Cold Plasma Technology Based Eco-Friendly Food Packaging Biomaterials
Abstract
1. Introduction
2. Cold Plasma Technology for Property Enhancements in Sustainable Packaging
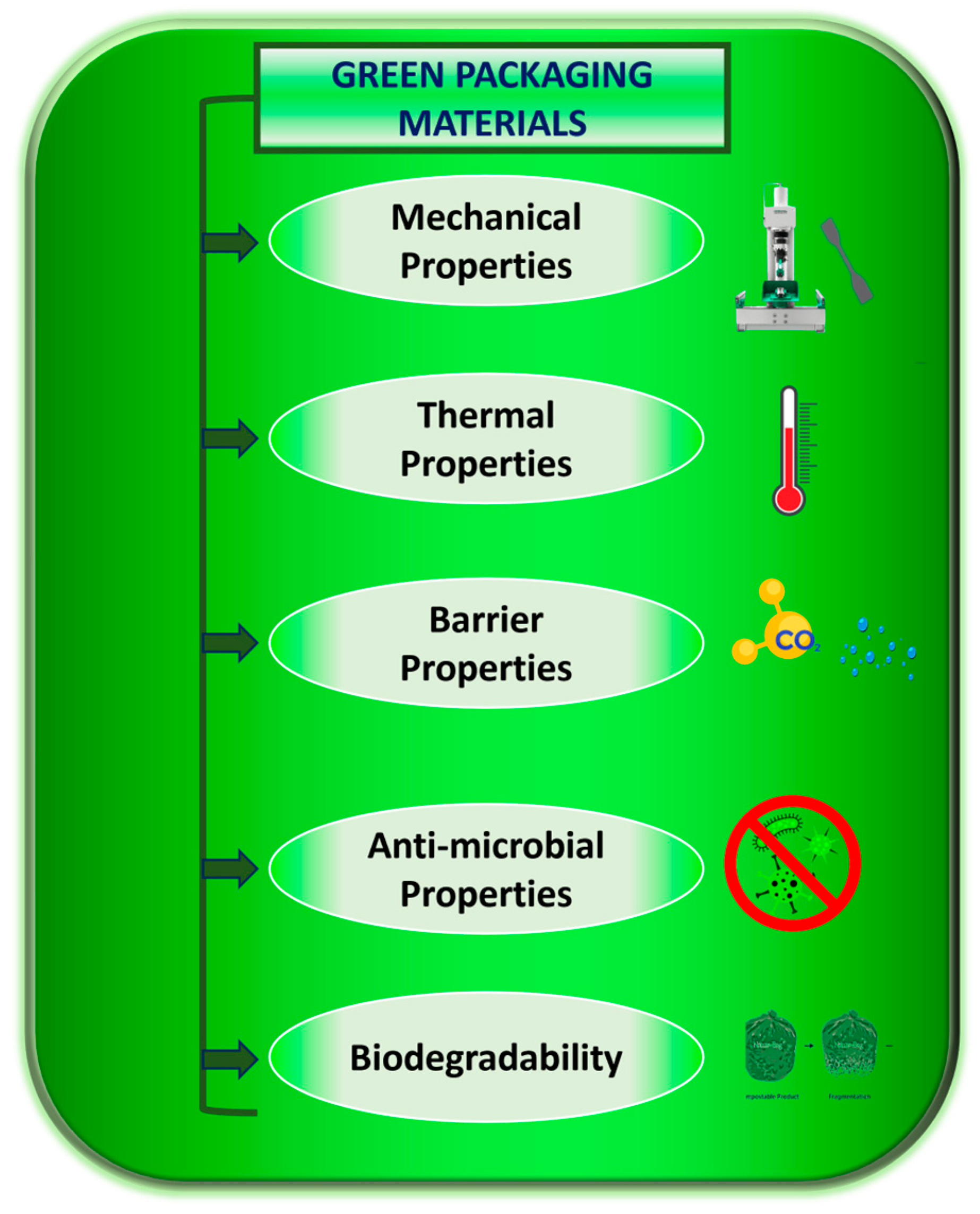
2.1. Surface Properties of Materials
2.2. Barrier Properties of Materials
2.3. Contact Angle for Wettability
2.4. Mechanical Properties of Materials
2.5. Thermal Properties of Materials
2.6. Surface Chemistry Properties
2.7. Anti-Microbial Properties of Materials
2.8. Biodegradability of Materials
3. Plasma Technology in Food Packaging
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thomas, S.; Mozetic, M.; Cvelbar, U.; Špatenka, P.; Praveen, K.M. Non-Thermal Plasma Technology for Polymeric Materials; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-813152-7. [Google Scholar]
- Karthik, C.; Rajalakshmi, S.; Thomas, S.; Thomas, V. Intelligent Polymeric Biomaterials Surface Driven by Plasma Processing. Curr. Opin. Biomed. Eng. 2023, 26, 100440. [Google Scholar] [CrossRef]
- Das, D.; Panesar, P.S.; Saini, C.S.; Kennedy, J.F. Improvement in Properties of Edible Film through Non-Thermal Treatments and Nanocomposite Materials: A Review. Food Packag. Shelf Life 2022, 32, 100843. [Google Scholar] [CrossRef]
- Joseph, B.; Ninan, N.; Visalakshan, R.M.; Denoual, C.; Bright, R.; Kalarikkal, N.; Grohens, Y.; Vasilev, K.; Thomas, S. Insights into the Biomechanical Properties of Plasma Treated 3D Printed PCL Scaffolds Decorated with Gold Nanoparticles. Compos. Sci. Technol. 2021, 202, 108544. [Google Scholar] [CrossRef]
- Silva, E.G.S.; Cardoso, S.; Bettencourt, A.F.; Ribeiro, I.A.C. Latest Trends in Sustainable Polymeric Food Packaging Films. Foods 2023, 12, 168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, F.; Li, C.; An, H.; Wan, T.; Zhang, P. Application of Chitosan and Its Derivative Polymers in Clinical Medicine and Agriculture. Polymers 2022, 14, 958. [Google Scholar] [CrossRef] [PubMed]
- Elashry, S.; ELsaeed, H.; El-Siragy, N.M. Microwave Plasma Discharge-Assisted Surface Modification of PVA Films: Coatings and Food Packaging. Eur. Phys. J. Plus 2022, 137, 1252. [Google Scholar] [CrossRef]
- Conrads, H.; Schmidt, M. Plasma Generation and Plasma Sources. Plasma Sources Sci. Technol. 2000, 9, 441–454. [Google Scholar] [CrossRef]
- Izdebska, J. Corona Treatment; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780323374682. [Google Scholar]
- Sasmazel, H.T.; Alazzawi, M.; Alsahib, N.K.A. Atmospheric Pressure Plasma Surface Treatment of Polymers and Influence on Cell Cultivation. Molecules 2021, 26, 1665. [Google Scholar] [CrossRef]
- Luo, J.; Yan, W.; Nasiru, M.M.; Zhuang, H.; Zhou, G.; Zhang, J. Evaluation of Physicochemical Properties and Volatile Compounds of Chinese Dried Pork Loin Curing with Plasma-Treated Water Brine. Sci. Rep. 2019, 9, 13793. [Google Scholar] [CrossRef]
- Rotan, M.; Zhuk, M.; Glaum, J. Activation of Ferroelectric Implant Ceramics by Corona Discharge Poling. J. Eur. Ceram. Soc. 2020, 40, 5402–5409. [Google Scholar] [CrossRef]
- Wais, S.I.; Mohammed, P.A. Influence of Magnetic Field on Characteristics of Corona Discharge in Wire-Cylinder Electrodes Configuration. Plasma 2021, 4, 764–779. [Google Scholar] [CrossRef]
- Lisco, F.; Shaw, A.; Wright, A.; Walls, J.M.; Iza, F. Atmospheric-Pressure Plasma Surface Activation for Solution Processed Photovoltaic Devices. Sol. Energy 2017, 146, 287–297. [Google Scholar] [CrossRef]
- Schütze, A.; Jeong, J.Y.; Babayan, S.E.; Park, J.; Selwyn, G.S.; Hicks, R.F. The Atmospheric-Pressure Plasma Jet: A Review and Comparison to Other Plasma Sources. IEEE Trans. Plasma Sci. 1998, 26, 1685–1694. [Google Scholar] [CrossRef]
- Bennett, C.; Ngamrung, S.; Ano, V.; Umongno, C.; Mahatheeranont, S.; Jakmunee, J.; Nisoa, M.; Leksakul, K.; Sawangrat, C.; Boonyawan, D. Comparison of Plasma Technology for the Study of Herbicide Degradation. RSC Adv. 2023, 13, 14078–14088. [Google Scholar] [CrossRef] [PubMed]
- Fridman, A. Physics and Applications of the Gliding Arc Discharge. In Proceedings of the 31st IEEE International Conference on Plasma Science, Baltimore, MD, USA, 1 July 2004. [Google Scholar] [CrossRef]
- Onyshchenko, I. Atmospheric Pressure Plasma Jet for Multipurpose Plasma Activation of Polymeric Substrates. Ph.D. Thesis, Ghent University, Gent, Belgium, 2019. [Google Scholar]
- Tucker, B.S.; Baker, P.A.; Xu, K.G.; Vohra, Y.K.; Thomas, V. Atmospheric pressure plasma jet: A facile method to modify the intimal surface of polymeric tubular conduits. J. Vac. Sci. Technol. A 2018, 36, 04F404. [Google Scholar] [CrossRef]
