Synthesis and Characterization of Maghemite Nanoparticles Functionalized with Poly(Sodium 4-Styrene Sulfonate) Saloplastic and Its Acute Ecotoxicological Impact on the Cladoceran Daphnia magna
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of γ-Fe2O3@PSSNa Core–Shell Arrangement
2.2. Characterization
2.3. D. magna Culture
2.4. D. magna Exposure Protocol and LC50 Acute Toxicity Determination
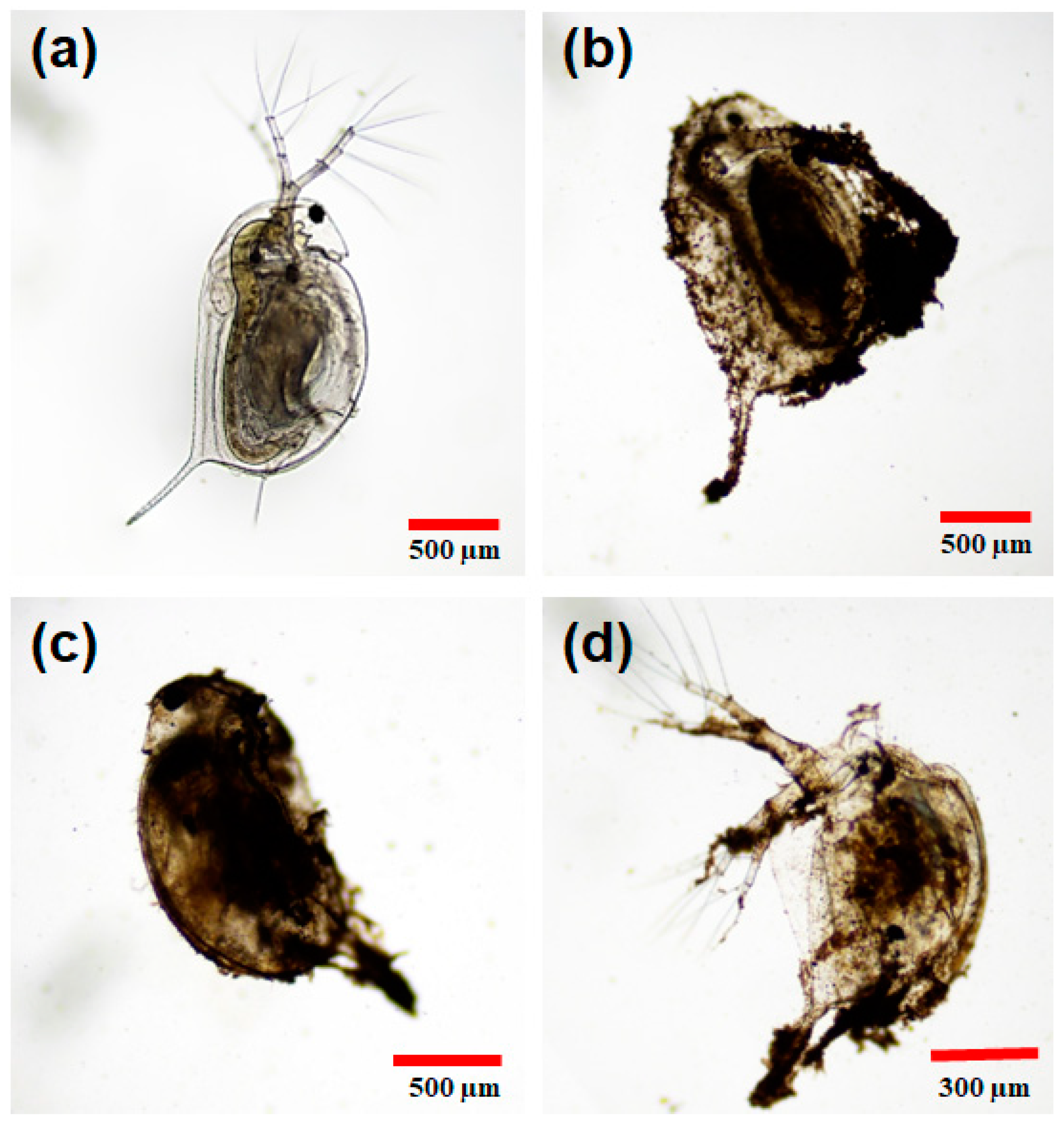
2.5. Morphological Evaluation in D. magna
3. Results and Discussion
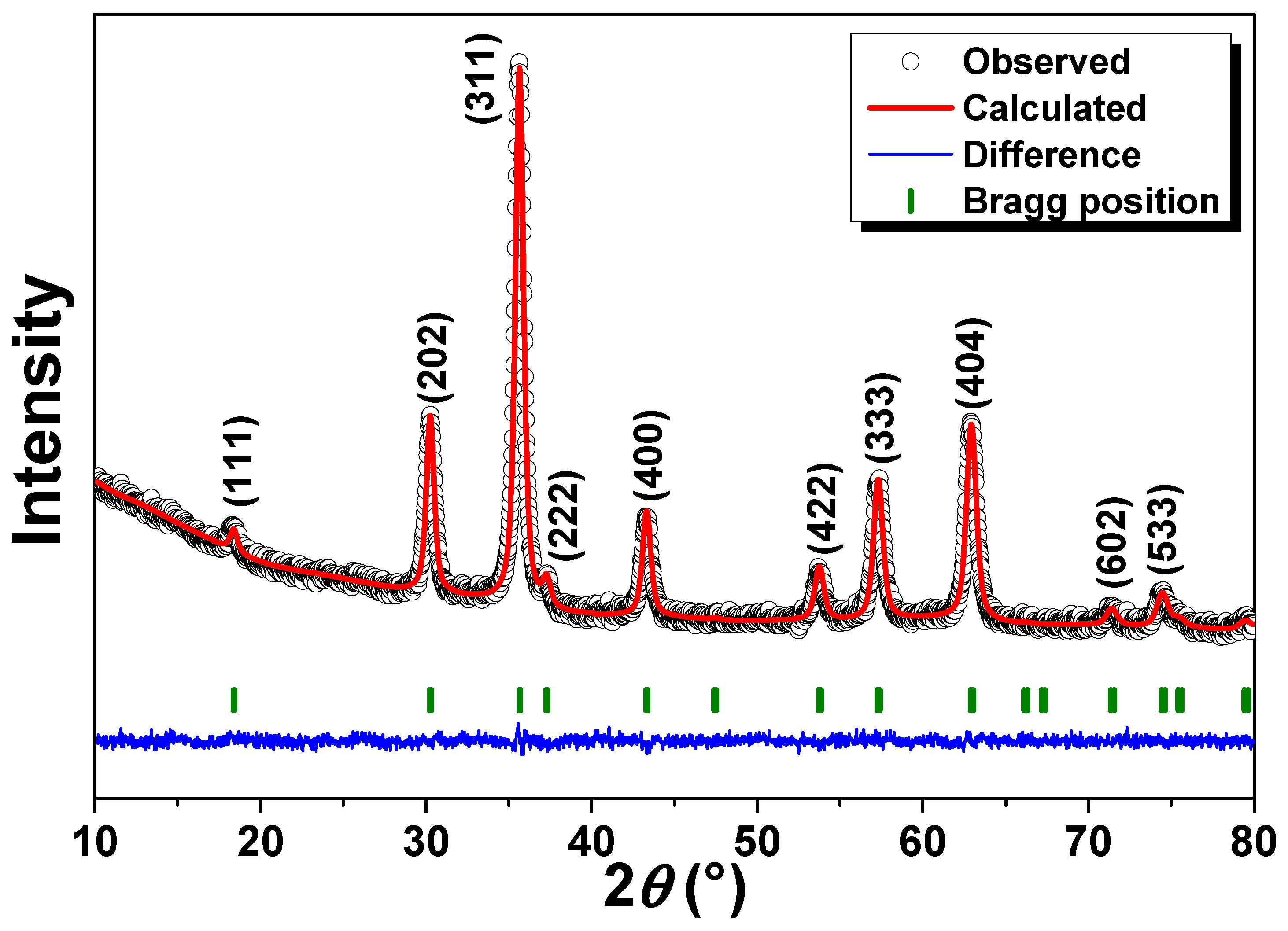
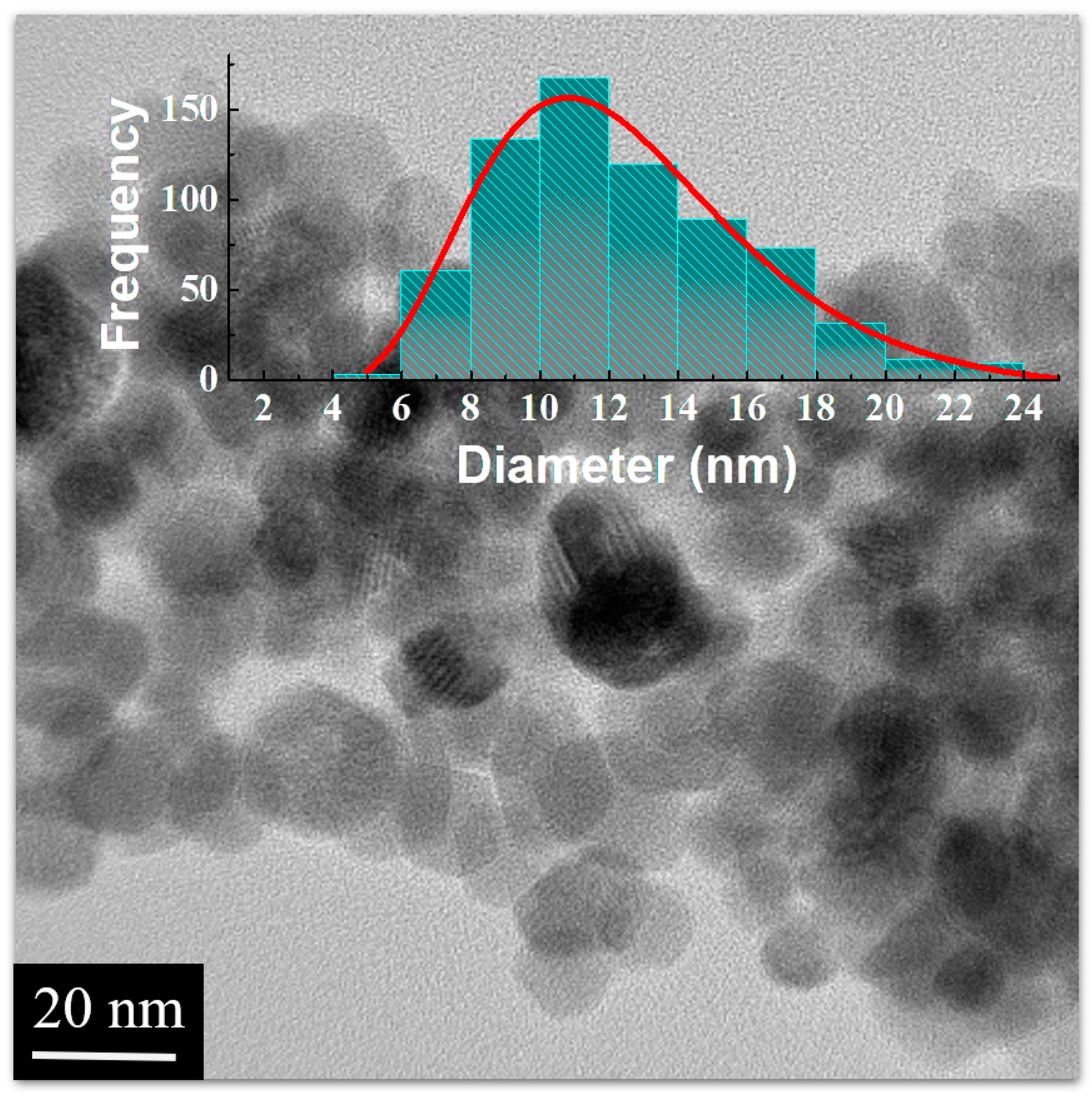
3.1. Rietveld Refinement and TEM Analysis
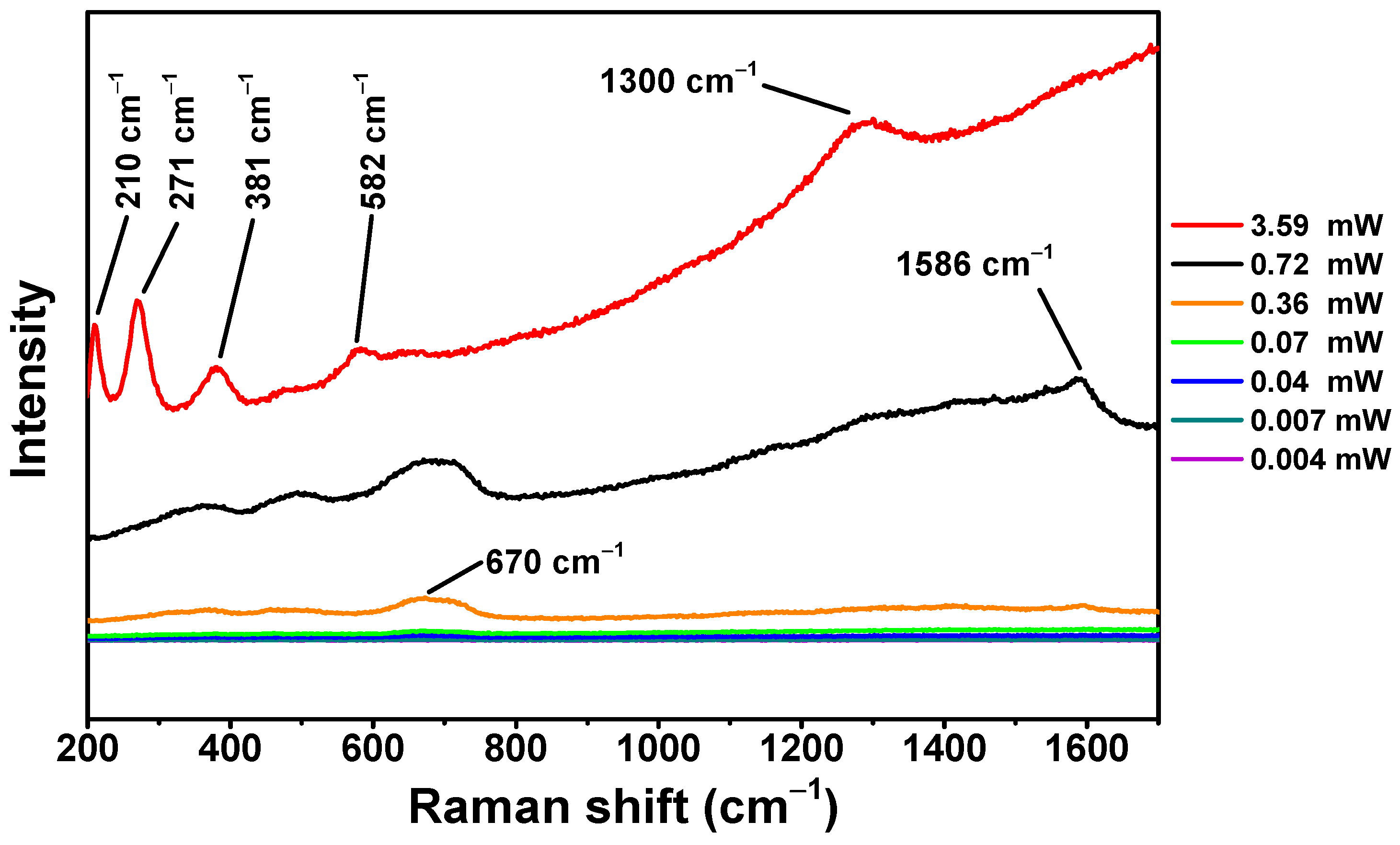
3.2. Raman Analysis
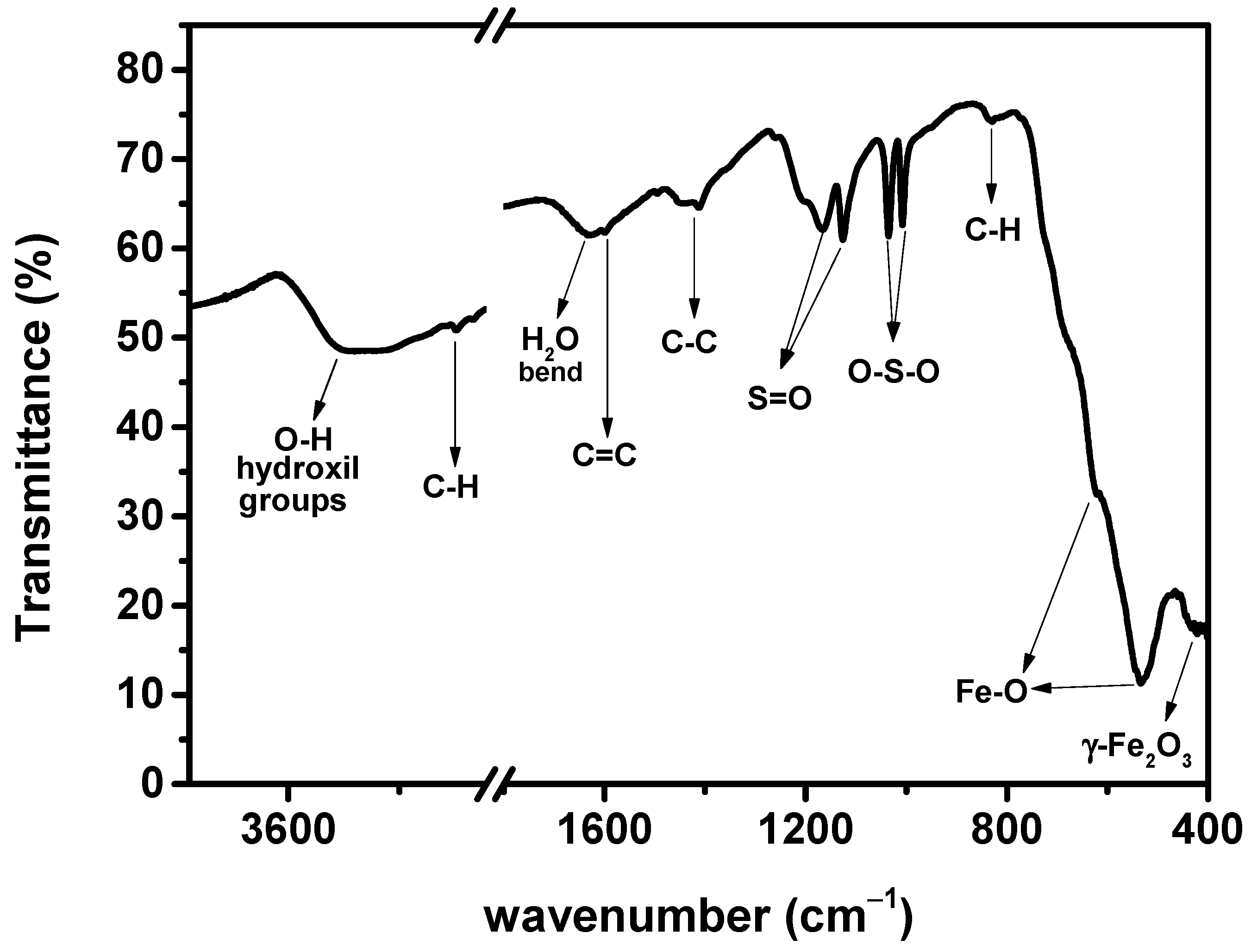
3.3. FTIR and Optical UV Vis Analysis
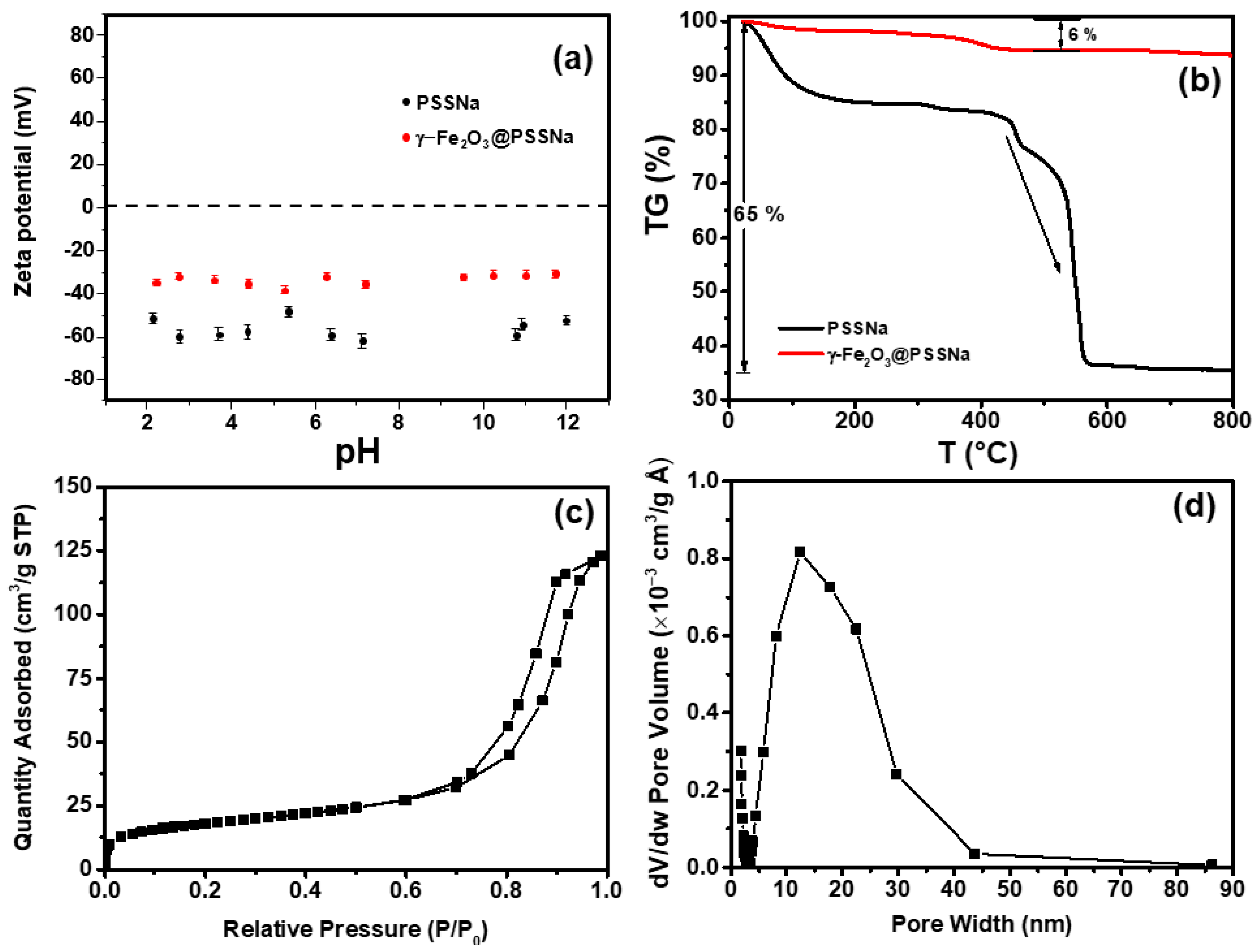
3.4. Colloidal, Thermal, and Textural Properties
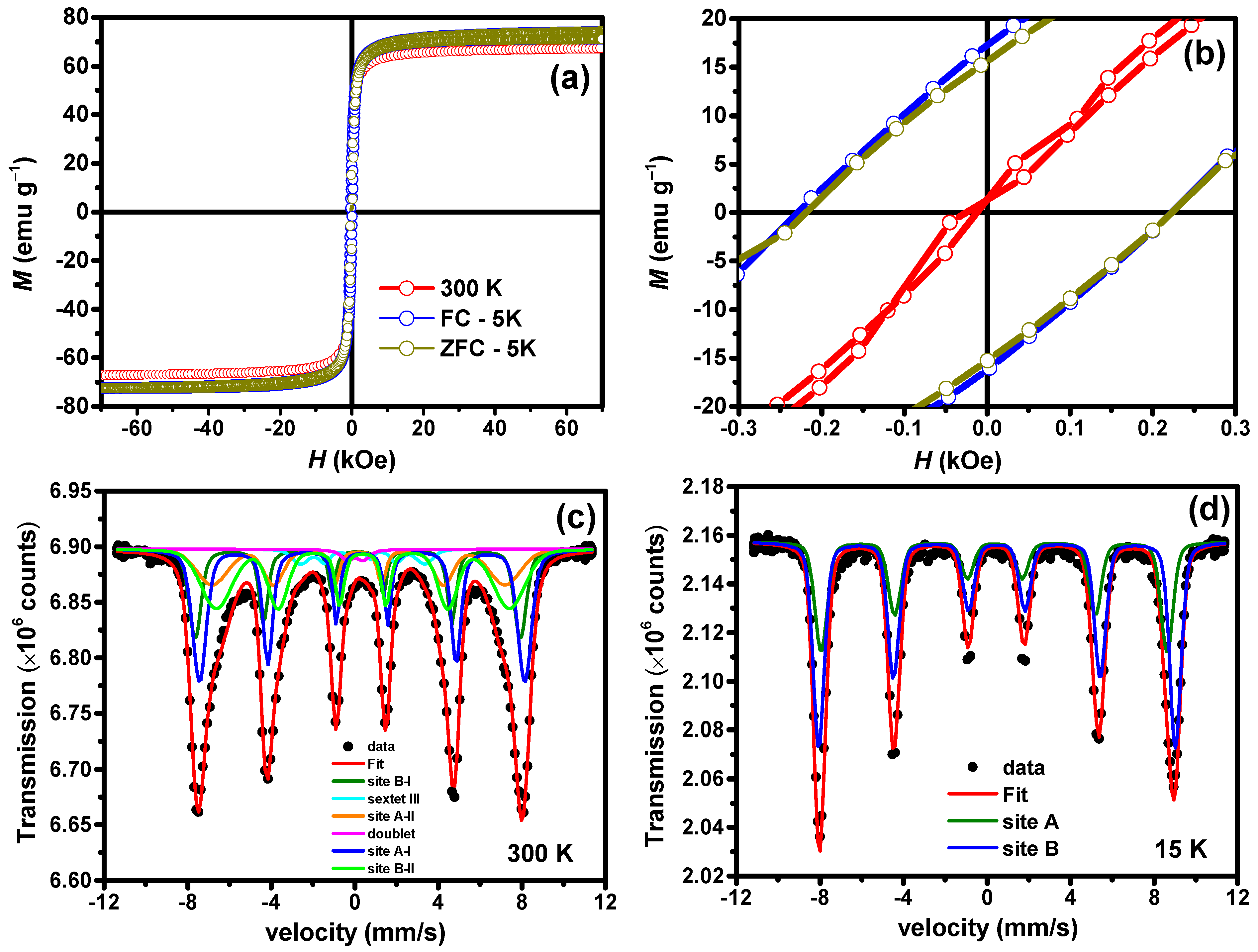
3.5. Magnetic Studies
3.6. Ecotoxicological Analysis in D. magna
3.6.1. LC50 of γ-Fe2O3@PSSNa Nanohybrid
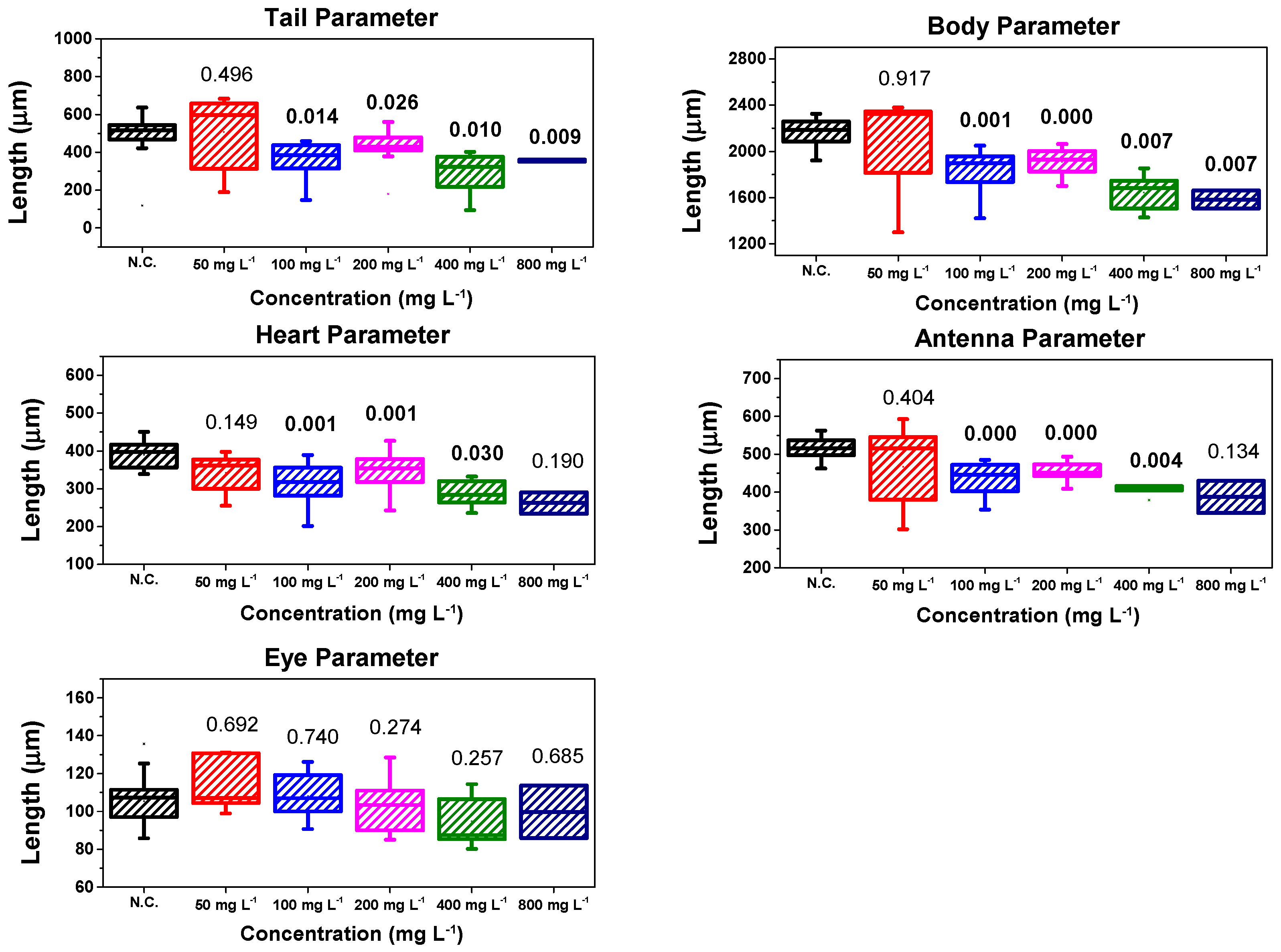
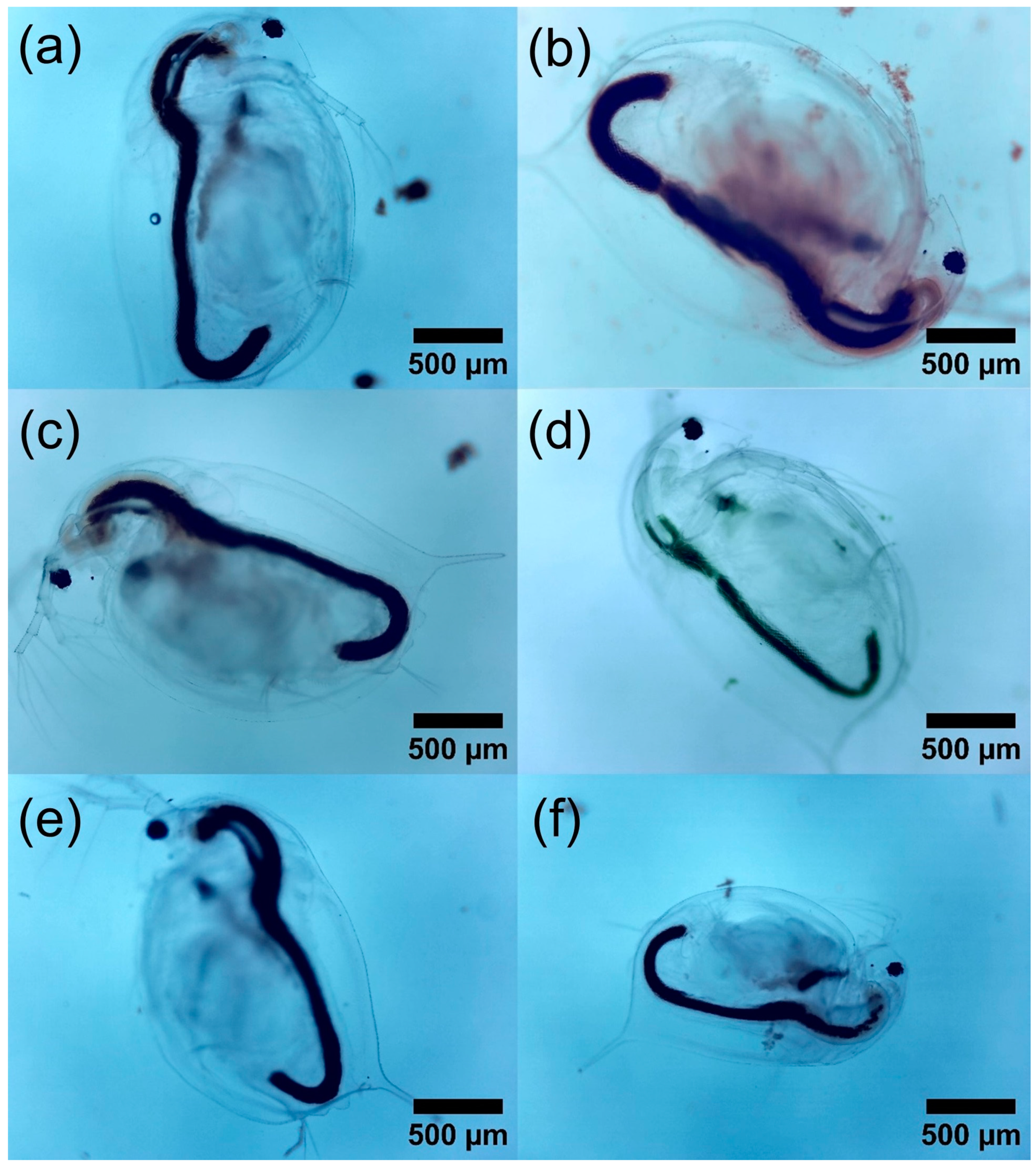
3.6.2. Morphological Analysis in D. magna
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Fu, Z.; Xi, S. The Effects of Heavy Metals on Human Metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Lawal, K.K.; Ekeleme, I.K.; Onuigbo, C.M.; Ikpeazu, V.O.; Obiekezie, S.O. A Review on the Public Health Implications of Heavy Metals. WJARR 2021, 10, 255–265. [Google Scholar] [CrossRef]
- Jalili, C.; Kazemi, M.; Cheng, H.; Mohammadi, H.; Babaei, A.; Taheri, E.; Moradi, S. Associations between Exposure to Heavy Metals and the Risk of Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Crit. Rev. Toxicol. 2021, 51, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Kumar, R.; Mittal, S.; Sahoo, P.K.; Vaid, U. Ground/Drinking Water Contaminants and Cancer Incidence: A Case Study of Rural Areas of South West Punjab, India. Hum. Ecol. Risk Assess. 2021, 27, 205–226. [Google Scholar] [CrossRef]
- Ramos-Guivar, J.A.; Checca-Huaman, N.R.; Litterst, F.J.; Passamani, E.C. Synergetic Effect between Zeolite 5 A and Maghemite Nanoparticles for Fast Lead Uptake from the Peruvian River Cumbaza: Study of Surface Adsorption Mechanism Using X-ray Photoelectron Spectroscopy. Appl. Surf. Sci. 2023, 18, 100489. [Google Scholar] [CrossRef]
