A Color-Detectable Vitamin C Controlled-Release System Fabricated Using Electrospinning
Abstract
1. Introduction
2. Experimental
2.1. Materials and Equipment
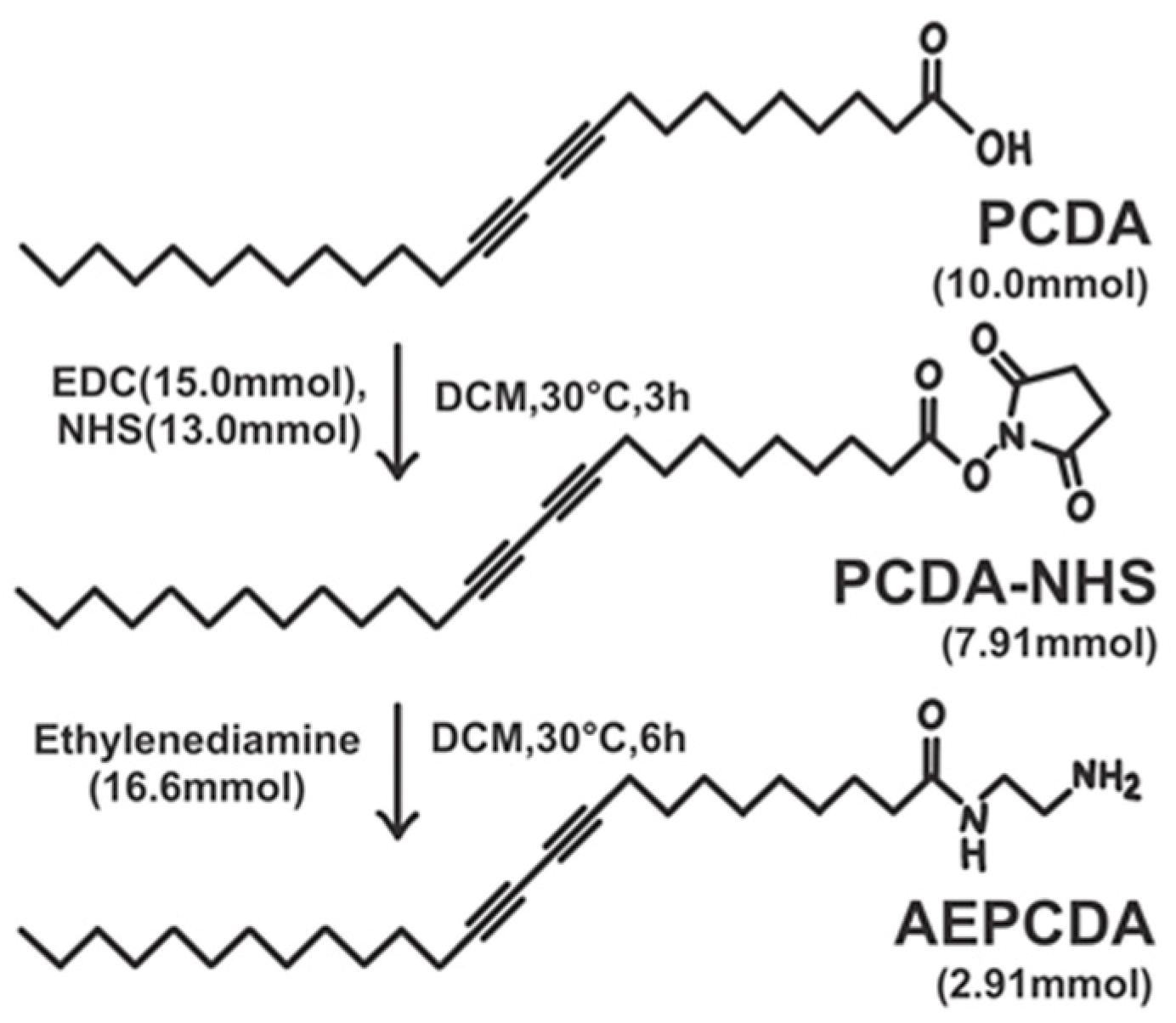
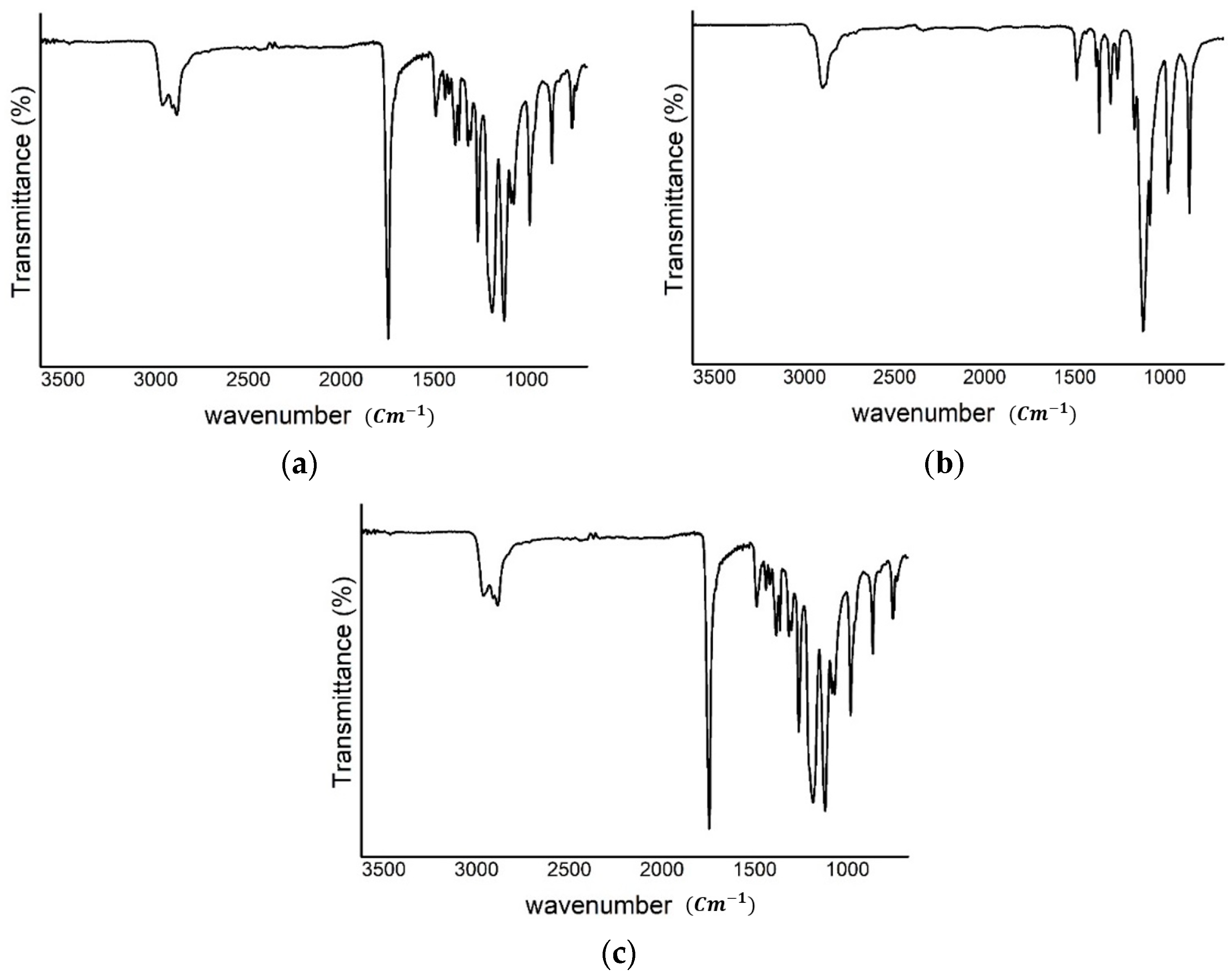
2.2. Synthesis of N-(2-Aminoethyl)pentacosa-10,12-diynamide (AEPCDA)
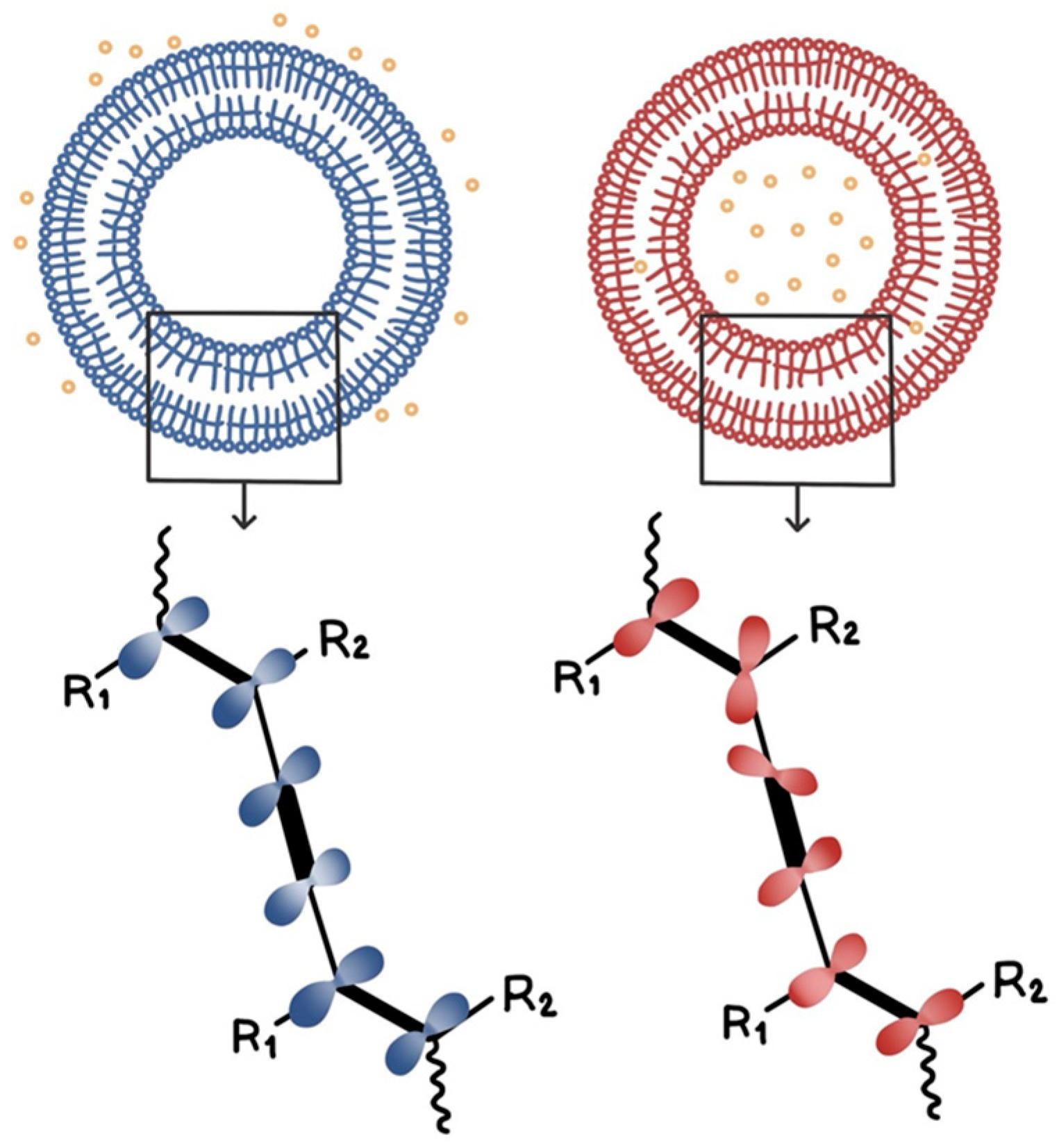
2.3. Formation of PDA Vesicle Solution
2.4. Electrospinning
2.5. Vitamin C Release
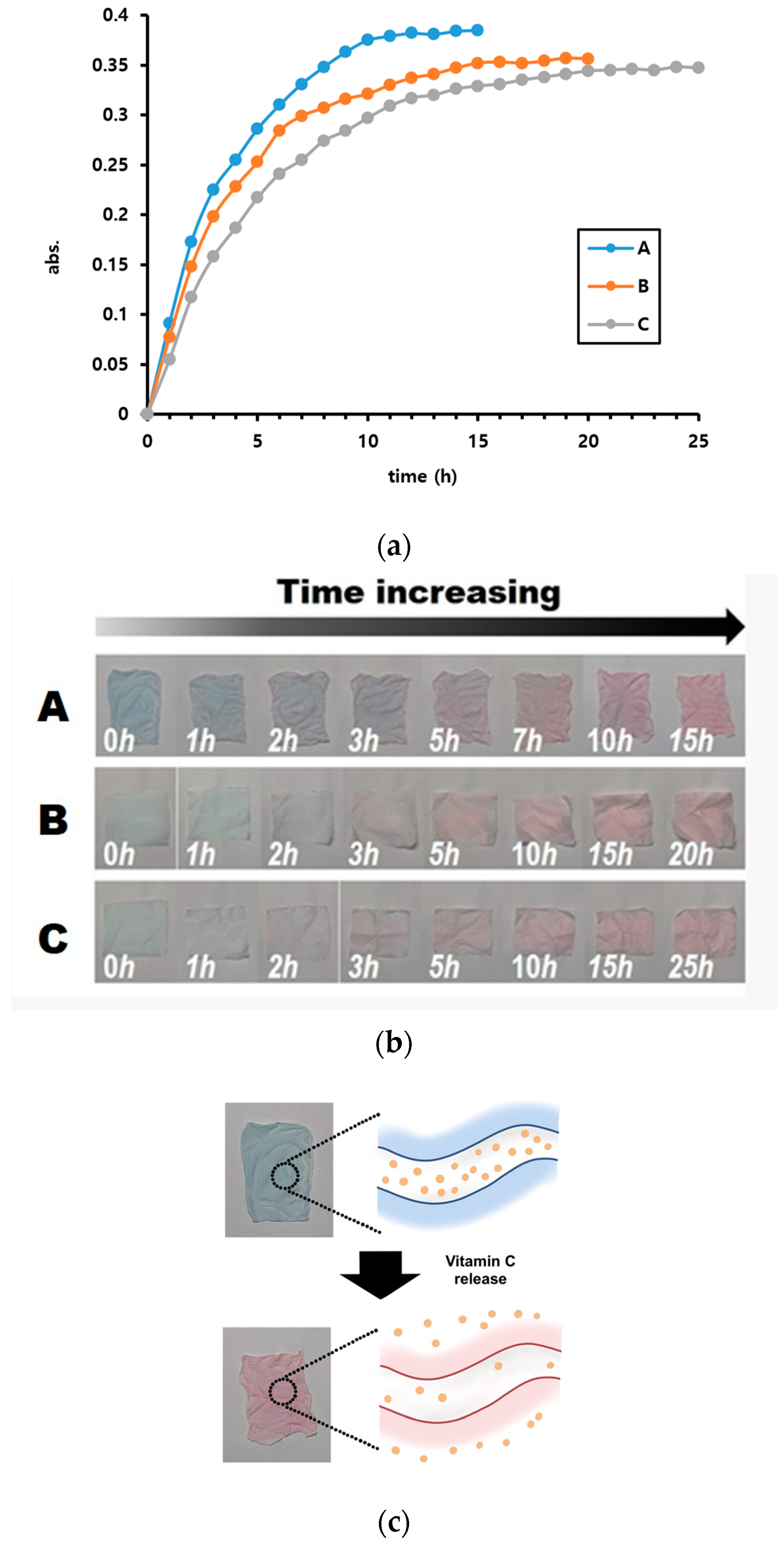
3. Results and Discussion
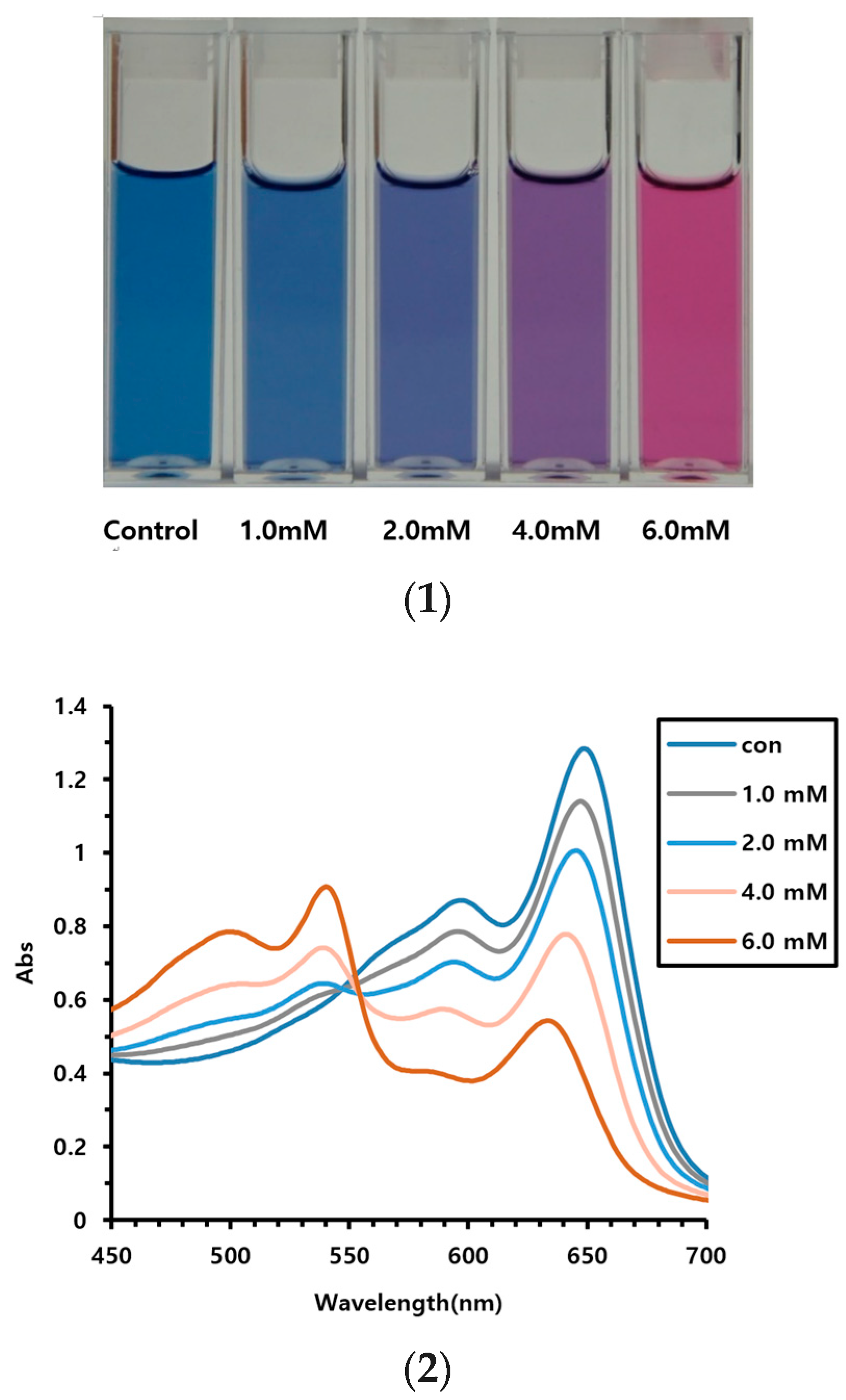
3.1. Vitamin C Detection Using AEPCDA Vesicle Solution
3.2. Detecting Vitamin C Release from Microfiber Mat
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boo, Y.C. Ascorbic acid (vitamin C) as a cosmeceutical to increase dermal collagen for skin antiaging purposes: Emerging combination therapies. Antioxidants 2022, 11, 1663. [Google Scholar] [CrossRef]
