Waterborne Intumescent Fire-Retardant Polymer Composite Coatings: A Review
Abstract
1. Introduction
2. Formulation of Waterborne Intumescent Fire-Retardant Polymer Composite Coatings
2.1. Intumescent Fire-Retardant Systems
2.2. Waterborne Film-Forming Matrices
2.2.1. Waterborne Acrylic-Resin-Based Intumescent Fire-Retardant Coatings
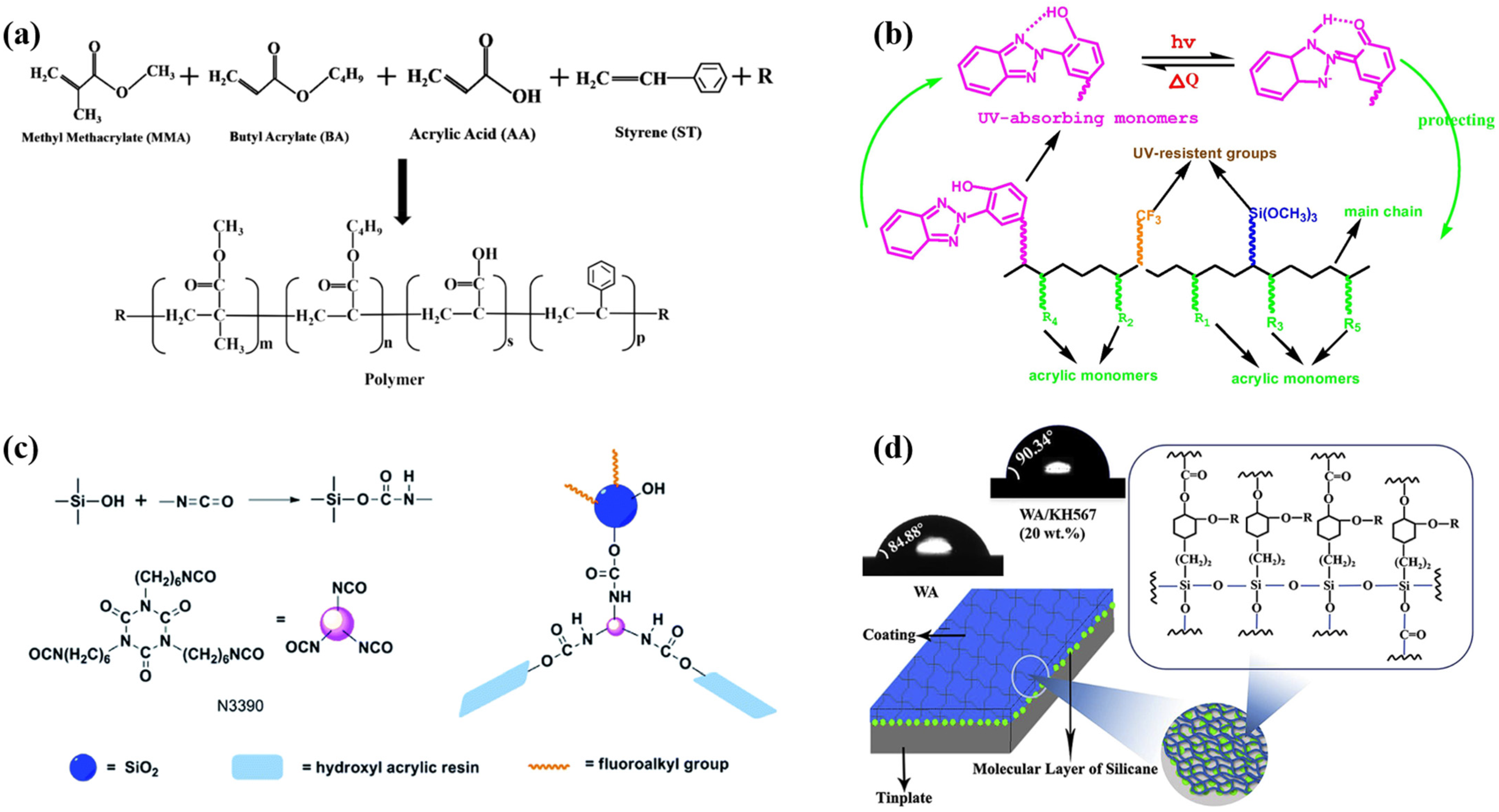
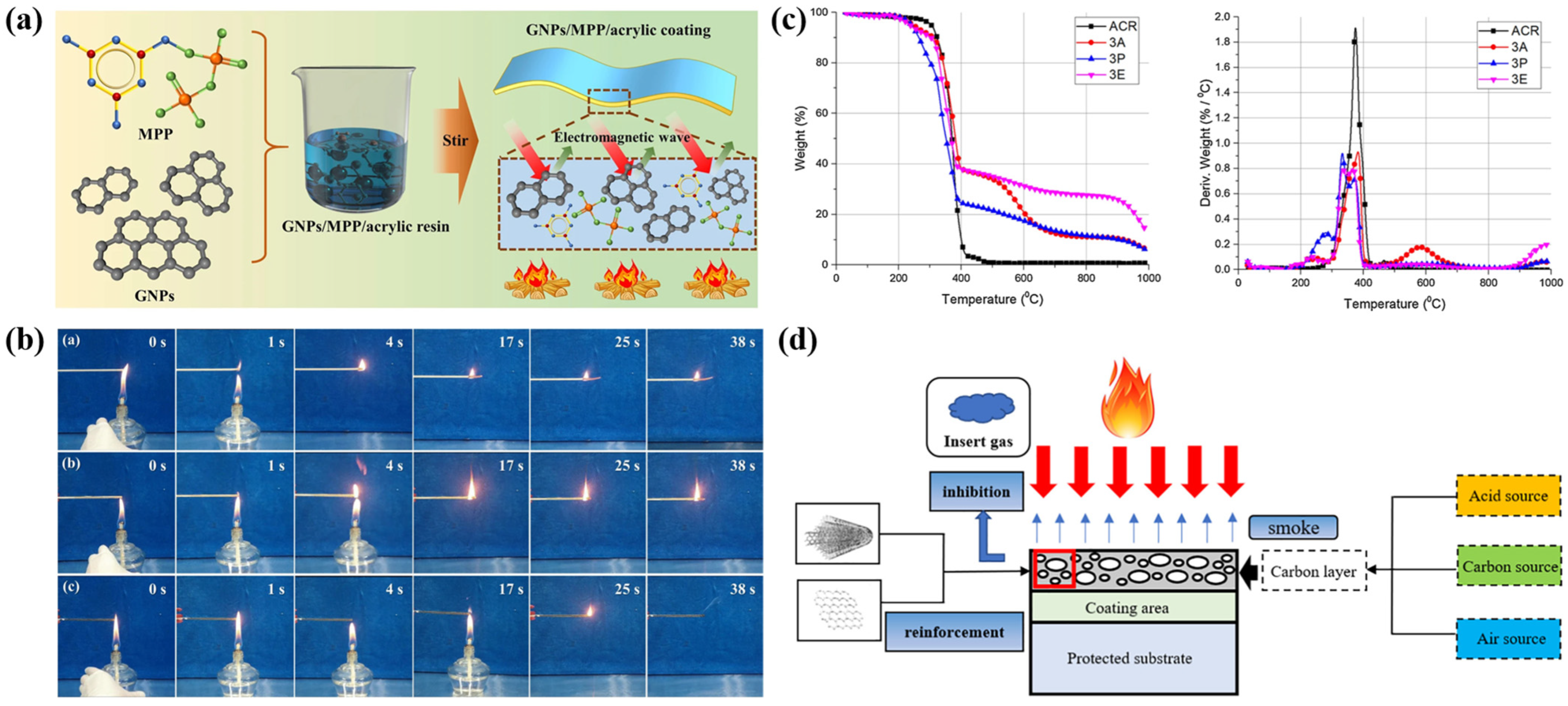
2.2.2. Waterborne Epoxy-Resin-Based Intumescent Fire-Retardant Coatings
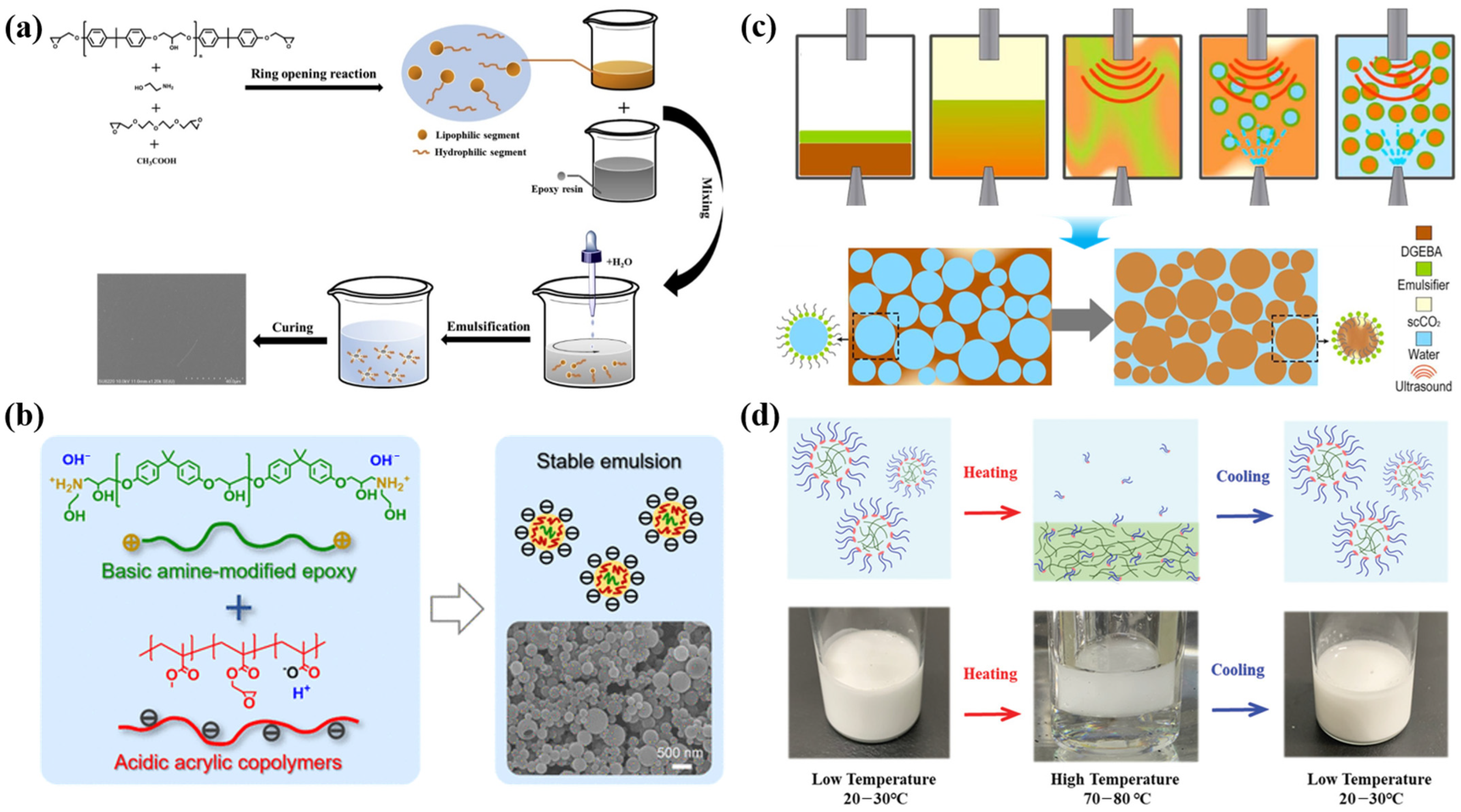
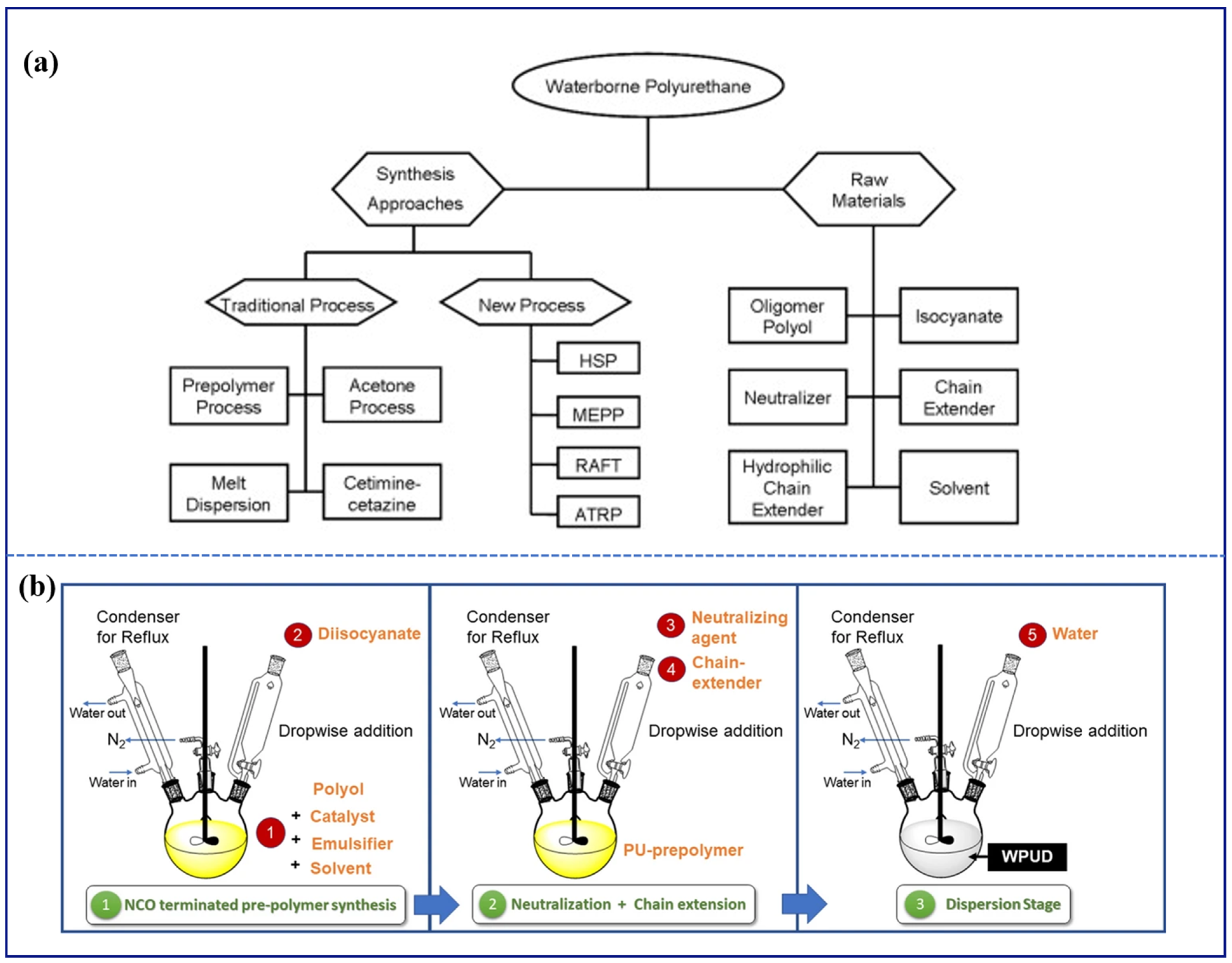
2.2.3. Waterborne Polyurethane-Based Intumescent Fire-Retardant Coatings
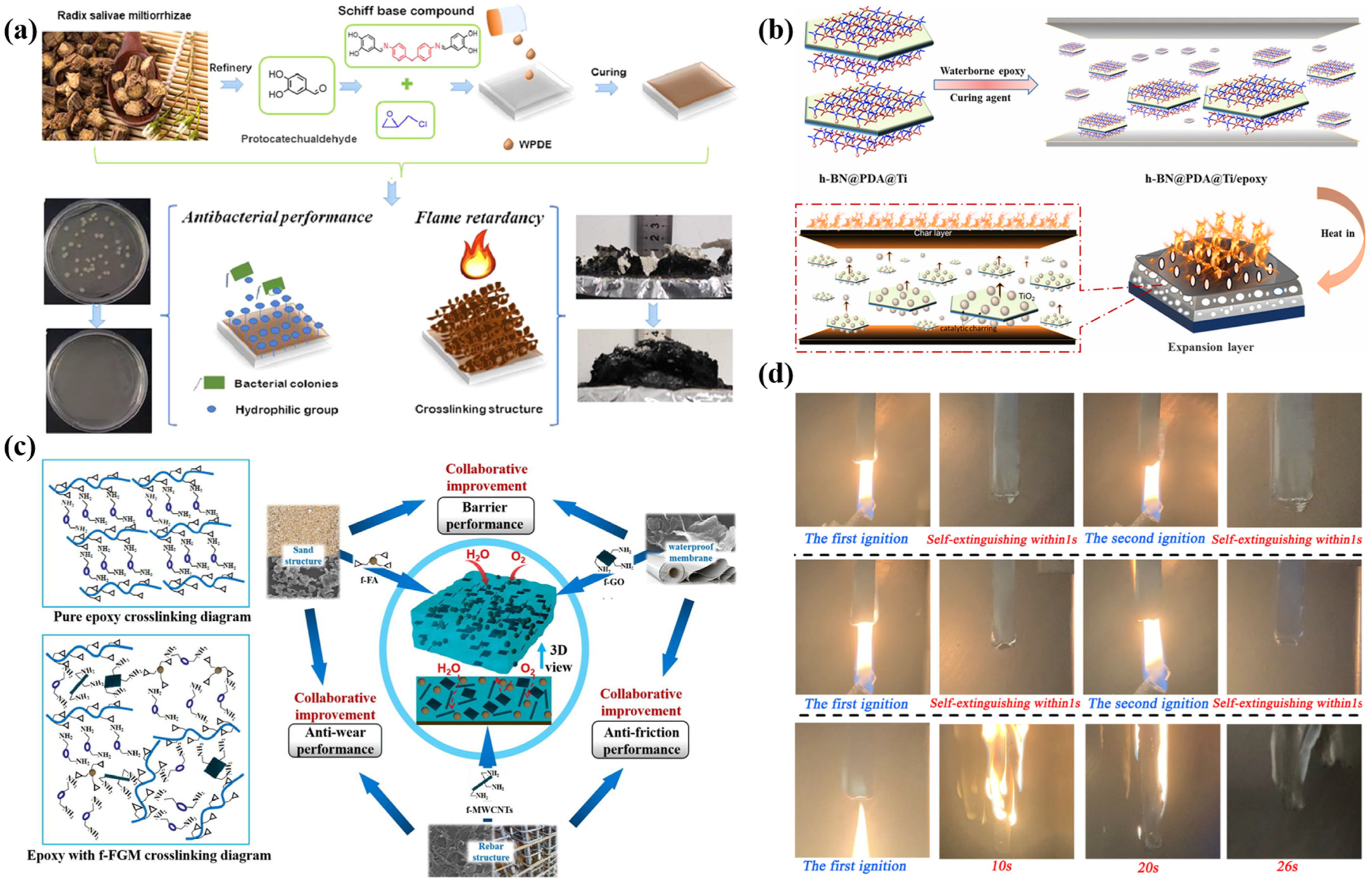
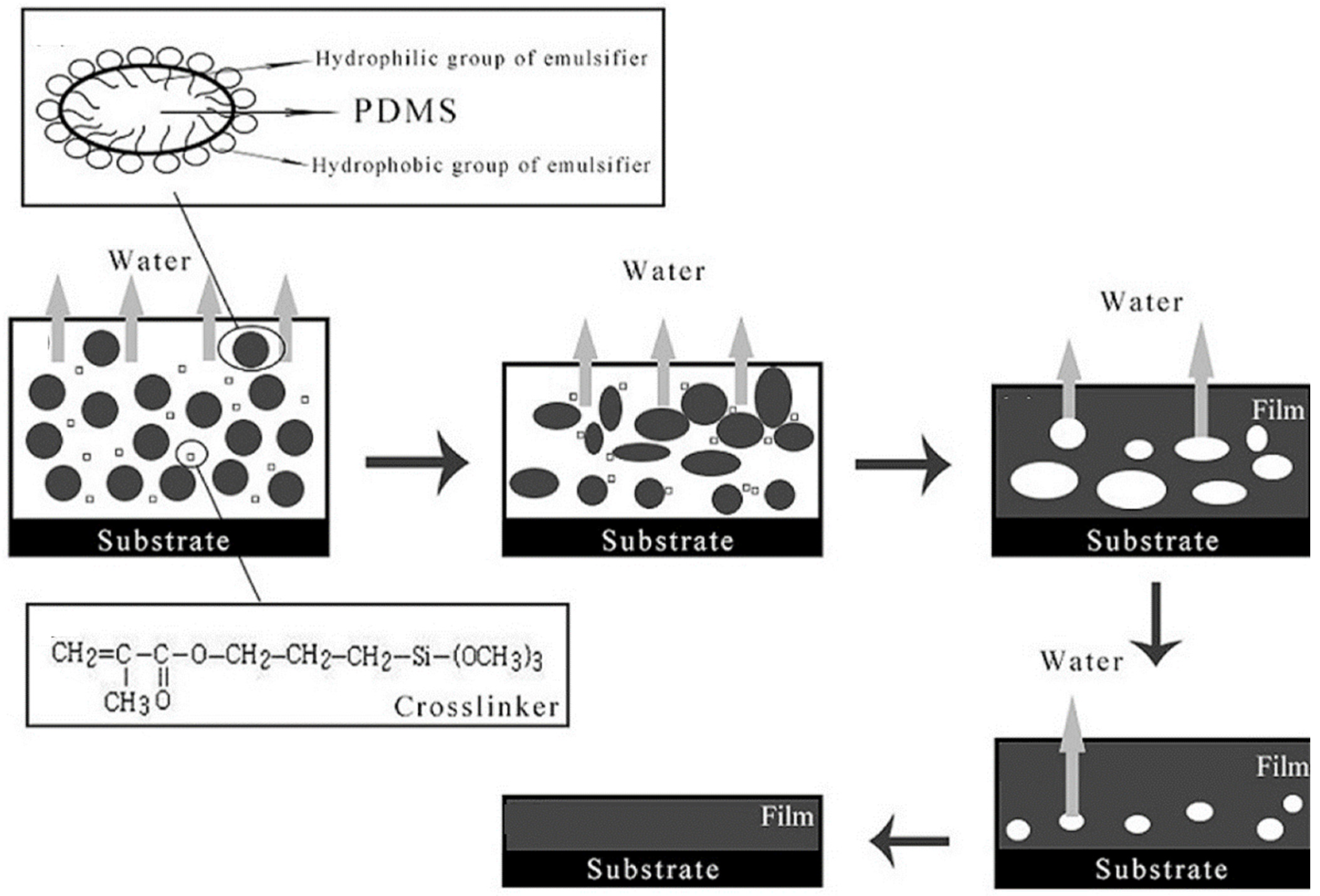
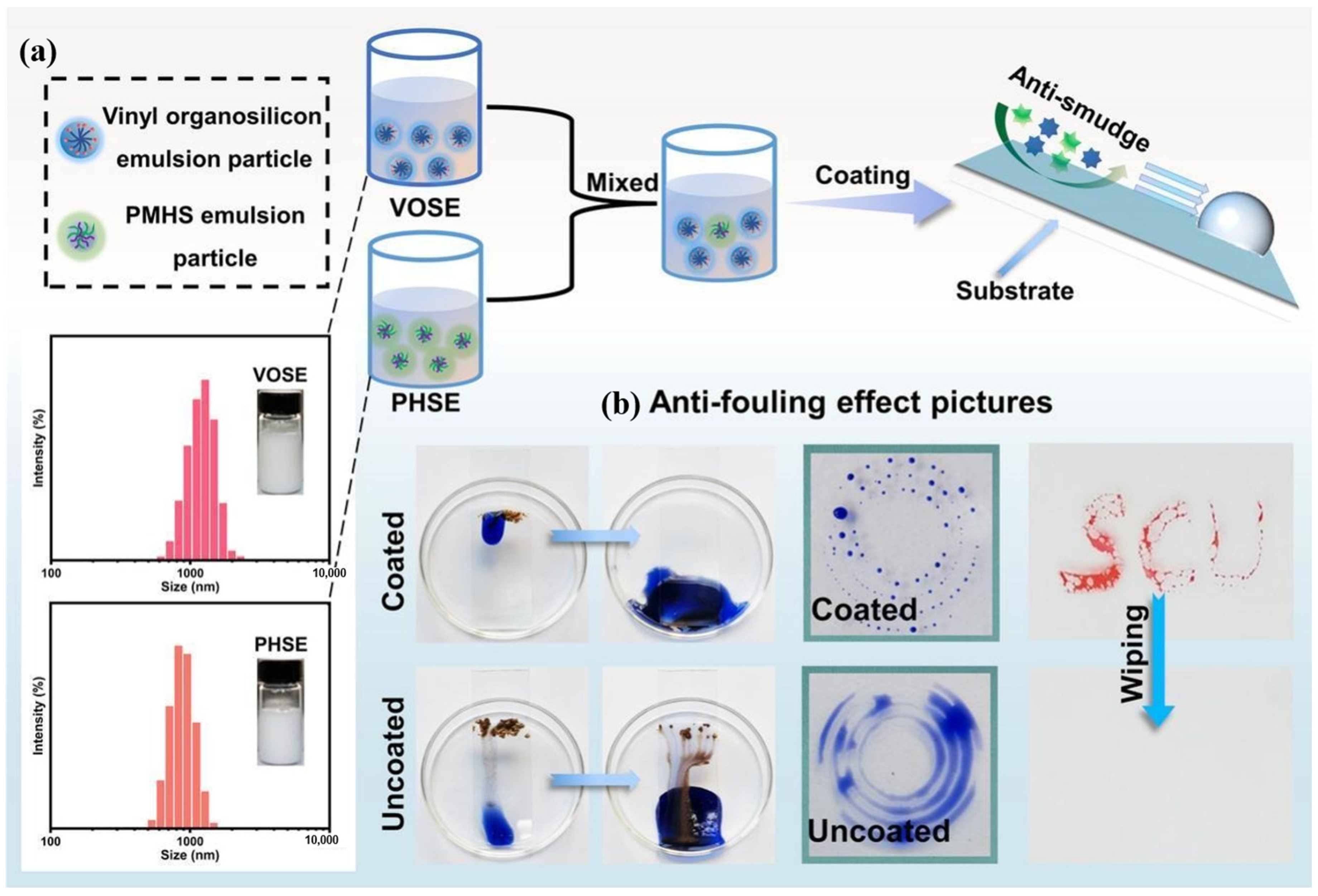
2.2.4. Waterborne Silicone-Based Intumescent Fire-Retardant Coatings
2.3. Functional Fillers
2.3.1. Inorganic Fillers
2.3.2. Organic Fillers
2.4. Additives
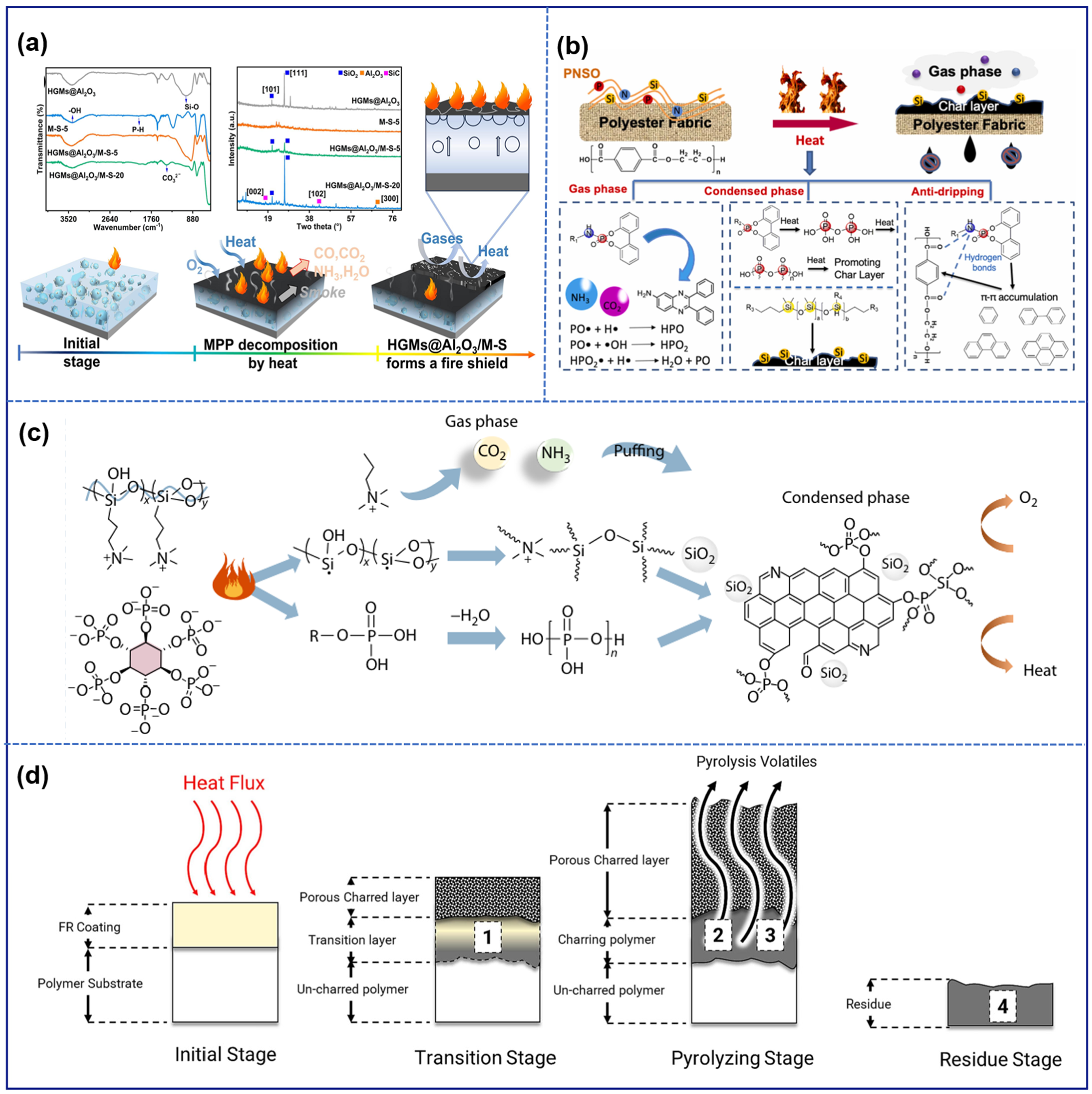
3. Intumescent Flame-Retardant Mechanism
3.1. Condensed-Phase Mechanism
3.2. Gas-Phase Mechanism
4. Applications of Waterborne Intumescent Fire-Retardant Polymer Composite Coatings
4.1. Wood
4.2. Foam
4.3. Fabric
4.4. Steel
4.5. Cable
5. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Ng, Y.H.; Zope, I.S.; Dasari, A.; Tan, K.H. Correlating the Performance of a Fire-Retardant Coating across Different Scales of Testing. Polymers 2020, 12, 2271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, A.-N.; He, S.-M.; Zheng, G.-Q.; Zeng, F.-R.; Wang, Y.-Z.; Liu, B.-W.; Zhao, H.-B. Biomimetic Nanoporous Transparent Universal Fire-Resistant Coatings. ACS Appl. Mater. Interfaces 2024, 16, 19519–19528. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Li, L.; Chen, L.; Kong, Q.; Chen, M.; Chen, C.; Zhang, Q.; Jiang, J. Biomaterials in Intumescent Fire-Retardant Coatings: A Review. Prog. Org. Coat. 2024, 192, 108483. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, G.; Dong, S.; Zhang, Q.; Kong, J. Study on Preparation and Fire-Retardant Mechanism Analysis of Intumescent Flame-Retardant Coatings. Surf. Coat. Technol. 2007, 201, 7835–7841. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Wang, X. Influence of Nano-Silica on the Flame Retardancy and Smoke Suppression Properties of Transparent Intumescent Fire-Retardant Coatings. Prog. Org. Coat. 2017, 112, 319–329. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.-W. Eco-Friendly Polymer Nanocomposite Coatings for Next-Generation Fire Retardants for Building Materials. Polymers 2024, 16, 2045. [Google Scholar] [CrossRef]
- Rabajczyk, A.; Zielecka, M.; Popielarczyk, T.; Sowa, T. Nanotechnology in Fire Protection—Application and Requirements. Materials 2021, 14, 7849. [Google Scholar] [CrossRef] [PubMed]
- Yew, M.C.; Ramli Sulong, N.H. Fire-Resistive Performance of Intumescent Flame-Retardant Coatings for Steel. Mater. Des. 2012, 34, 719–724. [Google Scholar] [CrossRef]
- Wen, O.Y.; Tohir, M.Z.M.; Yeaw, T.C.S.; Razak, M.A.; Zainuddin, H.S.; Hamid, M.R.A. Fire-Resistant and Flame-Retardant Surface Finishing of Polymers and Textiles: A State-of-the-Art Review. Prog. Org. Coat. 2023, 175, 107330. [Google Scholar] [CrossRef]
- Dhineshbabu, N.R.; Bose, S. UV Resistant and Fire Retardant Properties in Fabrics Coated with Polymer Based Nanocomposites Derived from Sustainable and Natural Resources for Protective Clothing Application. Compos. Part B Eng. 2019, 172, 555–563. [Google Scholar] [CrossRef]
- Jia, S.; Deng, S.; Lu, Y.; Wu, Y.; Qing, Y. A New Insight into the Design of Robust Superhydrophobic and Fire Retardant Wood: Breaking the Conflicting Requirement on Adhesives. Chem. Eng. J. 2023, 475, 146240. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, M.; Zhang, Z.; Su, X.; Li, Y.; Han, Z.; Lv, X.; He, J.; Li, H.; Zheng, Z.; et al. Biomimetic Construction of Green, Fire-Proof and Super-Hydrophobic Multifunctionality-Integrated Coatings via One-Step Spraying Method for Steel Structures. Colloids Surf. Physicochem. Eng. Asp. 2024, 683, 133056. [Google Scholar] [CrossRef]
- Qiang, X.; Shu, Y.; Jiang, X.; Xiao, Y.; Jin, P. Mechanical Behavior of Carbon Fiber-Reinforced Polymer/Steel Bonded Joints after Large-Space Fires and Thermal Conductivity of Non-Intumescent Coatings. J. Build. Eng. 2024, 86, 108803. [Google Scholar] [CrossRef]
- Cai, G.; Wu, J.; Guo, J.; Wan, Y.; Zhou, Q.; Zhang, P.; Yu, X.; Wang, M. A Novel Inorganic Aluminum Phosphate-Based Flame Retardant and Thermal Insulation Coating and Performance Analysis. Materials 2023, 16, 4498. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhou, H.; Yan, L.; Jia, H. Comparative Study of the Fire Protection Performance and Thermal Stability of Intumescent Fire-Retardant Coatings Filled with Three Types of Clay Nano-Fillers. Fire Mater. 2020, 44, 112–120. [Google Scholar] [CrossRef]
