Synthesis of a Novel Zwitterionic Hypercrosslinked Polymer for Highly Efficient Iodine Capture from Water
Abstract
1. Introduction
2. Experimental Section
2.1. General Remarks
2.1.1. Materials
2.1.2. Characterization
2.2. Synthesis of Materials
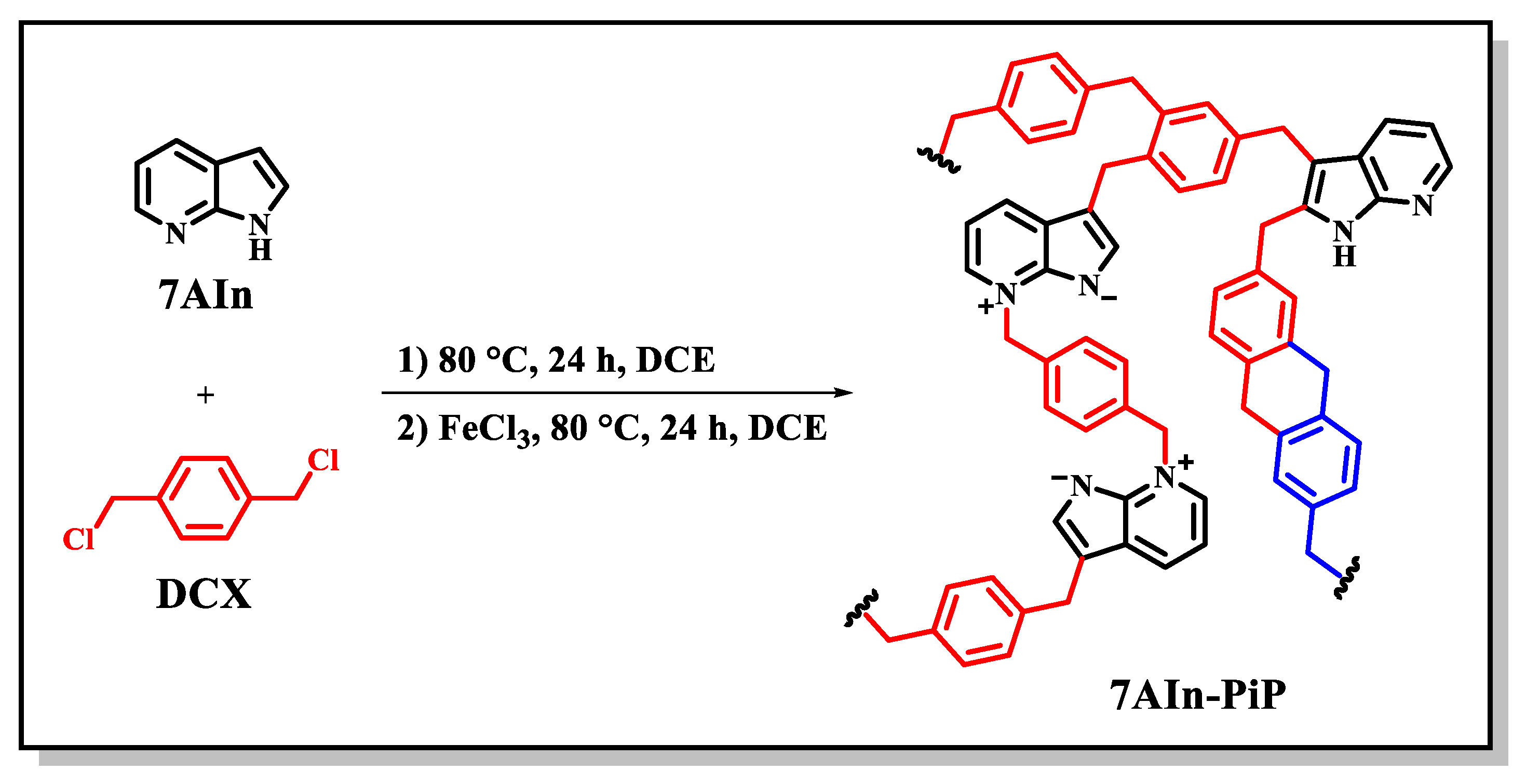
2.2.1. Synthesis of 7AIn-PiP
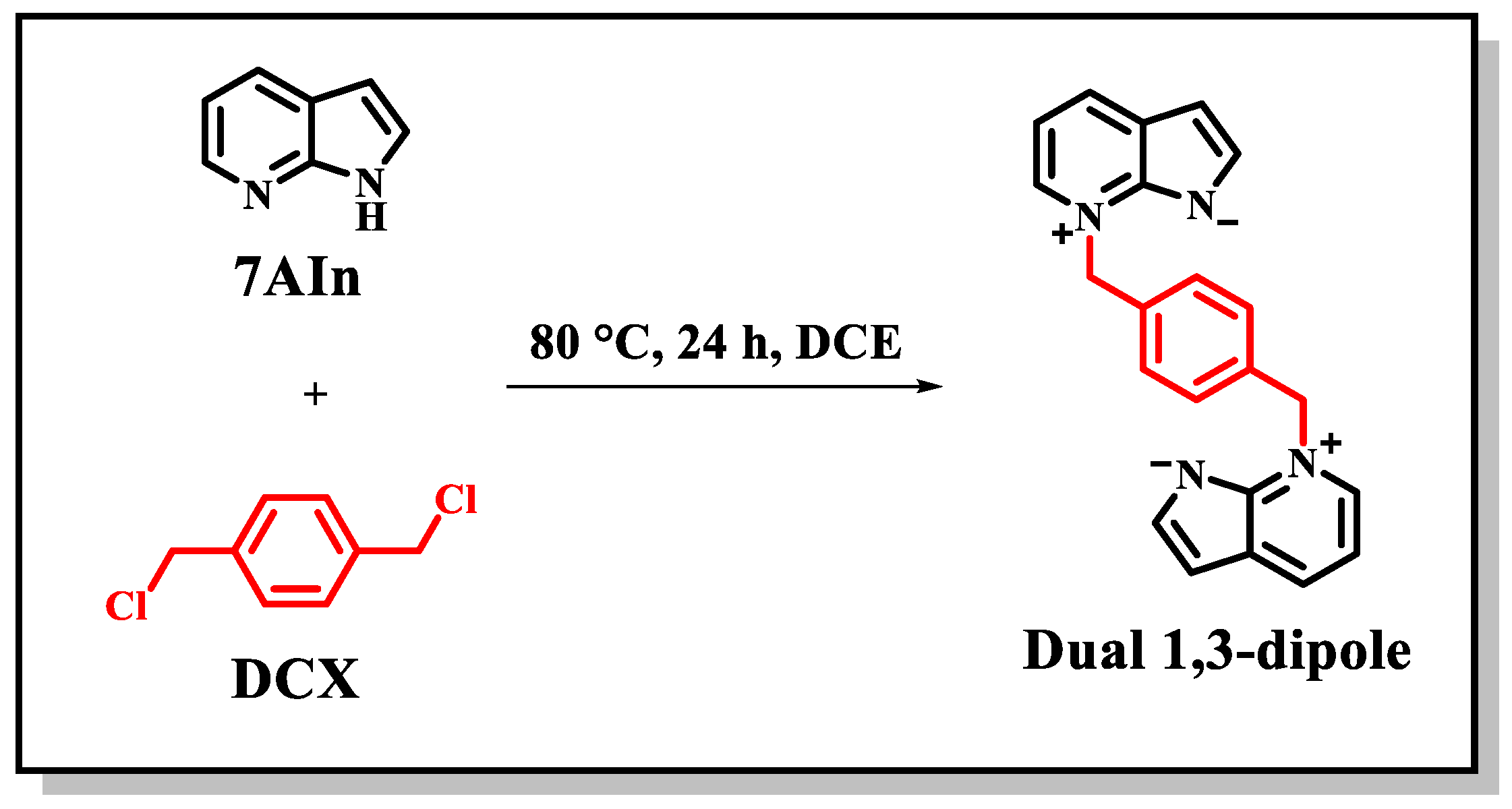
2.2.2. Synthesis of the Dual 1,3-Dipole
2.3. I2 Adsorption Experiments of 7AIn-PiP in I2 Aqueous Solution
2.3.1. I2 Adsorption Experiments of 7AIn-PiP in Saturated I2 Aqueous Solution
2.3.2. Recycling Tests of 7AIn-PiP in Saturated I2 Aqueous Solution
2.3.3. I2 Adsorption Experiments of 7AIn-PiP in Unsaturated I2 Aqueous Solution
3. Results and Discussion
3.1. Characterization and Analysis of Materials
3.1.1. Pore Properties and I2 Adsorption Results of a Batch of Polymers
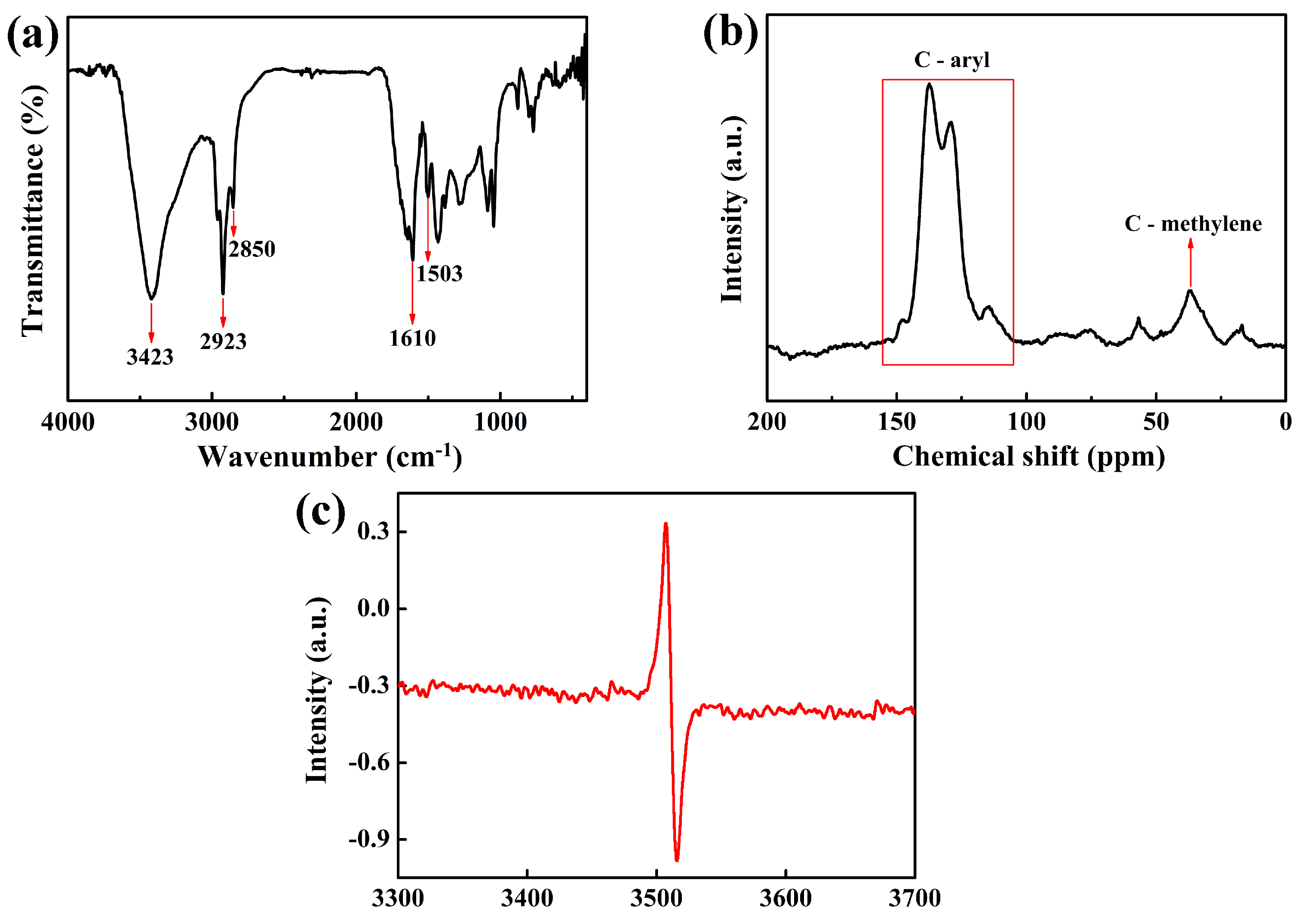
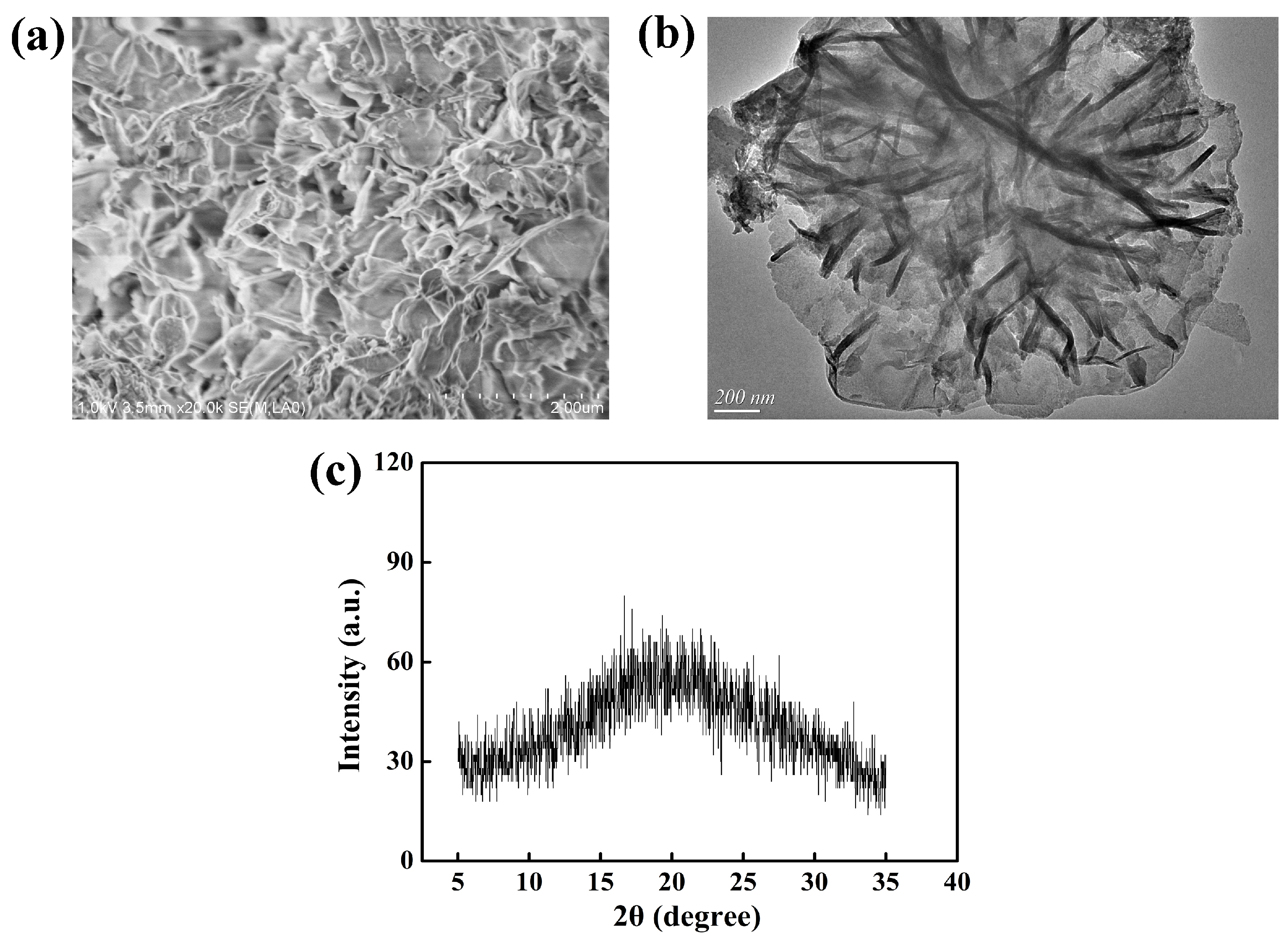
3.1.2. Characterization of 7AIn-PiP
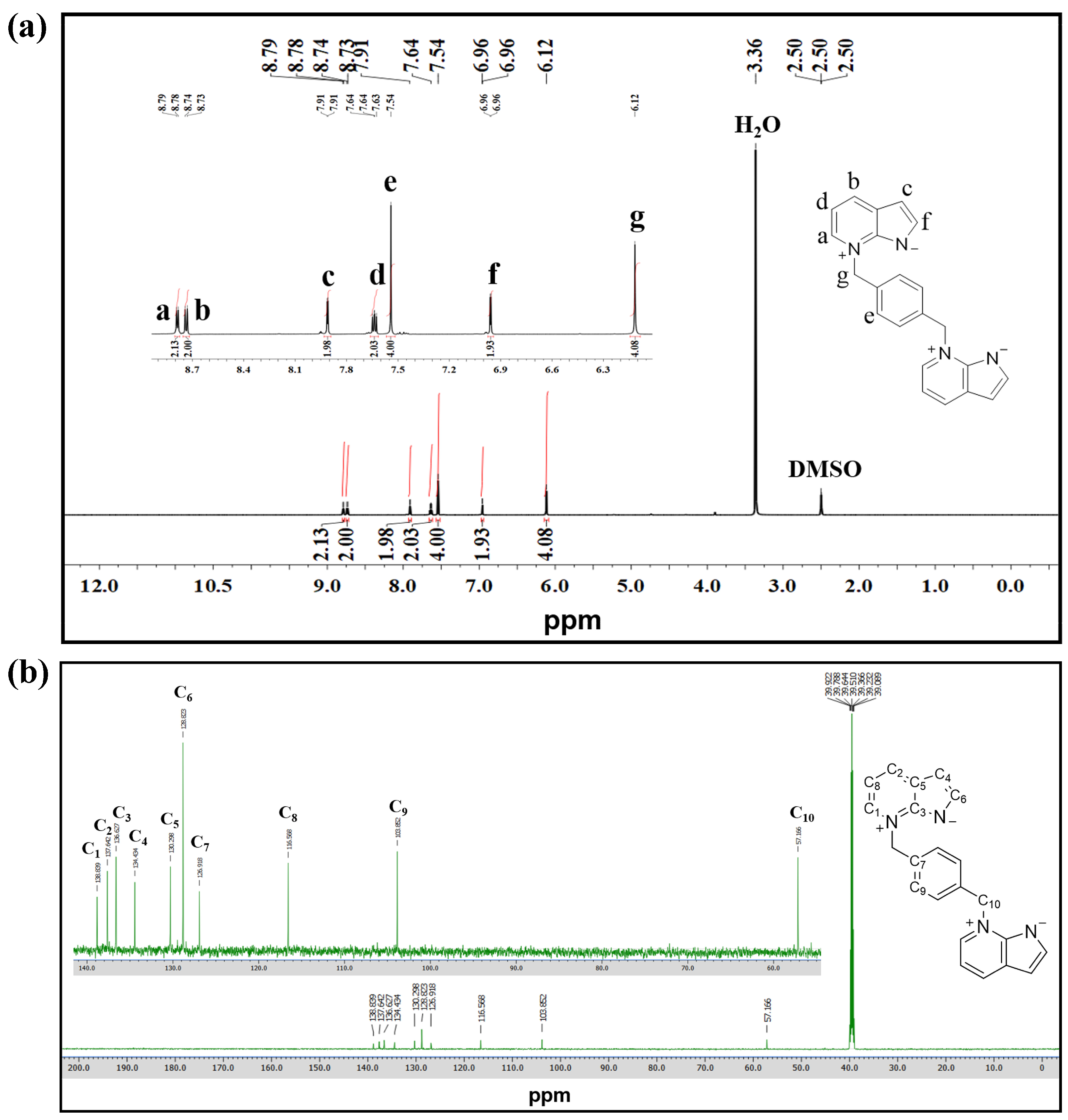
3.1.3. Characterization of the Dual 1,3-Dipole
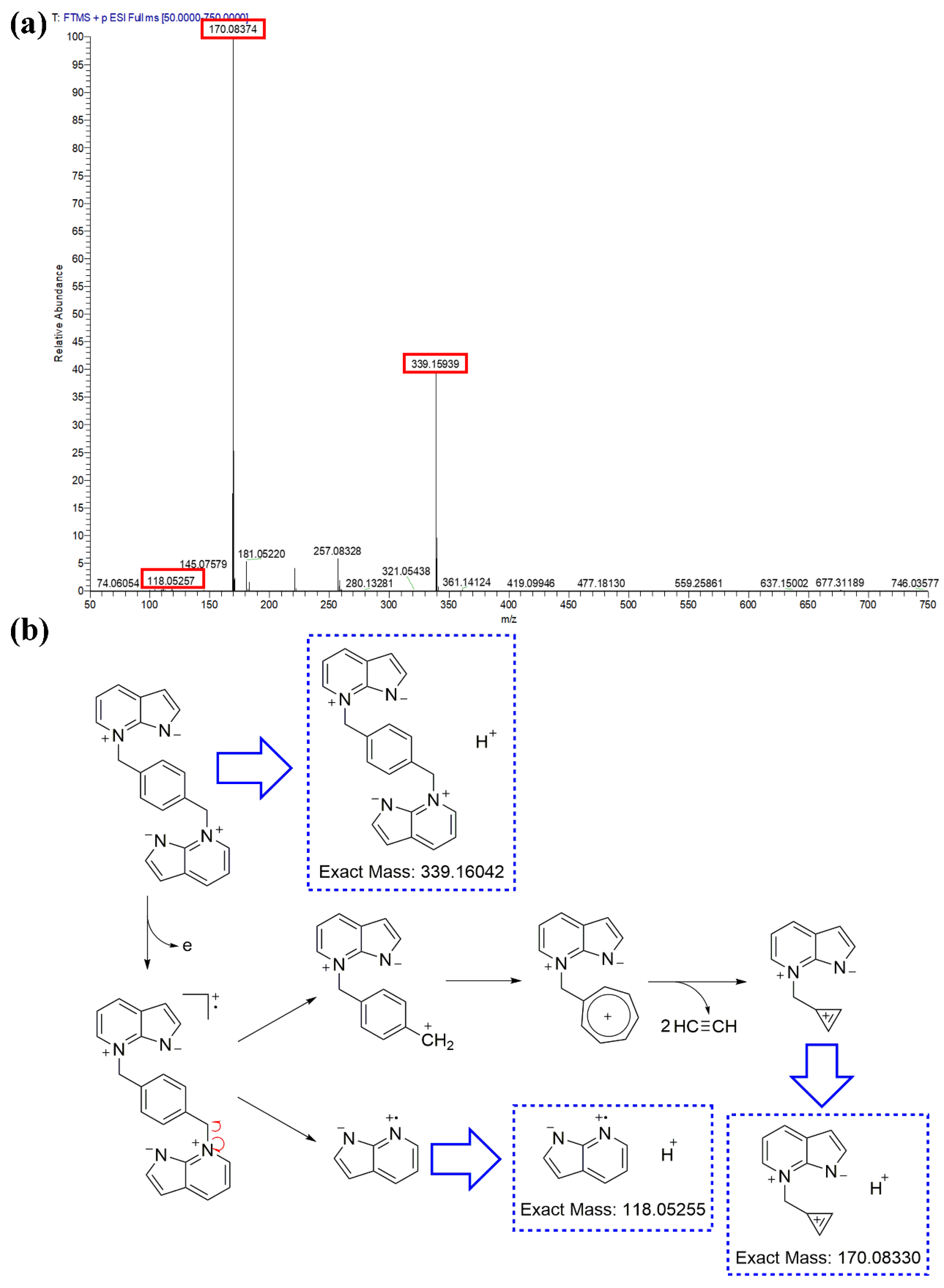
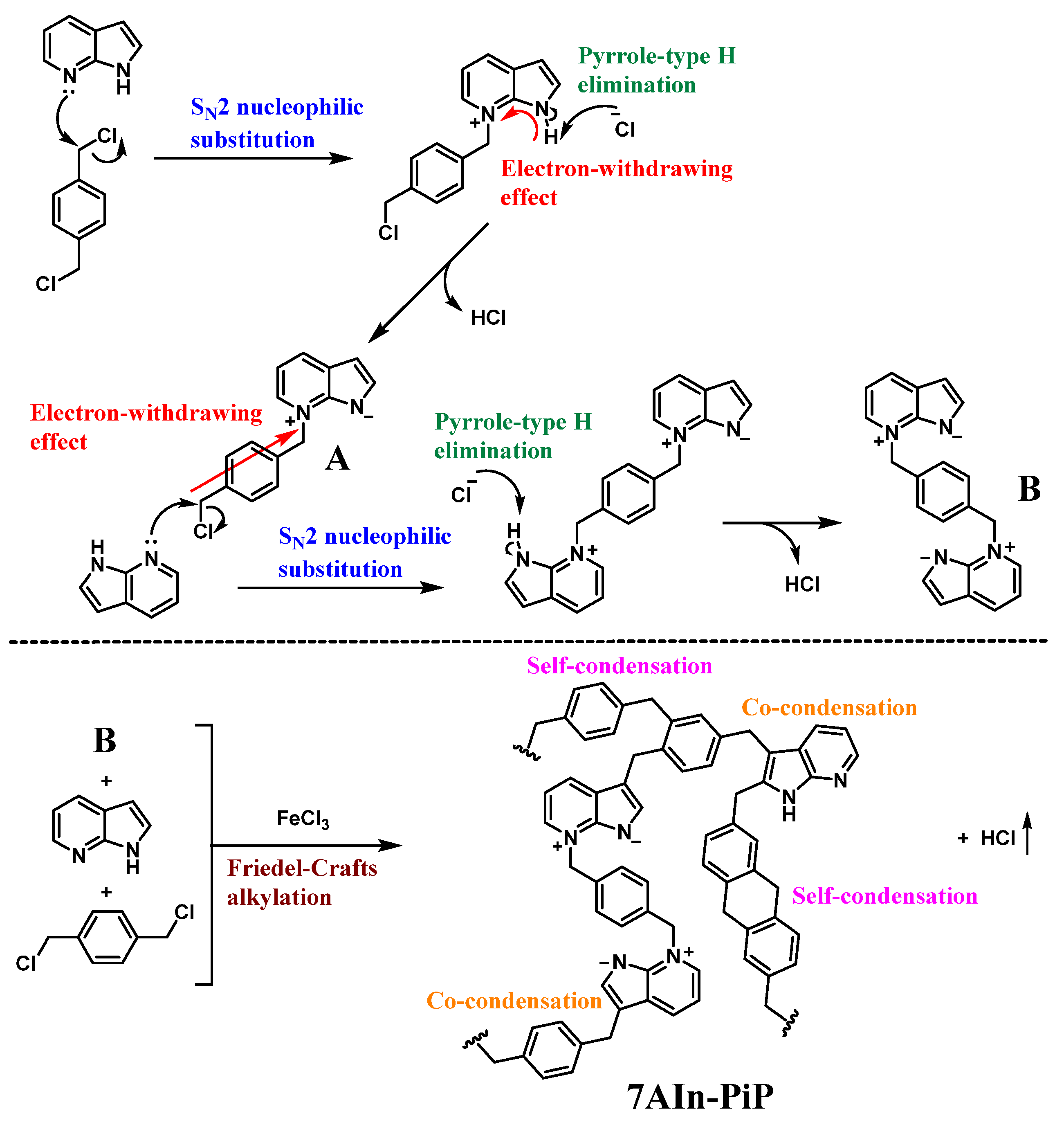
3.1.4. Formation Mechanism of the Dual 1,3-Dipole and Polymer 7AIn-PiP
3.2. I2 Adsorption Property of 7AIn-PiP
3.2.1. Evaluation of I2 Adsorption Property of 7AIn-PiP in Saturated I2 Aqueous Solution
3.2.2. Analysis of Adsorption Kinetics of 7AIn-PiP in Saturated I2 Aqueous Solution
3.2.3. Characterization of I2@7AIn-PiP
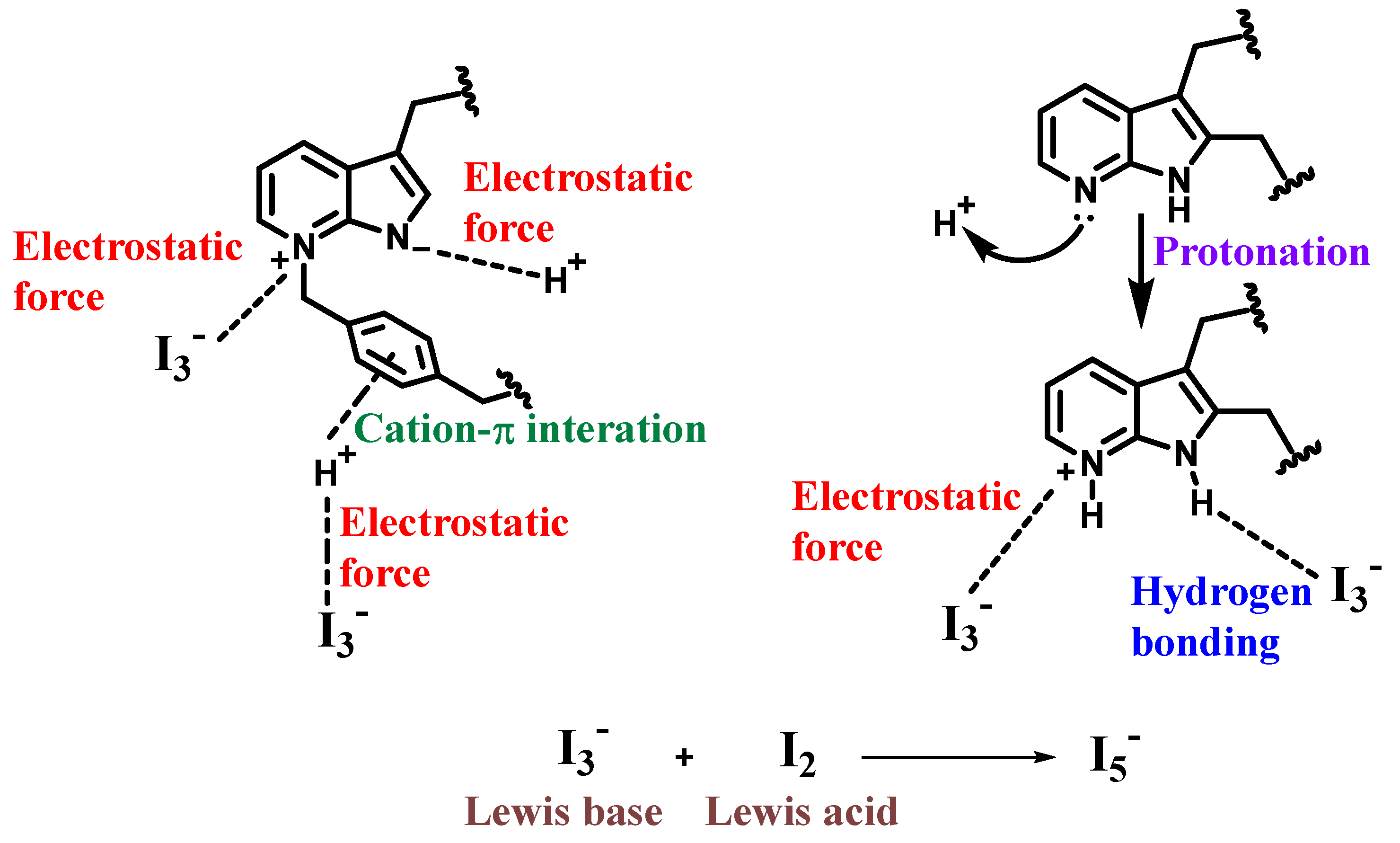
3.2.4. Possible I2 Adsorption Mechanism of 7AIn-PiP in Saturated I2 Aqueous Solution
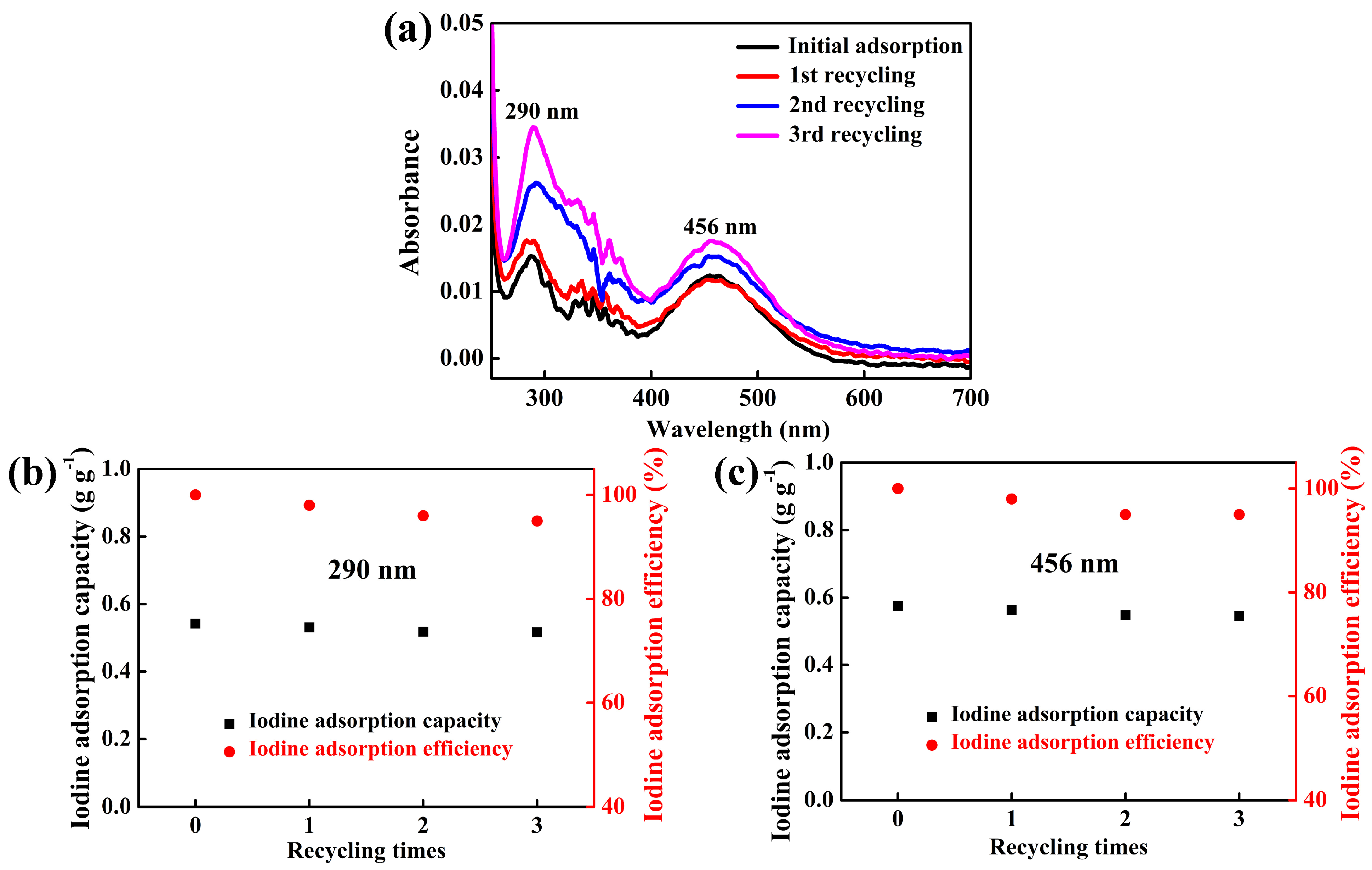
3.2.5. Regeneration Property of 7AIn-PiP in Saturated I2 Aqueous Solution
3.2.6. New Adsorption Phenomenon of 7AIn-PiP in Unsaturated I2 Aqueous Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, D.Y.; Zhou, Q.F.; Yin, Y.G.; Lu, D.W.; Hu, L.G.; Richmond, R.H.; Moon, H.B.; Yan, B.; Jiang, G.B. Implications of Fukushima’s radioactive water discharge on global environmental sustainability. Environ. Sci. Technol. 2024, 58, 3061–3064. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Jang, K.B.; Woo, T.H. Analysis of purification in radioactively contaminated ocean water near the Fukushima nuclear accident site. Energy. Sci. Eng. 2023, 11, 3310–3316. [Google Scholar] [CrossRef]
