Comparison of Printable Biomaterials for Use in Neural Tissue Engineering: An In Vitro Characterization and In Vivo Biocompatibility Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Biomaterials for 3D Printing
2.2. Scaffold Design and 3D Printing Process
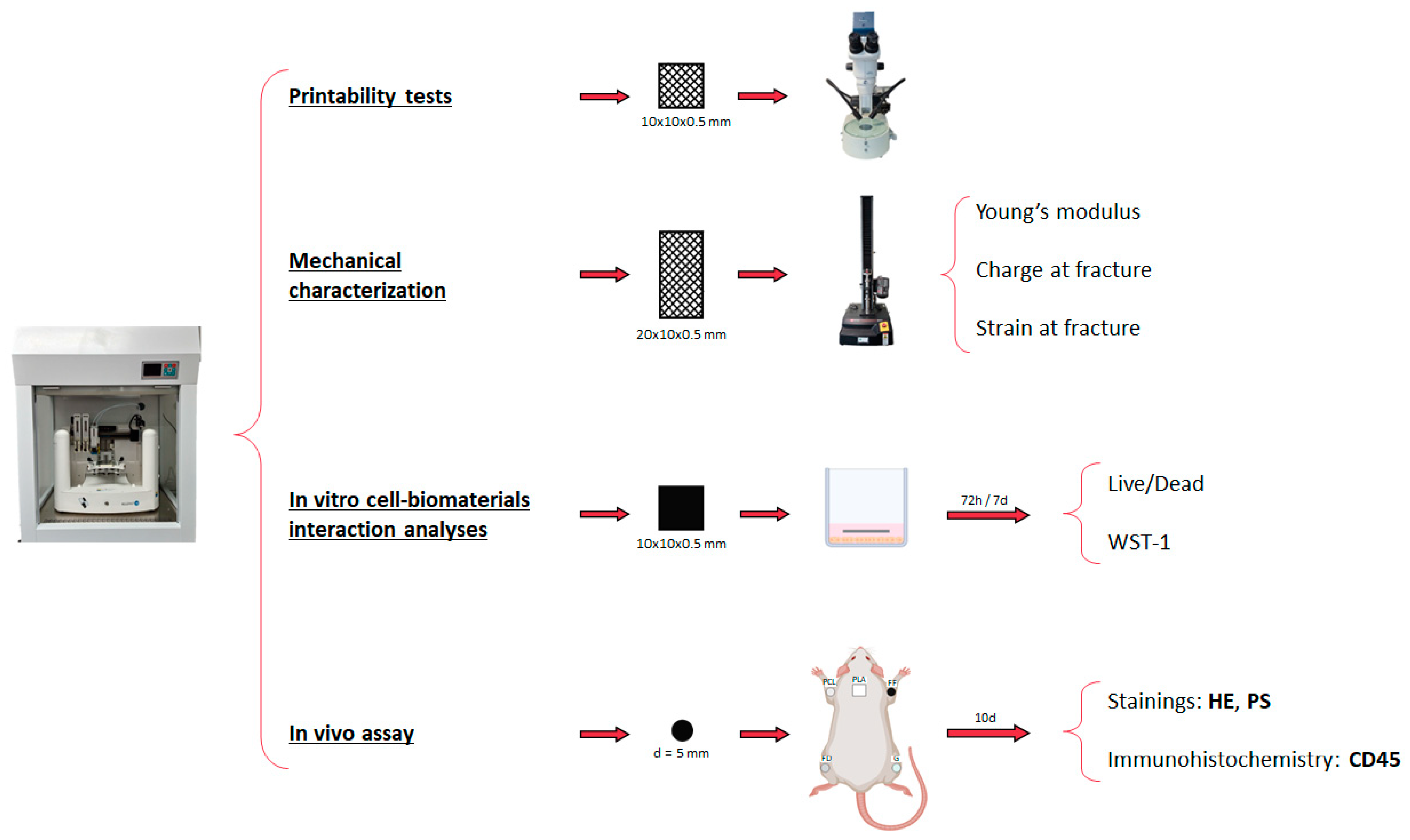
2.3. Printability Tests
2.4. Mechanical Characterization
2.5. In Vitro Cell-Biomaterial Interaction Analyses
2.6. In Vivo Assay
2.6.1. Surgical Procedure
2.6.2. Histological Analyses
2.7. Quantitative and Statistical Analyses
3. Results
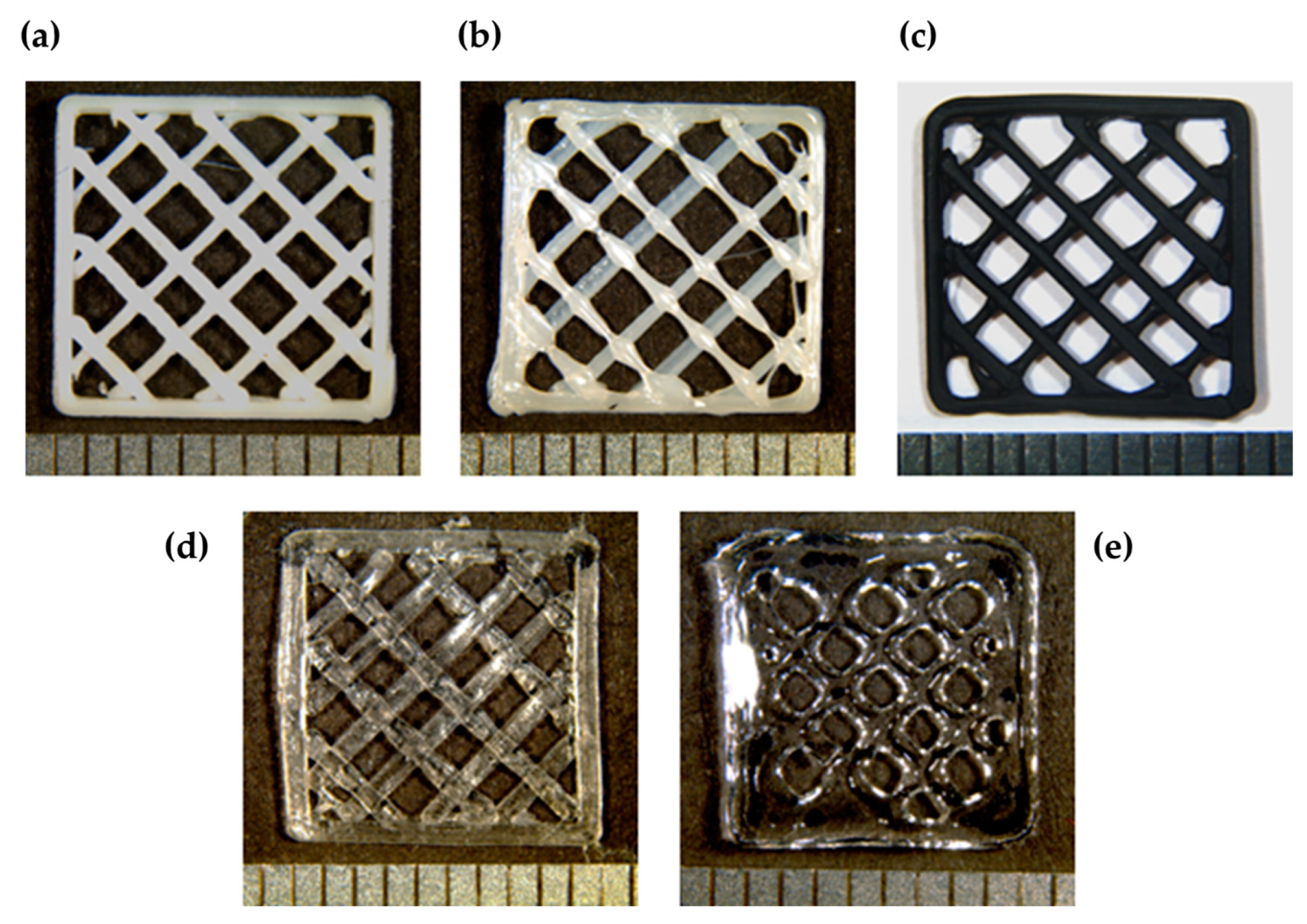
3.1. Printability
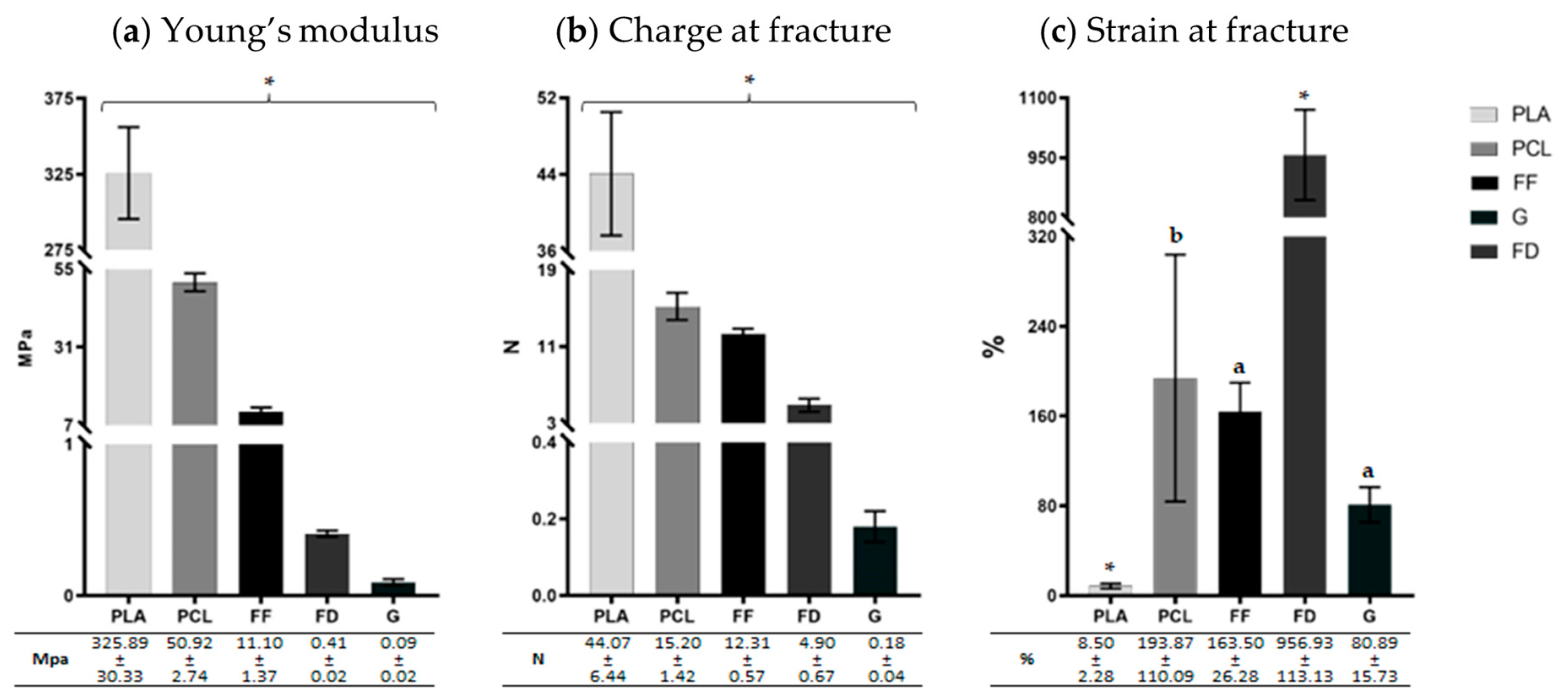
3.2. Mechanical Characterization
3.3. In Vitro Cell–Biomaterial Interaction Analyses
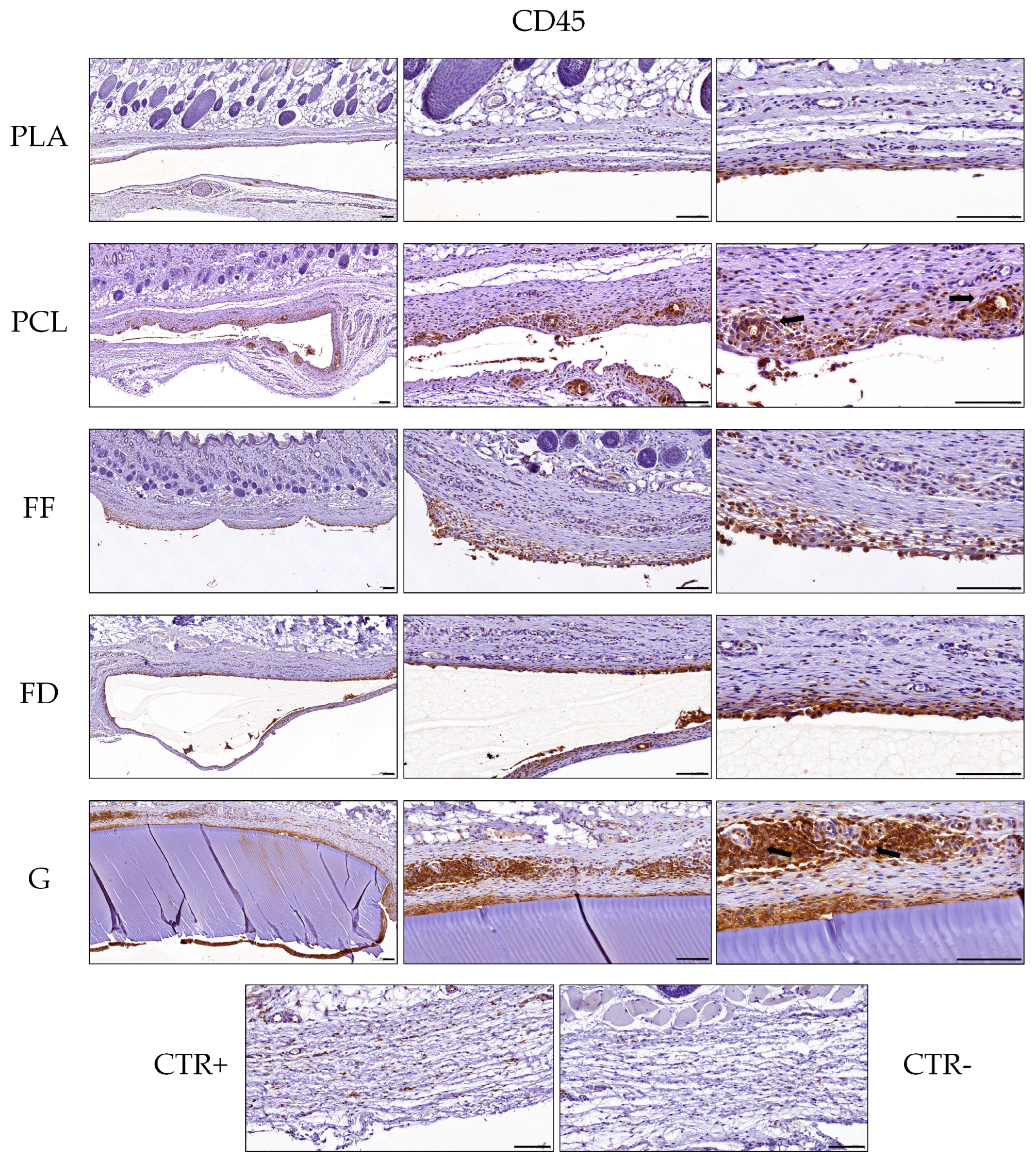
3.4. Histology of Implanted 3D-printed Scaffolds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collaborators, G.B.D.N. Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 6, 309–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, M.; Zhi, J.; Wu, S.; Wang, Y.; Pei, F. Research Hotspots and Trends of Peripheral Nerve Injuries Based on Web of Science From 2017 to 2021: A Bibliometric Analysis. Front. Neurol. 2022, 13, 872261. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Hu, S.; Wang, P.; Kang, H.; Peng, R.; Dong, Y.; Li, F. Spinal Cord Injury: The Global Incidence, Prevalence, and Disability From the Global Burden of Disease Study 2019. Spine 2022, 47, 1532–1540. [Google Scholar] [CrossRef]
- Carriel, V.; Garzon, I.; Alaminos, M.; Cornelissen, M. Histological assessment in peripheral nerve tissue engineering. Neural Regen. Res. 2014, 9, 1657–1660. [Google Scholar] [CrossRef]
- Kehoe, S.; Zhang, X.F.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef]
- Koffler, J.; Zhu, W.; Qu, X.; Platoshyn, O.; Dulin, J.N.; Brock, J.; Graham, L.; Lu, P.; Sakamoto, J.; Marsala, M.; et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019, 25, 263–269. [Google Scholar] [CrossRef]
- Costachescu, B.; Niculescu, A.G.; Dabija, M.G.; Teleanu, R.I.; Grumezescu, A.M.; Eva, L. Novel Strategies for Spinal Cord Regeneration. Int. J. Mol. Sci. 2022, 23, 4552. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J. Advances in tissue engineering. J. Pediatr. Surg. 2016, 51, 8–12. [Google Scholar] [CrossRef]
- Weinhart, M.; Hocke, A.; Hippenstiel, S.; Kurreck, J.; Hedtrich, S. 3D organ models-Revolution in pharmacological research? Pharmacol. Res. 2019, 139, 446–451. [Google Scholar] [CrossRef]
- Chandra, P.K.; Soker, S.; Atala, A. Chapter 1—Tissue engineering: Current status and future perspectives. In Principles of Tissue Engineering, 5th ed.; Lanza, R., Langer, R., Vacanti, J.P., Atala, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–35. [Google Scholar]