- Shao, X.; Du, Y.; Zheng, X.; Wang, J.; Wang, Y.; Zhao, S.; Xin, Z.; Li, L. Reduced Fire Hazards of Expandable Polystyrene Building Materials via Intumescent Flame-Retardant Coatings. J. Mater. Sci. 2020, 55, 7555–7572. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, H.-B.; Cheng, J.-B.; Li, W.; Zhang, J.; Wang, Y.-Z. A Green, Durable and Effective Flame-Retardant Coating for Expandable Polystyrene Foams. Chem. Eng. J. 2022, 440, 135807. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Huang, K.; Wang, Y.; Zheng, X.; Wang, J.; Du, Y.; Jiang, L.; Zhao, S. A Facile Strategy to Fabricate Intumescent Fire-Retardant and Smoke Suppression Protective Coatings for Natural Rubber. Polym. Test. 2020, 90, 106689. [Google Scholar] [CrossRef]
- Liang, W.; Bonsu, A.O.; Lv, H.; Yang, B.; Liu, C.; Deng, J. Intumescent Coating Thickness Effect on the Post-Heat Mechanical Properties of Composites. Structures 2020, 28, 2056–2072. [Google Scholar] [CrossRef]
- Häßler, D.; Mund, M.; Daus, L.-H.; Hothan, S.; Schaumann, P.; Schartel, B. Durability of Intumescent Coatings and Recommendations for Test Concepts for a Working Life of More than 10 Years. Fire Saf. J. 2024, 146, 104173. [Google Scholar] [CrossRef]
- Lucherini, A.; Maluk, C. Intumescent Coatings Used for the Fire-Safe Design of Steel Structures: A Review. J. Constr. Steel Res. 2019, 162, 105712. [Google Scholar] [CrossRef]
- Yin, X.; Pang, H.; Luo, Y.; Zhang, B. Eco-Friendly Functional Two-Component Flame-Retardant Waterborne Polyurethane Coatings: A Review. Polym. Chem. 2021, 12, 5400–5411. [Google Scholar] [CrossRef]
- Nørgaard, K.P.; Dam-Johansen, K.; Català, P.; Kiil, S. Investigation of Char Strength and Expansion Properties of an Intumescent Coating Exposed to Rapid Heating Rates. Prog. Org. Coat. 2013, 76, 1851–1857. [Google Scholar] [CrossRef]
- Zhong, F.; Lu, M.; Chen, C.; Liu, L.; Yang, X. Phytic Acid Cross-Linked Copper Ions Anchored to BN Surface to Enhance the Fire Performance of Waterborne Epoxy Intumescent Coatings. Colloids Surf. Physicochem. Eng. Asp. 2023, 665, 131275. [Google Scholar] [CrossRef]
- Qu, H.; Feng, M.; Li, M.; Tian, D.; Zhang, Y.; Chen, X.; Li, G. Enhancing the Carbonation and Chloride Resistance of Concrete by Nano-Modified Eco-Friendly Water-Based Organic Coatings. Mater. Today Commun. 2023, 37, 107284. [Google Scholar] [CrossRef]
- Ou, M.; Cui, J.; Zhao, Z.; Li, R.; Guan, H.; Liu, L.; Jiao, C.; Chen, X. Solvent-Free Intumescent Fire Protection Epoxy Coatings with Excellent Smoke Suppression, Toxicity Reduction, and Durability Enabled by a Micro/Nano-Structured P/N/Si-Containing Flame Retardant. Prog. Org. Coat. 2023, 183, 107762. [Google Scholar] [CrossRef]
- Strassburger, D.; Silveira, M.R.; Baldissera, A.F.; Ferreira, C.A. Performance of Different Water-Based Resins in the Formulation of Intumescent Coatings for Passive Fire Protection. J. Coat. Technol. Res. 2023, 20, 201–221. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Li, Y.; Li, Z.; Liu, S. Effects and Mechanisms of Ultralow Concentrations of Different Types of Graphene Oxide Flakes on Fire Resistance of Water-Based Intumescent Coatings. Coatings 2024, 14, 162. [Google Scholar] [CrossRef]
- Mac, V.P.; Do, M.T.; Nguyen, A.H.; Dao, P.H.; Nguyen, T.V.; Pham, C.N.; Nguyen, T.A. A Water-Based Flame-Retardant Coating with Cenospheres. J. Coat. Technol. Res. 2024. [Google Scholar] [CrossRef]
- Mariappan, T.; Kamble, A.; Naik, S.M. An Investigation of Primer Adhesion and Topcoat Compatibility on the Waterborne Intumescent Coating to Structural Steel. Prog. Org. Coat. 2019, 131, 371–377. [Google Scholar] [CrossRef]
- Hussain, A.; Landry, V.; Blanchet, P.; Hoang, D.-T.; Dagenais, C. Fire Performance of Intumescent Waterborne Coatings with Encapsulated APP for Wood Constructions. Coatings 2021, 11, 1272. [Google Scholar] [CrossRef]
- Wang, K.; Le, H. The Development of Cement-Based, Intumescent and Geopolymer Fire-Retardation Coatings for Metal Structures: A Review. Coatings 2023, 13, 495. [Google Scholar] [CrossRef]
- Baena, J.C.; Wang, C.; Kabir, I.I.; Khalid, A.; Nazir, M.T.; Yuen, A.C.Y.; Ahmad, F.; Yeoh, G.H. Fire Behaviour of Waterborne Intumescent Coatings on Timber Substrate for Bushfire Exposure. Fire Saf. J. 2023, 140, 103836. [Google Scholar] [CrossRef]
- Lee, Y.X.; Ahmad, F.; Karuppanan, S.; Sumby, C.; Shahed, C.A.; Ramli, S.H. Thermo-Mechanical Performance of Wolframite Mineral Reinforced Siloxane-Modified Epoxy-Based Intumescent Coating for Structural Steel. Polym. Adv. Technol. 2024, 35, e6279. [Google Scholar] [CrossRef]
- Shree, R.; Naik, R.B.; Naik, R.; Gunasekaran, G.; Nimje, R.; Ratna, D. Dual Curing Agents for Optimized Flame-Retardancy and Physico-Mechanical Properties of Cycloaliphatic Epoxy Coating. Mater. Chem. Phys. 2023, 295, 127136. [Google Scholar] [CrossRef]
- Yi, L.; Feng, S.; Wang, Z.; Ding, Y.; Chu, T.; Zhuang, Y. A Comprehensive Model to Predict the Fire Performance of Intumescent Fire-Retardant Coating on Steel Substrate. J. Build. Eng. 2024, 95, 110127. [Google Scholar] [CrossRef]
- Gravit, M.; Shabunina, D.; Shcheglov, N. Thermal Characteristics of Epoxy Fire-Retardant Coatings under Different Fire Regimes. Fire 2023, 6, 420. [Google Scholar] [CrossRef]