- Pinotti, C.N.; Ramos-Guivar, J.A.; Proveti, J.R.; Canchanya-Huaman, Y.; Arias-Contreras, M.A.; Checca-Huaman, N.R.; Passamani, E.C. Fractal-like Kinetics for Enhanced Boron Adsorption on Heterogeneous Magnetic Composite Surfaces. Mater. Chem. Phys. 2023, 308, 128313. [Google Scholar] [CrossRef]
- Peng, Q.; Zhao, H.; Chen, G.; Yang, Q.; Cao, X.; Xiong, S.; Liu, Q. Synthesis of Novel Magnetic Pitch-based Hypercrosslinked Polymers as Adsorbents for Effective Recovery of Ag+ with High Selectivity. J. Environ. Manag. 2023, 339, 117763. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Hu, Y.; Xu, G.; Li, M.; Zhu, Y.; Jiang, L.; Tu, Y.; Zhu, X.; Xie, X.; Li, A. Green Synthesis of a Magnetic β-cyclodextrin Polymer for Rapid Removal of Organic Micro-pollutants and Heavy Metals from Dyeing Wastewater. Environ. Res. 2020, 180, 108796. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Zhou, Y.; Chen, Y.; Liu, X.; Wang, J. Toxicological Effects of Microplastics and Heavy Metals on the Daphnia magna. Sci. Total Environ. 2020, 746, 141254. [Google Scholar] [CrossRef] [PubMed]
- Tatarazako, N.; Oda, S. The Water Flea Daphnia magna (Crustacea, Cladocera) as a Test Species for Screening and Evaluation of Chemicals with Endocrine Disrupting Effects on Crustaceans. Ecotoxicology 2007, 16, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Herold, B.C.; Bourne, N.; Marcellino, D.; Kirkpatrick, R.; Strauss, D.M.; Zaneveld, L.J.; Waller, D.P.; Anderson, R.A.; Chany, C.J.; Barham, B.J.; et al. Poly (Sodium 4-Styrene Sulfonate): An Effective Candidate Topical Antimicrobial for the Prevention of Sexually Transmitted Diseases. J. Infect. Dis. 2000, 181, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Botwina, P.; Obłoza, M.; Szczepański, A.; Szczubiałka, K.; Nowakowska, M.; Pyrć, K. In Vitro Inhibition of Zika Virus Replication with Poly (Sodium 4-Styrenesulfonate). Viruses 2020, 12, 926. [Google Scholar] [CrossRef] [PubMed]
- Farias-Cepeda, L.; Herrera-Ordonez, J.; Estevez, M.; Luna-Barcenas, G.; Rosales-Marines, L. New Insights on Surfactant-Free Styrene Emulsion Polymerization in the Presence of Sodium Styrene Sulfonate. Colloid Polym. Sci. 2016, 294, 1571–1576. [Google Scholar] [CrossRef]
- Corr, S.A.; Gun’ko, Y.K.; Tekoriute, R.; Meledandri, C.J.; Brougham, D.F. Poly (Sodium-4-Styrene) Sulfonate− Iron Oxide Nanocomposite Dispersions with Controlled Magnetic Resonance Properties. J. Phys. Chem. C 2008, 112, 13324–13327. [Google Scholar] [CrossRef]
