Fabrication and Characterization of Dissolving Microneedles Containing Oryza sativa L. Extract Complex for Enhancement of Transfollicular Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
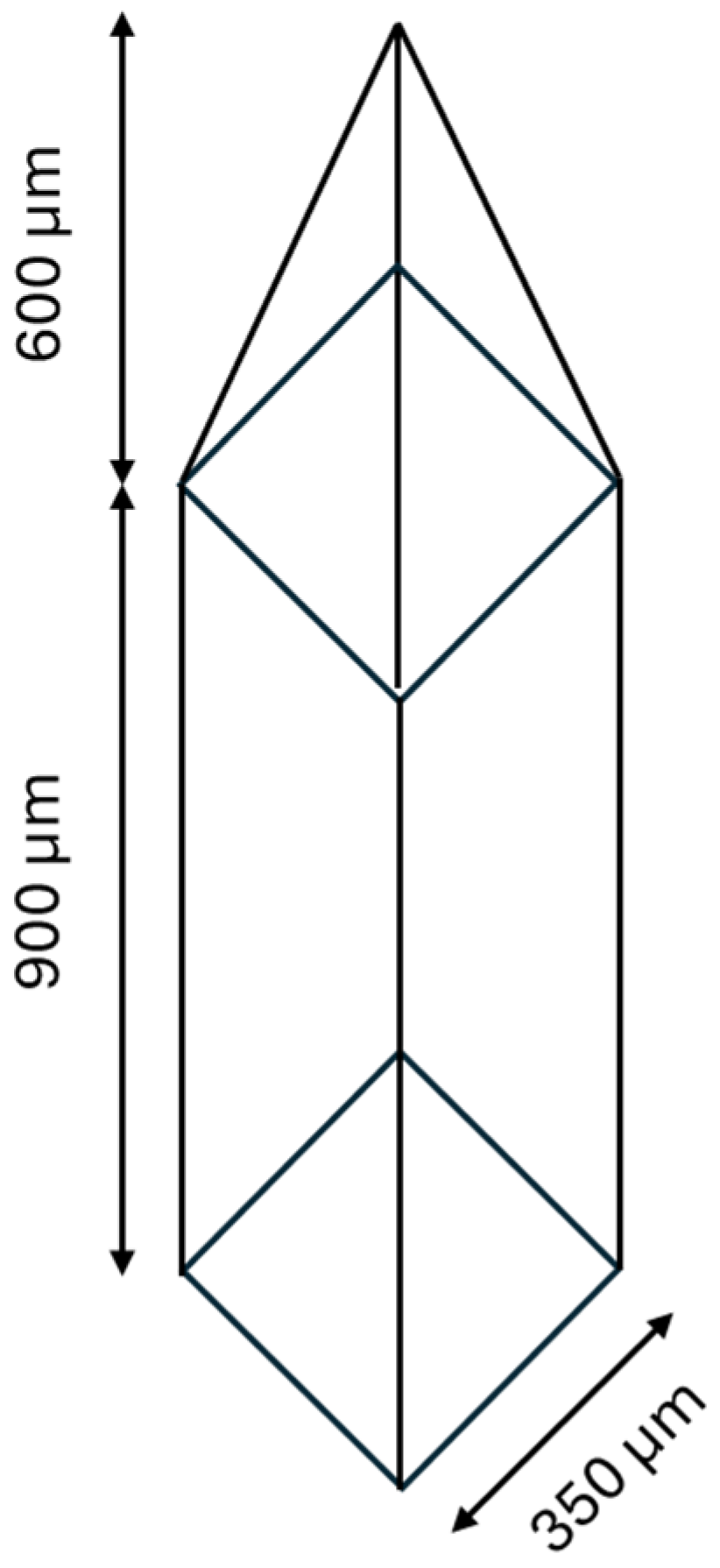
2.2. Fabrication of Microneedle Patch (Master Mold) Reverse PDMS Microneedle Mold
2.3. Preparation of PVP K90/HPMC E50 Microneedles
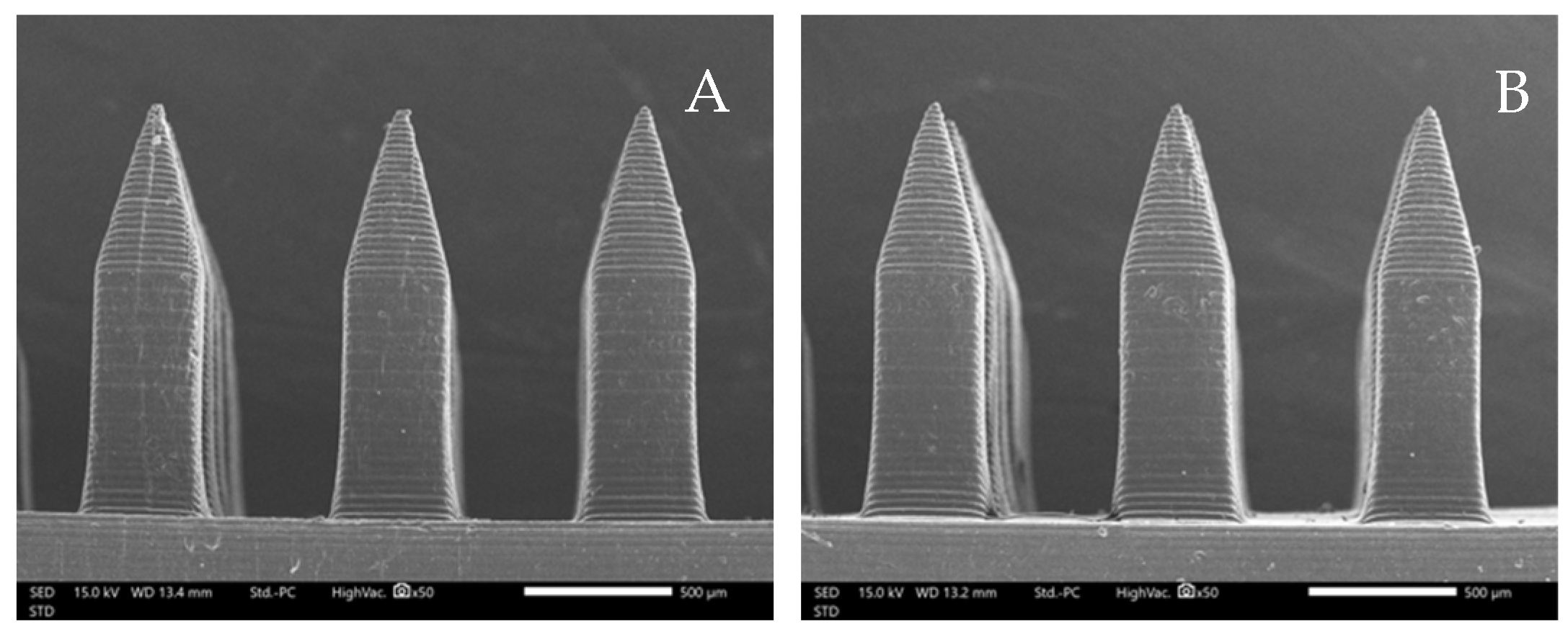
2.4. Morphological Structure Investigation
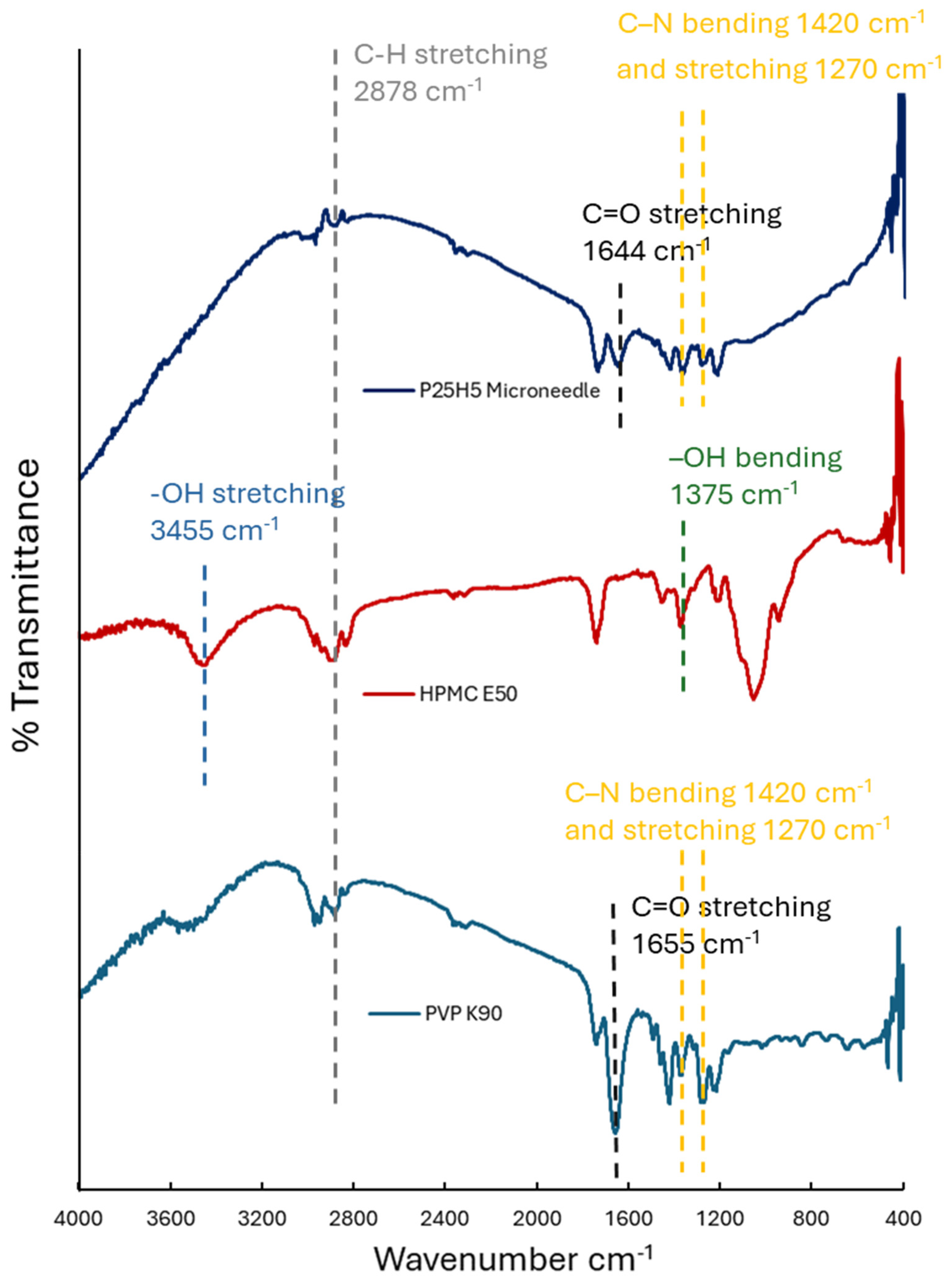
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Mechanical Strength
- H1 is the initial height of the microneedle (µm);
- H2 is the height of the microneedle after the test (µm).