- Zang, X.; Liu, W.; Wu, D.; Pan, X.; Zhang, W.; Bian, H.; Shen, R. Contemporary Fire Safety Engineering in Timber Structures: Challenges and Solutions. Fire 2024, 7, 2. [Google Scholar] [CrossRef]
- Bifulco, A.; Imparato, C.; Climaco, I.; Battegazzore, D.; Perrella, M.; Vitiello, G.; Aronne, A.; Malucelli, G. Multifunctional Fire-Resistant and Flame-Triggered Shape Memory Epoxy Nanocomposites Containing Carbon Dots. Chem. Eng. J. 2024, 484, 149327. [Google Scholar] [CrossRef]
- Tang, W.; Qian, L.; Prolongo, S.G.; Wang, D.-Y. Dendritic Copolymers from P-, N- and Si-Based Monomer and Melamine Phosphate Generate Thermal Deformation Toughening and a Rapid Charring Flame Retardant Effect in Polypropylene. Chem. Eng. J. 2023, 471, 144716. [Google Scholar] [CrossRef]
- Yuan, J.; Zhu, Z.; Wang, Y.; Yin, X.; Lin, X. Multi-Functional Solvent-Free SiO2 Nanofluid Simultaneously Improve Major Properties and Fluidity of Epoxy Resin: A New Strategy beyond Nanofillers. Polym. Degrad. Stab. 2023, 210, 110308. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.; Fu, L.; Xu, P.; Rao, G.; Xiao, W.; Wang, L.; Shi, Y. Interface Engineering of Multi-Component Core-Shell Flame Retardant towards Enhancing Fire Safety of Thermoplastic Polyurethane and Mechanism Investigation. Appl. Mater. Today 2024, 38, 102233. [Google Scholar] [CrossRef]
- Yeoh, G.H.; De Cachinho Cordeiro, I.M.; Wang, W.; Wang, C.; Yuen, A.C.Y.; Chen, T.B.Y.; Vargas, J.B.; Mao, G.; Garbe, U.; Chua, H.T. Carbon-Based Flame Retardants for Polymers: A Bottom-up Review. Adv. Mater. 2024, 2403835. [Google Scholar] [CrossRef]
- Wang, W.; He, J.; Li, C.; Zhang, N.; He, X.; Li, Q.; Sun, J.; Jiang, R.; Lei, Z.; Liu, Z.-H. Polyvinyl Alcohol/Montmorillonite/Magnesium Diboride Fibers with Superior Flame Retardancy, Strength, and Flexibility. Chem. Eng. J. 2023, 462, 142261. [Google Scholar] [CrossRef]
- Shen, Y.-B.; Yu, K.-X.; Wang, Y.-J.; Qu, Y.-H.; Pan, L.-Q.; Cao, C.-F.; Cao, K.; Gao, J.-F.; Shi, Y.; Song, P.; et al. Color Adjustable, Mechanically Robust, Flame-Retardant and Weather-Resistant TiO2/MMT/CNF Hierarchical Nanocomposite Coatings toward Intelligent Fire Cyclic Warning and Protection. Compos. Part B Eng. 2024, 271, 111159. [Google Scholar] [CrossRef]
- Ju, Y.; Hua, J.; Gu, Y.; Chen, H. Construction of High-Branched Derivatives Based on Melamine for Highly Effective Defoaming and Antifoaming. J. Colloid Interface Sci. 2023, 650, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Kowalik, J.; Tworek, M. Investigation of the Properties of a Water-Based Acrylic Dispersion Modified with an Ionic Liquid, Surfactant, and Thickener. Environ. Sci. Pollut. Res. 2023, 1–14. [Google Scholar] [CrossRef]
- Mucci, V.L.; Hormaiztegui, M.E.V.; Amalvy, J.I.; Aranguren, M.I. Formulation, Structure and Properties of Waterborne Polyurethane Coatings: A Brief Review. J. Adhes. Sci. Technol. 2024, 38, 489–516. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Zaghloul, M.M.Y.; Fuseini, M. Recent Progress in Epoxy Nanocomposites: Corrosion, Structural, Flame Retardancy and Applications—A Comprehensive Review. Polym. Adv. Technol. 2023, 34, 3438–3472. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Li, M.-C.; Zhang, S.; Zhou, W.; Mei, C.; Pan, M. Bioinspired, Stable Adhesive Ti3C2Tx MXene-Based Coatings towards Fire Warning, Smoke Suppression and VOCs Removal Smart Wood. Chem. Eng. J. 2023, 452, 139360. [Google Scholar] [CrossRef]
- Puri, R.G.; Khanna, A.S. Intumescent Coatings: A Review on Recent Progress. J. Coat. Technol. Res. 2017, 14, 1–20. [Google Scholar] [CrossRef]
- Anees, S.M.; Dasari, A. A Review on the Environmental Durability of Intumescent Coatings for Steels. J. Mater. Sci. 2018, 53, 124–145. [Google Scholar] [CrossRef]
- Lim, K.-S.; Bee, S.-T.; Sin, L.T.; Tee, T.-T.; Ratnam, C.T.; Hui, D.; Rahmat, A.R. A Review of Application of Ammonium Polyphosphate as Intumescent Flame Retardant in Thermoplastic Composites. Compos. Part B Eng. 2016, 84, 155–174. [Google Scholar] [CrossRef]
- Taviot-Guého, C.; Prévot, V.; Forano, C.; Renaudin, G.; Mousty, C.; Leroux, F. Tailoring Hybrid Layered Double Hydroxides for the Development of Innovative Applications. Adv. Funct. Mater. 2018, 28, 1703868. [Google Scholar] [CrossRef]
- Rabajczyk, A.; Zielecka, M.; Gniazdowska, J. Application of Nanotechnology in Extinguishing Agents. Materials 2022, 15, 8876. [Google Scholar] [CrossRef] [PubMed]
- An, W.; Ma, J.; Xu, Q.; Zhang, H. Nanoarchitectonics of Flame Retardant Leather: Current Status and Future Perspectives. Compos. Part Appl. Sci. Manuf. 2023, 165, 107327. [Google Scholar] [CrossRef]
- Yao, H.; Li, L.; Li, W.; Qi, D.; Fu, W.; Wang, N. Application of Nanomaterials in Waterborne Coatings: A Review. Resour. Chem. Mater. 2022, 1, 184–200. [Google Scholar] [CrossRef]
