Enhanced Antibacterial Activity of Vancomycin Loaded on Functionalized Polyketones
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Aliphatic Polyketones (PK)
2.2. Synthesis of PKSK and PKT by Paal–Knorr Modification of PK with Potassium Sulfanilate or Sodium Taurinate
2.3. Loading of Vancomycin on PKSK or PKT
2.4. Vancomycin Release
2.5. Antibacterial Activity
3. Results and Discussion
3.1. Synthesis of Post-Functionalized PK
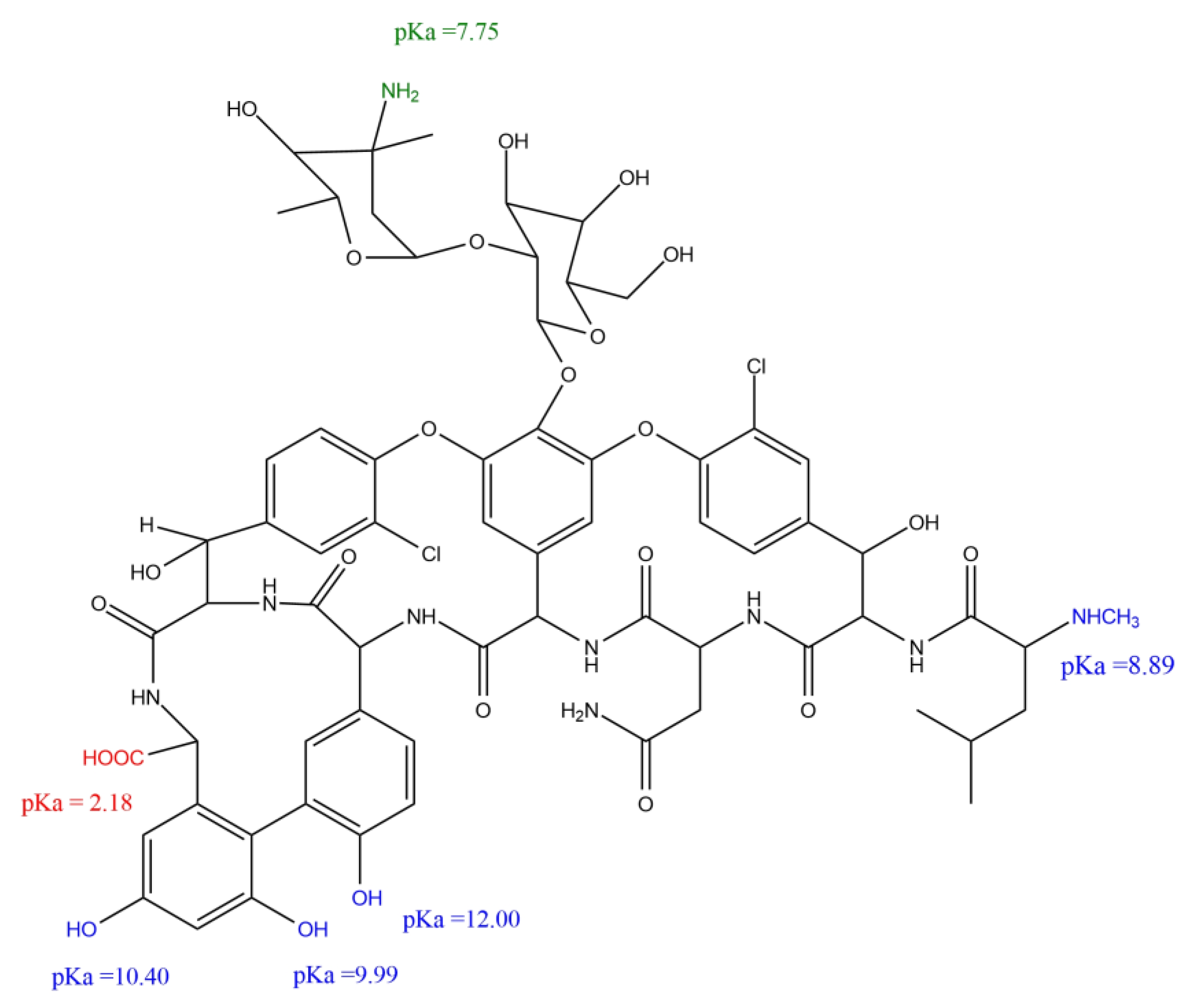
3.2. Influence of pH on Vancomycin Loading
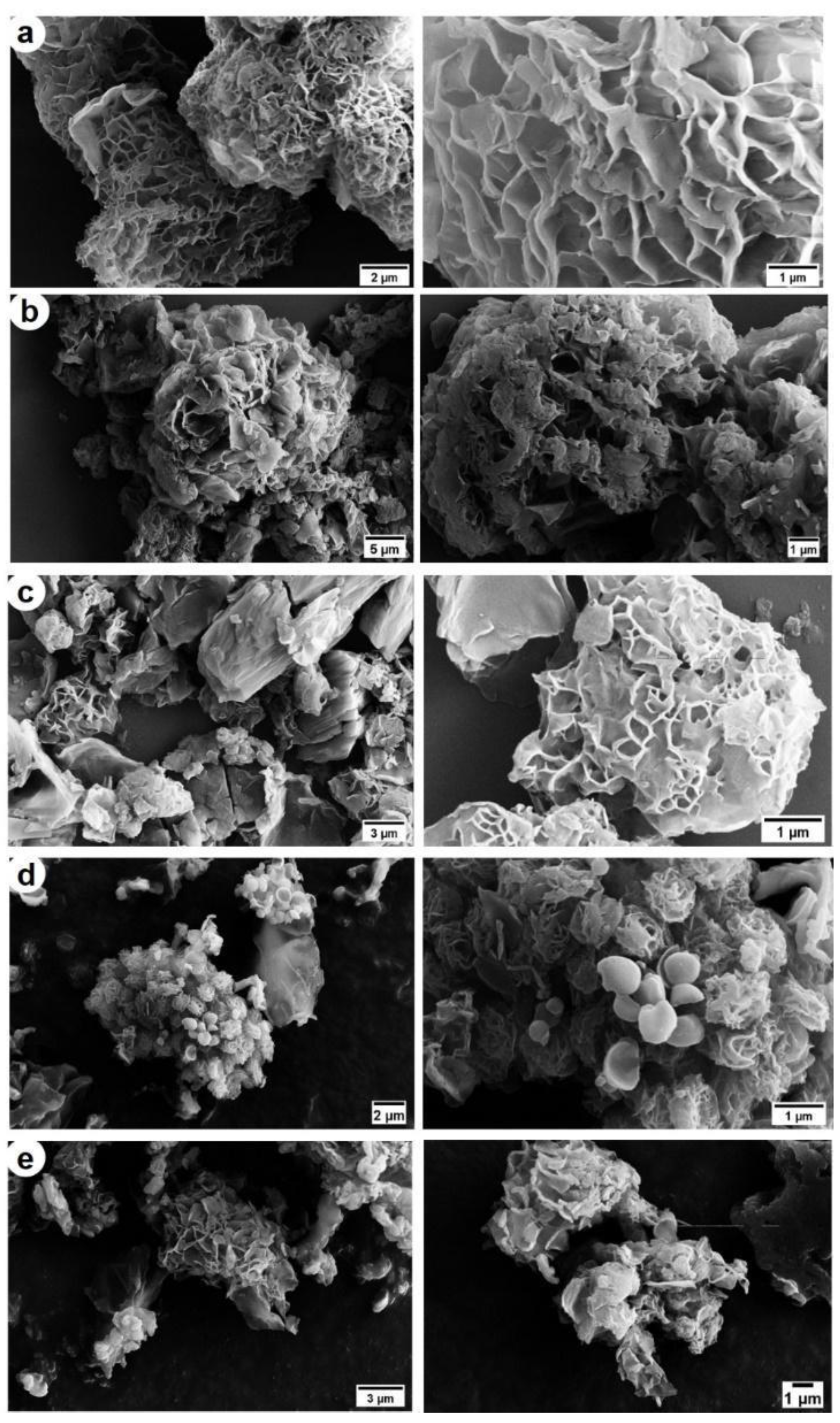
3.3. PKT-VCM and PKSK-VCM Characterisation
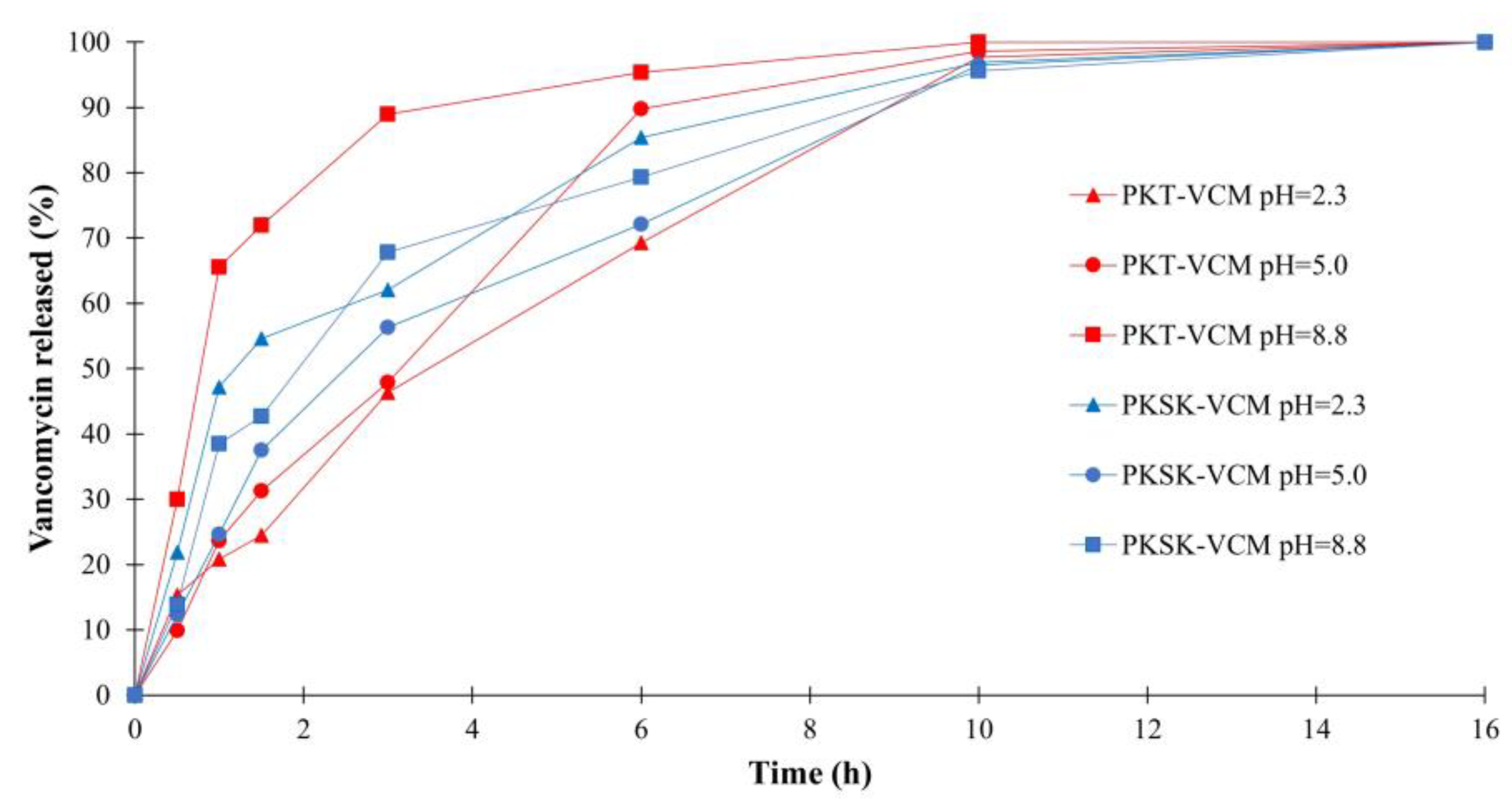
3.4. Release of Vancomycin from PK Polymers
3.5. Antimicrobial Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morandini, A.; Spadati, E.; Leonetti, B.; Sole, R.; Gatto, V.; Rizzolio, F.; Beghetto, V. Sustainable triazine-derived quaternary ammonium salts as antimicrobial agents. RSC Adv. 2021, 11, 28092–28096. [Google Scholar] [CrossRef]
- Reece, R.; Beckwith, C.G. The Infectious Diseases Specialist, At Risk of Extinction. J. Infect. Dis. 2023, 228, 1649–1651. [Google Scholar] [CrossRef] [PubMed]
- Momoh, A.O.; Asowata-Ayodele, A.M.; Olayemi, O.; David-Momoh, T. The comparative antimicrobial effects of castor, garlic, benniseed and bitter cola extracts on microorganisms isolated from hospitals’ wards. Microbes Infect. 2023; in press. [Google Scholar]
- Shree, P.; Singh, C.K.; Sodhi, K.K.; Surya, J.N.; Singh, D.K. Bactericidal and biofilm eradication efficacy of a fluorinated benzimidazole derivative, TFBZ, against methicillin-resistant Staphylococcus aureus. Med. Microecol. 2023, 16, 100084. [Google Scholar] [CrossRef]
- Liu, Z.; Deshazer, H.; Rice, A.J.; Chen, K.; Zhou, C.; Kallenbach, N.R. Multivalent Antimicrobial Peptides as Therapeutics: Design Principles and Structural Diversities. J. Med. Chem. 2006, 49, 3436–3439. [Google Scholar] [CrossRef]
- Bruni, G.; Maggi, L.; Tammaro, L.; Lorenzo, R.D.; Friuli, V.; D’Aniello, S.; Maietta, M.; Berbenni, V.; Milanese, C.; Girella, A.; et al. Electrospun fibers as potential carrier systems for enhanced drug release of perphenazine. Int. J. Pharm. 2016, 511, 190–197. [Google Scholar] [CrossRef]
- Gupta, A.; Makabenta, J.M.V.; Schlüter, F.; Landis, R.F.; Das, R.; Cuppels, M.; Rotello, V.M. Functionalized Polymers Enhance Permeability of Antibiotics in Gram-negative MDR Bacteria and Biofilms for Synergistic Antimicrobial Therapy. Adv. Ther. 2020, 3, 2000005. [Google Scholar] [CrossRef] [PubMed]
- Soto, S.M. Role of Efflux Pumps in the Antibiotic Resistance of Bacteria Embedded in a Biofilm. Virulence 2013, 4, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, V.K.; Pathania, R. Efflux pump inhibitors for bacterial pathogens: From bench to bedside Indian. J. Med. Res. 2019, 149, 129–145. [Google Scholar] [CrossRef]
- Wang, T.; Rong, F.; Tang, Y.; Li, M.; Feng, T.; Zhou, Q.; Li, P.; Huang, W. Targeted polymer-based antibiotic delivery system: A promising option for treating bacterial infections via macromolecular approaches. Prog. Polym. Sci. 2021, 16, 101389. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R.; Xiao, X.; Wang, Z. Molecules that Inhibit Bacterial Resistance Enzymes. Molecules 2019, 24, 43. [Google Scholar] [CrossRef]
- De Pascale, G.; Wright, G.D. Antibiotic resistance by enzyme inactivation: From mechanisms to solutions. Chembiochem 2010, 11, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Zgurskaya, H.I.; Rybenkov, V.V. Permeability barriers of Gram-negative pathogens. Ann. N. Y. Acad. Sci. 2020, 1459, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Montanaro, L.; Campoccia, D.; Arciola, C.R. Advancements in molecular epidemiology of implant infections and future perspectives. Biomaterials 2007, 28, 5155–5168. [Google Scholar] [CrossRef]