- Willott, J.D.; Lindhoud, S.; de Vos, W.M. Hot-pressing Polyelectrolyte Complexes into Tunable Dense Saloplastics. Polymer 2022, 242, 124583. [Google Scholar] [CrossRef]
- Nasser, F.; Lynch, I. Updating Traditional Regulatory Tests for Use with Novel Materials: Nanomaterial Toxicity Testing with Daphnia magna. Saf. Sci. 2019, 118, 497–504. [Google Scholar] [CrossRef]
- Shin, H.K.; Seo, M.; Shin, S.E.; Kim, K.Y.; Park, J.W.; No, K.T. Meta-analysis of Daphnia magna Nanotoxicity Experiments in Accordance with Test Guidelines. Environ. Sci. Nano 2018, 5, 765–775. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Bownik, A.; Dudka, J.; Kowal, K.; Ślaska, B. Daphnia magna Model in the Toxicity Assessment of Pharmaceuticals: A Review. Sci. Total Environ. 2021, 763, 143038. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Guivar, J.A.; Morales, M.A.; Litterst, F.J. Suppression of Exchange Bias Effect in Maghemite Nanoparticles Functionalized with H2Y. J. Magn. Magn. Mater. 2016, 420, 324–335. [Google Scholar] [CrossRef]
- Putz, H.; Brandeburg, K. Match!—Phase Identification from Powder Diffraction. Available online: https://www.crystalimpact.de/match (accessed on 27 May 2024).
- Rueda-Vellasmin, R.; Checca-Huaman, N.R.; Passamani, E.C.; Litterst, F.J.; Ramos-Guivar, J.A. Mössbauer Studies of Core-Single-Shell and Core-Double-Shell Polymer Functionalized Magnetic Nanoparticles. Hyperfine Interact. 2022, 243, 27. [Google Scholar] [CrossRef]
- Klencsár, Z. Mosswinn 4.0i, Revision, 13 January 2024. Available online: http://www.mosswinn.hu/ (accessed on 27 May 2024).
- Moyano-Arocutipa, M.F.; Zarria-Romero, J.Y.; Huertas-Chambilla, M.Y.; Checca-Huaman, N.R.; Pino, J.; Passamani, E.C.; Ramos-Guivar, J.A. In Situ and after Synthesis of Magnetic Nanoarchitectures Grown onto Zeolite Type 5A/CTAB Frameworks and Their Ecotoxicological Properties. Cryst. Growth Des. 2023, 23, 2951–2970. [Google Scholar] [CrossRef]
- Tamanaha-Vegas, C.A.; Zarria-Romero, J.Y.; Greneche, J.M.; Passamani, E.C.; Ramos-Guivar, J.A. Surface Magnetic Properties of a Ternary Nanocomposite and Its Ecotoxicological Properties in Daphnia magna. Adv. Powder Technol. 2022, 33, 103395. [Google Scholar] [CrossRef]
- Ramos-Guivar, J.A.; Flores-Cano, D.A.; Passamani, E.C. Differentiating Nanomaghemite and Nanomagnetite and Discussing Their Importance in Arsenic and Lead Removal from Contaminated Effluents: A Critical Review. Nanomaterials 2021, 11, 2310. [Google Scholar] [CrossRef] [PubMed]