- Birania, S.; Attkan, A.K.; Kumar, S.; Kumar, N.; Singh, V.K. Cold Plasma in Food Processing and Preservation: A Review. J. Food Process Eng. 2022, 45, e14110. [Google Scholar] [CrossRef]
- Lee, H.J.; Jung, H.; Choe, W.; Ham, J.S.; Lee, J.H.; Jo, C. Inactivation of Listeria Monocytogenes on Agar and Processed Meat Surfaces by Atmospheric Pressure Plasma Jets. Food Microbiol. 2011, 28, 1468–1471. [Google Scholar] [CrossRef]
- Edelblute, C.M.; Malik, M.A.; Heller, L.C. Surface-Dependent Inactivation of Model Microorganisms with Shielded Sliding Plasma Discharges and Applied Air Flow. Bioelectrochemistry 2015, 103, 22–27. [Google Scholar] [CrossRef]
- Mok, C.; Song, D.-M. Low-Pressure Discharge Plasma Inactivation of Salmonella Typhymurium and Sanitation of Egg. Food Eng. Prog. 2013, 17, 245–250. [Google Scholar] [CrossRef]
- Liu, X.; Hong, F.; Guo, Y.; Zhang, J.; Shi, J. Sterilization of Staphylococcus Aureus by an Atmospheric Non-Thermal Plasma Jet. Plasma Sci. Technol. 2013, 15, 439–442. [Google Scholar] [CrossRef]
- Fricke, K.; Reuter, S.; Schroder, D.; Schulz-Von Der Gathen, V.; Weltmann, K.D.; Von Woedtke, T. Investigation of Surface Etching of Poly(Ether Ether Ketone) by Atmospheric-Pressure Plasmas. IEEE Trans. Plasma Sci. 2012, 40, 2900–2911. [Google Scholar] [CrossRef]
- Nishime, T.M.C.; Wagner, R.; Kostov, K.G. Study of Modified Area of Polymer Samples Exposed to a He Atmospheric Pressure Plasma Jet Using Different Treatment Conditions. Polymers 2020, 12, 1028. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K.G.; Nishime, T.M.C.; Castro, A.H.R.; Toth, A.; Hein, L.R.O. Surface Modification of Polymeric Materials by Cold Atmospheric Plasma Jet. Appl. Surf. Sci. 2014, 314, 367–375. [Google Scholar] [CrossRef]
- Cools, P.; Asadian, M.; Nicolaus, W.; Declercq, H.; Morent, R.; De Geyter, N. Surface Treatment of PEOT/PBT (55/45) with a Dielectric Barrier Discharge in Air, Helium, Argon and Nitrogen at Medium Pressure. Materials 2018, 11, 391. [Google Scholar] [CrossRef] [PubMed]
- Fricke, K.; Steffen, H.; Von Woedtke, T.; Schröder, K.; Weltmann, K.D. High Rate Etching of Polymers by Means of an Atmospheric Pressure Plasma Jet. Plasma Process. Polym. 2011, 8, 51–58. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Soltani Firouz, M.; Mohi-Alden, K.; Omid, M. A Critical Review on Intelligent and Active Packaging in the Food Industry: Research and Development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef]
- Matouk, Z.; Torriss, B.; Rincón, R.; Dorris, A.; Beck, S.; Berry, R.M.; Chaker, M. Functionalization of Cellulose Nanocrystal Films Using Non-Thermal Atmospheric–Pressure Plasmas. Appl. Surf. Sci. 2020, 511, 145566. [Google Scholar] [CrossRef]
- Vassallo, E.; Pedroni, M.; Aloisio, M.; Chen, H.; Firpo, G.; Pietralunga, S.M.; Ripamonti, D. Plasma Deposition to Improve Barrier Performance of Biodegradable and Recyclable Substrates Intended for Food Packaging. Plasma 2022, 5, 451–461. [Google Scholar] [CrossRef]
- Sheikhi, Z.; Hosseini, S.M.; Khani, M.R.; Farhoodi, M.; Abdolmaleki, K.; Shokri, B.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Treatment of Starch Films with a Glow Discharge Plasma in Air and O2 at Low Pressure. Food Sci. Technol. Int. 2021, 27, 276–285. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; O’Neill, L.; Tiwari, B.K.; Bourke, P.; Cullen, P.J. Characterization of Dielectric Barrier Discharge Atmospheric Air Cold Plasma Treated Gelatin Films. Food Packag. Shelf Life 2015, 6, 61–67. [Google Scholar] [CrossRef]
- Moosavi, M.H.; Khani, M.R.; Shokri, B.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Modifications of Protein-Based Films Using Cold Plasma. Int. J. Biol. Macromol. 2020, 142, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Ledari, S.A.; Milani, J.M.; Lanbar, F.S. Improving Gelatin-Based Emulsion Films with Cold Plasma Using Different Gases. Food Sci. Nutr. 2020, 8, 6487–6496. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, C.; Pandey, I.; Pandey, H.; Ramteke, P.W.; Pandey, A.C.; Mishra, S.B.; Patil, S. Electrospun Nanofibrous Scaffold as a Potential Carrier of Antimicrobial Therapeutics for Diabetic Wound Healing and Tissue Regeneration; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780323527279. [Google Scholar]
- Honarvar, Z.; Farhoodi, M.; Khani, M.R.; Mohammadi, A.; Shokri, B.; Ferdowsi, R.; Shojaee-Aliabadi, S. Application of Cold Plasma to Develop Carboxymethyl Cellulose-Coated Polypropylene Films Containing Essential Oil. Carbohydr. Polym. 2017, 176, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Sun, N.N.; Chau, C.F. Application of Corona Electrical Discharge Plasma on Modifying the Physicochemical Properties of Banana Starch Indigenous to Taiwan. J. Food Drug Anal. 2018, 26, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Farris, S.; Introzzi, L.; Biagioni, P.; Holz, T.; Schiraldi, A.; Piergiovanni, L. Wetting of Biopolymer Coatings: Contact Angle Kinetics and Image Analysis Investigation. Langmuir 2011, 27, 7563–7574. [Google Scholar] [CrossRef] [PubMed]