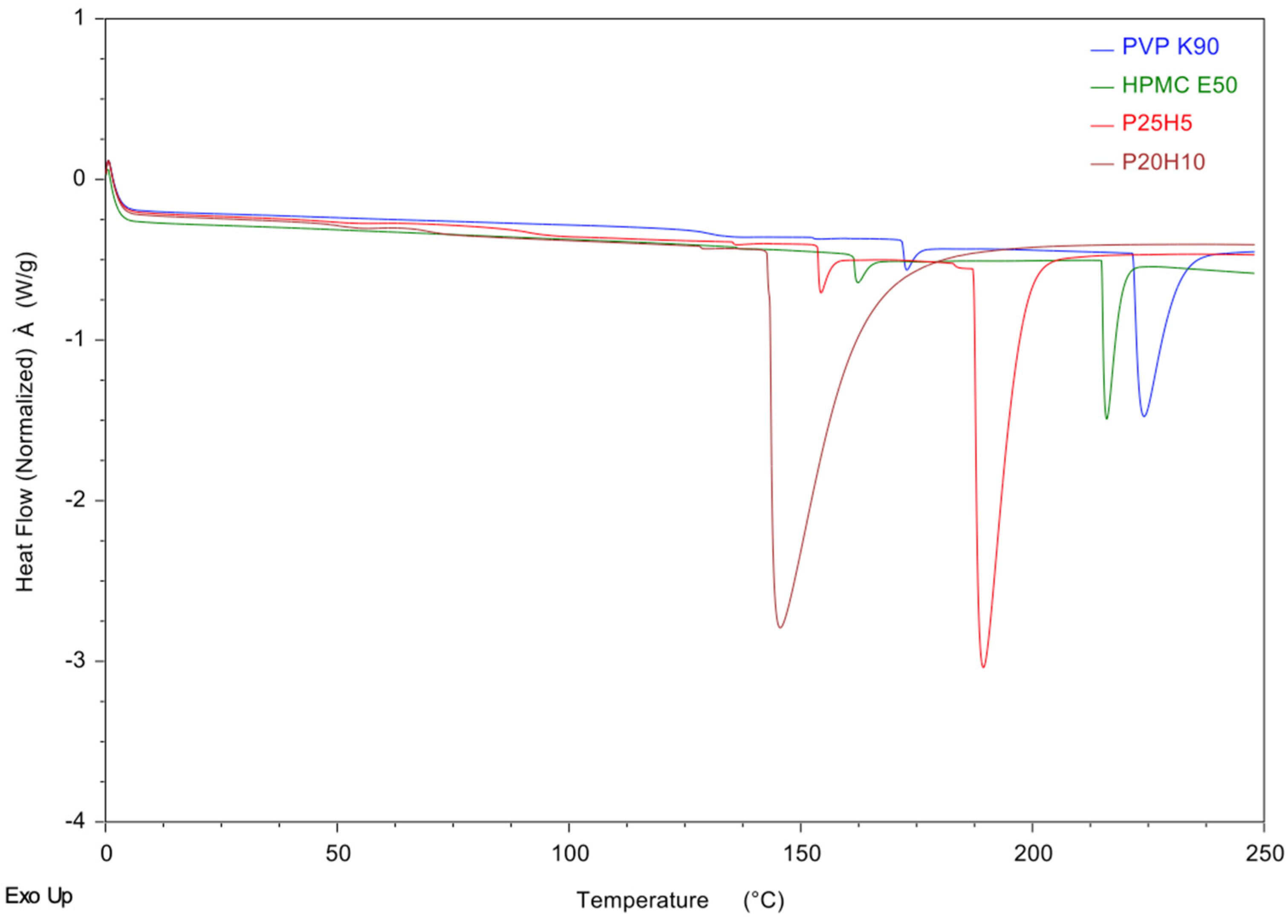
2.7. Differential Scanning Calorimetry (DSC) Studies
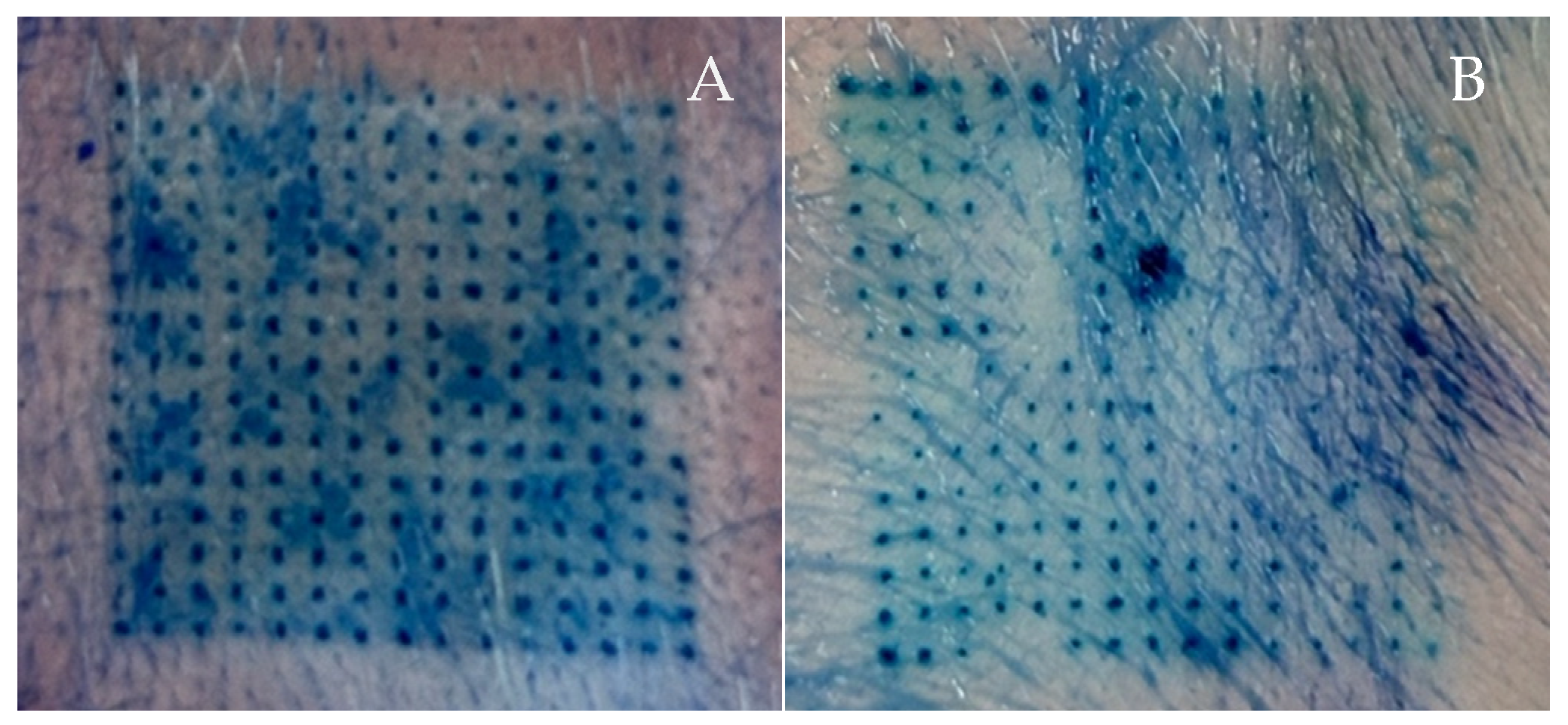
2.8. Ex Vivo Skin Insertion Test
2.9. Dissolution of Microneedles
2.10. Preparation and Characterization of Microneedles Containing Oryza sativa L. Extract Complex
2.11. Drug-Loading Content
2.12. Transfollicular Penetration Studies of Skin by Franz Diffusion Cells
2.13. Cell Viability Assay
2.14. Statistical Analysis
3. Results and Discussion
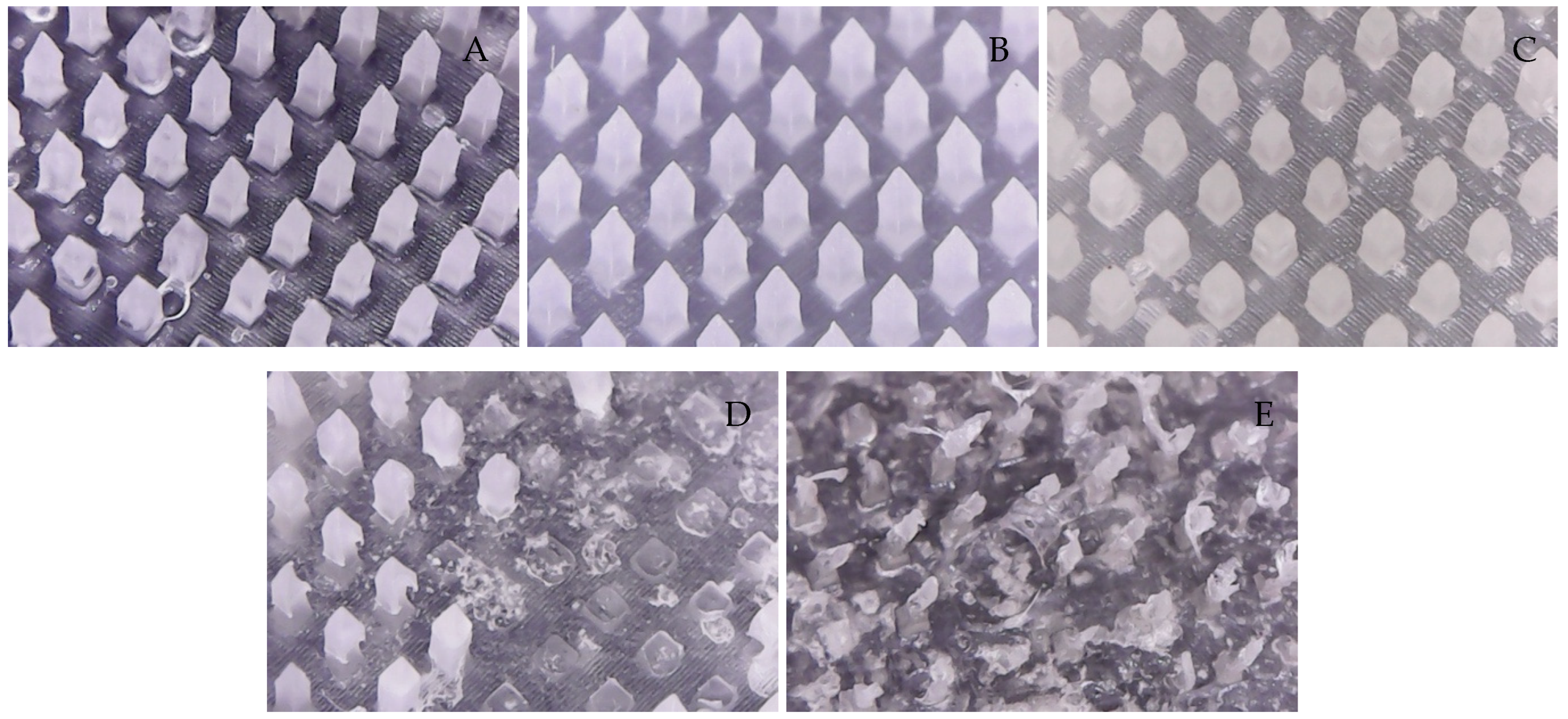
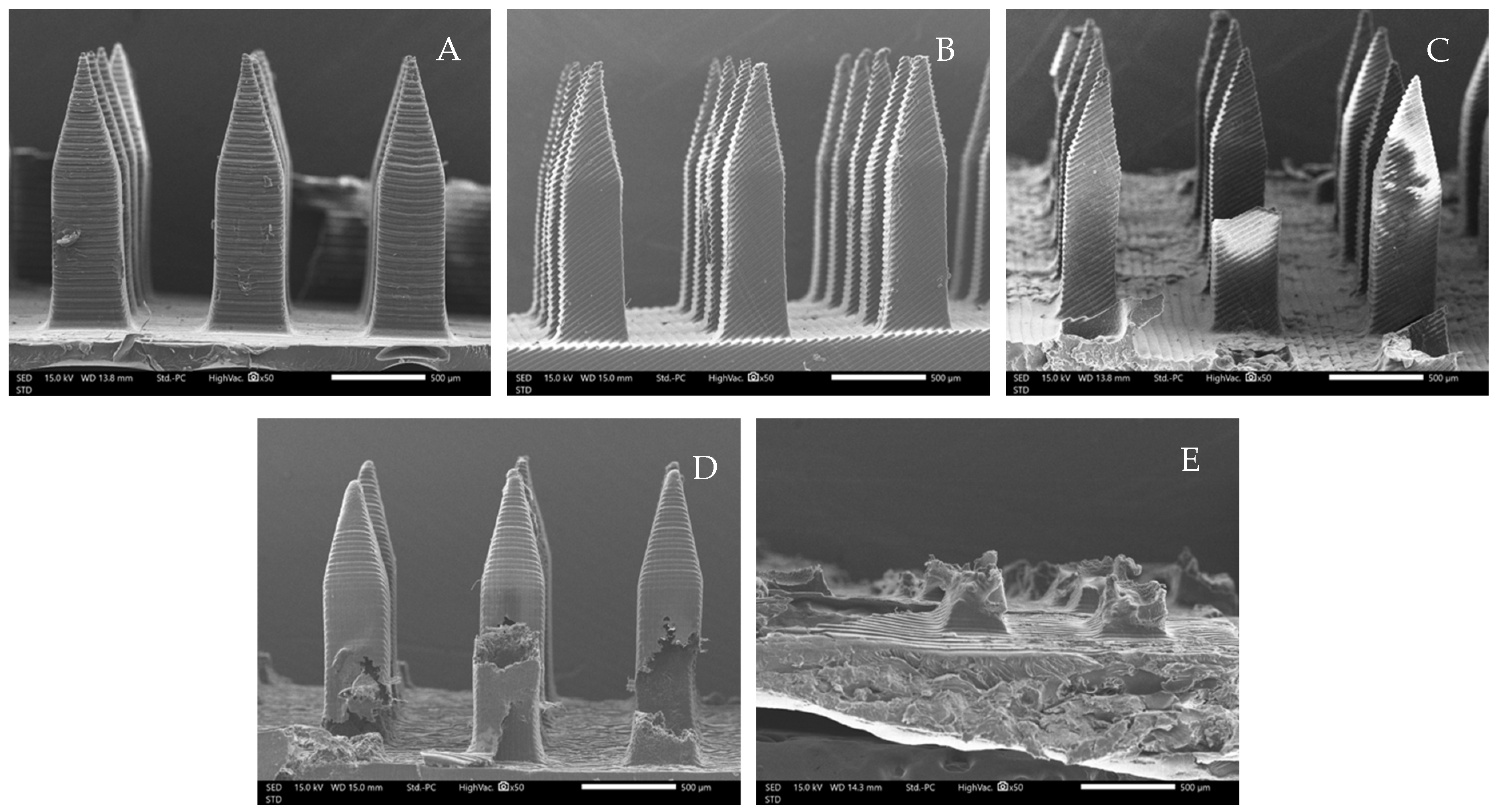
3.1. Morphological Structure of Microneedles
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. Mechanical Test
3.4. Differential Scanning Calorimetry (DSC) Studies
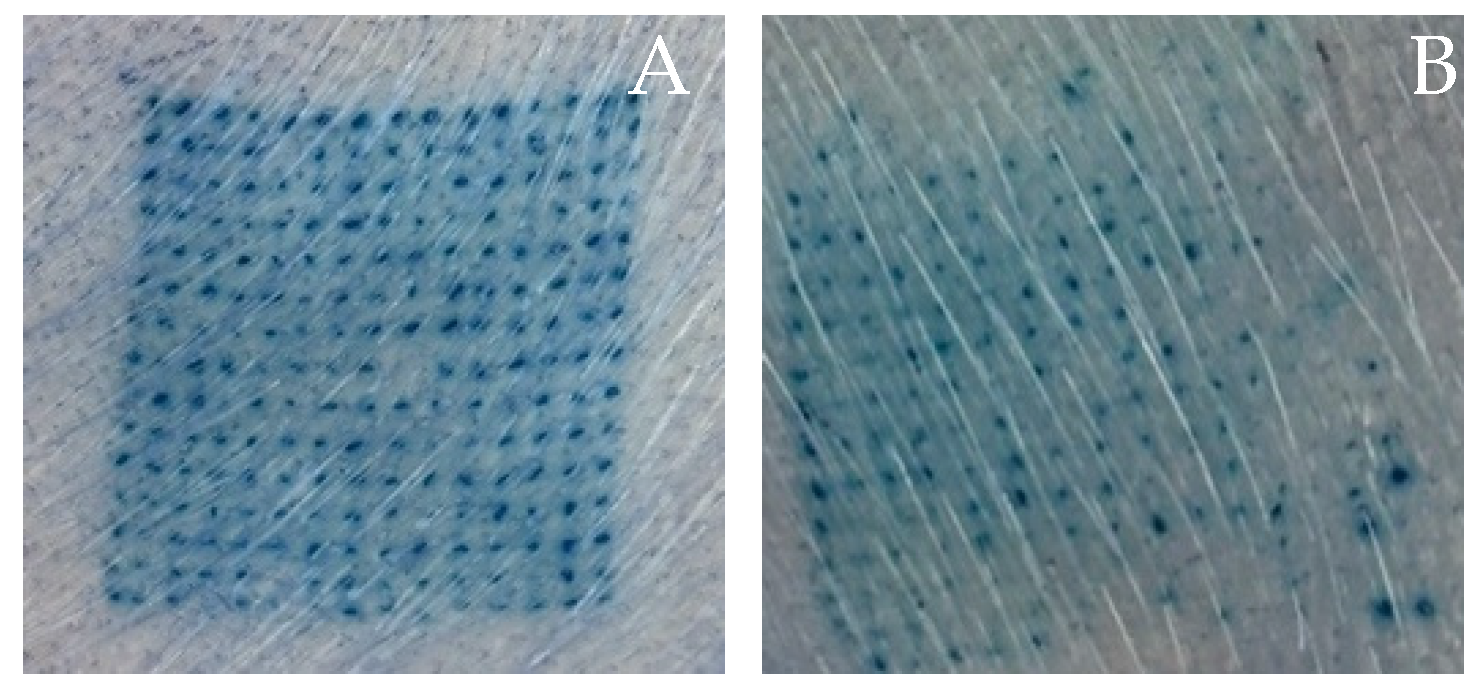
3.5. Ex Vivo Skin Insertion Test
3.6. Dissolution of Microneedles
3.7. Characterization of Microneedles Containing Oryza sativa L. Extract Complex
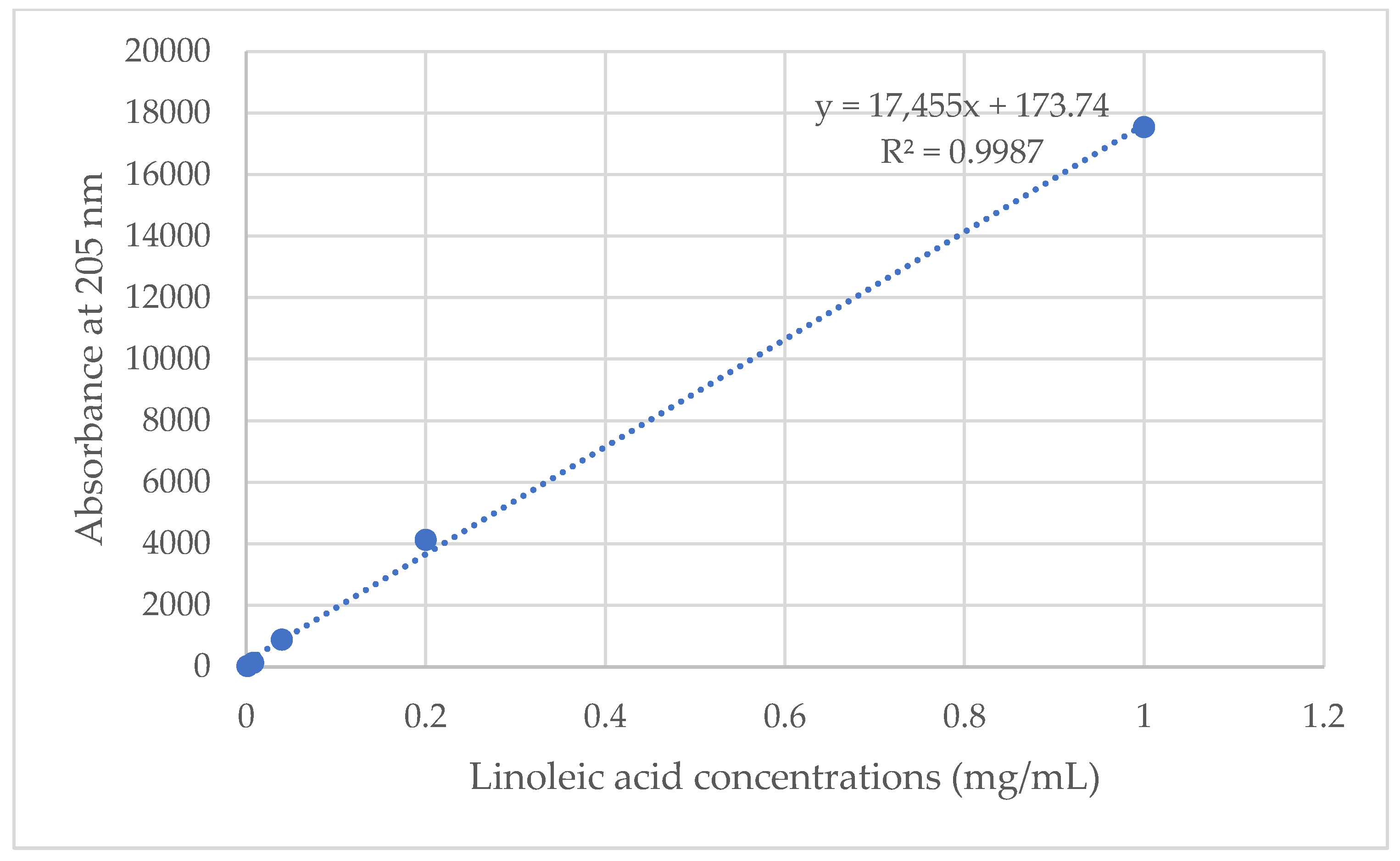
3.8. Drug-Loading Content
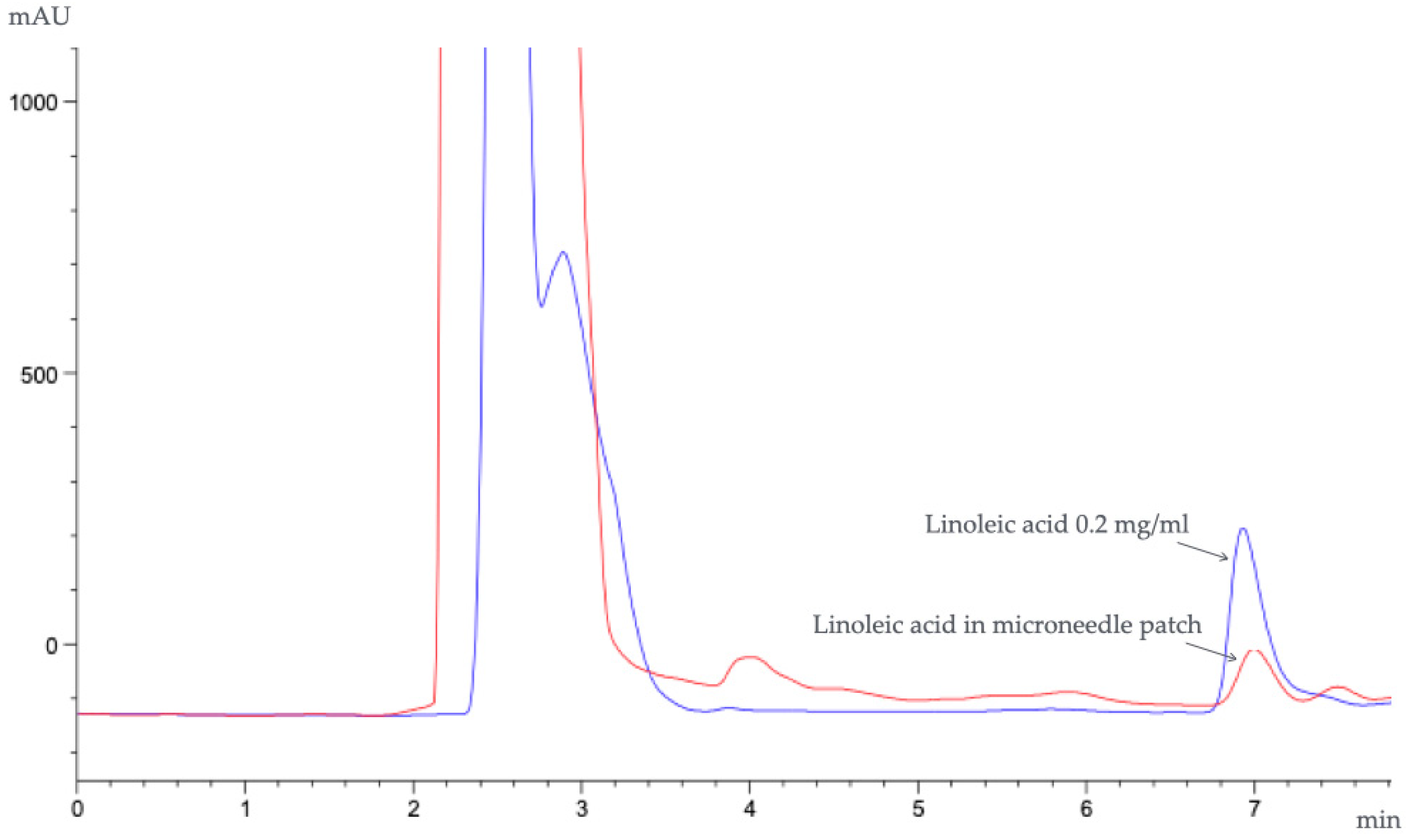
3.9. Transfollicular Penetration Test by Franz Diffusion Cells
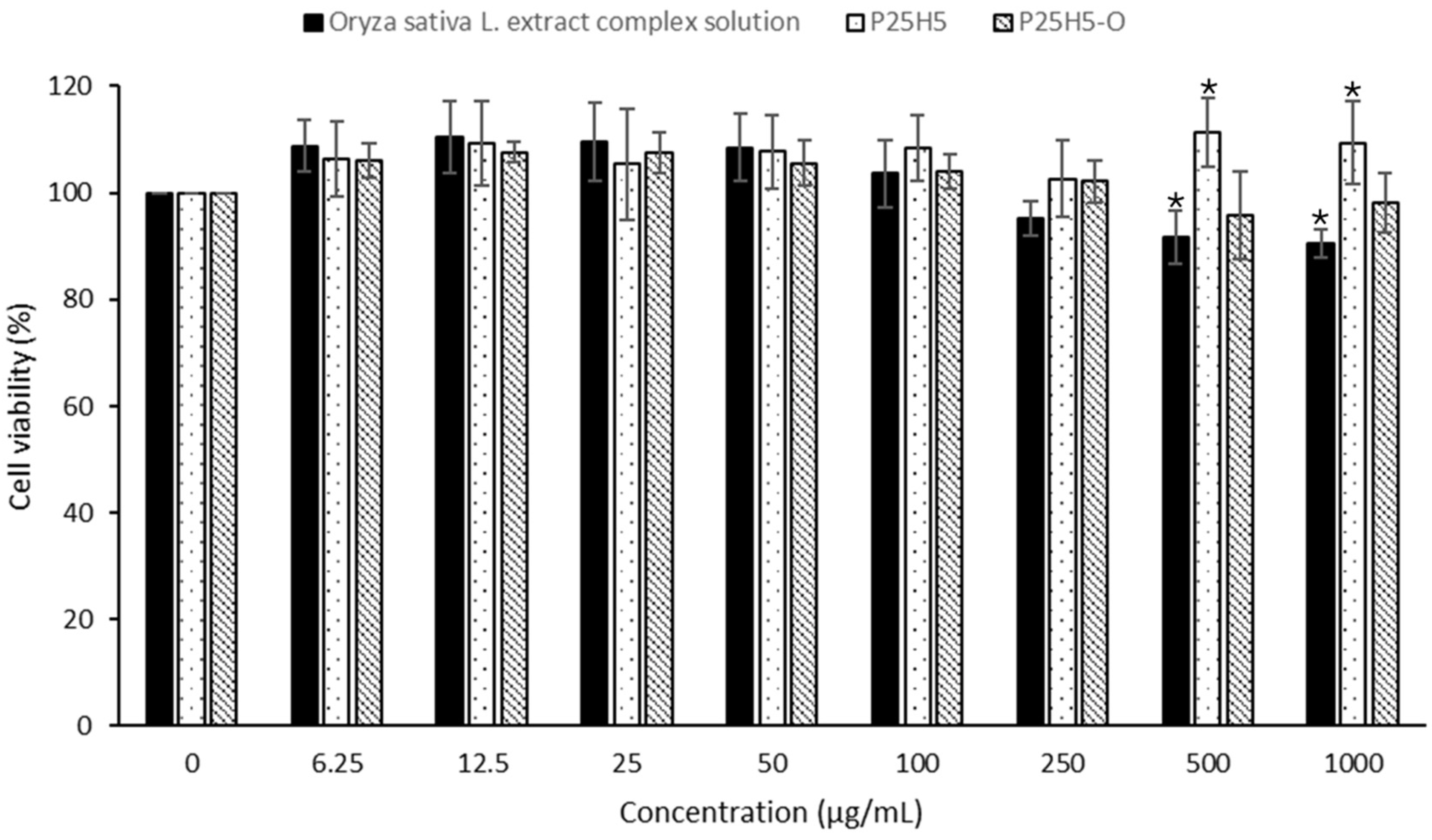
3.10. Cell Viability Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aqil, M.; Sultana, Y.; Ali, A. Transdermal delivery of β-blockers. Expert Opin. Drug Deliv. 2006, 3, 405–418. [Google Scholar] [CrossRef]
- Dharadhar, S.; Majumdar, A.; Dhoble, S.; Patravale, V. Microneedles for transdermal drug delivery: A systematic review. Drug Dev. Ind. Pharm. 2019, 45, 188–201. [Google Scholar] [CrossRef]
- He, J.; Zhang, Z.; Zheng, X.; Li, L.; Qi, J.; Wu, W.; Lu, Y. Design and evaluation of dissolving microneedles for enhanced dermal delivery of propranolol hydrochloride. Pharmaceutics 2021, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.R.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010, 17, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hou, M.; Yang, R.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Transdermal delivery of therapeutics through dissolvable gelatin/sucrose films coated on PEGDA microneedle arrays with improved skin permeability. J. Mater. Chem. B 2019, 7, 7515–7524. [Google Scholar] [CrossRef]
- Avcil, M.; Çelik, A. Microneedles in drug delivery: Progress and challenges. Micromachines 2021, 12, 1321. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Shyu, V.B.H.; Chen, C.T. Dissolving microneedle patches for transdermal insulin delivery in diabetic mice: Potential for clinical applications. Materials 2018, 11, 1625. [Google Scholar] [CrossRef]
- Leone, M.; Mönkäre, J.; Bouwstra, J.A.; Kersten, G. Dissolving microneedle patches for dermal vaccination. Pharm. Res. 2017, 34, 2223–2240. [Google Scholar] [CrossRef]