- Dearman, B.L.; Boyce, S.T.; Greenwood, J.E. Advances in Skin Tissue Bioengineering and the Challenges of Clinical Translation. Front. Surg. 2021, 8, 640879. [Google Scholar] [CrossRef] [PubMed]
- Rico-Sanchez, L.; Garzon, I.; Gonzalez-Andrades, M.; Ruiz-Garcia, A.; Punzano, M.; Lizana-Moreno, A.; Munoz-Avila, J.I.; Sanchez-Quevedo, M.D.C.; Martinez-Atienza, J.; Lopez-Navas, L.; et al. Successful development and clinical translation of a novel anterior lamellar artificial cornea. J. Tissue Eng. Regen. Med. 2019, 13, 2142–2154. [Google Scholar] [CrossRef] [PubMed]
- Carriel, V.; Alaminos, M.; Garzon, I.; Campos, A.; Cornelissen, M. Tissue engineering of the peripheral nervous system. Expert Rev. Neurother. 2014, 14, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, T.; Li, Y. 3D Printing and Bioprinting Nerve Conduits for Neural Tissue Engineering. Polymers 2020, 12, 1637. [Google Scholar] [CrossRef] [PubMed]
- Bedir, T.; Ulag, S.; Ustundag, C.B.; Gunduz, O. 3D bioprinting applications in neural tissue engineering for spinal cord injury repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110741. [Google Scholar] [CrossRef]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; de Aguilar, J.G.; et al. Current Status of Therapeutic Approaches against Peripheral Nerve Injuries: A Detailed Story from Injury to Recovery. Int. J. Biol. Sci. 2020, 16, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.S.; Cui, F.Z.; Zhang, W.; Feng, Q.L. Hierarchically biomimetic bone scaffold materials: Nano-HA/collagen/PLA composite. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 69, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J. Biomed. Sci. 2009, 16, 108. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U. Novel biopolymer-based sustainable composites for food packaging applications: A narrative review. Food Packag. Shelf 2022, 33, 100892. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.L.; Yavitt, B.M.; Wheeler, M.D.; Kolwich, J.L.; Donovan, L.N.; Sit, C.S.; Hatzikiriakos, S.G.; Jalsa, N.K.; MacQuarrie, S.L.; Kerton, F.M. Biochar as a sustainable and renewable additive for the production of Poly(ε-caprolactone) composites. Sustain. Chem. Pharm. 2022, 25, 100586. [Google Scholar] [CrossRef]
- Kim, G.J.; Lee, K.J.; Choi, J.W.; An, J.H. Modified Industrial Three-Dimensional Polylactic Acid Scaffold Cell Chip Promotes the Proliferation and Differentiation of Human Neural Stem Cells. Int. J. Mol. Sci. 2022, 23, 2204. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, D.N.; Pinto, G.B.A.; Cartarozzi, L.P.; de Oliveira, A.L.R.; Bovolato, A.L.C.; de Carvalho, M.; da Silva, J.V.L.; Dernowsek, J.A.; Golim, M.; Barraviera, B.; et al. 3D-printed nerve guidance conduits multi-functionalized with canine multipotent mesenchymal stromal cells promote neuroregeneration after sciatic nerve injury in rats. Stem Cell Res. Ther. 2021, 12, 303. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Mullins, M.E.; Cregg, J.M.; Hurtado, A.; Oudega, M.; Trombley, M.T.; Gilbert, R.J. Creation of highly aligned electrospun poly-L-lactic acid fibers for nerve regeneration applications. J. Neural Eng. 2009, 6, 016001. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Yuan, W.; Xu, J.; Tong, W.; Mi, J.; Ho, P.C.; Chow, D.H.K.; Li, Y.; Yao, H.; Li, X.; et al. Magnesium-Encapsulated Injectable Hydrogel and 3D-Engineered Polycaprolactone Conduit Facilitate Peripheral Nerve Regeneration. Adv. Sci. 2022, 9, e2202102. [Google Scholar] [CrossRef]
- Singh, A.; Asikainen, S.; Teotia, A.K.; Shiekh, P.A.; Huotilainen, E.; Qayoom, I.; Partanen, J.; Seppala, J.; Kumar, A. Biomimetic Photocurable Three-Dimensional Printed Nerve Guidance Channels with Aligned Cryomatrix Lumen for Peripheral Nerve Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 43327–43342. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Chang, C.W.; Wang, J.; Bupphathong, S.; Huang, W.; Lin, C.H. 3D-Bioprinted GelMA Scaffold with ASCs and HUVECs for Engineering Vascularized Adipose Tissue. ACS Appl. Bio Mater. 2024, 7, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Ullah, I.; Liu, X.; Mehmood, S.; Wang, L.; Ma, F.; Ullah, S.; Lu, Z.; Wang, Z.; Pei, R. GelMA-catechol coated FeHAp nanorods functionalized nanofibrous reinforced bio-instructive and mechanically robust composite hydrogel scaffold for bone tissue engineering. Biomater. Adv. 2023, 155, 213696. [Google Scholar] [CrossRef]
- Qian, Y.; Gong, J.; Lu, K.; Hong, Y.; Zhu, Z.; Zhang, J.; Zou, Y.; Zhou, F.; Zhang, C.; Zhou, S.; et al. DLP printed hDPSC-loaded GelMA microsphere regenerates dental pulp and repairs spinal cord. Biomaterials 2023, 299, 122137. [Google Scholar] [CrossRef]
- Sakr, M.A.; Sakthivel, K.; Hossain, T.; Shin, S.R.; Siddiqua, S.; Kim, J.; Kim, K. Recent trends in gelatin methacryloyl nanocomposite hydrogels for tissue engineering. J. Biomed. Mater. Res. A 2022, 110, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Recreus Conductive Filaflex—Electrically Conductive TPU Filament with Shore Hardness 92A. Available online: https://recreus.com/gb/filaments/3-21-filaflex-conductivo.html#/1-colour-black/2-diameter-175_mm/3-weight-500_gr (accessed on 5 April 2024).
- McMillan, A.H.; Thomee, E.K.; Dellaquila, A.; Nassman, H.; Segura, T.; Lesher-Perez, S.C. Rapid Fabrication of Membrane-Integrated Thermoplastic Elastomer Microfluidic Devices. Micromachines 2020, 11, 731. [Google Scholar] [CrossRef]