- Sim, M.; Hong, S.; Jung, S.; Kim, J.S.; Goo, Y.T.; Chun, W.Y.; Shin, D.M. Vitamin C supplementation promotes mental vitality in healthy young adults: Results from a cross-sectional analysis and a randomized, double-blind, placebo-controlled trial. Eur. J. Nutr. 2022, 61, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Giannakourou, M.C.; Taoukis, P.S. Effect of alternative preservation steps and storage on vitamin C stability in fruit and vegetable products: Critical review and kinetic modelling approaches. Foods 2021, 10, 2630. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Ramasamy, M.; Willis, N.C.; Kim, D.S.; Anderson, W.A.; Tam, K.C. Encapsulation and controlled release of vitamin C in modified cellulose nanocrystal/chitosan nanocapsules. Curr. Res. Food Sci. 2021, 4, 215–223. [Google Scholar] [CrossRef]
- Bacha, K.; Chemotti, C.; Monboisse, J.C.; Robert, A.; Furlan, A.L.; Smeralda, W.; Damblon, C.; Estager, J.; Brassart-Pasco, S.; Mbakidi, J.P.; et al. Encapsulation of vitamin C by glycerol-derived dendrimers, their interaction with biomimetic models of stratum corneum and their cytotoxicity. Molecules 2022, 27, 8022. