Abstract
Successful preparation of PVA/SrTiO3/CNT polymer nanocomposite films was accomplished via the solution casting method. The structural, optical, and thermal properties of the films were tested by XRD, SEM, FTIR, TGA, and UV-visible spectroscopy. Inclusion of the SrTiO3/CNT nanofillers with a maximum of 1 wt% drastically improved the optical and thermal properties of PVA films. SrTiO3 has a cubic crystal structure, and its average crystal size was found to be 28.75 nm. SEM images showed uniform distribution in the sample with 0.3 wt% of SrTiO3/CNTs in the PVA film, while some agglomerations appeared in the samples of higher SrTiO3/CNT content, i.e., at 0.7 and 1.0 wt%, in the PVA polymer films. The inclusion of SrTiO3/CNTs improved the thermal stability of PVA polymer films. The direct and indirect optical band gaps of the PVA films decreased when increasing the mass of the SrTiO3/CNTs, while the single-oscillator energy (E0) and dispersion energy (Ed) increased. The films’ refractive indices were gradually increased upon increasing the nanofillers’ weight. In addition, improvements in the optical susceptibility and nonlinear refractive indices’ values were also obtained. These films are qualified for optoelectronic applications due to their distinct optical and thermal properties.
1. Introduction
Polymers have garnered significant attention from researchers owing to their unique physical and chemical properties, coupled with their affordability, easy fabrication, stability across intermediate temperatures, transparency to visible and infrared wavelengths, and more [1,2,3]. Certain polymers like polyvinyl chloride (PVC) and polyvinyl alcohol (PVA) exhibit high transparency, enabling them to transmit a wide range of wavelengths, making them advantageous in various applications. PVA polymers are known for their ease of fabrication, high hydrophilicity, non-toxicity, availability, and dielectric strength, as well as their distinct optical, physical, and chemical properties [4,5,6]. Hence, PVA polymer holds promise for various applications due to the aforementioned features. Moreover, the properties of these polymers can be further enhanced through doping with suitable nanoparticles or nanofillers [7]. The properties of dopants, including their small size, optimal shape, and expansive surface area, serve as key factors that bolster the properties of the polymer [6].
Various studies have been conducted on polymer nanocomposites; however, the optical properties of PVA polymer have not yet been deeply investigated. The optical and structural properties of PVA were investigated when incorporating a low percentage of graphene oxide (GO) up to 1 wt%. The inclusion of GO into the PVA films reduced their direct and indirect optical band gaps [6]. Several studies have investigated the impact of GO on the properties of PVA polymer [8,9,10].
The structural, dielectric, and optical characteristics of PVA polymer were investigated upon incorporation of Ag-BaTiO3 nanofillers. The resulting PVA-Ag-BaTiO3 films exhibited favorable dielectric and optical characteristics, suggesting their suitability for electric and optoelectronic applications [11]. The structural and optical properties of PVA when doped with PANI/Ag nanoparticles have been studied, and its optical properties have a direct dependency on the nanoparticles’ mass [12].
Strontium titanate (SrTiO3) has been employed as a dopant for polymer films due to its distinct characteristics, i.e., a high dielectric constant, low dielectric loss, and minimal leakage current. With a relatively wide band gap energy of approximately 3.2 eV and a cubic perovskite structure at room temperature, SrTiO3 is anticipated to improve the thermal and optical performance of PVA polymer films when used as a dopant [3,13,14]. Hence, previously the thermal and dielectric properties of undoped PVA and doped PVA with SrTiO3 were examined. Despite the relatively high percentage of SrTiO3, ranging from 5 to 15 wt%, the PVA/SrTiO3 nanocomposites exhibited favorable performance [15].
On other hand, CNTs are expected to cause light scattering within PVA films, thus increasing the path length of photons, particularly in the UV–vis region. CNTs are sheets of graphene formed into tubes which are widely used for structural enhancements. CNTs are hundred-folds stronger than steel [16,17]. The inclusion of SrTiO3 into PVA can cause photon scattering. The presence of SrTiO3/CNTs in a polymer film is also expected to enhance the thermal stability of the polymer. A recent study has shown a positive impact on the optical properties of PVDF polymer when doped with SrTiO3/CNTs [17]. Therefore, using SrTiO3/CNTs as dopants in PVA films has a high potential to improve their optical and thermal stability. The influence of SrTiO3/CNTs on PVA films has not yet been investigated. Therefore, this work studies the impact of SrTiO3/CNT mixtures as dopants on the structural, thermal, and optical properties of PVA polymer films. The PVA polymer films doped with (0.0, 0.3, 0.7, and 1.0 wt%) SrTiO3/CNTs were synthesized via the solution casting method. The structural, optical, and thermal properties of these films were measured. Therein, various characterization techniques, i.e., XRD, SEM, TGA, and UV-visible spectroscopy, were utilized. The thermal and optical performance of PVA films were improved upon the inclusion of SrTiO3/CNTs. The distinct properties of PVA/SrTiO3/CNT polymer nanocomposite films indicate that the films have high potential to be included in optoelectronic applications.
2. Experimental
2.1. Materials
Polyvinyl alcohol (PVA), carbon nanotubes (CNTs), and strontium titanate (SrTiO3) nanopowder were purchased from Nanografi locating in Ankara, Turkey. Ethanol absolute was purchased from Sigma-Aldrich, Burlington, MA, USA.
2.2. Preparation Method and Characterization
A mixture of 0.2 g CNTs and 0.8 g SrTiO3 nanopowder was dissolved into 50 mL of ethanol for an hour. The mixture was exposed to ultrasonic waves for an hour and then dried inside an electric oven under 50 °C. Finally, the leftover product was finely ground. The solution casting technique was utilized to process the polymer films. As such, 1 g of PVA was added to 80 mL of deionized water under magnetic stirring for an hour at ambient temperature to be dissolved. Different contents of SrTiO3/CNTs, i.e., 0.003, 0.007, and 0.01 g, were added to the PVA solution samples and stirred regularly for an hour. The resulting solutions, each with a volume of ~80 mL, were poured into glass petri dishes and left to dry in an oven under 80 °C for 48 h. Then, the polymer films were peeled off and taken for characterization. The thickness and dimensions of these films were almost the same, where the average of the thickness was nearly 112 µm. A Shimadzu diffractometer (Kyoto, Japan) with model of 7000 and λ of CuKα radiation = 1.54056 Å scanning in the range of 2θ = 10–80° was used to show the XRD patterns of the polymer films that consisted of the pure PVA and mixed PVA with 0.3, 0.7, and 1 wt% SrTiO3/CNTs. A Shimadzu spectrometer (FTIR–Tracer 100, Kyoto, Japan) scanning wavenumbers from 399 to 4000 cm−1 was utilized to record ATR spectra. A Thermo Fisher Quattro ESEM (Thermo Fisher Scientific, Waltham, MA, USA) was also used to produce FESEM micrographs of our samples. This advanced technique was crucial as a source of morphology and microstructure information about the polymer films. The mass loss of the polymer nanocomposite films was examined as function of temperature in the range of 30–600 °C by a Shimadzu TGA-51 Thermogravimetric Analyzer (Kyoto, Japan). A Cary 60 UV–vis spectrophotometer, Agilent (Santa Clara, CA, USA) at a wavelength scan 190–1000 nm was used to provide optical measurements.