- Zhou, W.N.; Lavendomme, R.; Zhang, D.W. Recent progress in iodine capture by macrocycles and cages. Chem. Commun. 2024, 60, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Cui, D.; Zhang, S.R.; Xu, Y.H.; Jiang, D.L. Iodine capture in porous organic polymers and metal-organic frameworks materials. Mater. Horiz. 2019, 6, 1571–1595. [Google Scholar] [CrossRef]
- Yu, Y.N.; Yin, Z.; Cao, L.H.; Ma, Y.M. Organic porous solid as promising iodine capture materials. J. Incl. Phenom. Macrocycl. Chem. 2022, 102, 395–427. [Google Scholar] [CrossRef]
- Sun, Q.; Aguila, B.; Ma, S.Q. Opportunities of porous organic polymers for radionuclide sequestration. Trends Chem. 2019, 1, 292–303. [Google Scholar] [CrossRef]
- Zheng, Z.Y.; Lin, Q.Y.; Gao, Y.; Ji, X.F.; Sessler, J.L.; Wang, H.Y. Recent advances in iodine adsorption from water. Coordin. Chem. Rev. 2024, 511, 215860. [Google Scholar] [CrossRef]
- Sun, J.K.; Antonietti, M.; Yuan, J.Y. Nanoporous ionic organic networks: From synthesis to materials applications. Chem. Soc. Rev. 2016, 45, 6627–6656. [Google Scholar] [CrossRef]
- Xu, D.; Guo, J.N.; Yan, F. Porous ionic polymers: Design, synthesis, and applications. Prog. Polym. Sci. 2018, 79, 121–143. [Google Scholar] [CrossRef]
- Zhang, P.H.; Wang, Z.F.; Cheng, P.; Chen, Y.; Zhang, Z.J. Design and application of ionic covalent organic frameworks. Coordin. Chem. Rev. 2021, 438, 213873. [Google Scholar] [CrossRef]
- Chen, D.Y.; Ma, T.T.; Zhao, X.Y.; Jing, X.F.; Zhao, R.; Zhu, G.S. Multi-functionalization integration into the electrospun nanofibers exhibiting effective iodine capture from water. ACS Appl. Mater. Interfaces 2022, 14, 47126–47135. [Google Scholar] [CrossRef]
- Sen, A.; Sharma, S.; Dutta, S.; Shirolkar, M.M.; Dam, G.K.; Let, S.; Ghosh, S.K. Functionalized ionic porous organic polymers exhibiting high iodine uptake from both the vapor and aqueous medium. ACS Appl. Mater. Interfaces 2021, 13, 34188–34196. [Google Scholar] [CrossRef] [PubMed]
