Novel Approach for Cardioprotection: In Situ Targeting of Metformin via Conductive Hydrogel System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CSMA and Pam-GelMA
2.3. Cytotoxicity Assay
2.4. Rheological Testing of Hydrogels
2.5. Live/Dead Staining
2.6. Detection of Metf Release
2.7. JC-1 Staining
2.8. ROS Detection
2.9. Establishment of Rat Model
2.10. Echocardiographic Analysis
2.11. Histological Analysis
2.12. Statistical Analysis
3. Results and Discussion
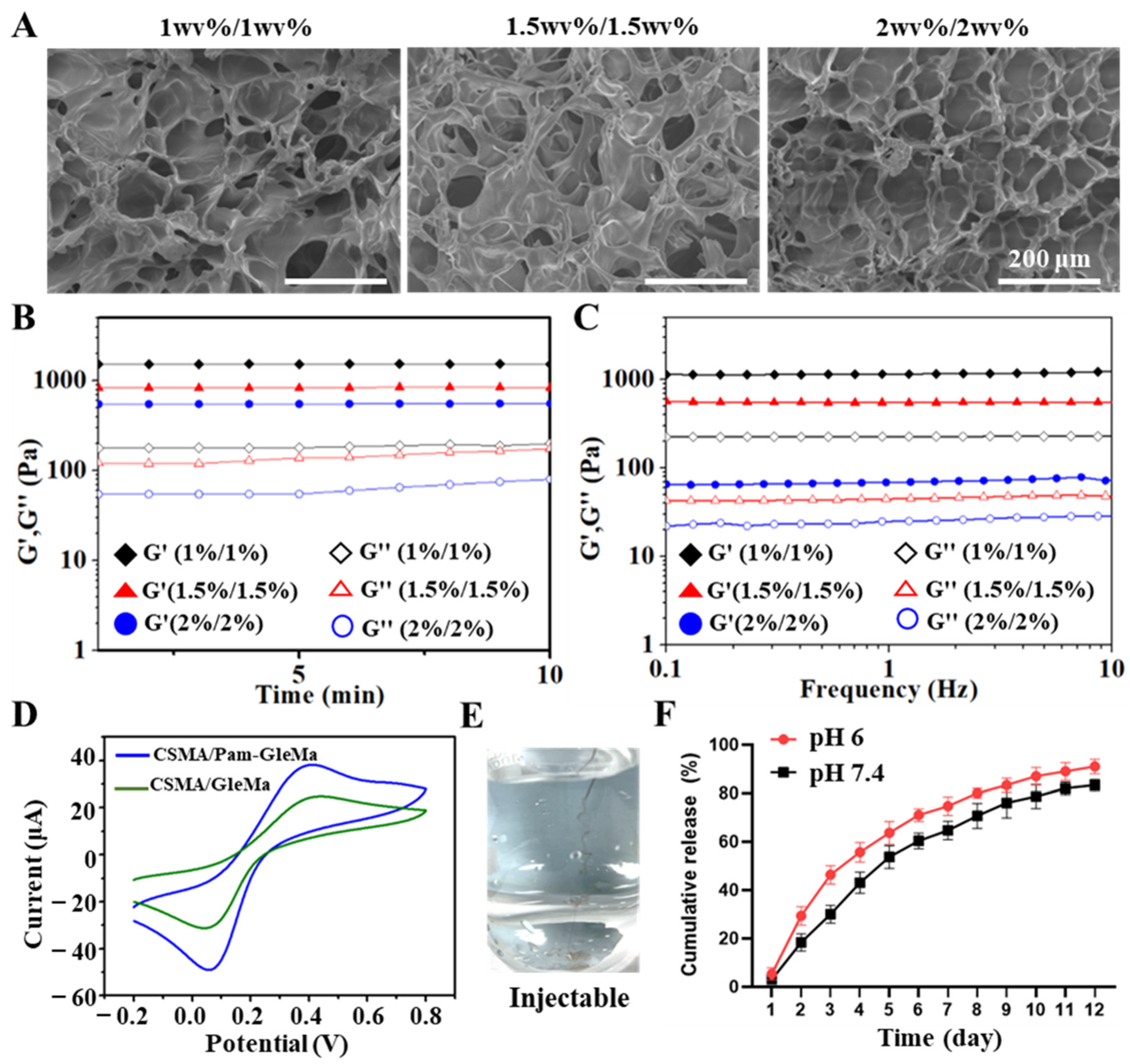
3.1. Preparation of CSMA/Pam-GelMA Hydrogels
3.2. Characterization of CSMA/Pam-GelMA Hydrogels
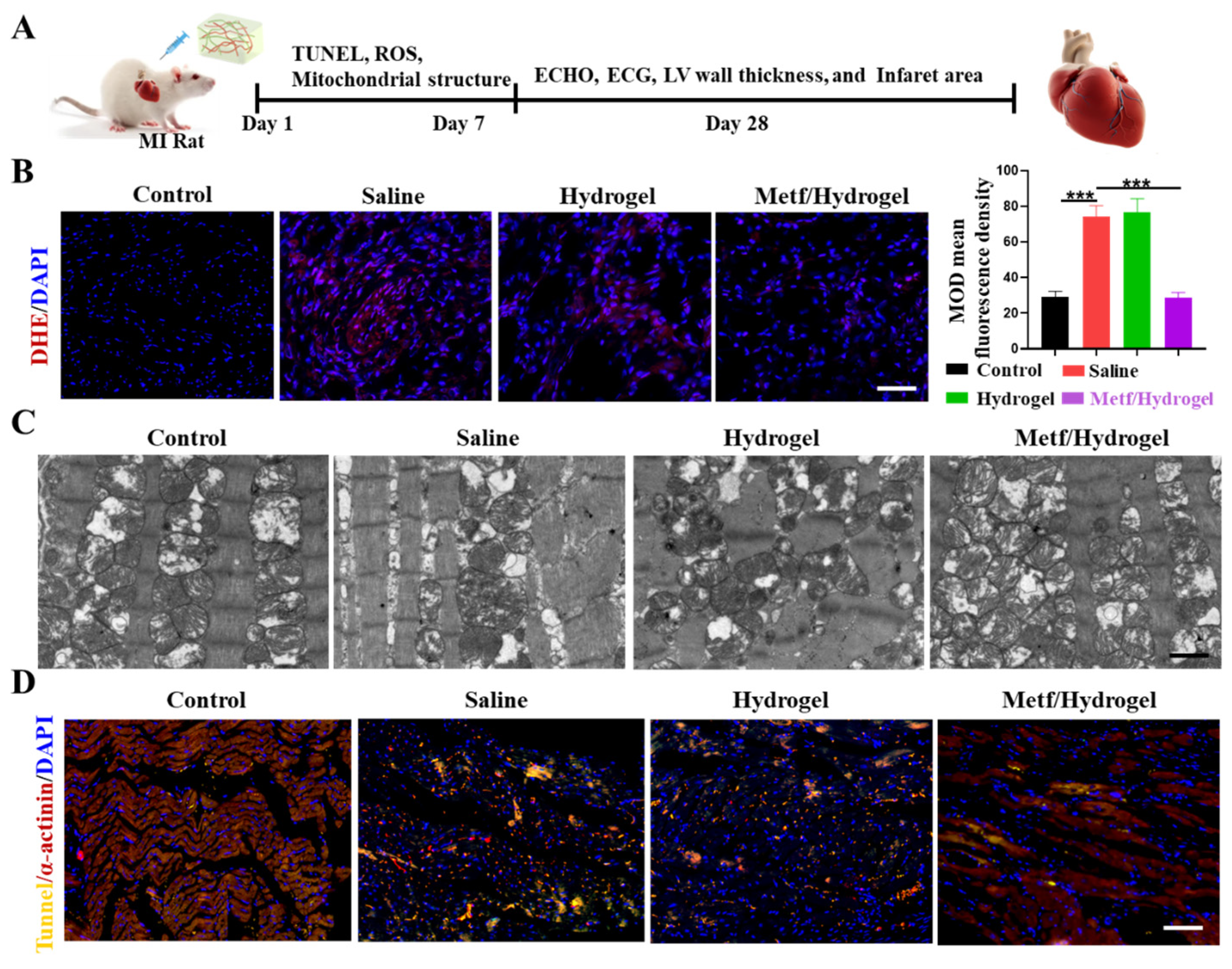
3.3. Effect of Metf/Hydrogel on ROS-Induced CM Activity
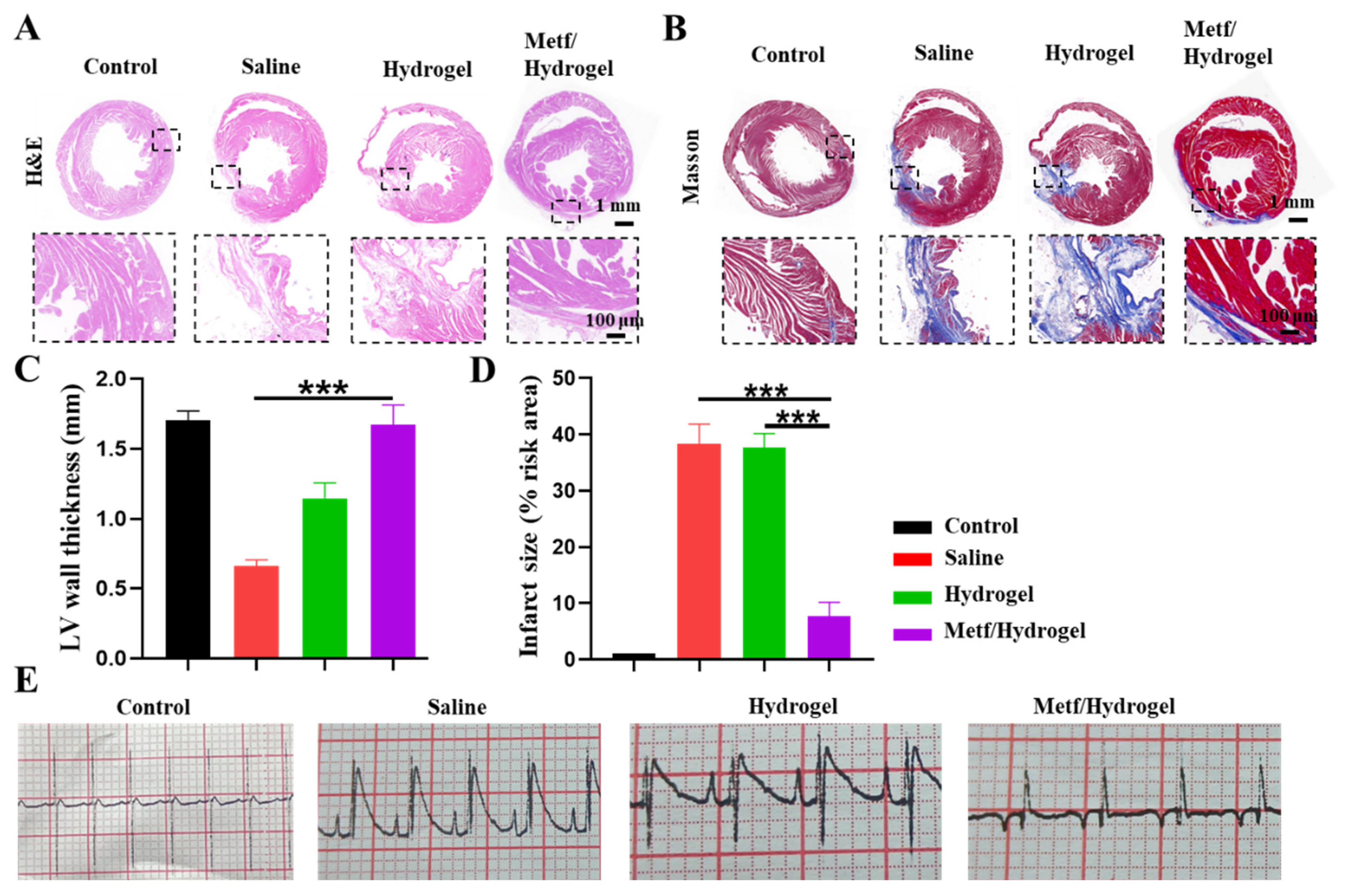
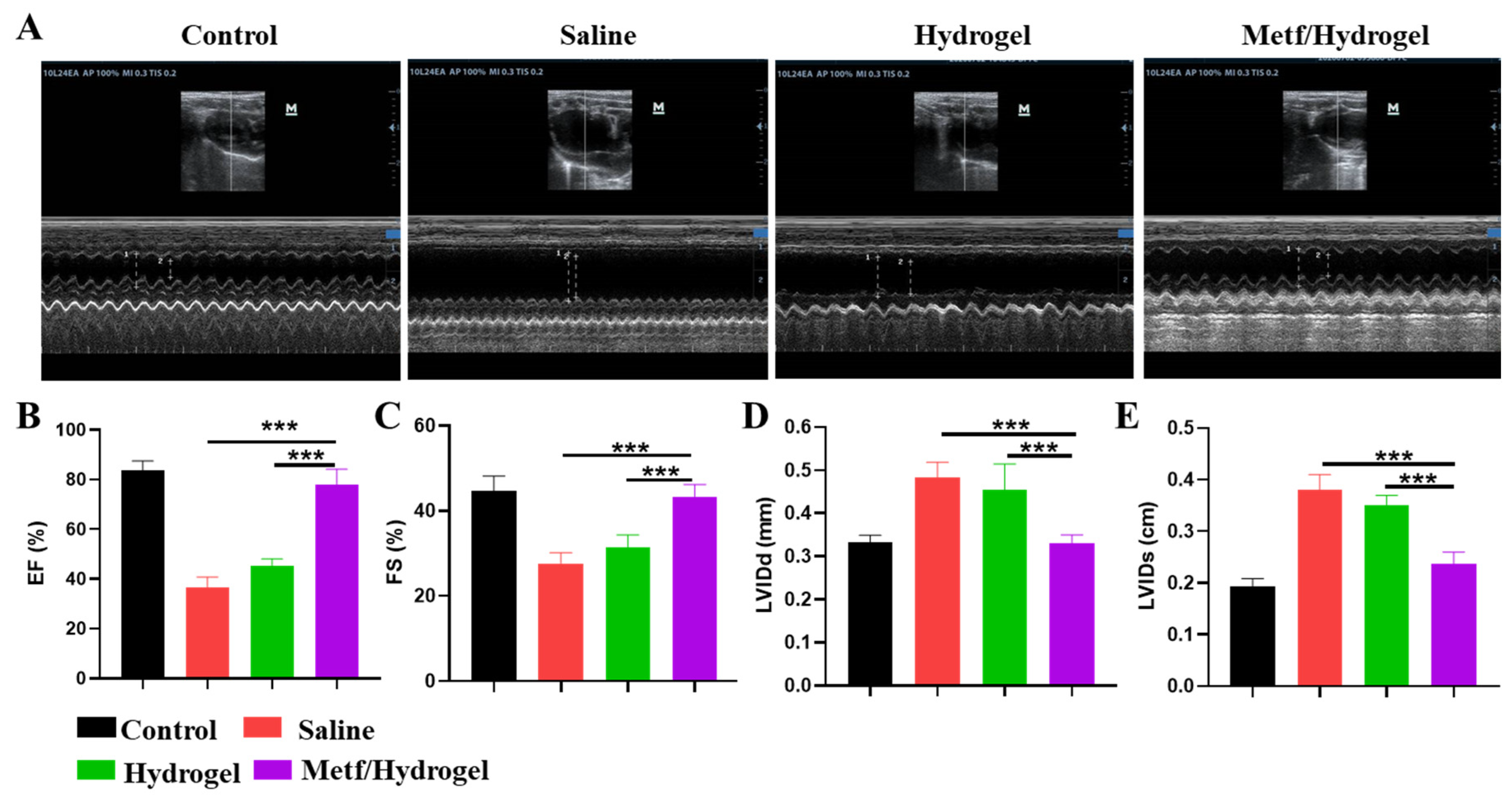
3.4. Effect of Metf/Hydrogel on Heart Structure and Function in Rats with MI
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Christensen, D.M.; Strange, J.E.; El-Chouli, M.; Falkentoft, A.C.; Malmborg, M.; Nouhravesh, N.; Gislason, G.; Schou, M.; Torp-Pedersen, C.; Sehested, T.S.G. Temporal Trends in Noncardiovascular Morbidity and Mortality Following Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2023, 82, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.T.; Khera, R.; Lau, G.; Qiu, F.; Wang, Y.; Austin, P.C.; Koh, M.; Lin, Z.; Lee, D.S.; Wijeysundera, H.C.; et al. Readmission and Mortality After Hospitalization for Myocardial Infarction and Heart Failure. J. Am. Coll. Cardiol. 