- Al-Japairai, K.A.S.; Mahmood, S.; Almurisi, S.H.; Venugopal, J.R.; Hilles, A.R.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef]
- Lee, I.-C.; Wu, Y.-C.; Tsai, S.-W.; Chen, C.-H.; Wu, M.-H. Fabrication of two-layer dissolving polyvinylpyrrolidone microneedles with different molecular weights for in vivo insulin transdermal delivery. RSC Adv. 2017, 7, 5067–5075. [Google Scholar] [CrossRef]
- Chanabodeechalermrung, B.; Chaiwarit, T.; Chaichit, S.; Udomsom, S.; Baipaywad, P.; Worajittiphon, P.; Jantrawut, P. HPMC/PVP K90 Dissolving Microneedles Fabricated from 3D-Printed Master Molds: Impact on Microneedle Morphology, Mechanical Strength, and Topical Dissolving Property. Polymers 2024, 16, 452. [Google Scholar] [CrossRef] [PubMed]
- Aung, N.N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Pamornpathomkul, B. HPMC/PVP dissolving microneedles: A promising delivery platform to promote trans-epidermal delivery of alpha-arbutin for skin lightening. AAPS Pharmscitech. 2020, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Richter, H.; Meinke, M.; Sterry, W.; Patzelt, A. Which skin model is the most appropriate for the investigation of topically applied substances into the hair follicles? Skin Pharmacol. Physiol. 2010, 23, 47–52. [Google Scholar] [CrossRef]
- Barry, B. Drug delivery routes in skin: A novel approach. Adv. Drug Deliv. Rev. 2002, 54, S31–S40. [Google Scholar] [CrossRef] [PubMed]
- Price, V.H. Alopecia areata: Clinical aspects. J. Investig. Dermatol. 1991, 96, 68S. [Google Scholar] [CrossRef]
- Manosroi, A.; Ruksiriwanich, W.; Abe, M.; Manosroi, W.; Manosroi, J. Transfollicular enhancement of gel containing cationic niosomes loaded with unsaturated fatty acids in rice (Oryza sativa) bran semi-purified fraction. Eur. J. Pharm. Biopharm. 2012, 81, 303–313. [Google Scholar] [CrossRef]
- Linsaenkart, P.; Ruksiriwanich, W.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Sommano, S.R.; Prom-U-Thai, C.; Jamjod, S.; Arjin, C. Natural Melanogenesis Inhibitor, Antioxidant, and Collagen Biosynthesis Stimulator of Phytochemicals in Rice Bran and Husk Extracts from Purple Glutinous Rice (Oryza sativa L. cv. Pieisu 1 CMU) for Cosmetic Application. Plants 2023, 12, 970. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Linsaenkart, P.; Khantham, C.; Muangsanguan, A.; Sringarm, K.; Jantrawut, P.; Prom-U-Thai, C.; Jamjod, S.; Yamuangmorn, S.; Arjin, C. Regulatory effects of thai rice by-product extracts from Oryza sativa L. cv. Bue Bang 3 CMU and Bue Bang 4 CMU on melanin production, nitric oxide secretion, and steroid 5α-reductase inhibition. Plants 2023, 12, 653. [Google Scholar] [CrossRef]
