Wound Dressing with Electrospun Core-Shell Nanofibers: From Material Selection to Synthesis
Abstract
1. Introduction
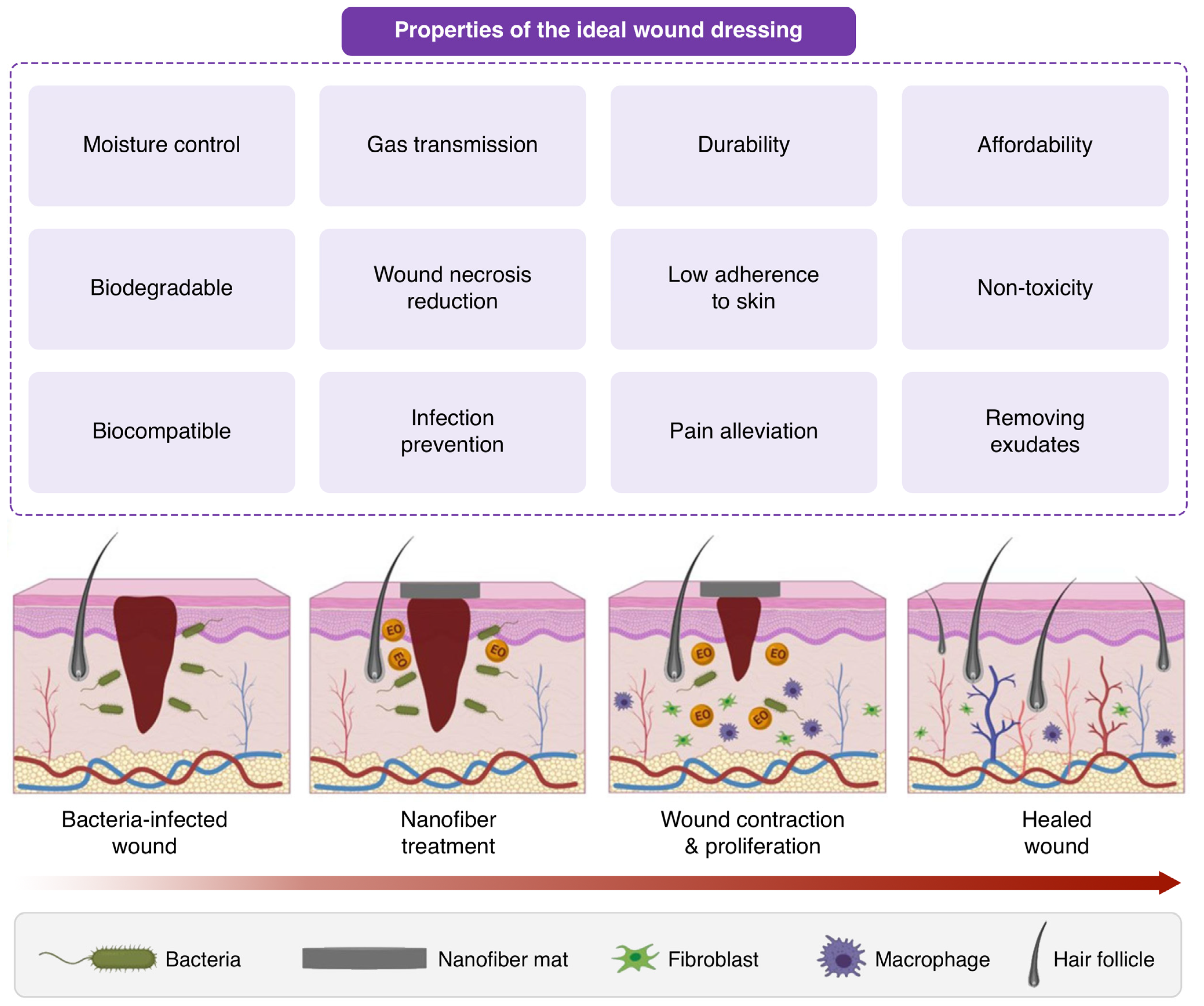
2. Wound Dressing: An Emerging Field of Study
Ideal Wound Dressing
3. Nanofibers
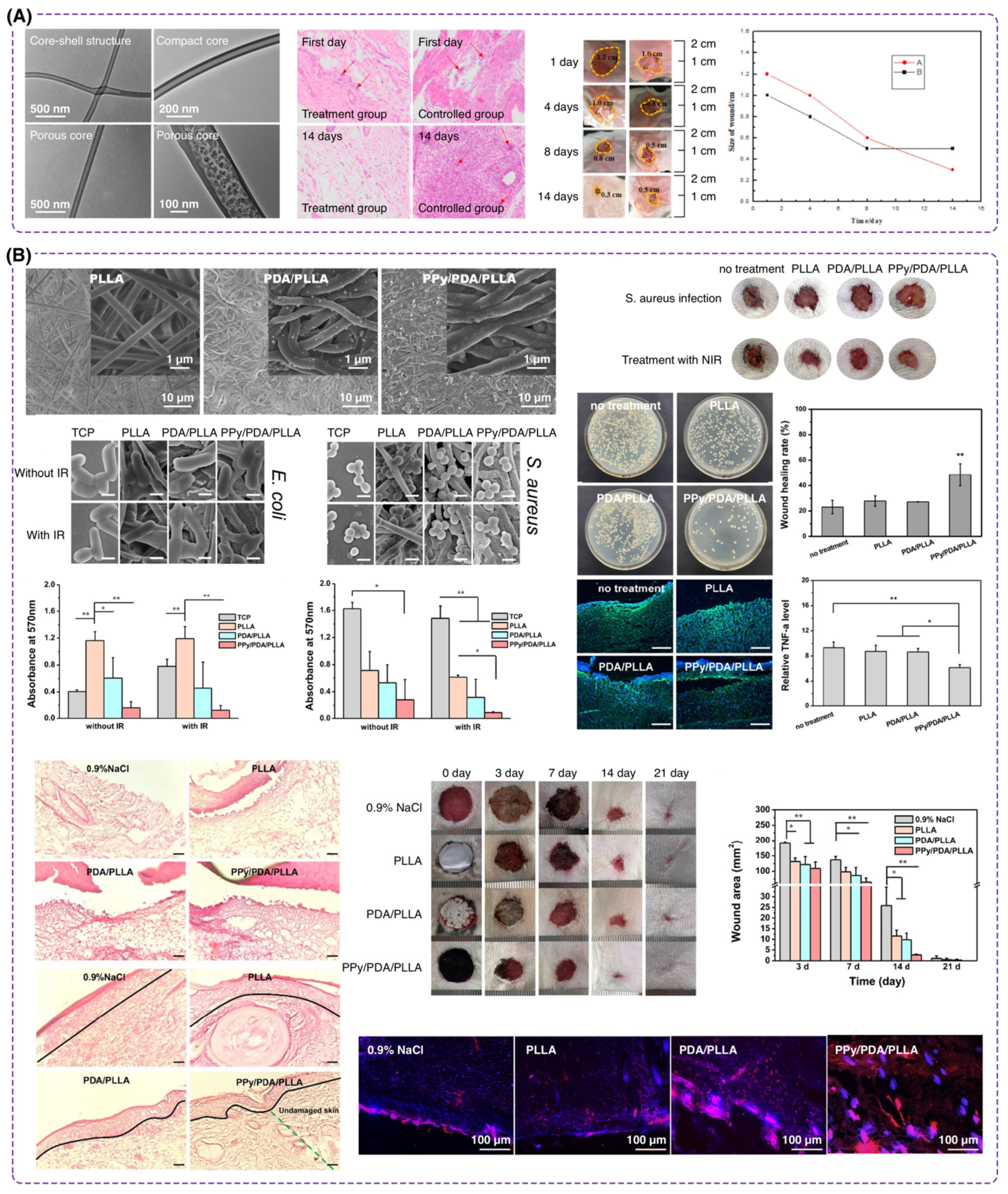
3.1. Core-Shell Nanofibers
3.2. Porous Nanofibers

3.3. Composite Nanofibers
3.4. Functionalized Nanofibers
3.5. Hollow Nanofibers
3.6. Solid Nanofibers
4. Materials Selection
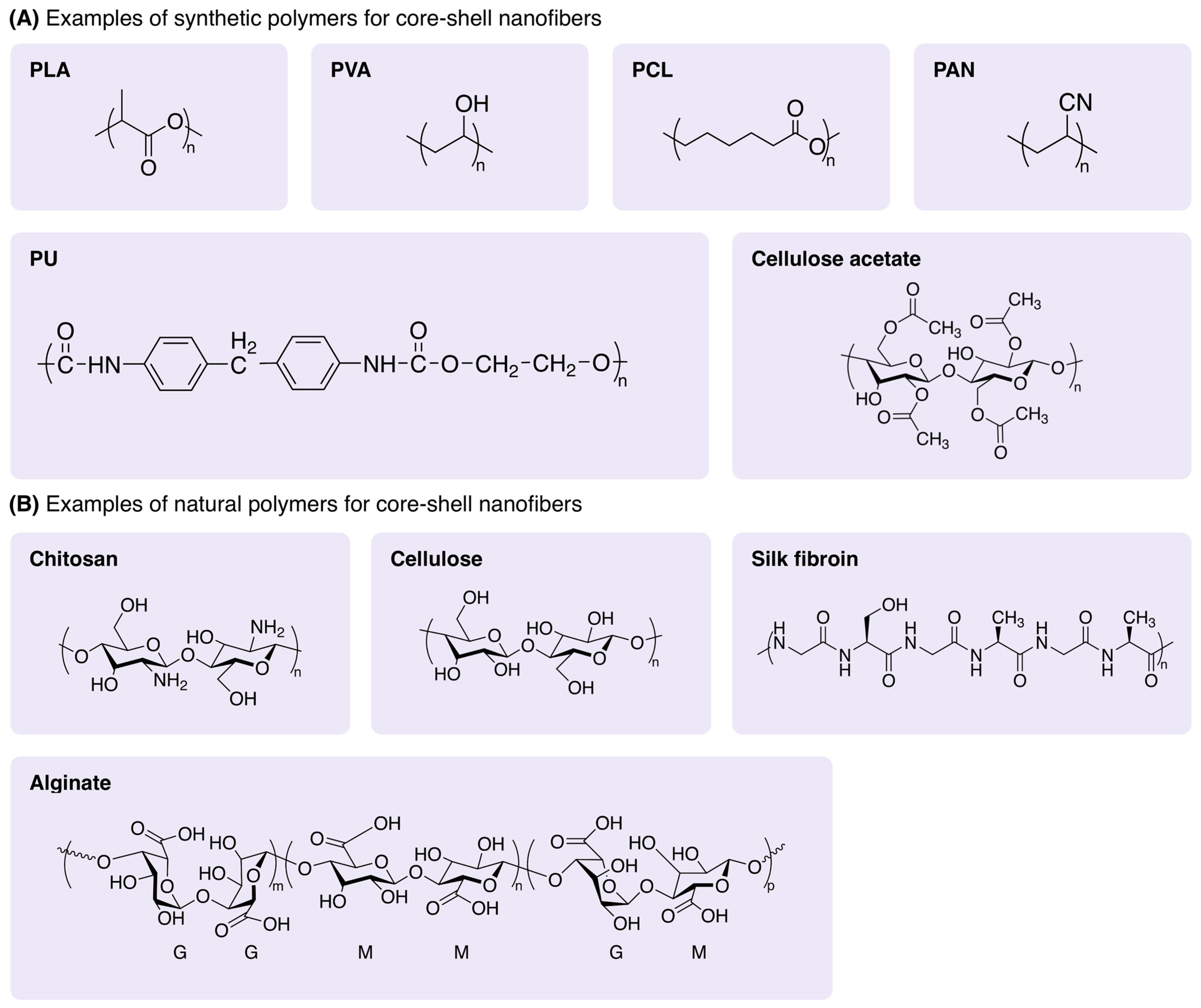
4.1. Materials for Core-Shell Nanofibers
4.1.1. Synthetic Polymers
4.1.2. Natural Polymers
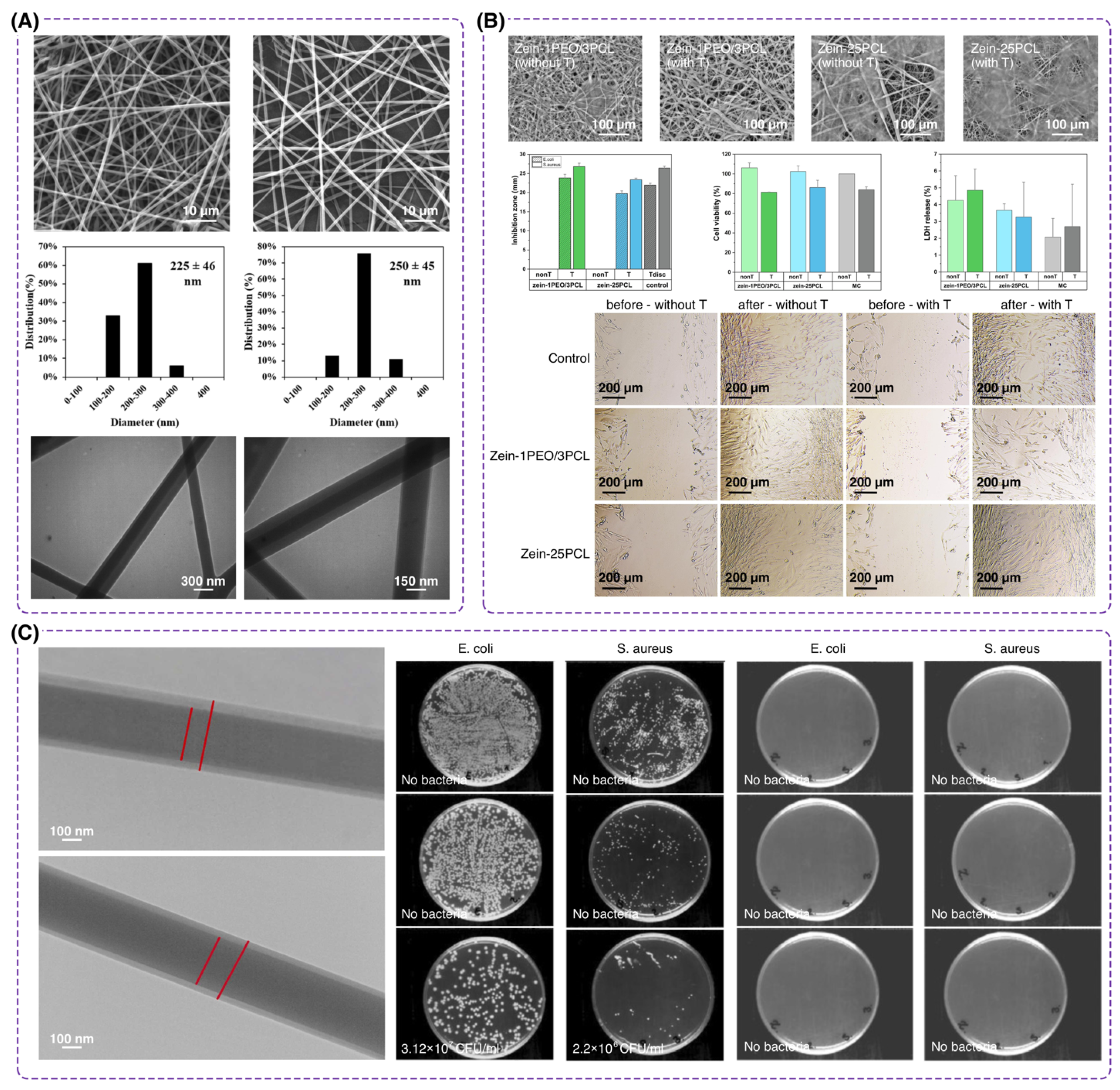
4.1.3. Antibacterial Materials
5. Processing Methods
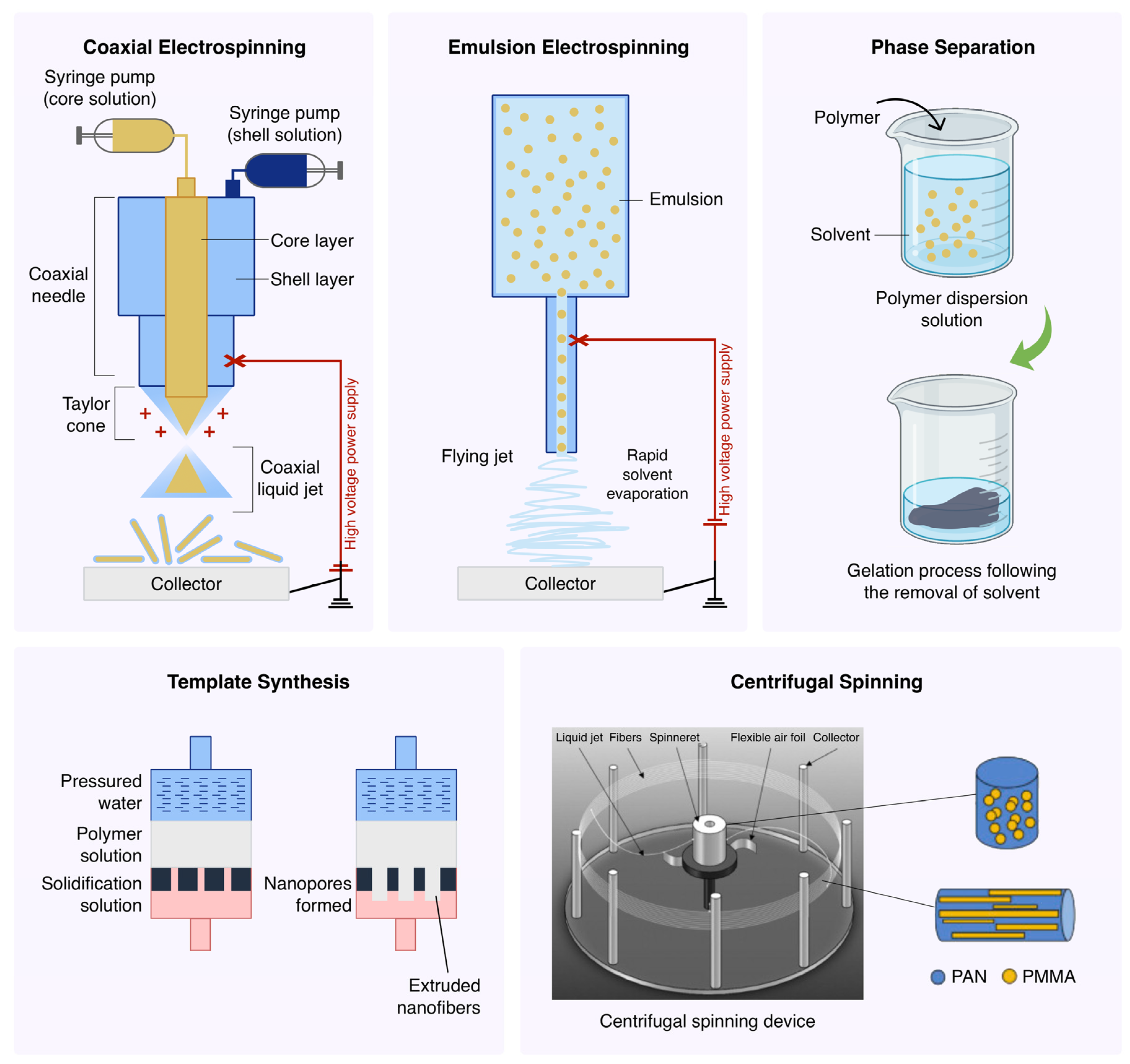
5.1. Coaxial Electrospinning
5.2. Emulsion Electrospinning
| Method | Pros | Cons |
|---|---|---|
| Coaxial electrospinning |
|
|
| Emulsion electrospinning |
|
|
| Phase separation |
|
|
| Template synthesis |
|
|
| Centrifugal spinning |
|
|
5.3. Template Synthesis
5.4. Centrifugal Spinning
5.5. Phase Separation
6. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Ovington, L.G. Advances in wound dressings. Clin. Dermatol. 2007, 25, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.; Grey, J.E.; Harding, K.G. Wound dressings. Br. Med. J. 2006, 332, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Paul, W. Advances in Wound Healing Materials; Smithers Rapra: Gaithersburg, MD, USA, 2015. [Google Scholar]
- Aramwit, P. Introduction to biomaterials for wound healing. Wound Health Biomater. 2016, 2, 3–38. [Google Scholar] [CrossRef]
- MacEwan, M.R.; MacEwan, S.; Kovacs, T.R.; Batts, J. What Makes the Optimal Wound Healing Material? A Review of Current Science and Introduction of a Synthetic Nanofabricated Wound Care Scaffold. Cureus 2017, 9, e1736. [Google Scholar] [CrossRef] [PubMed]
- Raju, N.R.; Silina, E.; Stupin, V.; Manturova, N.; Chidambaram, S.B.; Achar, R.R. Multifunctional and Smart Wound Dressings—A Review on Recent Research Advancements in Skin Regenerative Medicine. Pharmaceutics 2022, 14, 1574. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Khalili, S.; Khorasani, S.N.; Neisiany, R.E.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Tran, H.Q.; Shahriar, S.M.S.; Yan, Z.; Xie, J. Recent Advances in Functional Wound Dressings. Adv. Wound Care 2023, 12, 399–427. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Le, T.T.N.; Nguyen, A.T.; Le, H.N.T.; Pham, T.T. Biomedical materials for wound dressing: Recent advances and applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef]
- Dong, R.; Guo, B. Smart wound dressings for wound healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Kenry; Lim, C.T. Nanofiber technology: Current status and emerging developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar] [CrossRef]
- Abdullah, M.F.; Nuge, T.; Andriyana, A.; Ang, B.C.; Muhamad, F. Core-Shell Fibers: Design, Roles, and Controllable Release Strategies in Tissue Engineering and Drug Delivery. Polymers 2019, 11, 2008. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Zeng, L.; Qiao, Z.; Liu, X.; Liu, H.; Zhang, J.; Ding, J. Fabrication of Electrospun Polymer Nanofibers with Diverse Morphologies. Molecules 2019, 24, 834. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Tanioka, A. Functionality in Electrospun Nanofibrous Membranes Based on Fiber’s Size, Surface Area, and Molecular Orientation. Membranes 2011, 1, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Naseri, E.; Ahmadi, A. A review on wound dressings: Antimicrobial agents, biomaterials, fabrication techniques, and stimuli-responsive drug release. Eur. Polym. J. 2022, 173, 111293. [Google Scholar] [CrossRef]
- Broussard, K.C.; Powers, J.G. Wound Dressings: Selecting the Most Appropriate Type. Am. J. Clin. Dermatol. 2013, 14, 449–459. [Google Scholar] [CrossRef]
- Zaman, H.U.; Islam, J.M.M.; Khan, M.A.; Khan, R.A. Physico-mechanical properties of wound dressing material and its biomedical application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Nanomaterials for Wound Dressings: An Up-to-Date Overview. Molecules 2020, 25, 2699. [Google Scholar] [CrossRef]
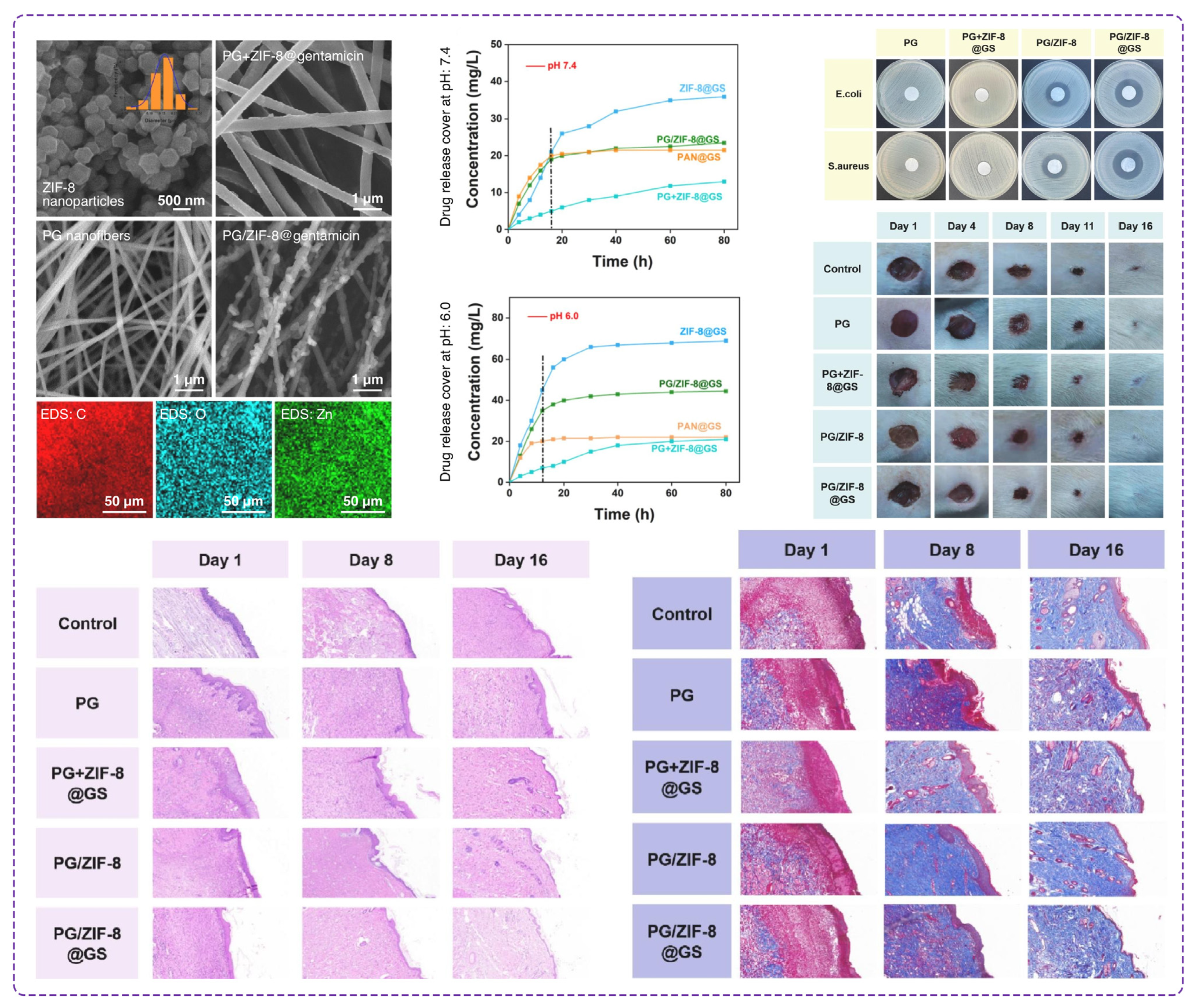
- Zhao, K.; Lu, Z.-H.; Zhao, P.; Kang, S.-X.; Yang, Y.-Y.; Yu, D.-G. Modified tri–axial electrospun functional core-shell nanofibrous membranes for natural photodegradation of antibiotics. Chem. Eng. J. 2021, 425, 131455. [Google Scholar] [CrossRef]
- Zhou, J.; Yi, T.; Zhang, Z.; Yu, D.-G.; Liu, P.; Wang, L.; Zhu, Y. Electrospun Janus core (ethyl cellulose//polyethylene oxide) @ shell (hydroxypropyl methyl cellulose acetate succinate) hybrids for an enhanced colon-targeted prolonged drug absorbance. Adv. Compos. Hybrid Mater. 2023, 6, 189. [Google Scholar] [CrossRef]
- Sun, Z.; Zussman, E.; Yarin, A.L.; Wendorff, J.H.; Greiner, A. Compound Core-Shell Polymer Nanofibers by Co-Electrospinning. Adv. Mater. 2003, 15, 1929–1932. [Google Scholar] [CrossRef]
- Yang, J.; Xu, L. Electrospun Nanofiber Membranes with Various Structures for Wound Dressing. Materials 2023, 16, 6021. [Google Scholar] [CrossRef]
- Abdelhakeem, E.; Monir, S.; Teaima, M.H.M.; Rashwan, K.O.; El-Nabarawi, M. State-of-the-Art Review of Advanced Electrospun Nanofiber Composites for Enhanced Wound Healing. AAPS PharmSciTech 2023, 24, 246. [Google Scholar] [CrossRef] [PubMed]
- Valachová, K.; El Meligy, M.A.; Šoltés, L. Hyaluronic acid and chitosan-based electrospun wound dressings: Problems and solutions. Int. J. Biol. Macromol. 2022, 206, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Behere, I.; Ingavle, G. In vitro and in vivo advancement of multifunctional electrospun nanofiber scaffolds in wound healing applications: Innovative nanofiber designs, stem cell approaches, and future perspectives. J. Biomed. Mater. Res. Part A 2022, 110, 443–461. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Nature-Derived and Synthetic Additives to poly(ɛ-Caprolactone) Nanofibrous Systems for Biomedicine; an Updated Overview. Front. Chem. 2022, 9, 809676. [Google Scholar] [CrossRef]
- Deshmukh, S.B.; Kathiresan, M.; Kulandainathan, M.A. A review on biopolymer-derived electrospun nanofibers for biomedical and antiviral applications. Biomater. Sci. 2022, 10, 4424–4442. [Google Scholar] [CrossRef]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef]
- Agarwal, A.; Rao, G.K.; Majumder, S.; Shandilya, M.; Rawat, V.; Purwar, R.; Verma, M.; Srivastava, C.M. Natural protein-based electrospun nanofibers for advanced healthcare applications: Progress and challenges. 3 Biotech 2022, 12, 92. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci. 2019, 19, e1800256. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Shahsavari, S.; Sarrafzadeh, M.H.; Larijani, B.; Dorkoosh, F.A.; Haghpanah, V.; Khorramizadeh, M.R. Cellulose acetate electrospun nanofibers for drug delivery systems: Applications and recent advances. Carbohydr. Polym. 2018, 198, 131–141. [Google Scholar] [CrossRef]
- Zare, M.R.; Khorram, M.; Barzegar, S.; Asadian, F.; Zareshahrabadi, Z.; Saharkhiz, M.J.; Ahadian, S.; Zomorodian, K. Antimicrobial core-shell electrospun nanofibers containing Ajwain essential oil for accelerating infected wound healing. Int. J. Pharm. 2021, 603, 120698. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, K.; Kotaki, M.; Zhang, Y.; Mo, X.; Ramakrishna, S. Recent Advances in Polymer Nanofibers. J. Nanosci. Nanotechnol. 2004, 4, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Deka, M. Nanofiber Reinforced Composite Polymer Electrolyte Membranes. In Nanofibers; BoD—Books on Demand: Norderstedt, Germany, 2010. [Google Scholar] [CrossRef]
