheng, J.; Xiong, Y. Method of Increasing Hydroxymethyl Content of Hexahydroxymethyl Melamine. CN104592138A, 6 May 2015. [Google Scholar]



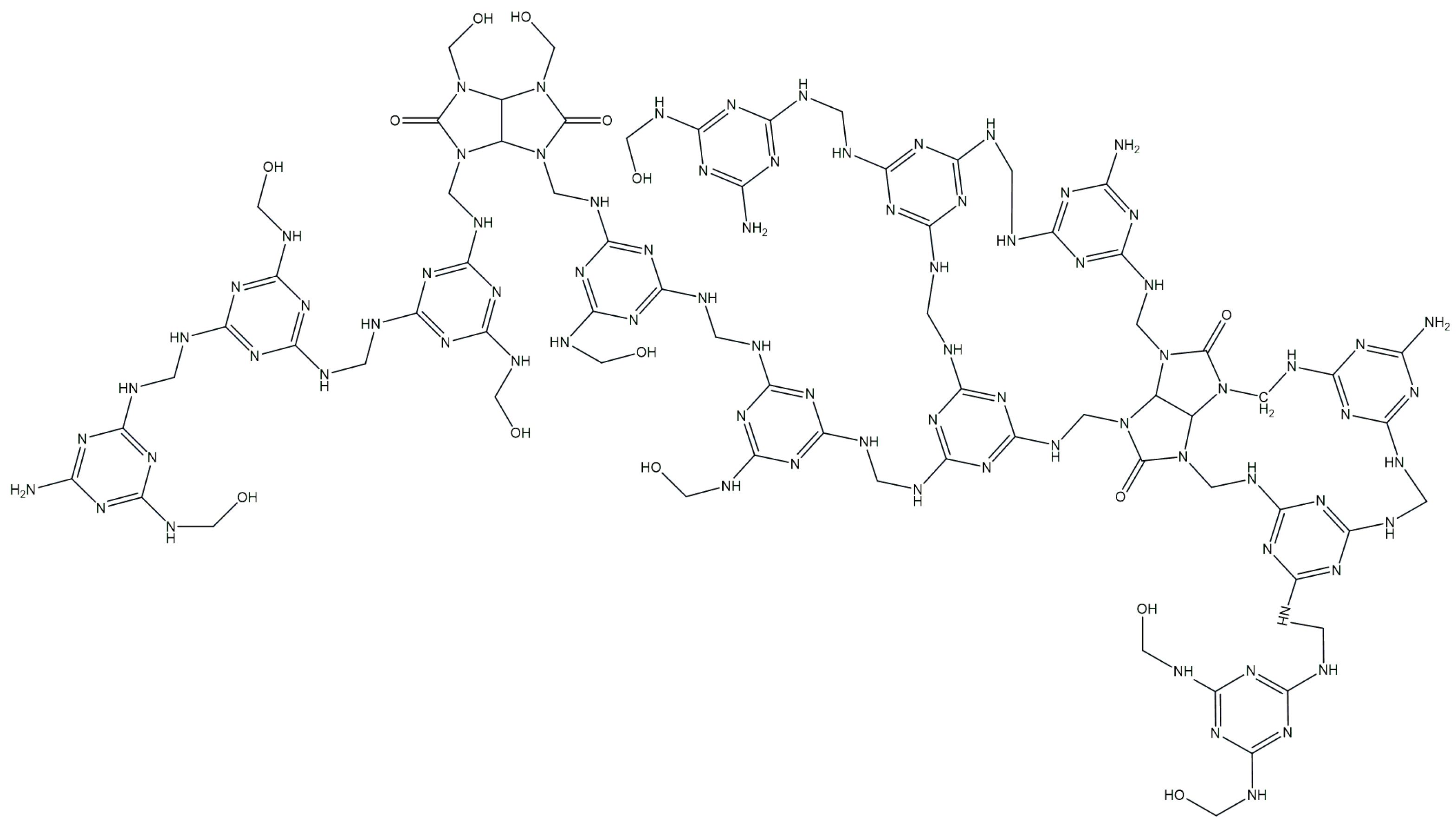
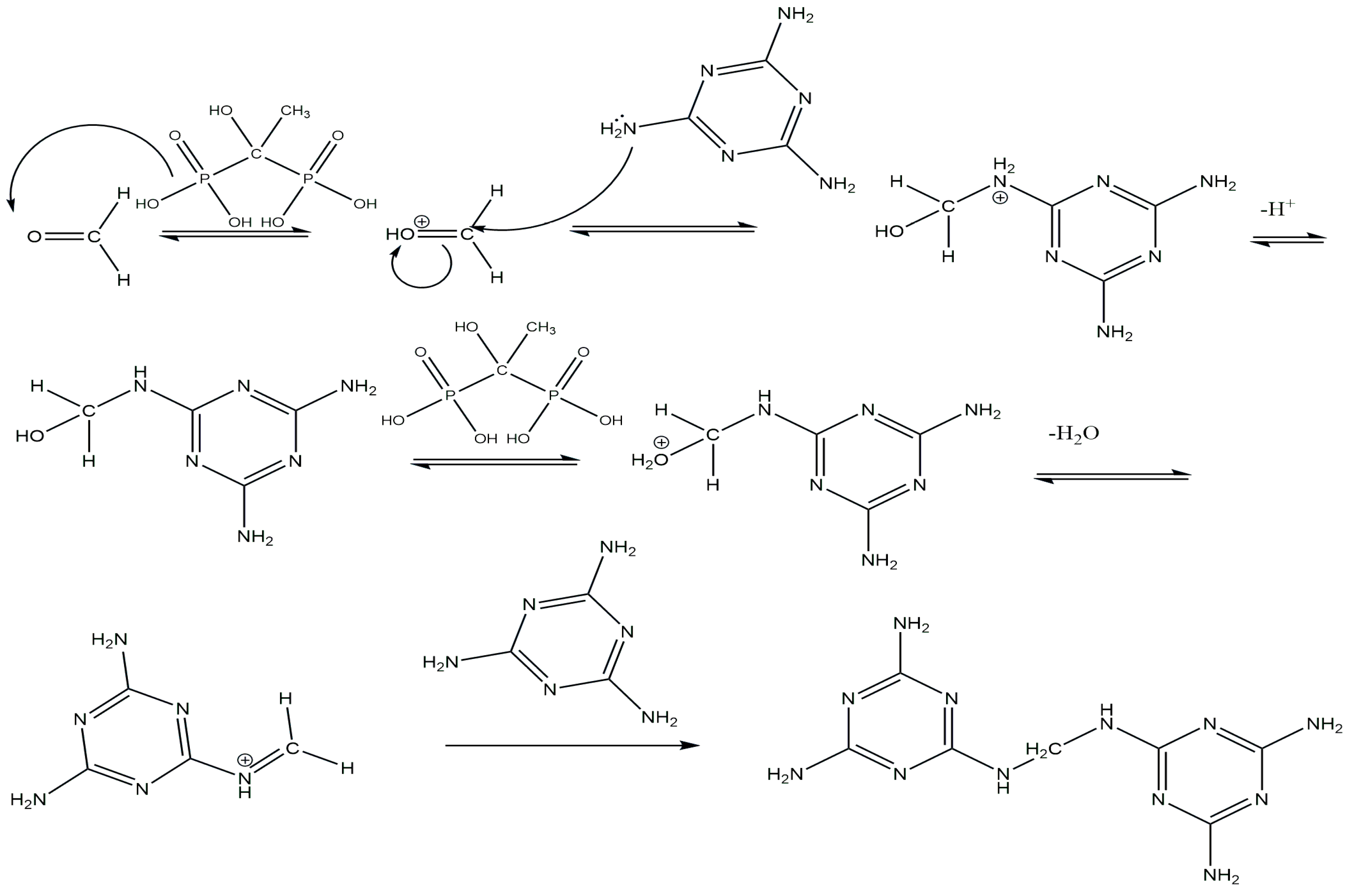
| Sample | Plasticizer | Method | Xavg, % |
|---|---|---|---|
| Sample 1 | HCl conc. | SP | 1.29 |
| Sample 1 | HCl conc. | FL | 1.40 |
| Sample 2 | HEDP cr. | SP | 1.26 |
| Sample 2 | HEDP cr. | FL | 1.34 |
| Sample 3 | HCl dil. | SP | 1.15 |
| Sample 3 | HCl dil. | FL | 1.28 |
| Sample 4 | HEDP sol. | SP | 1.24 |
| Sample 4 | HEDP sol. | FL | 1.22 |
| Sample 5 | Without plasticizer | SP | 1.53 |
| Sample 5 | Without plasticizer | FL | 1.62 |
| Plasticizer | Content of Methylol Groups and Formaldehyde, % |
|---|---|
| HCl conc. | 24.7 |
| HEDP cr. | 2.9 |
| HCl dil. | 15.5 |
| HEDP sol. | 1.7 |
| Without plasticizer | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanasheva, N.; Ukhov, A.; Malkov, V.S.; Gubankov, A.; Sergazina, S.; Issabayeva, M.A.; Mashan, T.; Kolpek, A.; Ryskaliyeva, R.; Bakibaev, A.; et al. The Synthesis of a New Glycoluryl–Melamine–Formaldehyde Polymer under the Action of HEDP and the Investigation of the Content of Methylol Groups and Free Formaldehyde. Polymers 2024, 16, 2877. https://doi.org/10.3390/polym16202877