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Farshi, P.; Ahmadi, P.; Ahmadi, A.; Yousefi, M.; Ghorbani, M.; Hosseini, S.M. Encapsulation of vitamins using nanoliposome: Recent advances and perspectives. Adv. Pharm. Bull. 2023, 13, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Susa, F.; Pisano, R. Advances in ascorbic Acid (vitamin C) manufacturing: Green extraction techniques from natural sources. Processes 2023, 11, 3167. [Google Scholar] [CrossRef]
- Khaldia, S.; Lamia, B.; Yasmina, K.; Lahcene, B. Preparation, characterization and antioxidant activity of microspheres made of cellulose triacetate (CTA) to control the release of vitamin C. J. Chem. Technol. Biotechnol. 2020, 95, 1800–1807. [Google Scholar] [CrossRef]
- Maurya, V.K.; Shakya, A.; McClements, D.J.; Srinivasan, R.; Bashir, K.; Ramesh, T.; Lee, J.; Sathiyamoorthi, E. Vitamin C fortification: Need and recent trends in encapsulation technologies. Front. Nutr. 2023, 10, 1229243. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.J.; Shin, Y.J.; Hwang, S.W.; Shin, J.S. Microencapsulation of imidazole curing agent by solvent evaporation method using W/O/W emulsion. J. Appl. Polym. Sci. 2013, 129, 1036–1044. [Google Scholar] [CrossRef]