- Abebe, G.M. Detection of Biofilm Formation and Antibiotic Resistance in Klebsiella Oxytoca and Klebsiella Pneumoniae from Animal Origin Foods. Int. J. Microbiol. 2020, 5, 120. [Google Scholar] [CrossRef]
- Das, A.; Patro, S.; Simnani, F.Z.; Singh, D.; Sinha, A.; Kumari, K.; Rao, P.V.; Singh, S.; Kaushik, N.K.; Panda, P.K.; et al. Biofilm modifiers: The disparity in paradigm of oral biofilm ecosystem. Biomed. Pharmacother 2023, 164, 114966. [Google Scholar] [CrossRef] [PubMed]
- Venter, H.; Henningsen, M.L.; Begg, S.L. Antimicrobial resistance in healthcare, agriculture and the environment: The biochemistry behind the headlines. Essays Biochem. 2017, 61, 1–10. [Google Scholar] [CrossRef]
- Rehman, S. A parallel and silent emerging pandemic: Antimicrobial resistance (AMR) amid COVID-19 pandemic. J. Infect. Public Health. 2023, 16, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A. Osek, Antibiotic Resistance in Bacteria-A Review. J. Antibiot. 2022, 11, 1079. [Google Scholar] [CrossRef]
- Chin, K.W.; Michelle Tiong, H.L.; Luang-In, V.; Ma, N.L. The Role of Five-Membered Heterocycles in the Molecular Structure of Antibacterial Drugs Used in Therapy. Environ. Adv. 2023, 11, 100331. [Google Scholar] [CrossRef]
- Morandini, A.; Leonetti, B.; Riello, P.; Sole, R.; Gatto, V.; Caligiuri, I.; Rizzolio, F.; Beghetto, V. Synthesis and Antimicrobial Evaluation of Bis-morpholine Triazine Quaternary Ammonium Salts. ChemMedChem 2021, 16, 3172–3176. [Google Scholar] [CrossRef] [PubMed]
- Malaekeh-Nikouei, B.; Fazly Bazzaz, B.S.; Mirhadi, E.; Tajani, A.S.; Khameneh, B. The role of nanotechnology in combating biofilm-based antibiotic resistance. J. Drug Deliv. Sci. Technol. 2020, 60, 101880. [Google Scholar] [CrossRef]
- Birk, S.E.; Boisen, A.; Nielsen, L.H. Polymeric nano- and microparticulate drug delivery systems for treatment of biofilms. Adv. Drug Deliv. Rev. 2021, 174, 30–52. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.; Phung, A.N.; Škalko-Basnet, N.; Obuobi, S. Smart delivery systems for microbial biofilm therapy: Dissecting design, drug release and toxicological features. J. Control Release 2023, 354, 394–416. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, Z.; Ali, A.A.; Al-Sayah, M.H. Liposomes-Based Drug Delivery Systems of Anti-Biofilm Agents to Combat Bacterial Biofilm Formation. Antibiotics 2023, 12, 875. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, K.; Srinivasan, S.; Shanmugam, A. Review of the efficacy of nanoparticle-based drug delivery systems for cancer treatment. J. Biomed. Technol. 2024, 5, 109–122. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnology 2018, 16, 71. [Google Scholar] [CrossRef]
- Al-Hussaniy, H.A.; Аlmajidi, Y.Q.; Oraibi, A.I.; Alkarawi, A.H. Nanoemulsions as medicinal components in insoluble medicines. Pharmacia 2023, 70, 537–547. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles' Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Zhang, H.; Zhuang, Y.; Shen, H.; Chen, Y.; Zhao, Y.; Chen, B.; Xiao, Z.; Dai, J. Aligned Scaffolds with Biomolecular Gradients for Regenerative Medicine. Polymers 2019, 11, 341. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Ding, H.; Tan, P.; Fu, S.; Tian, X.; Zhang, H.; Ma, X.; Gu, Z.; Luo, K. Preparation and application of pH-responsive drug delivery systems. J. Control Release 2022, 348, 206–238. [Google Scholar] [CrossRef]