- Song, A.Y.; Oh, Y.A.; Roh, S.H.; Kim, J.H.; Min, S.C. Cold Oxygen Plasma Treatments for the Improvement of the Physicochemical and Biodegradable Properties of Polylactic Acid Films for Food Packaging. J. Food Sci. 2016, 81, E86–E96. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Guo, P.; Chen, Y.; Chen, G.Y.; Ji, H.; Ran, Y.; Li, S.H.; Chen, Y. Surface Modification via Atmospheric Cold Plasma (ACP): Improved Functional Properties and Characterization of Zein Film. Ind. Crops Prod. 2018, 115, 124–133. [Google Scholar] [CrossRef]
- Huhtamäki, T.; Tian, X.; Korhonen, J.T.; Ras, R.H.A. Surface-Wetting Characterization Using Contact-Angle Measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Gardner, D.J.; Shen, W. Contact Angles and Wettability of Cellulosic Surfaces: A Review of Proposed Mechanisms and Test Strategies. BioResources 2015, 10, 8657–8749. [Google Scholar] [CrossRef]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the Integrity of Natural Biopolymer Films Used in Food Packaging by Crosslinking Approach: A Review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef]
- Chen, G.; Dong, S.; Zhao, S.; Li, S.; Chen, Y. Improving Functional Properties of Zein Film via Compositing with Chitosan and Cold Plasma Treatment. Ind. Crops Prod. 2019, 129, 318–326. [Google Scholar] [CrossRef]
- Goiana, M.L.; de Brito, E.S.; Alves Filho, E.G.; Miguel, E.d.C.; Fernandes, F.A.N.; de Azeredo, H.M.C.; Rosa, M.d.F. Corn Starch Based Films Treated by Dielectric Barrier Discharge Plasma. Int. J. Biol. Macromol. 2021, 183, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Sheikhi, Z.; Mirmoghtadaie, L.; Khani, M.R.; Farhoodi, M.; Beikzadeh, S.; Abdolmaleki, K.; Kazemian-Bazkiaee, F.; Shokri, B.; Shojaee-Aliabadi, S. Physicochemical Characterization of Argon Plasma-Treated Starch Film. J. Agric. Sci. Technol. 2020, 22, 999–1008. [Google Scholar]
- Abdulkareem, A.; Kasak, P.; Nassr, M.G.; Mahmoud, A.A.; Al-Ruweidi, M.K.A.A.; Mohamoud, K.J.; Hussein, M.K.; Popelka, A. Surface Modification of Poly(Lactic Acid) Film via Cold Plasma Assisted Grafting of Fumaric and Ascorbic Acid. Polymers 2021, 13, 3717. [Google Scholar] [CrossRef]
- Romani, V.P.; Olsen, B.; Pinto Collares, M.; Meireles Oliveira, J.R.; Prentice-Hernández, C.; Guimarães Martins, V. Improvement of Fish Protein Films Properties for Food Packaging through Glow Discharge Plasma Application. Food Hydrocoll. 2019, 87, 970–976. [Google Scholar] [CrossRef]
- Oh, Y.A.; Roh, S.H.; Min, S.C. Cold Plasma Treatments for Improvement of the Applicability of Defatted Soybean Meal-Based Edible Film in Food Packaging. Food Hydrocoll. 2016, 58, 150–159. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; O’Neill, L.; Tiwari, B.K.; Bourke, P.; Cullen, P.J. Physicochemical Characterization of Plasma-Treated Sodium Caseinate Film. Food Res. Int. 2014, 66, 438–444. [Google Scholar] [CrossRef]
- Arolkar, G.A.; Salgo, M.J.; Kelkar-Mane, V.; Deshmukh, R.R. The Study of Air-Plasma Treatment on Corn Starch/Poly(ε-Caprolactone) Films. Polym. Degrad. Stab. 2015, 120, 262–272. [Google Scholar] [CrossRef]
- Ucar, Y.; Ceylan, Z.; Durmus, M.; Tomar, O.; Cetinkaya, T. Application of Cold Plasma Technology in the Food Industry and Its Combination with Other Emerging Technologies. Trends Food Sci. Technol. 2021, 114, 355–371. [Google Scholar] [CrossRef]
- Lee, K.T. Quality and Safety Aspects of Meat Products as Affected by Various Physical Manipulations of Packaging Materials. Meat Sci. 2010, 86, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Onaizi, S.A.; Leong, S.S.J. Tethering Antimicrobial Peptides: Current Status and Potential Challenges. Biotechnol. Adv. 2011, 29, 67–74. [Google Scholar] [CrossRef]
- Véronique, C.O.M.A. Bioactive Packaging Technologies for Extended Shelf Life of Meat-Based Products. Meat Sci. 2008, 78, 90–103. [Google Scholar] [CrossRef]
- Kerry, J.P.; O’Grady, M.N.; Hogan, S.A. Past, Current and Potential Utilisation of Active and Intelligent Packaging Systems for Meat and Muscle-Based Products: A Review. Meat Sci. 2006, 74, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.W.; Hou, C.Y.; Hsieh, C.C.; Chang, C.K.; Wu, Y.S.; Hsieh, C.W. Preparation of Antimicrobial Active Packaging Film by Capacitively Coupled Plasma Treatment. Lwt 2020, 117, 108612. [Google Scholar] [CrossRef]