- Nizori, A.; Bui, L.T.T.; Jiec, F.; Smalld, D.M. Spray-drying microencapsulation of ascorbic acid: Impact of varying loading content on physicochemical properties of microencapsulated powders. J. Sci. Food Agric. 2020, 100, 4165–4171. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.J.; Kim, J.G.; Shin, J.S. Microencapsulation of imidazole curing agents by spray-drying method using W/O emulsion. J. Appl. Polym. Sci. 2012, 126, E108–E115. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun nanofibers: New concepts, materials, and applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Progress in the field of electrospinning for tissue engineering applications. Adv. Mater. 2009, 21, 3343–3351. [Google Scholar] [CrossRef] [PubMed]
- Yubo Liu, Y.; Chen, X.; Liu, Y.; Gao, Y.; Liu, P. Electrospun coaxial fibers to optimize the release of poorly water-soluble drug. Polymers 2022, 14, 469. [Google Scholar] [CrossRef]
- Eskitoros-Togay, S.M.; Bulbul, Y.E.; Dilsiz, N. Controlled release of doxycycline within core/shell poly(ε-caprolactone)/poly(ethylene oxide) fibers via coaxial electrospinning. J. Appl. Polym. Sci. 2020, 137, 49273. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Zhu, Y.; Wang, L.; Yu, D.G.; Liu, L.Y. Tri-layer core–shell fibers from coaxial electrospinning for a modified release of metronidazole. Pharmaceutics 2023, 15, 2561. [Google Scholar] [CrossRef]
- Fang, F.; Meng, F.; Luo, L. Recent advances on polydiacetylene-based smart materials for biomedical applications. Mater. Chem. Front. 2020, 4, 1089–1104. [Google Scholar] [CrossRef]
- Shin, M.J.; Shin, J.S. A molecularly imprinted polymer undergoing a color change depending on the concentration of bisphenol A. Microchim. Acta 2020, 187, 44. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Shin, M.J. Electrospun coaxial microfiber-based water detecting sensor using expansion pressure mechanism. Polym. Eng. Sci. 2023, 63, 167–175. [Google Scholar] [CrossRef]