- Andrady, A.L. Science and Technology of Polymer Nanofibers; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Yarin, A.L.; Pourdeyhimi, B.; Ramakrishna, S. Fundamentals and Applications of Micro- and Nanofibers; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Huang, Y.; Song, J.; Yang, C.; Long, Y.; Wu, H. Scalable manufacturing and applications of nanofibers. Mater. Today Proc. 2019, 28, 98–113. [Google Scholar] [CrossRef]
- Yoon, K.; Hsiao, B.S.; Chu, B. Functional nanofibers for environmental applications. J. Mater. Chem. 2008, 18, 5326–5334. [Google Scholar] [CrossRef]
- Leung, V.; Ko, F. Biomedical applications of nanofibers. Polym. Adv. Technol. 2011, 22, 350–365. [Google Scholar] [CrossRef]
- Fang, J.; Niu, H.; Lin, T.; Wang, X. Applications of electrospun nanofibers. Sci. Bull. 2008, 53, 2265–2286. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef]
- Jin, L.; Wang, T.; Feng, Z.-Q.; Leach, M.K.; Wu, J.; Mo, S.; Jiang, Q. A facile approach for the fabrication of core-shell PEDOT nanofiber mats with superior mechanical properties and biocompatibility. J. Mater. Chem. B 2013, 1, 1818–1825. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Zhu, Y.; Wang, L.; Yu, D.-G.; Liu, L.-Y. Tri-Layer Core-Shell Fibers from Coaxial Electrospinning for a Modified Release of Metronidazole. Pharmaceutics 2023, 15, 2561. [Google Scholar] [CrossRef]
- Ding, Y.; Dou, C.; Chang, S.; Xie, Z.; Yu, D.-G.; Liu, Y.; Shao, J. Core-Shell Eudragit S100 Nanofibers Prepared via Triaxial Electrospinning to Provide a Colon-Targeted Extended Drug Release. Polymers 2020, 12, 2034. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Song, W.; Zhang, Y.; Yu, D.-G.; Liu, Y. Electrospun Core (HPMC–Acetaminophen)-Shell (PVP–Sucralose) Nanohybrids for Rapid Drug Delivery. Gels 2022, 8, 357. [Google Scholar] [CrossRef]
- Kaviannasab, E.; Semnani, D.; Khorasani, S.N.; Varshosaz, J.; Khalili, S.; Ghahreman, F. Core-shell nanofibers of poly (ε–caprolactone) and Polyvinylpyrrolidone for drug delivery system. Mater. Res. Express 2019, 6, 115015. [Google Scholar] [CrossRef]
- Huang, C.; Wang, M.; Yu, S.; Yu, D.-G.; Bligh, S.W.A. Electrospun Fenoprofen/Polycaprolactone @ Tranexamic Acid/Hydroxyapatite Nanofibers as Orthopedic Hemostasis Dressings. Nanomaterials 2024, 14, 646. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.; Gao, Y.; Yu, D.-G.; Liu, P. Elaborate design of shell component for manipulating the sustained release behavior from core-shell nanofibres. J. Nanobiotechnol. 2022, 20, 244. [Google Scholar] [CrossRef] [PubMed]
- Alishahi, M.; Khorram, M.; Asgari, Q.; Davani, F.; Goudarzi, F.; Emami, A.; Arastehfar, A.; Zomorodian, K. Glucantime-loaded electrospun core-shell nanofibers composed of poly (ethylene oxide)/gelatin-poly(vinyl alcohol)/chitosan as dressing for cutaneous leishmaniasis. Int. J. Biol. Macromol. 2020, 163, 288–297. [Google Scholar] [CrossRef]
- Afshar, S.; Rashedi, S.; Nazockdast, H.; Ghazalian, M. Preparation and characterization of electrospun poly(lactic acid)-chitosan core-shell nanofibers with a new solvent system. Int. J. Biol. Macromol. 2019, 138, 1130–1137. [Google Scholar] [CrossRef]
- Rashedi, S.; Afshar, S.; Rostami, A.; Ghazalian, M.; Nazockdast, H. Co-electrospun poly(lactic acid)/gelatin nanofibrous scaffold prepared by a new solvent system: Morphological, mechanical and in vitro degradability properties. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 545–553. [Google Scholar] [CrossRef]
- Maryam Salehi Esfandarani, M.S.J. Producing porous nanofibers. In Proceedings of the 2nd International Conference, Olomouc, Czech Republic, 12–14 October 2010. [Google Scholar]
- Niloufar Sabetzadeh, A.A.G. How porous nanofibers have enhanced the engineering of advanced materials: A review. J. Text. Polym. 2017, 5, 3–21. [Google Scholar]
- Khajavi, R.; Abbasipour, M. Electrospinning as a versatile method for fabricating coreshell, hollow and porous nanofibers. Sci. Iran. 2012, 19, 2029–2034. [Google Scholar] [CrossRef]
- Wang, P.; Lv, H.; Cao, X.; Liu, Y.; Yu, D.-G. Recent Progress of the Preparation and Application of Electrospun Porous Nanofibers. Polymers 2023, 15, 921. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hou, L.; Yue, G.; Li, H.; Zhang, J.; Liu, J.; Miao, B.; Wang, N.; Bai, J.; Cui, Z.; et al. Progress of Fabrication and Applications of Electrospun Hierarchically Porous Nanofibers. Adv. Fiber Mater. 2022, 4, 604–630. [Google Scholar] [CrossRef]
- Chen, L.; Song, Y.; Liu, W.; Dong, H.; Wang, D.; Liu, J.; Liu, Q.; Chen, X. MOF-based nanoscale Pt catalyst decorated SnO2 porous nanofibers for acetone gas detection. J. Alloys Compd. 2022, 893, 162322. [Google Scholar] [CrossRef]
- Li, Z.; Liu, S.; Song, S.; Xu, W.; Sun, Y.; Dai, Y. Porous ceramic nanofibers as new catalysts toward heterogeneous reactions. Compos. Commun. 2019, 15, 168–178. [Google Scholar] [CrossRef]
- Beck, R.J.; Zhao, Y.; Fong, H.; Menkhaus, T.J. Electrospun lignin carbon nanofiber membranes with large pores for highly efficient adsorptive water treatment applications. J. Water Process. Eng. 2017, 16, 240–248. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, D.; Sønderskov, S.M.; Xia, D.; Wu, X.; Liang, C.; Dong, M. Tannic acid-functionalized 3D porous nanofiber sponge for antibiotic-free wound healing with enhanced hemostasis, antibacterial, and antioxidant properties. J. Nanobiotechnol. 2023, 21, 190. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, X. Fabrication of porous carbon nanofibers and their application as anode materials for rechargeable lithium-ion batteries. Nanotechnology 2009, 20, 155705. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Feng, Y.; Huang, Z.-M.; Ramakrishna, S.; Lim, C.T. Fabrication of porous electrospun nanofibres. Nanotechnology 2006, 17, 901–908. [Google Scholar] [CrossRef]
- Cao, X.; Chen, W.; Zhao, P.; Yang, Y.; Yu, D.-G. Electrospun Porous Nanofibers: Pore−Forming Mechanisms and Applications for Photocatalytic Degradation of Organic Pollutants in Wastewater. Polymers 2022, 14, 3990. [Google Scholar] [CrossRef]
- Širc, J.; Hobzová, R.; Kostina, N.; Munzarová, M.; Juklíčková, M.; Lhotka, M.; Kubinová, S.; Zajícová, A.; Michálek, J. Morphological Characterization of Nanofibers: Methods and Application in Practice. J. Nanomater. 2012, 2012, 327369. [Google Scholar] [CrossRef]
- Lee, B.-S.; Jeon, S.-Y.; Park, H.; Lee, G.; Yang, H.-S.; Yu, W.-R. New Electrospinning Nozzle to Reduce Jet Instability and Its Application to Manufacture of Multi-layered Nanofibers. Sci. Rep. 2014, 4, 6758. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, Y.; Song, Y. Core-Shell Nanofibers: Nano Channel and Capsule by Coaxial Electrospinning. In Nanofibers; Kumar, A., Ed.; BoD—Books on Demand: Norderstedt, Germany, 2010. [Google Scholar]
- Zhuo, H.T.; Hu, J.L.; Chen, S.J. Coaxial electrospun polyurethane core-shell nanofibers for shape memory and antibacterial nanomaterials. Express Polym. Lett. 2011, 5, 182–187. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Tung, S.-H. One-Step Electrospinning To Produce Nonsolvent-Induced Macroporous Fibers with Ultrahigh Oil Adsorption Capability. Macromolecules 2017, 50, 2528–2534. [Google Scholar] [CrossRef]
- Liu, W.; Huang, C.; Jin, X. Tailoring the grooved texture of electrospun polystyrene nanofibers by controlling the solvent system and relative humidity. Nanoscale Res. Lett. 2014, 9, 350. [Google Scholar] [CrossRef]
