Unveiling the Impact of Gelation Temperature on the Rheological and Microstructural Properties of Type A Gelatin Hydrogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Type A Gelatin
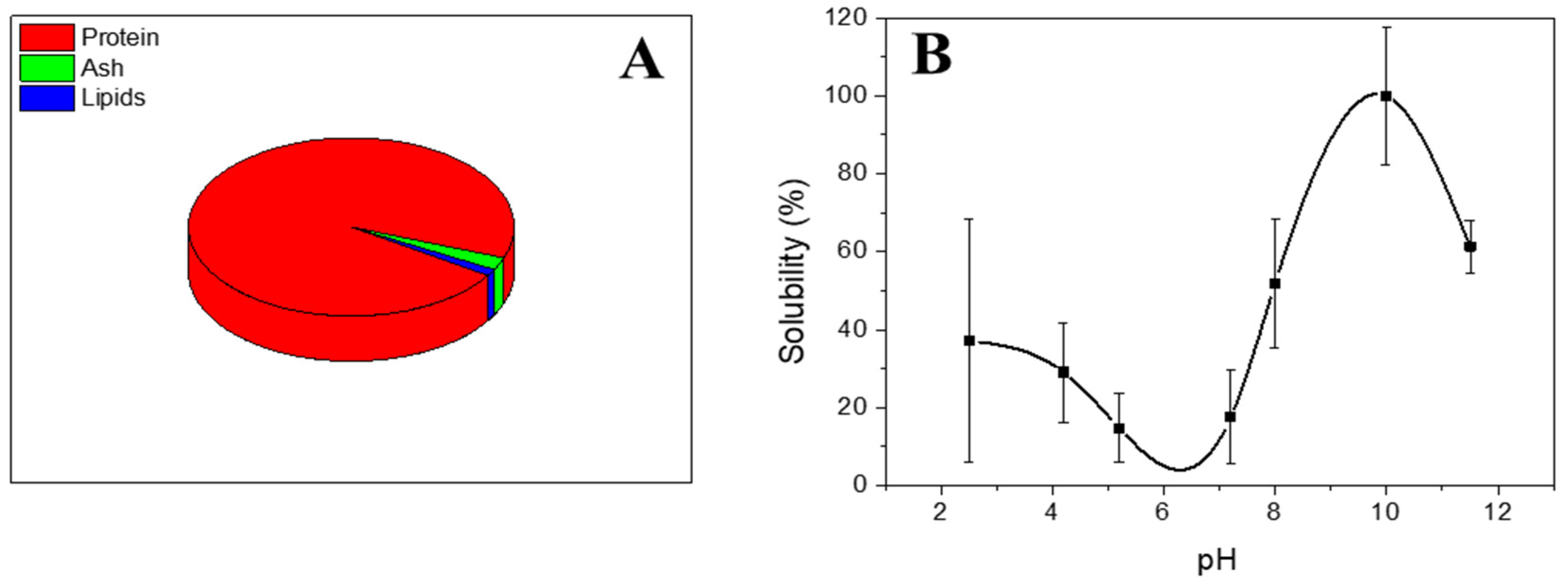
2.2.1. Chemical Composition
2.2.2. Protein Solubility
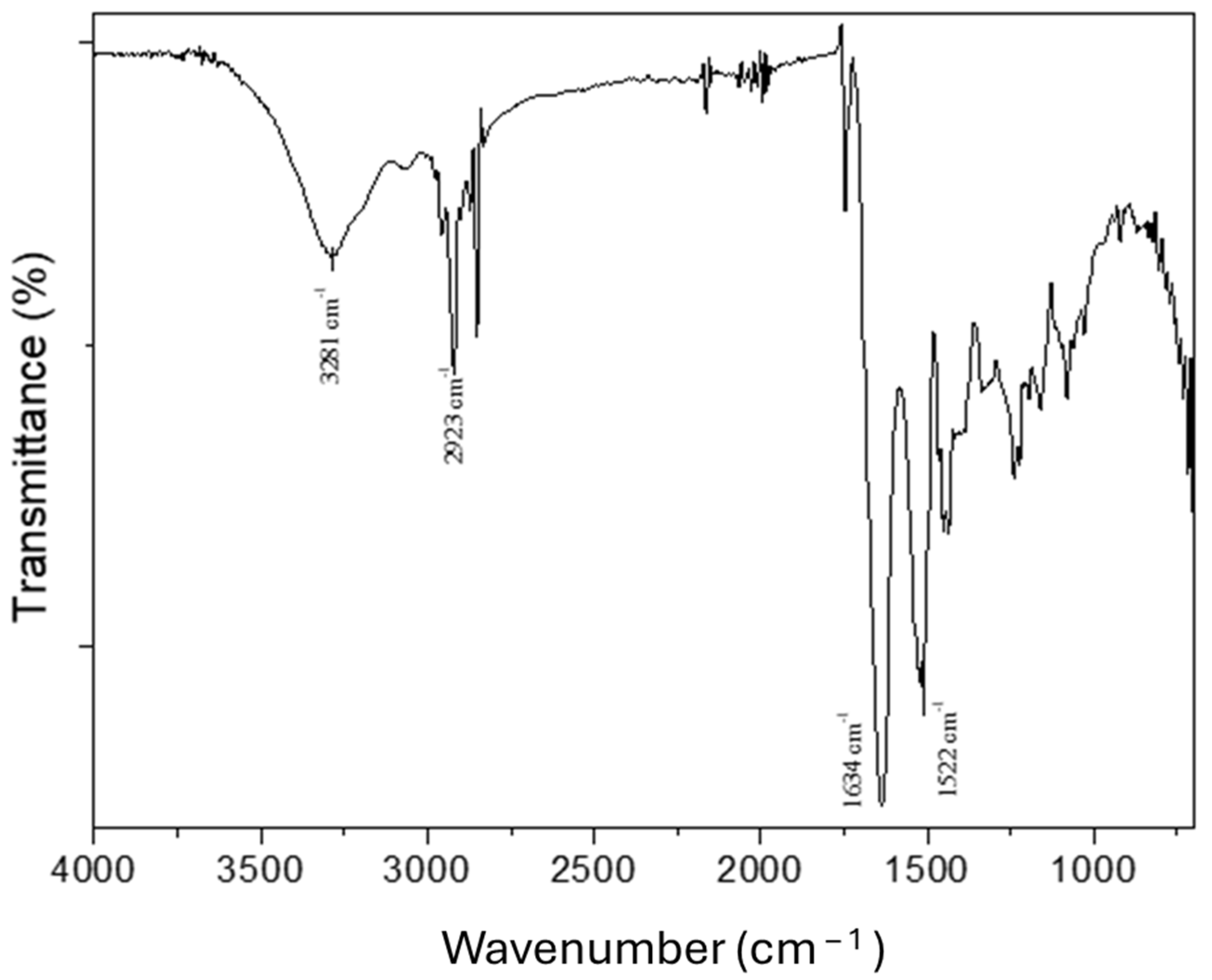
2.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.3. Formation of Hydrogels