- Jie, K.C.; Chen, H.; Zhang, P.F.; Guo, W.; Li, M.J.; Yang, Z.Z.; Dai, S. A benzoquinone-derived porous hydrophenazine framework for efficient and reversible iodine capture. Chem. Commun. 2018, 54, 12706–12709. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, A.M.; Zhou, M.; Xu, Y.Y.; Zhang, Y.; He, Q. Nonporous amorphous super adsorbents for highly effective and selective adsorption of iodine in water. Nat. Commun. 2023, 14, 5388. [Google Scholar] [CrossRef] [PubMed]
- Avais, M.; Chattopadhyay, S. Porous polyaminoamides via an exotemplate synthesis approach for ultrahigh multimedia iodine adsorption. J. Mater. Chem. A 2022, 10, 20090–20100. [Google Scholar] [CrossRef]
- Li, X.M.; Chen, G.; Jia, Q. One-pot synthesis of viologen-based hypercrosslinked polymers for efficient volatile iodine capture. Micropor. Mesopor. Mat. 2019, 279, 186–192. [Google Scholar] [CrossRef]
- Raja, A.A.; Yavuz, C.T. Charge induced formation of crystalline network polymers. RSC Adv. 2014, 4, 59779–59784. [Google Scholar] [CrossRef]
- Zhang, P.F.; Qiao, Z.A.; Jiang, X.G.; Veith, G.M.; Dai, S. Nanoporous ionic organic networks: Stabilizing and supporting gold nanoparticles for catalysis. Nano. Lett. 2015, 15, 823–828. [Google Scholar] [CrossRef]
- Chen, G.J.; Zhou, Y.; Wang, X.C.; Li, J.; Xue, S.; Liu, Y.Q.; Wang, Q.; Wang, J. Construction of porous cationic frameworks by crosslinking polyhedral oligomeric silsesquioxane units with N-heterocyclic linkers. Sci. Rep. 2015, 5, 11236. [Google Scholar] [CrossRef]
- Kim, K.; Buyukcakir, O.; Coskun, A. Diazapyrenium-based porous cationic polymers for colorimetric amine sensing and capture from CO2 scrubbing conditions. RSC Adv. 2016, 6, 77406–77409. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Khamizov, R.K. A pseudo-second order kinetic equation for sorption processes. Russ. J. Phys. Chem. A 2020, 94, 171–176. [Google Scholar] [CrossRef]
- Qian, X.; Zhu, Z.Q.; Sun, H.X.; Ren, F.; Mu, P.; Liang, W.D.; Chen, L.H.; Li, A. Capture and reversible storage of volatile iodine by novel conjugated microporous polymers containing thiophene units. ACS Appl. Mater. Interfaces 2016, 8, 21063–21069. [Google Scholar] [CrossRef] [PubMed]