- De Vietro, N.; Conte, A.; Incoronato, A.L.; Del Nobile, M.A.; Fracassi, F. Aerosol-Assisted Low Pressure Plasma Deposition of Antimicrobial Hybrid Organic-Inorganic Cu-Composite Thin Films for Food Packaging Applications. Innov. Food Sci. Emerg. Technol. 2017, 41, 130–134. [Google Scholar] [CrossRef]
- Goddard, J.M.; Hotchkiss, J.H. Polymer Surface Modification for the Attachment of Bioactive Compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Ceylan, H.G.; Atasoy, A.F. New Bioactive Edible Packing Systems: Synbiotic Edible Films/Coatings as Carries of Probiotics and Prebiotics. Food Bioprocess Technol. 2023, 16, 1413–1428. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Shafique, B.; Aadil, R.M.; Manzoor, M.F.; Cheng, J.H. Modification in Cellulose Films through Ascent Cold Plasma Treatment and Polymerization for Food Products Packaging. Trends Food Sci. Technol. 2023, 134, 162–176. [Google Scholar] [CrossRef]
- Rashvand, M.; Matera, A.; Altieri, G.; Genovese, F.; Nikzadfar, M.; Feyissa, A.H.; Renzo, G.C.; Carlo, G.; Renzo, D. Effect of Dielectric Barrier Discharge Cold Plasma on the Bio-Nanocomposite Film and Its Potential to Preserve the Quality of Strawberry under Modified Atmosphere Packaging. Res. Sq. 2023. preprint. [Google Scholar] [CrossRef]
- Rashvand, M.; Abbaszadeh, R. Effect of Cold Plasma on the Firmness of Olive Fruit in Packaging and Atmospheric Space. J. Packag. Technol. Res. 2019, 3, 253–259. [Google Scholar] [CrossRef]
- Glicerina, V.; Siroli, L.; Gottardi, D.; Ticchi, N.; Capelli, F.; Accorsi, R.; Gherardi, M.; Minelli, M.; Fiorini, M.; Andrisano, V.; et al. Influence of an Innovative, Biodegradable Active Packaging on the Quality of Sunflower Oil and “Pesto” Sauce during Storage. Appl. Food Res. 2023, 3, 100313. [Google Scholar] [CrossRef]
- Akhavan-Mahdavi, S.; Mirzazadeh, M.; Alam, Z.; Solaimanimehr, S. The Effect of Chitosan Coating Combined with Cold Plasma on the Quality and Safety of Pistachio during Storage. Food Sci. Nutr. 2023, 11, 4296–4307. [Google Scholar] [CrossRef] [PubMed]
- Tahsiri, Z.; Hedayati, S.; Niakousari, M. Improving the Functional Properties of Wild Almond Protein Isolate Films by Persian Gum and Cold Plasma Treatment. Int. J. Biol. Macromol. 2023, 229, 746–751. [Google Scholar] [CrossRef]
- Li, Z.; Deng, S.; Chen, J. Surface Modification via Dielectric Barrier Discharge Atmospheric Cold Plasma (DBD–ACP): Improved Functional Properties of Soy Protein Film. Foods 2022, 11, 1196. [Google Scholar] [CrossRef]
- da Fonseca de Albuquerque, M.D.; Bastos, D.C.; Ţălu, Ş.; Matos, R.S.; Pires, M.A.; Salerno, M.; da Fonseca Filho, H.D.; Simão, R.A. Vapor Barrier Properties of Cold Plasma Treated Corn Starch Films. Coatings 2022, 12, 1006. [Google Scholar] [CrossRef]
- Goiana, M.L.; Mattos, A.L.A.; de Azeredo, H.M.C.; de Freitas Rosa, M.; Fernandes, F.A.N. Influence of Dielectric Barrier Discharge Cold Plasma Treatment on Starch, Gelatin, and Bacterial Cellulose Biodegradable Polymeric Films. Polymers 2022, 14, 5215. [Google Scholar] [CrossRef] [PubMed]
- Yudhistira, B.; Sulaimana, A.S.; Punthi, F.; Chang, C.K.; Lung, C.T.; Santoso, S.P.; Gavahian, M.; Hsieh, C.W. Cold Plasma-Based Fabrication and Characterization of Active Films Containing Different Types of Myristica Fragrans Essential Oil Emulsion. Polymers 2022, 14, 1618. [Google Scholar] [CrossRef]
- Capelli, F.; Tappi, S.; Gritti, T.; De Aguiar Saldanha Pinheiro, A.C.; Laurita, R.; Tylewicz, U.; Spataro, F.; Braschi, G.; Lanciotti, R.; Galindo, F.G.; et al. Decontamination of Food Packages from SARS-CoV-2 RNA with a Cold Plasma-Assisted System. Appl. Sci. 2021, 11, 4177. [Google Scholar] [CrossRef]
- Sharmin, N.; Sone, I.; Walsh, J.L.; Sivertsvik, M.; Fernández, E.N. Effect of Citric Acid and Plasma Activated Water on the Functional Properties of Sodium Alginate for Potential Food Packaging Applications. Food Packag. Shelf Life 2021, 29, 100733. [Google Scholar] [CrossRef]
- Cui, H.; Yang, X.; Abdel-Samie, M.A.; Lin, L. Cold Plasma Treated Phlorotannin/Momordica Charantia Polysaccharide Nanofiber for Active Food Packaging. Carbohydr. Polym. 2020, 239, 116214. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Q.; Luo, Y.; Murad, M.S.; Zhu, L.; Mu, G. Improved Packing Performance and Structure-Stability of Casein Edible Films by Dielectric Barrier Discharges (DBD) Cold Plasma. Food Packag. Shelf Life 2020, 24, 100471. [Google Scholar] [CrossRef]