- Lin, J.; Ding, B.; Yu, J.; Hsieh, Y. Direct Fabrication of Highly Nanoporous Polystyrene Fibers via Electrospinning. ACS Appl. Mater. Interfaces 2010, 2, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yan, J.; Miao, Y.-E.; Huang, Y.; Liu, T. Catalytic and antibacterial activities of green-synthesized silver nanoparticles on electrospun polystyrene nanofiber membranes using tea polyphenols. Compos. Part B Eng. 2015, 79, 217–223. [Google Scholar] [CrossRef]
- Ma, L.; Ma, S.; Kang, H.; Shen, X.; Wang, T.; Jiang, X.; Chen, Q. Preparation of Ag-doped ZnO-SnO2 hollow nanofibers with an enhanced ethanol sensing performance by electrospinning. Mater. Lett. 2017, 209, 188–192. [Google Scholar] [CrossRef]
- Zaarour, B.; Zhu, L.; Huang, C.; Jin, X. Controlling the Secondary Surface Morphology of Electrospun PVDF Nanofibers by Regulating the Solvent and Relative Humidity. Nanoscale Res. Lett. 2018, 13, 285. [Google Scholar] [CrossRef]
- Sawicka, K.M.; Gouma, P. Electrospun composite nanofibers for functional applications. J. Nanopart. Res. 2006, 8, 769–781. [Google Scholar] [CrossRef]
- Sahay, R.; Kumar, P.S.; Sridhar, R.; Sundaramurthy, J.; Venugopal, J.; Mhaisalkar, S.G.; Ramakrishna, S. Electrospun composite nanofibers and their multifaceted applications. J. Mater. Chem. 2012, 22, 12953–12971. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Ghasemi-Mobarakeh, L.; Ramakrishna, S. Electrospun Composite Nanofibers for Tissue Regeneration. J. Nanosci. Nanotechnol. 2011, 11, 3039–3057. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Tobías, H.; Morales, G.; Grande, D. Comprehensive review on electrospinning techniques as versatile approaches toward antimicrobial biopolymeric composite fibers. Mater. Sci. Eng. C 2019, 101, 306–322. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, Y.; Duan, G.; Mei, C.; Greiner, A.; Agarwal, S. Electrospun nanofiber reinforced composites: A review. Polym. Chem. 2018, 9, 2685–2720. [Google Scholar] [CrossRef]
- Toriello, M.; Afsari, M.; Shon, H.K.; Tijing, L.D. Progress on the Fabrication and Application of Electrospun Nanofiber Composites. Membranes 2020, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, Z.; Zhang, J.; Cao, J.; Wang, S.; Tian, Q.; Gao, M.; Xu, Q. Fabrication of PVA/graphene oxide/TiO2 composite nanofibers through electrospinning and interface sol–gel reaction: Effect of graphene oxide on PVA nanofibers and growth of TiO2. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 318–325. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Shenashen, M.A.; Elmarakbi, A. Advances in polymer/inorganic nanocomposite fabrics for lightweight and high-strength armor and ballistic-proof materials. Chem. Eng. J. 2024, 493, 152422. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Shenashen, M.A.; Higazy, S.A.; Elmarakbi, A. Progress in biomimetic leverages for marine antifouling using nanocomposite coatings. J. Mater. Chem. B 2020, 8, 3701–3732. [Google Scholar] [CrossRef]
- Wang, J.; Cai, N.; Chan, V.; Zeng, H.; Shi, H.; Xue, Y.; Yu, F. Antimicrobial hydroxyapatite reinforced-polyelectrolyte complex nanofibers with long-term controlled release activity for potential wound dressing application. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126722. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Staszak, K.; Woźniak-Budych, M.J.; Litowczenko, J.; Maciejewska, B.M.; Jurga, S. Surface functionalization—The way for advanced applications of smart materials. Co-Ord. Chem. Rev. 2021, 436, 213846. [Google Scholar] [CrossRef]
- Bucci, R.; Vaghi, F.; Erba, E.; Romanelli, A.; Gelmi, M.L.; Clerici, F. Peptide grafting strategies before and after electrospinning of nanofibers. Acta Biomater. 2021, 122, 82–100. [Google Scholar] [CrossRef]
- Huang, Z.; Daniels, R.H.; Enzerink, R.-J.; Hardev, V.; Sahi, V.; Goodman, S.B. Effect of Nanofiber-Coated Surfaces on the Proliferation and Differentiation of Osteoprogenitors In Vitro. Tissue Eng. Part A 2008, 14, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Nhlapo, N.; Dzogbewu, T.C.; de Smidt, O. Nanofiber Polymers for Coating Titanium-Based Biomedical Implants. Fibers 2022, 10, 36. [Google Scholar] [CrossRef]
- Kulkarni, D.; Musale, S.; Panzade, P.; Paiva-Santos, A.C.; Sonwane, P.; Madibone, M.; Choundhe, P.; Giram, P.; Cavalu, S. Surface Functionalization of Nanofibers: The Multifaceted Approach for Advanced Biomedical Applications. Nanomaterials 2022, 12, 3899. [Google Scholar] [CrossRef] [PubMed]
- Eatemadi, A.; Daraee, H.; Zarghami, N.; Melat Yar, H.; Akbarzadeh, A. Nanofiber: Synthesis and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Srikar, R.; Gambaryan-Roisman, T.; Steffes, C.; Stephan, P.; Tropea, C.; Yarin, A. Nanofiber coating of surfaces for intensification of drop or spray impact cooling. Int. J. Heat Mass Transf. 2009, 52, 5814–5826. [Google Scholar] [CrossRef]
- Fu, X.; Du, W.; Dou, H.; Fan, Y.; Xu, J.; Tian, L.; Zhao, J.; Ren, L. Nanofiber Composite Coating with Self-Healing and Active Anticorrosive Performances. ACS Appl. Mater. Interfaces 2021, 13, 57880–57892. [Google Scholar] [CrossRef]
- Zhu, T.; Yu, K.; Bhutto, M.A.; Guo, X.; Shen, W.; Wang, J.; Chen, W.; El-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Synthesis of RGD-peptide modified poly(ester-urethane) urea electrospun nanofibers as a potential application for vascular tissue engineering. Chem. Eng. J. 2017, 315, 177–190. [Google Scholar] [CrossRef]
- Li, L.; Peng, S.; Lee, J.K.Y.; Ji, D.; Srinivasan, M.; Ramakrishna, S. Electrospun hollow nanofibers for advanced secondary batteries. Nano Energy 2017, 39, 111–139. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Direct Fabrication of Composite and Ceramic Hollow Nanofibers by Electrospinning. Nano Lett. 2004, 4, 933–938. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Z.; Wang, L. Hollow fibers: From fabrication to applications. Chem. Commun. 2021, 57, 9166–9177. [Google Scholar] [CrossRef]
- Hajimohammadi, M.; Soltani, P.; Semnani, D.; Taban, E.; Fashandi, H. Nonwoven fabric coated with core-shell and hollow nanofiber membranes for efficient sound absorption in buildings. J. Affect. Disord. 2022, 213, 108887. [Google Scholar] [CrossRef]
- Wu, S.; Shi, W.; Li, K.; Cai, J.; Xu, C.; Gao, L.; Lu, J.; Ding, F. Chitosan-based hollow nanofiber membranes with polyvinylpyrrolidone and polyvinyl alcohol for efficient removal and filtration of organic dyes and heavy metals. Int. J. Biol. Macromol. 2023, 239, 124264. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Cui, J.; Qu, Q.; Wang, Y.; Zhang, J.; Xiong, R.; Ma, W.; Huang, C. Multistructured Electrospun Nanofibers for Air Filtration: A Review. ACS Appl. Mater. Interfaces 2021, 13, 23293–23313. [Google Scholar] [CrossRef]
- Moghe, A.K.; Gupta, B.S. Co-axial Electrospinning for Nanofiber Structures: Preparation and Applications. Polym. Rev. 2008, 48, 353–377. [Google Scholar] [CrossRef]
- Tesárek, P.; Ryparová, P.; Rácová, Z.; Králík, V.; Němeček, J.; Kromka, A.; Nežerka, V. Mechanical Properties of Single and Double-Layered PVA Nanofibers. Key Eng. Mater. 2014, 586, 261–264. [Google Scholar] [CrossRef]
- Olmos, D.; González-Benito, J. Polymeric Materials with Antibacterial Activity: A Review. Polymers 2021, 13, 613. [Google Scholar] [CrossRef]
- Luo, H.; Yin, X.-Q.; Tan, P.-F.; Gu, Z.-P.; Liu, Z.-M.; Tan, L. Polymeric antibacterial materials: Design, platforms and applications. J. Mater. Chem. B 2021, 9, 2802–2815. [Google Scholar] [CrossRef]