- Khantham, C.; Ruksiriwanich, W.; Sringarm, K.; Prom-U-Thai, C.; Jamjod, S.; Arjin, C.; Muangsanguan, A.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y. Effects of bioactive composition in Oryza sativa L. cv. KDML105 bran extract on gene expression related to hair cycle in human hair follicle dermal papilla cells. Agronomy 2023, 13, 295. [Google Scholar] [CrossRef]
- Khantham, C.; Linsaenkart, P.; Chaitep, T.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; Prom-U-Thai, C. Antioxidation, anti-inflammation, and regulation of SRD5A gene expression of Oryza sativa cv. Bue Bang 3 CMU husk and bran extracts as androgenetic alopecia molecular treatment substances. Plants 2022, 11, 330. [Google Scholar] [CrossRef]
- Chanabodeechalermrung, B.; Chaiwarit, T.; Udomsom, S.; Rachtanapun, P.; Piboon, P.; Jantrawut, P. Determination of vat-photopolymerization parameters for microneedles fabrication and characterization of HPMC/PVP K90 dissolving microneedles utilizing 3D-printed mold. Sci. Rep. 2024, 14, 16174. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Huang, Y.; Kang, Y.; Dai, G.; Liu, Z.; Xu, K.; Zhong, W. The effects of molecular weight of hyaluronic acid on transdermal delivery efficiencies of dissolving microneedles. Eur. J. Pharm. Sci. 2022, 168, 106075. [Google Scholar] [CrossRef] [PubMed]
- Qiang, N.; Liu, Z.; Lu, M.; Yang, Y.; Liao, F.; Feng, Y.; Liu, G.; Qiu, S. Preparation and properties of polyvinylpyrrolidone/sodium carboxymethyl cellulose soluble microneedles. Materials 2023, 16, 3417. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-C.; He, J.-S.; Tsai, M.-T.; Lin, K.-C. Fabrication of a novel partially dissolving polymer microneedle patch for transdermal drug delivery. J. Mater. Chem. B 2015, 3, 276–285. [Google Scholar] [CrossRef]
- Sullivan, S.P.; Murthy, N.; Prausnitz, M.R. Minimally invasive protein delivery with rapidly dissolving polymer microneedles. Adv. Mater. 2008, 20, 933–938. [Google Scholar] [CrossRef]
- Sainakham, M.; Manosroi, A.; Abe, M.; Manosroi, W.; Manosroi, J. Potent in vivo anticancer activity and stability of liposomes encapsulated with semi-purified Job’s tear (Coix lacryma-jobi Linn.) extracts on human colon adenocarcinoma (HT-29) xenografted mice. Drug Deliv. 2016, 23, 3399–3407. [Google Scholar] [CrossRef]
- Manosroi, J.; Chankhampan, C.; Chaikul, P.; Manosroi, W.; Manosroi, A. Transfollicular enhancement of methyl myristate loaded in cationic niosomes incorporated in hair lotion. J. Microencapsul. 2016, 33, 585–594. [Google Scholar] [CrossRef]
- Siqueira, P.; Siqueira, É.; De Lima, A.E.; Siqueira, G.; Pinzón-Garcia, A.D.; Lopes, A.P.; Segura, M.E.C.; Isaac, A.; Pereira, F.V.; Botaro, V.R. Three-dimensional stable alginate-nanocellulose gels for biomedical applications: Towards tunable mechanical properties and cell growing. Nanomaterials 2019, 9, 78. [Google Scholar] [CrossRef]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef]