- Kim, J.H.; Mun, C.; Ma, J.; Park, S.G.; Lee, S.; Kim, C.S. Simple Fabrication of Transparent, Colorless, and Self-Disinfecting Polyethylene Terephthalate Film via Cold Plasma Treatment. Nanomaterials 2020, 10, 949. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, Y.; Jin, N.; Li, J.; Dong, S.; Li, S.; Zhang, Z.; Chen, Y. Zein Films with Porous Polylactic Acid Coatings via Cold Plasma Pre-Treatment. Ind. Crops Prod. 2020, 150, 112382. [Google Scholar] [CrossRef]
- Heidemann, H.M.; Dotto, M.E.R.; Laurindo, J.B.; Carciofi, B.A.M.; Costa, C. Cold Plasma Treatment to Improve the Adhesion of Cassava Starch Films onto PCL and PLA Surface. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123739. [Google Scholar] [CrossRef]
- Lin, L.; Liao, X.; Cui, H. Cold Plasma Treated Thyme Essential Oil/Silk Fibroin Nanofibers against Salmonella Typhimurium in Poultry Meat. Food Packag. Shelf Life 2019, 21, 100337. [Google Scholar] [CrossRef]
- Pillai, R.R.; Adhikari, K.R.; Gardner, S.; Sunilkumar, S.; Sanas, S.; Mohammad, H.; Thomas, V. Inkjet-printed plasma-functionalized polymer-based capacitive sensor for PAHs. Mater. Today Commun. 2023, 35, 105659. [Google Scholar] [CrossRef]
- Vijayan, V.M.; Walker, M.; Morris, J.J.; Thomas, V. Recent mitigation strategies in engineered healthcare materials towards antimicrobial applications. Curr. Opin. Biomed. Eng. 2022, 22, 100377. [Google Scholar] [CrossRef]
- Tucker, B.S.; Aliakbarshirazi, S.; Vijayan, V.M.; Thukkaram, M.; De Geyter, N.; Thomas, V. Nonthermal plasma processing for nanostructured biomaterials and tissue engineering scaffolds: A mini review. Curr. Opin. Biomed. Eng. 2021, 17, 100259. [Google Scholar] [CrossRef]
- Tucker, B.S.; Vijayan, V.M.; Vohra, Y.K.; Thomas, V. Novel magneto-plasma processing for enhanced modification of electrospun biomaterials. Mater. Lett. 2019, 250, 96–98. [Google Scholar] [CrossRef]
- Vijayan, V.M.; Tucker, B.S.; Baker, P.A.; Vohra, Y.K.; Thomas, V. Non-equilibrium hybrid organic plasma processing for superhydrophobic PTFE surface towards potential bio-interface applications. Colloids Surf B Biointerfaces 2019, 183, 110463. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.M.; Tucker, B.S.; Hwang, P.T.J.; Bobba, P.S.; Jun, H.-W.; Catledge, S.A.; Vohra, Y.K.; Thomas, V. Non-equilibrium organosilane plasma polymerization for modulating the surface of PTFE towards potential blood contact applications. J. Mater. Chem. B 2020, 8, 2814–2825. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.M.; Tucker, B.S.; Dimble, P.S.; Vohra, Y.K.; Thomas, V. Dusty-Plasma-Assisted Synthesis of Silica Nanoparticles for in Situ Surface Modification of 3D-Printed Polymer Scaffolds. ACS Appl. Nano Mater. 2020, 3, 7392–7396. [Google Scholar] [CrossRef]
- Vijayan, V.M.; Walker, M.; Pillai, R.R.; Moreno, G.H.; Vohra, Y.K.; Morris, J.J.; Thomas, V. Plasma Electroless Reduction: A Green Process for Designing Metallic Nanostructure Interfaces onto Polymeric Surfaces and 3D Scaffolds. ACS Appl. Mater. Interfaces 2022, 14, 25065–25079. [Google Scholar] [CrossRef] [PubMed]
- Tucker, B.S.; Surolia, R.; Baker, P.A.; Vohra, Y.; Antony, V.; Thomas, V. Low-Temperature Air Plasma Modification of Electrospun Soft Materials and Bio-interfaces. In TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings; Springer International Publishing: Cham, Switzerland, 2019; pp. 819–826. [Google Scholar]
- Bradford, J.P.; Tucker, B.; Hernandez-Moreno, G.; Charles, P.; Thomas, V. Low-temperature inductively coupled plasma as a method to promote biomineralization on 3D printed poly (lactic acid) scaffolds. J. Mater. Sci. 2021, 56, 14717–14728. [Google Scholar] [CrossRef]
- Bradford, J.P.; Hernandez-Moreno, G.; Pillai, R.R.; Hernandez-Nichols, A.L.; Thomas, V. Low-Temperature Plasmas Improving Chemical and Cellular Properties of Poly (Ether Ether Ketone) Biomaterial for Biomineralization. Materials 2023, 17, 171. [Google Scholar] [CrossRef]
- Karthik, C.; Sarngadharan, S.C.; Thomas, V. Low-Temperature Plasma Techniques in Biomedical Applications and Therapeutics: An Overview. Int. J. Mol. Sci. 2023, 25, 524. [Google Scholar] [CrossRef]
- Deslima, C.; Vinoy, V.; Renjith, P.; Vijayan, V. Application of low-temperature plasma treatment for rapid and efficient polydopamine coating on 3D-printed polymer scaffolds. MRS Commun. 2023, 13, 1163–1170. [Google Scholar] [CrossRef]

| CAP Generation Methods | Schematic Diagram | Features | Reference |
|---|---|---|---|
| Dielectric Barrier Discharge (DBD) Plasma | 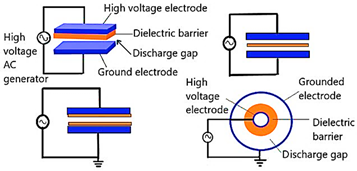 | Planar or cylindrical consist of an insulating electrode and a grounded electrode. The DBDs are scalable, efficient, and have short processing times [10,11]. They also consume less energy. The high ignition voltage and the narrow discharge gap height, which are both connected with plasma homogeneity, are the main downsides, though. | Reprinted from [10] under under the terms of the Creative Commons CC-BY license and copyright permission from Elsevier |