- Yadav, S.K.; Yadav, B.; Kumar Gupta, M.; Harish, S. A Comprehensive Review on Solid Dispersion Technique to Enhance the Solubility and Bioavailability of Poorly Water-Soluble Drugs. Int. J. Pharm. Res. 2023, 14, 106–117. Available online: https://www.ijppronline.com/index.php/IJPPR/article/view/313 (accessed on 10 May 2024). [CrossRef]
- Sole, R.; Buranello, C.; Di Michele, A.; Beghetto, V. Boosting physical-mechanical properties of adipic acid/chitosan films by DMTMM cross-linking. Int. J. Biol. Macromol. 2022, 209, 2009–2019. [Google Scholar] [CrossRef]
- Singh, S.; Alrobaian, M.M.; Molugulu, N.; Agrawal, N.; Numan, A.; Kesharwani, P. Pyramid-Shaped PEG-PCL-PEG Polymeric-Based Model Systems for Site-Specific Drug Delivery of Vancomycin with Enhance Antibacterial Efficacy. ACS Omega 2020, 5, 11935–11945. [Google Scholar] [CrossRef]
- Park, M.R.; Seo, B.B.; Song, S.C. Dual ionic interaction system based on polyelectrolyte complex and ionic, injectable, and thermosensitive hydrogel for sustained release of human growth hormone. Biomaterials 2013, 34, 1327–1336. [Google Scholar] [CrossRef]
- Palleria, C.; Di Paolo, A.; Giofrè, C.; Caglioti, C.; Leuzzi, G.; Siniscalchi, A.; De Sarro, G.; Gallelli, L. Pharmacokinetic drug-drug interaction and their implication in clinical management. J. Pharm. Sci. Res. 2013, 18, 601–610. [Google Scholar]
- Stipa, P.; Marano, S.; Galeazzi, R.; Minnelli, C.; Mobbili, G.; Laudadio, E. Prediction of drug-carrier interactions of PLA and PLGA drug-loaded nanoparticles by molecular dynamics simulations. Eur. Polym. J. 2021, 147, 110292. [Google Scholar] [CrossRef]
- Al Ragib, A.; Chakma, R.; Dewan, K.; Islam, T.; Kormoker, T.; Idris, A.M. Current advanced drug delivery systems: Challenges and potentialities. Int. J. Drug Deliv. Technol. 2022, 76, 103727. [Google Scholar] [CrossRef]
- Beghetto, V.; Gatto, V.; Conca, S.; Bardella, N.; Scrivanti, A. Polyamidoamide dendrimers and cross-linking agents for stabilized bioenzymatic resistant metal-free bovine collagen. Molecules 2019, 24, 3611–3622. [Google Scholar] [CrossRef]
- Wang, M.-Q.; Zou, H.; Liu, W.-B.; Liu, N.; Wu, Z.-Q. Bottlebrush Polymers Based on RAFT and the “C1” Polymerization Method: Controlled Synthesis and Application in Anticancer Drug Delivery. ACS Macro Lett. 2022, 11, 179–185. [Google Scholar] [CrossRef]
- Wang, C.; Zou, H.; Liu, N.; Wu, Z.-Q. Recent Advances in Polyallenes: Preparation, Self-Assembly, and Stimuli-Responsiveness. Chem. Asian J. 2021, 16, 3864. [Google Scholar] [CrossRef]
- Zhao, S.-Q.; Hu, G.; Xu, X.-H.; Kang, S.-M.; Liu, N.; Wu, Z.-Q. Synthesis of Redox-Responsive Core Cross-Linked Micelles Carrying Optically Active Helical Poly(phenyl isocyanide) Arms and Their Applications in Drug Delivery. ACS Macro Lett. 2018, 7, 1073–1079. [Google Scholar] [CrossRef]
- Larson, N.; Ghandehari, H. Polymeric Conjugates for Drug Delivery. Chem. Mater. 2012, 24, 840–853. [Google Scholar] [CrossRef]
- Irby, D.; Du, C.; Li, F. Lipid–Drug Conjugate for Enhancing Drug Delivery. Mol. Pharm. 2017, 14, 1325–1338. [Google Scholar] [CrossRef]
- Dalela, M.; Shrivastav, T.G.; Kharbanda, S.; Singh, H. pH-Sensitive Biocompatible Nanoparticles of Paclitaxel-Conjugated Poly(styrene-co-maleic acid) for Anticancer Drug Delivery in Solid Tumors of Syngeneic Mice. ACS Appl. Mater. Interfaces 2015, 7, 26530–26548. [Google Scholar] [CrossRef]
- Wu, F.; Jin, T. Polymer-Based Sustained-Release Dosage Forms for Protein Drugs, Challenges, and Recent Advances. AAPS PharmSciTech 2008, 9, 1218–1229. [Google Scholar] [CrossRef]