- Kida, D.; Gładysz, O.; Szulc, M.; Zborowski, J.; Junka, A.; Janeczek, M.; Lipińska, A.; Skalec, A.; Karolewicz, B. Development and evaluation of a polyvinylalcohol-cellulose derivative-based film with povidone-iodine predicted for wound treatment. Polymers 2020, 12, 1271. [Google Scholar] [CrossRef]
- Huang, S.-W.; Lin, Y.-F.; Li, Y.-X.; Hu, C.-C.; Chiu, T.-C. Synthesis of fluorescent carbon dots as selective and sensitive probes for cupric ions and cell imaging. Molecules 2019, 24, 1785. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, C.; Sugumaran, A.; Krishnaswami, V.; Palanichamy, R.; Velayutham, R.; Natesan, S. Development and evaluation of polyvinylpyrrolidone K90 and poloxamer 407 self-assembled nanomicelles: Enhanced topical ocular delivery of artemisinin. Polymers 2021, 13, 3038. [Google Scholar] [CrossRef]
- Wang, L.; Dong, W.; Xu, Y. Synthesis and characterization of hydroxypropyl methylcellulose and ethyl acrylate graft copolymers. Carbohydr. Polym. 2007, 68, 626–636. [Google Scholar] [CrossRef]
- Filimon, A.; Onofrei, M.D.; Bargan, A.; Stoica, I.; Dunca, S. Bioactive materials based on hydroxypropyl methylcellulose and silver nanoparticles: Structural-morphological characterization and antimicrobial testing. Polymers 2023, 15, 1625. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.S.; Xu, Y.W.; Wang, Y.C.; Wang, L.; Zhang, Y.D.; Hang, T.; Chen, H.J.; Xie, X.; Yang, B.R. Electrostatic assembly of ultraviolet-curable cellulose-coated silver nanowires as transparent electrodes for nanogenerator. Appl. Phys. Express 2018, 11, 075002. [Google Scholar] [CrossRef]
- Ning, X.; Wiraja, C.; Lio, D.C.S.; Xu, C. A double-layered microneedle platform fabricated through frozen spray-coating. Adv. Healthc. Mater. 2020, 9, 2000147. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Song, J.E.; Jun, S.-H.; Park, S.-G.; Kang, N.-G. Sugar-triggered burst drug releasing poly-lactic acid (PLA) microneedles and its fabrication based on solvent-casting approach. Pharmaceutics 2022, 14, 1758. [Google Scholar] [CrossRef]
- Shim, W.S.; Hwang, Y.M.; Park, S.G.; Lee, C.K.; Kang, N.G. Role of polyvinylpyrrolidone in dissolving microneedle for efficient transdermal drug delivery: In vitro and clinical studies. Bull. Korean Chem. Soc. 2018, 39, 789–793. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Sabri, A.H.B.; Moreno-Castellanos, N.; Utomo, E.; Cárcamo-Martínez, Á.; Domínguez-Robles, J.; Wardoyo, L.A.H.; Donnelly, R.F. Soluplus®-based dissolving microarray patches loaded with colchicine: Towards a minimally invasive treatment and management of gout. Biomater. Sci. 2022, 10, 5838–5855. [Google Scholar] [CrossRef]
