Degradation of Polymer Materials in the Environment and Its Impact on the Health of Experimental Animals: A Review
Abstract
1. Introduction
2. Common Polymeric Materials and Their Degradation Products
2.1. Common Polymeric Materials
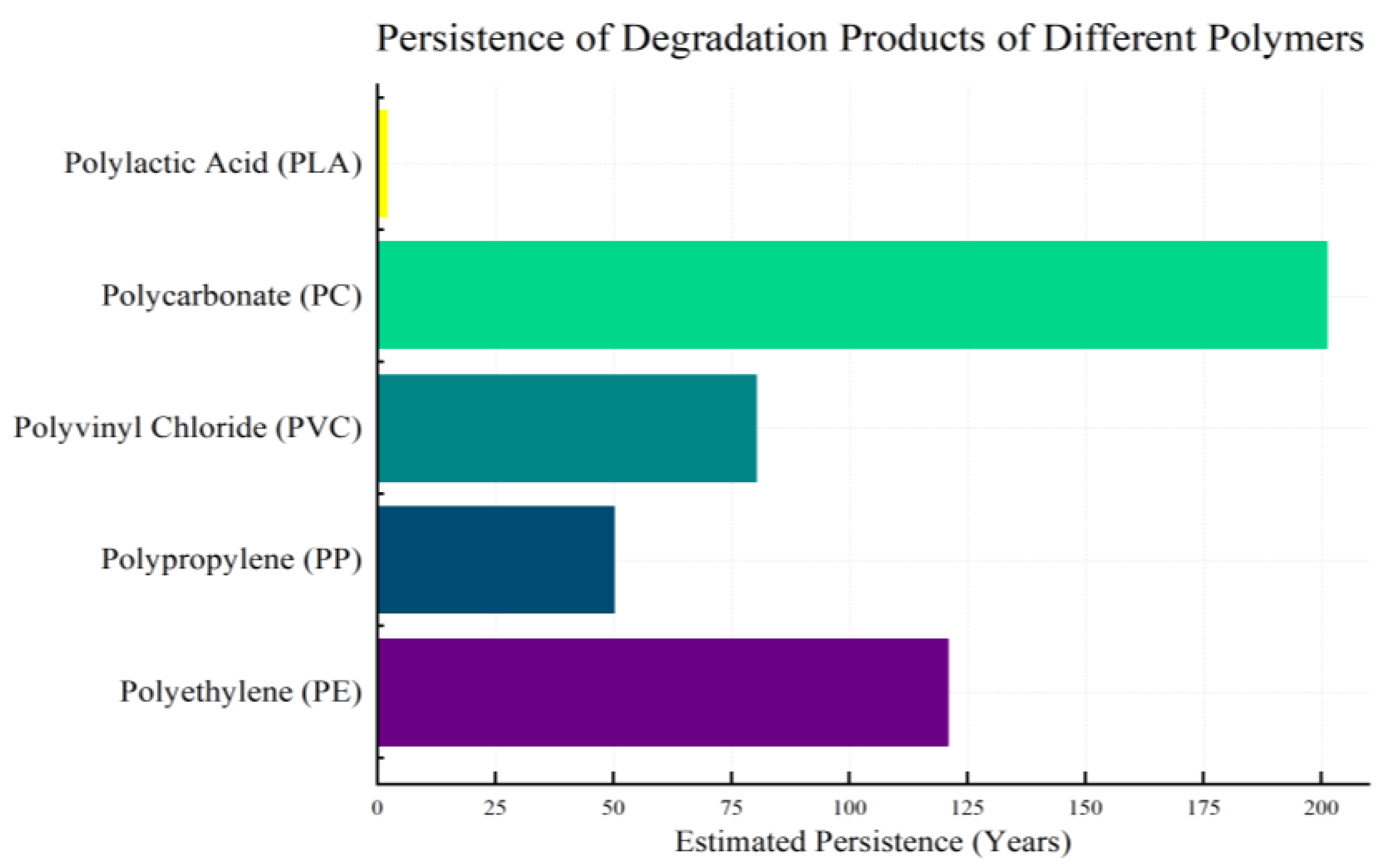
2.2. Persistence of Polymeric Degradation Products
2.3. Transformation of Polymeric Degradation Products
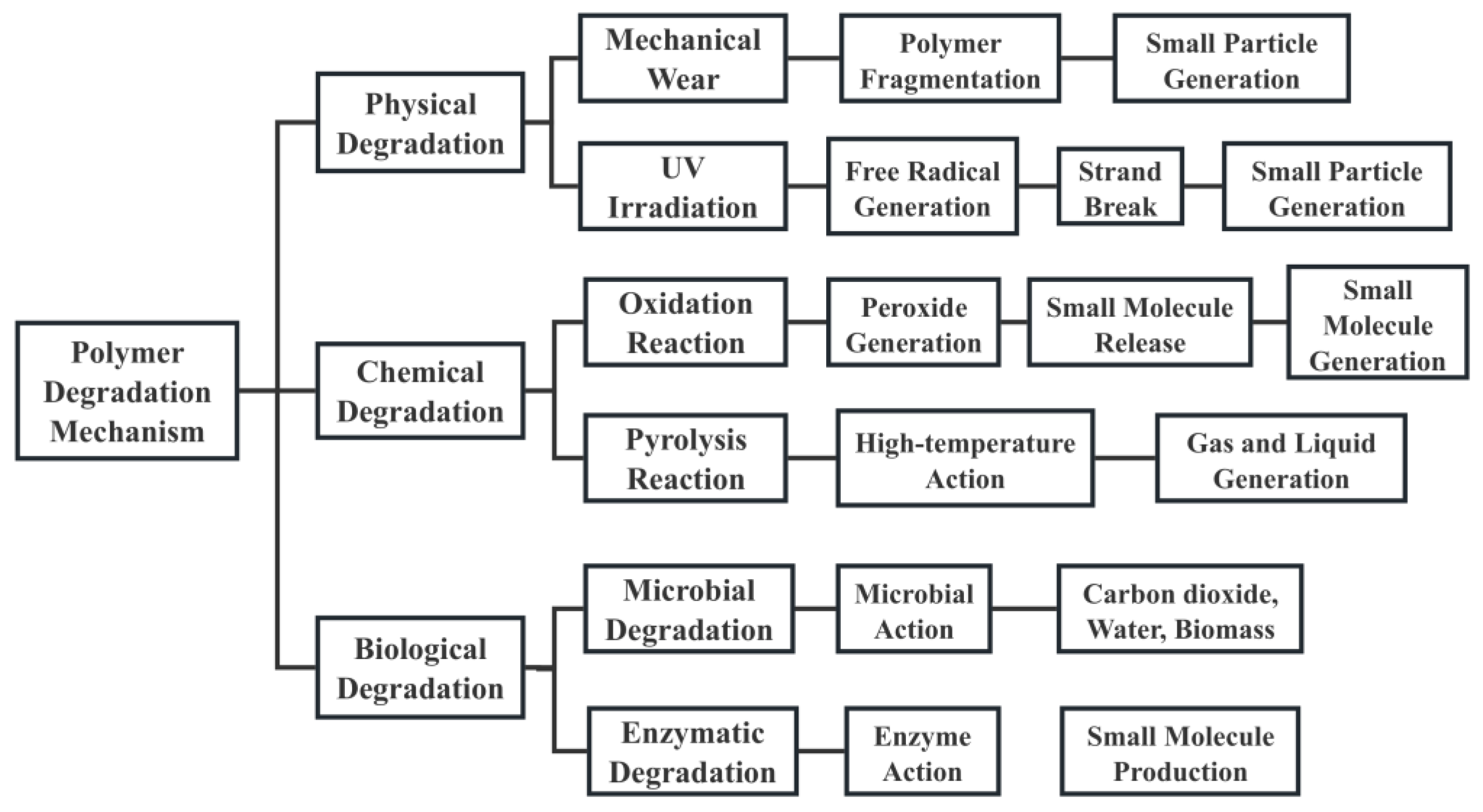
3. Mechanisms of Polymeric Material Degradation
3.1. Physical Degradation
3.2. Chemical Degradation
3.2.1. Photochemical Degradation
3.2.2. Thermo-Oxidative Degradation
3.2.3. Hydrolytic Degradation
3.3. Biological Degradation
3.4. Environmental Factors Affecting Degradation
3.5. Other Factors
4. Environmental Distribution and Pollution of Polymeric Degradation Products
4.1. Distribution in Different Environmental Compartments
4.1.1. Soil
4.1.2. Water Bodies
4.1.3. Atmosphere
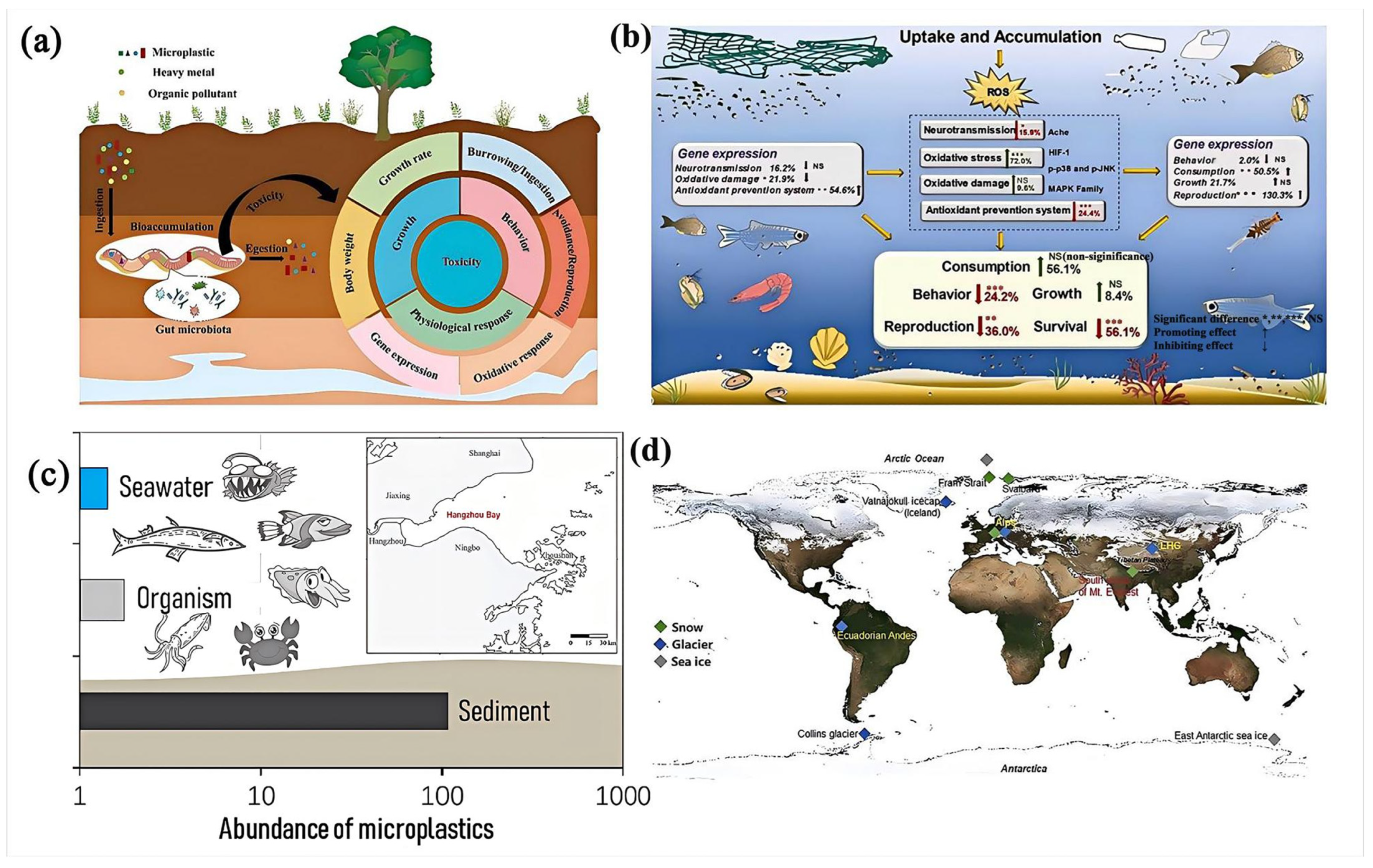
4.2. Pollution Levels and Trends
4.2.1. Global and Regional Variations
4.2.2. Microplastics (MPs)
4.3. Environmental Impact
4.3.1. Ecosystem and Animal Health
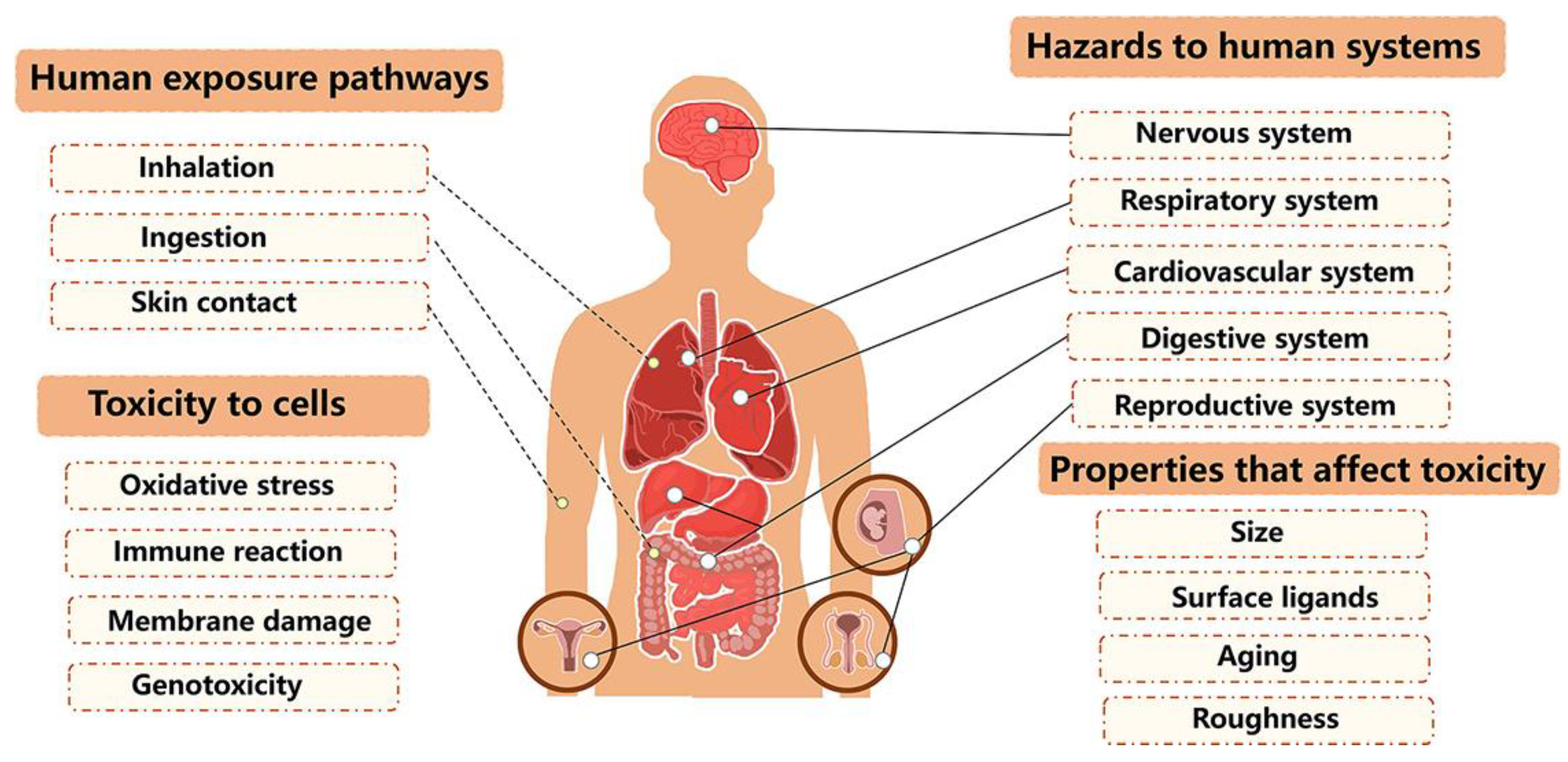
4.3.2. Human Health Concerns
4.4. Current Status of Polymer Recycling
4.5. Mitigation Strategies
4.5.1. Policy and Regulation
4.5.2. Public Awareness and Education
4.5.3. Technological Innovations
4.5.4. Monitoring and Regulation
5. Health Effects of Polymeric Material Degradation Products on Experimental Animals
5.1. Organ-Specific Toxicity
5.1.1. Liver Toxicity
5.1.2. Kidney Toxicity
5.2. Immunotoxicity and Inflammatory Responses
5.2.1. Immune System Modulation
5.2.2. Inflammatory Responses
5.3. Genotoxicity and Carcinogenicity
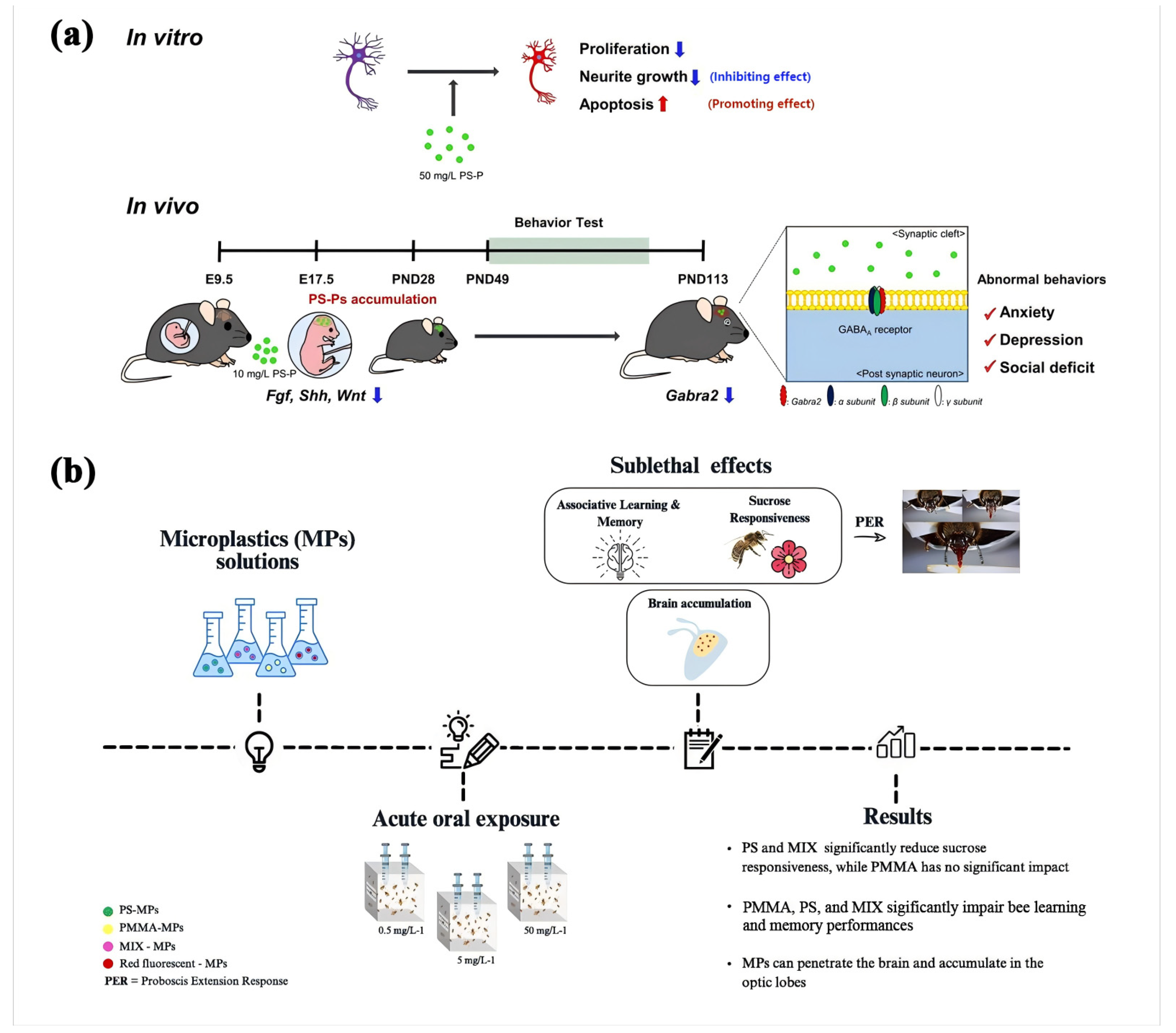
5.4. Behavioral and Neurological Effects
5.4.1. Anxiety and Depression
5.4.2. Cognitive Function
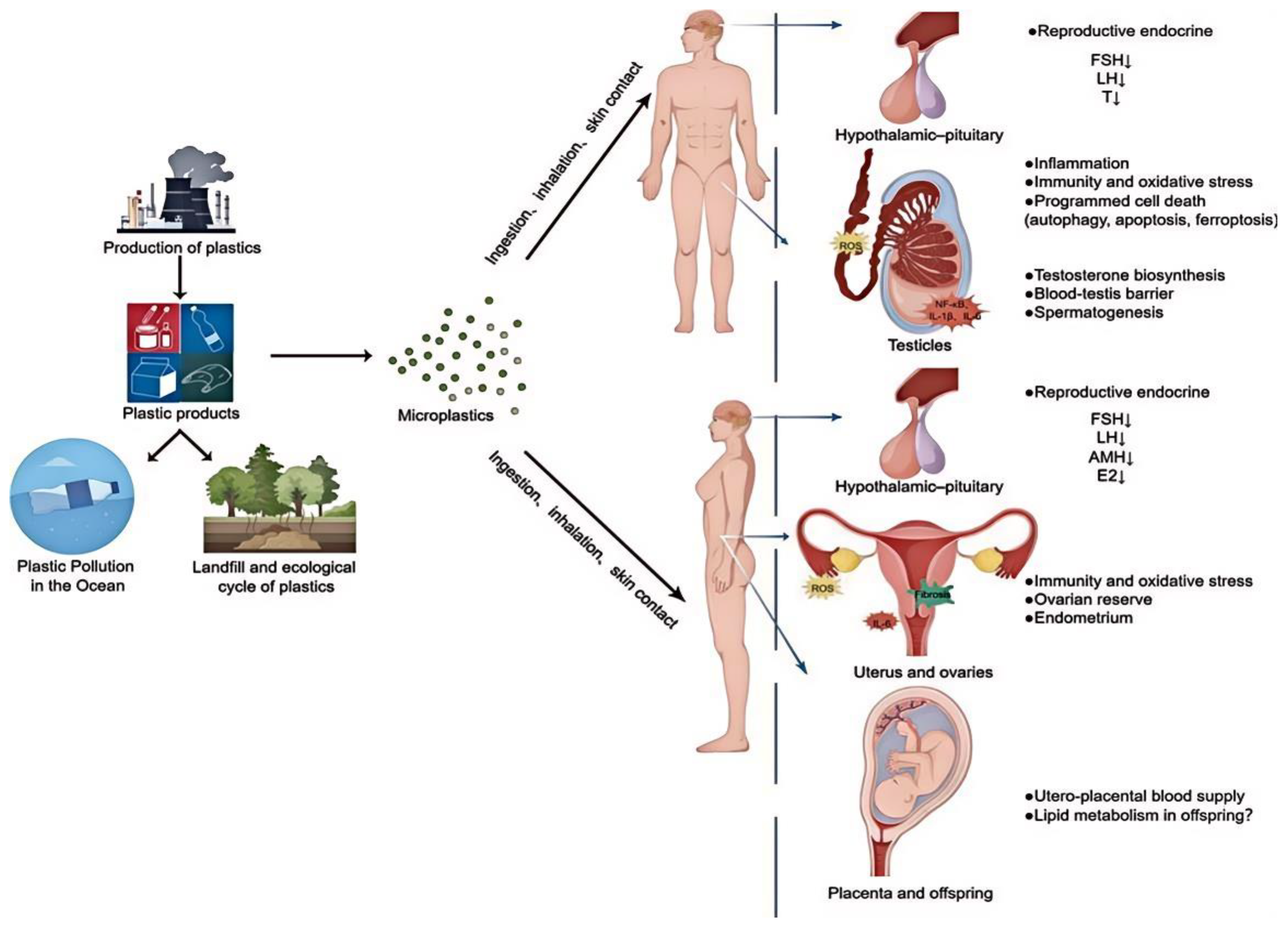
5.5. Metabolic and Endocrine Disruption
5.5.1. Metabolic Disorders
5.5.2. Endocrine Disruption
5.6. Developmental and Reproductive Effects
5.6.1. Developmental Toxicity
5.6.2. Fertility and Reproduction
5.7. Long-Term Health Implications
5.7.1. Cumulative Effects
5.7.2. Transgenerational Effects
6. Exposure of Experimental Animals and Impact Assessment
7. Comprehensive Impact of Polymeric Material Degradation on Environmental Health
7.1. Ecological Disruptions
7.1.1. Habitat Alterations
7.1.2. Biodiversity Loss
7.2. Chemical Contamination
7.2.1. Leaching of Additives
7.2.2. Toxicological Impacts
7.2.3. Persistent Organic Pollutants
7.3. Long-Term Sustainability Challenges
7.4. Major Progress in Polymer Removal
8. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Austin, H.P.; Allen, M.D.; Donohoe, B.S.; Rorrer, N.A.; Kearns, F.L.; Silveira, R.L.; Pollard, B.C.; Dominick, G.; Duman, R.; El Omari, K. Characterization and engineering of a plastic-degrading aromatic polyesterase. Proc. Natl. Acad. Sci. USA 2018, 115, E4350–E4357. [Google Scholar] [CrossRef] [PubMed]
- Neu, L.; Hammes, F. Feeding the building plumbing microbiome: The importance of synthetic polymeric materials for biofilm formation and management. Water 2020, 12, 1774. [Google Scholar] [CrossRef]
- Zarei, M.; Lee, G.; Lee, S.G.; Cho, K. Advances in biodegradable electronic skin: Material progress and recent applications in sensing, robotics and human—Machine interfaces. Adv. Mater. 2023, 35, 2203193. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Protection. disinfection. and immunization for healthcare during the COVID-19 pandemic: Role of natural and synthetic macromolecules. Sci. Total Environ. 2021, 776, 145989. [Google Scholar] [CrossRef]
- Fortună, M.E.; Ungureanu, E.; Jităreanu, D.C.; Țopa, D.C.; Harabagiu, V. Effects of hybrid polymeric material based on polycaprolactone on the environment. Materials 2022, 15, 4868. [Google Scholar] [CrossRef] [PubMed]
- Pinaeva, L.G.; Noskov, A.S. Biodegradable biopolymers: Real impact to environment pollution. Sci. Total Environ. 2024, 947, 174445. [Google Scholar] [CrossRef] [PubMed]
- Gałko, G.; Sajdak, M. Trends for the thermal degradation of polymeric materials: Analysis of available techniques, issues, and opportunities. Appl. Sci. 2022, 12, 9138. [Google Scholar] [CrossRef]
- Liu, X.; Hua, X.; Wu, H. Degradation Behavior of Poly (Lactic Acid) during Accelerated Photo-Oxidation: Insights into Structural Evolution and Mechanical Properties. J. Polym. Environ. 2024, 32, 3810–3821. [Google Scholar] [CrossRef]
- Deng, S.; Chen, A.; Chen, W.; Lai, J.; Pei, Y.; Wen, J.; Yang, C.; Luo, J.; Zhang, J.; Lei, C. Fabrication of biodegradable and biocompatible functional polymers for anti-infection and augmenting wound repair. Polymers 2022, 15, 120. [Google Scholar] [CrossRef]
- Marlina, D.; Sato, H. Study on the higher-order structure and hydrogen bonding of biodegradable polymer by low-frequency vibrational spectroscopy. IOP Conf. Ser. Mater. Sci. Eng. 2020, 835, 012004. [Google Scholar] [CrossRef]
- Wilkes, R.A.; Aristilde, L. Degradation and metabolism of synthetic plastics and associated products by Pseudomonas sp.: Capabilities and challenges. J. Appl. Microbiol. 2017, 123, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Vila-Costa, M.; Martínez-Varela, A.; Rivas, D.; Martinez, P.; Pérez-López, C.; Zonja, B.; Montemurro, N.; Tauler, R.; Barceló, D.; Ginebreda, A. Advanced analytical. chemometric, and genomic tools to identify polymer degradation products and potential microbial consumers in wastewater environments. Chem. Eng. J. 2022, 442, 136175. [Google Scholar] [CrossRef]
- El Assimi, T.; Beniazza, R.; Raihane, M.; Youcef, H.B.; El Meziane, A.; Kricheldorf, H.; Lahcini, M. Overview on progress in polysaccharides and aliphatic polyesters as coating of water-soluble fertilizers. J. Coat. Technol. Res. 2022, 19, 989–1007. [Google Scholar] [CrossRef]
- Heidarzadeh, M.; Abdi, N.; Varvani, J.; Ahmadi, A.; Toranjzar, H. Zoning of some physicochemical parameters in the sediments of Meighan wetland in Iran: Response to urbanization. industrial, and agricultural activities. Environ. Monit. Assess. 2023, 195, 894. [Google Scholar] [CrossRef]
- Österlund, H.; Blecken, G.; Lange, K.; Marsalek, J.; Gopinath, K.; Viklander, M. Microplastics in urban catchments: Review of sources. pathways, and entry into stormwater. Sci. Total Environ. 2023, 858, 159781. [Google Scholar] [CrossRef]
- Bhagwat, G.; Tran, T.K.A.; Lamb, D.; Senathirajah, K.; Grainge, I.; Connor, W.O.; Juhasz, A.; Palanisami, T. Biofilms enhance the adsorption of toxic contaminants on plastic microfibers under environmentally relevant conditions. Environ. Sci. Technol. 2021, 55, 8877–8887. [Google Scholar] [CrossRef]
- Malafeev, K.V.; Apicella, A.; Incarnato, L.; Scarfato, P. Understanding the Impact of Biodegradable Microplastics on Living Organisms Entering the Food Chain: A Review. Polymers 2023, 15, 3680. [Google Scholar] [CrossRef]