- Bassas-Galia, M.; Follonier, S.; Pusnik, M.; Zinn, M. Natural polymers: A source of inspiration. In Bioresorbable Polymers for Biomedical Applications; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar] [CrossRef]
- Chen, R.; Huang, C.; Ke, Q.; He, C.; Wang, H.; Mo, X. Preparation and characterization of coaxial electrospun thermoplastic polyurethane/collagen compound nanofibers for tissue engineering applications. Colloids Surf. B Biointerfaces 2010, 79, 315–325. [Google Scholar] [CrossRef]
- Naeimirad, M.; Zadhoush, A.; Kotek, R.; Neisiany, R.E.; Khorasani, S.N.; Ramakrishna, S. Recent advances in core/shell bicomponent fibers and nanofibers: A review. J. Appl. Polym. Sci. 2018, 135, 46265. [Google Scholar] [CrossRef]
- Scheirs, J.; Long, T.E. (Eds.) Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters; Polymer Science & Technology General; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Rehm, B.H.A. Polyester synthases: Natural catalysts for plastics. Biochem. J. 2003, 376, 15–33. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- Mehta, R.; Kumar, V.; Bhunia, H.; Upadhyay, S.N. Synthesis of Poly(Lactic Acid): A Review. J. Macromol. Sci. Part C Polym. Rev. 2005, 45, 325–349. [Google Scholar] [CrossRef]
- Teixeira, S.; Eblagon, K.M.; Miranda, F.; Pereira, M.F.R.; Figueiredo, J.L. Towards Controlled Degradation of Poly(lactic) Acid in Technical Applications. C 2021, 7, 42. [Google Scholar] [CrossRef]
- Ghaffari-Bohlouli, P.; Zahedi, P.; Shahrousvand, M. Performance evaluation of poly (l-lactide-co-D, l-lactide)/poly (acrylic acid) blends and their nanofibers for tissue engineering applications. Int. J. Biol. Macromol. 2019, 122, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhu, X.; Wang, N.; Zhang, X.; Yang, D.; Nie, J.; Ma, G. Biodegradable core-shell electrospun nanofibers based on PLA and γ-PGA for wound healing. Eur. Polym. J. 2019, 116, 30–37. [Google Scholar] [CrossRef]
- Suner, S.C.; Oral, A.; Yildirim, Y. Design of Poly(lactic) acid/gelatin core-shell bicomponent systems as a potential wound dressing material. J. Mech. Behav. Biomed. Mater. 2024, 150, 106255. [Google Scholar] [CrossRef]
- Xiong, F.; Wei, S.; Sheng, H.; Wu, S.; Liu, Z.; Cui, W.; Sun, Y.; Wu, Y.; Li, B.; Xuan, H.; et al. Three-layer core-shell structure of polypyrrole/polydopamine/poly(l-lactide) nanofibers for wound healing application. Int. J. Biol. Macromol. 2022, 222, 1948–1962. [Google Scholar] [CrossRef] [PubMed]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Spagnoli, C.; Cowman, M.K. Poly(vinyl alcohol) as versatile biomaterial for potential biomedical applications. J. Mater. Sci. Mater. Med. 2003, 14, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Ben Halima, N. Poly(vinyl alcohol): Review of its promising applications and insights into biodegradation. RSC Adv. 2016, 6, 39823–39832. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of Poly(vinyl alcohol) and Natural Polymers. Polym. Rev. 2018, 58, 247–287. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1451–1457. [Google Scholar] [CrossRef]
- Barzegar, S.; Zare, M.R.; Shojaei, F.; Zareshahrabadi, Z.; Koohi-Hosseinabadi, O.; Saharkhiz, M.J.; Iraji, A.; Zomorodian, K.; Khorram, M. Core-shell chitosan/PVA-based nanofibrous scaffolds loaded with Satureja mutica or Oliveria decumbens essential oils as enhanced antimicrobial wound dressing. Int. J. Pharm. 2021, 597, 120288. [Google Scholar] [CrossRef] [PubMed]
- Najafiasl, M.; Osfouri, S.; Azin, R.; Zaeri, S. Alginate-based electrospun core/shell nanofibers containing dexpanthenol: A good candidate for wound dressing. J. Drug Deliv. Sci. Technol. 2020, 57, 101708. [Google Scholar] [CrossRef]
- Maleki, H.; Mathur, S.; Klein, A. Antibacterial Ag containing core-shell polyvinyl alcohol-poly (lactic acid) nanofibers for biomedical applications. Polym. Eng. Sci. 2020, 60, 1221–1230. [Google Scholar] [CrossRef]
- Martin, A.; Cai, J.; Schaedel, A.-L.; van der Plas, M.; Malmsten, M.; Rades, T.; Heinz, A. Zein-polycaprolactone core-shell nanofibers for wound healing. Int. J. Pharm. 2022, 621, 121809. [Google Scholar] [CrossRef]
- Ghazalian, M.; Afshar, S.; Rostami, A.; Rashedi, S.; Bahrami, S.H. Fabrication and characterization of chitosan-polycaprolactone core-shell nanofibers containing tetracycline hydrochloride. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128163. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Guarino, V.; Gentile, G.; Sorrentino, L.; Ambrosio, L. Polycaprolactone: Synthesis, Properties, and Applications. Encycl. Polym. Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Yan, E.; Jiang, J.; Yang, X.; Fan, L.; Wang, Y.; An, Q.; Zhang, Z.; Lu, B.; Wang, D.; Zhang, D. pH-sensitive core-shell electrospun nanofibers based on polyvinyl alcohol/polycaprolactone as a potential drug delivery system for the chemotherapy against cervical cancer. J. Drug Deliv. Sci. Technol. 2020, 55, 101455. [Google Scholar] [CrossRef]
- Moradipour, P.; Limoee, M.; Janfaza, S.; Behbood, L. Core-Shell Nanofibers Based on Polycaprolactone/Polyvinyl Alcohol and Polycaprolactone/Collagen for Biomedical Applications. J. Pharm. Innov. 2021, 17, 911–920. [Google Scholar] [CrossRef]
- Sadeghi, E.; Zebarjad, S.M.; Khademi, F.; Bagherzadeh, E. Enhancing structural strength and improving cell survival through Polycaprolactone/(gelatin/hydroxyapatite) Core-Shell nanofibers for tissue engineering. Polym. Compos. 2022, 43, 7379–7389. [Google Scholar] [CrossRef]
- Bahmani, E.; Dizaji, B.F.; Talaei, S.; Koushkbaghi, S.; Yazdani, H.; Abadi, P.G.; Akrami, M.; Shahrousvand, M.; Jazi, F.S.; Irani, M. Fabrication of poly(e-caprolactone)/paclitaxel (core)/chitosan/zein/multi-walled carbon nanotubes/doxorubicin (shell) nanofibers against MCF-7 breast cancer. Polym. Adv. Technol. 2023, 34, 789–799. [Google Scholar] [CrossRef]
- Raina, N.; Pahwa, R.; Khosla, J.K.; Gupta, P.N.; Gupta, M. Polycaprolactone-based materials in wound healing applications. Polym. Bull. 2021, 79, 7041–7063. [Google Scholar] [CrossRef]
- Hashemi, S.-S.; Saadatjo, Z.; Mahmoudi, R.; Delaviz, H.; Bardania, H.; Rajabi, S.-S.; Rafati, A.; Zarshenas, M.M.; Barmak, M.J. Preparation and evaluation of polycaprolactone/chitosan/Jaft biocompatible nanofibers as a burn wound dressing. Burns 2022, 48, 1690–1705. [Google Scholar] [CrossRef]
- Fischer, S.; Thümmler, K.; Volkert, B.; Hettrich, K.; Schmidt, I.; Fischer, K. Properties and Applications of Cellulose Acetate. Macromol. Symp. 2008, 262, 89–96. [Google Scholar] [CrossRef]
- Wsoo, M.A.; Shahir, S.; Bohari, S.P.M.; Nayan, N.H.M.; Razak, S.I.A. A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: A new perspective. Carbohydr. Res. 2020, 491, 107978. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Park, W.H. Antimicrobial cellulose acetate nanofibers containing silver nanoparticles. Carbohydr. Polym. 2006, 65, 430–434. [Google Scholar] [CrossRef]
- Khalf, A.; Singarapu, K.; Madihally, S.V. Cellulose acetate core-shell structured electrospun fiber: Fabrication and characterization. Cellulose 2015, 22, 1389–1400. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Liu, P. Electrospun Core–Sheath Nanofibers with a Cellulose Acetate Coating for the Synergistic Release of Zinc Ion and Drugs. Mol. Pharm. 2023, 21, 173–182. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- de Souza, F.M.; Kahol, P.K.; Gupta, R.K. Introduction to Polyurethane Chemistry; American Chemical Society: Washington, DC, USA, 2021. [Google Scholar]