- Jubb, A.M.; Allen, H.C. Vibrational Spectroscopic Characterization of Hematite, Maghemite, and Magnetite Thin Films Produced by Vapor Deposition. ACS Appl. Mater. Interface 2010, 2, 2804–2812. [Google Scholar] [CrossRef]
- Hanesch, M. Raman Spectroscopy of Iron Oxides and (Oxy) Hydroxides at Low Laser Power and Possible Applications in Environmental Magnetic Studies. Geophys. J. Int. 2009, 177, 941–948. [Google Scholar] [CrossRef]
- Ramos-Guivar, J.A.; Zarria-Romero, J.Y.; Castro-Merino, I.L.; Greneche, J.M.; Passamani, E.C. Improvement of the Thermal Stability of Nanomaghemite by Functionalization with Type 5A Zeolite and Magnetic Properties Studied by In-Field 57Fe Mössbauer Measurements. J. Magn. Magn. Mater. 2022, 552, 169241. [Google Scholar] [CrossRef]
- Mendili, Y.E.; Grasset, F.; Randrianantoandro, N.; Nerambourg, N.; Greneche, J.M.; Bardeau, J.F. Improvement of Thermal Stability of Maghemite Nanoparticles Coated with Oleic Acid and Oleylamine Molecules: Investigations under Laser Irradiation. J. Phys. Chem. C 2015, 119, 10662–10668. [Google Scholar] [CrossRef]
- Ramos-Guivar, J.A.; Gonzalez-Gonzalez, J.C.; Litterst, F.J.; Passamani, E.C. Rietveld Refinement, μ-Raman, X-ray Photoelectron, and Mossbauer Studies of Metal Oxide Nanoparticles Growth on Multiwall Carbon Nanotubes and Graphene Oxide. Cryst. Growth Des. 2021, 21, 2128–2141. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Brown, D.R.; Dale, J.A.; Plant, S. Raman Spectroscopy of Sulfonated Polystyrene Resins. Vib. Spectros. 2000, 24, 213–224. [Google Scholar] [CrossRef]
- De Faria, D.L.; Silva, S.V.; De Oliveira, M.T. Raman Microspectroscopy of Some Iron Oxides and Oxyhydroxides. J. Raman Spectros. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Ramos-Guivar, J.A.; Passamani, E.C.; Litterst, J. Superspin Glass State in Functionalized Zeolite 5A-Maghemite Nanoparticles. AIP Adv. 2021, 11, 035223. [Google Scholar] [CrossRef]
- Ogholbeyg, A.B.; Kianvash, A.; Hajalilou, A.; Abouzari-Lotf, E.; Zarebkohan, A. Cytotoxicity Characteristics of Green Assisted-Synthesized Superparamagnetic Maghemite (γ-Fe2O3) Nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 12135–12143. [Google Scholar] [CrossRef]
- Rodriguez, A.F.R.; Rocha, C.O.; Piazza, R.D.; Dos Santos, C.C.; Morales, M.A.; Faria, F.S.E.D.V.; Iqbal, M.Z.; Barbosa, L.; Chaves, Y.O.; Mariuba, L.A.; et al. Synthesis, Characterization and Applications of Maghemite Beads Functionalized with Rabbit Antibodies. Nanotechnology 2018, 29, 365701. [Google Scholar] [CrossRef] [PubMed]