- Tsochatzis, E.D.; Gika, H.; Theodoridis, G.; Maragou, N.; Thomaidis, N.; Corredig, M. Microplastics and nanoplastics: Exposure and toxicological effects require important analysis considerations. Heliyon 2024, 10, e32261. [Google Scholar] [CrossRef]
- Roman, L.; Schuyler, Q.; Wilcox, C.; Hardesty, B.D. Plastic pollution is killing marine megafauna. but how do we prioritize policies to reduce mortality. Conserv. Lett. 2021, 14, e12781. [Google Scholar] [CrossRef]
- Li, J.; Zheng, X.; Liu, X.; Zhang, L.; Zhang, S.; Li, Y.; Zhang, W.; Li, Q.; Zhao, Y.; Chen, X. Effect and mechanism of microplastics exposure against microalgae: Photosynthesis and oxidative stress. Sci. Total Environ. 2023, 905, 167017. [Google Scholar] [CrossRef]
- Alijagic, A.; Hedbrant, A.; Persson, A.; Larsson, M.; Engwall, M.; Särndahl, E. NLRP3 inflammasome as a sensor of micro-and nanoplastics immunotoxicity. Front. Immunol. 2023, 14, 1178434. [Google Scholar] [CrossRef] [PubMed]
- Çobanoğlu, H.; Belivermiş, M.; Sıkdokur, E.; Kılıç, Ö.; Çayır, A. Genotoxic and cytotoxic effects of polyethylene microplastics on human peripheral blood lymphocytes. Chemosphere 2021, 272, 129805. [Google Scholar] [CrossRef] [PubMed]
- Liebgott, C.; Chaib, I.; Doyen, P.; Robert, H.; Eutamene, H.; Duflos, G.; Reynaud, S.; Grassl, B.; Mercier-Bonin, M. Fate and impact of nanoplastics in the human digestive environment after oral exposure: A common challenge for toxicology and chemistry. Trends Analyt. Chem. 2023, 166, 117175. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Chen, Q.; Jin, L.; Peng, R. Research progress of zebrafish model in aquatic ecotoxicology. Water 2023, 15, 1735. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Nag, R.; Cummins, E. Ranking of potential hazards from microplastics polymers in the marine environment. J. hazard.Mater. 2022, 429, 128399. [Google Scholar]
- Lin, H.; Li, X.; Gao, H.; Hu, W.; Yu, S.; Li, X.; Lei, L.; Yang, F. The role of gut microbiota in mediating increased toxicity of nano-sized polystyrene compared to micro-sized polystyrene in mice. Chemosphere 2024, 358, 142275. [Google Scholar] [CrossRef]
- Ajonuma, L.; Bamiro, S.; Adeyinka, R.; Fapohunda, D.; Makanjuola, S. P-100 Prolonged exposure to polyethylene glycol (peg) 6000 severely affects the reproductive system of male Wistar rats. Hum. Reprod. 2024, 39, 108474. [Google Scholar] [CrossRef]
- Varg, J.E.; Svanbäck, R. Multi stress system: Microplastics in freshwater and their effects on host microbiota. Sci. Total Environ. 2023, 856, 159106. [Google Scholar] [CrossRef]
- Bandow, N.; Aitken, M.D.; Geburtig, A.; Kalbe, U.; Piechotta, C.; Schoknecht, U.; Simon, F.-G.; Stephan, I. Using environmental simulations to test the release of hazardous substances from polymer-based products: Are realism and pragmatism mutually exclusive objectives. Materials 2020, 13, 2709. [Google Scholar] [CrossRef]
- Zhorin, V.; Kiselev, M.; Kotenev, V. Melting and Crystallization of High-and Low-Density Polyethylenes in Mixtures with Some Oxides and Ionic Crystals after Intensive Plastic Deformation. Prot. Met. Phys. Chem. Surf. 2021, 57, 289–296. [Google Scholar] [CrossRef]
- Budhiraja, V.; Urh, A.; Horvat, P.; Krzan, A. Synergistic adsorption of organic pollutants on weathered polyethylene microplastics. Polymers 2022, 14, 2674. [Google Scholar] [CrossRef] [PubMed]
- Najafi, N.; Koupai, J.A.; Karevan, M.; Mostajeran, M. High-density polyethylene/zinc oxide nanocomposite with antibacterial and anti-UV radiation properties to reduce evaporation from free surface waters. Polym. Compos. 2022, 43, 7616–7632. [Google Scholar] [CrossRef]
- Fu, Y.W.; Sun, W.F.; Wang, X. UV-initiated crosslinking reaction mechanism and electrical breakdown performance of crosslinked polyethylene. Polymers 2020, 12, 420. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.; Hameed, B. Green hydroxyapatite-zeolite catalyst derived from steel waste as an effective catalyst for the hydrocarbon production via co-catalytic pyrolysis of sugarcane bagasse and high-density polyethylene. Catal. Commun. 2023, 184, 106795. [Google Scholar] [CrossRef]
- Tiboni, M.; Curzi, G.; Aluigi, A.; Casettari, L. An easy 3D printing approach to manufacture vertical diffusion cells for in vitro release and permeation studies. J. Drug Deliv. Sci. Technol. 2021, 65, 102661. [Google Scholar] [CrossRef]
- Melekhina, V.Y.; Vlasova, A.V.; Ilyin, S.O. Asphaltenes from Heavy Crude Oil as Ultraviolet Stabilizers against Polypropylene Aging. Polymers 2023, 15, 4313. [Google Scholar] [CrossRef]
- Liu, X.; Yang, R. Conversion among photo-oxidative products of polypropylene in solid, liquid and gaseous states. BMC Chem. 2020, 14, 44. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Aljohani, M.; Al-bonayan, A.M.; Alsharief, H.H.; Alrefaee, S.H.; Almotairy, A.R.Z.; El-Metwaly, N.M. Development of rare-earth doped aluminate embedded into electrospun polyvinyl chloride nanofibers for information encryption. React. Funct. Polym. 2023, 188, 105609. [Google Scholar] [CrossRef]
- Li, A.; Huang, B.Q.; Zhang, W.L.; Ding, Y.M.; Zhou, R. Experimental study on pyrolysis gas products of chlorinated polyvinyl chloride and its smoke properties during combustion. J. Therm. Anal. Calorim. 2021, 147, 8213–8224. [Google Scholar] [CrossRef]
- Wang, B.; Lu, Y.H.; Lu, Y.W. Organic tin, calcium–zinc and titanium composites as reinforcing agents and its effects on the thermal stability of polyvinyl chloride. J. Therm. Anal. Calorim. 2020, 142, 671–683. [Google Scholar] [CrossRef]
- Li, Y.; Yu, W.; Li, Y.; Ding, Y.; Liu, H.; Zhang, Y.; Guo, M. Graphene nanosheet stabilized Pickering emulsion and its polystyrene nanocomposites. J. Appl. Polym. Sci. 2023, 140, e54018. [Google Scholar] [CrossRef]
- Rohrbach, S.; Gkoutselis, G.; Mauel, A.; Telli, N.; Senker, J.; Ho, A.; Rambold, G.; Horn, M.A. Setting new standards: Multiphasic analysis of microplastic mineralization by fungi. Chemosphere 2024, 349, 141025. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, X.; Jiang, R.; You, J.; Ouyang, G. New insights into the photo-degraded polystyrene microplastic: Effect on the release of volatile organic compounds. J. Hazard. Mater. 2022, 431, 128523. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Zhang, M.Q.; Hu, C.; Xiao, D.; Wang, M.; Ma, D. Catalytic oxidation of polystyrene to aromatic oxygenates over a graphitic carbon nitride catalyst. Nat. Commun. 2022, 13, 4809. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R. Polyethylene terephthalate (PET) in the packaging industry. Polym. Test. 2020, 90, 106707. [Google Scholar] [CrossRef]