2020, 75, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich-Nikitin, I.; Kirshenbaum, L.A. Circadian regulated control of myocardial ischemia-reperfusion injury. Trends Cardiovasc. Med. 2024, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Algoet, M.; Janssens, S.; Himmelreich, U.; Gsell, W.; Pusovnik, M.; Van den Eynde, J.; Oosterlinck, W. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc. Med. 2023, 33, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Q.; Chen, S.P.; Sun, J.; Wang, X.M.; Chen, N.; Zhou, Y.Q.; Tian, Y.K.; Ye, D.W. Berberine protects against ischemia-reperfusion injury: A review of evidence from animal models and clinical studies. Pharmacol. Res. 2019, 148, 104385. [Google Scholar] [CrossRef]
- Zhou, L.; Tang, S.; Li, F.; Wu, Y.; Li, S.; Cui, L.; Luo, J.; Yang, L.; Ren, Z.; Zhang, J.; et al. Ceria nanoparticles prophylactic used for renal ischemia-reperfusion injury treatment by attenuating oxidative stress and inflammatory response. Biomaterials 2022, 287, 121686. [Google Scholar] [CrossRef] [PubMed]
- He, S.F.; Jin, S.Y.; Yang, W.; Pan, Y.L.; Huang, J.; Zhang, S.J.; Zhang, L.; Zhang, Y. Cardiac μ-opioid receptor contributes to opioid-induced cardioprotection in chronic heart failure. Br. J. Anaesth. 2018, 121, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Lin, X.; Shen, L.; Feng, Y. The protective effect of herbal polysaccharides on ischemia-reperfusion injury. Int. J. Biol. Macromol. 2016, 92, 431–440. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Zhang, L.; Li, X.; Zhou, Z.; Jiao, L.; Shao, Y.; Li, M.; Leng, B.; Zhou, Y.; et al. Metformin Protects against H(2)O(2)-Induced Cardiomyocyte Injury by Inhibiting the miR-1a-3p/GRP94 Pathway. Mol. Ther. Nucleic Acids 2018, 13, 189–197. [Google Scholar] [CrossRef]