Kanasheva N, Ukhov A, Malkov VS, Gubankov A, Sergazina S, Issabayeva MA, Mashan T, Kolpek A, Ryskaliyeva R, Bakibaev A, et al. The Synthesis of a New Glycoluryl–Melamine–Formaldehyde Polymer under the Action of HEDP and the Investigation of the Content of Methylol Groups and Free Formaldehyde. Polymers. 2024; 16(20):2877. https://doi.org/10.3390/polym16202877
Chicago/Turabian StyleKanasheva, Nurdana, Arthur Ukhov, Victor S. Malkov, Alexander Gubankov, Samal Sergazina, Manar A. Issabayeva, Togzhan Mashan, Ainagul Kolpek, Roza Ryskaliyeva, Abdigali Bakibaev, and et al. 2024. "The Synthesis of a New Glycoluryl–Melamine–Formaldehyde Polymer under the Action of HEDP and the Investigation of the Content of Methylol Groups and Free Formaldehyde" Polymers 16, no. 20: 2877. https://doi.org/10.3390/polym16202877
APA StyleKanasheva, N., Ukhov, A., Malkov, V. S., Gubankov, A., Sergazina, S., Issabayeva, M. A., Mashan, T., Kolpek, A., Ryskaliyeva, R., Bakibaev, A., & Yerkassov, R. (2024). The Synthesis of a New Glycoluryl–Melamine–Formaldehyde Polymer under the Action of HEDP and the Investigation of the Content of Methylol Groups and Free Formaldehyde. Polymers, 16(20), 2877. https://doi.org/10.3390/polym16202877







