Modification of the Dielectric and Thermal Properties of Organic Frameworks Based on Nonterminal Epoxy Liquid Crystal with Silicon Dioxide and Titanium Dioxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Technique
3. Results and Discussion
3.1. Curing Conditions
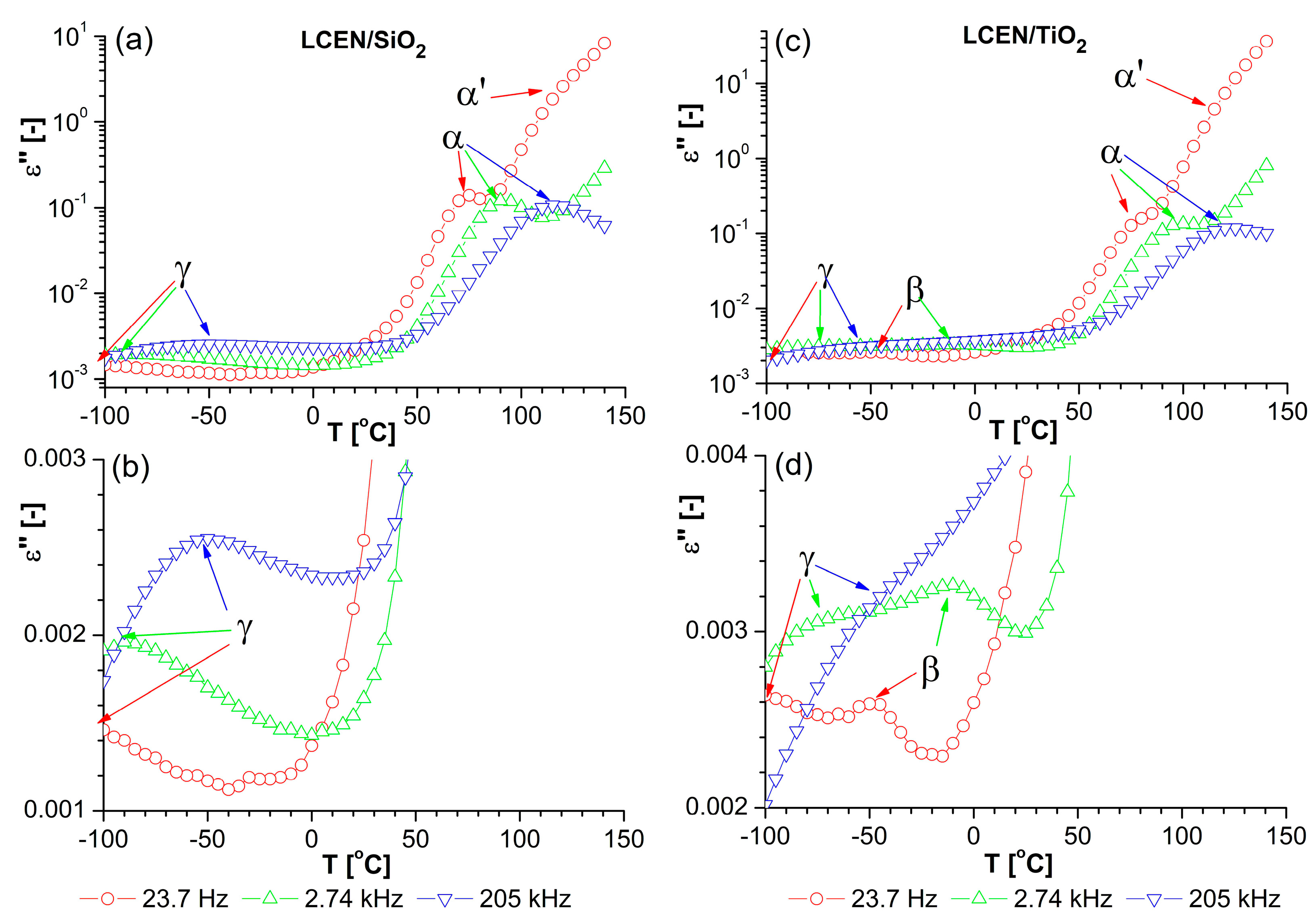
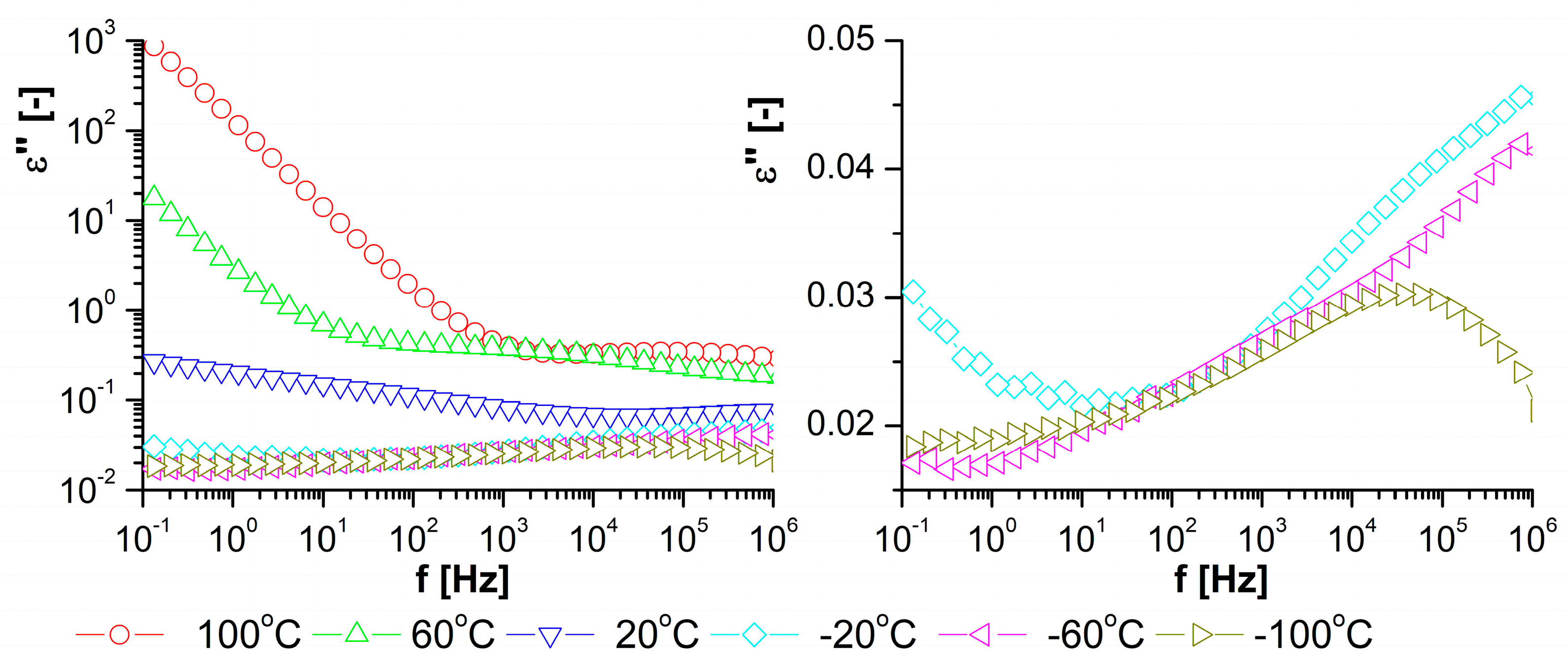
3.2. Dielectric Observation of the Examined Materials
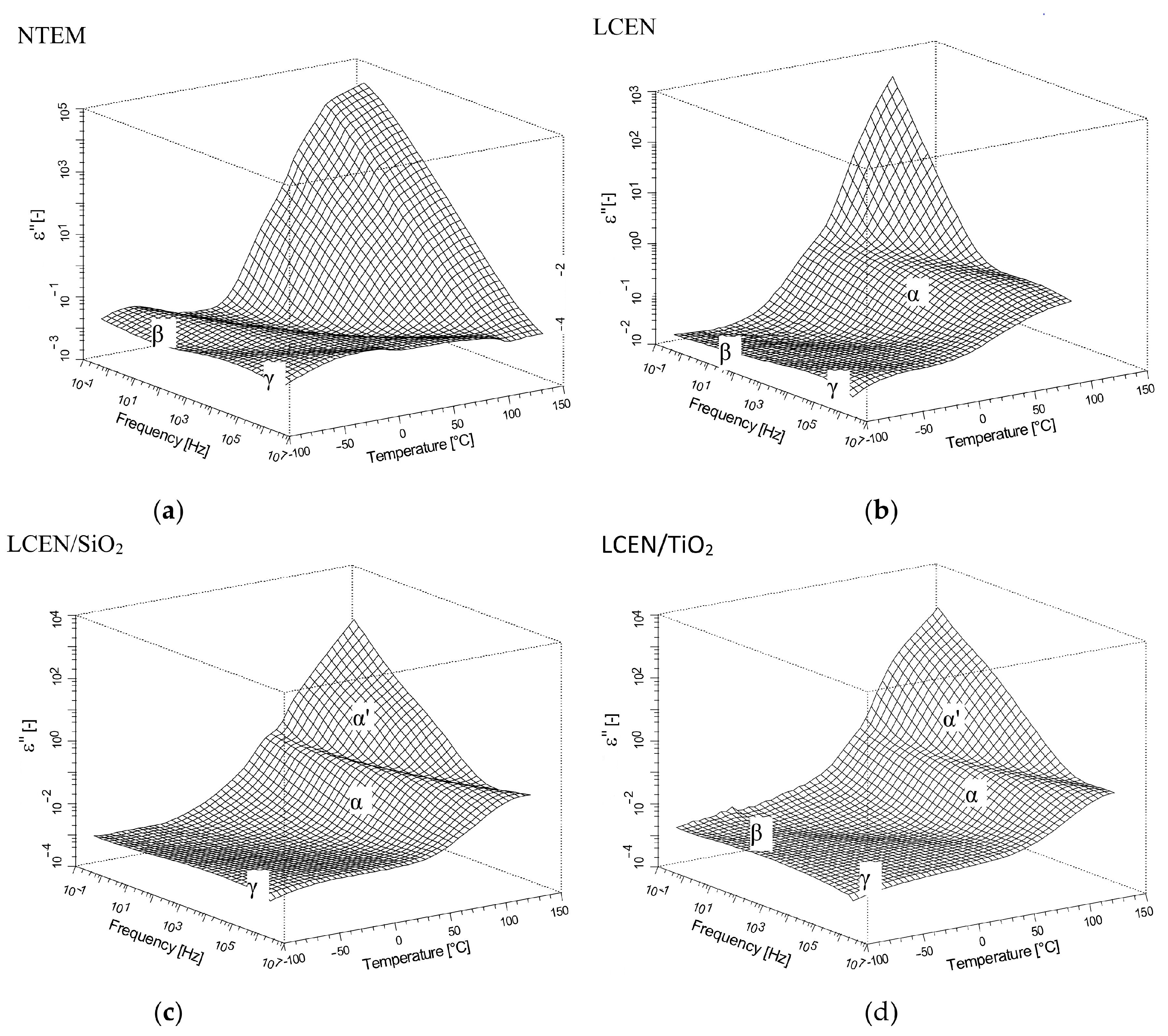
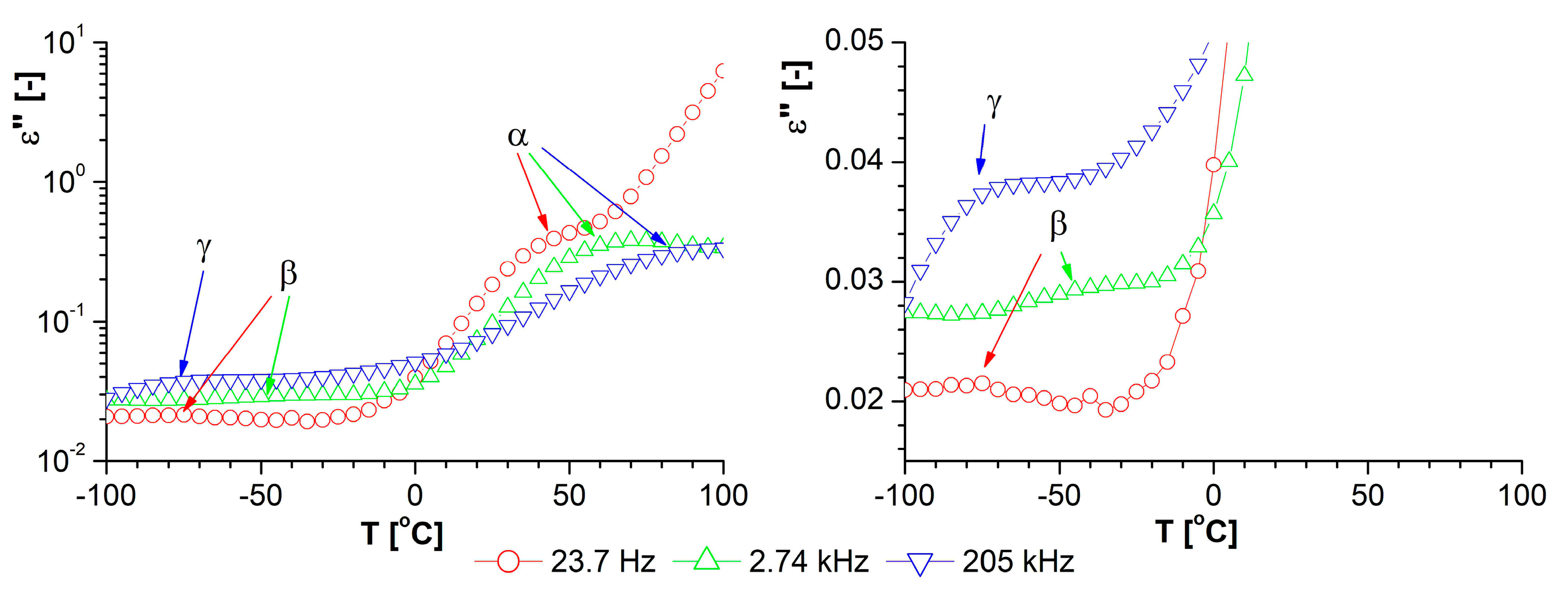
3.3. Properties of the Obtained Organic/Inorganic Matrices in Dielectric Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Wang, C.; Campbell, G.A.; Earls, J.D.; Priester, J.D. Effects of liquid crystalline structure formation on the curing kinetics of an epoxy resin. J. Polym. Sci. A Polym. Chem. 1997, 35, 1105–1124. [Google Scholar] [CrossRef]