- An, S.H.; Zhu, X.; He, Y.Y.; Yang, L.; Wang, H.; Jin, S.B.; Hu, J.; Liu, H.L. Porosity modulation in two-dimensional covalent organic frameworks leads to enhanced iodine adsorption performance. Ind. Eng. Chem. Res. 2019, 58, 10495–10502. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Q.B.; Liu, Y.C.; Meng, X.Y.; Ye, Y.; Liu, X.H.; Song, X.W.; Liang, Z.Q. Multifunctional conjugated microporous polymers with pyridine unit for efficient iodine sequestration, exceptional tetracycline sensing and removal. J. Hazard. Mater. 2020, 387, 121949. [Google Scholar] [CrossRef]
- Liu, C.; Jin, Y.C.; Yu, Z.H.; Gong, L.; Wang, H.L.; Yu, B.Q.; Zhang, W.; Jiang, J.Z. Transformation of porous organic cages and covalent organic frameworks with efficient iodine vapor capture performance. J. Am. Chem. Soc. 2022, 144, 12390–12399. [Google Scholar] [CrossRef]
- Luo, D.; He, Y.L.; Tian, J.Y.; Sessler, J.L.; Chi, X.D. Reversible iodine capture by nonporous adaptive crystals of a bipyridine cage. J. Am. Chem. Soc. 2022, 144, 113–117. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Ge, R.L.; Song, X.D.; Xing, X.Q.; Jiang, Q.K.; Lu, H.; Hao, C.; Guo, X.W.; Gao, Y.N.; et al. A 3D covalent organic framework with exceptionally high iodine capture capability. Chem. Eur. J. 2018, 24, 585–589. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, J.; Xiong, S.H.; Lu, P.G.; Tang, J.T.; He, J.Q.; Javaid, M.U.; Pan, C.Y.; Yu, G.P. Ferrocene-based porous organic polymers for high-affinity iodine capture. Chem. Eng. J. 2020, 380, 122420. [Google Scholar] [CrossRef]
- Wang, H.; Hu, H.P.; Peng, Q.F. Superior removal of iodine via cyclophosphazene-based conjugation-enriched cross-linking hybrid polymers. Colloids Surf. A 2021, 627, 127185. [Google Scholar]
- Huang, Y.L.; Li, W.; Xu, Y.W.; Ding, M.; Ding, J.; Zhang, Y.; Wang, Y.H.; Chen, S.Y.; Jin, Y.D.; Xia, C.Q. Rapid iodine adsorption from vapor phase and solution by a nitrogen-rich covalent piperazine-triazine-based polymer. New J. Chem. 2021, 45, 5363–5370. [Google Scholar] [CrossRef]
- Svensson, P.H.; Kloo, L. Synthesis, structure, and bonding in polyiodide and metal iodide-iodine systems. Chem. Rev. 2003, 103, 1649–1684. [Google Scholar] [CrossRef] [PubMed]
- Li, D.W.; Zhou, L.; Li, M.; Yang, J.F.; Yao, Z.W.; Zhang, L.; Meng, Z.; Yang, L.M.; Shi, H.; Tang, H.; et al. Selective capture of palladium by protonation-armed pyridine nitrogen in extreme water environments. Chem. Eng. J. 2023, 466, 143235. [Google Scholar] [CrossRef]