- Conroy, S.; Zhang, X. Theoretical insights into chemical recycling of polyethylene terephthalate (PET). Polym. Degrad. Stab. 2024, 223, 110729. [Google Scholar] [CrossRef]
- Washihira, N.; Murakami, M.; Nakamura, M.; Fujii, S.; Matsushima, T.; Asahara, H.; Kishida, A.; Tanabe, T.; Kimura, T.; Kobayashi, M. Application of a genetically engineered macrophage cell line for evaluating cellular effects of UV/US-treated poly (ethylene terephthalate) microplastics. Colloids Surf. B 2024, 234, 113735. [Google Scholar] [CrossRef]
- Djapovic, M.; Milivojevic, D.; Ilic-Tomic, T.; Lješević, M.; Nikolaivits, E.; Topakas, E.; Maslak, V.; Nikodinovic-Runic, J. Synthesis and characterization of polyethylene terephthalate (PET) precursors and potential degradation products: Toxicity study and application in discovery of novel PETases. Chemosphere 2021, 275, 130005. [Google Scholar] [CrossRef]
- Acierno, D.; Graziosi, L.; Patti, A. Puncture Resistance and UV aging of Nanoparticle-Loaded Waterborne Polyurethane-Coated Polyester Textiles. Materials 2023, 16, 6844. [Google Scholar] [CrossRef]
- Salgado, C.A.; Vidigal, P.M.P.; Vanetti, M.C.D. Biodegradation of polyurethanes by Staphylococcus warneri and by microbial co-culture. Chemosphere 2024, 359, 142169. [Google Scholar] [CrossRef]
- Gunawan, N.R.; Tessman, M.; Zhen, D.; Johnson, L.; Evans, P.; Clements, S.M.; Pomeroy, R.S.; Burkart, M.D.; Simkovsky, R.; Mayfield, S.P. Biodegradation of renewable polyurethane foams in marine environments occurs through depolymerization by marine microorganisms. Sci. Total Environ. 2022, 850, 158761. [Google Scholar] [CrossRef] [PubMed]
- Orts, J.M.; Parrado, J.; Pascual, J.A.; Orts, A.; Cuartero, J.; Tejada, M.; Ros, M. Polyurethane foam residue biodegradation through the Tenebrio molitor digestive tract: Microbial communities and enzymatic activity. Polymers 2022, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- Motta, A.; La Mantia, F.P.; Ascione, L.; Mistretta, M.C. Theoretical study on the decomposition mechanism of bisphenol A polycarbonate induced by the combined effect of humidity and UV irradiation. J. Mol. Graph. Model 2020, 99, 107622. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudian, M.H.; Mesdaghinia, A.; Mahvi, A.H.; Nasseri, S.; Nabizadeh, R.; Dehghani, M.H. Photocatalytic degradation of bisphenol a from aqueous solution using bismuth ferric magnetic nanoparticle: Synthesis, characterization and response surface methodology-central composite design modeling, J. Environ. Health Sci. Eng. 2022, 20, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakami, Y.M.N.; Wahab, M.A.; Yildirir, E.; Ates, F. Thermal degradation kinetics, thermodynamics and pyrolysis behaviour of polycarbonate by TGA and Py-GC/MS. J. Energy Inst. 2024, 113, 101499. [Google Scholar]
- Kim, S.; Lee, H.S.; Yang, W.; Kwon, E.E. Recovery of lactic acid from biodegradable straw waste through a CO2-assisted thermochemical process. J. CO2 Util. 2022, 64, 102164. [Google Scholar] [CrossRef]
- Cañado, N.; Lizundia, E.; Akizu-Gardoki, O.; Minguez, R.; Lekube, B.; Arrillaga, A.; Iturrondobeitia, M. 3D printing to enable the reuse of marine plastic waste with reduced environmental impacts. J. Ind. Ecol. 2022, 26, 2092–2107. [Google Scholar] [CrossRef]
- Kaur, R.; Chauhan, I. Biodegradable plastics: Mechanisms of degradation and generated bio microplastic impact on soil health. Biodegradation 2024, 1–30. [Google Scholar] [CrossRef]
- Song, X.Y.; Liu, F.S.; Yu, S.T. Kinetics of poly(3-hydroxybutyrate) hydrolysis using acidic functionalized ionic liquid as catalyst, Catal. Today 2016, 276, 145–149. [Google Scholar] [CrossRef]
- Peller, J.R.; Mezyk, S.P.; Shidler, S.; Castleman, J.; Kaiser, S.; Faulkner, R.F.; Pilgrim, C.D.; Wilson, A.; Martens, S.; Horne, G.P. Facile nanoplastics formation from macro and microplastics in aqueous media. Environ. Pollut. 2022, 313, 120171. [Google Scholar] [CrossRef]
- Pothiraj, C.; Gokul, T.A.; Kumar, K.R.; Ramasubramanian, A.; Palanichamy, A.; Venkatachalam, K.; Pastorino, P.; Barcelò, D.; Balaji, P.; Faggio, C. Vulnerability of microplastics on marine environment: A review. Ecol. Indic. 2023, 155, 111058. [Google Scholar] [CrossRef]
- Fauser, P.; Vorkamp, K.; Strand, J. Residual additives in marine microplastics and their risk assessment—A critical review. Mar. Pollut. Bul. 2022, 177, 113467. [Google Scholar] [CrossRef] [PubMed]
- Habumugisha, T.; Zhang, Z.; Uwizewe, C.; Yan, C.; Ndayishimiye, J.C.; Rehman, A.; Zhang, X. Toxicological review of micro-and nano-plastics in aquatic environments: Risks to ecosystems, food web dynamics and human health. Ecotoxicol. Environ. Saf. 2024, 278, 116426. [Google Scholar] [CrossRef] [PubMed]
- Zappaterra, F.; Renzi, M.; Piccardo, M.; Spennato, M.; Asaro, F.; Di Serio, M.; Vitiello, R.; Turco, R.; Todea, A.; Gardossi, L. Understanding marine biodegradation of bio-based oligoesters and plasticizers. Polymers 2023, 15, 1536. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.M.; Feng, J.; Chen, J.; Delgado-Baquerizo, M.; Zhang, Q.; Zhou, X.-Q.; Yuan, Y.; Feng, S.; Zhang, K.; Liu, Y.-R. Microbial assemblies associated with temperature sensitivity of soil respiration along an altitudinal gradient. Sci. Total Environ. 2022, 820, 153257. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Wang, Y.; Li, Y.; Lin, L.; Chen, Y.; Shen, J. Occurrence, degradation pathways, and potential synergistic degradation mechanism of microplastics in surface water: A review. Curr. Pollut. Rep. 2023, 9, 312–326. [Google Scholar] [CrossRef]
- Kumar, A.; Youssef, G. Coexistence of Hardening and Softening Phenomena in Elastomeric Polymers under Nano-Impact Loading. Macromol. Mater. Eng. 2024, 2400134. [Google Scholar] [CrossRef]
- Yang, F.; Tchekwagep, J.J.K.; Wang, S.; Huang, S.; Cheng, X. The Effect of Extensive Heat Exposure on the Mechanical Properties of Polymer-Modified Sulfoaluminate Cement Repair Mortar. Case Stud. Constr. Mater. 2024, 20, e03348. [Google Scholar] [CrossRef]
- Vyshnava Kumar, G.; Rao, G.B.; Marshal, S.J.J.; Bannaravuri, P.K.; Joel, S.S.; Calvin, G.J. Effect of Bamboo Leaf and Pista Shell Powder Particles on Mechanical and Wear Behaviour of Polymer Composites. J. Inst. of Eng. 2023, 105, 911–921. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Z.; Li, H.; Teng, J.; Xu, L. Shape memory epoxy polymer (SMEP) composite mechanical properties enhanced by introducing graphene oxide (GO) into the matrix. Materials 2019, 12, 1107. [Google Scholar] [CrossRef]
- Riahinezhad, M.; Esmizadeh, E.; Lopez-Carreon, I.; Gaur, A.; Lu, H.; Lacasse, M.A. Crack Length of Elastomeric Sealants and Their Service Life in Contrasting Canadian Climates: Effects of Climate Change. Polymers 2024, 16, 2039. [Google Scholar] [CrossRef] [PubMed]