- Huang, K.Y.; Que, J.Q.; Hu, Z.S.; Yu, Y.W.; Zhou, Y.Y.; Wang, L.; Xue, Y.J.; Ji, K.T.; Zhang, X.M. Metformin suppresses inflammation and apoptosis of myocardiocytes by inhibiting autophagy in a model of ischemia-reperfusion injury. Int. J. Biol. Sci. 2020, 16, 2559–2579. [Google Scholar] [CrossRef]
- Leech, T.; Chattipakorn, N.; Chattipakorn, S.C. The beneficial roles of metformin on the brain with cerebral ischaemia/reperfusion injury. Pharmacol. Res. 2019, 146, 104261. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Picón-César, M.J.; Molina-Vega, M.; Suárez-Arana, M.; González-Mesa, E.; Sola-Moyano, A.P.; Roldan-López, R.; Romero-Narbona, F.; Olveira, G.; Tinahones, F.J.; González-Romero, S. Metformin for gestational diabetes study: Metformin vs insulin in gestational diabetes: Glycemic control and obstetrical and perinatal outcomes: Randomized prospective trial. Am. J. Obstet. Gynecol. 2021, 225, 517.e511–517.e517. [Google Scholar] [CrossRef] [PubMed]
- Vera, I.M.; Grilo Ruivo, M.T.; Lemos Rocha, L.F.; Marques, S.; Bhatia, S.N.; Mota, M.M.; Mancio-Silva, L. Targeting liver stage malaria with metformin. JCI Insight 2019, 4, e127441. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Lipska, K.J.; Mayo, H.; Bailey, C.J.; McGuire, D.K. Metformin in patients with type 2 diabetes and kidney disease: A systematic review. JAMA 2014, 312, 2668–2675. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef]