- Schiebel, J.; Gaspari, R.; Sandner, A.; Ngo, K.; Gerber, H.D.; Cavalli, A.; Ostermann, A.; Heine, A.; Klebe, G. Charges shift protonation: Neutron diffraction reveals that aniline and 2- aminopyridine become protonated upon binding to trypsin. Angew. Chem. Int. Ed. 2017, 56, 4887–4890. [Google Scholar] [CrossRef] [PubMed]
- Pašalić, H.; Aquino, A.J.A.; Tunega, D.; Haberhauer, G.; Gerzabek, M.H.; Lischka, H. Cation-π interactions in competition with cation microhydration: A theoretical study of alkali metal cation-pyrene complexes. J. Mol. Model. 2017, 23, 131. [Google Scholar] [CrossRef]
- Kumar, N.; Gaur, A.S.; Sastry, G.N. A perspective on the nature of cation-π interactions. J. Chem. Sci. 2021, 133, 97. [Google Scholar] [CrossRef]
- Huang, M.; Yang, L.; Li, X.Y.; Chang, G.J. An indole-derived porous organic polymer for the efficient visual colorimetric capture of iodine in aqueous media via the synergistic effects of cation-π and electrostatic forces. Chem. Commun. 2020, 56, 1401–1404. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.L.; Wang, Y.; Wei, C.T.; Ai, K.L.; Lu, L.H. Hydrogen bond-mediated strong adsorbent-I3− interactions enable high-efficiency radioiodine capture. Mater. Horiz. 2019, 6, 1517–1525. [Google Scholar] [CrossRef]
- Schlamadinger, D.E.; Daschbach, M.M.; Gokel, G.W.; Kim, J.E. UV resonance Raman study of cation-π interactions in an indole crown ether. J. Raman Spectrosc. 2011, 42, 633–638. [Google Scholar] [CrossRef]
- Cripps, R.; Venuat, L.; Bruchertseifer, H. Quick analytical method for the determination of iodide and iodate ions in aqueous solutions. J. Radioanal. Nucl. Chem. 2003, 256, 357–360. [Google Scholar] [CrossRef]
- Bruchertseifer, H.; Cripps, R.; Guentay, S.; Jaeckel, B. Analysis of iodine species in aqueous solutions. Anal. Bioanal. Chem. 2003, 375, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Gottardi, W. Redox-potentiometric/titrimetric analysis of aqueous iodine solutions. Fresenius J. Anal. Chem. 1998, 362, 263–269. [Google Scholar] [CrossRef]