3. Result and Discussion
3.1. Structural Characterization
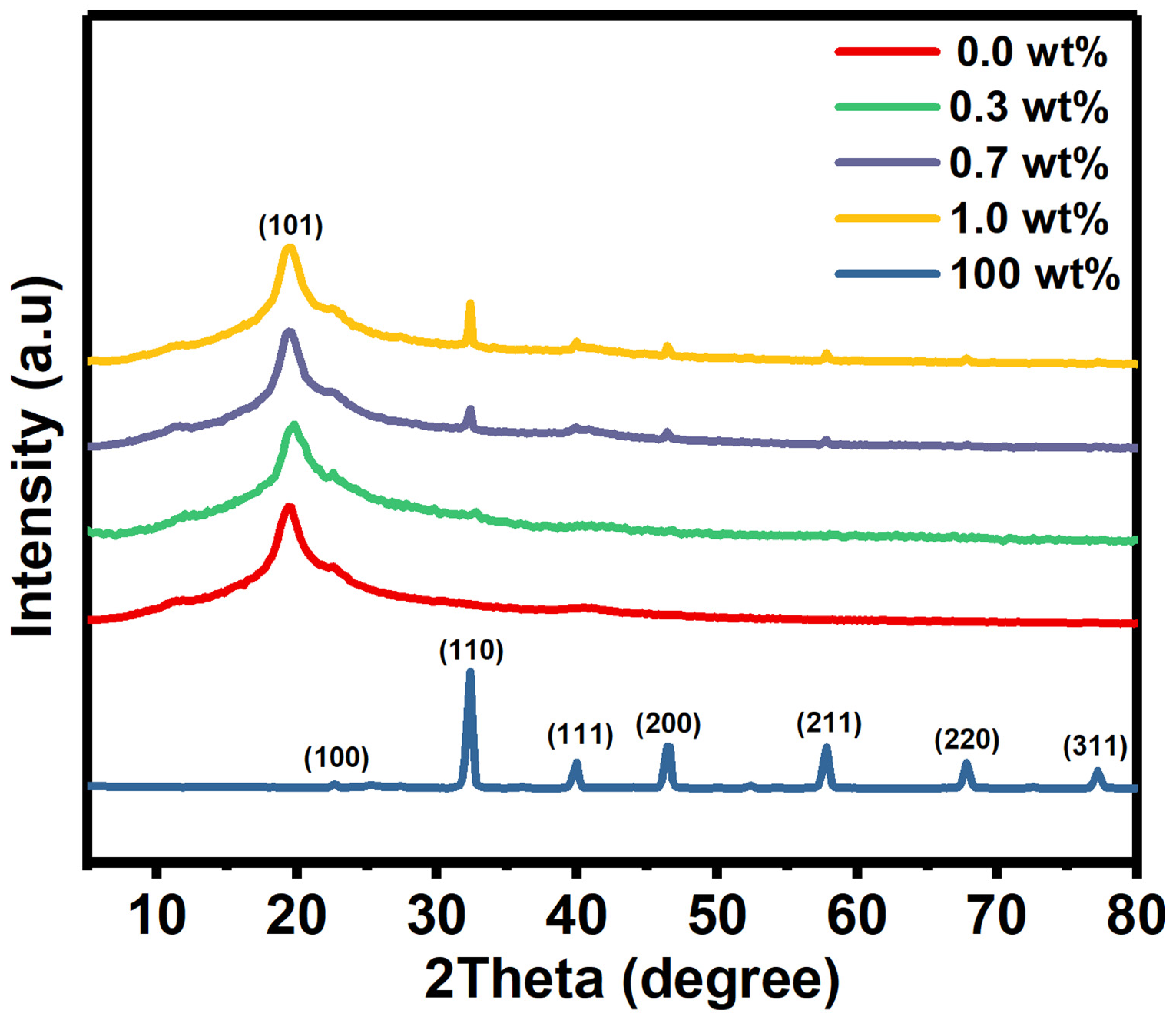
XRD as a well-known identification technique, was used to obtain information about the crystal structure of the films. Here, the XRD patterns of pure PVA film, SrTiO3/CNTs, and doped PVA with 0.3, 0.7, and 1 wt% SrTiO3/CNTs are shown in Figure 1. A broad peak centered at 2θ = 19.4° was observed, resulting from the (101) plane of PVA in its monoclinic crystal structure [18]. The small peak appearing at 2θ = 40.6° indicates the semi-crystallinity structure of the PVA film [19,20]. The molecular strong interaction by intermolecular hydrogen bonding formed the nature of the PVA’s crystallinity [20].
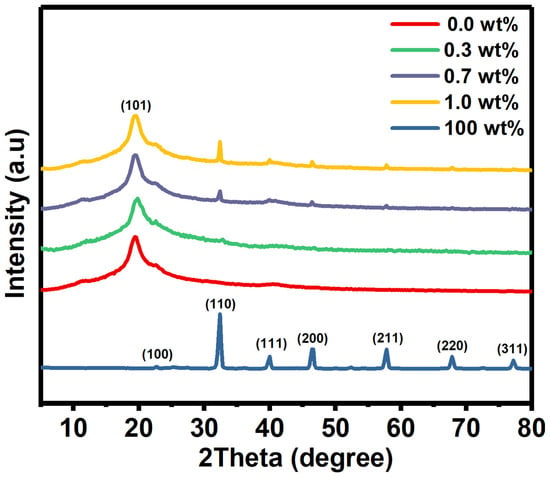
Figure 1.
The XRD diffraction peaks of pure PVA and doped PVA with SrTiO3/CNTs.
The SrTiO3/CNT film has various peaks located at 2θ = 22.66°, 32.3°, 39.95°, 46.5°, 57.77°, 67.86°, and 77.25° which are assigned to the (100), (110), (111), (200), (211), (220), and (310) planes [3]. It is obvious that when increasing the SrTiO3/CNT content from 0.3 to 1 wt% in the PVA polymer films, the intensity at 2θ = 32.3° gradually increases as expected, since that peak is related to the (110) plane of SrTiO3. Here and based on XRD patterns, the SrTiO3 has a cubic crystal structure, as reported in JCPDS card (35–0734) [21].
The average crystal size of the nanoparticles can be calculated by the Debye–Scherrer method (see Equation (1)).
D is the average crystal size, while and are the wavelength of the X-ray and full width at half maximum, respectively. The SrTiO3 crystal size is an average of 28.75 nm.
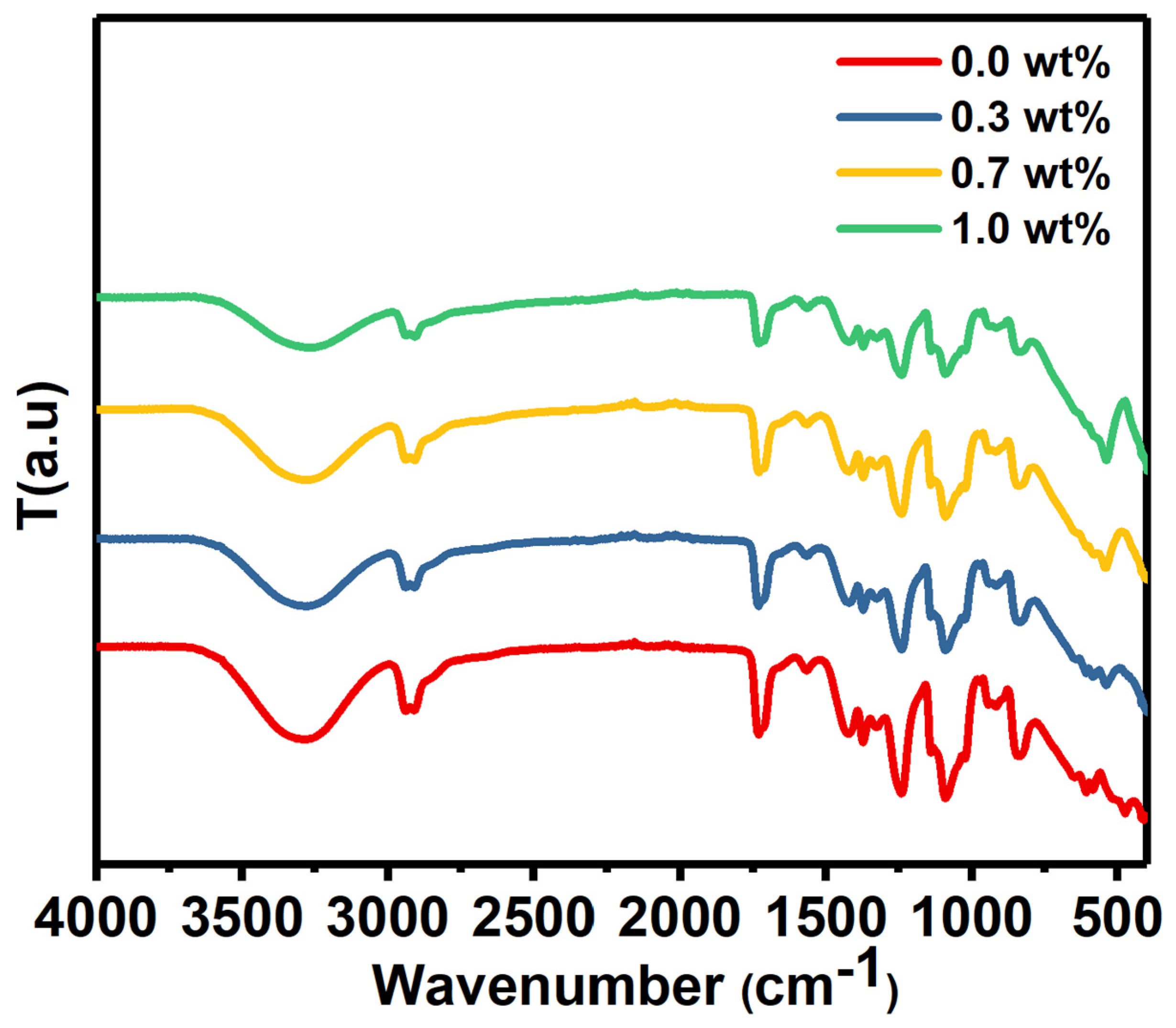
The FTIR spectrum of PVA/SrTiO3/CNTs was measured from 400 cm−1 to 4000 cm−1, as depicted in Figure 2.
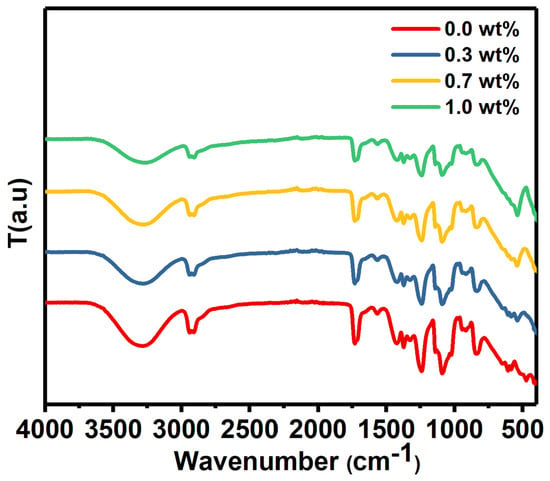
Figure 2.