- Konstantinova, A.; Yudaev, P.; Shapagin, A.; Panfilova, D.; Palamarchuk, A.; Chistyakov, E. Non-Flammable Epoxy Composition Based on Epoxy Resin DER-331 and 4-(β-Carboxyethenyl)Phenoxy-Phenoxycyclotriphosphazenes with Increased Adhesion to Metals. Sci 2024, 6, 30. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Z.; Sun, C.; Xu, M.; Li, B. A Novel Macromolecular Phosphorus-Nitrogen Containing Flame Retardant for Polycarbonate. Polym. Degrad. Stab. 2024, 220, 110648. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, H.; Lin, X.; Xiang, S.; Feng, X.; Wan, C. Surface Flame-Retardant Systems of Rigid Polyurethane Foams: An Overview. Materials 2023, 16, 2728. [Google Scholar] [CrossRef]
- Li, S.-N.; Zeng, Z.-F.; He, X.-F.; Xu, Z.-C.; Luo, Y.-H.; Ni, Q.-Y.; Peng, L.; Gong, L.-X.; Li, Y.; Jiang, B. Mechanically Flexible and Flame-Retardant Cellulose Nanofibril-Based Films Integrated with MXene and Chitosan. Soft Sci. 2022, 2, 21. [Google Scholar] [CrossRef]
- Jin, W.-J.; He, W.-L.; Gu, L.; Cheng, X.-W.; Guan, J.-P. An Eco-Friendly and Intumescent P/N/S-Containing Flame Retardant Coating for Polyamide 6 Fabric. Eur. Polym. J. 2022, 180, 111610. [Google Scholar] [CrossRef]
- Wu, W.-S.; Ni, Y.-P.; Chen, L.; Fu, T.; Wang, X.-L.; Wang, Y.-Z. Trinity Effect of Potassium Sulfonate-Benzimidozale towards Self-Intumescent Flame-Retarded Polyester with Low Fire Hazards. Chem. Eng. J. 2022, 429, 132121. [Google Scholar] [CrossRef]
- Cao, C.-F.; Yu, B.; Chen, Z.-Y.; Qu, Y.-X.; Li, Y.-T.; Shi, Y.-Q.; Ma, Z.-W.; Sun, F.-N.; Pan, Q.-H.; Tang, L.-C.; et al. Fire Intumescent, High-Temperature Resistant, Mechanically Flexible Graphene Oxide Network for Exceptional Fire Shielding and Ultra-Fast Fire Warning. Nano-Micro Lett. 2022, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Chetehouna, K.; Settar, A.; Rousseau, J.; Roudaut, C.; Lemée, L.; Aboura, Z. Kinetic Analysis of the Thermal Degradation of an Intumescent Fire Retardant Coated Green Biocomposite. Thermochim. Acta 2022, 711, 179211. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, F.; Yusoff, P.S.M.M. Effect of Boric Acid and Melamine on the Intumescent Fire-Retardant Coating Composition for the Fire Protection of Structural Steel Substrates. J. Appl. Polym. Sci. 2013, 128, 2983–2993. [Google Scholar] [CrossRef]
- van der Veen, I.; de Boer, J. Phosphorus Flame Retardants: Properties, Production, Environmental Occurrence, Toxicity and Analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yang, W.; Yuen, A.C.Y.; Cordeiro, I.M.D.C.; Qiu, S.; Zhang, J.; Wu, W.; Hu, Y.; Yeoh, G.H. A Novel Green IFR System: Design of a Self-Assembled Peanut Shell-Based Flame Retardant and Its Fire Performance in EP. Prog. Org. Coat. 2023, 174, 107277. [Google Scholar] [CrossRef]
- Yin, H.; Yuan, D.; Cai, X. Red Phosphorus Acts as Second Acid Source to Form a Novel Intumescent-Contractive Flame-Retardant System on ABS. J. Therm. Anal. Calorim. 2013, 111, 499–506. [Google Scholar] [CrossRef]
- Gavgani, J.N.; Adelnia, H.; Mir Mohamad Sadeghi, G.; Zafari, F. Intumescent Flame Retardant Polyurethane/Starch Composites: Thermal, Mechanical, and Rheological Properties. J. Appl. Polym. Sci. 2014, 131, 41158. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, Y.; Wang, X.; Chen, Z.; Xu, Z. Preparation of a Chitosan-Based Flame-Retardant Synergist and Its Application in Flame-Retardant Polypropylene. J. Appl. Polym. Sci. 2014, 131, 40845. [Google Scholar] [CrossRef]
- Mizera, K.; Sałasińska, K.; Ryszkowska, J.; Kurańska, M.; Kozera, R. Effect of the Addition of Biobased Polyols on the Thermal Stability and Flame Retardancy of Polyurethane and Poly(Urea)Urethane Elastomers. Materials 2021, 14, 1805. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Li, J. Regulating Effects of Nitrogenous Bases on the Char Structure and Flame Retardancy of Polypropylene/Intumescent Flame Retardant Composites. ACS Sustain. Chem. Eng. 2017, 5, 2375–2383. [Google Scholar] [CrossRef]
- Lu, S.; Chen, X.; Zhang, B.; Lu, Z.; Jiang, W.; Fang, X.; Li, J.; Liu, B.; Ding, T.; Xu, Y. Synergistic Modification of Polyformaldehyde by Biobased Calcium Magnesium Bi-Ionic Melamine Phytate with Intumescent Flame Retardant. Polymers 2024, 16, 614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Song, L.; Lu, H.; Wang, J.; Hu, Y. The Thermal Property and Flame Retardant Mechanism of Intumescent Flame Retardant Paraffin System with Metal. Ind. Eng. Chem. Res. 2010, 49, 6003–6009. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Ni, A.; Ding, A.; Han, X.; Sun, Z. The Effects of a Macromolecular Charring Agent with Gas Phase and Condense Phase Synergistic Flame Retardant Capability on the Properties of PP/IFR Composites. Materials 2018, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yu, B.; Song, P.; Maluk, C.; Wang, H. Surface-Coating Engineering for Flame Retardant Flexible Polyurethane Foams: A Critical Review. Compos. Part B Eng. 2019, 176, 107185. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, L.; Fan, Z.; Yu, Y.; Liu, R. Bio-Based Coating of Phytic Acid, Chitosan, and Biochar for Flame-Retardant Cotton Fabrics. Polym. Degrad. Stab. 2022, 199, 109898. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Zhu, Y.-C.; Song, S.; Li, S.-N.; Aarons, J.; Tang, L.-C.; Bae, J. Polysulfide-Inhibiting, Thermotolerant and Nonflammable Separators Enabled by DNA Co-Assembled CNT/MXene Networks for Stable High-Safety Li–S Batteries. Compos. Part B Eng. 2023, 251, 110465. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Peng, B.; Ren, Y.; Cheng, B.; Ding, C.; Su, X.; He, J.; Lin, S. Flame Retardant Cellulosic Fabrics via Layer-by-Layer Self-Assembly Double Coating with Egg White Protein and Phytic Acid. J. Clean. Prod. 2020, 243, 118641. [Google Scholar] [CrossRef]