- Kashaninejad, N.; Nguyen, N.T. Microfluidic solutions for biofluids handling in on-skin wearable systems. Lab Chip 2023, 23, 913–937. [Google Scholar] [CrossRef] [PubMed]
- Carriel, V.; Scionti, G.; Campos, F.; Roda, O.; Castro, B.; Cornelissen, M.; Garzon, I.; Alaminos, M. In vitro characterization of a nanostructured fibrin agarose bio-artificial nerve substitute. J. Tissue Eng. Regen. Med. 2017, 11, 1412–1426. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, O.D.; El Soury, M.; Gonzalez-Quevedo, D.; Sanchez-Porras, D.; Chato-Astrain, J.; Campos, F.; Carriel, V. Histological, Biomechanical, and Biological Properties of Genipin-Crosslinked Decellularized Peripheral Nerves. Int. J. Mol. Sci. 2021, 22, 674. [Google Scholar] [CrossRef] [PubMed]
- Berasain, J.; Avila-Fernandez, P.; Cardenas-Perez, R.; Canaves-Llabres, A.I.; Etayo-Escanilla, M.; Alaminos, M.; Carriel, V.; Garcia-Garcia, O.D.; Chato-Astrain, J.; Campos, F. Genipin crosslinking promotes biomechanical reinforcement and pro-regenerative macrophage polarization in bioartificial tubular substitutes. Biomed. Pharmacother. 2024, 174, 116449. [Google Scholar] [CrossRef]
- Gonzalez-Quevedo, D.; Diaz-Ramos, M.; Chato-Astrain, J.; Sanchez-Porras, D.; Tamimi, I.; Campos, A.; Campos, F.; Carriel, V. Improving the regenerative microenvironment during tendon healing by using nanostructured fibrin/agarose-based hydrogels in a rat Achilles tendon injury model. Bone Jt. J. 2020, 102-B, 1095–1106. [Google Scholar] [CrossRef]
- Martin-Piedra, M.A.; Garzon, I.; Oliveira, A.C.; Alfonso-Rodriguez, C.A.; Carriel, V.; Scionti, G.; Alaminos, M. Cell viability and proliferation capability of long-term human dental pulp stem cell cultures. Cytotherapy 2014, 16, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Durand-Herrera, D.; Campos, F.; Jaimes-Parra, B.D.; Sanchez-Lopez, J.D.; Fernandez-Valades, R.; Alaminos, M.; Campos, A.; Carriel, V. Wharton’s jelly-derived mesenchymal cells as a new source for the generation of microtissues for tissue engineering applications. Histochem. Cell Biol. 2018, 150, 379–393. [Google Scholar] [CrossRef]
- Carriel, V.; Campos, F.; Aneiros-Fernandez, J.; Kiernan, J.A. Tissue Fixation and Processing for the Histological Identification of Lipids. Methods Mol. Biol. 2017, 1560, 197–206. [Google Scholar] [CrossRef]
- Sanchez-Porras, D.; Bermejo-Casares, F.; Carmona, R.; Weiss, T.; Campos, F.; Carriel, V. Tissue Fixation and Processing for the Histological Identification of Lipids. Methods Mol. Biol. 2023, 2566, 175–186. [Google Scholar] [CrossRef]