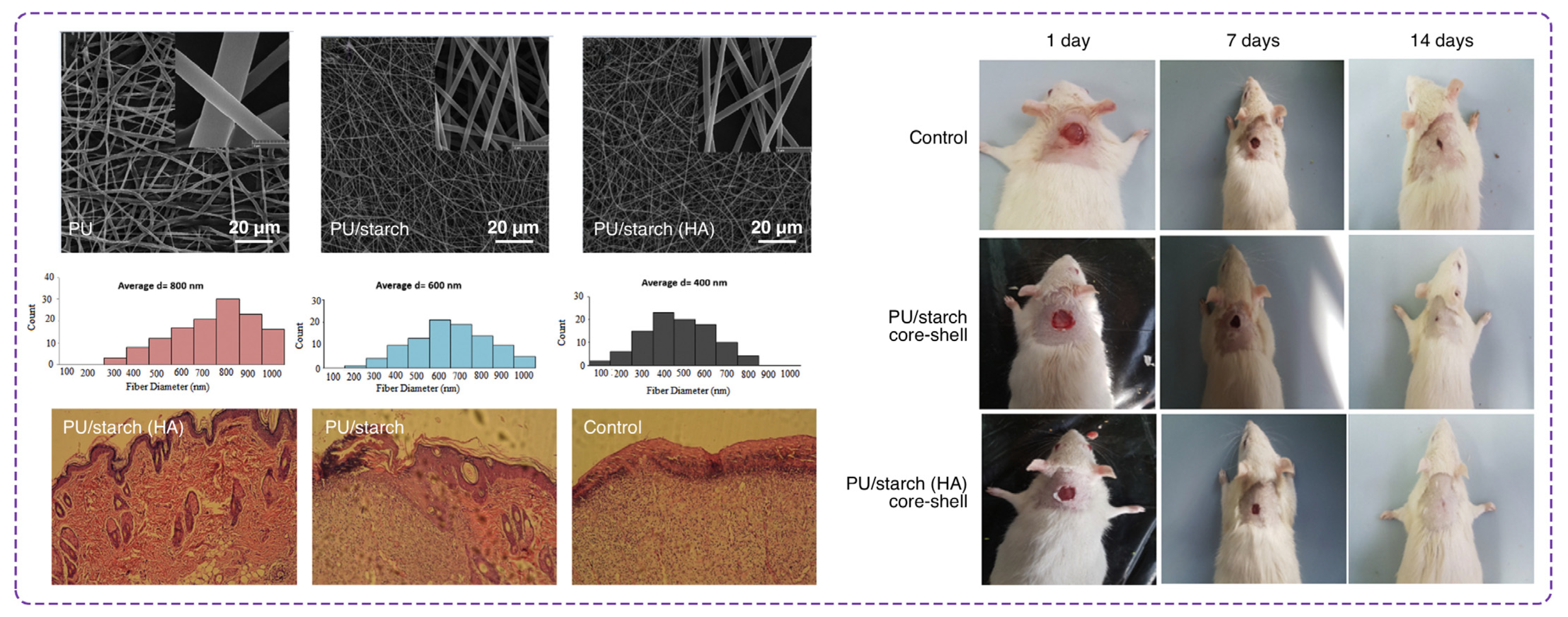
- Movahedi, M.; Asefnejad, A.; Rafienia, M.; Khorasani, M.T. Potential of novel electrospun core-shell structured polyurethane/starch (hyaluronic acid) nanofibers for skin tissue engineering: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2020, 146, 627–637. [Google Scholar] [CrossRef]
- Maleknia, L.; Dilamian, M.; Pilehrood, M.K.; Sadeghi-Aliabadi, H.; Hekmati, A.H. Preparation, process optimization and characterization of core-shell polyurethane/chitosan nanofibers as a potential platform for bioactive scaffolds. Res. Pharm. Sci. 2018, 13, 273–282. [Google Scholar] [CrossRef]
- Currie, S.; Shariatzadeh, F.J.; Singh, H.; Logsetty, S.; Liu, S. Highly Sensitive Bacteria-Responsive Membranes Consisting of Core-Shell Polyurethane Polyvinylpyrrolidone Electrospun Nanofibers for In Situ Detection of Bacterial Infections. ACS Appl. Mater. Interfaces 2020, 12, 45859–45872. [Google Scholar] [CrossRef]
- Wang, T.; Kumar, S. Electrospinning of polyacrylonitrile nanofibers. J. Appl. Polym. Sci. 2006, 102, 1023–1029. [Google Scholar] [CrossRef]
- Nataraj, S.; Yang, K.; Aminabhavi, T. Polyacrylonitrile-based nanofibers—A state-of-the-art review. Prog. Polym. Sci. 2012, 37, 487–513. [Google Scholar] [CrossRef]
- Wang, J.; Pan, K.; He, Q.; Cao, B. Polyacrylonitrile/polypyrrole core/shell nanofiber mat for the removal of hexavalent chromium from aqueous solution. J. Hazard. Mater. 2013, 244–245, 121–129. [Google Scholar] [CrossRef]
- Miao, F.; Shao, C.; Li, X.; Lu, N.; Wang, K.; Zhang, X.; Liu, Y. Flexible solid-state supercapacitors based on freestanding electrodes of electrospun polyacrylonitrile@polyaniline core-shell nanofibers. Electrochim. Acta 2015, 176, 293–300. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Ni, X.; Liu, G.; Cheng, W.; Han, G. Coaxial Electrospinning and Characterization of Core-Shell Structured Cellulose Nanocrystal Reinforced PMMA/PAN Composite Fibers. Materials 2017, 10, 572. [Google Scholar] [CrossRef]
- Huang, J.; Cao, Y.; Huang, Z.; Imbraguglio, S.A.; Wang, Z.; Peng, X.; Guo, Z. Comparatively Thermal and Crystalline Study of Poly(methyl-methacrylate)/Polyacrylonitrile Hybrids: Core-Shell Hollow Fibers, Porous Fibers, and Thin Films. Macromol. Mater. Eng. 2016, 301, 1327–1336. [Google Scholar] [CrossRef]
- Wu, X.-M.; Branford-White, C.J.; Yu, D.-G.; Chatterton, N.P.; Zhu, L.-M. Preparation of core-shell PAN nanofibers encapsulated α-tocopherol acetate and ascorbic acid 2-phosphate for photoprotection. Colloids Surf. B Biointerfaces 2011, 82, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Emam, M.H.; Elezaby, R.S.; Swidan, S.A.; Loutfy, S.A.; Hathout, R.M. Cerium Oxide Nanoparticles/Polyacrylonitrile Nanofibers as Impervious Barrier against Viral Infections. Pharmaceutics 2023, 15, 1494. [Google Scholar] [CrossRef]
- Kumar, S.K.S.; Prakash, C. Characterization of electrospun polyurethane/polyacrylonitrile nanofiber for protective textiles. Iran. Polym. J. 2021, 30, 1263–1271. [Google Scholar] [CrossRef]
- Yang, J.; Liu, C.-L.; Ding, Y.-N.; Sun, T.-C.; Bai, X.-H.; Cao, Z.-K.; Ramakrishna, S.; Zhang, J.; Long, Y.-Z. Synergistic antibacterial polyacrylonitrile/gelatin nanofibers coated with metal-organic frameworks for accelerating wound repair. Int. J. Biol. Macromol. 2021, 189, 698–704. [Google Scholar] [CrossRef]
- Haktaniyan, M.; Bradley, M. Polymers showing intrinsic antimicrobial activity. Chem. Soc. Rev. 2022, 51, 8584–8611. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, X.; Hua, T.; Fu, J.; Koo, M.; Chan, W.; Poon, T. Chitosan Natural Polymer Material for Improving Antibacterial Properties of Textiles. ACS Appl. Bio Mater. 2021, 4, 4014–4038. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Zarrintaj, P.; Oftadeh, M.O.; Keramati, F.; Fouladiha, H.; Sohrabi-Jahromi, S.; Ziraksaz, Z. Tissue engineering; strategies, tissues, and biomaterials. Biotechnol. Genet. Eng. Rev. 2017, 33, 144–172. [Google Scholar] [CrossRef]
- Hafdani, F.N.; Sadeghinia, N. A review on application of chitosan as a natural antimicrobial. Int. J. Med. Health Biomed. Bioeng. Pharm. Eng. 2011, 5, 46–50. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.T.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Peh, K.; Khan, T.; Ch’ng, H. Mechanical, bioadhesive strength and biological evaluations of chitosan films for wound dressing. J. Pharm. Pharm. Sci. 2000, 3, 303–311. [Google Scholar]
- Azad, A.K.; Sermsintham, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan membrane as a wound-healing dressing: Characterization and clinical application. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 69, 216–222. [Google Scholar] [CrossRef]
- Keirouz, A.; Radacsi, N.; Ren, Q.; Dommann, A.; Beldi, G.; Maniura-Weber, K.; Rossi, R.M.; Fortunato, G. Nylon-6/chitosan core/shell antimicrobial nanofibers for the prevention of mesh-associated surgical site infection. J. Nanobiotechnol. 2020, 18, 51. [Google Scholar] [CrossRef]
- Hasanbegloo, K.; Banihashem, S.; Dizaji, B.F.; Bybordi, S.; Farrokh-Eslamlou, N.; Abadi, P.G.-S.; Jazi, F.S.; Irani, M. Paclitaxel-loaded liposome-incorporated chitosan (core)/poly(ε-caprolactone)/chitosan (shell) nanofibers for the treatment of breast cancer. Int. J. Biol. Macromol. 2023, 230, 123380. [Google Scholar] [CrossRef]
- Rajasekaran, R.; Dutta, A.; Ray, P.G.; Seesala, V.S.; Ojha, A.K.; Dogra, N.; Roy, S.; Banerjee, M.; Dhara, S. High fibroin-loaded silk-PCL electrospun fiber with core-shell morphology promotes epithelialization with accelerated wound healing. J. Mater. Chem. B 2022, 10, 9622–9638. [Google Scholar] [CrossRef]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W.; et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Kasoju, N.; Bora, U. Silk Fibroin in Tissue Engineering. Adv. Health Mater. 2012, 1, 393–412. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, X.; Gunawidjaja, R.; Lin, Y.; Gupta, M.K.; Kaplan, D.L.; Naik, R.R.; Tsukruk, V.V. Mechanical Properties of Robust Ultrathin Silk Fibroin Films. Adv. Funct. Mater. 2007, 17, 2229–2237. [Google Scholar] [CrossRef]
- Wang, M.; Jin, H.J.; Kaplan, D.L.; Rutledge, G.C. Mechanical Properties of Electrospun Silk Fibers. Macromolecules 2004, 37, 6856–6864. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Reis, R.L.; Ramakrishna, S.; Kundu, S.C. Functionalized silk fibroin nanofibers as drug carriers: Advantages and challenges. J. Control. Release 2020, 321, 324–347. [Google Scholar] [CrossRef]
- Long, Y.; Cheng, X.; Tang, Q.; Chen, L. The antigenicity of silk-based biomaterials: Sources, influential factors and applications. J. Mater. Chem. B 2021, 9, 8365–8377. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Saeb, M.R.; Zarrintaj, P.; Kundu, S.C.; Khademhosseini, A. Silk fibroin scaffolds for common cartilage injuries: Possibilities for future clinical applications. Eur. Polym. J. 2019, 115, 251–267. [Google Scholar] [CrossRef]
- Patil, P.P.; Reagan, M.R.; Bohara, R.A. Silk fibroin and silk-based biomaterial derivatives for ideal wound dressings. Int. J. Biol. Macromol. 2020, 164, 4613–4627. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of Silk Fibroin Use in Wound Dressings. Trends Biotechnol. 2018, 36, 907–922. [Google Scholar] [CrossRef]