- Daou, T.J.; Greneche, J.-M.; Lee, S.-J.; Lee, S.; Lefevre, C.; Bégin-Colin, S.; Pourroy, G. Spin Canting of Maghemite Studied by NMR and In-Field Mössbauer Spectrometr. J. Phys. Chem. 2010, 114, 8794–8799. [Google Scholar] [CrossRef]
- Brijmojan, S.B.; Swier, S.; Weiss, R.A.; Shaw, M.T. Synthesis and Characterization of Cross-linked Sulfonated Polystyrene Nanoparticles. Ind. Eng. Chem. Res. 2005, 44, 8039–8045. [Google Scholar] [CrossRef]
- Funda, S.; Ohki, T.; Liu, Q.; Hossain, J.; Ishimaru, Y.; Ueno, K.; Shirai, H. Correlation between the Fine Structure of Spin-Coated PEDOT: PSS and the Photovoltaic Performance of Organic/Crystalline-Silicon Heterojunction Solar Cells. J. Appl. Sci. 2016, 120, 033103. [Google Scholar] [CrossRef]
- Karri, S.N.; Srinivasan, P. Synthesis of PEDOT:PSS using benzoyl peroxide as an alternative oxidizing agent for ESD coating and electro-active material in supercapacitor. Mater. Sci. Energy Technol. 2019, 2, 208–2015. [Google Scholar] [CrossRef]
- Cristovan, F.H.; Nascimento, C.M.; Bell, M.J.V.; Laureto, E.; Duarte, J.L.; Dias, I.F.L.; Cruz, W.O.; Marletta, A. Synthesis and Optical Characterization of Polystyrene Sulfonate Films Doped with Nd(III). Chem. Phys. 2006, 326, 514–520. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences, and Uses; Wiley-vch: Weinheim, Germany, 2003. [Google Scholar] [CrossRef]
- Barakat, N.A. Synthesis and Characterization of Maghemite Iron Oxide (γ-Fe2O3) Nanofibers: Novel Semiconductor with Magnetic Feature. J. Mater. Sci. 2012, 47, 6237–6245. [Google Scholar] [CrossRef]
- Mirza, I.M.; Ali, K.; Sarfraz, A.K.; Ali, A.; Ul Haq, A. A Study of Dielectric, Optical and Magnetic Characteristics of Maghemite Nanocrystallites. Mater. Chem. Phys. 2015, 164, 183–187. [Google Scholar] [CrossRef]
- Zhai, C.; Niu, Y.; Liu, J.; Yang, T. Effect of Octadecylamine Polyoxyethylene Ether on the Adsorption Feature of Sodium Polystyrene Sulfonate on the SiC Surface and the Relevant Dispersion Stability of Slurry. Coll. Surf. A 2022, 633, 127799. [Google Scholar] [CrossRef]