- Ramadon, D.; Permana, A.D.; Courtenay, A.J.; McCrudden, M.T.; Tekko, I.A.; McAlister, E.; Anjani, Q.K.; Utomo, E.; McCarthy, H.O.; Donnelly, R.F. Development, evaluation, and pharmacokinetic assessment of polymeric microarray patches for transdermal delivery of vancomycin hydrochloride. Mol. Pharm. 2020, 17, 3353–3368. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, P.; Niu, W.; Fang, R.; Chen, H.; An, Y.; Wang, W.; Jiang, C.; Ye, J. Preparation and evaluation of dissolving tofacitinib microneedles for effective management of rheumatoid arthritis. Eur. J. Pharm. Sci. 2023, 188, 106518. [Google Scholar] [CrossRef] [PubMed]
- Unni, A.; Mathew, M.M.; Manathanath, M.; Jacob, S.; Sankaranarayanan, P.; Vasu, S.T.; Panicker, U.G. Biocompatibility evaluation of nano-hydroxyapatite modified hydroxypropyl methylcellulose/polyvinylpyrrolidone blends. Polym. Bull. 2024, 81, 3439–3458. [Google Scholar] [CrossRef]
- Zafar, N.; Mahmood, A.; Sarfraz, R.M.; Elaissari, A. Simvastatin loaded dissolvable microneedle patches with improved pharmacokinetic performance. Micromachines 2022, 13, 1304. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, M.; Song, W.; Zhang, Y.; Yu, D.-G.; Liu, Y. Electrospun core (HPMC–acetaminophen)–shell (PVP–sucralose) nanohybrids for rapid drug delivery. Gels 2022, 8, 357. [Google Scholar] [CrossRef]
- Jin, C.; Wu, F.; Hong, Y.; Shen, L.; Lin, X.; Zhao, L.; Feng, Y. Updates on applications of low-viscosity grade Hydroxypropyl methylcellulose in coprocessing for improvement of physical properties of pharmaceutical powders. Carbohydr. Polym. 2023, 311, 120731. [Google Scholar] [CrossRef]
- Karavas, E.; Georgarakis, E.; Bikiaris, D. Application of PVP/HPMC miscible blends with enhanced mucoadhesive properties for adjusting drug release in predictable pulsatile chronotherapeutics. Eur. J. Pharm. Biopharm. 2006, 64, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Pünnel, L.C.; Lunter, D.J. Film-forming systems for dermal drug delivery. Pharmaceutics 2021, 13, 932. [Google Scholar] [CrossRef]
- Ryu, H.S.; Jeong, J.; Lee, C.M.; Lee, K.S.; Lee, J.-N.; Park, S.-M.; Lee, Y.-M. Activation of hair cell growth factors by linoleic acid in Malva verticillata seed. Molecules 2021, 26, 2117. [Google Scholar] [CrossRef]
- Shukla, T.; Upmanyu, N.; Agrawal, M.; Saraf, S.; Saraf, S.; Alexander, A. Biomedical applications of microemulsion through dermal and transdermal route. Biomed. Pharmacother. 2018, 108, 1477–1494. [Google Scholar] [CrossRef]
 Solution formulation,
Solution formulation,  microneedle formulation.
microneedle formulation.