- Mansour, A.; Romani, M.; Acharya, A.B.; Rahman, B.; Verron, E.; Badran, Z. Drug Delivery Systems in Regenerative Medicine: An Updated Review. Pharmaceutics 2023, 15, 695. [Google Scholar] [CrossRef]
- Asadi, N.; Del Bakhshayesh, A.R.; Davaran, S.; Akbarzadeh, A. Common biocompatible polymeric materials for tissue engineering and regenerative medicine. Mater. Chem. Phys. 2020, 242, 122528. [Google Scholar] [CrossRef]
- Pei, X.; Wang, J.; Cong, Y.; Fu, J. Recent progress in polymer hydrogel bioadhesives. J. Polym. Sci. 2021, 59, 1312–1337. [Google Scholar] [CrossRef]
- Xu, R.; Fang, Y.; Zhang, Z.; Cao, Y.; Yan, Y.; Gan, L.; Xu, J.; Zhou, G. Recent Advances in Biodegradable and Biocompatible Synthetic Polymers Used in Skin Wound Healing. Materials 2023, 16, 5459. [Google Scholar] [CrossRef]
- Zehetmaier, P.C.; Vagin, S.I.; Rieger, B. Functionalization of aliphatic polyketones. MRS Bull. 2013, 38, 239–244. [Google Scholar] [CrossRef]
- Bartsch, G.C.; Malinova, V.; Volkmer, B.E.; Hautmann, R.E.; Rieger, B. Biokompatibilität von CO-Alkene-Polymeren mit aus urologischen Geweben isolierten primären Zellen und undifferenzierten Zellen. BJU Int. 2007, 99, 447–453. [Google Scholar] [CrossRef]
- Knorr, L. Synthese von pyrrolderivaten. Ber. Dtsch. Chem. Ges. 1884, 17, 1635–1642. [Google Scholar] [CrossRef]
- Brubaker, M.M.; Coffman, D.D.; Hoehn, H.H. Synthesis and Characterization of Ethylene/Carbon Monoxide Copolymers, A New Class of Polyketones. J. Am. Chem. Soc. 1952, 74, 1509–1515. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Wu, S.W. Methyl Fluorosulphonyldifluoroacetate; a New Trifluoromethylating Agent. J. Chem. Soc. Chem. Commun. 1989, 11, 705–706. [Google Scholar] [CrossRef]
- Green, M.J.; Lucy, A.R.; Lu, S.; Paton, R. Functionalisation of alkene–carbon monoxide alternating copolymers via transketalisation reactions. J. Chem. Soc. Chem. Commun. 1994, 18, 2063. [Google Scholar] [CrossRef]
- Lu, S.; Paton, R.M.; Green, M.J.; Lucy, A.R. Synthesis and characterization of polyketoximes derived from alkene-carbon monoxide copolymers. Eur. Pol. J. 1996, 32, 1285. [Google Scholar] [CrossRef]
- Khansawai, P.; Paton, R.M.; Reed, D. Polyketones as alternating copolymers of carbon monoxide. Chem. Commun. 1999, 73, 1297. [Google Scholar] [CrossRef]
- Nozaki, K.; Kosaka, N.; Graubner, V.M.; Hiyama, T. Methylenation of an Optically Active γ-Polyketone: Synthesis of a New Class of Hydrocarbon Polymers with Main-Chain Chirality. Macromolecules 2001, 34, 6167–6168. [Google Scholar] [CrossRef]
- Reuter, P.; Fuhrmann, R.; Mucke, A.; Voegele, J.; Rieger, B.; Franke, R. PCO/alkene copolymers as a promising class of biocompatible materials, 1. Examination of the in vitro toxicity. Macromol. Biosci. 2003, 3, 123. [Google Scholar] [CrossRef]
- Matteoli, U.; Beghetto, V.; Scrivanti, A.; Aversa, M.; Bertoldini, M.; Bovo, S. An alternative stereoselective synthesis of (R)- and (S)-Rosaphen® via asymmetric catalytic hydrogenation. Chirality 2011, 23, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Araya-Hermosilla, R.; Lima, G.M.R.; Raffa, P.; Fortunato, G.; Pucci, A.; Flores, M.E.; Moreno-Villoslada, I.; Broekhuis, A.A.; Picchioni, F. Intrinsic self-healing thermoset through covalent and hydrogen bonding interactions. Eur. Polym. J. 2016, 81, 186–197. [Google Scholar] [CrossRef]
- Ratna, D. Handbook of Thermoset Resins; Smithers Rapra: Shawbury, UK, 2009. [Google Scholar]
- Vavasori, A.; Ronchin, L. Polyketones: Synthesis and Applications. In Encyclopedia of Polymer Science and Technology; Wiley: Hoboken, NJ, USA, 2017; pp. 1–41. [Google Scholar]