- Hadisi, Z.; Farokhi, M.; Bakhsheshi-Rad, H.R.; Jahanshahi, M.; Hasanpour, S.; Pagan, E.; Dolatshahi-Pirouz, A.; Zhang, Y.S.; Kundu, S.C.; Akbari, M. Hyaluronic Acid (HA)-Based Silk Fibroin/Zinc Oxide Core-Shell Electrospun Dressing for Burn Wound Management. Macromol. Biosci. 2020, 20, e1900328. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef] [PubMed]
- Aarstad, O.A.; Tøndervik, A.; Sletta, H.; Skjåk-Bræk, G. Alginate Sequencing: An Analysis of Block Distribution in Alginates Using Specific Alginate Degrading Enzymes. Biomacromolecules 2011, 13, 106–116. [Google Scholar] [CrossRef]
- Yang, J.-S.; Xie, Y.-J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Norouzi, M.-R.; Ghasemi-Mobarakeh, L.; Itel, F.; Schoeller, J.; Fashandi, H.; Borzi, A.; Neels, A.; Fortunato, G.; Rossi, R.M. Emulsion electrospinning of sodium alginate/poly(ε-caprolactone) core/shell nanofibers for biomedical applications. Nanoscale Adv. 2022, 4, 2929–2941. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Valipouri, A.; Ravandi, S.A.H.; Kouhi, M.; Mobarakeh, L.G. Fabrication, characterization, and drug release study of vitamin C–loaded alginate/polyethylene oxide nanofibers for the treatment of a skin disorder. Polym. Adv. Technol. 2019, 30, 2447–2457. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Okoro, G.; Lin, I.-H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Chang, C.-C.; Kuo, T.-R. Emerging Trends in Nanomaterials for Antibacterial Applications. Int. J. Nanomed. 2021, 16, 5831–5867. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M. Antibacterial Activity of Nanomaterials. Nanomaterials 2018, 8, 359. [Google Scholar] [CrossRef]
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Ma, H.; Wallis, L.K.; Diamond, S.; Li, S.; Canas-Carrell, J.; Parra, A. Impact of solar UV radiation on toxicity of ZnO nanoparticles through photocatalytic reactive oxygen species (ROS) generation and photo-induced dissolution. Environ. Pollut. 2014, 193, 165–172. [Google Scholar] [CrossRef]
- Hensley, K.; A Robinson, K.; Gabbita, S.; Salsman, S.; A Floyd, R. Reactive oxygen species, cell signaling, and cell injury. Free Radic. Biol. Med. 2000, 28, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, C.M.; Gambetti, S.; Dondi, A.; Cervellati, C. Oxygen, Reactive Oxygen Species and Tissue Damage. Curr. Pharm. Des. 2004, 10, 1611–1626. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Martínez-Castañón, G.A.; Niño-Martínez, N.; Martínez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanopart. Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Samberg, M.E.; Orndorff, P.E.; Monteiro-Riviere, N.A. Antibacterial efficacy of silver nanoparticles of different sizes, surface conditions and synthesis methods. Nanotoxicology 2011, 5, 244–253. [Google Scholar] [CrossRef]
- Helmlinger, J.; Sengstock, C.; Groß-Heitfeld, C.; Mayer, C.; Schildhauer, T.A.; Köller, M.; Epple, M. Silver nanoparticles with different size and shape: Equal cytotoxicity, but different antibacterial effects. RSC Adv. 2016, 6, 18490–18501. [Google Scholar] [CrossRef]
- Ahmad, J.; Wen, X.; Li, F.; Wang, B. Novel triangular silver nanoparticle modified membranes for enhanced antifouling performance. RSC Adv. 2019, 9, 6733–6744. [Google Scholar] [CrossRef]
- Singh, S.; Bharti, A.; Meena, V.K. Green synthesis of multi-shaped silver nanoparticles: Optical, morphological and antibacterial properties. J. Mater. Sci. Mater. Electron. 2015, 26, 3638–3648. [Google Scholar] [CrossRef]
- Li, Y.; Yan, Y.; Wang, J.; Li, L.; Tang, F. Preparation of silver nanoparticles decorated mesoporous silica nanorods with photothermal antibacterial property. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129242. [Google Scholar] [CrossRef]
- Van Dong, P.; Ha, C.H.; Binh, L.T.; Kasbohm, J. Chemical synthesis and antibacterial activity of novel-shaped silver nanoparticles. Int. Nano Lett. 2012, 2, 9. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Vo, Q.K.; Phung, D.D.; Nguyen, Q.N.V.; Thi, H.H.; Thi, N.H.N.; Thi, P.P.N.; Bach, L.G.; Van Tan, L. Controlled Synthesis of Triangular Silver Nanoplates by Gelatin–Chitosan Mixture and the Influence of Their Shape on Antibacterial Activity. Processes 2019, 7, 873. [Google Scholar] [CrossRef]
- Helmlinger, J.; Prymak, O.; Loza, K.; Gocyla, M.; Heggen, M.; Epple, M. On the Crystallography of Silver Nanoparticles with Different Shapes. Cryst. Growth Des. 2016, 16, 3677–3687. [Google Scholar] [CrossRef]
- Sayed, R.; Saad, H.; Hagagy, N. Silver nanoparticles: Characterization and antibacterial properties. Rend. Lince- Sci. Fis. E Nat. 2018, 29, 81–86. [Google Scholar] [CrossRef]
- Long, Y.M.; Hu, L.G.; Yan, X.T.; Zhao, X.C.; Zhou, Q.F.; Cai, Y.; Jiang, G. Surface ligand controls silver ion release of nanosilver and its antibacterial activity against Escherichia coli. Int. J. Nanomed. 2017, 2017, 3193–3206. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, C.; Wang, X.; Liu, D. Release Strategies of Silver Ions from Materials for Bacterial Killing. ACS Appl. Bio Mater. 2021, 4, 3985–3999. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial Activity and Mechanism of Action of Zinc Oxide Nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef]
- Narayanan, P.M.; Wilson, W.S.; Abraham, A.T.; Sevanan, M. Synthesis, Characterization, and Antimicrobial Activity of Zinc Oxide Nanoparticles Against Human Pathogens. BioNanoScience 2012, 2, 329–335. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.L.; Abuçafy, M.P.; Manaia, E.B.; Junior, J.A.O.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship between Structure And Antimicrobial Activity Of Zinc Oxide Nanoparticles: An Overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef]
- Rekha, K.; Nirmala, M.; Nair, M.G.; Anukaliani, A. Structural, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide nanoparticles. Phys. B Condens. Matter 2010, 405, 3180–3185. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.d.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Choudhury, S.R.; Ordaz, J.; Lo, C.-L.; Damayanti, N.P.; Zhou, F.; Irudayaraj, J. From the Cover: Zinc oxide Nanoparticles-Induced Reactive Oxygen Species Promotes Multimodal Cyto- and Epigenetic Toxicity. Toxicol. Sci. 2017, 156, 261–274. [Google Scholar] [CrossRef]
- Yu, K.-N.; Yoon, T.-J.; Minai-Tehrani, A.; Kim, J.-E.; Park, S.J.; Jeong, M.S.; Ha, S.-W.; Lee, J.-K.; Kim, J.S.; Cho, M.-H. Zinc oxide nanoparticle induced autophagic cell death and mitochondrial damage via reactive oxygen species generation. Toxicol. In Vitro 2013, 27, 1187–1195. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, C.; Chen, X.; Yang, Z. Zinc oxide nanoparticles induce renal toxicity through reactive oxygen species. Food Chem. Toxicol. 2016, 90, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, J.; Cai, L.; Wu, Y.; Ji, M.; Jiang, H.; Chen, J. Dissection of the antibacterial mechanism of zinc oxide nanoparticles with manipulable nanoscale morphologies. J. Hazard. Mater. 2022, 430, 128436. [Google Scholar] [CrossRef] [PubMed]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Umasuthan, N.; Mohan, R.; Lee, J.; Kim, S.-J. Antibacterial Activity of Graphene Oxide Nanosheets. Sci. Adv. Mater. 2012, 4, 1111–1117. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Ren, X.; Tan, X.; Hayat, T.; Alsaedi, A.; Cheng, C.; Chen, C. Impact of graphene oxide on the antibacterial activity of antibiotics against bacteria. Environ. Sci. Nano 2017, 4, 1016–1024. [Google Scholar] [CrossRef]