- Carriel, V.S.; Aneiros-Fernandez, J.; Arias-Santiago, S.; Garzon, I.J.; Alaminos, M.; Campos, A. A novel histochemical method for a simultaneous staining of melanin and collagen fibers. J. Histochem. Cytochem. 2011, 59, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Dexter, F. Wilcoxon-Mann-Whitney test used for data that are not normally distributed. Anesth. Analg. 2013, 117, 537–538. [Google Scholar] [CrossRef] [PubMed]
- Moshayedi, P.; Ng, G.; Kwok, J.C.; Yeo, G.S.; Bryant, C.E.; Fawcett, J.W.; Franze, K.; Guck, J. The relationship between glial cell mechanosensitivity and foreign body reactions in the central nervous system. Biomaterials 2014, 35, 3919–3925. [Google Scholar] [CrossRef]
- Gu, B.K.; Choi, D.J.; Park, S.J.; Kim, Y.J.; Kim, C.H. 3D Bioprinting Technologies for Tissue Engineering Applications. Adv. Exp. Med. Biol. 2018, 1078, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, M.; Chen, Z.; Zhang, T.; Huang, J.; Dai, J.; Zhang, Z. 3D bioprinted neural tissue constructs for spinal cord injury repair. Biomaterials 2021, 272, 120771. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Qian, Y.; Fan, C. Biomimicry in 3D printing design: Implications for peripheral nerve regeneration. Regen. Med. 2021, 16, 683–701. [Google Scholar] [CrossRef] [PubMed]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Zhang, X.; Sun, Y.; Gao, Q.; Fu, J.; Cai, X.; He, Y. Printability during projection-based 3D bioprinting. Bioact. Mater. 2022, 11, 254–267. [Google Scholar] [CrossRef]
- Arguchinskaya, N.V.; Isaeva, E.V.; Kisel, A.A.; Beketov, E.E.; Lagoda, T.S.; Baranovskii, D.S.; Yakovleva, N.D.; Demyashkin, G.A.; Komarova, L.N.; Astakhina, S.O.; et al. Properties and Printability of the Synthesized Hydrogel Based on GelMA. Int. J. Mol. Sci. 2023, 24, 2121. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Tashman, J.W.; Shiwarski, D.J.; Feinberg, A.W. A high performance open-source syringe extruder optimized for extrusion and retraction during FRESH 3D bioprinting. HardwareX 2021, 9, e00170. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, S.; Anand, S.; Shah, T.; Tasoglu, S. Bioprinting for Neural Tissue Engineering. Trends Neurosci. 2018, 41, 31–46. [Google Scholar] [CrossRef] [PubMed]
- El Soury, M.; Garcia-Garcia, O.D.; Moretti, M.; Perroteau, I.; Raimondo, S.; Lovati, A.B.; Carriel, V. Comparison of Decellularization Protocols to Generate Peripheral Nerve Grafts: A Study on Rat Sciatic Nerves. Int. J. Mol. Sci. 2021, 22, 2389. [Google Scholar] [CrossRef]
- Garcia-Garcia, O.D.; El Soury, M.; Campos, F.; Sanchez-Porras, D.; Geuna, S.; Alaminos, M.; Gambarotta, G.; Chato-Astrain, J.; Raimondo, S.; Carriel, V. Comprehensive ex vivo and in vivo preclinical evaluation of novel chemo enzymatic decellularized peripheral nerve allografts. Front. Bioeng. Biotechnol. 2023, 11, 1162684. [Google Scholar] [CrossRef]