The FTIR spectrum of pure PVA and PVA doped with SrTiO3/CNTs.
The FTIR spectrum had a broad absorption band centered at nearly 3270 cm−1 for pure PVA film and doped PVA with different masses of SrTiO3/CNTs. These bands originated from –OH stretching vibration [22]. C–H stretching and out-of-plane C–H stretching caused the peaks located around 2920 and 1426 cm−1, respectively [23]. C–O stretching vibration caused the band at 1090 cm−1 [24].
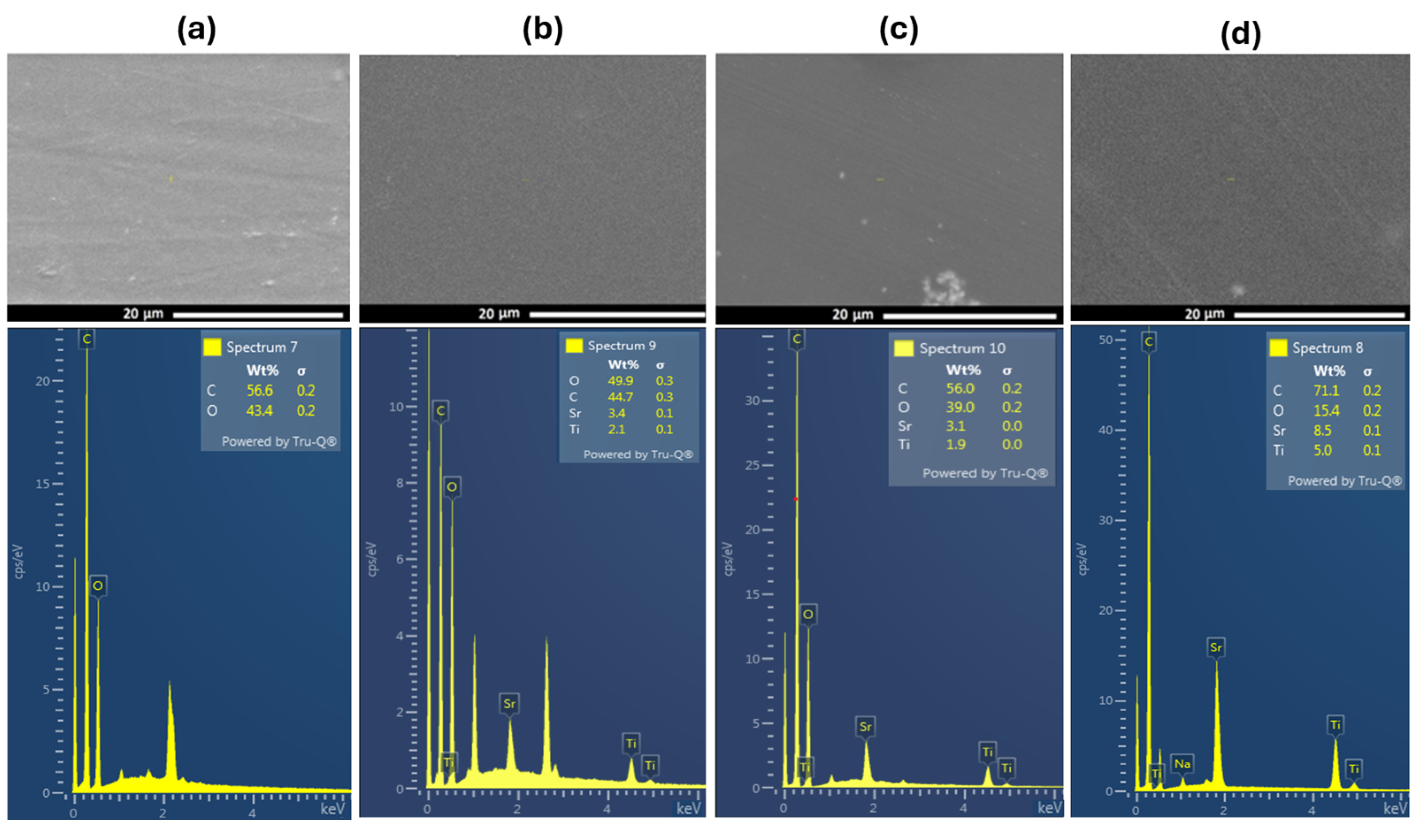
The scanning electron micrograph images of PVA and PVA doped with SrTiO3/CNTs are shown in Figure 3. A SEM image of pure PVA film is shown in Figure 3a. The inclusion of a small content of SrTiO3/CNTs, i.e., 0.3 wt%, showed uniform distribution in the PVA polymer film, as depicted in Figure 3b. However, when the dopants’ content increased to 0.7 and 1 wt%, some agglomerations were formed, as shown in the bottom of the SEM images in Figure 3c,d. The formation of agglomerations can be attributed to the dopants and the conditions of the polymer film preparation [18]. The increased concentration of nanoparticles could prevent their uniform dispersion and interfacial interaction between polymer chains and nanoparticles [3,18,25]. The crosslinking between PVA and SrTiO3/CNTs is assigned to either strong hydrogen bonds or the increased viscosity of the polymer nanocomposites during the preparation process, and such phenomena have been previously reported in [3,26,27]. Figure 3a–d shows the EDS analysis of the included elements in PVA films incorporating 0.3, 0.7, and 1 wt% of SrTiO3/CNT samples, respectively. The EDX analysis evidences the increment of the SrTiO3 ratios in the prepared PVA polymer films. The corresponding elemental distribution mapping is illustrated in Figure S1 (ESI).
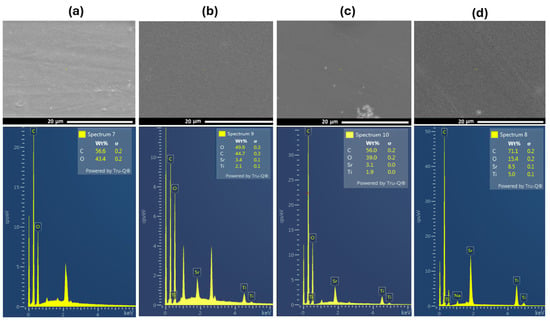
Figure 3.
SEM scans of (a) pure PVA film and (b–d) PVA films doped with SrTiO3/CNTs.
3.2. Thermal Stability
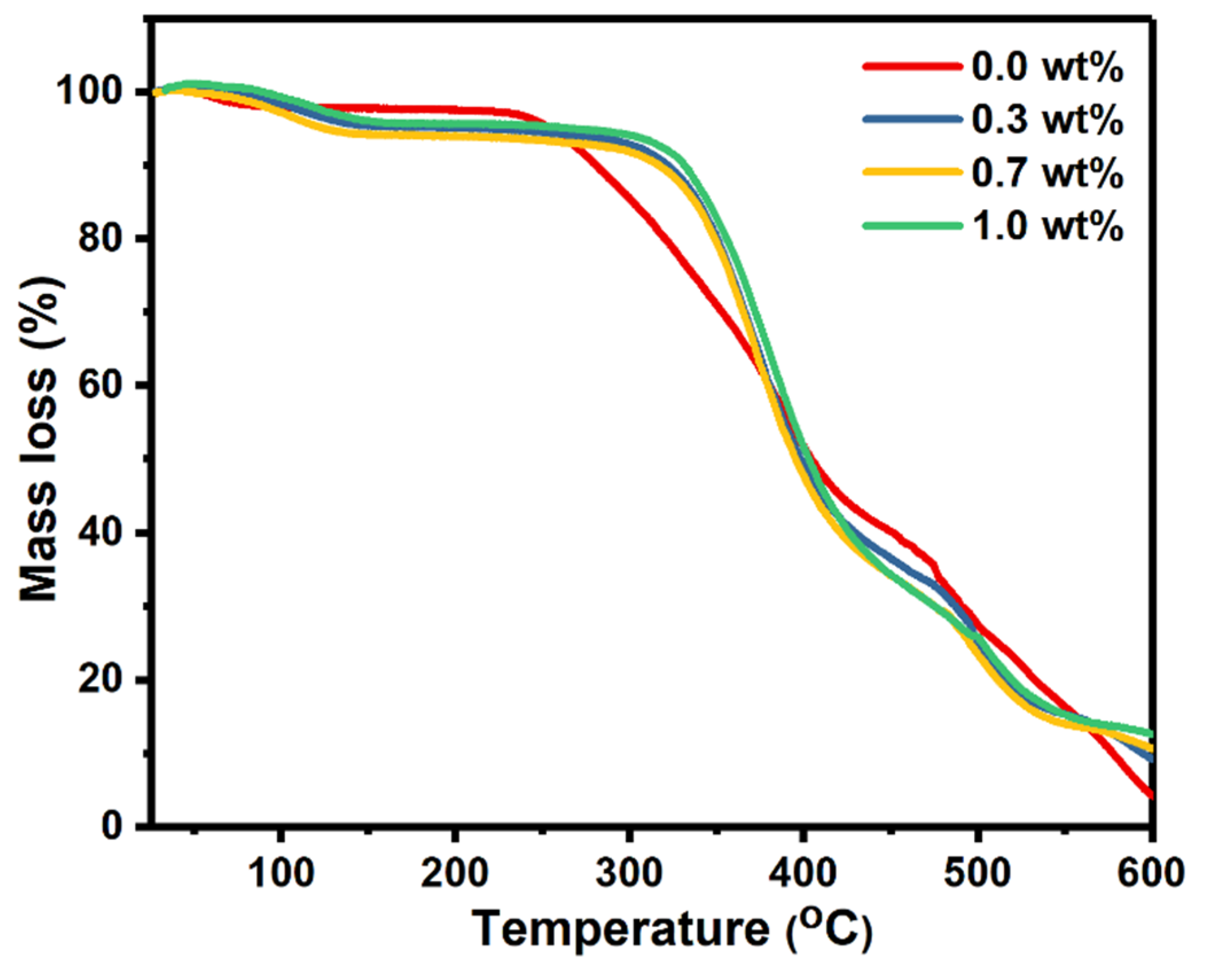
TGA analysis was used to investigate the thermal stability of the polymer nanocomposites under different temperatures. The mass losses of pure PVA and PVA doped with SrTiO3/CNT samples were plotted against the temperature, as shown in Figure 4.