2.4. Hydrogel Characterization
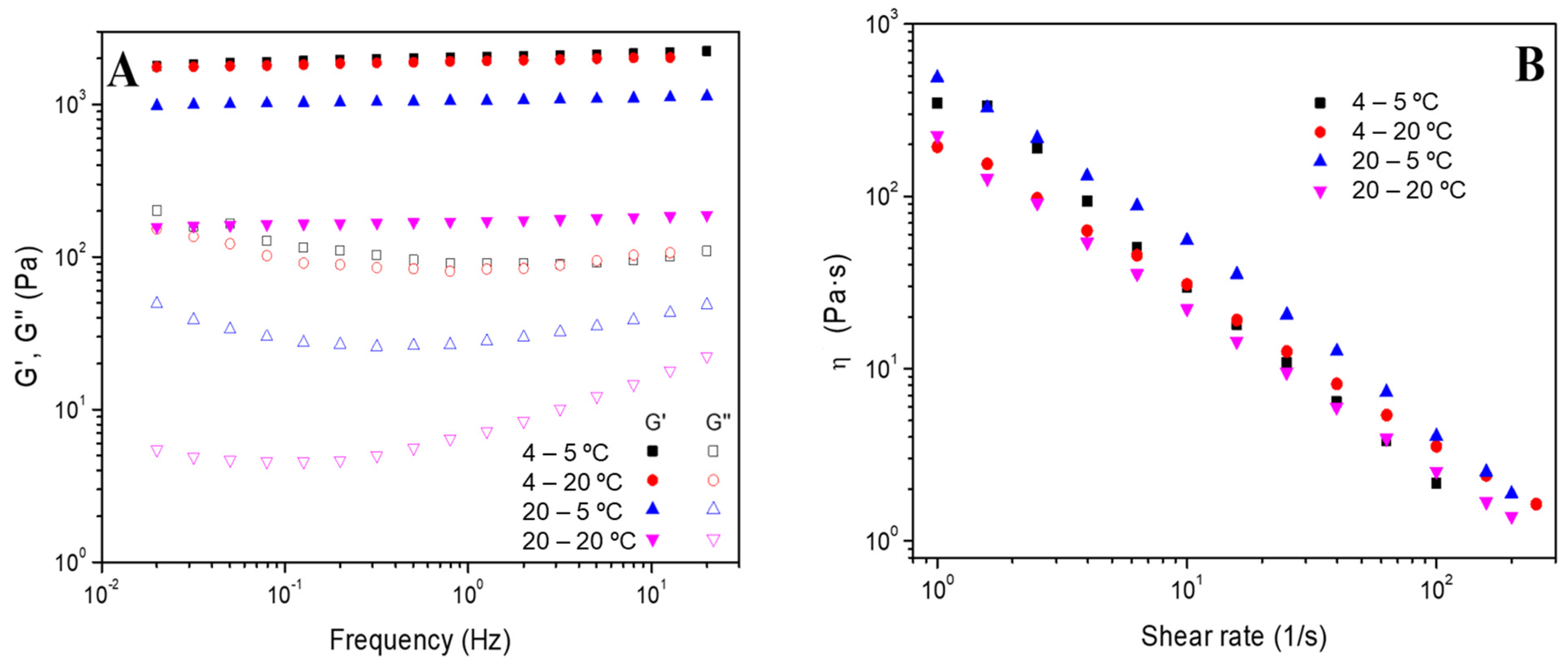
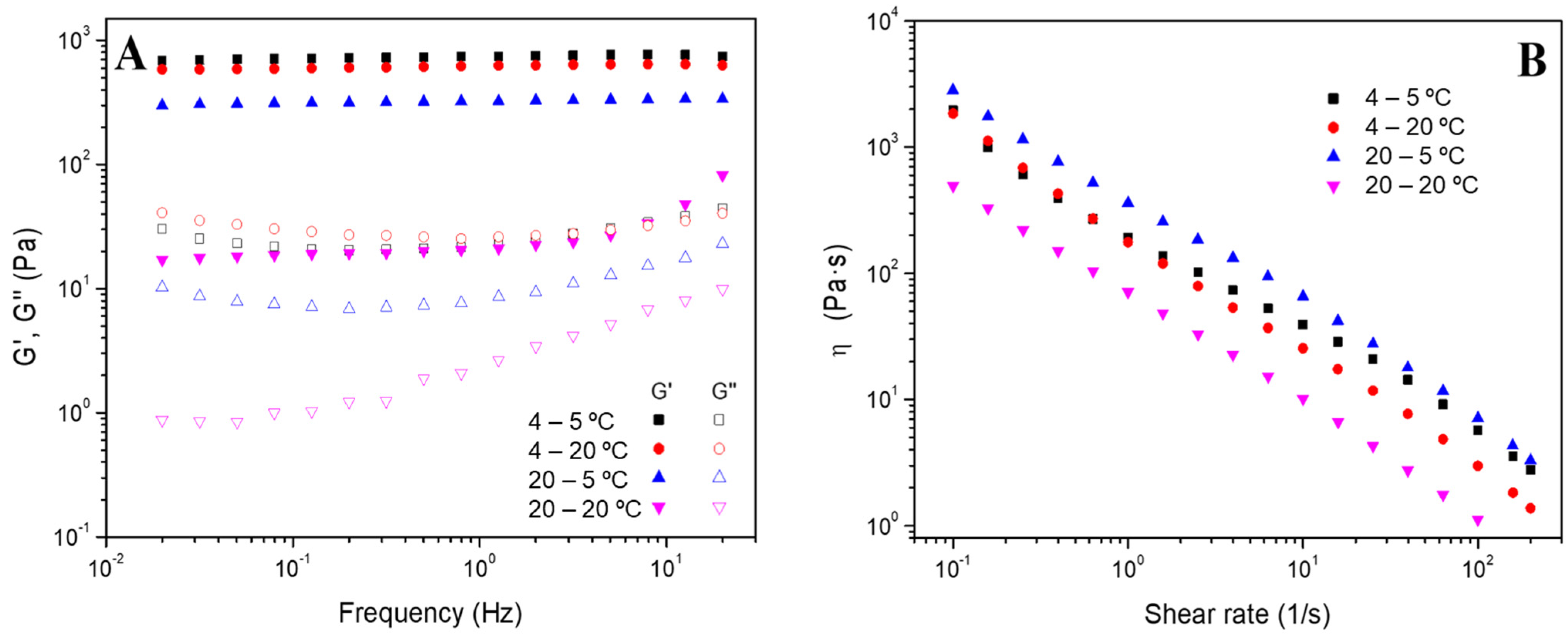
2.4.1. Rheological Evaluation
- Strain sweep tests: Measurements among 0.1% and 100% strain at a consistent frequency of 1 Hz and different temperatures (5 and 20 °C) were performed to obtain the linear viscoelastic range (interval where the elastic and viscous moduli are independent of the strain applied) and the critical strain (the maximum strain supported by the sample within the linear viscoelastic range).
- Frequency sweep tests: The measurements were performed in a frequency interval between 0.02 and 20 Hz at a particular strain for each system (inside the linear viscoelastic range) and different temperatures (5 and 20 °C). In these evaluations, the elastic and viscous moduli (G′ and G″, respectively) were acquired, together with the loss tangent (tan δ = G″/G′). Furthermore, the values for G’ and tan δ at 1 Hz (G′1 and tan δ1) were selected and tabulated.
- Flow curves: These tests were performed between 0.1 and 200 s−1 of shear rate and at a fixed strain of 3%. These tests enable the classification of the systems as Newtonian, shear thinning or shear thickening according to the dependence of viscosity (η) on shear rate [34].