- Naassaoui, I.; Aschi, A. Influence of Temperature and Salt on Coacervation in an Aqueous Mixture of Poly-L-Lysine (PLL) and Poly-(Sodium Styrene Sulfonate) (PSSNA). Eur. Biophys. J. 2021, 50, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, Z.; Jachimska, B.; Jasiński, T.; Warszyński, P.; Wasilewska, M. Structure of Poly (Sodium 4-Styrenesulfonate)(PSS) in Electrolyte Solutions: Theoretical Modeling and Measurements. Coll. Surf. A 2009, 343, 96–103. [Google Scholar] [CrossRef]
- Nurdin, I.; Ridwan; Satriananda. The Effect of pH and Time on the Stability of Superparamagnetic Maghemite Nanoparticle Suspensions. MATEC Web Conf. 2016, 39, 01001. [Google Scholar] [CrossRef]
- Ma, L.; He, Y.; Luo, P.; Zhang, L.; Yu, Y. Automatic Dispersion, Long-Term Stability of Multi-Walled Carbon Nanotubes in High Concentration Electrolytes. J. Nanopart. Res. 2018, 20, 45. [Google Scholar] [CrossRef]
- Harris, C.M.; Miller, S.G.; Andresen, K.; Thompson, L.J. Quantitative Measurement of Sodium Polystyrene Sulfonate Adsorption onto CTAB Capped Gold Nanoparticles Reveals Hard and Soft Coronas. J. Colloid Interface Sci. 2018, 510, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Jordan, N.; Ritter, A.; Foerstendorf, H.; Scheinost, A.C.; Weiß, S.; Heim, K.; Grenzer, J.; Mücklich, A.; Reuther, H. Adsorption Mechanism of Selenium(VI) onto Maghemite. GCA 2013, 103, 63–75. [Google Scholar] [CrossRef]
- Chen, B.W.; He, Y.C.; Sung, S.Y.; Le, T.T.H.; Hsieh, C.L.; Chen, J.Y.; Wei, Z.H.; Yao, D.J. Synthesis and Characterization of Magnetic Nanoparticles Coated with Polystyrene Sulfonic Acid for Biomedical Applications. Sci. Technol. Adv. Mater. 2020, 21, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Niemark, A.V.; Olivier, J.P.; Rodríguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Mejía, M.E.; Pariona, N.; Bravo, J.A.; Ramos-Guivar, J.A.; Mtz-Enriquez, A.I. Synthesis and Characterization of Maghemite Submicron Particles: Novel Adsorbent for Arsenic Removal. Hyperfine Interact. 2023, 244, 8. [Google Scholar] [CrossRef]
- Zakharova, I.N.; Shipilin, M.A.; Alekseev, V.P.; Shipilin, A.M. Mössbauer Study of Maghemite Nanoparticles. Technol. Phys. Lett. 2012, 38, 55–58. [Google Scholar] [CrossRef]
- García, A.; Espinosa, R.; Delgado, L.; Casals, E.; González, E.; Puntes, V.; Barata, C.; Font, X.; Sánchez, A. Acute Toxicity of Cerium Oxide, Titanium Oxide and Iron Oxide Nanoparticles Using Standardized Tests. Desalination 2011, 269, 136–141. [Google Scholar] [CrossRef]
- Magro, M.; De Liguoro, M.; Franzago, E.; Baratella, D.; Vianello, F. The Surface Reactivity of Iron Oxide Nanoparticles as a Potential Hazard for Aquatic Environments: A Study on Daphnia magna Adults and Embryos. Sci. Rep. 2018, 8, 13017. [Google Scholar] [CrossRef] [PubMed]