| Corona Discharge Plasma | 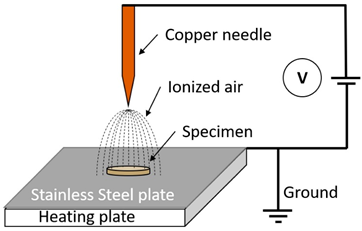 | A runnel of charged particles, including ions and electrons, is called a corona, and it is accelerated by an electric field. It is created when a gaseous space gap, such as one containing air or another gas, is exposed to a voltage high enough to cause a series of high-velocity particle collisions with neutral molecules, leading to the creation of more ions. One of the noticeable advantages of corona discharge is the energy consumption to breakdown the gas is very low. Also, since the supply voltage is very less than DBD, it is very safe to use. But compared to the DBD plasma, overall, the processing effect of corona discharge is very weak, and the concentration of charge can cause electron damage soon [12,13]. | Reprinted from [12], under under the terms of the Creative Commons CC-BY license |
| Atmospheric Pressure Plasma (APP) | 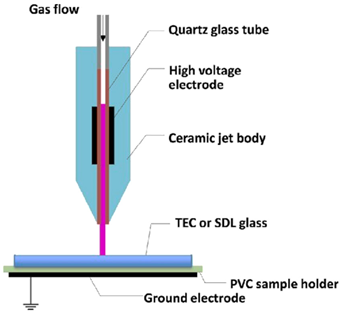 | A neutral gas under an electrical field is the primary source to produce the APP. A gas is excited using direct current or alternating current at frequencies varying from low to several GHz while it is under atmospheric pressure. For plasma creation, the chosen plasma gas is often accomplished between two plates. Continuous treatments can be done using APP. So, this process is very cost effective and strong. The initial setup for the process is not economical [14,15]. | Reprinted from [14] under under the terms of the Creative Commons CC-BY license |
| Gliding Arc Discharge Plasma |  | An atmospheric pressure arc discharge known as a “gliding arc” can produce very high levels of electron density, current, and power as well as a relatively low temperature and an enhanced electric field, which are common of cold atmospheric plasmas. Improved damping ratio is one of the important advantages of gliding arc discharge plasma [16,17]. In some cases, severe overheating can be a potential drawback of this method. This can cause heating of the medium | Reprinted from [16] under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence. |
| Radio Frequency (RF) discharge Plasma | 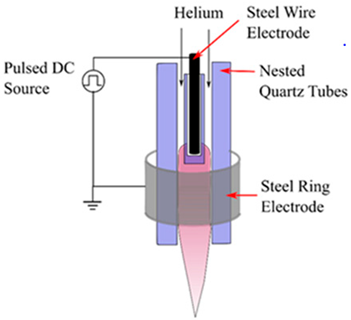 | A needle electrode’s tip generates plasma, which spreads outside the ceramic nozzle to a grounded ring electrode attached to the two RF voltage electrodes (of which a frequency of 13.56 MHz is typically utilized). The spatial spread of RF plasma is not constrained by electrodes. It will prevent metallic vapours from contaminating the plasma. RF plasmas are widely recognized for producing plasmas with high electron densities [18]. | Reprinted from [19] |
| No. | Plasma Treatment Conditions | Matrix and Fillers; Composite Type | Applications | Effect of Plasma Treatment on Properties | References |
|---|---|---|---|---|---|
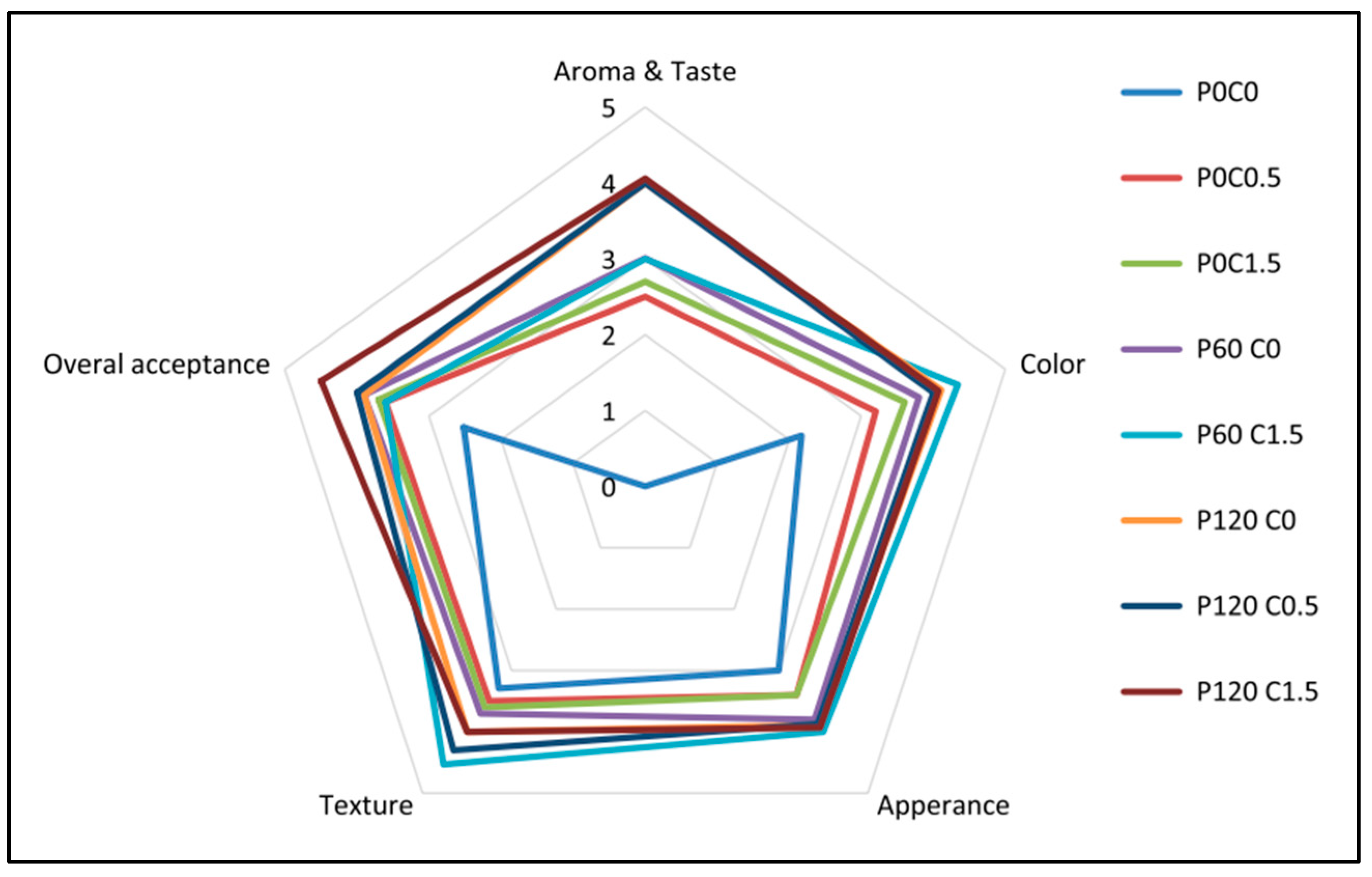
| 1. | Dielectric barrier discharge (DBD) cold plasma, for 5, 10 and 15 min. Maximum transmission power: 50 W; Voltage: 15 kV; Current: 10 mA; Frequency: 50 kHz; Power source: DC pulse type with pulse width modulation (PWM) | Chitosan + cellulose nanoparticles; Films | Packaging of strawberry | For films: Improved mechanical properties (TS & EAB), water vapour permeability, oxygen transmission rate, moisture content and water contact angle. For substrates: Enhanced mechanical properties (firmness and Young’s modulus), chemical attributes (pH, soluble solid content and total ascorbic acid), physical characteristics (weight loss and colour features), microbial activities (bacteria, yeast and mould) | [65] |
| 2. | Open-air DBD cold plasma. Peak voltage: 20 kV; Frequency: 20 kHz | Polylactic acid multilayer films | Active packaging of sunflower oil and “pesto” sauce; Biodegradable multilayer active packaging, to extend food products shelf-life and/or maintain high quality levels of oily foods during storage. | Immobilization of oxygen scavenger agent (ascorbic acid); Decreased oxidation kinetics; Better and more stable quality characteristics in terms of colorimetric, microbiological and textural parameters | [67] |
| 3. | DBD cold plasma, for 60 and 120 s. Gas source: Air; Argon gas type, oxygen gas pressure of 0.4 millibars equivalent to 0.3 Torr and power of 89 watts equivalent to radiometric waves | Chitosan solution | Preservation of quality and safety (shelf life) of pistachios during storage | Significant reduction in the amount of aflatoxin, mold and yeast after 120 days; Physicochemical characteristics of pistachios did not change significantly; No adverse effect on the sensory characteristics of pistachios | [68] |