2.4.2. Morphological Evaluation
2.5. Statistical Analysis
3. Results and Discussions
3.1. Characterization of Type A Gelatin
3.2. Characterization of Gelatin-Based Hydrogels
3.2.1. Effect of the Gelation Temperature
3.2.2. Effect of the Addition of Tetracycline
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prajapati, S.K.; Jain, A.; Jain, A.; Jain, S. Biodegradable Polymers and Constructs: A Novel Approach in Drug Delivery. Eur. Polym. J. 2019, 120, 109191. [Google Scholar] [CrossRef]
- Salari, N.; Faraji, F.; Torghabeh, F.M.; Faraji, F.; Mansouri, K.; Abam, F.; Shohaimi, S.; Akbari, H.; Mohammadi, M. Polymer-Based Drug Delivery Systems for Anticancer Drugs: A Systematic Review. Cancer Treat. Res. Commun. 2022, 32, 100605. [Google Scholar] [CrossRef] [PubMed]
- Dethe, M.R.; A, P.; Ahmed, H.; Agrawal, M.; Roy, U.; Alexander, A. PCL-PEG Copolymer Based Injectable Thermosensitive Hydrogels. J. Control. Release 2022, 343, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef]
- Rana, D.; Desai, N.; Salave, S.; Karunakaran, B.; Giri, J.; Benival, D.; Gorantla, S.; Kommineni, N. Collagen-Based Hydrogels for the Eye: A Comprehensive Review. Gels 2023, 9, 643. [Google Scholar] [CrossRef]
- Shultz, R.; Zhong, Y. Hydrogel-Based Local Drug Delivery Strategies for Spinal Cord Repair. Neural Regen. Res. 2021, 16, 247–253. [Google Scholar] [PubMed]
- Xie, Y.; Liu, M.; Cai, C.; Ye, C.; Guo, T.; Yang, K.; Xiao, H.; Tang, X.; Liu, H. Recent Progress of Hydrogel-Based Local Drug Delivery Systems for Postoperative Radiotherapy. Front. Oncol. 2023, 13, 1027254. [Google Scholar] [CrossRef]
- Zhao, Y.; Ran, B.; Xie, X.; Gu, W.; Ye, X.; Liao, J. Developments on the Smart Hydrogel-Based Drug Delivery System for Oral Tumor Therapy. Gels 2022, 8, 741. [Google Scholar] [CrossRef]
- Webber, M.J.; Pashuck, E.T. (Macro)Molecular Self-Assembly for Hydrogel Drug Delivery. Adv. Drug Deliv. Rev. 2021, 172, 275–295. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in Drug Delivery: Progress and Challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- McKenzie, M.; Betts, D.; Suh, A.; Bui, K.; Kim, L.D.; Cho, H. Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs. Molecules 2015, 20, 20397–20408. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-Hydrogel: A Hybrid Biomaterial System for Localized Drug Delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef] [PubMed]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Fabrication and Characterization of Hydrogels Based on Gelatinised Collagen with Potential Application in Tissue Engineering. Polymers 2020, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
- Geanaliu-Nicolae, R.E.; Andronescu, E. Blended Natural Support Materials—Collagen Based Hydrogels Used in Biomedicine. Materials 2020, 13, 5641. [Google Scholar] [CrossRef] [PubMed]
- Copes, F.; Pien, N.; Van Vlierberghe, S.; Boccafoschi, F.; Mantovani, D. Collagen-Based Tissue Engineering Strategies for Vascular Medicine. Front. Bioeng. Biotechnol. 2019, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Petros, S.; Tesfaye, T.; Ayele, M. A Review on Gelatin Based Hydrogels for Medical Textile Applications. J. Eng. 2020, 2020, 8866582. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Martínez-Puig, D.; Costa-Larrión, E.; Rubio-Rodríguez, N.; Gálvez-Martín, P. Collagen Supplementation for Joint Health: The Link between Composition and Scientific Knowledge. Nutrients 2023, 15, 1332. [Google Scholar] [CrossRef]