- Liu, B.-W.; Zhao, H.-B.; Wang, Y.-Z. Advanced Flame-Retardant Methods for Polymeric Materials. Adv. Mater. 2022, 34, 2107905. [Google Scholar] [CrossRef]
- Canosa, G.; Alfieri, P.V.; Giudice, C.A. Hybrid Intumescent Coatings for Wood Protection against Fire Action. Ind. Eng. Chem. Res. 2011, 50, 11897–11905. [Google Scholar] [CrossRef]
- Zafar, F.; Ghosal, A.; Sharmin, E.; Chaturvedi, R.; Nishat, N. A Review on Cleaner Production of Polymeric and Nanocomposite Coatings Based on Waterborne Polyurethane Dispersions from Seed Oils. Prog. Org. Coat. 2019, 131, 259–275. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Y.; Wang, Y.; Ban, T.; Zhang, Y.; Zhang, J.; Zhu, X. Preparation and Characteristics of Crosslinked Fluorinated Acrylate Modified Waterborne Polyurethane for Metal Protection Coating. Prog. Org. Coat. 2021, 158, 106371. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, W.; Xu, P.; Cai, X.; Dong, W.; Chen, M.; Du, M.; Liu, T.; Jan Lemstra, P.; Ma, P. The Bonding Strength, Water Resistance and Flame Retardancy of Soy Protein-Based Adhesive by Incorporating Tailor-Made Core–Shell Nanohybrid Compounds. Chem. Eng. J. 2022, 428, 132390. [Google Scholar] [CrossRef]
- Song, F.; Liu, T.; Fan, Q.; Li, D.; Ou, R.; Liu, Z.; Wang, Q. Sustainable, High-Performance, Flame-Retardant Waterborne Wood Coatings via Phytic Acid Based Green Curing Agent for Melamine-Urea-Formaldehyde Resin. Prog. Org. Coat. 2022, 162, 106597. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.; Wei, S.; Yue, Y.; Dong, L.; Yu, L.; He, G. Preparation and Application of the Water-Based Acrylic Self-Polishing Resin: Good Storage Stability, Good Film-Forming Matrix of Marine Antifouling Coatings. J. Appl. Polym. Sci. 2024, 141, e55532. [Google Scholar] [CrossRef]
- Haddadi, S.A.; A Ramazani, S.A.; Mahdavian, M.; Taheri, P.; Mol, J.M.C.; Gonzalez-Garcia, Y. Self-Healing Epoxy Nanocomposite Coatings Based on Dual-Encapsulation of Nano-Carbon Hollow Spheres with Film-Forming Resin and Curing Agent. Compos. Part B Eng. 2019, 175, 107087. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, Z.; Xiong, D.; Pan, L.; Liu, Y. Preparation and Properties of a Novel Low Crystallinity Cross-Linked Network Waterborne Polyurethane for Water-Based Ink. Prog. Org. Coat. 2019, 133, 161–168. [Google Scholar] [CrossRef]
- Brachaczek, W. The Modelling Technology of Protective Silicone Coatings in Terms of Selected Physical Properties: Hydrophobicity, Scrub Resistance and Water Vapour Diffusion. Prog. Org. Coat. 2014, 77, 859–867. [Google Scholar] [CrossRef]
- Jiao, C.; Sun, L.; Shao, Q.; Song, J.; Hu, Q.; Naik, N.; Guo, Z. Advances in Waterborne Acrylic Resins: Synthesis Principle, Modification Strategies, and Their Applications. ACS Omega 2021, 6, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Yan, Z.; Hao, L.; Elnaggar, A.Y.; El-Bahy, S.M.; Zhang, F.; Azab, I.H.E.; Shao, Q.; Mersal, G.A.M.; Wang, J.; et al. Improving Water Resistance and Mechanical Properties of Waterborne Acrylic Resin Modified by Octafluoropentyl Methacrylate. J. Mater. Sci. 2023, 58, 1452–1464. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, X.; Li, H.; Zhang, S.; Zhou, T.; Xie, H. Modification of Nano-Hybrid Silicon Acrylic Resin with Anticorrosion and Hydrophobic Properties. Polym. Test. 2020, 82, 106287. [Google Scholar] [CrossRef]
- Mehravar, S.; Ballard, N.; Tomovska, R.; Asua, J.M. Polyurethane/Acrylic Hybrid Waterborne Dispersions: Synthesis, Properties and Applications. Ind. Eng. Chem. Res. 2019, 58, 20902–20922. [Google Scholar] [CrossRef]
- Rahman, O.U.; Kashif, M.; Ahmad, S. Nanoferrite Dispersed Waterborne Epoxy-Acrylate: Anticorrosive Nanocomposite Coatings. Prog. Org. Coat. 2015, 80, 77–86. [Google Scholar] [CrossRef]
- Lei, H.; He, D.; Guo, Y.; Tang, Y.; Huang, H. Synthesis and Characterization of UV-Absorbing Fluorine-Silicone Acrylic Resin Polymer. Appl. Surf. Sci. 2018, 442, 71–77. [Google Scholar] [CrossRef]
- Xue, F.; Jia, D.; Li, Y.; Jing, X. Facile Preparation of a Mechanically Robust Superhydrophobic Acrylic Polyurethane Coating. J. Mater. Chem. A 2015, 3, 13856–13863. [Google Scholar] [CrossRef]
- Jiao, C.; Shao, Q.; Wu, M.; Zheng, B.; Guo, Z.; Yi, J.; Zhang, J.; Lin, J.; Wu, S.; Dong, M.; et al. 2-(3,4-Epoxy) Ethyltriethoxysilane-Modified Waterborne Acrylic Resin: Preparation and Property Analysis. Polymer 2020, 190, 122196. [Google Scholar] [CrossRef]