| 4. | Atmospheric air cold plasma treatment for 5, 10 and 15 min in the excitation mode. Input voltage: 6.2 kV; Power level: 60 kW; Pulse frequency: 10 kHz | Wild almond protein isolate (WAPI) + Persian gum (PG); Films | Edible films in food packaging | Progressively improved mechanical properties (increased thickness, TS and EAB); No significant effect on WVP and solubility; Surface roughness directly proportional to plasma treatment time, but surface remained integrated; Best results obtained for films with 10 min treatment; Properties tend to deteriorate after 15 min treatment | [69] |
| 5. | Dielectric Barrier Discharge Atmospheric Cold Plasma (DBD–ACP); Fixed exposure time (3 min) with varying voltages of 10, 20, 30, 40, and 50 kV; Fixed voltage (30 kV) with varying exposure times (1, 2, 3, 4, 5 min) | Soy protein films | Edible packaging and food preservation | Increased water interactive properties and thermostability; Decreased surface roughness; Effects of different ACP treatment times too | [70] |
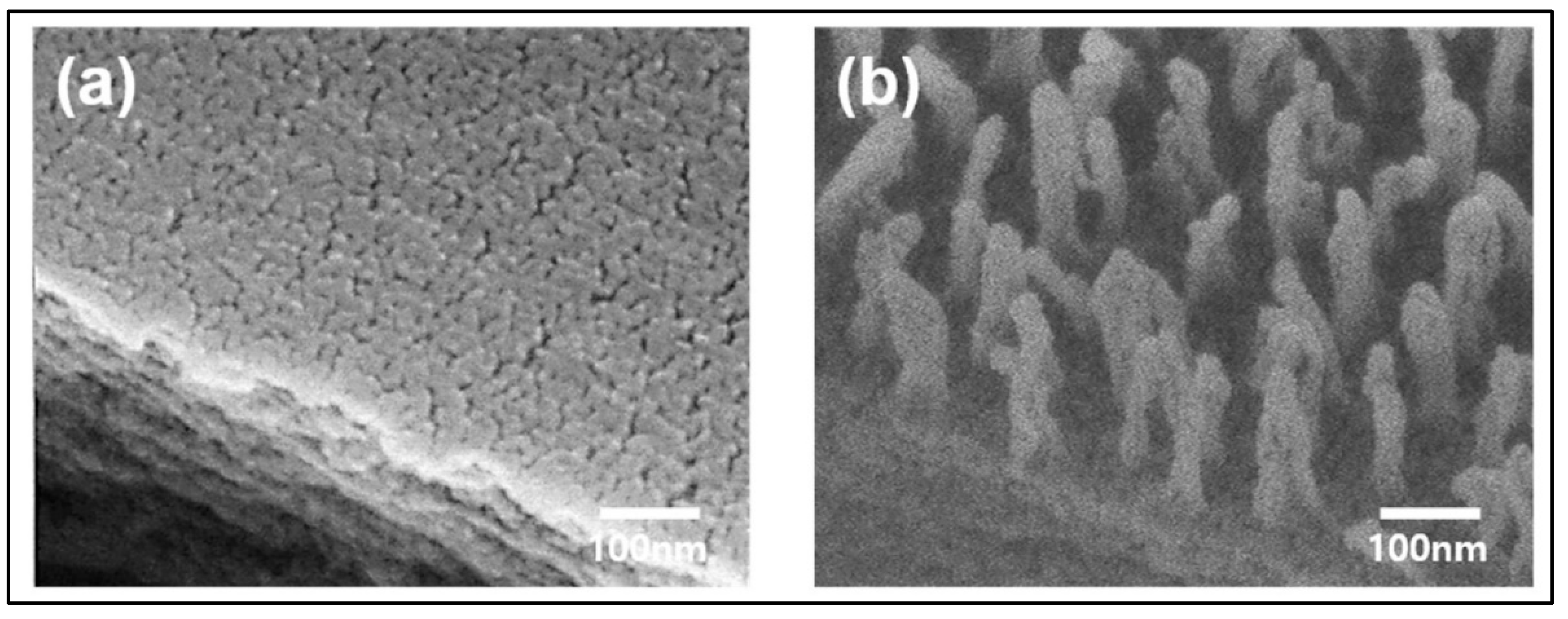
| 6. | Cold plasma based on helium. Glow discharge reactor at 13.56 MHz. Chamber vacuum: <8 Pa. Treatment with He: Self-bias voltage −100 V; Treatment time: 10 min. Treatment with HMDSO: Self-bias voltage −60 V; Treatment time: 20 min | Hexamethyldisilox-ane (HMDSO) treated extruded corn starch films | Barrier films for food packaging and pharmaceutical products | More homogeneous coating and smaller granules; Increased hydrophobicity, but roughness created by helium plasma was not effective in increasing the water contact angle of the modified surface; No much effect on water vapour permeation; Significant reduction in absorbed water content, mostly due to the formation of a barrier to water absorption of around 80%; Physical barrier to water, while allowing permeation to water vapour | [71] |
| 7. | DBD cold plasma treatment. Voltage: 20 kV; Excitation frequencies: 50, 400 and 900 Hz; Treatment time: 5 min | Starch, gelatin and bacterial cellulose films | Sustainable and biodegradable alternatives for plastic packaging | Improved hydrophobicity, surface morphology, tensile strength, and elasticity module; Reduced water solubility; Pronounced changes for starch films at low excitation frequency (50 Hz) of plasma, and for gelatin and bacterial cellulose films at high excitation frequency (900 Hz) | [72] |
| 8. | Cold plasma treatment. Vacuum plasma reactor. Frequency: 13.56 MHz; Pressure: 0.0643 Torr; Power: 30 W; Treatment time: 60 s | LDPE + Myristica fragrans Essential Oil (MFEO); Films | Active food packaging material | Cold plasma treatment improved the properties of LDPE films by facilitating MFEO coating | [73] |
| 9. | Surface dielectric barrier discharge (SDBD) plasma from Plasma Assisted Sanitation System (PASS) for 5 and 10 min. Gas: Environmental air; Relative humidity: 20–40%; Voltage: 1–20 kV; Frequency: 1–20 kHz; Tunable duty cycle: 1–100%. Imposed voltage: 6 kV; Frequency: 5 kHz; Fixed duty cycle: 100% | Polyethylene terephthalate (PET) trays (350 microns thick) and polypropylene (PP) film (69 microns thick) | Newly developed plasma sanitation system for food packaging decontamination from SARS-CoV-2 RNA | Plasma treatment decontaminated virus, without significantly affecting the properties of packaging and food substrate; 5-min treatment reduced detected RNA for both surfaces, but to different extents. Indicated that interaction between reactive species and viral genetic material is affected by the matrix; 10-min treatment completely degraded RNA molecules from both surfaces | [74] |
| 10. | Plasma activated water (PAW) produced using surface barrier discharge (SBD) sourced high voltage cold plasma (CP). Sinusoidal signal frequency: 18 kHz; Atmospheric pressure; Plasma-inducing gas: Room air | Sodium alginate films | Food packaging | Increased TS, tensile modulus, EAB, LVE region and storage modulus; No intersection between G′ & G″; Showed shear thinning properties or non-Newtonian behaviour; decreased WVTR | [75] |
| 11. | Cold plasma treatment. Treatment time: 30 s; Power: 350 W; Nitrogen flow rate: 100 standard cubic centimeters/min (sccm) | Momordica charantia polysaccharide (MCP) nanofibre + Phlorotannin (PT); Electrospun nanofibre membranes | Active food packaging | Increased release efficiency of PT, resulting in an increase in antibacterial and anti-oxidant activities, without the alteration of chemical structure | [76] |