- Kapoor, D.; Verma, K.; Jain, S.; Sharma, S. Gelatin-Based Hydrogels for Drug Delivery: A Recent Update. In Biomaterial-Based Hydrogels; Springer: Singapore, 2024; pp. 67–87. ISBN 978-981-99-8826-6. [Google Scholar]
- Nejat, H.; Rabiee, M.; Varshochian, R.; Tahriri, M.; Jazayeri, H.E.; Rajadas, J.; Ye, H.; Cui, Z.; Tayebi, L. Preparation and Characterization of Cardamom Extract-Loaded Gelatin Nanoparticles as Effective Targeted Drug Delivery System to Treat Glioblastoma. React. Funct. Polym. 2017, 120, 46–56. [Google Scholar] [CrossRef]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef]
- Padhi, J.R. Preparation and Characterization of Novel Gelatin and Carrageenan Based Hydrogels for Topical Delivery. Doctoral Dissertation, Biju Patnaik University of Technology Rourkela Odisha, Odisha, India, 2015. [Google Scholar]
- Andreazza, R.; Morales, A.; Pieniz, S.; Labidi, J. Gelatin-Based Hydrogels: Potential Biomaterials for Remediation. Polymers 2023, 15, 1026. [Google Scholar] [CrossRef]
- Mehdi-Sefiani, H.; Perez-Puyana, V.; Ostos, F.J.; Sepúlveda, R.; Romero, A.; Rafii-El-Idrissi Benhnia, M.; Chicardi, E. Type-A Gelatin-Based Hydrogel Infiltration and Degradation in Titanium Foams as a Potential Method for Localised Drug Delivery. Polymers 2023, 15, 275. [Google Scholar] [CrossRef] [PubMed]
- Hermida-Merino, C.; Cabaleiro, D.; Lugo, L.; Valcarcel, J.; Vázquez, J.A.; Bravo, I.; Longo, A.; Salloum-Abou-jaoude, G.; Solano, E.; Gracia-Fernández, C.; et al. Characterization of Tuna Gelatin-Based Hydrogels as a Matrix for Drug Delivery. Gels 2022, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, H.; Persson, S.; Caram-Lelham, N. How Interactions between Drugs and Agarose-Carrageenan Hydrogels Influence the Simultaneous Transport of Drugs. J. Control. Release 1999, 59, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Svirskis, D.; Waterhouse, G.I.N.; Wu, Z. Hydroxypropyl Methylcellulose Bioadhesive Hydrogels for Topical Application and Sustained Drug Release: The Effect of Polyvinylpyrrolidone on the Physicomechanical Properties of Hydrogel. Pharmaceutics 2023, 15, 2360. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2005; ISBN 9780935584752. [Google Scholar]
- Liang, Q.; Wang, L.; Sun, W.; Wang, Z.; Xu, J.; Ma, H. Isolation and Characterization of Collagen from the Cartilage of Amur Sturgeon (Acipenser Schrenckii). Process Biochem. 2014, 49, 318–323. [Google Scholar] [CrossRef]
- Perez-Puyana, V.M.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A.; Perez-Puyana, V.M.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Highly Porous Protein-Based 3D Scaffolds with Different Collagen Concentrates for Potential Application in Tissue Engineering. J. Appl. Polym. Sci. 2019, 136, 47954. [Google Scholar] [CrossRef]
- Markwell, M.A.K.; Haas, S.M.; Bieber, L.L.; Tolbert, N.E. A Modification of the Lowry Procedure to Simplify Protein Determination in Membrane and Lipoprotein Samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Rubio-Valle, J.F.; Romero, A.; Ostos, F.J.; Rafii-El-Idrissi Benhnia, M.; Perez-Puyana, V. Biocompatible and Thermoresistant Hydrogels Based on Collagen and Chitosan. Polymers 2022, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.M. Developments in Food Proteins. In Soy Proteins; Applied Science Publishers: London, UK, 1983; pp. 67–108. [Google Scholar]
- Duconseille, A.; Astruc, T.; Quintana, N.; Meersman, F.; Sante-Lhoutellier, V. Gelatin Structure and Composition Linked to Hard Capsule Dissolution: A Review. Food Hydrocoll. 2015, 43, 360–376. [Google Scholar] [CrossRef]