- Markovičová, L.; Zatkalíková, V. The effect of UV aging on structural polymers. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; p. 012004. [Google Scholar]
- Yoo, D.; Oh, S.; Han, Y.; Jeong, J.; Jung, S.; Park, K. Change of mechanical properties of e-PTFE support by electrochemical degradation in polymer electrolyte membrane fuel cell. Korean J. Chem. Eng. 2024, 41, 2433–2440. [Google Scholar] [CrossRef]
- Wang, N.; Gu, A.; Lei, Y. Characterization and evaluation of UV-irradiation aging of urushi by micro-UV/Py-GC/MS system. Sci. China Technol. Sci. 2023, 66, 2258–2270. [Google Scholar] [CrossRef]
- Zweifel, H. Degradation of Polymers by Photooxidation. Chimia 1993, 47, 390. [Google Scholar] [CrossRef]
- Li, W.; Zhao, W.; Zhu, H.; Li, Z.-J.; Wang, W. State of the art in the photochemical degradation of (micro) plastics: From fundamental principles to catalysts and applications. J. Mater. Chem. A 2023, 11, 2503–2527. [Google Scholar] [CrossRef]
- Watanabe, R.; Nakamura, S.; Sugahara, A.; Kishi, M.; Sato, H.; Hagihara, H.; Shinzawa, H. Revealing Molecular-Scale Structural Changes in Polymer Nanocomposites during Thermo-Oxidative Degradation Using Evolved Gas Analysis with High-Resolution Time-of-Flight Mass Spectrometry Combined with Principal Component Analysis and Kendrick Mass Defect Analysis. Anal. Chem. 2024, 96, 2628–2636. [Google Scholar]
- Quintana, A.; Celina, M.C. Overview of DLO modeling and approaches to predict heterogeneous oxidative polymer degradation. Polym. Degrad. Stab. 2018, 149, 173–191. [Google Scholar] [CrossRef]
- Papanikolaou, K.G.; Wu, J.; Huber, G.W.; Mavrikakis, M. Mechanistic insights into the pyrolysis of poly (vinyl chloride). J. Polym. Res. 2023, 30, 83. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Hoye, T.R. Poly (4-ketovalerolactone) from levulinic acid: Synthesis and hydrolytic degradation. Macromolecules 2020, 53, 4952–4959. [Google Scholar] [CrossRef]
- Wang, M.; Qiu, Y.; Chen, T.; Zhao, L. The catalytic mechanism and limiting factor of polyamide hydrolysis for chemical recycling: The classic hydrolysis of polyamide 4. J. Polym. Res. 2023, 30, 186. [Google Scholar] [CrossRef]
- Fukushima, K.; Hakozaki, S.; Lang, R.; Haga, Y.; Nakai, S.; Narumi, A.; Tanaka, M.; Kato, T. Hydrolyzable and biocompatible aliphatic polycarbonates with ether-functionalized side chains attached via amide linkers. Polym. J. 2024, 56, 431–442. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, W.; Valencak, T.G.; Zhang, Y.; Liu, G.; Ren, D. Biodegradation of conventional plastics: Candidate organisms and potential mechanisms. Sci. Total Environ. 2023, 885, 163908. [Google Scholar] [CrossRef] [PubMed]
- Sui, B.; Wang, T.; Fang, J.; Hou, Z.; Shu, T.; Lu, Z.; Liu, F.; Zhu, Y. Recent advances in the biodegradation of polyethylene terephthalate with cutinase-like enzymes. Front. Microbiol. 2023, 14, 1265139. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.R.; Cha, Q.Q.; Liu, S.S.; Wang, S.Y.; Li, P.Y.; Li, C.Y.; Wang, P.; Chen, X.L.; Tian, J.-W.; Xin, Y. Phytoplankton-derived polysaccharides and microbial peptidoglycans are key nutrients for deep-sea microbes in the Mariana Trench. Microbiome 2024, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Mrigwani, A.; Thakur, B.; Guptasarma, P. Conversion of polyethylene terephthalate into pure terephthalic acid through synergy between a solid-degrading cutinase and a reaction intermediate-hydrolysing carboxylesterase. Green Chem. 2022, 24, 6707–6719. [Google Scholar] [CrossRef]
- Kaur, R.; Goyal, D. Biodegradation of Butachlor by Bacillus altitudinis and Identification of Metabolites. Curr. Microbiol. 2020, 77, 2602–2612. [Google Scholar] [CrossRef]
- Kim, J.R.; Thelusmond, J.R.; Albright III, V.C.; Chai, Y. Exploring structure-activity relationships for polymer biodegradability by microorganisms. Sci. Total Environ. 2023, 890, 164338. [Google Scholar] [CrossRef]
- Rezaei, Z.; Moghimi, H. Fungal-bacterial consortia: A promising strategy for the removal of petroleum hydrocarbons. Ecotoxicol. Environ. Saf. 2024, 280, 116543. [Google Scholar] [CrossRef]
- Laolu-Balogun, E.; Owen, S.; Read, S.; Shipway, P.; Voisey, K. Effect of humidity and oxygen on friction, wear and durability of a polymer-bonded molybdenum disulfide (MoS2)-based dry film lubricant (DFL) coating system in large amplitude fretting. Wear 2024, 552–553, 205426. [Google Scholar] [CrossRef]
- He, S.Y.; Jia, M.Y.; Xiang, Y.P.; Song, B.; Xiong, W.P.; Cao, J.; Peng, H.H.; Yang, Y.; Wang, W.J.; Yang, Z.H.; et al. Biofilm on microplastics in aqueous environment: Physicochemical properties and environmental implications. J. Hazard. Mater. 2021, 424, 127286. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Y.; Chen, B.; Dong, G.; Zhao, Y.; Zhang, B. Marine biodegradation of plastic films by Alcanivorax under various ambient temperatures: Bacterial enrichment, morphology alteration, and release of degradation products. Sci. Total Environ. 2024, 917, 170527. [Google Scholar] [CrossRef] [PubMed]
- Christ, N.; Scheuring, B.M.; Schelleis, C.; Liebig, W.V.; Montesano, J.; Weidenmann, K.A.; Hohe, J. Characterization and simulation of the interface between a continuous and discontinuous carbon fiber reinforced thermoplastic by using the climbing drum peel test considering humidity. Polymers 2024, 16, 976. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ye, L.; Liu, H.; Wei, Q.; Zhang, X.; Zheng, Z.; Li, Z.; Lu, S. Flexible and transparent polylactic acid/hydrophobically modified nanobagasse cellulose/tannic acid/MXene films with highly efficient ultraviolet shielding. Compos. Sci. Technol. 2024, 251, 110550. [Google Scholar] [CrossRef]
- Ge, J.; Wang, M.; Liu, P.; Zhang, Z.; Peng, J.; Guo, X. A systematic review on the aging of microplastics and the effects of typical factors in various environmental media. Trends Anal. Chem. 2023, 162, 117025. [Google Scholar] [CrossRef]
- Cabeza, C.; van Lier, J.B.; van der Steen, P. Effects of thermal and enzymatic pre-treatments on the solubilisation of extracellular polymeric substances (EPS) and subsequent anaerobic digestion of microalgae-bacterial biomass. Algal Res. 2023, 72, 103130. [Google Scholar] [CrossRef]
- Ariza-Tarazona, M.C.; Siligardi, C.; Carreón-López, H.A.; Valdéz-Cerda, J.E.; Pozzi, P.; Kaushik, J.; Villarreal-Chiu, J.F.; Cedillo-González, E.I. Low environmental impact remediation of microplastics: Visible-light photocatalytic degradation of PET microplastics using bio-inspired C,N-TiO2/SiO2 photocatalysts. Mar. Pollut. Bull. 2023, 193, 115206. [Google Scholar] [CrossRef]
- Jiang, Z.; Erol, O.; Chatterjee, D.; Xu, W.; Hibino, N.; Romer, L.H.; Kang, S.H.; Gracias, D.H. Direct ink writing of poly (tetrafluoroethylene) (PTFE) with tunable mechanical properties. ACS Appl. Mater. Interfaces 2019, 11, 28289–28295. [Google Scholar] [CrossRef]