- Araya-Hermosilla, E.; Moreno-Villoslada, I.; Araya-Hermosilla, R.; Flores, M.E.; Raffa, P.; Biver, T.; Pucci, A.; Picchioni, F.; Mattoli, V. pH-Responsive Polyketone/5,10,15,20-Tetrakis-(Sulfonatophenyl)Porphyrin Supramolecular Submicron Colloidal Structures. Polymers 2020, 12, 2017. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, L.; Wu, Z.-Q. Alkyne-Palladium(II)-Catalyzed Living Polymerization of Isocyanides: An Exploration of Diverse Structures and Functions. Acc. Chem. Res. 2021, 54, 3953–3967. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, L.; Wu, Z.-Q. Helix-Induced Asymmetric Self-Assembly of π-Conjugated Block Copolymers: From Controlled Syntheses to Distinct Properties. Acc. Chem. Res. 2023, 56, 2954–2967. [Google Scholar] [CrossRef]
- Agostinelli, E.; Belli, F.; Tempera, G.; Mura, A.; Floris, G.; Toniolo, L.; Vavasori, A.; Fabris, S.; Momo, F.; Stevanato, R. Polyketone polymer: A new support for direct enzyme immobilization. J. Biotechnol. 2007, 127, 670–678. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.; Parlanti, P.; Gemmi, M.; Mattoli, V.; Di Pietro, S.; Iacopini, D.; Granchi, C.; Turchi, B.; Fratini, F.; Di Bussolo, V.; et al. Functionalized aliphatic polyketones with germicide activity. RSC Adv. 2022, 12, 35358–35366. [Google Scholar] [CrossRef]
- Cetinkaya, Y.; Falk, P.; Mayhall, C.G. Vancomycin-Resistant Enterococci Clin. Microbiol. Rev. 2000, 13, 686–707. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.P. Vancomycin: A History. Clin. Infect. Dis. 2006, 42, S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Dinu, V.; Lu, Y.; Weston, N.; Lithgo, R.; Coupe, H.; Channell, G.; Adams, G.G.; Torcello Gómez, A.; Sabater, C.; Mackie, A.; et al. The antibiotic vancomycin induces complexation and aggregation of gastrointestinal and submaxillary mucins. Sci. Rep. 2020, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J. Old and modern antibiotic structures with potential for today’s infections. ADMET DMPK 2022, 10, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Ottonello, A.; Wyllie, J.A.; Yahiaoui, O.; Sun, S.; Koelln, R.A.; Homer, J.A.; Johnson, R.M.; Murray, E.; Williams, P.; Bolla, J.R.; et al. Shapeshifting bullvalene-linked vancomycin dimers as effective antibiotics against multidrug-resistant gram-positive bacteria. Proc. Natl. Acad. Sci. USA 2023, 120, e22087371. [Google Scholar] [CrossRef] [PubMed]
- Willems, R.P.J.; Van Dijk, K.; Vehreschild, M.J.G.T.; Biehl, L.M.; Ket, J.C.F.; Remmelzwaal, S.; Vandenbroucke-Grauls, C.M.J.E. Incidence of infection with multidrug-resistant Gram-negative bacteria and vancomycin-resistant enterococci in carriers: A systematic review and meta-regression analysis. Lancet Infect. Dis. 2023, 23, 719–731. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, R.F.; Leong, K.W.C.; Cumming, V.; Van Hal, S. Vancomycin-resistant Enterococcus faecium and the emergence of new sequence types associated with hospital infection. J. Res. Microbiol. 2023, 174, 104046. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hetjens, L.; Wolter, N.; Li, H.; Shi, X.; Pich, A. Charge-reversible and biodegradable chitosan-based microgels for lysozyme-triggered release of vancomycin. J. Adv. Res. 2023, 43, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Mejuto, A.; Magariños, B.; Ferreira-Gonçalves, T.; Starbird-Pérez, R.; Álvarez-Lorenzo, C.; Reis, C.P.; Ardao, I.; García-González, C.A. Vancomycin-loaded methylcellulose aerogel scaffolds for advanced bone tissue engineering. Carbohydr. Polym. 2024, 324, 121536. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Hu, Y.; Li, X. Polymer mechanochemistry in drug delivery: From controlled release to precise activation. J. Control Release 2024, 365, 259–273. [Google Scholar] [CrossRef]