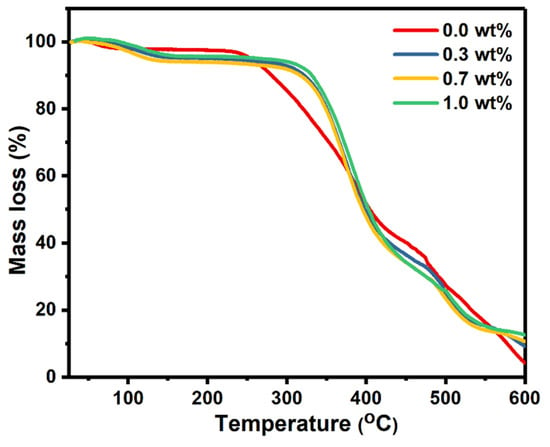
Figure 4.
TGA scans of pure PVA films and PVA doped with SrTiO3/CNTs.
It is obvious that there were three decomposition stages for all the polymer films. The first small degradation stage occurred in the range of 80–145 °C and is assigned to the evaporation of the solvent. For the pure PVA film, the second degradation stage occurred at 235–417 °C, while the third degradation stage began at 475 °C and continued up to the end of the scale at 600 °C. The doped PVA films with SrTiO3/CNTs also had two degradation stages at 310–424 °C and 500–550 °C. Incorporating the SrTiO3/CNTs into the PVA delayed the thermal degradation, since the second degradation stage of pure PVA began at 235 °C while the doped PVA remained stable up to 310 °C. It is noticeable that small additions of SrTiO3/CNTs with a maximum of 1 wt% kept the PVA films stable for 75 °C more than pure PVA. Therefore, it is obvious the thermal stability of the PVA films was clearly improved when incorporating SrTiO3/CNTs.
3.3. Optical Measurements
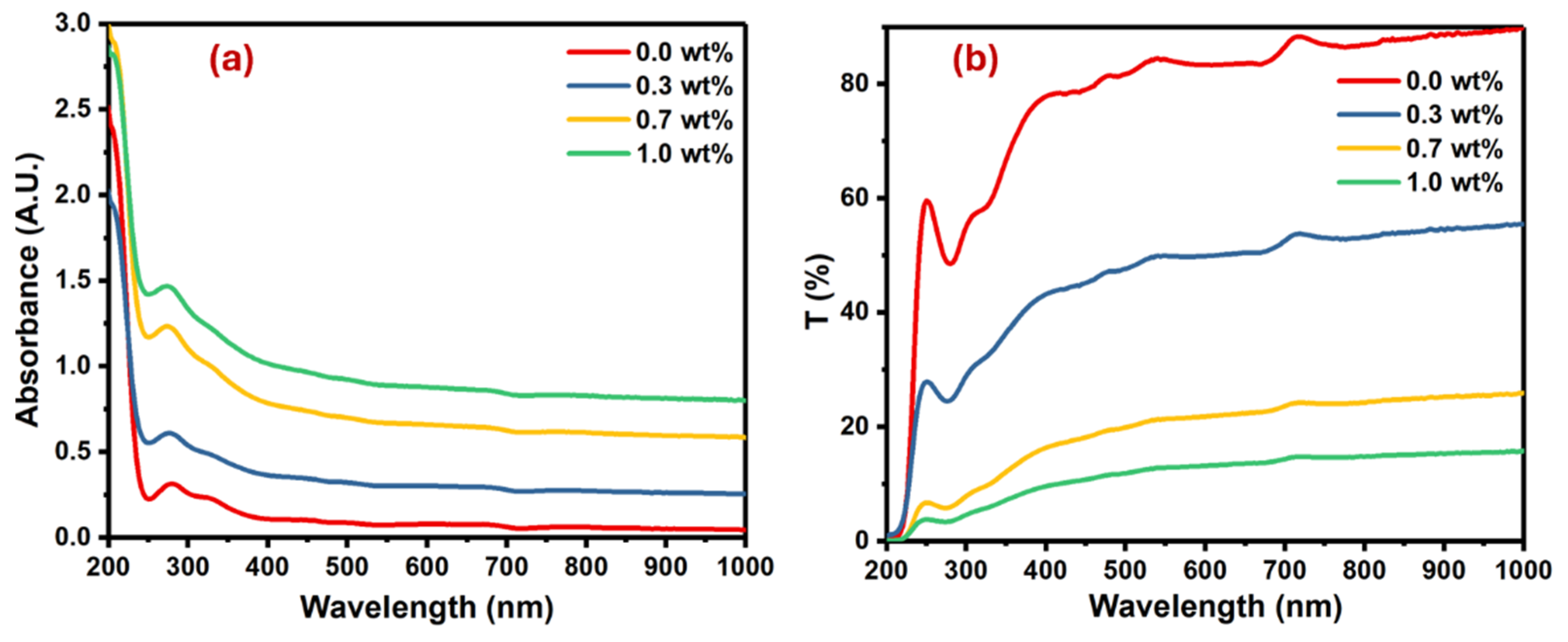
The optical properties of pure PVA and PVA doped with different masses of SrTiO3/CNTs were investigated, and the results of their optical absorbance and transmittance spectra are shown in Figure 5.
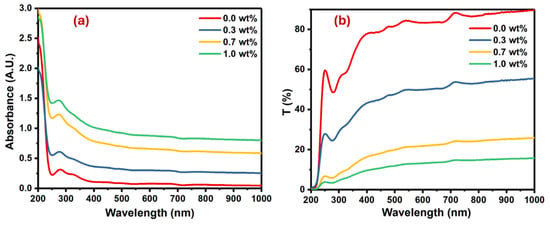
Figure 5.
Plots for the PVA films doped with SrTiO3/CNTs: (a) absorbance versus wavelength and (b) transmittance versus wavelength.
The pure PVA film had an absorption band at 280 nm because of the π→π* interband electronic transitions [28,29]. Upon the addition of SrTiO3/CNTs into the PVA film, the absorption band slightly blue shifted due to band gap widening, as shown in Figure 5a. The magnitude of the blue shift intensified with higher concentrations of SrTiO3/CNTs, peaking at 6 nm when 1 wt% SrTiO3/CNTs was present. This significant increase suggests the successful preparation of polymer nanocomposites. The increase in the blue shift with higher concentrations of SrTiO3/CNTs can be attributed to the interactions between the nanoparticles and the polymer. As the concentration of SrTiO3/CNTs increases, there is a greater incorporation of these nanoparticles into the polymer matrix. This incorporation alters the optical properties of the nanocomposite material, leading to the absorption or emission wavelengths shifting towards the blue region of the spectrum. The peak shift reaching its maximum at 1 wt% SrTiO3/CNTs indicates optimal nanoparticle dispersion and interaction within the polymer matrix, reflecting the successful preparation of the nanocomposites. Moreover, as the concentration of SrTiO3/CNTs increased, it caused a band defect formation in the PVA film [30]. Figure 5b shows the transmittance of undoped PVA as well as doped PVA with SrTiO3/CNTs.