- Jung, S.H.; Yeon, J.W.; Kang, Y.; Song, K. Determination of triiodide ion concentration using UV-Visible spectrophotometry. Asian J. Chem. 2014, 26, 4084–4086. [Google Scholar] [CrossRef]
- Moreno, C.; Baeza-Romero, M.T.; Sanz, M.; GálvezÓscar, A.V.L.; Ianni, J.C.; Espíldora, E. Iodide conversion to iodate in aqueous and solid aerosols exposed to ozone. Phys. Chem. Chem. Phys. 2020, 22, 5625–5637. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.W.; Song, L.N.; Wang, Y.S.; Bai, T.H.; Long, C.M.; Wu, M.M.; Feng, Y.; Mi, J. Three novel indole-based porous organic polymers for efficient iodine capture in water. J. Radioanal. Nucl. Ch. 2023, 332, 4271–4290. [Google Scholar] [CrossRef]
- Xiong, S.H.; Tang, X.; Pan, C.Y.; Li, L.; Tang, J.T.; Yu, G.P. Carbazole-bearing porous organic polymers with a mulberry-like morphology for efficient iodine capture. ACS Appl. Mater. Interfaces. 2019, 11, 27335–27342. [Google Scholar] [CrossRef]
- Chen, D.Y.; Fu, Y.; Yu, W.G.; Yu, G.P.; Pan, C.Y. Versatile adamantane-based porous polymers with enhanced microporosity for efficient CO2 capture and iodine removal. Chem. Eng. J. 2018, 334, 900–906. [Google Scholar] [CrossRef]
- Zeng, Z.Y.; Lou, Z.C.; Hu, L.R.; Dou, W.T.; Zhao, X.L.; Li, X.D.; Fang, J.F.; Qian, X.H.; Yang, H.B.; Xu, L. Metal-organic cages for efficient capture and convenient detection of iodine from vapor and aqueous solutions. Chem. Eng. J. 2024, 496, 154091. [Google Scholar] [CrossRef]
- Das, M.; Sarkar, S.K.; Patra, Y.S.; Manna, A.; Mukherjee, S.; Das, S. Soft self-templating approach-derived covalent triazine framework with bimodal nanoporosity for efficient radioactive iodine capture for safe nuclear energy. ACS Appl. Nano Mater. 2022, 5, 8783–8793. [Google Scholar] [CrossRef]
- Jiao, J.M.; Xie, W.; Li, Y.M.; Lin, C.; Jiang, J.L.; Wang, L.Y. Effective iodine capture behavior by a censer-shaped macrocycle in vapor phase and aqueous solution. Chem. Eur. J. 2022, 28, e202201933. [Google Scholar] [CrossRef]
- Jin, P.Y.; Liang, W.T.; Rong, Y.Q.; Wu, W.A.H.; Gou, M.; Tang, Y.Q.; Yang, C. Remarkable iodine uptake by aniline-based macrocyclic arenes through a reverse dissolution mechanism. J. Mater. Chem. A. 2023, 11, 11126–11132. [Google Scholar] [CrossRef]