- Wang, J.; Gao, X.; Boarino, A.; Célerse, F.; Corminboeuf, C.; Klok, H.-A. Mechanical Acceleration of Ester Bond Hydrolysis in Polymers. Macromolecules 2022, 55, 10145–10152. [Google Scholar] [CrossRef]
- Laurel, M.; MacKinnon, D.; Becker, J.; Terracciano, R.; Drain, B.; Houck, H.A.; Becer, C.R. Degradable thioester core-crosslinked star-shaped polymers. Polym. Chem. 2022, 13, 5579–5589. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Wahbi, M.; Kasmi, N.; Papageorgiou, G.Z.; Bikiaris, D.N. Effect of additives on the thermal and thermo-oxidative stability of poly (ethylene furanoate) biobased polyester. Thermochim. Acta 2020, 686, 178549. [Google Scholar] [CrossRef]
- Plota, A.; Masek, A. Analysis of the aging and stabilization processes in cyclic polyolefins containing various natural or synthetic stabilizers. Polymers 2023, 273, 125879. [Google Scholar] [CrossRef]
- Chiarcos, R.; Sparnacci, K.; Antonioli, D.; Ivaldi, C.; Gianotti, V.; Po, R.; Biagini, P.; Losio, S.; Laus, M. Catalyst residues severely impact the thermal stability and degradation mechanism of polycarbonates: How to turn a flaw into an opportunity. European Polym. J. 2024, 214, 113148. [Google Scholar] [CrossRef]
- Queraltó, A.; Frohnhoven, R.; Mathur, S.; Gómez, A. Intrinsic piezoelectric characterization of BiFeO3 nanofibers and its implications for energy harvesting. Appl. Surf. Sci. 2020, 509, 144760. [Google Scholar] [CrossRef]
- Teijido, R.; Ruiz-Rubio, L.; Echaide, A.G.; Vilas-Vilela, J.L.; Lanceros-Mendez, S.; Zhang, Q. State of the art and current trends on layered inorganic-polymer nanocomposite coatings for anticorrosion and multi-functional applications. Prog. Org. Coat. 2022, 163, 106684. [Google Scholar] [CrossRef]
- Oh, S.M.; Lee, C.H.; Kim, S.Y. Processing method determines the long-term stability of particle dispersions in concentrated nanoparticle/polymer suspensions. Soft Matter 2022, 18, 841–848. [Google Scholar] [CrossRef]
- Lapuk, S.E.; Ponomareva, M.A.; Galukhin, A.V.; Mukhametzyanov, T.A.; Schick, C.; Gerasimov, A.V. Glass transition kinetics and physical aging of polyvinylpyrrolidones with different molecular masses. Macromolecules 2022, 55, 4516–4522. [Google Scholar] [CrossRef]
- Biale, G.; La Nasa, J.; Mattonai, M.; Corti, A.; Castelvetro, V.; Modugno, F. Seeping plastics: Potentially harmful molecular fragments leaching out from microplastics during accelerated ageing in seawater. Water Res. 2022, 219, 118521. [Google Scholar] [CrossRef]
- Li, Q.; Yan, J.; Li, Y.; Liu, Y.; Andom, O.; Li, Z. Microplastics alter cadmium accumulation in different soil-plant systems: Revealing the crucial roles of soil bacteria and metabolism. J. Hazard. Mater. 2024, 474, 134768. [Google Scholar] [CrossRef]
- Cui, W.; Gao, P.; Zhang, M.; Wang, L.; Sun, H.; Liu, C. Adverse effects of microplastics on earthworms: A critical review. Sci. Total Environ. 2022, 850, 158041. [Google Scholar] [CrossRef]
- Han, Y.; Lian, F.; Xiao, Z.; Gu, S.; Cao, X.; Wang, Z.; Xing, B. Potential toxicity of nanoplastics to fish and aquatic invertebrates: Current understanding, mechanistic interpretation, and meta-analysis. J. Hazard. Mater. 2022, 427, 127870. [Google Scholar] [CrossRef]
- Nunes, B.Z.; Huang, Y.; Ribeiro, V.V.; Wu, S.; Holbech, H.; Moreira, L.B.; Xu, E.G.; Castro, I.B. Microplastic contamination in seawater across global marine protected areas boundaries. Environ. Pollut. 2023, 316, 120692. [Google Scholar] [CrossRef] [PubMed]
- Scales, B.S.; Hassenrück, C.; Moldaenke, L.; Hassa, J.; Rückert-Reed, C.; Rummel, C.; Völkner, C.; Rynek, R.; Busche, T.; Kalinowski, J. Hunting for pigments in bacterial settlers of the Great Pacific Garbage Patch. Environ. Microbiol. 2024, 26, e16639. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Wu, P.; Pan, G.; Li, J.; Jin, H. Microplastics in seawater, sediment, and organisms from Hangzhou Bay. Mar. Pollut. Bull. 2022, 181, 113940. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, M.; Pastorino, P.; Lesa, D.; Renzi, M.; Anselmi, S.; Prearo, M.; Pizzul, E. Microplastics accumulation in functional feeding guilds and functional habit groups of freshwater macrobenthic invertebrates: Novel insights in a riverine ecosystem. Sci. Total Environ. 2022, 804, 150207. [Google Scholar] [CrossRef]
- Kyriakoudes, G.; Turner, A. Suspended and deposited microplastics in the coastal atmosphere of southwest England. Chemosphere 2023, 343, 140258. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, T.; Kang, S.; Shi, H.; Mai, L.; Allen, D.; Allen, S. Current status and future perspectives of microplastic pollution in typical cryospheric regions. Earth-Sci. Rev. 2022, 226, 103924. [Google Scholar] [CrossRef]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef]
- Napper, I.E.; Baroth, A.; Barrett, A.C.; Bhola, S.; Chowdhury, G.W.; Davies, B.F.; Duncan, E.M.; Kumar, S.; Nelms, S.E.; Niloy, M.N.H. The distribution and characterisation of microplastics in air, surface water and sediment within a major river system. Sci. Total Environ. 2023, 901, 166640. [Google Scholar] [CrossRef]
- Tong, H.; Zhong, X.; Duan, Z.; Yi, X.; Cheng, F.; Xu, W.; Yang, X. Micro-and nanoplastics released from biodegradable and conventional plastics during degradation: Formation, aging factors, and toxicity. Sci. Total Environ. 2022, 833, 155275. [Google Scholar] [CrossRef]
- Sin, L.T.; Balakrishnan, V.; Bee, S.-T.; Bee, S.-L. A review of the current state of microplastic pollution in South Asian countries. Sustainability 2023, 15, 6813. [Google Scholar] [CrossRef]
- Zolotova, N.; Kosyreva, A.; Dzhalilova, D.; Fokichev, N.; Makarova, O. Harmful effects of the microplastic pollution on animal health: A literature review. PeerJ 2022, 10, e13503. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Xu, P.; Zhou, W.; Xu, X.; Li, H.; He, W.; Yue, W.; Zhang, L.; Ding, D.; Suo, A. Impacts of conventional and biodegradable microplastics on juvenile Lates calcarifer: Bioaccumulation, antioxidant response, microbiome, and proteome alteration. Mar. Pollut. Bull. 2022, 179, 113744. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.Z.; Luo, X.Q.; Li, J.L.; Liu, Z.; Duan, L.; Deng, Q.Q.; Chen, C.; Tang, S.; Li, W.J.; Wang, P. Deciphering the pathogenic risks of microplastics as emerging particulate organic matter in aquatic ecosystem. J. Hazard. Mater. 2024, 474, 134728. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Iqbal, B.; Khan, I.; Khan, A.R.; Jho, E.H.; Salam, A.; Zhou, H.; Zhao, X.; Li, G.; Du, D. Microplastic contamination in the agricultural soil—Mitigation strategies, heavy metals contamination, and impact on human health: A review. Plant Cell Re. 2024, 43, 65. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, N.P.; Fennell, T.R.; Johnson, L.M. Unintended human ingestion of nanoplastics and small microplastics through drinking water, beverages, and food sources. NanoImpact 2021, 21, 100302. [Google Scholar] [CrossRef]