- Zheng, Z.; Tan, Y.; Li, Y.; Liu, Y.; Yi, G.; Yu, C.Y.; Wei, H. Biotherapeutic-loaded injectable hydrogels as a synergistic strategy to support myocardial repair after myocardial infarction. J. Control. Release Off. J. Control. Release Soc. 2021, 335, 216–236. [Google Scholar] [CrossRef]
- Zheng, Z.; Yu, C.; Wei, H. Injectable Hydrogels as Three-Dimensional Network Reservoirs for Osteoporosis Treatment. Tissue Eng. Part B Rev. 2021, 27, 430–454. [Google Scholar] [CrossRef]
- Zheng, Z.; Lei, C.; Liu, H.; Jiang, M.; Zhou, Z.; Zhao, Y.; Yu, C.Y.; Wei, H. A ROS-Responsive Liposomal Composite Hydrogel Integrating Improved Mitochondrial Function and Pro-Angiogenesis for Efficient Treatment of Myocardial Infarction. Adv. Healthc. Mater. 2022, 11, e2200990. [Google Scholar] [CrossRef]
- Zheng, Z.; Guo, Z.; Zhong, F.; Wang, B.; Liu, L.; Ma, W.; Yu, C.Y.; Wei, H. A dual crosslinked hydrogel-mediated integrated peptides and BMSC therapy for myocardial regeneration. J. Control. Release Off. J. Control. Release Soc. 2022, 347, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bei, Z.; Li, T.; Qian, Z. An injectable conductive hydrogel with dual responsive release of rosmarinic acid improves cardiac function and promotes repair after myocardial infarction. Bioact. Mater. 2023, 29, 132–150. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Sun, J.; Wang, J.; He, S.; Liu, Z.; Xie, J.; Yu, C.Y.; Wei, H. Enhancing myocardial infarction treatment through bionic hydrogel-mediated spatial combination therapy via mtDNA-STING crosstalk modulation. J. Control. Release Off. J. Control. Release Soc. 2024, 371, 570–587. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, X.; Zhang, J.; Shen, S.; Yin, W.; Ye, G.; Wang, L.; Hou, H.; Qiu, X. A tunable self-healing ionic hydrogel with microscopic homogeneous conductivity as a cardiac patch for myocardial infarction repair. Biomaterials 2021, 273, 120811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Y.; Qian, S.; Qian, X.; Jiao, J.; Ma, B.; Chen, J.; Cheng, H.; Li, X.; Lin, Y.; et al. Injectable and Conductive Nanomicelle Hydrogel with α-Tocopherol Encapsulation for Enhanced Myocardial Infarction Repair. ACS Nano 2024, 18, 10216–10229. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Dong, J.; Li, W.; Li, Z.; Gao, R.; Liu, X.; Wang, J.; Su, Q.; Wen, B.; Ouyang, W.; et al. Extracellular Matrix/Glycopeptide Hybrid Hydrogel as an Immunomodulatory Niche for Endogenous Cardiac Repair after Myocardial Infarction. Adv. Sci. 2023, 10, e2301244. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hong, H.; Ajiteru, O.; Sultan, M.T.; Lee, Y.J.; Lee, J.S.; Lee, O.J.; Lee, H.; Park, H.S.; Choi, K.Y.; et al. 3D bioprinted silk fibroin hydrogels for tissue engineering. Nat. Protoc. 2021, 16, 5484–5532. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Gu, J.; Li, L.; Yu, W.; Yin, S.; Qin, M.; Jiang, Q.; Wang, W.; Cao, Y. Hydrogel tapes for fault-tolerant strong wet adhesion. Nat. Commun. 