- Nectow, A.R.; Marra, K.G.; Kaplan, D.L. Biomaterials for the development of peripheral nerve guidance conduits. Tissue Eng. Part B Rev. 2012, 18, 40–50. [Google Scholar] [CrossRef]
- Clarke, E.C. Spinal Cord Mechanical Properties. Stud. Mechanobiol. Tissue Eng. Biomater. 2011, 3, 25–40. [Google Scholar] [CrossRef]
- Bilston, L.E.; Thibault, L.E. The mechanical properties of the human cervical spinal cord in vitro. Ann. Biomed. Eng. 1996, 24, 67–74. [Google Scholar] [CrossRef]
- Ichihara, K.; Taguchi, T.; Sakuramoto, I.; Kawano, S.; Kawai, S. Mechanism of the spinal cord injury and the cervical spondylotic myelopathy: New approach based on the mechanical features of the spinal cord white and gray matter. J. Neurosurg. 2003, 99, 278–285. [Google Scholar] [CrossRef]
- Fabbri, R.; Cacopardo, L.; Ahluwalia, A.; Magliaro, C. Advanced 3D Models of Human Brain Tissue Using Neural Cell Lines: State-of-the-Art and Future Prospects. Cells 2023, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Gregory, H.; Phillips, J.B. Materials for peripheral nerve repair constructs: Natural proteins or synthetic polymers? Neurochem. Int. 2021, 143, 104953. [Google Scholar] [CrossRef]
- Hsiao, D.; Hsu, S.H.; Chen, R.S.; Chen, M.H. Characterization of designed directional polylactic acid 3D scaffolds for neural differentiation of human dental pulp stem cells. J. Formos. Med. Assoc. 2020, 119, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qin, S.H.; He, M.; Zhou, D.F.; Qin, Q.D.; Wang, H. Current applications of poly(lactic acid) composites in tissue engineering and drug delivery. Compos. Part B-Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Xu, R.; Fang, Y.; Zhang, Z.; Cao, Y.; Yan, Y.; Gan, L.; Xu, J.; Zhou, G. Recent Advances in Biodegradable and Biocompatible Synthetic Polymers Used in Skin Wound Healing. Materials 2023, 16, 5459. [Google Scholar] [CrossRef]
- Lachaux, J.; Salmon, H.; Loisel, F.; Arouche, N.; Ochoa, I.; Fernandez, L.L.; Uzan, G.; Mercier, O.; Veres, T.; Roy, E. Soft Thermoplastic Elastomer for Easy and Rapid Spin-Coating Fabrication of Microfluidic Devices with High Hydrophilization and Bonding Performances. Adv. Mater. Technol. 2019, 4, 1800308. [Google Scholar] [CrossRef]
- Van den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lee, M.J. Quick tips for interpreting cell death experiments. Nat. Cell Biol. 2023, 25, 1720–1723. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Fournier, E.; Passirani, C.; Montero-Menei, C.N.; Benoit, J.P. Biocompatibility of implantable synthetic polymeric drug carriers: Focus on brain biocompatibility. Biomaterials 2003, 24, 3311–3331. [Google Scholar] [CrossRef]