- Shreeraj, G.; Sah, A.; Sarkar, S.; Giri, A.; Sahoo, A.; Patra, A. Structural modulation of nitrogen-rich covalent organic frameworks for iodine capture. Langmuir. 2023, 39, 16069–16078. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yu, Z.C.; Zhang, T.T.; Pan, D.W.; Dai, J.J.; Li, Q.; Tao, Z.; Xiao, X. A cucurbit[8]uril-based supramolecular framework material for reversible iodine capture in the vapor phase and solution. Small 2024, 20, 2308175. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.Q.; Li, Z.W.; Lin, F.; Tang, R.Z.; Zhang, W.Q.; Liu, H.W.; Ouyang, G.F.; Tan, Y. The paradigm for exceptional iodine capture by nonporous amorphous electron-deficient cyclophanes. J. Hazard. Mater. 2024, 465, 133449. [Google Scholar] [CrossRef]
- Li, B.; Wang, B.; Huang, X.Y.; Dai, L.; Cui, L.; Li, J.; Jia, X.S.; Li, C.J. Terphen[n]arenes and quaterphen[n]arenes (n=3-6): One-c supramolecular gels, and iodine capture. Angew. Chem. Int. Ed. 2019, 58, 3885–3889. [Google Scholar] [CrossRef]
- Lin, Y.X.; Jiang, X.F.; Kim, S.T.; Alahakoon, S.B.; Hou, X.S.; Zhang, Z.Y.; Thompson, C.M.; Smaldone, R.A.; Ke, C.F. An elastic hydrogen-bonded cross-linked organic framework for effective iodine capture in water. J. Am. Chem. Soc. 2017, 139, 7172–7175. [Google Scholar] [CrossRef]
- Zheng, Z.Y.; Lin, Q.Y.; Xie, L.H.; Chen, X.L.; Zhou, H.; Lin, K.H.; Zhang, D.S.; Chi, X.D.; Sessler, J.L.; Wang, H.Y. Macrocycle polymeric networks based on a chair-like calix[4]pyrrole for the rapid and efficient adsorption of iodine from water. J. Mater. Chem. A. 2023, 11, 13399–13408. [Google Scholar] [CrossRef]
- Cao, J.J.; Zhu, H.T.Z.; Shangguan, L.Q.; Liu, Y.Z.; Liu, P.R.; Li, Q.; Wu, Y.T.; Huang, F.H. A pillar[5]arene-based 3D polymer network for efficient iodine capture in aqueous solution. Polym. Chem. 2021, 12, 3517–3521. [Google Scholar] [CrossRef]
- Gao, R.; An, B.H.; Zhou, C.; Zhang, X. Synthesis of a triazaisotruxene-based porous organic polymer and its application in iodine capture. Molecules 2022, 27, 8722. [Google Scholar] [CrossRef]

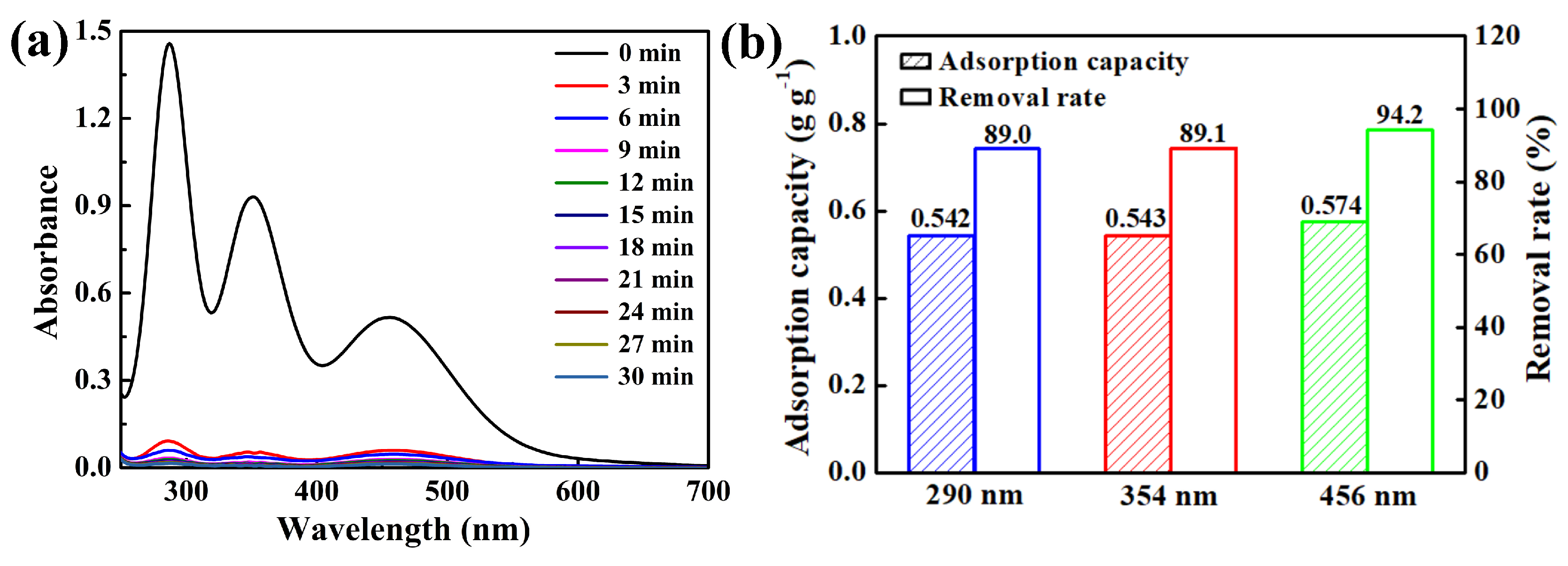
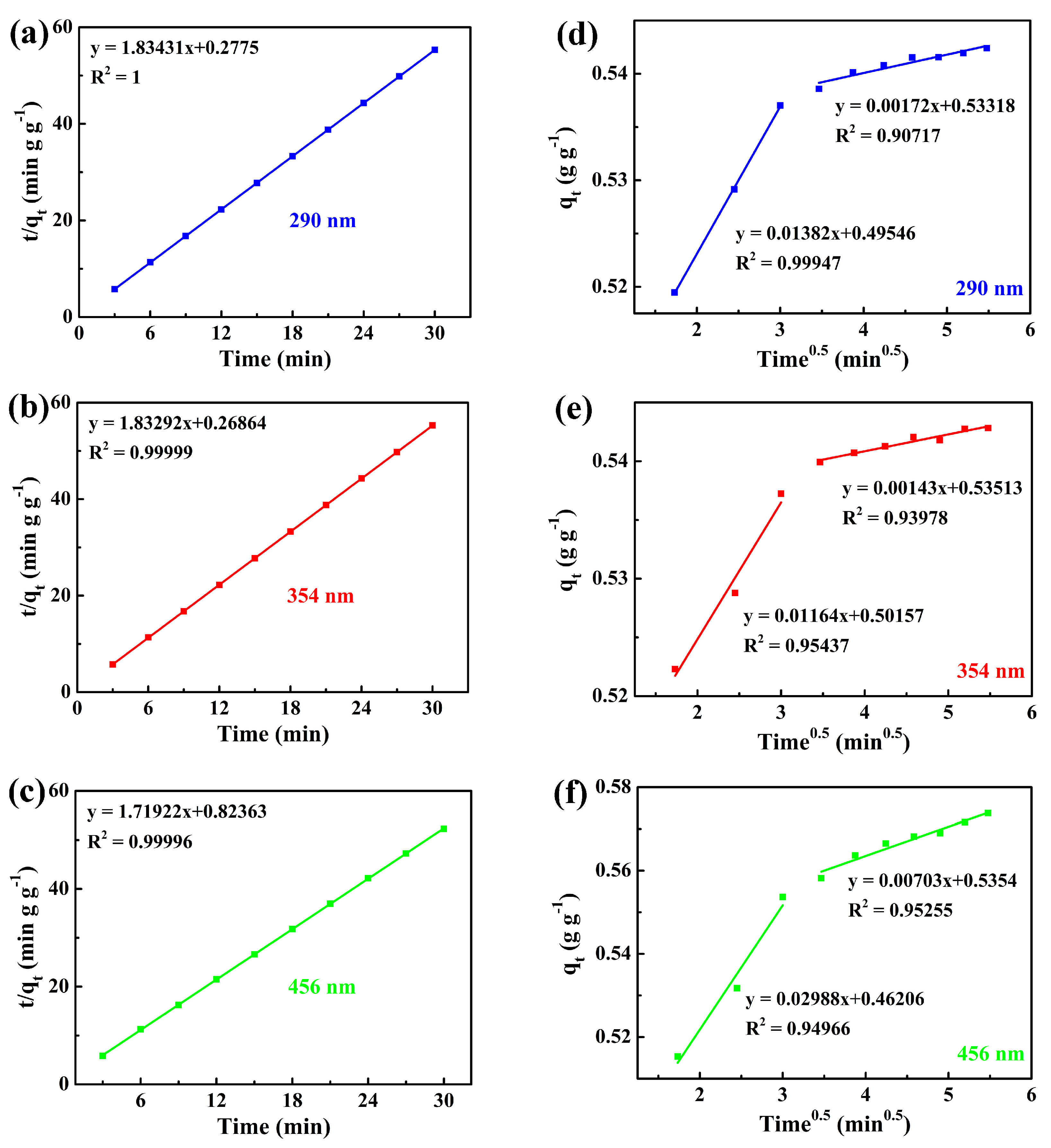
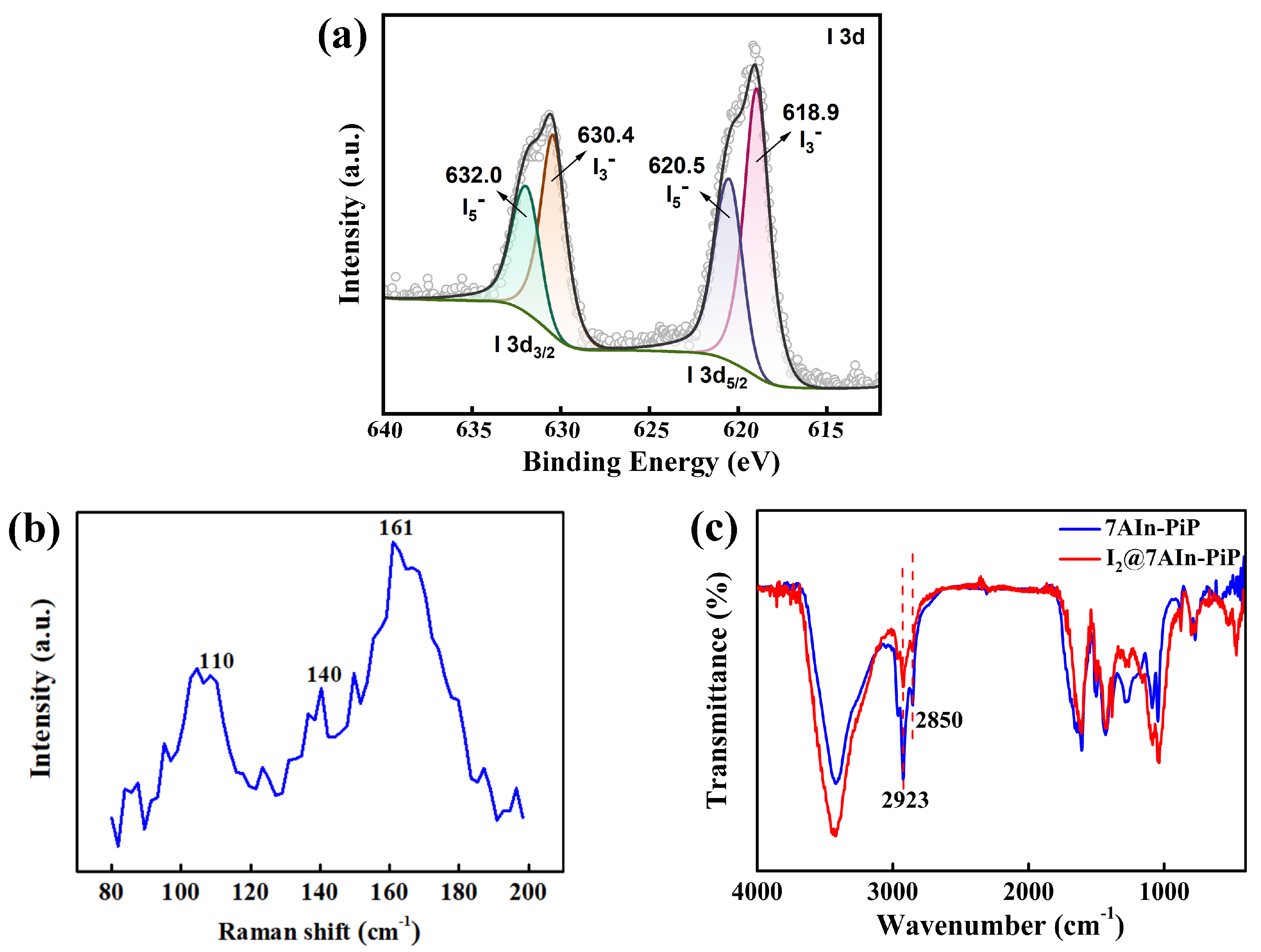
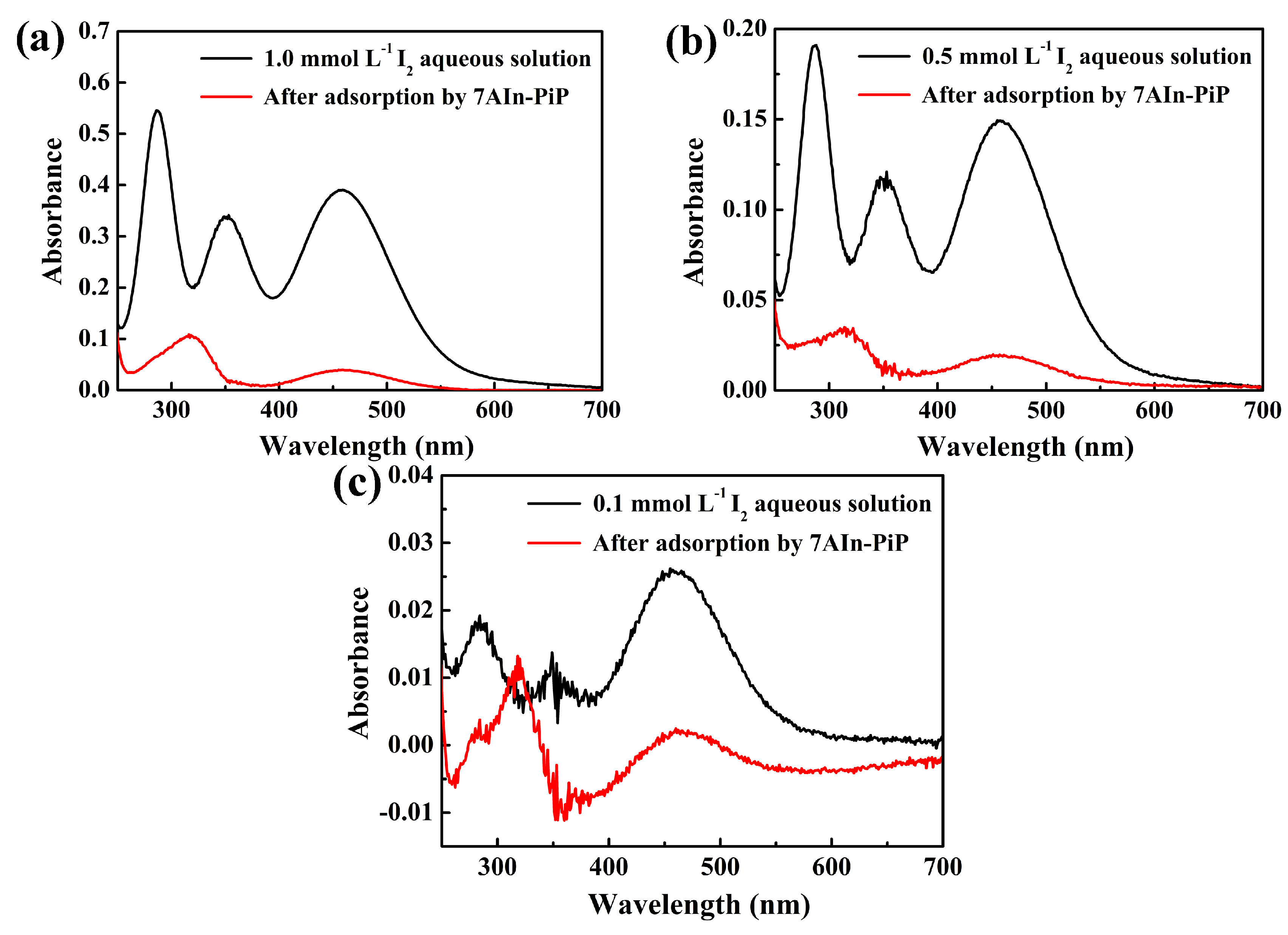
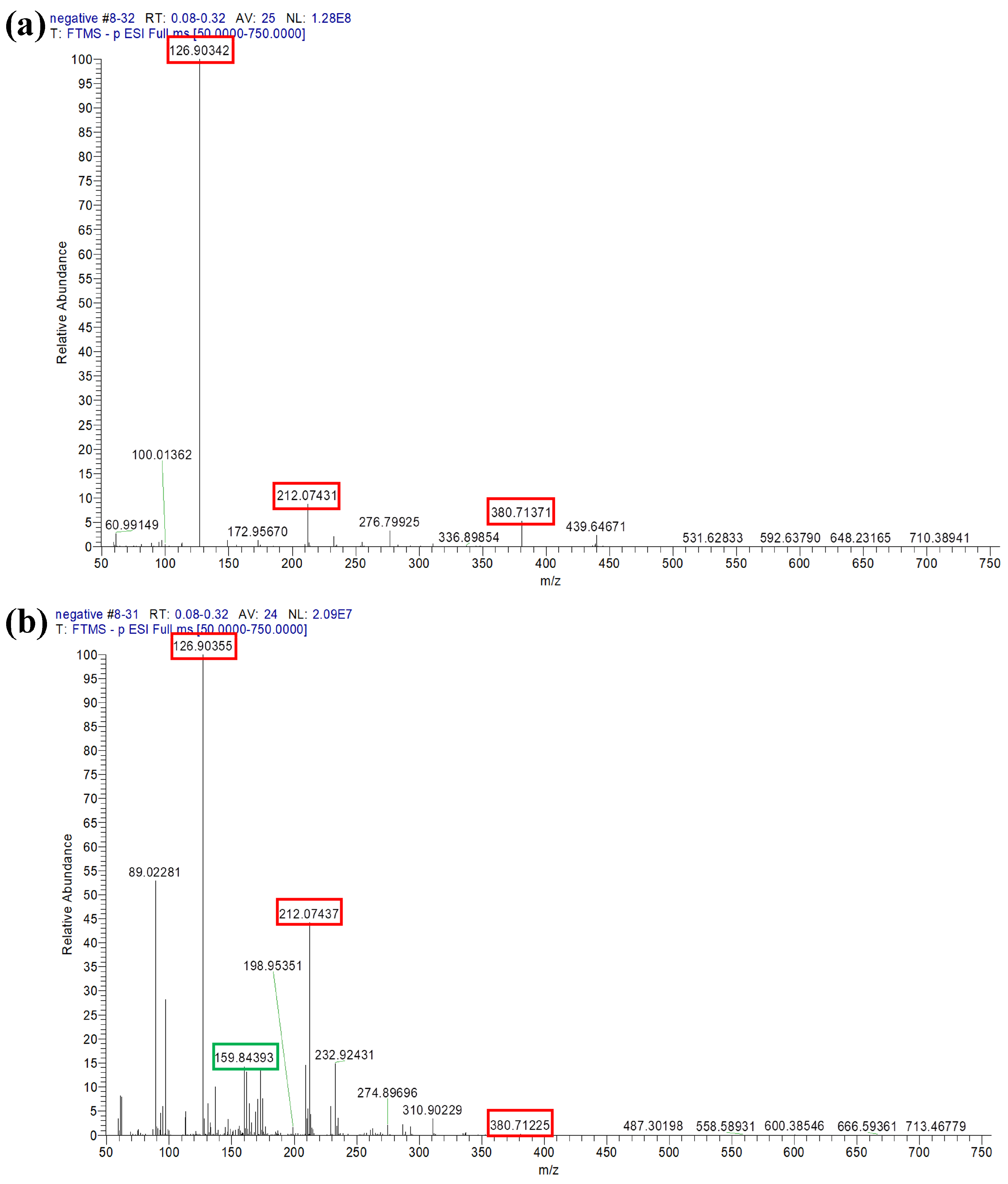