- Ruiz, J.C.; Alvarez-Lorenzo, C.; Taboada, P.; Burillo, G.; Bucio, E.; De Prijck, K.; Nelis, H.J.; Coenye, T.; Concheiro, A. Polypropylene grafted with smart polymers (PNIPAAm/PAAc) for loading and controlled release of vancomycin. Eur. J. Pharm. Biopharm. 2008, 70, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Zakeri-Milani, P.; Loveymi, B.D.; Jelvehgari, M.; Valizadeh, H. The characteristics and improved intestinal permeability of vancomycin PLGA-nanoparticles as colloidal drug delivery system. Colloids Surf. B. 2013, 103, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Yousry, C.; Elkheshen, S.A.; El-laithy, H.M.; Essam, T.; Fahmy, R.H. Studying the influence of formulation and process variables on Vancomycin-loaded polymeric nanoparticles as potential carrier for enhanced ophthalmic delivery. Eur. J. Pharm. Sci. 2017, 100, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Hassan, D.; Omolo, C.A.; Fasiku, V.O.; Mocktar, C.; Govender, T. Novel chitosan-based pH-responsive lipid-polymer hybrid nanovesicles (OLA-LPHVs) for delivery of vancomycin against methicillin-resistant Staphylococcus aureus infections. Int. J. Biol. Macromol. 2020, 147, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Thamvasupong, P.; Viravaidya-Pasuwat, K. Controlled Release Mechanism of Vancomycin from Double-Layer Poly-L-Lactic Acid-Coated Implants for Prevention of Bacterial Infection. Polymers 2022, 14, 3493. [Google Scholar] [CrossRef]
- Sahiner, M.; Yilmaz, A.S.; Ayyala, R.S.; Sahiner, N. Carboxymethyl Chitosan Microgels for Sustained Delivery of Vancomycin and Long-Lasting Antibacterial Effects. Gels 2023, 9, 708. [Google Scholar] [CrossRef]
- Liu, W.-B.; Gao, R.-T.; Zhou, L.; Liu, N.; Chen, Z.; Wu, Z.-Q. Combination of vancomycin and guanidinium-functionalized helical polymers for synergistic antibacterial activity and biofilm ablation. Chem. Sci. 2022, 13, 10375–10382. [Google Scholar] [CrossRef]
- Sezer, A.D.; Kazak Sarılmışer, H.; Rayaman, E.; Çevikbaş, A.; Öner Akbuğa, E.T.J. Development and characterization of vancomycin-loaded levan-based microparticular system for drug delivery. Pharm. Dev. Technol. 2017, 22, 627–634. [Google Scholar] [CrossRef]
- Vinod, L.A.; Rajendran, D.; Shivashankar, M.; Chandrasekaran, N. Surface interaction of vancomycin with polystyrene microplastics and its effect on human serum albumin. Int. J. Biol. Macromol. 2024, 256, 128491. [Google Scholar] [CrossRef]
- Vavasori, A.; Ronchin, L.; Quartarone, G.; Tortato, C. The catalytic copolymerization of ethene with carbon monoxide efficiently carried out in water/dichloromethane/sodium dodecylsulfate emulsion. Mod. Res. Catal. 2013, 2, 93–99. [Google Scholar] [CrossRef]
- Vavasori, A.; Toniolo, L. Carbon monoxide-ethylene copolymerization catalyzed by a Pd(AcO)2/dppp/TsOH1 system: The promoting effect of water and of the acid. J. Mol. Catal. A Chem. 1996, 110, 13–23. [Google Scholar] [CrossRef]
- Ataollahi, N.; Girardi, F.; Cappelletto, E.; Vezzù, K.; Di Noto, V.; Scardi, P.; Callone, E.; Di Maggio, R. Chemical modification and structural rearrangements of polyketone-based polymer membrane. J. Appl. Polym. Sci. 2017, 134, 45485. [Google Scholar] [CrossRef]
- Ataollahi, N.; Vezzù, K.; Nawn, G.; Pace, G.; Cavinato, G.; Girardi, F.; Scardi, P.; Di Noto, V.; Di Maggio, R. A Polyketone-based Anion Exchange Membrane for Electrochemical Applications: Synthesis and Characterization. Electrochim. Acta. 2017, 226, 148–157. [Google Scholar] [CrossRef]