- Wang, W.; Do, A.T.N.; Kwon, J.H. Ecotoxicological effects of micro-and nanoplastics on terrestrial food web from plants to human beings. Sci. Total Environ. 2022, 834, 155333. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Li, H.; Ma, R.; Yu, Z.; Li, L.; Xiang, M.; Chen, X.; Hua, X.; Yu, Y. A review of toxicity induced by persistent organic pollutants (POPs) and endocrine-disrupting chemicals (EDCs) in the nematode Caenorhabditis elegans. J. Environ. Manag. 2019, 237, 519–525. [Google Scholar] [CrossRef]
- Liu, Z.; You, X.Y. Recent progress of microplastic toxicity on human exposure base on in vitro and in vivo studies. Sci. Total Environ. 2023, 903, 166766. [Google Scholar] [CrossRef]
- Chang, S.H. Plastic waste as pyrolysis feedstock for plastic oil production: A review. Sci. Total Environ. 2023, 877, 162719. [Google Scholar] [CrossRef]
- van Der Marel, E.R. Trading plastic waste in a global economy: Soundly regulated by the Basel Convention? J. Environ. Law 2022, 34, 477–497. [Google Scholar] [CrossRef]
- Miguel, I.; Santos, A.; Venâncio, C.; Oliveira, M. Knowledge, concerns and attitudes towards plastic pollution: An empirical study of public perceptions in Portugal. Sci. Total Environ. 2024, 906, 167784. [Google Scholar] [CrossRef] [PubMed]
- Damayanti, D.; Saputri, D.R.; Marpaung, D.S.S.; Yusupandi, F.; Sanjaya, A.; Simbolon, Y.M.; Asmarani, W.; Ulfa, M.; Wu, H.S. Current prospects for plastic waste treatment. Polymers 2022, 14, 3133. [Google Scholar] [CrossRef] [PubMed]
- Amesho, K.T.; Chinglenthoiba, C.; Samsudin, M.S.; Lani, M.N.; Pandey, A.; Desa, M.N.M.; Suresh, V. Microplastics in the environment: An urgent need for coordinated waste management policies and strategies. J. Environ. Manag. 2023, 344, 118713. [Google Scholar] [CrossRef] [PubMed]
- Goodman, K.E.; Hua, T.; Sang, Q.X.A. Effects of polystyrene microplastics on human kidney and liver cell morphology, cellular proliferation, and metabolism. ACS Omega 2022, 7, 34136–34153. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, A.A.; Alnasser, S.M.; Shalaby, A.M.; Alabiad, M.A.; Abd-Almotaleb, N.A.; Alorini, M.; Jaber, F.A.; Khayal, E.E.-S. Polystyrene microplastic particles induced hepatotoxic injury via pyroptosis, oxidative stress, and fibrotic changes in adult male albino rats; the therapeutic role of silymarin. Toxicol. Mech. Methods 2023, 33, 512–528. [Google Scholar] [CrossRef]
- Liang, X.; Liang, J.; Zhang, S.; Yan, H.; Luan, T. Di-2-ethylhexyl phthalate disrupts hepatic lipid metabolism in obese mice by activating the LXR/SREBP-1c and PPAR-α signaling pathways. Sci. Total Environ. 2024, 914, 169919. [Google Scholar] [CrossRef]
- Li, X.; Feng, L.; Kuang, Q.; Wang, X.; Yang, J.; Niu, X.; Gao, L.; Huang, L.; Luo, P.; Li, L. Microplastics cause hepatotoxicity in diabetic mice by disrupting glucolipid metabolism via PP2A/AMPK/HNF4A and promoting fibrosis via the Wnt/β-catenin pathway. Environ. Toxicol. 2024, 39, 1018–1030. [Google Scholar] [CrossRef]
- Tao, J.; Deng, P.; Lin, M.; Chen, C.; Ma, Q.; Yang, L.; Zhang, W.; Luo, Y.; Chen, S.; Pi, H. Long-term exposure to polystyrene microplastics induces hepatotoxicity by altering lipid signatures in C57BL/6J mice. Chemosphere 2024, 347, 140716. [Google Scholar] [CrossRef]
- Su, H.Y.; Lai, C.S.; Lee, K.H.; Chiang, Y.W.; Chen, C.C.; Hsu, P.C. Prenatal exposure to low-dose di-(2-ethylhexyl) phthalate (DEHP) induces potentially hepatic lipid accumulation and fibrotic changes in rat offspring. Ecotoxicol. Environ. Saf. 2024, 269, 115776. [Google Scholar] [CrossRef]
- Li, S.; Gu, X.; Zhang, M.; Jiang, Q.; Xu, T. Di (2-ethylhexyl) phthalate and polystyrene microplastics co-exposure caused oxidative stress to activate NF-κB/NLRP3 pathway aggravated pyroptosis and inflammation in mouse kidney. Sci. Total Environ. 2024, 926, 171817. [Google Scholar] [CrossRef]
- Xiong, X.; Gao, L.; Chen, C.; Zhu, K.; Luo, P.; Li, L. The microplastics exposure induce the kidney injury in mice revealed by RNA-seq. Ecotoxicol. Environ. Saf. 2023, 256, 114821. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.R.; Yan, K.; Nguyen, W.; Rawle, D.J.; Tang, B.; Larcher, T.; Suhrbier, A. Microplastics dysregulate innate immunity in the SARS-CoV-2 infected lung. Front. Immun. 2024, 15, 1382655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, Y.; Jing, L.; Zhao, H. Microplastics induced inflammation in the spleen of developmental Japanese quail (Coturnix japonica) via ROS-mediated p38 MAPK and TNF signaling pathway activation1. Environ. Pollut. 2024, 341, 122891. [Google Scholar] [CrossRef] [PubMed]
- Sarma, D.K.; Dubey, R.; Samarth, R.M.; Shubham, S.; Chowdhury, P.; Kumawat, M.; Verma, V.; Tiwari, R.R.; Kumar, M. The biological effects of polystyrene nanoplastics on human peripheral blood lymphocytes. Nanomaterials 2022, 12, 1632. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Zhang, Y.; Zhang, X.; Zhang, G.; Jin, C.; Yang, J.; Wu, S.; Lu, X. Bisphenol A and Di (2-Ethylhexyl) Phthalate promote pulmonary carcinoma in female rats via estrogen receptor beta: In vivo and in silico analysis. Ecotoxicol. Environ. Saf. 2023, 250, 114496. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, D.; Wan, Z.; Wei, Z.; Chen, Z.; Wang, Y.; Han, X.; Chen, Y. Exposure to different surface-modified polystyrene nanoparticles caused anxiety, depression, and social deficit in mice via damaging mitochondria in neurons. Sci. Total Environ. 2024, 919, 170739. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Lee, S.H.; Moon, H.J.; So, Y.H.; Lee, H.R.; Lee, E.-H.; Jung, E.-M. Exposure to polystyrene particles causes anxiety-, depression-like behavior and abnormal social behavior in mice. J. Hazard. Mater. 2023, 454, 131465. [Google Scholar] [CrossRef]
- Pasquini, E.; Ferrante, F.; Passaponti, L.; Pavone, F.S.; Costantini, I.; Baracchi, D. Microplastics reach the brain and interfere with honey bee cognition. Sci. Total Environ. 2024, 912, 169362. [Google Scholar] [CrossRef]
- Shi, C.; Han, X.; Guo, W.; Wu, Q.; Yang, X.; Wang, Y.; Tang, G.; Wang, S.; Wang, Z.; Liu, Y. Disturbed Gut-Liver axis indicating oral exposure to polystyrene microplastic potentially increases the risk of insulin resistance. Environ. Inter. 2022, 164, 107273. [Google Scholar] [CrossRef]
- Biemann, R.; Blüher, M.; Isermann, B. Exposure to endocrine-disrupting compounds such as phthalates and bisphenol A is associated with an increased risk for obesity. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101546. [Google Scholar] [CrossRef]