- Liang, C.; Du, Y.; Wang, Y.; Ma, A.; Huang, S.; Ma, Z. Intumescent Fire-Retardant Coatings for Ancient Wooden Architectures with Ideal Electromagnetic Interference Shielding. Adv. Compos. Hybrid Mater. 2021, 4, 979–988. [Google Scholar] [CrossRef]
- Ng, Y.H.; Dasari, A.; Tan, K.H.; Qian, L. Intumescent Fire-Retardant Acrylic Coatings: Effects of Additive Loading Ratio and Scale of Testing. Prog. Org. Coat. 2021, 150, 105985. [Google Scholar] [CrossRef]
- Zhan, W.; Ni, L.; Gu, Z.; Cui, F.; Jiang, J.; Chen, L. The Influences of Graphene and Carbon Nanotubes on Properties of Waterborne Intumescent Fire Resistive Coating. Powder Technol. 2021, 385, 572–579. [Google Scholar] [CrossRef]
- Capricho, J.C.; Fox, B.; Hameed, N. Multifunctionality in Epoxy Resins. Polym. Rev. 2020, 60, 1–41. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, S.; Wang, L.; Guo, C.; Wang, X.; Wang, D.; Zhao, Z.-B.; Yang, K.; Ma, Y.; Li, W. Understanding the Role of Epoxy Emulsifiers in Water-Borne Epoxy Coatings with the Aggregation-Induced Emission Approach. Prog. Org. Coat. 2022, 170, 106987. [Google Scholar] [CrossRef]
- Elmore, J.D.; Kincaid, D.S.; Komar, P.C.; Nielsen, J.E. Waterborne Epoxy Protective Coatings for Metal. J. Coat. Technol. 2002, 74, 63–72. [Google Scholar] [CrossRef]
- Ai, D.; Mo, R.; Wang, H.; Lai, Y.; Jiang, X.; Zhang, X. Preparation of Waterborne Epoxy Dispersion and Its Application in 2K Waterborne Epoxy Coatings. Prog. Org. Coat. 2019, 136, 105258. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, D.; Xu, M.; Xu, Y. Mechanistic Investigation on the Formation of Epoxy Resin Multi-Hollow Spheres Prepared by a Phase Inversion Emulsification Technique. Macromol. Rapid Commun. 2000, 21, 574–578. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Huang, Y.-C.; Wu, C.-H.; Lin, H.-W.; Chiu, W.-Y.; Jeng, R.-J.; Tung, S.-H. Waterborne Epoxy/Acrylic Resins Stabilized through the Neutralization of Basic Amine-Modified Epoxy and Acidic Acrylic Copolymers. ACS Appl. Polym. Mater. 2024, 6, 828–836. [Google Scholar] [CrossRef]
- Gao, H.; Hu, G.; Liu, K.; Wu, L. Preparation of Waterborne Dispersions of Epoxy Resin by Ultrasonic-Assisted Supercritical CO2 Nanoemulsification Technique. Ultrason. Sonochem. 2017, 39, 520–527. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Hsu, S.; Lin, T.; Liao, Y.; Chiu, W.; Lin, H.; Wu, C.; Jeng, R.; Tung, S. Amphiphilic Thermoresponsive Poly(Hydroxyaminoethers) as Effective Emulsifiers for Preparation of Waterborne Epoxy Resins. Macromol. Mater. Eng. 2022, 307, 2100668. [Google Scholar] [CrossRef]
- Muhammed Raji, A.; Hambali, H.U.; Khan, Z.I.; Binti Mohamad, Z.; Azman, H.; Ogabi, R. Emerging Trends in Flame Retardancy of Rigid Polyurethane Foam and Its Composites: A Review. J. Cell. Plast. 2023, 59, 65–122. [Google Scholar] [CrossRef]
- Ji, J.; Huang, S.; Liu, S.; Yuan, Y.; Zhao, J.; Zhang, S. A Novel Biomass-Derived Schiff Base Waterborne Epoxy Coating for Flame Retardation and Anti-Bacteria. Polym. Degrad. Stab. 2022, 199, 109910. [Google Scholar] [CrossRef]
- Yang, Z.; Xiao, G.; Chen, C.; Chen, C.; Wang, M.; Zhong, F.; Zeng, S.; Lin, L. Synergistic Decoration of Organic Titanium and Polydopamine on Boron Nitride to Enhance Fire Resistance of Intumescent Waterborne Epoxy Coating. Colloids Surf. Physicochem. Eng. Asp. 2021, 621, 126561. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Liu, S.; Luo, H.; Fan, W.; Liu, Z.; Liu, F.; Wang, H. Anti-Corrosion and Wear-Resistant Coating of Waterborne Epoxy Resin by Concrete- like Three-Dimensional Functionalized Framework Fillers. Chem. Eng. Sci. 2021, 242, 116748. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Yang, N.; Hou, Y.; Chen, D.; Liu, J.; Cai, G.; Wang, M. The Effect of Different Diluents and Curing Agents on the Performance of Epoxy Resin-Based Intumescent Flame-Retardant Coatings. Materials 2024, 17, 348. [Google Scholar] [CrossRef]
- Dall Agnol, L.; Dias, F.T.G.; Ornaghi, H.L.; Sangermano, M.; Bianchi, O. UV-Curable Waterborne Polyurethane Coatings: A State-of-the-Art and Recent Advances Review. Prog. Org. Coat. 2021, 154, 106156. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Fang, C.; Li, S.; Cheng, Y.; Lei, W.; Meng, X. Recent Advances in Synthesis of Waterborne Polyurethane and Their Application in Water-Based Ink: A Review. J. Mater. Sci. Technol. 2015, 31, 708–722. [Google Scholar] [CrossRef]
- Vaidya, S.M.; Jadhav, S.M.; Patil, M.J.; Mestry, S.U.; Mahajan, U.R.; Mhaske, S.T. Recent Developments in Waterborne Polyurethane Dispersions (WPUDs): A Mini-Review on Thermal and Mechanical Properties Improvement. Polym. Bull. 2022, 79, 5709–5745. [Google Scholar] [CrossRef]
- Du, W.; Jin, Y.; Lai, S.; Shi, L.; Shen, Y.; Pan, J. Urethane-Silica Functionalized Graphene Oxide for Enhancing Mechanical Property and Fire Safety of Waterborne Polyurethane Composites. Appl. Surf. Sci. 2019, 492, 298–308. [Google Scholar] [CrossRef]