- Nyska, A.; Schiffenbauer, Y.S.; Brami, C.T.; Maronpot, R.R.; Ramot, Y. Histopathology of biodegradable polymers: Challenges in interpretation and the use of a novel compact MRI for biocompatibility evaluation. Polym. Adv. Technol. 2014, 25, 461–467. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef] [PubMed]
- Bos, R.R.M.; Rozema, F.R.; Boering, G.; Nijenhuis, A.J.; Pennings, A.J.; Verwey, A.B.; Nieuwenhuis, P.; Jansen, H.W.B.; Debruijn, W.C. Degradation of and Tissue Reaction to Biodegradable Poly(L-Lactide) for Use as Osteosynthesis. Adv. Biomat. 1992, 10, 405–411. [Google Scholar]
- Christen, M.O.; Vercesi, F. Polycaprolactone: How a Well-Known and Futuristic Polymer Has Become an Innovative Collagen-Stimulator in Esthetics. Clin. Cosmet. Investig. Dermatol. 2020, 13, 31–48. [Google Scholar] [CrossRef]
- Kim, J.A.; Van Abel, D. Neocollagenesis in human tissue injected with a polycaprolactone-based dermal filler. J. Cosmet. Laser Ther. 2015, 17, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S. Changes in Dermal Thickness in Biopsy Study of Histologic Findings After a Single Injection of Polycaprolactone-Based Filler into the Dermis. Aesthet. Surg. J. 2019, 39, Np484–Np494. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer-Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Martin, K.E.; Garcia, A.J. Macrophage phenotypes in tissue repair and the foreign body response: Implications for biomaterial-based regenerative medicine strategies. Acta Biomater. 2021, 133, 4–16. [Google Scholar] [CrossRef]
- Bova, L.; Maggiotto, F.; Micheli, S.; Giomo, M.; Sgarbossa, P.; Gagliano, O.; Falcone, D.; Cimetta, E. A Porous Gelatin Methacrylate-Based Material for 3D Cell-Laden Constructs. Macromol. Biosci. 2023, 23, e2200357. [Google Scholar] [CrossRef]
- Monteiro, N.; Thrivikraman, G.; Athirasala, A.; Tahayeri, A.; Franca, C.M.; Ferracane, J.L.; Bertassoni, L.E. Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dent. Mater. 2018, 34, 389–399. [Google Scholar] [CrossRef]
- Sensharma, P.; Madhumathi, G.; Jayant, R.D.; Jaiswal, A.K. Biomaterials and cells for neural tissue engineering: Current choices. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Elkhoury, K.; Morsink, M.; Sanchez-Gonzalez, L.; Kahn, C.; Tamayol, A.; Arab-Tehrany, E. Biofabrication of natural hydrogels for cardiac, neural, and bone Tissue engineering Applications. Bioact. Mater. 2021, 6, 3904–3923. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, R.; Santhamoorthy, M.; Alagumalai, K.; Haldhar, R.; Raorane, C.J.; Raj, V.; Kim, S.C. Novel Approach in Biodegradation of Synthetic Thermoplastic Polymers: An Overview. Polymers 2022, 14, 4271. [Google Scholar] [CrossRef] [PubMed]