| 12. | DBD cold plasma. Voltage changed group adjusted at a changed treatment of 0, 30, 40, 50, 60 and 70 V under the duration of 60 s. Time changed group subjected to a sustaining time of 0, 15, 30, 45, 60, 90 and 120 s under the voltage of 50 V; Current: 2 ± 0.2 A | Casein edible films | Packaging material | Crystalloid migration and casein aggregation (via SEM) leading to reinforcement of structure stability; Slight change in crystal structure (via XRD); Stable state of molecular structure (via FTIR); Remarkable improvement in packing characters (including mechanical and barrier properties); Slight modifications of colour and transparency; Rearrangement in order of protein chains | [77] |
| 13. | Carbon tetrafluoride (CF4) reactive-ion etching (RIE) using 13.56 MHz radio-frequency plasma equipment. Flow rate: 3 sccm; Working pressure: 3.0 × 10−2 Torr; Treatment time: 4 min; Power: 100 W | Transparent, colourless and self-disinfecting polyethylene terephthalate (PET) film that mimics the surface structure of Progomphus obscurus (sand dragon) wing, physically killing the attached bacteria | Antibacterial overcoating with good optical properties for contactable surfaces in private and public interior spaces and packaging applications | Introduction of nanopillars; Improved optical properties (transparency and colourlessness); Notable enhancement in antibacterial activity against S. aureus and E. coli by activating or strengthening physical biocidal action | [78] |
| 14. | Cold plasma (CP) generated by dielectric barrier discharges (DBD) plasma reactor. Voltage: 60 V; Current: 1.5 A. Short-term treatment time: 60 s; Long-term treatment time: 120 s | CP pre-treated zein films + Porous PLA layer coating by breath figure self-assembly | Biodegradable packaging | Better-ordered porous structure after coating with PLA; Induced compatibility between zein and PLA molecules, by changing the protein conformation and by enhancing the intermolecular hydrogen bonding interactions; Significant improvement in surface hydrophobicity, fracture resistance, water vapor barrier, and thermal stability; Improved UV barrier and excellent biodegradability; Potential to enhance adhesion and improve functionalities of porous coating on other biopolymer materials | [79] |
| 15. | DBD atmospheric air cold plasma (at ambient temperature and atmospheric pressure). Plasma discharge frequency: 50 Hz; Voltage: 31 kV; Treatment time: 1, 5, 10, 15 and 20 min | Polycaprolactone (PCL) or poly(lactic acid) (PLA) and cassava starch multilayers | Multilayer packaging materials | Increased hydrophilicity and surface roughness; Improved adhesion between layers, zeta potential, delamination resistance, etc. | [80] |
| 16. | Cold plasma treatment. Power: 400 W; Treatment time: 4 min; Nitrogen flow rate: 100 sccm | Silk fibroin nanofibers + Cold plasma treated thyme essential oil (TO) composite films, post-treated with cold plasma | Effective antimicrobial packaging to increase shelf life of foods | Increased antibacterial activity by increasing TO release amount, due to surface modification, but without affecting chemical composition of the films; Decreased number of Salmonella Typhimurium in chicken and duck meat | [81] |
| 17. | DBD-50 cold plasma reactor. Power: 100 W; Treatment time: 30, 60, 90, 120 and 150 s | Zein + Chitosan films | Food and pharmaceutical packaging materials | Improved wettability, TS, EAB, water vapour barrier and thermal stability; Secondary structure of zein molecules became ordered; Rougher surface morphology, increased surface free energy and enhanced hydrogen bond interactions between zein and chitosan after plasma treatment (optimum range: 60–90 s) | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karthik, C.; Mavelil-Sam, R.; Thomas, S.; Thomas, V. Cold Plasma Technology Based Eco-Friendly Food Packaging Biomaterials. Polymers 2024, 16, 230. https://doi.org/10.3390/polym16020230
Karthik C, Mavelil-Sam R, Thomas S, Thomas V. Cold Plasma Technology Based Eco-Friendly Food Packaging Biomaterials. Polymers. 2024; 16(2):230. https://doi.org/10.3390/polym16020230
Chicago/Turabian StyleKarthik, Chandrima, Rubie Mavelil-Sam, Sabu Thomas, and Vinoy Thomas. 2024. "Cold Plasma Technology Based Eco-Friendly Food Packaging Biomaterials" Polymers 16, no. 2: 230. https://doi.org/10.3390/polym16020230
APA StyleKarthik, C., Mavelil-Sam, R., Thomas, S., & Thomas, V. (2024). Cold Plasma Technology Based Eco-Friendly Food Packaging Biomaterials. Polymers, 16(2), 230. https://doi.org/10.3390/polym16020230