- Baumann, J.; Köser, J.; Arndt, D.; Filser, J. The Coating Makes the Difference: Acute Effects of Iron Oxide Nanoparticles on Daphnia magna. Sci. Total Environ. 2014, 484, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Zarria-Romero, J.Y.; Ocampo-Anticona, J.A.; Pinotti, C.N.; Passamani, E.C.; Checca-Huaman, N.R.; Castro-Merino, I.L.; Pino, J.; Shiga, B.; Ramos-Guivar, J.A. Ecotoxicological Properties of Functionalized Magnetic Graphene Oxide and Multiwall Carbon Nanotubes in Daphnia magna. Ceram. Int. 2023, 49, 15200–15212. [Google Scholar] [CrossRef]
- Farsi, L.; Sabzalipour, S.; Khodadadi, M.; Fard, N.J.H.; Jamali-Sheini, F. The Ecotoxicity of Nanoparticles Co2O3 and Fe2O3 on Daphnia magna in Freshwater. J. Water Chem. Technol. 2021, 43, 509–516. [Google Scholar] [CrossRef]
- Shariati, F.; Poordeljoo, T.; Zanjanchi, P. The Acute Toxicity of SiO2 and Fe3O4 Nanoparticles on Daphnia magna. Silicon 2020, 12, 2941–2946. [Google Scholar] [CrossRef]
- Nikitin, O.V.; Nasyrova, E.I.; Kuzmin, R.S.; Minnegulova, L.M.; Latypova, V.Z. Effects of Polystyrene Microparticles on the Morphofunctional Parameters of Daphnia magna. ББК 2013, 6, 117. [Google Scholar]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.A.; Cedervall, T. Brain Damage and Behavioural Disorders in Fish Induced by Plastic Nanoparticles Delivered through the Food Chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Villa, F.; Checca-Huaman, N.R.; Ramos-Guivar, J.A. Ecotoxicological Properties of Titanium Dioxide Nanomorphologies in Daphnia magna. Nanomaterials 2023, 13, 927. [Google Scholar] [CrossRef] [PubMed]
- Martin-Folgar, R.; Esteban-Arranz, A.; Negri, V.; Morales, M. Graphene Oxides (GOs) with Different Lateral Dimensions and Thicknesses Affect the Molecular Response in Chironomus riparius. Nanomaterials 2023, 13, 967. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.S.; Lu, C.Y.; Chang, S.H. Evaluation of Acute Toxicity and Teratogenic Effects of Plant Growth Regulators by Daphnia magna Embryo Assay. J. Hazard. Mater. 2011, 190, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Saito, H.; Niikura, Y.; Shigeoka, T.; Nakano, Y. Embryonic Development Assay with Daphnia magna: Application to Toxicity of Aniline Derivatives. Chemosphere 2001, 45, 487–495. [Google Scholar] [CrossRef] [PubMed]