 Solution formulation,
Solution formulation,  microneedle formulation.
microneedle formulation.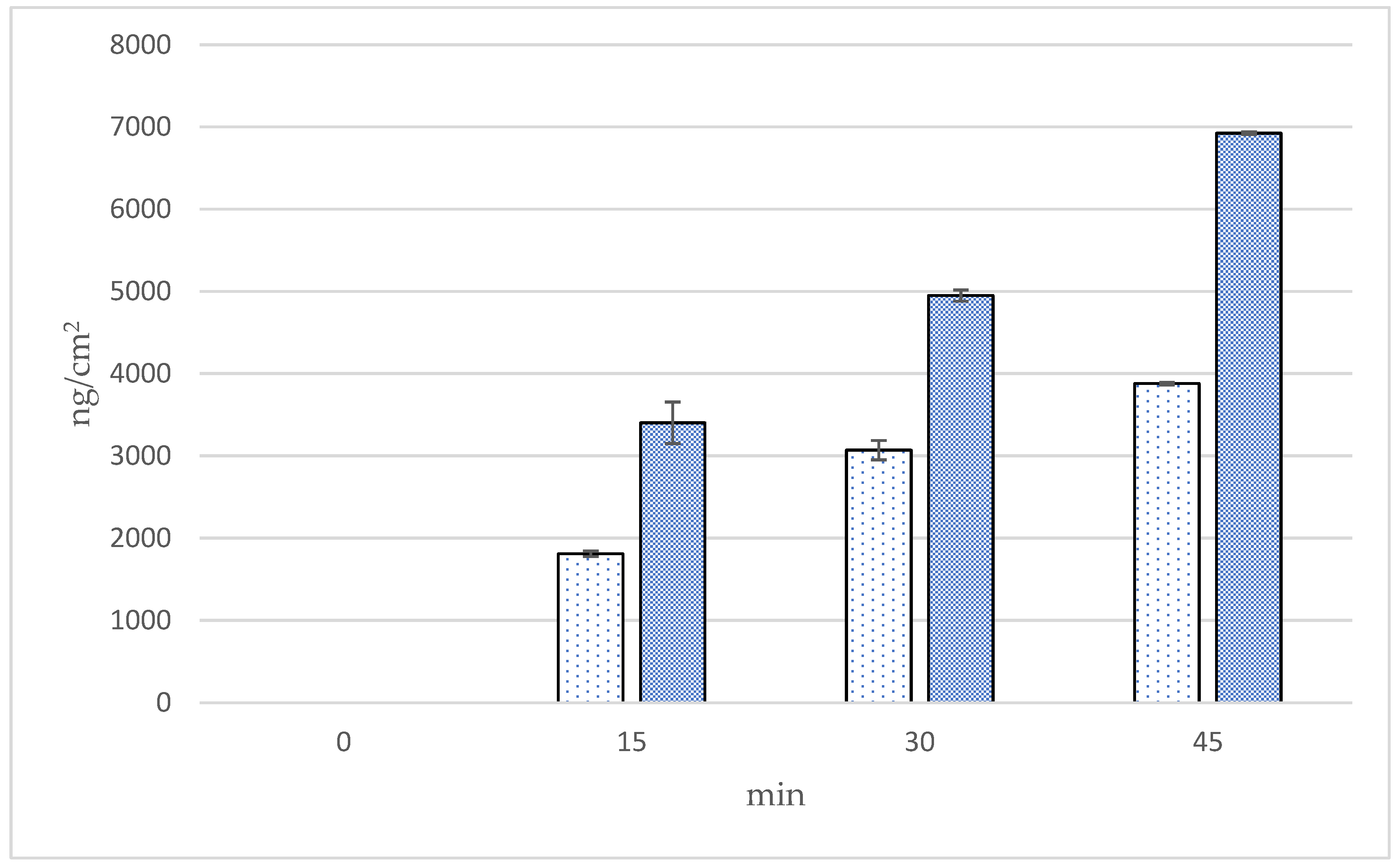
 Solution formulation,
Solution formulation,  microneedle formulation.
microneedle formulation.
 Solution formulation,
Solution formulation,  microneedle formulation.
microneedle formulation.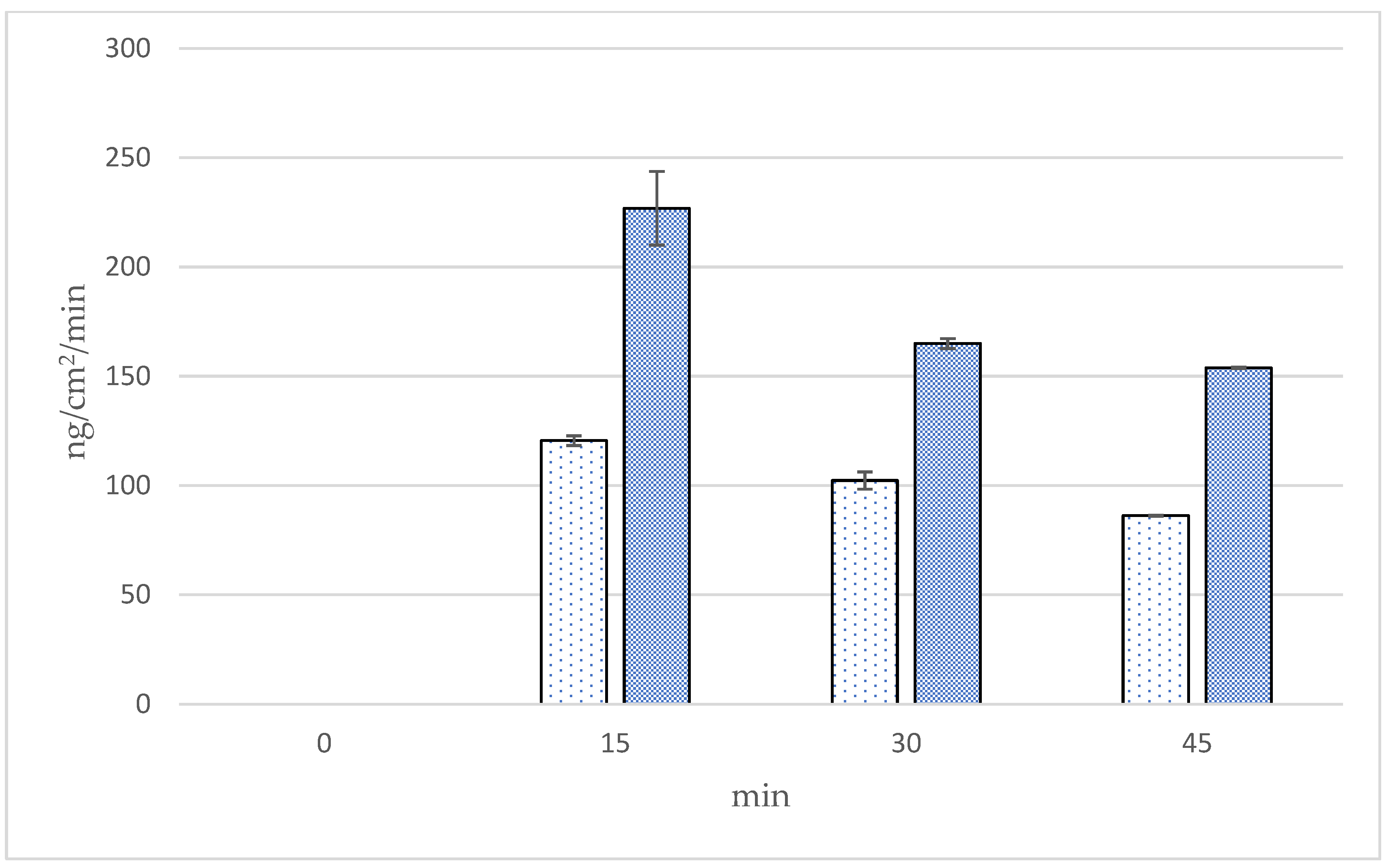
 Solution formulation,
Solution formulation,  microneedle formulation.
microneedle formulation.