- Wang, S.; Du, X.; Jiang, Y.; Xu, J.; Zhou, M.; Wang, H.; Cheng, X.; Du, Z. Synergetic Enhancement of Mechanical and Fire-Resistance Performance of Waterborne Polyurethane by Introducing Two Kinds of Phosphorus–Nitrogen Flame Retardant. J. Colloid Interface Sci. 2019, 537, 197–205. [Google Scholar] [CrossRef]
- Zielecka, M.; Rabajczyk, A.; Cygańczuk, K.; Pastuszka, Ł.; Jurecki, L. Silicone Resin-Based Intumescent Paints. Materials 2020, 13, 4785. [Google Scholar] [CrossRef]
- Gardelle, B.; Duquesne, S.; Vandereecken, P.; Bellayer, S.; Bourbigot, S. Resistance to Fire of Intumescent Silicone Based Coating: The Role of Organoclay. Prog. Org. Coat. 2013, 76, 1633–1641. [Google Scholar] [CrossRef]
- Pham, S.T.; Tieu, A.K.; Sencadas, V.; Joseph, P.; Arun, M.; Cortie, D. Thermoresponsive Hybrid Colloidal Capsules as an Inorganic Additive for Fire-Resistant Silicone-Based Coatings. Ind. Eng. Chem. Res. 2022, 61, 13104–13116. [Google Scholar] [CrossRef]
- Somasundaran, P.; Mehta, S.C.; Purohit, P. Silicone Emulsions. Adv. Colloid Interface Sci. 2006, 128–130, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Z.; Qi, Y. Effect of Emulsifier on the Structure and Properties of Waterborne Silicone Antifouling Coating. Coatings 2020, 10, 168. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Luo, H.; Yao, A.; Chen, X.; Zhang, M.; Huang, Q.; Xiang, J.; Chen, Y.; Fan, H. Construction and Performance of Waterborne Organosilicon Anti-Fouling Coating Based on Hydrosilylation. Prog. Org. Coat. 2023, 185, 107918. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, G.; Yang, J. Influences of Silicone Emulsion on Fire Protection of Waterborne Intumescent Fire-Resistive Coating. J. Coat. Technol. Res. 2014, 11, 231–237. [Google Scholar] [CrossRef]
- Nasir, K.M.; Sulong, N.H.R.; Johan, M.R.; Afifi, A.M. Synergistic Effect of Industrial- and Bio-Fillers Waterborne Intumescent Hybrid Coatings on Flame Retardancy, Physical and Mechanical Properties. Prog. Org. Coat. 2020, 149, 105905. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J. Benign Design of Intumescent Flame Retardant Coating Incorporated Various Carbon Sources. Constr. Build. Mater. 2020, 236, 117433. [Google Scholar] [CrossRef]
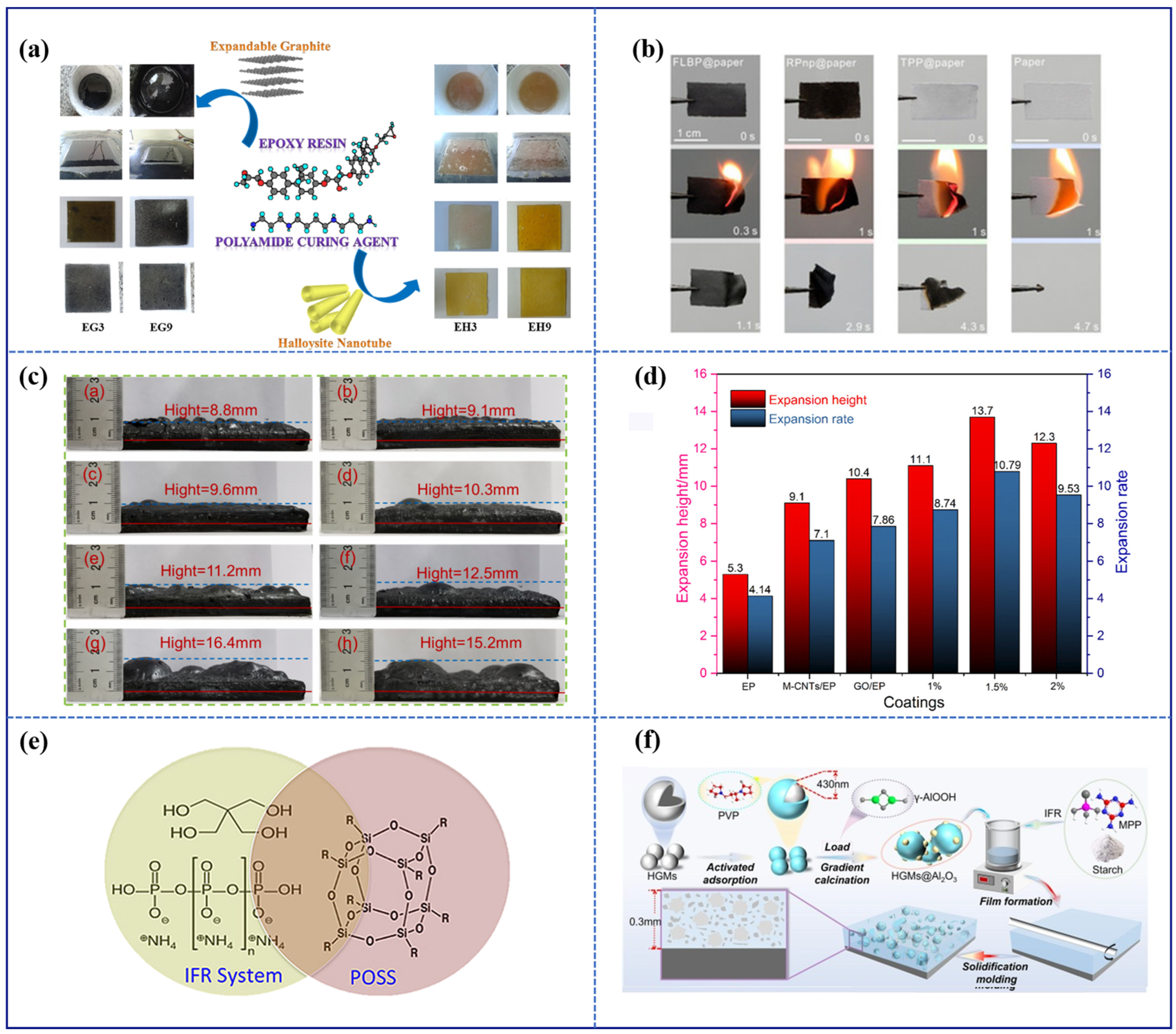
- Vahabi, H.; Jouyandeh, M.; Cochez, M.; Khalili, R.; Vagner, C.; Ferriol, M.; Movahedifar, E.; Ramezanzadeh, B.; Rostami, M.; Ranjbar, Z.; et al. Short-Lasting Fire in Partially and Completely Cured Epoxy Coatings Containing Expandable Graphite and Halloysite Nanotube Additives. Prog. Org. Coat. 2018, 123, 160–167. [Google Scholar] [CrossRef]
- Zhang, T.; Xie, H.; Xie, S.; Hu, A.; Liu, J.; Kang, J.; Hou, J.; Hao, Q.; Liu, H.; Ji, H. A Superior Two-Dimensional Phosphorus Flame Retardant: Few-Layer Black Phosphorus. Molecules 2023, 28, 5062. [Google Scholar] [CrossRef]
- Chen, C.; Xiao, G.; Zhong, F.; Dong, S.; Yang, Z.; Chen, C.; Wang, M.; Zou, R. Synergistic Effect of Carbon Nanotubes Bonded Graphene Oxide to Enhance the Flame Retardant Performance of Waterborne Intumescent Epoxy Coatings. Prog. Org. Coat. 2022, 162, 106598. [Google Scholar] [CrossRef]
- Turgut, G.; Dogan, M.; Tayfun, U.; Ozkoc, G. The Effects of POSS Particles on the Flame Retardancy of Intumescent Polypropylene Composites and the Structure-Property Relationship. Polym. Degrad. Stab. 2018, 149, 96–111. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, G.; Gao, L.; Cui, W.; Wang, Y. Preparation and Performance of Intumescent Water-Based Coatings with Both Thermal Insulation and Flame Retardant Functions. Chem. Eng. J. 2024, 480, 148165. [Google Scholar] [CrossRef]
- Cai, W.; Wang, B.-B.; Wang, X.; Zhu, Y.-L.; Li, Z.-X.; Xu, Z.-M.; Song, L.; Hu, W.-Z.; Hu, Y. Recent Progress in Two-Dimensional Nanomaterials Following Graphene for Improving Fire Safety of Polymer (Nano)Composites. Chin. J. Polym. Sci. 2021, 39, 935–956. [Google Scholar] [CrossRef]
- Wan, M.; Shi, C.; Qian, X.; Qin, Y.; Jing, J.; Che, H.; Ren, F.; Li, J.; Yu, B. Interface Assembly of Flower-like Ni-MOF Functional MXene towards the Fire Safety of Thermoplastic Polyurethanes. Compos. Part Appl. Sci. Manuf. 2022, 163, 107187. [Google Scholar] [CrossRef]
- Song, K.; Pan, Y.-T.; Zhang, J.; Song, P.; He, J.; Wang, D.-Y.; Yang, R. Metal–Organic Frameworks–Based Flame-Retardant System for Epoxy Resin: A Review and Prospect. Chem. Eng. J. 2023, 468, 143653. [Google Scholar] [CrossRef]
- Gaggero, G.; Delucchi, M.; Di Tanna, G.; Lagazzo, A.; Vicini, S.; Botter, R. Effect of Different Alginate Salts on the Rheological and Tensile Properties of Waterborne Paints. Prog. Org. Coat. 2022, 163, 106676. [Google Scholar] [CrossRef]
- Ysiwata-Rivera, A.P.; Hernández-Hernández, E.; Cadenas-Pliego, G.; Ávila-Orta, C.A.; González-Morones, P.; Jesús, J.A.V.; Cuara-Díaz, E.; Gallardo-Vega, C.A.; Mata-Padilla, J.M. Effect of Modified Hexagonal Boron Nitride Nanoparticles on the Emulsion Stability, Viscosity and Electrochemical Behavior of Nanostructured Acrylic Coatings for the Corrosion Protection of AISI 304 Stainless Steel. Coatings 2020, 10, 488. [Google Scholar] [CrossRef]