- Perreault, F.; de Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Olborska, A.; Janas-Naze, A.; Kaczmarek, Ł.; Warga, T.; Halin, D.S.C. Antibacterial Effect of Graphene and Graphene Oxide as a Potential Material for Fiber Finishes. Autex Res. J. 2020, 20, 506–516. [Google Scholar] [CrossRef]
- Zolezzi, C.; Ihle, C.F.; Angulo, C.; Palma, P.; Palza, H. Effect of the Oxidation Degree of Graphene Oxides on their Adsorption, Flocculation, and Antibacterial Behavior. Ind. Eng. Chem. Res. 2018, 57, 15722–15730. [Google Scholar] [CrossRef]
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012, 71, 114–116. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef] [PubMed]
- Sayed, F.A.-Z.; Eissa, N.G.; Shen, Y.; Hunstad, D.A.; Wooley, K.L.; Elsabahy, M. Morphologic design of nanostructures for enhanced antimicrobial activity. J. Nanobiotechnol. 2022, 20, 536. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.K.; Kumar, V.; Pandey, V.; Naraian, R.; Gopal, R. Bacterial killing efficacy of synthesized rod shaped cuprous oxide nanoparticles using laser ablation technique. SN Appl. Sci. 2019, 1, 1426. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, G.; Rawat, M. A Brief Review on Synthesis and Characterization of Copper Oxide Nanoparticles and its Applications. J. Bioelectron. Nanotechnol. 2016, 1, 9. [Google Scholar]
- Oruç, Ç.; Altındal, A. Structural and dielectric properties of CuO nanoparticles. Ceram. Int. 2017, 43, 10708–10714. [Google Scholar] [CrossRef]
- Amiri, M.R.; Alavi, M.; Taran, M.; Kahrizi, D. Antibacterial, antifungal, antiviral, and photocatalytic activities of TiO2 nanoparticles, nanocomposites, and bio-nanocomposites: Recent advances and challenges. J. Public Health Res. 2022, 11, 22799036221104151. [Google Scholar] [CrossRef]
- Vargas, M.A.; Rodríguez-Páez, J.E. Amorphous TiO2 nanoparticles: Synthesis and antibacterial capacity. J. Non-Cryst. Solids 2017, 459, 192–205. [Google Scholar] [CrossRef]
- Anandgaonker, P.; Kulkarni, G.; Gaikwad, S.; Rajbhoj, A. Synthesis of TiO2 nanoparticles by electrochemical method and their antibacterial application. Arab. J. Chem. 2019, 12, 1815–1822. [Google Scholar] [CrossRef]
- Thakur, N.; Thakur, N.; Kumar, A.; Thakur, V.K.; Kalia, S.; Arya, V.; Kumar, A.; Kumar, S.; Kyzas, G.Z. A critical review on the recent trends of photocatalytic, antibacterial, antioxidant and nanohybrid applications of anatase and rutile TiO2 nanoparticles. Sci. Total Environ. 2024, 914, 169815. [Google Scholar] [CrossRef]
- Clément, L.; Hurel, C.; Marmier, N. Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants—Effects of size and crystalline structure. Chemosphere 2013, 90, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.-J.; Chen, S.; Yang, J.-H.; Gong, X.-G.; Yan, Y.; Wei, S.-H. Effective band gap narrowing of anatase TiO2 by strain along a soft crystal direction. Appl. Phys. Lett. 2010, 96, 221901. [Google Scholar] [CrossRef]
- Chen, C.; Dirican, M.; Zhang, X. Centrifugal Spinning—High Rate Production of Nanofibers. In Electrospinning: Nanofabrication and Applications; William Andrew Publishing: Norwich, NY, USA, 2019. [Google Scholar] [CrossRef]
- Han, D.; Steckl, A.J. Coaxial Electrospinning Formation of Complex Polymer Fibers and their Applications. Chempluschem 2019, 84, 1453–1497. [Google Scholar] [CrossRef]
- Yoon, J.; Yang, H.-S.; Lee, B.-S.; Yu, W.-R. Recent Progress in Coaxial Electrospinning: New Parameters, Various Structures, and Wide Applications. Adv. Mater. 2018, 30, e1704765. [Google Scholar] [CrossRef] [PubMed]
- Yarin, A.L. Coaxial electrospinning and emulsion electrospinning of core-shell fibers. Polym. Adv. Technol. 2011, 22, 310–317. [Google Scholar] [CrossRef]
- Qu, H.; Wei, S.; Guo, Z. Coaxial electrospun nanostructures and their applications. J. Mater. Chem. A 2013, 1, 11513. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, J.; Yu, G.; Cardenas, R.; Wei, S.; Wujcik, E.K.; Guo, Z. Coaxial electrospun fibers: Applications in drug delivery and tissue engineering. WIREs Nanomed. Nanobiotechnol. 2016, 8, 654–677. [Google Scholar] [CrossRef] [PubMed]
- Raheja, A.; Chandra, T.S.; Natarajan, T.S. Design of a low cost spinneret assembly for coaxial electrospinning. Appl. Phys. Lett. 2015, 106, 254101. [Google Scholar] [CrossRef]
- Huang, Z.-X.; Wu, J.-W.; Wong, S.-C.; Qu, J.-P.; Srivatsan, T.S. The technique of electrospinning for manufacturing core-shell nanofibers. Mater. Manuf. Process. 2018, 33, 202–219. [Google Scholar] [CrossRef]
- Wang, C.; Yan, K.-W.; Lin, Y.-D.; Hsieh, P.C.H. Biodegradable Core/Shell Fibers by Coaxial Electrospinning: Processing, Fiber Characterization, and Its Application in Sustained Drug Release. Macromolecules 2010, 43, 6389–6397. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, F.; Zhang, H. Emulsion electrospinning: Fundamentals, food applications and prospects. Trends Food Sci. Technol. 2018, 80, 175–186. [Google Scholar] [CrossRef]
- Angeles, M.; Cheng, H.; Velankar, S.S. Emulsion electrospinning: Composite fibers from drop breakup during electrospinning. Polym. Adv. Technol. 2008, 19, 728–733. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Wang, C.; Tong, S.N.; Tse, Y.H.; Wang, M. Conventional Electrospinning vs. Emulsion Electrospinning: A Comparative Study on the Development of Nanofibrous Drug/Biomolecule Delivery Vehicles. Adv. Mater. Res. 2012, 410, 118–121. [Google Scholar] [CrossRef]
- Spano, F.; Quarta, A.; Martelli, C.; Ottobrini, L.; Rossi, R.M.; Gigli, G.; Blasi, L. Fibrous scaffolds fabricated by emulsion electrospinning: From hosting capacity to in vivo biocompatibility. Nanoscale 2016, 8, 9293–9303. [Google Scholar] [CrossRef] [PubMed]
- Nikmaram, N.; Roohinejad, S.; Hashemi, S.; Koubaa, M.; Barba, F.J.; Abbaspourrad, A.; Greiner, R. Emulsion-based systems for fabrication of electrospun nanofibers: Food, pharmaceutical and biomedical applications. RSC Adv. 2017, 7, 28951–28964. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A. On the way to clean and safe electrospinning—Green electrospinning: Emulsion and suspension electrospinning. Polym. Adv. Technol. 2011, 22, 372–378. [Google Scholar] [CrossRef]
- Xu, X.; Zhuang, X.; Chen, X.; Wang, X.; Yang, L.; Jing, X. Preparation of Core-Sheath Composite Nanofibers by Emulsion Electrospinning. Macromol. Rapid Commun. 2006, 27, 1637–1642. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Cui, W.; Zhou, S.; Tan, R.; Wang, C. Structural stability and release profiles of proteins from core-shell poly (DL-lactide) ultrafine fibers prepared by emulsion electrospinning. J. Biomed. Mater. Res. Part A 2008, 86, 374–385. [Google Scholar] [CrossRef]
- El-Toni, A.M.; Habila, M.A.; Labis, J.P.; Alothman, Z.A.; Alhoshan, M.; Elzatahry, A.A.; Zhang, F. Design, synthesis and applications of core–shell, hollow core, and nanorattle multifunctional nanostructures. Nanoscale 2016, 8, 2510–2531. [Google Scholar] [CrossRef]
- Liu, Y.; Goebl, J.; Yin, Y. Templated synthesis of nanostructured materials. Chem. Soc. Rev. 2013, 42, 2610–2653. [Google Scholar] [CrossRef] [PubMed]
- Wade, T.L.; Wegrowe, J.-E. Template synthesis of nanomaterials. Eur. Phys. J. Appl. Phys. 2005, 29, 3–22. [Google Scholar] [CrossRef]
- Park, B.; Choi, J.; Park, J.-W. Cellulose Nanofiber-Templated Synthesis of Polypyrrole-Polyaniline Core-Shell Composites. Macromol. Res. 2022, 30, 836–841. [Google Scholar] [CrossRef]
- Wei, W.; Yang, Z. Template Synthesis of Hierarchically Structured Composites. Adv. Mater. 2008, 20, 2965–2969. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Goebl, J.; Yin, Y. Self-templated synthesis of hollow nanostructures. Nano Today 2009, 4, 494–507. [Google Scholar] [CrossRef]