- Chen, G.; Xiong, S.; Jing, Q.; van Gestel, C.A.; van Straalen, N.M.; Roelofs, D.; Sun, L.; Qiu, H. Maternal exposure to polystyrene nanoparticles retarded fetal growth and triggered metabolic disorders of placenta and fetus in mice. Sci. Total Environ. 2023, 854, 158666. [Google Scholar] [CrossRef] [PubMed]
- Rong, W.; Chen, Y.; Xiong, Z.; Zhao, H.; Li, T.; Liu, Q.; Song, J.; Wang, X.; Liu, Y.; Liu, S. Effects of combined exposure to polystyrene microplastics and 17α-Methyltestosterone on the reproductive system of zebrafish. Theriogenology 2024, 215, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, Y.; Li, G.; Xiong, Y.; Zhang, Y.; Zhang, M. The hidden threat: Unraveling the impact of microplastics on reproductive health. Sci. Total Environ. 2024, 935, 173177. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Ma, T.; Sha, X.; Liu, Z.; Zhou, Y.; Meng, X.; Chen, Y.; Han, X.; Ding, J. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 2021, 401, 123430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Zhao, Y.; Zhao, J.; Yu, T.; Yao, Y.; Zhao, R.; Yu, R.; Liu, J.; Su, J. Reproductive toxicity of microplastics in female mice and their offspring from induction of oxidative stress. Environ. Pollut. 2023, 327, 121482. [Google Scholar] [CrossRef]
- Qiu, Y.; Luo, L.; Yang, Y.; Kong, Y.; Li, Y.; Wang, D. Potential toxicity of nanopolystyrene on lifespan and aging process of nematode Caenorhabditis elegans. Sci. Total Environ. 2020, 705, 135918. [Google Scholar] [CrossRef]
- Li, H.; Jiang, Y.; Gu, Y.; Chen, C.; Yu, J.; Wang, C.; Shi, C.; Pan, R.; Chen, H. Environmentally persistent free radicals on photoaging microplastics shortens longevity via inducing oxidative stress in Caenorhabditis elegans. Chemosphere 2024, 361, 142560. [Google Scholar] [CrossRef]
- Zaheer, J.; Kim, H.; Ko, I.O.; Jo, E.-K.; Choi, E.-J.; Lee, H.-J.; Shim, I.; Woo, H.-j.; Choi, J.; Kim, G.-H. Pre/post-natal exposure to microplastic as a potential risk factor for autism spectrum disorder. Environ. Inter. 2022, 161, 107121. [Google Scholar] [CrossRef]
- Teng, M.; Zhao, X.; Wang, C.; Wang, C.; White, J.C.; Zhao, W.; Zhou, L.; Duan, M.; Wu, F. Polystyrene nanoplastics toxicity to zebrafish: Dysregulation of the brain–intestine–microbiota axis. ACS Nano 2022, 16, 8190–8204. [Google Scholar] [CrossRef]
- Vattanasit, U.; Kongpran, J.; Ikeda, A. Airborne microplastics: A narrative review of potential effects on the human respiratory system. Sci. Total Environ. 2023, 904, 166745. [Google Scholar] [CrossRef]
- Borgatta, M.; Breider, F. Inhalation of Microplastics—A Toxicological Complexity. Toxics 2024, 12, 358. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gomes, D.; Jin, L.; Mathis, S.P.; Li, X.; Rouchka, E.C.; Bodduluri, H.; Conklin, D.J.; Toole, T.E.O. Polystyrene bead ingestion promotes adiposity and cardiometabolic disease in mice. Ecotoxicol. Environ. Saf. 2022, 232, 113239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, C.; Liu, Y.; Li, W.; Li, S.; Peng, L.; Kang, L.; Ullah, S.; Gong, Z.; Li, Z. Microplastics are detected in human gallstones and have the ability to form large cholesterol-microplastic heteroaggregates. J. Hazard. Mater. 2024, 467, 133631. [Google Scholar] [CrossRef] [PubMed]
- Abafe, O.A.; Harrad, S.; Abdallah, M.A.-E. Assessment of human dermal absorption of flame retardant additives in polyethylene and polypropylene microplastics using 3D human skin equivalent models. Environ. Int. 2024, 186, 108635. [Google Scholar] [CrossRef] [PubMed]
- Lett, Z.; Hall, A.; Skidmore, S.; Alves, N.J. Environmental microplastic and nanoplastic: Exposure routes and effects on coagulation and the cardiovascular system. Environ. Pollut. 2021, 291, 118190. [Google Scholar] [CrossRef]
- Cappello, T.; De Marco, G.; Conti, G.O.; Giannetto, A.; Ferrante, M.; Mauceri, A.; Maisano, M. Time-dependent metabolic disorders induced by short-term exposure to polystyrene microplastics in the Mediterranean mussel Mytilus galloprovincialis. Ecotoxicol. Environ. Saf. 2021, 209, 111780. [Google Scholar] [CrossRef]
- Gaspar, L.; Bartman, S.; Coppotelli, G.; Ross, J.M. Acute exposure to microplastics induced changes in behavior and inflammation in young and old mice. Inter. J. Mol. Sci. 2023, 24, 12308. [Google Scholar] [CrossRef]
- Bao, R.Q.; Cheng, Z.R.; Peng, L.C.; Mehmood, T.; Gao, L.; Zhuo, S.C.; Wang, L.; Su, Y.Y. Effects of biodegradable and conventional microplastics on the intestine, intestinal community composition, and metabolic levels in tilapia (Oreochromis mossambicus). Aquat. Toxicol. 2023, 265, 106745. [Google Scholar] [CrossRef]
- Song, Z.; Wu, H.; Fang, X.; Feng, X.; Zhou, L. The cardiovascular toxicity of polystyrene microplastics in rats: Based on untargeted metabolomics analysis. Front. Pharmacol. 2024, 15, 1336369. [Google Scholar] [CrossRef]
- Vasse, G.F.; Melgert, B.N. Microplastic and plastic pollution: Impact on respiratory disease and health. Eur. Res. Rev. 2024, 33, 230226. [Google Scholar] [CrossRef]
- Luo, T.; Wang, D.; Zhao, Y.; Li, X.; Yang, G.; Jin, Y. Polystyrene microplastics exacerbate experimental colitis in mice tightly associated with the occurrence of hepatic inflammation. Sci. Total Environ. 2022, 844, 156884. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, J.; Tretola, M.; Seifert, J.; Brugger, D. Interactions between microplastics and the gastrointestinal microbiome. Ital. J. Anim. Sci. 2024, 23, 1044–1056. [Google Scholar] [CrossRef]
- Kaseke, T.; Lujic, T.; Cirkovic Velickovic, T. Nano-and microplastics migration from plastic food packaging into dairy products: Impact on nutrient digestion, absorption, and metabolism. Foods 2023, 12, 3043. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Rathman, J.F.; Magdziarz, T.; Mostrag, A.; Kulkarni, S.; Barton-Maclaren, T.S. Do similar structures have similar no observed adverse effect level (NOAEL) values? Exploring chemoinformatics approaches for estimating NOAEL bounds and uncertainties. Chem. Res. Toxicol. 2020, 34, 616–633. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.D.; Elanjickal, A.I.; Mankar, J.S.; Krupadam, R.J. Assessment of cancer risk of microplastics enriched with polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2020, 398, 122994. [Google Scholar] [CrossRef]
- Song, J.; Chen, X.; Li, S.; Tang, H.; Dong, S.; Wang, M.; Xu, H. The environmental impact of mask-derived microplastics on soil ecosystems. Sci. Total Environ. 2024, 912, 169182. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, W.; Zhang, Z.; Grossart, H.-P.; Gadd, G.M. Microplastics provide new microbial niches in aquatic environments. Appl. Microbiol. Biotechnol. 2020, 104, 6501–6511. [Google Scholar] [CrossRef]
- Ng, P.L.; Kinn-Gurzo, S.S.; Chan, K.Y.K. Microplastics impede larval urchin selective feeding. Sci. Total Environ. 2022, 838, 155770. [Google Scholar] [CrossRef]