2021, 12, 7156. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Qian, M.; Zhang, Y.; Liu, Q.; Midgley, A.C.; Liu, Y.; Che, Y.; Hou, J.; Zhao, Q. An Injectable Dual-Function Hydrogel Protects Against Myocardial Ischemia/Reperfusion Injury by Modulating ROS/NO Disequilibrium. Adv. Sci. 2022, 9, e2105408. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Liu, K.; Wang, K.; Fang, L.; Weng, L.T.; Zhang, H.; Tang, Y.; Ren, F.; Zhao, C.; et al. Mussel-Inspired Adhesive and Tough Hydrogel Based on Nanoclay Confined Dopamine Polymerization. ACS Nano 2017, 11, 2561–2574. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Li, J.; Nie, Y.; Zheng, Z. Novel Approach for Cardioprotection: In Situ Targeting of Metformin via Conductive Hydrogel System. Polymers 2024, 16, 2226. https://doi.org/10.3390/polym16152226
Tan Y, Li J, Nie Y, Zheng Z. Novel Approach for Cardioprotection: In Situ Targeting of Metformin via Conductive Hydrogel System. Polymers. 2024; 16(15):2226. https://doi.org/10.3390/polym16152226
Chicago/Turabian StyleTan, Ying, Jierong Li, Yali Nie, and Zhi Zheng. 2024. "Novel Approach for Cardioprotection: In Situ Targeting of Metformin via Conductive Hydrogel System" Polymers 16, no. 15: 2226. https://doi.org/10.3390/polym16152226
APA StyleTan, Y., Li, J., Nie, Y., & Zheng, Z. (2024). Novel Approach for Cardioprotection: In Situ Targeting of Metformin via Conductive Hydrogel System. Polymers, 16(15), 2226. https://doi.org/10.3390/polym16152226