- Sole, R.; Gatto, V.; Conca, S.; Bardella, N.; Morandini, A.; Beghetto, V. Sustainable Triazine-Based Dehydro-Condensation Agents for Amide Synthesis. Molecules 2021, 26, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Toncelli, C.; Schoonhoven, M.J.; Broekhuis, A.A.; Picchioni, F. Paal-Knorr kinetics in waterborne polyketone-based formulations as modulating cross-linking tool in electrodeposition coatings. Mater. Des. 2016, 108, 718–724. [Google Scholar] [CrossRef]
- Scrivanti, A.; Sole, R.; Bortoluzzi, M.; Beghetto, V.; Bardella, N.; Dolmella, A. Synthesis of new triazolyl-oxazoline chiral ligands and study of their coordination to Pd(II) metal centers. Inorganica Chim. Acta 2019, 498, 119129. [Google Scholar] [CrossRef]
- Ferreira, I.S.; Bettencourt, A.F.; Gonçalves, L.M.D.; Kasper, S.; Bétrisey, B.; Kikhney, J.; Moter, A.; Trampuz, A.; Almeida, A.J. Activity of daptomycin- and vancomycin-loaded poly-epsilon-caprolactone microparticles against mature staphylococcal biofilms. Nanomed. J. 2015, 10, 4351–4366. [Google Scholar] [CrossRef]
- Le Ray, A.-M.; Chiffoleau, S.; Iooss, P.; Grimandi, G.; Gouyette, A.; Daculsi, G.; Merle, C. Vancomycin encapsulation in biodegradable poly(ε-caprolactone) microparticles for bone implantation. Influence of the formulation process on size, drug loading, in vitro release and cytocompatibility. Biomaterials 2003, 24, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Ebrahimi, P.; Hadianamrei, R. Optimization of particle size and encapsulation efficiency of vancomycin nanoparticles by response surface methodology. Pharm. Dev. Technol. 2014, 19, 987–998. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Mocktar, C.; Sikwal, D.R.; Sonawane, S.J.; Kathiravan, M.K.; Skelton, A.; Govender, T. Ion pairing with linoleic acid simultaneously enhances encapsulation efficiency and antibacterial activity of vancomycin in solid lipid nanoparticles. Colloids Surf. B Biointerfaces 2014, 117, 303–311. [Google Scholar] [CrossRef]
- Takacs-Novak, K.; Noszal, B.; Tòkés-Kovesdi, M.; Sz6sz, G. Acid-base properties and proton-speciation of vancomycin. Int. J. Pharm. 1993, 89, 261–263. [Google Scholar] [CrossRef]
- Flores-Rojas, G.G.; Vázquez, E.; López-Saucedo, F.; Buendia-Gonzalez, L.; Vera-Graziano, R.; Mendizabal, E.; Bucio, E. Lignocellulosic membrane grafted with 4-vinylpiridine using radiation chemistry: Antimicrobial activity of loaded vancomycin. Cellulose 2023, 30, 3853–3868. [Google Scholar] [CrossRef]
- Bil, M.; Jurczyk-Kowalska, M.; Kopeć, K.; Heljak, M. Study of Correlation between Structure and Shape-Memory Effect/Drug-Release Profile of Polyurethane/Hydroxyapatite Composites for Antibacterial Implants. Polymers 2023, 15, 938. [Google Scholar] [CrossRef] [PubMed]
- Avila-Novoa, M.G.; Solis-Velazquez, O.A.; Guerrero-Medina, P.J.; González-Gómez, J.P.; González-Torres, B.; Velázquez-Suárez, N.Y.; Martínez-Chávez, L.; Martínez-Gonzáles, N.E.; De la Cruz-Color, L.; Ibarra-Velázquez, L.M.; et al. Genetic and compositional analysis of biofilm formed by Staphylococcus aureus isolated from food contact surfaces. Front. Microbiol. 2022, 13, 1001700. [Google Scholar] [CrossRef]
- Beghetto, V.; Gatto, V.; Samiolo, R.; Scolaro, C.; Brahimi, S.; Facchin, M.; Visco, A. Plastics today: Key challenges and EU strategies towards carbon neutrality: A review. Environ. Pollut. 2023, 334, 122102. [Google Scholar] [CrossRef]

| PKSK | PKSK-VCM pH 2.3 | PKSK-VCM pH 5 | PKSK-VCM pH 8.8 | Functional Groups | Note |
|---|---|---|---|---|---|
| Wavenumbers (cm−1) | |||||
| 685 | x | x | x | ẟ C=C ẟ C=C-H | Strong |