- Lin, W.; Luo, H.; Wu, J.; Liu, X.; Cao, B.; Liu, Y.; Yang, P.; Yang, J. Polystyrene microplastics enhance the microcystin-LR-induced gonadal damage and reproductive endocrine disruption in zebrafish. Sci. Total Environ. 2023, 876, 162664. [Google Scholar] [CrossRef]
- Lim, Y.K.; Lee, M.; Hong, S.; Baek, S.H. Laboratory evaluation of floating marine plastic debris as a potential vector for transportation of the harmful benthic dinoflagellate Fukuyoa koreansis. J. Appl. Phycol. 2022, 34, 2515–2521. [Google Scholar] [CrossRef]
- Burgos-Aceves, M.A.; Abo-Al-Ela, H.G.; Faggio, C. Impact of phthalates and bisphenols plasticizers on haemocyte immune function of aquatic invertebrates: A review on physiological, biochemical, and genomic aspects. J. Hazard. Mater. 2021, 419, 126426. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, I.; Lorigo, M.; Cairrao, E. Update about the disrupting-effects of phthalates on the human reproductive system. Mol. Reprod. Dev. 2021, 88, 650–672. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.S.; Brownlow, A.; Baillie, A.; Barber, J.L.; Barnett, J.; Davison, N.J.; Deaville, R.; Ten Doeschate, M.; Penrose, R.; Perkins, M. Evaluation of a marine mammal status and trends contaminants indicator for European waters. Sci. Total Environ. 2023, 866, 161301. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, J.F.; Lu, Y.; Hua, D.; Wang, X.; Zou, X.H. Biodegradable microplastics (BMPs): A new cause for concern? Environ. Sci. Pollut. Res. 2021, 28, 66511–66518. [Google Scholar] [CrossRef]

| Publication Date | Degradation Mechanism | Research Methods | Key Findings | Ref. |
|---|---|---|---|---|
| January 2023 | Thermo-oxidative degradation | Thermogravimetric analysis | Found that high temperature can alter the thermal degradation performance of PVC. | [79] |
| June 2023 | Photocatalytic degradation | Thermogravimetric analysis, visible light irradiation test | The effectiveness of bio-inspired C,N-TiO2/SiO2 photocatalysts in the degradation of PET microplastics was discovered. | [97] |
| January 2024 | Hydrolytic degradation | Hydrolysis test, platelet adhesion test | Demonstrated the effects of an amide linker on the hydration and hydrolytic properties of APCs. | [82] |
| August 2022 | Enzymatic degradation | Establishment of enzyme reaction system, enzyme catalyzed degradation | Described the synergistic effect of keratinase hydrolysis carboxylesterase in PET degradation. | [86] |
| May 2023 | Microbial degradation | Microbial cultivation screening, biodegradation test | Explored the effect of the structure–activity relationship of microorganisms on the biodegradability of polymers. | [88] |
| Environmental Conditions | Main Degradation Pathways | Degradation Products | Persistence |
|---|---|---|---|
| Water | Hydrolytic degradation, UV degradation | Microplastics, nanoplastics, low molecular weight compounds | Medium to high persistence, possibly several years or even longer |
| Soil | Microbial degradation, chemical degradation | Organic acids, CO2, methane, microplastics | Low to moderate persistence |
| Air | Thermal degradation, photo-oxidative degradation | CO2, low molecular weight volatile organic compounds | Low to moderate persistence |
| Sea | Hydrolytic degradation, UV degradation | Microplastics, nanoplastics, low molecular weight compounds | High persistence, ranging from years to decades |
| Time Period | Process Description | Impact Analysis |
|---|---|---|
| Day 0 | Environmental exposure, polymer release into the environment (such as landfill and industrial emissions). | Polymers begin to be exposed to nature and are affected by factors such as ultraviolet radiation, temperature, and humidity. |
| Day 1–30 | Initial degradation, physical and chemical degradation, begins. | Molecular weight decreases and surface degradation occurs. |
| Day 30–90 | Further degradation of polymers produces intermediate products (small molecule compounds or microplastic particles). | Intermediate products may enter the environment and affect ecosystems. |
| Day 90–180 | Biodegradable polymer products (microorganisms or enzymes) generate smaller molecules or harmless substances. | Degradation is deeper under biological action, and some substances may be toxic. |
| Day 180–Year 1 | Partial incomplete degradation products accumulate in the environment and may be transferred upwards through the food chain. | The long-term accumulation of products, which may enter water sources, the soil, and the atmosphere, affecting the health of animals and plants. |
| Year 1–5 | Degradation products or microplastics are ingested by animals through the food chain, affecting their physiological functions. | Potential toxic effects, including visceral damage, metabolic disorders, etc. |
| Polymer Type | Degradation Products | Exposure Pathway | Observed Effects | Relevance to Human Health |
|---|---|---|---|---|
| Polylactic acid (PLA) | CO2, H2O | Oral administration, injection, skin contact | Changes in weight and blood biochemical indicators | Less restricted and generally considered a relatively safe degradation product. |
| Polybutylene succinate (PBS) | Succinic acid, butanediol | Oral administration, injection | Liver and kidney damage, blood toxicity | Further research is needed on the potential impact of long-term exposure on human health. |
| Polyethylene (PE) | Microplastic particles | Inhalation, skin contact | Allergic reactions, respiratory system issues | May have a significant impact on individuals with allergies. |
| Polypropylene (PP) | Succinic acid, butanediol | Inhalation, injection | Immune system response, nervous system damage | May lead to immune and neurological health issues. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yin, Z.; Xiang, S.; Yan, H.; Tian, H. Degradation of Polymer Materials in the Environment and Its Impact on the Health of Experimental Animals: A Review. Polymers 2024, 16, 2807. https://doi.org/10.3390/polym16192807
Zhang X, Yin Z, Xiang S, Yan H, Tian H. Degradation of Polymer Materials in the Environment and Its Impact on the Health of Experimental Animals: A Review. Polymers. 2024; 16(19):2807. https://doi.org/10.3390/polym16192807
Chicago/Turabian StyleZhang, Xiyu, Zhenxing Yin, Songbai Xiang, Huayu Yan, and Hailing Tian. 2024. "Degradation of Polymer Materials in the Environment and Its Impact on the Health of Experimental Animals: A Review" Polymers 16, no. 19: 2807. https://doi.org/10.3390/polym16192807
APA StyleZhang, X., Yin, Z., Xiang, S., Yan, H., & Tian, H. (2024). Degradation of Polymer Materials in the Environment and Its Impact on the Health of Experimental Animals: A Review. Polymers, 16(19), 2807. https://doi.org/10.3390/polym16192807