| Parent System | Mean Particle Size in Aqueous Media (nm) | NPs Source | Exposition Time (h) | LC50 (mg L−1) | Reference |
|---|---|---|---|---|---|
| Fe3O4 NPs (6 nm) | n.d. | Synthesized | 48 | 2.3 | [55] |
| SAMN * (11 (2) nm) | 5–20 | Synthesized | 48 | 1.25–40 | [56] |
| PVP-IONP (6.1 (6) nm) | 82.3 | Synthesized | 48 | 9750 | [57] |
| MWCNTs-γ-Fe2O3 (13.8 (6) nm for γ-Fe2O3) | n.d. | Synthesized | 24 | 381.8 | [58] |
| GO-γ-Fe2O3 (10.4 (2) nm for γ-Fe2O3) | n.d. | Synthesized | 24 | 0.9 | [58] |
| Fe2O3 (20–40 nm) | n.d. | Commercial | 96 | 163.21 | [59] |
| NP-Fe3O4 (<20 nm) | n.d. | Synthesized | 48 | 977.24 | [60] |
| PS (<75 μm) | n.d. | Commercial | 48 | 78.94 | [61] |
| PAO2N (52 nm) | 56 | Commercial | <24 | <75 | [62] |
| γ-Fe2O3@PSSNa (11(2) nm) | 232.2 | Synthesized | 24 | 533(5) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Guivar, J.A.; Rueda-Vellasmin, R.; Manrique-Castillo, E.V.; Mendoza-Villa, F.; Checca-Huaman, N.-R.; Passamani, E.C. Synthesis and Characterization of Maghemite Nanoparticles Functionalized with Poly(Sodium 4-Styrene Sulfonate) Saloplastic and Its Acute Ecotoxicological Impact on the Cladoceran Daphnia magna. Polymers 2024, 16, 1581. https://doi.org/10.3390/polym16111581
Ramos-Guivar JA, Rueda-Vellasmin R, Manrique-Castillo EV, Mendoza-Villa F, Checca-Huaman N-R, Passamani EC. Synthesis and Characterization of Maghemite Nanoparticles Functionalized with Poly(Sodium 4-Styrene Sulfonate) Saloplastic and Its Acute Ecotoxicological Impact on the Cladoceran Daphnia magna. Polymers. 2024; 16(11):1581. https://doi.org/10.3390/polym16111581
Chicago/Turabian StyleRamos-Guivar, Juan A., Renzo Rueda-Vellasmin, Erich V. Manrique-Castillo, F. Mendoza-Villa, Noemi-Raquel Checca-Huaman, and Edson C. Passamani. 2024. "Synthesis and Characterization of Maghemite Nanoparticles Functionalized with Poly(Sodium 4-Styrene Sulfonate) Saloplastic and Its Acute Ecotoxicological Impact on the Cladoceran Daphnia magna" Polymers 16, no. 11: 1581. https://doi.org/10.3390/polym16111581
APA StyleRamos-Guivar, J. A., Rueda-Vellasmin, R., Manrique-Castillo, E. V., Mendoza-Villa, F., Checca-Huaman, N.-R., & Passamani, E. C. (2024). Synthesis and Characterization of Maghemite Nanoparticles Functionalized with Poly(Sodium 4-Styrene Sulfonate) Saloplastic and Its Acute Ecotoxicological Impact on the Cladoceran Daphnia magna. Polymers, 16(11), 1581. https://doi.org/10.3390/polym16111581