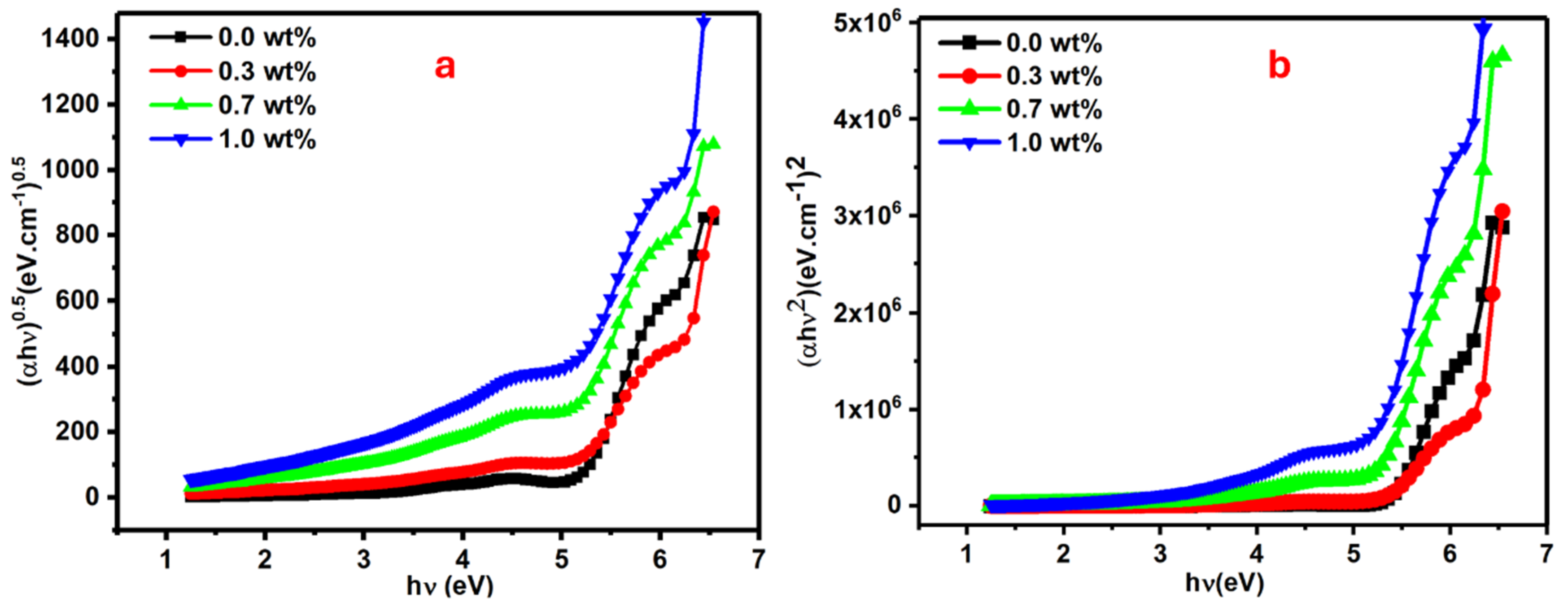
It is obvious the transmittance decreased with the increasing mass of SrTiO3/CNTs. This observation is typical, since the inclusion and dispersion of nanoparticles in the polymer films results in the scattering of incident photons. The optical band gap in the polymer film is usually obtained by the Tauc equation (see Equation (2)) [31,32]:
where α is a coefficient of absorption and B is a constant, while hυ indicates incident photon energy. The n value will be 0.5 in the case of direct allowed transitions and 2 in case of indirect allowed transitions. The direct and indirect band gap values can be estimated from the interception of the extended straight line of the curve to the zero absorption axis in Figure 6a,b.
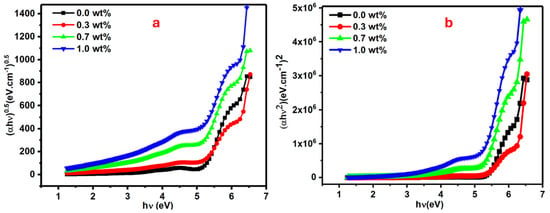
Figure 6.
Plots for the PVA films doped with SrTiO3/CNTs: (a) (∝hυ)0.5 vs. hυ and (b) (∝hυ)2 vs. hυ for the PVA/SrTiO3/CNT films.
The direct optical band gap for pure and doped PVA with 0.3, 0.7, and 1 wt% SrTiO3/CNTs gradually decreased to 5.06, 4.86, 4.76, and 4.50 eV, respectively. The tiny mass of the dopant in the range of 0.3–1 wt% caused drastic changes in the value of the direct band gap. The indirect optical band gap for pure and doped PVA with 0.3, 0.7 and 1 wt% SrTiO3/CNTs gradually decreased to 5.31, 5.10, 5.08, and 4.92 eV, respectively.
Edir and Eind values as listed in Table 1 are decreased with the increments of SrTiO3/CNTs. The substantial shift in the energy band gap of PVA, ranging from 5.06 to 4.50 eV, suggests that SrTiO3/CNT nanoparticles induce modifications in the electronic structure of the PVA matrix. This alteration is attributed to the localized electronic states formed by the incorporated SrTiO3/CNT nanoparticles within the optical band gap of PVA, functioning as trapping and recombination centers. Consequently, the observed change in the optical band gap occurs. Additionally, the decrease in the optical band gap may be attributed to an increase in the degree of disorder in the samples, resulting from changes in the polymer structure [33,34,35,36,37].

Table 1.
Optical parameters of SrTiO3/CNT doped PVA films.
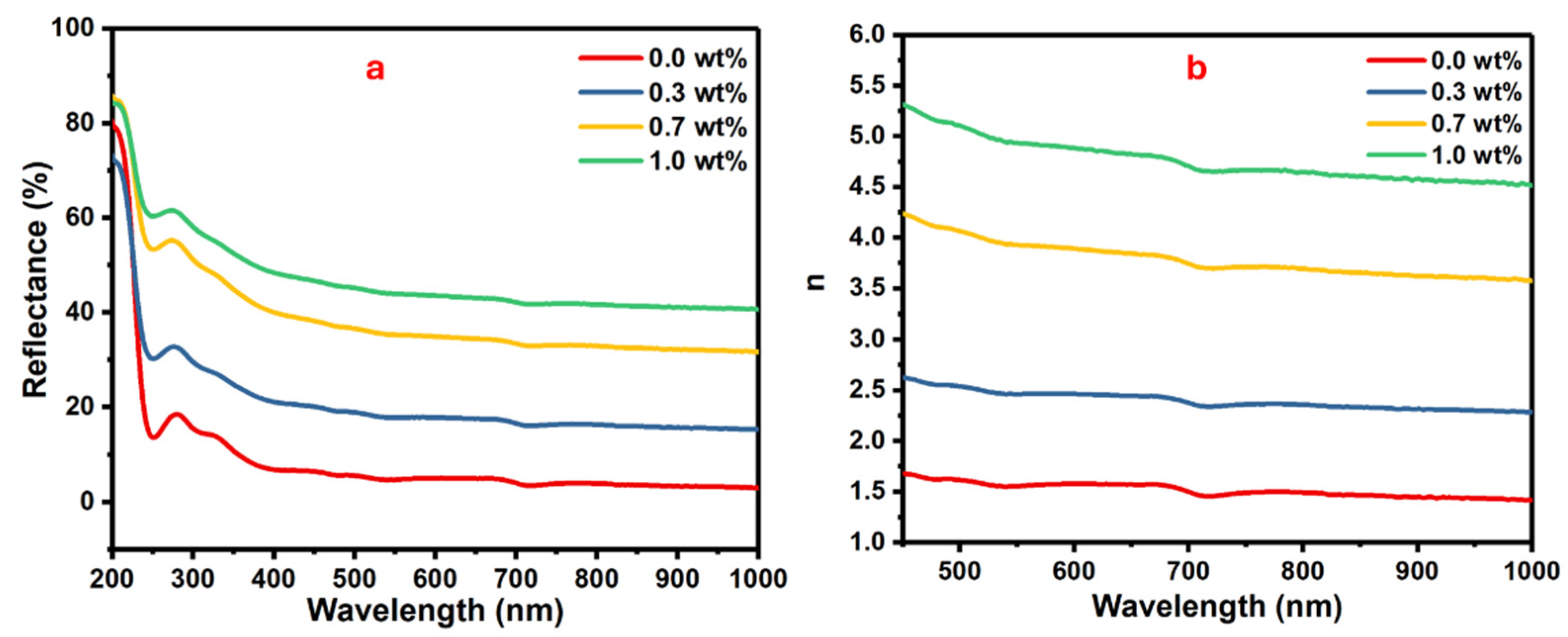
Investigating the refractive index of the polymer nanocomposite films is meaningful for determining their suitable applications. The films with the advantage of a high refractive index and electrical performance are qualified for optoelectronic applications. Some factors, i.e., molecular structure, nanofillers, film thickness, etc., can influence a film’s refractive index [38].
The reflectance and refractive index as function of wavelength are plotted in Figure 7. Reflectance gives information about the amount of reflected light from a surface with respect to incident light.
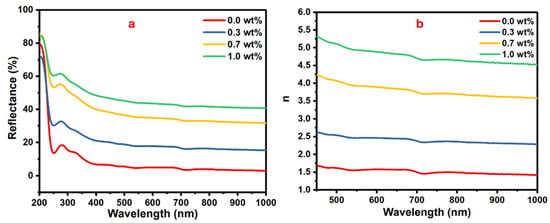
Figure 7.
The (a) reflectance vs. wavelength and (b) the refractive index vs. wavelength for the PVA/SrTiO3/CNT polymer films.
The changes in the reflectance against the wavelength for the samples of PVA doped with different masses of SrTiO3/CNTs are shown in Figure 7a. Equation (3) shows the relationship between reflectance (R) and refractive index (n) [39,40].
where the extinction coefficient is represented by (k ). The refractive indices are gradually increased with the increasing mass of SrTiO3/CNTs, as displayed in Figure 6b. The enhancement of the refractive index is attributed to the formation of a large cluster from the gathered SrTiO3/CNT nanoparticles [41]. The polymer films with a high high-refractive-index are qualified for optical device applications, i.e., optical coatings, anti-reflection screens, etc.