- Shin, M.J. Relationship of color change to permeation of target compound in polydiacetylene vesicle system. J. Appl. Polym. Sci. 2021, 138, 51192. [Google Scholar] [CrossRef]
- Kim, C.; Hong, C.; Lee, K. Structures and strategies for enhanced sensitivity of polydiacetylene(PDA) based biosensor platforms. Biosens. Bioelectron. 2021, 181, 113120. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.; Kim, J. Effect of the molecular size of analytes on polydiacetylene chromism. Adv. Funct. Mater. 2010, 20, 1397–1403. [Google Scholar] [CrossRef]
- Charoenthai, N.; Pattanatornchai, T.; Wacharasindhu, S.; Sukwattanasintt, M.; Traiphol, R. Roles of head group architecture and side chain length on colorimetric response of polydiacetylene vesicles to temperature, ethanol and pH. J. Colloid Interface Sci. 2011, 360, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Wacharasindhu, S.; Montha, S.; Boonyiseng, J.; Potisatityuenyong, A.; Phollookin, C.; Tumcharern, G.; Sukwattanasintt, M. Thermochromism in liquid crystalline polydiacetylenes. Macromolecules 2010, 43, 716–724. [Google Scholar] [CrossRef]


| Type of Nozzle | Position | Gauge (G) | Diameter (mm) | |
|---|---|---|---|---|
| ID | OD | |||
| DC | core | 25 | 0.26 | 0.52 |
| shell | 18 | 0.92 | 1.28 | |
| Flow Rate (Shell) | Average Diameter | |
|---|---|---|
| A | 1.5 mL/h | 3.9 ± 0.6 μm |
| B | 2.0 mL/h | 4.8 ± 0.6 μm |
| C | 2.5 mL/h | 5.7 ± 0.7 μm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, M.J. A Color-Detectable Vitamin C Controlled-Release System Fabricated Using Electrospinning. Polymers 2024, 16, 1347. https://doi.org/10.3390/polym16101347
Shin MJ. A Color-Detectable Vitamin C Controlled-Release System Fabricated Using Electrospinning. Polymers. 2024; 16(10):1347. https://doi.org/10.3390/polym16101347
Chicago/Turabian StyleShin, Min Jae. 2024. "A Color-Detectable Vitamin C Controlled-Release System Fabricated Using Electrospinning" Polymers 16, no. 10: 1347. https://doi.org/10.3390/polym16101347
APA StyleShin, M. J. (2024). A Color-Detectable Vitamin C Controlled-Release System Fabricated Using Electrospinning. Polymers, 16(10), 1347. https://doi.org/10.3390/polym16101347