- Duffy, P.; McMahon, S.; Wang, X.; Keaveney, S.; O’Cearbhaill, E.D.; Quintana, I.; Rodríguez, F.J.; Wang, W.X. Synthetic bioresorbable poly-α-hydroxyesters as peripheral nerve guidance conduits; a review of material properties, design strategies and their efficacy to date. Biomater. Sci. 2019, 7, 4912–4943. [Google Scholar] [CrossRef]
- Vach Agocsova, S.; Culenova, M.; Birova, I.; Omanikova, L.; Moncmanova, B.; Danisovic, L.; Ziaran, S.; Bakos, D.; Alexy, P. Resorbable Biomaterials Used for 3D Scaffolds in Tissue Engineering: A Review. Materials 2023, 16, 4267. [Google Scholar] [CrossRef]
- Chansoria, P.; Asif, S.; Polkoff, K.; Chung, J.; Piedrahita, J.A.; Shirwaiker, R.A. Characterizing the Effects of Synergistic Thermal and Photo-Cross-Linking during Biofabrication on the Structural and Functional Properties of Gelatin Methacryloyl (GelMA) Hydrogels. ACS Biomater. Sci. Eng. 2021, 7, 5175–5188. [Google Scholar] [CrossRef]



| Material | Printing Temperature (°C) | Flow Speed (mm/s) | Infill Speed (mm/s) | Nozzle Diameter (mm) | Extruder |
|---|---|---|---|---|---|
| PLA | 220 | 1.00 | 8 | 0.40 | Filament |
| PCL | 80 | 1.00 | 8 | 0.40 | Filament |
| FF | 249 | 1.00 | 6 | 0.80 | Filament |
| FD | 230 | 1.75 | 3 | 0.40 | Pellet |
| GelMA | 21–22 | 1.50 | 6 | 0.41 | Syringe 5 cc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etayo-Escanilla, M.; Campillo, N.; Ávila-Fernández, P.; Baena, J.M.; Chato-Astrain, J.; Campos, F.; Sánchez-Porras, D.; García-García, Ó.D.; Carriel, V. Comparison of Printable Biomaterials for Use in Neural Tissue Engineering: An In Vitro Characterization and In Vivo Biocompatibility Assessment. Polymers 2024, 16, 1426. https://doi.org/10.3390/polym16101426
Etayo-Escanilla M, Campillo N, Ávila-Fernández P, Baena JM, Chato-Astrain J, Campos F, Sánchez-Porras D, García-García ÓD, Carriel V. Comparison of Printable Biomaterials for Use in Neural Tissue Engineering: An In Vitro Characterization and In Vivo Biocompatibility Assessment. Polymers. 2024; 16(10):1426. https://doi.org/10.3390/polym16101426
Chicago/Turabian StyleEtayo-Escanilla, Miguel, Noelia Campillo, Paula Ávila-Fernández, José Manuel Baena, Jesús Chato-Astrain, Fernando Campos, David Sánchez-Porras, Óscar Darío García-García, and Víctor Carriel. 2024. "Comparison of Printable Biomaterials for Use in Neural Tissue Engineering: An In Vitro Characterization and In Vivo Biocompatibility Assessment" Polymers 16, no. 10: 1426. https://doi.org/10.3390/polym16101426
APA StyleEtayo-Escanilla, M., Campillo, N., Ávila-Fernández, P., Baena, J. M., Chato-Astrain, J., Campos, F., Sánchez-Porras, D., García-García, Ó. D., & Carriel, V. (2024). Comparison of Printable Biomaterials for Use in Neural Tissue Engineering: An In Vitro Characterization and In Vivo Biocompatibility Assessment. Polymers, 16(10), 1426. https://doi.org/10.3390/polym16101426