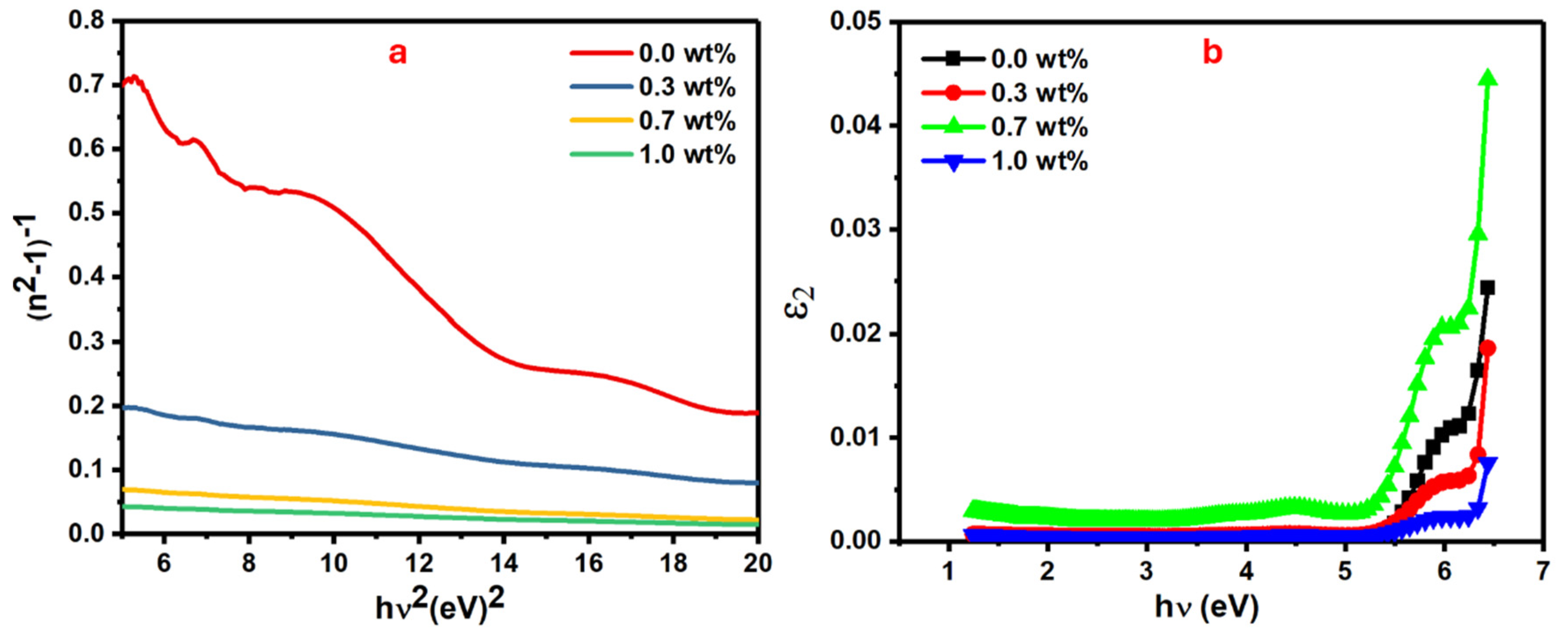
The material dispersion parameters, known as oscillator energy, dispersion energy, and transition moments, can be evaluated from refractive index dispersion as in Equation (4), which includes single-oscillator energy (E0) and dispersion energy (Ed) [42].
Figure 8a shows the plot of the (n2 − 1)−1 vs. (hυ)2 where E0 and Ed values are obtained from its slope and intercept. The Ed values directly increase from 4.69 to 92.57 eV when the mass of SrTiO3/CNTs increases from 0 to 1 wt%, while the E0 values show an incremental trend from 4.48 eV to 4.98, as listed in Table 2.
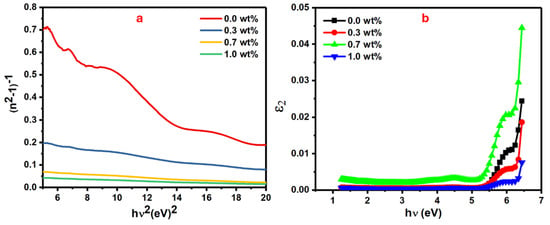
Figure 8.
CNT (a) (n2 − 1)−1 versus , and (b) 2 versus h.

Table 2.
Summarized dispersion parameters of PVA/SrTiO3/CNT polymer films.
The enhanced intermolecular interactions, which resulted from increments in polymer chain packing during orientation, caused a significant increase in Ed values [43]. In addition, the significant increase in Ed values could have occurred due to thermal fluctuations that originated from dispersion forces or long-range van der Waals [44] forces. The single oscillator model, in the case of zero photon energy (hυ = 0), can be utilized to obtain a polymer’s static refractive index (n0) [45];
The calculated n0 values increased from 1.43 to 4.42 when the dopant (SrTiO3/CNTs) mass increased from 0 wt% to 1 wt%. It is well known that polymer films which have a high refractive index are qualified to be applied in optoelectronic devices. The addition of SrTiO3/CNTs as a dopant increased the refractive index of the PVA polymer films, and hence increasing the amount of added dopant enhanced the optical properties of the films. Therefore, these prepared films could be employed in display screens, encapsulations of organic light-emitting diodes, image sensors, and fabrication of plastic lenses that are used in eyeglasses [46,47].
Figure 8b shows the plot of the ɛ2 as optical dielectric loss which is expressed by 2nk vs. hv for the doped PVA films with different masses of SrTiO3/CNTs [48]. From this figure, the interception of the extrapolated linear part of the curve along the hυ axis gives the values of the real band gap (Er), which is a crucial parameter in determining the recommended optical transitions [49].
The Er values are relatively close to the values of Eind along with all additions of the dopants. Due to these outcomes, the majority of optical transitions in these films follow the indirect transitions. The oscillator strength (f) of doped polymer films is another important parameter that affects their optical performance. There are some key factors that play major roles in tuning the oscillator strength (f) of the films, such as the chemical structure and the molecular mass of the polymer, film thickness, and molecules’ aggregation in the polymer film. Knowing these factors helps to produce polymer film with optimal optical properties for intended applications. Equation (5) can be used to calculate the oscillator strength of the polymer film [50];
The oscillator strength values of the PVA films are summarized in Table 2 and gradually increase with the additions of SrTiO3/CNTs. The molecular electronic structure depends on the chemical structure of the polymer films, and thus linear optical susceptibility (χ(1)) and third order nonlinear optical susceptibility (χ(3)) are controlled by their electronic structure. Some applications of the polymer films, i.e., optical data storage and optical switching, rely on their linear and nonlinear optical susceptibilities [51].
The χ(1) and χ(3) values of PVA/SrTiO3/CNT films summarized in Table 2 are calculated from Equation (7) [52];
The dopants can interact with the polymer and result in the enhancement of the local electric field as well as create new energy levels, and thus increase χ(3) [53]. The polarizability of the molecules is dependent on the chemical structure of the polymer molecules and therefore influences the nonlinear refractive index (n2) [54]. The n2 is a crucial parameter to determine the suitable applications for the prepared polymer films which can be calculated by Equation (8) [55,56];
It is obvious that as we increased the content of SrTiO3/CNTs, the polarizability of the polymer molecules enhanced and thus increased the nonlinear refractive index (n2), as reported in Table 2. The linear and nonlinear refractive indices of polymer films are improved for the PVA films with the additions of the SrTiO3/CNTs. Therefore, the prepared films have high potential to be used in optoelectronic applications. Some optical parameters of PVA/SrTiO3/CNT polymer films, i.e., Edir, Eind, n0, χ(3), and n2, were used for comparison with previous studies, as reported in Table 3. The value of Edir in this study is relatively smaller than the values of previous studies. The values of n0, χ(3), and n2 are greater than those reported in previous studies, which demonstrates the preference of PVA/SrTiO3/CNT polymer films for optoelectronic applications.

Table 3.
The optical parameters, i.e., Edir, Eind, n0, χ(3), and n2, of PVA/SrTiO3/CNT polymer films compared with optical parameters of other related polymer nanocomposites.
4. Conclusions
In conclusion, this study successfully fabricated PVA/SrTiO3/CNT polymer nanocomposite films using the solution casting method. The SrTiO3 nanoparticles exhibited a cubic crystal structure with an average crystal size of 28.75 nm. Incorporation of SrTiO3/CNTs up to 1 wt% resulted in an increase in the lattice parameter of SrTiO3 within the PVA polymer film from 3.91 Å to 3.95 Å. Additionally, the inclusion of SrTiO3/CNT nanofillers up to 1 wt% significantly improved the optical properties of the PVA films. Moreover, the thermal stability of the films was enhanced, with the onset of the second degradation stage increasing from 235 °C for pure PVA to 310 °C for doped PVA. SEM images showed a uniform distribution of 0.3 wt% SrTiO3/CNTs in the PVA polymer films, while some agglomerations were observed in films containing 0.7 and 1.0 wt% SrTiO3/CNTs. The direct and indirect optical band gaps of PVA films decreased with increasing mass percentage of SrTiO3/CNTs. Furthermore, the single-oscillator energy (E0), dispersion energy (Ed), and optical susceptibility values increased with increasing nanofiller weight, with calculated n0 values ranging from 1.43 to 4.42. Additionally, improvements in nonlinear refractive index values were observed due to the inclusion of SrTiO3/CNTs. These findings suggest that the fabricated films exhibit promising optical and thermal properties suitable for optoelectronic applications. Future research efforts will focus on investigating the electrical properties of PVA/SrTiO3/CNT polymer nanocomposite films through electrical measurements.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym16101392/s1. Figure S1: EDX elemental mapping analysis of the prepared samples (a) PVA, (b) PVA/SrTiO3/CNTs, (3wt%) (c) PVA/SrTiO3/CNTs (7wt%), and PVA/SrTiO3/CNTs(10wt%).