 Solution formulation,
Solution formulation,  microneedle formulation.
microneedle formulation.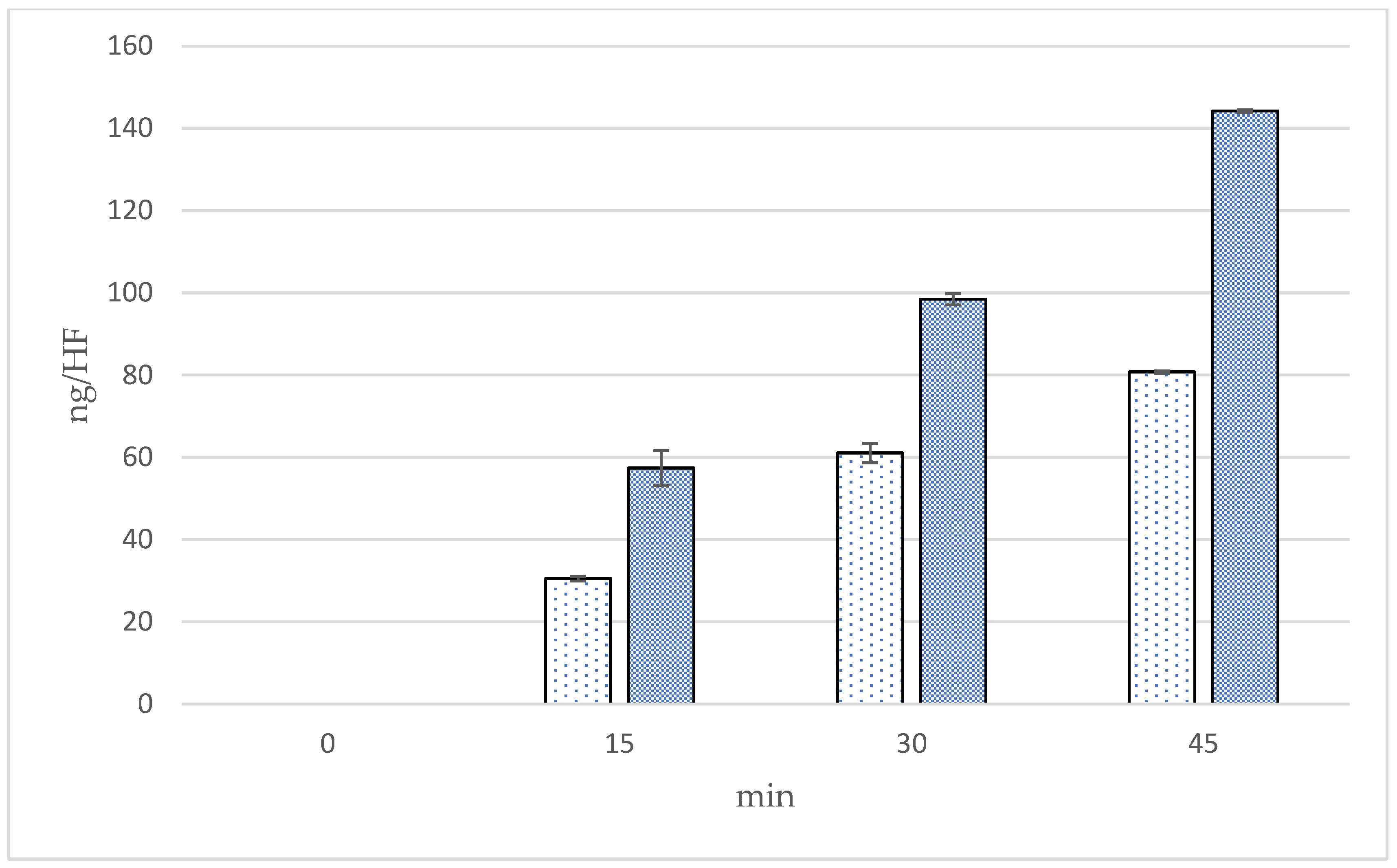
| Formulations | Composition | ||
|---|---|---|---|
| PVP K90 (g) | HPMC E50 (g) | DI Water/Anhydrous Ethanol Solution (g) | |
| P25H5 | 25 | 5 | 20 |
| P20H10 | 20 | 10 | 20 |
| P15H15 | 15 | 15 | 20 |
| P10H20 | 10 | 20 | 20 |
| P5H25 | 5 | 25 | 20 |
| Formulations | Height (µm) | Accuracy of Height (%) | Width (µm) | Accuracy of Width (%) |
|---|---|---|---|---|
| P25H5 | 1479.42 ± 19.06 a | 98.63 ± 1.27 a | 456.36 ± 7.41 a | 101.41 ± 1.65 a |
| P20H10 | 1475.01 ± 18.06 a | 98.33 ± 1.20 a | 391.18 ± 7.84 b | 86.93 ± 1.74 b |
| P15H15 | 870.86 ± 500.00 b | 58.06 ± 33.33 b | 326.14 ± 38.25 c | 72.07 ± 8.50 c |
| P10H20 | 393.63 ± 237.33 bc | 26.24 ± 15.82 bc | 383.98 ± 28.77 bc | 85.33 ± 6.39 bc |
| P5H25 | 326.12 ± 32.81 c | 21.74 ± 2.19 c | 377.29 ± 148.23 c | 83.84 ± 32.94 abc |
| Formulation | Force/Array (N) | Force/Needle (N) | Height Change (%) | Blue Dot (%) |
|---|---|---|---|---|
| P25H5 | 41.94 ± 3.06 a | 0.19 ± 0.01 a | 3.40 ± 2.04% a | 98.52 ± 1.43 a |
| P20H10 | 31.60 ± 1.60 b | 0.14 ± 0.01 b | 7.89 ± 1.74% b | 95.26 ± 2.45 b |
| Formulation | Time (min) | ||||
|---|---|---|---|---|---|
| 0 | 5 | 15 | 30 | 45 | |
| P25H5 |  |  |  |  |  |
| P20H10 |  |  |  |  |  |
| Formulation | Height (µm) | Accuracy of Height (%) | Width (µm) | Accuracy of Width (%) | Force/Array (N) | Force/Needle (N) | Height Change (%) | Blue Dot (%) |
|---|---|---|---|---|---|---|---|---|
| P25H5-O | 1417.26 ± 6.49 a | 94.48 ± 0.43 a | 456.63 ± 6.46 a | 101.47 ± 1.44 a | 26.52 ± 1.29 a | 0.12 ± 0.01 a | 8.22 ± 1.82 a | 93.04 ± 7.03 a |
| P20H10-O | 1415.29 ± 15.58 a | 94.35 ± 1.04 a | 411.45 ± 16.55 b | 91.43 ± 3.68 b | 17.75 ± 2.03 b | 0.08 ± 0.01 b | 16.30 ± 3.66 b | 53.63 ± 13.31 b |
| Formulations | Time (min) | Cumulative Amounts (ng/cm2) | Fluxes (ng/cm2/min) | Follicular Penetration per Hair Follicle (ng/HF) |
|---|---|---|---|---|
| Microneedle | 15 | 3402.87 ± 504.80 a | 226.86 ± 33.65 a | 57.36 ± 8.51 a |
| 30 | 4949.63 ± 134.61 b | 164.99 ± 4.49 a,b | 98.44 ± 2.68 b | |
| 45 | 6924.72 ± 33.65 c | 153.88 ± 0.75 b | 144.20 ± 0.70 c | |
| Solution | 15 | 1808.52 ± 67.31 a | 120.57 ± 4.49 a | 30.49 ± 1.13 a |
| 30 | 3069.72 ± 235.57 b | 102.32 ± 7.85 b | 61.05 ± 4.68 b | |
| 45 | 3878.80 ± 33.25 c | 86.20 ± 0.55 b,c | 80.77 ± 0.53 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiwarit, T.; Chanabodeechalermrung, B.; Jantrawut, P.; Ruksiriwanich, W.; Sainakham, M. Fabrication and Characterization of Dissolving Microneedles Containing Oryza sativa L. Extract Complex for Enhancement of Transfollicular Delivery. Polymers 2024, 16, 2377. https://doi.org/10.3390/polym16162377
Chaiwarit T, Chanabodeechalermrung B, Jantrawut P, Ruksiriwanich W, Sainakham M. Fabrication and Characterization of Dissolving Microneedles Containing Oryza sativa L. Extract Complex for Enhancement of Transfollicular Delivery. Polymers. 2024; 16(16):2377. https://doi.org/10.3390/polym16162377
Chicago/Turabian StyleChaiwarit, Tanpong, Baramee Chanabodeechalermrung, Pensak Jantrawut, Warintorn Ruksiriwanich, and Mathukorn Sainakham. 2024. "Fabrication and Characterization of Dissolving Microneedles Containing Oryza sativa L. Extract Complex for Enhancement of Transfollicular Delivery" Polymers 16, no. 16: 2377. https://doi.org/10.3390/polym16162377
APA StyleChaiwarit, T., Chanabodeechalermrung, B., Jantrawut, P., Ruksiriwanich, W., & Sainakham, M. (2024). Fabrication and Characterization of Dissolving Microneedles Containing Oryza sativa L. Extract Complex for Enhancement of Transfollicular Delivery. Polymers, 16(16), 2377. https://doi.org/10.3390/polym16162377