- Wang, H.; Li, R.; Wu, Q.; Fei, G.; Li, Y.; Zou, M.; Sun, L. Gemini Surfactant-Assisted Fabrication of Graphene Oxide/Polyaniline towards High-Performance Waterborne Anti-Corrosive Coating. Appl. Surf. Sci. 2021, 565, 150581. [Google Scholar] [CrossRef]
- Liang, C.; Zhao, P.; Gong, X.; Liu, H.; Yang, L.; Li, Q.; Lu, L. Investigation on the Mechanical Properties of CSA Cement-Based Coating and Its Application. Constr. Build. Mater. 2021, 305, 124724. [Google Scholar] [CrossRef]
- Kohse-Höinghaus, K. Combustion in the Future: The Importance of Chemistry. Proc. Combust. Inst. 2021, 38, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, J.; Guo, Y.; Meng, Q.; Chen, K.; Pan, B.; Zhou, X. Synthesis and Application of Silicone Modified Flame Retardant for Polyester Fabric. Colloids Surf. Physicochem. Eng. Asp. 2024, 680, 132679. [Google Scholar] [CrossRef]
- Zeng, X.-L.; Lan, X.-S.; Wang, Y.; Zhang, L.; Guo, D.-M.; Zhao, H.-B. Highly Transparent Fire-Resistant Coatings with Intumescent Three-Source Integration. Chin. J. Polym. Sci. 2024, 42, 907–915. [Google Scholar] [CrossRef]
- De Cachinho Cordeiro, I.M.; Chen, T.B.Y.; Yuen, A.C.Y.; Chen, Q.; Yang, W.; Wang, C.; Wang, W.; Chan, Q.N.; Zhang, J.; Yang, W.; et al. Characterising Flame-Retardant Mechanism of Phosphorous-Containing Intumescent Coating on Polyethylene via ReaxFF MD Simulations. Chem. Eng. J. 2024, 480, 148169. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Wang, J.; Liu, Y.; Hou, H.; Pan, R.; Zhou, X. Study of Thermal Pyrolysis Characteristics and Fire Extinguishing Performance of Novel Halon Alternatives for Aviation Applications. J. Therm. Anal. Calorim. 2024, 1–19. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Z.; Huang, J.; Wang, Y. A Flame Retardant Poly Vinyl Alcohol/Graphene Oxide/Phytic Acid Composite for a Quick Response and Ultra-Long Fire Alarm. J. Mater. Chem. A 2024, 12, 6050–6066. [Google Scholar] [CrossRef]
- Chen, C.; Wang, B.; Xiao, G.; Zhong, F.; Zhou, J.; Cao, M.; Yang, Z.; Chen, C.; Zou, R. Flower-like Ni-Al/LDH Synergistic Polyaniline Anchored to the Carbon Sphere Surface to Improve the Fire Performance of Waterborne Epoxy Coatings. Prog. Org. Coat. 2024, 186, 108068. [Google Scholar] [CrossRef]
- Li, X.-M.; Bai, W.-B.; Lin, Y.-C.; Ding, F.-C.; Jian, R.-K. Interfacial Coordination-Assisted Construction of Mechanically Robust, Fluorescent and Intumescent Flame Retardant Epoxy Resins. Chem. Eng. J. 2024, 479, 147549. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, J.; Liu, L.; Zheng, H.; Dai, J.; Tang, L.-C.; Song, P. A Highly Fire-Retardant Rigid Polyurethane Foam Capable of Fire-Warning. Compos. Commun. 2022, 29, 101046. [Google Scholar] [CrossRef]
- Wang, T.; Xu, J.; Zhan, Y.-J.; He, L.; Deng, J.; Fu, Z.-C.; Zhao, H.-B.; Chen, M.-J. Eco-Friendly and Facile Integrated Intumescent Polyelectrolyte Complex Coating with Universal Flame Retardancy and Smoke Suppression for Cotton and Its Blending Fabrics. ACS Sustain. Chem. Eng. 2023, 11, 4838–4849. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Z.; Huang, J.; Wang, Y. Water-Based Intumescent Fire Resistance Coating Containing Organic-Modified Glass Fiber for Steel Structure. J. Clean. Prod. 2024, 442, 140897. [Google Scholar] [CrossRef]
- Babu Aurtherson, P.; Hemanandh, J.; Devarajan, Y.; Mishra, R.; Abraham, B.C. Experimental Testing and Evaluation of Coating on Cables in Container Fire Test Facility. Nucl. Eng. Technol. 2022, 54, 1652–1656. [Google Scholar] [CrossRef]


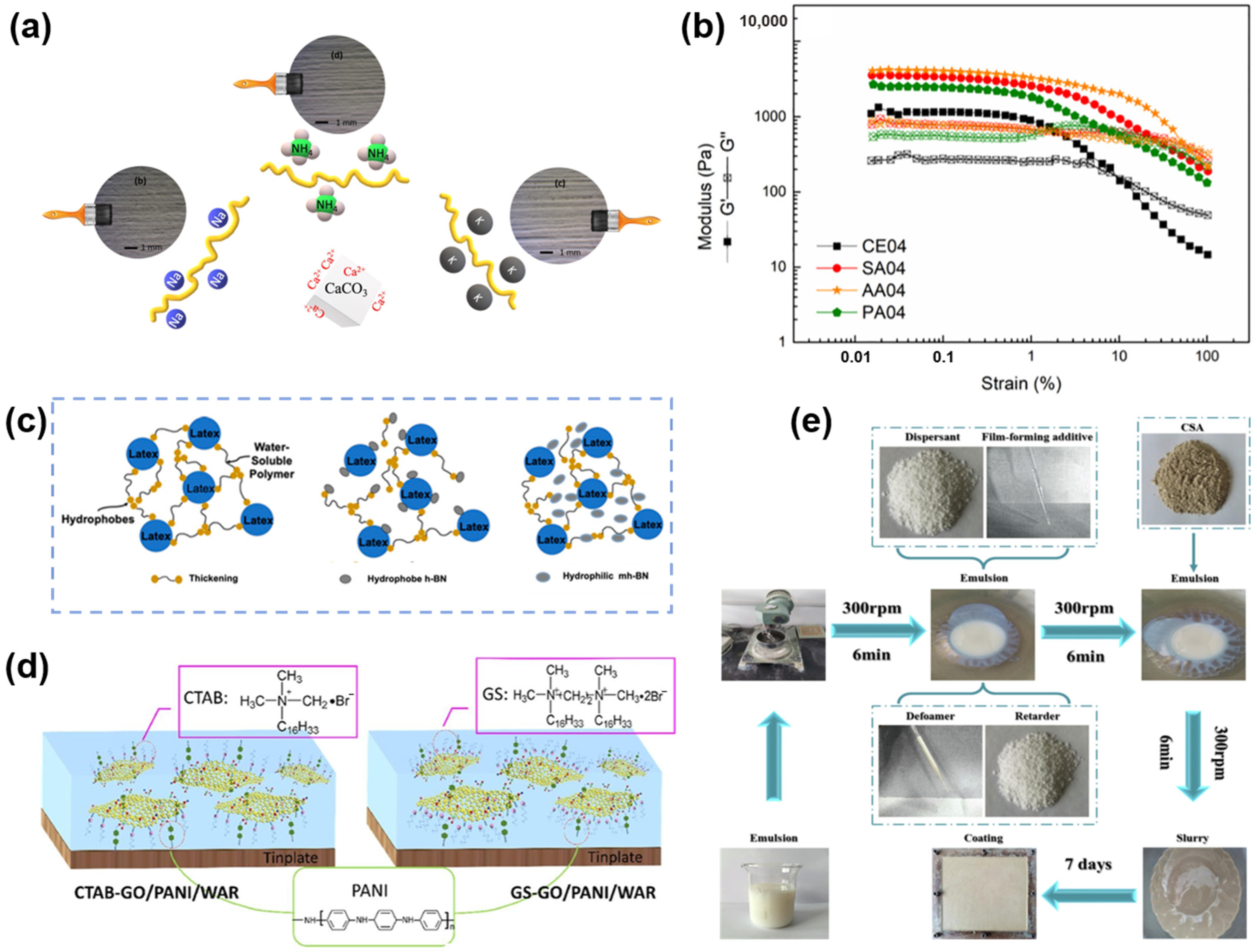
| Components | Commonly Used Substances | Role |
|---|---|---|
| Acid source | H3BO3, H3PO4, [(NH4)3PO4]n | Dehydrating agent |
| Carbon source | starch, chitosan, polyol | Charring agent |
| Gas source | Urea, melamine, chlorinated paraffin | Foaming agent |
| Film-forming matrices | Acrylic resin, epoxy resin, polyurethane, silicone | Continuous phase produces coating |
| Functional fillers | Alumina, kaolin, diatomite, silica fiber | Improving flame resistance |
| Additives | Defoamer, thickener | Enhancing the condition and effectiveness of coatings |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Cao, C.-F.; Chen, Z.-Y.; Liu, S.-C.; Bae, J.; Tang, L.-C. Waterborne Intumescent Fire-Retardant Polymer Composite Coatings: A Review. Polymers 2024, 16, 2353. https://doi.org/10.3390/polym16162353
Li Y, Cao C-F, Chen Z-Y, Liu S-C, Bae J, Tang L-C. Waterborne Intumescent Fire-Retardant Polymer Composite Coatings: A Review. Polymers. 2024; 16(16):2353. https://doi.org/10.3390/polym16162353
Chicago/Turabian StyleLi, Yang, Cheng-Fei Cao, Zuan-Yu Chen, Shuai-Chi Liu, Joonho Bae, and Long-Cheng Tang. 2024. "Waterborne Intumescent Fire-Retardant Polymer Composite Coatings: A Review" Polymers 16, no. 16: 2353. https://doi.org/10.3390/polym16162353
APA StyleLi, Y., Cao, C.-F., Chen, Z.-Y., Liu, S.-C., Bae, J., & Tang, L.-C. (2024). Waterborne Intumescent Fire-Retardant Polymer Composite Coatings: A Review. Polymers, 16(16), 2353. https://doi.org/10.3390/polym16162353