Funding
This work was funded by the Deanship of Graduate Studies and Scientific Research at Jouf University under grant No. (DGSSR-2023-02-02020).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The author declares no conflict of interest.
References
- Patel, V.K.; Kant, R.; Chauhan, P.S.; Bhattacharya, S. Introduction to Applications of Polymers and Polymer Composites. Trends Appl. Polym. Polym. Compos. 2022. [Google Scholar] [CrossRef]
- Brinson, H.F.; Brinson, L.C.; Brinson, H.F.; Brinson, L.C. Characteristics, applications and properties of polymers. Polym. Eng. Sci. Viscoelasticity Introd. 2015, 57–100. [Google Scholar]
- Alshammari, A.H.; Alshammari, M.; Alshammari, K.; Allam, N.K.; Taha, T.A. PVC/PVP/SrTiO3 polymer blend nanocomposites as potential materials for optoelectronic applications. Results Phys. 2023, 44, 106173. [Google Scholar] [CrossRef]
- Vera Garcia, P.F.; Guerrero Dimas, L.A.; Cedillo Portillo, J.J.; Martínez Anguiano, O.A.; Sáenz Galindo, A.; Narro Cespedes, R.I.; Acuña Vazquez, P.; Castañeda Facio, A. PVA Blends and Nanocomposites, Properties and Applications: A Review. Green-Based Nanocompos. Mater. Appl. 2023, 191–206. [Google Scholar]
- Zyoud, S.H.; Almoadi, A.; AlAbdulaal, T.H.; Alqahtani, M.S.; Harraz, F.A.; Al-Assiri, M.S.; Yahira, I.S.; Zahran, H.Y.; Mohammed, M.I.; Abdel-Wahab, M.S. Structural, Optical, and Electrical Investigations of Nd2O3-Doped PVA/PVP Polymeric Composites for Electronic and Optoelectronic Applications. Polymers 2023, 15, 1351. [Google Scholar] [CrossRef] [PubMed]
- Hurayra–Lizu, K.A.; Bari, M.W.; Gulshan, F.; Islam, M.R. GO based PVA nanocomposites: Tailoring of optical and structural properties of PVA with low percentage of GO nanofillers. Heliyon 2021, 7, e06983. [Google Scholar] [CrossRef]
- Benabid, F.Z.; Kharchi, N.; Zouai, F.; Mourad, A.H.I.; Benachour, D. Impact of co-mixing technique and surface modification of ZnO nanoparticles using stearic acid on their dispersion into HDPE to produce HDPE/ZnO nanocomposites. Polym. Polym. Compos. 2019, 27, 389–399. [Google Scholar] [CrossRef]
- Gahlot, S.; Kulshrestha, V.; Agarwal, G.; Jha, P.K. Synthesis and characterization of PVA/GO nanocomposite films. In Macromolecular Symposia; Wiley: Weinheim, Germany, 2018; Volume 357, pp. 173–177. [Google Scholar]
- Kashyap, S.; Pratihar, S.K.; Behera, S.K. Strong and ductile graphene oxide reinforced PVA nanocomposites. J. Alloys Compd. 2016, 684, 254–260. [Google Scholar] [CrossRef]
- Yahia, I.S.; Mohammed, M.I. Facile synthesis of graphene oxide/PVA nanocomposites for laser optical limiting: Band gap analysis and dielectric constants. J. Mater. Sci. Mater. Electron. 2018, 29, 8555–8563. [Google Scholar] [CrossRef]
- Oreibi, I.; Ali Habeeb, M.; Abdul Hamza, R.S. Polymer nanocomposites comprising PVA matrix and Ag–BaTiO3 nanofillers: A comparative study of structural, dielectric and optical characteristics for optics and quantum nanoelectronic applications. Opt. Quantum Electron. 2024, 56, 119. [Google Scholar] [CrossRef]
- Abdelhamied, M.M.; Atta, A.; Abdelreheem, A.M.; Farag, A.T.M.; El Okr, M.M. Synthesis and optical properties of PVA/PANI/Ag nanocomposite films. J. Mater. Sci. Mater. Electron. 2020, 31, 22629–22641. [Google Scholar] [CrossRef]
- Wang, D.; Ye, J.; Kako, T.; Kimura, T. Photophysical and photocatalytic properties of SrTiO3 doped with Cr cations on different sites. J. Phys. Chem. B 2006, 110, 15824–15830. [Google Scholar] [CrossRef]
- Botelho, C.N.; Falcão, S.S.; Soares RE, P.; Pereira, S.R.; de Menezes, A.S.; Kubota, L.T.; Damos, F.S.; Luz, R.C. Evaluation of a photoelectrochemical platform based on strontium titanate, sulfur doped carbon nitride and palladium nanoparticles for detection of SARS-CoV-2 spike glycoprotein S1. Biosens. Bioelectron. X 2022, 11, 100167. [Google Scholar] [CrossRef] [PubMed]
- Taha, T.A.; Alzara, M.A.A. Synthesis, thermal and dielectric performance of PVA-SrTiO3 polymer nanocomposites. J. Mol. Struct. 2021, 1238, 130401. [Google Scholar] [CrossRef]
- Venkatesan, D.; Aravind Kumar, J.; Mohana Prakash, R. Synthesis, Properties, and Applications of Polymer Nanocomposite Matrices. In Handbook of Polymer and Ceramic Nanotechnology; Springer International Publishing: Cham, Switzerland, 2021; pp. 465–485. [Google Scholar]
- Taha, T.A.M.; Alanazi, S.S.; El-Nasser, K.S.; Alshammari, A.H.; Ismael, A. Structure–Property Relationships in PVDF/SrTiO3/CNT Nanocomposites for Optoelectronic and Solar Cell Applications. Polymers 2024, 16, 736. [Google Scholar] [CrossRef]
- Alshammari, K.; Alashgai, T.; Alshammari, A.H.; Abdelhamied, M.M.; Alotibi, S.; Atta, A. Effects of Nd2O3 Nanoparticles on the Structural Characteristics and Dielectric Properties of PVA Polymeric Films. Polymers 2023, 15, 4084. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K.; Timmiati, S.N.; Masdar, M.S. New composite membrane poly (vinyl alcohol)/graphene oxide for direct ethanol–proton exchange membrane fuel cell. J. Appl. Polym. Sci. 2019, 136, 46928. [Google Scholar] [CrossRef]
- Ali, H.E.; Abdel-Aziz, M.M.; Algarni, H.; Yahia, I.S. The structure analysis and optical performance of PVA films doped with Fe3+-metal for UV-limiter, and optoelectronics. Mater. Res. Express 2019, 6, 085334. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Muhamad Sarih, N.; Mohamad, S.; Joon Ching, J. SrtiO3 nanocube-doped polyaniline nanocomposites with enhanced photocatalytic degradation of methylene blue under visible light. Polymers 2016, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Sedlařík, V.; Saha, N.; Kuřitka, I.; Saha, P. Preparation and characterization of poly (vinyl alcohol)/lactic acid compounded polymeric films. Int. J. Polym. Anal. Charact. 2006, 11, 253–270. [Google Scholar] [CrossRef]
- Suganthi, S.; Vignesh, S.; Kalyana Sundar, J.; Raj, V. Fabrication of PVA polymer films with improved antibacterial activity by fine-tuning via organic acids for food packaging applications. Appl. Water Sci. 2020, 10, 100. [Google Scholar] [CrossRef]
- Mohsin, M.; Hossin, A.; Haik, Y. Thermal and mechanical properties of poly (vinyl alcohol) plasticized with glycerol. J. Appl. Polym. Sci. 2011, 122, 3102–3109. [Google Scholar] [CrossRef]
- Althubiti, N.A.; Al-Harbi, N.; Sendi, R.K.; Atta, A.; Henaish, A.M. Surface characterization and electrical properties of low energy irradiated PANI/PbS polymeric nanocomposite materials. Inorganics 2023, 11, 74. [Google Scholar] [CrossRef]
- Kayış, A.; Kavgacı, M.; Yaykaşlı, H.; Kerli, S.; Eskalen, H. Investigation of structural, morphological, mechanical, thermal and optical properties of PVA-ZnO nanocomposites. Glass Phys. Chem. 2021, 47, 451–461. [Google Scholar] [CrossRef]
- AlAbdulaal, T.H.; Ali, H.E.; Ganesh, V.; Aboraia, A.M.; Khairy, Y.; Hegazy, H.H.; Soldatov, V.A.; Zahran, H.Y.; Abdel-Wahab, M.S.; Yahia, I.S. Investigating the structural morphology, linear/nonlinear optical characteristics of Nd2O3 doped PVA polymeric composite films: Kramers-Kroning approach. Phys. Scr. 2021, 96, 125831. [Google Scholar] [CrossRef]