| Characteristic Absorption Wavelength λmax (nm) | Experimental Adsorption Capacity at Equilibrium qe, exp (g g−1) | Parameters of Pseudo-Second-Order Model | ||
|---|---|---|---|---|
| qe, cal (g g−1) | k2 (g g−1 min−1) | R2 | ||
| 290 | 0.542 | 0.545 | 12.125 | 1.00000 |
| 354 | 0.543 | 0.546 | 12.506 | 0.99999 |
| 456 | 0.574 | 0.582 | 3.589 | 0.99996 |
| Characteristic Absorption Wavelength λmax (nm) | The First Stage | The Second Stage | ||||
|---|---|---|---|---|---|---|
| kp (g g−1 min−0.5) | C (g g−1) | R2 | kp (g g−1 min−0.5) | C (g g−1) | R2 | |
| 290 | 0.01382 | 0.49546 | 0.99947 | 0.00172 | 0.53318 | 0.90717 |
| 354 | 0.01164 | 0.50157 | 0.95437 | 0.00143 | 0.53513 | 0.93978 |
| 456 | 0.02988 | 0.46206 | 0.94966 | 0.00703 | 0.5354 | 0.95255 |
| Adsorbent | The Mass of Adsorbent (mg) | Iodine Adsorption Capacity of 1 g Adsorbent (g g−1) | Iodine Adsorption Efficiency (%) | ||
|---|---|---|---|---|---|
| 290 nm | 456 nm | 290 nm | 456 nm | ||
| Original 7AIn-PiP | 5.0 | 0.542 | 0.574 | 100 | 100 |
| After the 1st regeneration | 5.1 | 0.531 | 0.563 | 98.0 | 98.1 |
| After the 2nd regeneration | 5.2 | 0.518 | 0.548 | 95.6 | 95.5 |
| After the 3rd regeneration | 5.2 | 0.516 | 0.545 | 95.2 | 94.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Song, L.; Han, B.; Hu, J.; Li, Z.; Mi, J. Synthesis of a Novel Zwitterionic Hypercrosslinked Polymer for Highly Efficient Iodine Capture from Water. Polymers 2024, 16, 2846. https://doi.org/10.3390/polym16192846
Yu J, Song L, Han B, Hu J, Li Z, Mi J. Synthesis of a Novel Zwitterionic Hypercrosslinked Polymer for Highly Efficient Iodine Capture from Water. Polymers. 2024; 16(19):2846. https://doi.org/10.3390/polym16192846
Chicago/Turabian StyleYu, Jingwen, Luna Song, Bingying Han, Jiangliang Hu, Zhong Li, and Jie Mi. 2024. "Synthesis of a Novel Zwitterionic Hypercrosslinked Polymer for Highly Efficient Iodine Capture from Water" Polymers 16, no. 19: 2846. https://doi.org/10.3390/polym16192846
APA StyleYu, J., Song, L., Han, B., Hu, J., Li, Z., & Mi, J. (2024). Synthesis of a Novel Zwitterionic Hypercrosslinked Polymer for Highly Efficient Iodine Capture from Water. Polymers, 16(19), 2846. https://doi.org/10.3390/polym16192846