- Huczko, A. Template-based synthesis of nanomaterials. Appl. Phys. A 2000, 70, 365–376. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y. Centrifugal Spinning: An Alternative Approach to Fabricate Nanofibers at High Speed and Low Cost. Polym. Rev. 2014, 54, 677–701. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, J. Research on the development of the centrifugal spinning. MATEC Web Conf. 2017, 95, 07003. [Google Scholar] [CrossRef]
- Xu, H.; Yagi, S.; Ashour, S.; Du, L.; Hoque, M.E.; Tan, L. A Review on Current Nanofiber Technologies: Electrospinning, Centrifugal Spinning, and Electro-Centrifugal Spinning. Macromol. Mater. Eng. 2023, 308, 2200502. [Google Scholar] [CrossRef]
- Weitz, R.T.; Harnau, L.; Rauschenbach, S.; Burghard, M.; Kern, K. Polymer Nanofibers via Nozzle-Free Centrifugal Spinning. Nano Lett. 2008, 8, 1187–1191. [Google Scholar] [CrossRef]
- Luz, H.Z.; dos Santos, L.A.L. Centrifugal spinning for biomedical use: A review. Crit. Rev. Solid State Mater. Sci. 2023, 48, 519–534. [Google Scholar] [CrossRef]
- Noroozi, S.; Arne, W.; Larson, R.G.; Taghavi, S.M. A comprehensive mathematical model for nanofibre formation in centrifugal spinning methods. J. Fluid Mech. 2020, 892, A26. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, Z.; Lu, B.; Chen, B.; Lai, Z. The movement and forces of spinning solution in the nozzle during high-speed centrifugal spinning. J. Eng. Fibers Fabr. 2019, 14, 1558925019828207. [Google Scholar] [CrossRef]
- Xu, H.; Chen, H.; Li, X.; Liu, C.; Yang, B. A comparative study of jet formation in nozzle- and nozzle-less centrifugal spinning systems. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1547–1559. [Google Scholar] [CrossRef]
- Ma, H.; Chen, G.; Zhang, J.; Liu, Y.; Nie, J.; Ma, G. Facile fabrication of core-shell polyelectrolyte complexes nanofibers based on electric field induced phase separation. Polymer 2017, 110, 80–86. [Google Scholar] [CrossRef]
- Atkin, R.; Davies, P.; Hardy, J.; Vincent, B. Preparation of Aqueous Core/Polymer Shell Microcapsules by Internal Phase Separation. Macromolecules 2004, 37, 7979–7985. [Google Scholar] [CrossRef]
- Zhao, Y.; Döhler, D.; Lv, L.P.; Binder, W.H.; Landfester, K.; Crespy, D. Facile Phase-Separation Approach to Encapsulate Functionalized Polymers in Core-Shell Nanoparticles. Macromol. Chem. Phys. 2014, 215, 198–204. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, N. Effect of phase-separated patterns on the formation of core-shell structure. J. Mater. Sci. Technol. 2020, 38, 64–72. [Google Scholar] [CrossRef]




| Source Title | Year | Aim | References |
|---|---|---|---|
| Electrospun nanofiber membranes with various structures for wound dressing | 2023 | Reviewed the development and application of electrospun nanofiber membranes for wound dressings, focusing on structural design and the incorporation of therapeutic factors. | [23] |
| State-of-the-art review of advanced electrospun nanofiber composites for enhanced wound healing | 2023 | Presented the advancement in electrospun nanofiber composites for wound healing with an emphasis on morphologies and methods. | [24] |
| Hyaluronic acid and chitosan-based electrospun wound dressings: Problems and solutions | 2022 | Centered on the factors affecting the electrospinning of hyaluronic acid and chitosan for wound dressing applications including their biological roles and mechanisms. | [25] |
| In vitro and in vivo advancement of multifunctional electrospun nanofiber scaffolds in wound healing applications: Innovative nanofiber designs, stem cell approaches, and future perspectives | 2022 | Discussed nanofiber geometries with their potentials and stem cell approaches for electrospun nanofiber scaffolds. | [26] |
| Nature-derived and synthetic additives to poly(ɛ-caprolactone) nanofibrous systems for biomedicine: An updated overview | 2022 | Reviewed recent advancements in PCL nanofibers for biomedical and tissue engineering applications to improve their properties since 2017. | [27] |
| A review on biopolymer-derived electrospun nanofibers for biomedical and antiviral applications | 2022 | Reviewed the efficiency and optimization of electrospinning method in fabrication of multifunctional nanofibers for biomedical and tissue regeneration applications. | [28] |
| Review on nanoparticles and nanostructured materials: Bioimaging, biosensing, drug delivery, tissue engineering, antimicrobial, and agro-food applications | 2022 | Presented synthesis methods and applications of various nanomaterials in agricultural and biomedical related fields. | [29] |
| Natural protein-based electrospun nanofibers for advanced healthcare applications: Progress and challenges | 2022 | Highlighted the advancement and challenges in natural protein-based electrospun nanofibers for wound dressing, tissue engineering, and drug delivery applications. | [30] |
| Nanofibers for biomedical and healthcare applications | 2019 | Focused on the recent reports on fabrication, scaling-up challenges, and application of electrospun nanofibers, featuring their potential in drug delivery, wound healing, and tissue engineering applications. | [31] |
| Cellulose acetate electrospun nanofibers for drug delivery systems: Applications and recent advances | 2018 | Reviewed the methods, applications, and opportunities of cellulose acetate electrospun nanofibers in drug delivery systems. | [32] |
| Nanomaterial | Abbreviation | Structure | Dimension (nm) | Surface Area (m2/g) | Mechanism of Toxicity |
|---|---|---|---|---|---|
| Silver nanoparticles | AgNPs | Spherical | 10–20 | 20–50 | ROS, Ag+ release, inflammatory responses, genotoxicity, mitochondrial dysfunction |
| Zinc oxide | ZnO | Hexagonal | 10–30 | 10–50 | ROS, Zn2+ ion release, inflammatory responses, genotoxicity |
| Graphene oxide | GO | Sheet | 100–200 | 2630 | ROS, physical cell membrane disruption, adsorption of biomolecules and starvation |
| Copper oxide | CuO | Rod-like | 10–50 | 20–80 | ROS, Cu2+ release, inflammatory responses, binding to bacterial proteins and enzymes |
| Titanium oxide | TiO2 | Rod-like and anatase | 5–50 | 50–200 | ROS, photocatalytic activity, physical cell membrane disruption, adsorption biomolecules, genotoxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajabifar, N.; Rostami, A.; Afshar, S.; Mosallanezhad, P.; Zarrintaj, P.; Shahrousvand, M.; Nazockdast, H. Wound Dressing with Electrospun Core-Shell Nanofibers: From Material Selection to Synthesis. Polymers 2024, 16, 2526. https://doi.org/10.3390/polym16172526
Rajabifar N, Rostami A, Afshar S, Mosallanezhad P, Zarrintaj P, Shahrousvand M, Nazockdast H. Wound Dressing with Electrospun Core-Shell Nanofibers: From Material Selection to Synthesis. Polymers. 2024; 16(17):2526. https://doi.org/10.3390/polym16172526
Chicago/Turabian StyleRajabifar, Nariman, Amir Rostami, Shahnoosh Afshar, Pezhman Mosallanezhad, Payam Zarrintaj, Mohsen Shahrousvand, and Hossein Nazockdast. 2024. "Wound Dressing with Electrospun Core-Shell Nanofibers: From Material Selection to Synthesis" Polymers 16, no. 17: 2526. https://doi.org/10.3390/polym16172526
APA StyleRajabifar, N., Rostami, A., Afshar, S., Mosallanezhad, P., Zarrintaj, P., Shahrousvand, M., & Nazockdast, H. (2024). Wound Dressing with Electrospun Core-Shell Nanofibers: From Material Selection to Synthesis. Polymers, 16(17), 2526. https://doi.org/10.3390/polym16172526