- Sankhla, A.; Sharma, R.; Yadav, R.S.; Kashyap, D.; Kothari, S.L.; Kachhwaha, S. Biosynthesis and characterization of cadmium sulfide nanoparticles—An emphasis of zeta potential behavior due to capping. Mater. Chem. Phys. 2016, 170, 44–51. [Google Scholar] [CrossRef]
- Bhajantri, R.F.; Ravindrachary, V.; Harisha, A. Microstructural studies on BaCl2 doped poly(vinyl alcohol). Polymer 2006, 47, 3591–3598. [Google Scholar] [CrossRef]
- Heiba, Z.K.; Mohamed, M.B.; Imam, N.G. Fine-tune optical absorption and light emitting behavior of the CdS/PVA hybridized film nanocomposite. J. Mol. Struct. 2017, 1136, 321–329. [Google Scholar] [CrossRef]
- Mahmoud, W.E.; Al-Ghamdi, A.A.; Al-Agel, F. Synthesis and optical properties of poly (vinyl acetate)/bismuth oxide nanorods. Polym. Adv. Technol. 2011, 22, 2055–2061. [Google Scholar] [CrossRef]
- Mahmoud, W.E.; Shirbeeny, W.; Al-Ghamdi, A.A.; Al-Heniti, S. Synthesis and characterization of CdxZn1−xO nanoparticles-doped aryl poly ether ether ketone for novel application potentials. J. Appl. Polym. Sci. 2012, 125, 339–343. [Google Scholar] [CrossRef]
- Shanshool, H.M.; Yahaya, M.; Yunus, W.M.; Abdullah, I.Y. Investigation of energy band gap in polymer/ZnO nanocomposites. J. Mater. Sci. Mater. Electron. 2016, 27, 9804–9811. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Aziz, S.B.; Omer, K.M.; Salih, Y.M. Reducing the optical band gap of polyvinyl alcohol (PVA) based nanocomposite. J. Mater. Sci. Mater. Electron. 2015, 26, 5303–5309. [Google Scholar] [CrossRef]
- Muhammad, F.F.; Aziz, S.B.; Hussein, S.A. Effect of the dopant salt on the optical parameters of PVA:NaNO3 solid polymer electrolyte. J. Mater. Sci. Mater. Electron. 2015, 26, 521–529. [Google Scholar] [CrossRef]
- Rozra, J.; Saini, I.; Sharma, A.; Chandak, N.; Aggarwal, S.; Dhiman, R.; Sharma, P.K. Cu nanoparticles induced structural, optical and electrical modification in PVA. Mater. Chem. Phys. 2012, 134, 1121–1126. [Google Scholar] [CrossRef]
- Abdelaziz, M. Cerium (III) doping effects on optical and thermal properties of PVA films. Phys. B 2011, 406, 1300–1307. [Google Scholar] [CrossRef]
- Alhassan, S.; Alshammari, M.; Alshammari, K.; Alotaibi, T.; Alshammari, A.H.; Fawaz, Y.; Taha, T.A.; Henini, M. Preparation and Optical Properties of PVDF-CaFe2O4 Polymer Nanocomposite Films. Polymers 2023, 15, 2232. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, S.; Alshammari, K.; Alshammari, M.; Alotaibi, T.; Alshammari, A.H.; Fawaz, Y.; Taha, T.A.; Henini, M. Synthesis and optical properties of polyvinylidene difluoride nanocomposites comprising MoO3/g-C3N4. Results Phys. 2023, 48, 106403. [Google Scholar] [CrossRef]
- El-naggar, A.M.; Heiba, Z.K.; Mohamed, M.B.; Kamal, A.M.; Osman, M.M.; Albassam, A.A.; Lakshminarayana, G. Improvement of the optical characteristics of PVA/PVP blend with different concentrations of SnS2/Fe. J. Vinyl Addit. Technol. 2022, 28, 82–93. [Google Scholar] [CrossRef]
- Yasir, M.; Sai, T.; Sicher, A.; Scheffold, F.; Steiner, U.; Wilts, B.D.; Dufresne, E.R. Enhancing the Refractive Index of Polymers with a Plant-Based Pigment. Small 2021, 17, 2103061. [Google Scholar] [CrossRef]
- Štrbac, D.D.; Lukić, S.R.; Petrović, D.M.; Gonzalez-Leal, J.M.; Srinivasan, A. Single oscillator energy and dispersion energy of uniform thin chalcogenide films from Cu–As–S–Se system. J. Non-Cryst. Solids 2007, 353, 1466–1469. [Google Scholar] [CrossRef]
- Dutcher, J.R.; Dalnoki-Veress, K.; Nickel, B.G.; Roth, C.B. Instabilities in thin polymer films: From pattern formation to rupture. In Macromolecular Symposia; Wiley: Weinheim, Germany, 2000; Volume 159, pp. 143–150. [Google Scholar]
- Taha, T.A.; Hendawy, N.; El-Rabaie, S.; Esmat, A.; El-Mansy, M.K. Effect of NiO NPs doping on the structure and optical properties of PVC polymer films. Polym. Bull. 2019, 76, 4769–4784. [Google Scholar] [CrossRef]
- Higashihara, T.; Ueda, M. Recent Progress in High Refractive Index Polymers. Macromolecules 2015, 48, 1915–1929. [Google Scholar] [CrossRef]
- Tang, Y.; Cabrini, S.; Nie, J.; Pina-Hernandez, C. High-refractive index acrylate polymers for applications in nanoimprint lithography. Chin. Chem. Lett. 2020, 31, 256–260. [Google Scholar] [CrossRef]
- Deshpande, M.P.; Solanki, G.K.; Agarwal, M.K. Optical band gap in tungsten diselenide single crystals intercalated by indium. Mater. Lett. 2000, 43, 66–72. [Google Scholar] [CrossRef]
- Soliman, T.S.; Zaki, M.F.; Hessien, M.M.; Elkalashy, S.I. The structure and optical properties of PVA-BaTiO3 nanocomposite films. Opt. Mater. 2021, 111, 110648. [Google Scholar] [CrossRef]
- Aziz, S.B.; Rasheed, M.A.; Hussein, A.M.; Ahmed, H.M. Fabrication of polymer blend composites based on [PVA-PVP] (1 − x):(Ag2S) × (0.01 ≤ x ≤ 0.03) with small optical band gaps: Structural and optical properties. Mater. Sci. Semicond. Process. 2017, 71, 197–203. [Google Scholar] [CrossRef]
- Mondal, R.; Biswas, D.; Paul, S.; Das, A.S.; Chakrabarti, C.; Roy, D.; Bhattacharya, S.; Kabi, S. Investigation of microstructural, optical, physical properties and dielectric relaxation process of sulphur incorporated selenium–tellurium ternary glassy systems. Mater. Chem. Phys. 2021, 257, 123793. [Google Scholar] [CrossRef]
- Nalwa, H.S. Organic materials for third-order nonlinear optics. Adv. Mater. 1993, 5, 341–358. [Google Scholar] [CrossRef]
- Keru, G.; Ndungu, P.G.; Nyamori, V.O. A review on carbon nanotube/polymer composites for organic solar cells. Int. J. Energy Res. 2014, 38, 1635–1653. [Google Scholar] [CrossRef]
- Divya, R.; Manikandan, N.; Girisun, T.S.; Vinitha, G. Investigations on the structural, morphological, linear and third order nonlinear optical properties of manganese doped zinc selenide nanoparticles for optical limiting application. Opt. Mater. 2020, 100, 109641. [Google Scholar] [CrossRef]
- Bredas, J.L.; Adant, C.; Tackx, P.; Persoons, A.; Pierce, B.M. Third-order nonlinear optical response in organic materials: Theoretical and experimental aspects. Chem. Rev. 1994, 94, 243–278. [Google Scholar] [CrossRef]
- Innocenzi, P.; Lebeau, B. Organic–inorganic hybrid materials for non-linear optics. J. Mater. Chem. 2005, 15, 3821–3831. [Google Scholar] [CrossRef]
- Dhatarwal, P.; Sengwa, R.J. Investigation on the optical properties of (PVP/PVA)/Al2O3 nanocomposite films for green disposable optoelectronics. Phys. B Condens. Matter 2021, 613, 412989. [Google Scholar] [CrossRef]
- Rashad, M. Tuning optical properties of polyvinyl alcohol doped with different metal oxide nanoparticles. Opt. Mater. 2020, 105, 109857. [Google Scholar] [CrossRef]
- Abd-Elnaiem, A.M.; Rashad, M.; Hanafy, T.A.; Shaalan, N.M. Improvement of optical properties of functionalized polyvinyl alcohol-zinc oxide hybrid nanocomposites for wide UV optoelectronic applications. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2429–2444. [Google Scholar] [CrossRef]
- Alrowaili, Z.A.; Taha, T.A.; El-Nasser, K.S.; Donya, H. Significant enhanced optical parameters of PVA-Y2O3 polymer nanocomposite films. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3101–3110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).