| 813, 832 | 806 | 806 | 806 | ẟ C=C-H ẟ C=C | Weak |
| 1010, 1035, 1057 | 1015, 1035, 1057 | 1015, 1035, 1057 | 1015, 1035, 1057 | ν S=O ν C-N ẟ C=C-H | Change in relative intensity |
| 1124 | 1124 | 1124 | 1124 | ν C-N | |
| 1164 | ~1195 | ~1195 | ~1195 | ν C-N ν C=O | Sharp for PKSK, broad for all other samples |
| 1246, 1259 | 1260 | 1260 | 1260 | ν C=O ν C-N | Change in relative intensity |
| 1335 | 1335 | 1335 | 1335 | ν S=O ẟ C-H | |
| x | x | 1384 | 1384 | ν S=O | |
| 1408, 1426 | 1408, 1426 | 1408, 1426 | 1408, 1426 | ν S=O ν C=C-H | |
| 1499 | 1499 | 1499 | 1499 | ẟ C=C-H | Change in relative intensity |
| 1693 | 1693 | 1693 | 1693 | ν C=O | |
| 2852, 2912, 2963 | 2852, 2912, 2963 | 2852, 2912, 2963 | 2852, 2912, 2963 | ν C-H | |
| 3437 | 3437 | 3437 | 3437 | ν OH | |
| PKT | PKT-VCM pH 2.3 | PKT-VCM pH 5 | PKT-VCM pH 8.8 | Functional Groups | Note |
|---|---|---|---|---|---|
| Wavenumbers (cm−1) | |||||
| 812 | 812 | 812 | 812 | ẟ C=C-H ẟ C=C | |
| 1054 | 1023, 1054, 1095 | 1023, 1054, 1095 | 1023, 1054, 1095 | ν S=O ν C-N ẟ C=C-H | 1023 and 1095 shoulders of the central peak |
| 1198 | ~1198 | ~1198 | ~1198 | ν C-N ν C=O | Broader for PKT-VCM samples |
| 1259 | 1259 | 1259 | 1259 | ν C=O ν C-N | |
| 1336 | 1336 | 1336 | 1336 | ν S=O ẟ C-H | |
| x | 1384 | 1384 | 1384 | ν S=O | |
| 1409, 1427 | 1409, 1427 | 1409, 1427 | 1409, 1427 | ν S=O ν C=C-H | |
| 1470 | 1470 | 1470 | 1470 | ẟ C=C-H | Weak and broad change in relative intensity |
| 1693 | 1693 | 1693 | 1693 | ν C=O | |
| 2853, 2913, 2953 | 2852, 2918, 2962 | 2852, 2918, 2962 | 2852, 2918, 2962 | ν C-H | |
| ~3439 | ~3439 | ~3439 | ~3439 | ν OH | |
| Sample | pH | Elementals (%) | EE% | LC% | |
|---|---|---|---|---|---|
| N | S | ||||
| PKSK | - | 4.02 ± 0.01 | 9.31 ± 0.02 | - | - |
| PKSK-VCM | 2.3 | 4.36 ± 0.02 | 7.67 ± 0.01 | 41.25 ± 0.03 | 23.08 ± 0.01 |
| PKSK-VCM | 5.0 | 7.13 ± 0.01 | 2.37 ± 0.03 | 75.00 ± 0.02 | 31.03 ± 0.01 |
| PKSK-VCM | 8.8 | 5.86 ± 0.01 | 5.10 ± 0.01 | 66.66 ± 0.01 | 25.00 ± 0.02 |
| PKT | - | 1.66 ± 0.03 | 4.23 ± 0.02 | - | - |
| PKT-VCM | 2.3 | 2.10 ± 0.01 | 3.75 ± 0.03 | 23.08 ± 0.01 | 10.71 ± 0.02 |
| PKT-VCM | 5.0 | 7.73 ± 0.02 | 1.12 ± 0.01 | 80.00 ± 0.02 | 31.58 ± 0.01 |
| PKT-VCM | 8.8 | 3.66 ± 0.03 | 3.41 ± 0.03 | 42.67 ± 0.02 | 15.15 ± 0.03 |
| Sample | pH | MIC (µg/mL) |
|---|---|---|
| PKSK-VCM | 2.3 | 1.44 |
| PKSK-VCM | 5.0 | 0.23 |
| PKSK-VCM | 8.8 | 1.56 |
| PKT-VCM | 2.3 | 2.68 |
| PKT-VCM | 5.0 | 0.24 |
| PKT-VCM | 8.8 | 3.79 |
| VCM | 1.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rampazzo, R.; Vavasori, A.; Ronchin, L.; Riello, P.; Marchiori, M.; Saorin, G.; Beghetto, V. Enhanced Antibacterial Activity of Vancomycin Loaded on Functionalized Polyketones. Polymers 2024, 16, 1890. https://doi.org/10.3390/polym16131890
Rampazzo R, Vavasori A, Ronchin L, Riello P, Marchiori M, Saorin G, Beghetto V. Enhanced Antibacterial Activity of Vancomycin Loaded on Functionalized Polyketones. Polymers. 2024; 16(13):1890. https://doi.org/10.3390/polym16131890
Chicago/Turabian StyleRampazzo, Rachele, Andrea Vavasori, Lucio Ronchin, Pietro Riello, Martina Marchiori, Gloria Saorin, and Valentina Beghetto. 2024. "Enhanced Antibacterial Activity of Vancomycin Loaded on Functionalized Polyketones" Polymers 16, no. 13: 1890. https://doi.org/10.3390/polym16131890
APA StyleRampazzo, R., Vavasori, A., Ronchin, L., Riello, P., Marchiori, M., Saorin, G., & Beghetto, V. (2024). Enhanced Antibacterial Activity of Vancomycin Loaded on Functionalized Polyketones. Polymers, 16(13), 1890. https://doi.org/10.3390/polym16131890