- Ratanavaraporn, J.; Rangkupan, R.; Jeeratawatchai, H.; Kanokpanont, S.; Damrongsakkul, S. Influences of Physical and Chemical Crosslinking Techniques on Electrospun Type A and B Gelatin Fiber Mats. Int. J. Biol. Macromol. 2010, 47, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Sarika, P.R.; Pavithran, A.; James, N.R. Cationized Gelatin/Gum Arabic Polyelectrolyte Complex: Study of Electrostatic Interactions. Food Hydrocoll. 2015, 49, 176–182. [Google Scholar] [CrossRef]
- Pérez-Puyana, V.M. Development of Novel Scaffolds from Nanostructured Biopolymer Matrices with Applications in Tissue Engineering. Ph.D. Thesis, Universidad de Sevilla, Sevilla, Spain, 2019. [Google Scholar]
- Jing, F.Y.; Zhang, Y.Q. Unidirectional Nanopore Dehydration Induces an Anisotropic Polyvinyl Alcohol Hydrogel Membrane with Enhanced Mechanical Properties. Gels 2022, 8, 803. [Google Scholar] [CrossRef]
- Chen, Q.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. An Interpenetrating Alginate/Gelatin Network for Three-Dimensional (3D) Cell Cultures and Organ Bioprinting. Molecules 2020, 25, 756. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gong, J.P. Design Principles for Strong and Tough Hydrogels. Nat. Rev. Mater. 2024, 9, 380–398. [Google Scholar] [CrossRef]
- Raja, I.S.; Fathima, N.N. Porosity and Dielectric Properties as Tools to Predict Drug Release Trends from Hydrogels. Springerplus 2014, 3, 393. [Google Scholar] [CrossRef]
- Yang, M.R.; Cheng, Y.T.; Tsai, H.C.; Darge, H.F.; Huang, C.C.; Lin, S.Y. Hofmeister Effect-Based Soaking Strategy for Gelatin Hydrogels with Adjustable Gelation Temperature, Mechanical Properties, and Ionic Conductivity. Biomater. Adv. 2023, 152, 213504. [Google Scholar] [CrossRef]

| SYSTEMS | Critical Strain (%) | |
|---|---|---|
| − | + Tetracycline | |
| Hydrogel 4–5 °C | 32.4 ± 10.3 a | 102.7 ± 0.2 d |
| Hydrogel 4–20 °C | 14.8 ± 0.3 b | 99.9 ± 0.1 e |
| Hydrogel 20–5 °C | 104.4 ± 0.5 c | 103.7 ± 0.7 cf |
| Hydrogel 20–20 °C | 9.2 ± 6.7 b | 13.0 ± 3.0 b |
| Systems | G′1 (Pa) | tan δ1 (−) | ||
|---|---|---|---|---|
| − | +Tetracycline | − | +Tetracycline | |
| Hydrogel 4–5 °C | 1983 ± 91 a | 692 ± 42 d | 0.04 ± 0.01 A | 0.03 ± 0.01 A |
| Hydrogel 4–20 °C | 1928 ± 86 a | 518 ± 95 c | 0.03 ± 0.01 A | 0.09 ± 0.01 B |
| Hydrogel 20–5 °C | 1044 ± 1 b | 371 ± 52 c | 0.03 ± 0.01 A | 0.02 ± 0.01 A |
| Hydrogel 20–20 °C | 300 ± 101 c | 20 ± 5 e | 0.03 ± 0.01 A | 0.04 ± 0.01 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehdi-Sefiani, H.; Chicardi, E.; Romero, A.; Perez-Puyana, V.M. Unveiling the Impact of Gelation Temperature on the Rheological and Microstructural Properties of Type A Gelatin Hydrogels. Polymers 2024, 16, 1842. https://doi.org/10.3390/polym16131842
Mehdi-Sefiani H, Chicardi E, Romero A, Perez-Puyana VM. Unveiling the Impact of Gelation Temperature on the Rheological and Microstructural Properties of Type A Gelatin Hydrogels. Polymers. 2024; 16(13):1842. https://doi.org/10.3390/polym16131842
Chicago/Turabian StyleMehdi-Sefiani, Hanaa, E. Chicardi, A. Romero, and Victor M. Perez-Puyana. 2024. "Unveiling the Impact of Gelation Temperature on the Rheological and Microstructural Properties of Type A Gelatin Hydrogels" Polymers 16, no. 13: 1842. https://doi.org/10.3390/polym16131842
APA StyleMehdi-Sefiani, H., Chicardi, E., Romero, A., & Perez-Puyana, V. M. (2024). Unveiling the Impact of Gelation Temperature on the Rheological and Microstructural Properties of Type A Gelatin Hydrogels. Polymers, 16(13), 1842. https://doi.org/10.3390/polym16131842