- Castell, P.; Serra, A.; Galià, M.; Giamberini, M.; Carfagna, C. Anisotropic thermosets from liquid-crystalline azomethynic epoxy resins and primary aromatic diamines. J. Polym. Sci. A Polym. Chem. 2003, 41, 1–12. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jang, J. The effect of mesogenic length on the curing behavior and properties of liquid crystalline epoxy resins. Polymer 2006, 47, 3036–3042. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jang, J. Anisotropically ordered liquid crystalline epoxy network on carbon fiber surface. Polym. Bull. 2007, 59, 261–268. [Google Scholar] [CrossRef]
- Suzuki, N.; Kiba, S.; Yamauchi, Y. Fabrication of mesoporous silica KIT-6/polymer composite and its low thermal expansion property. Mater. Lett. 2011, 65, 544–547. [Google Scholar] [CrossRef]
- Paquet, C.; Kumacheva, E. Nanostructured polymers for photonics. Mater. Today 2008, 11, 48–56. [Google Scholar] [CrossRef]
- Agra-Kooijman, D.M.; Fisch, M.R.; Joshi, L.; Ren, W.; McMullan, P.J.; Griffin, A.C.; Kumar, S. Dual relaxation and structural changes under uniaxial strain in main-chain smectic-C liquid crystal elastomer. Phys. Chem. Chem. Phys. 2015, 17, 191–199. [Google Scholar] [CrossRef]
- Guan, Z.; Wang, L.; Bae, J. Advances in 4D printing of liquid crystalline elastomers: Materials, techniques, and applications. Mater. Horiz. 2022, 9, 1825–1849. [Google Scholar] [CrossRef] [PubMed]
- Douglas, E.P. Structure-property relationships in liquid crystalline thermosets. J. Macromol. Sci. Polym. Rev. 2006, 46, 127–141. [Google Scholar] [CrossRef]
- Shiota, A.; Ober, C.K. Rigid rod and liquid crystalline thermosets. Prog. Polym. Sci. 1997, 22, 975–1000. [Google Scholar] [CrossRef]
- Carfagna, C.; Amendola, C.E.; Giamberini, M. Liquid crystalline epoxy based thermosetting polymers. Prog. Polym. Sci. 1997, 22, 1607–1647. [Google Scholar] [CrossRef]
- Mohan, P. A critical review: The modification, properties, and applications of epoxy resins. Polym. Plast. Technol. Eng. 2013, 52, 107–125. [Google Scholar] [CrossRef]
- Jeong, I.; Bin Kim, C.; Kang, D.G.; Jeong, K.U.; Jang, S.G.; You, N.H.; Ahn, S.; Lee, D.S.; Goh, M. Liquid crystalline epoxy resin with improved thermal conductivity by intermolecular dipole–dipole interactions. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 708–715. [Google Scholar] [CrossRef]
- Zhao, M.; Yuan, K.; Wang, Y.; Li, G.; Guo, J.; Gu, L.; Hu, W.; Zhao, H.; Tang, Z. Metal–organic frameworks as selectivity regulators for hydrogenation reactions. Nature 2016, 539, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Khan, S.; Chauhan, S.; Biradar, A.M. Metal oxide-nanoparticles and liquid crystal composites: A review of recent progress. J. Mol. Liq. 2020, 297, 112052. [Google Scholar] [CrossRef]
- Chou, T.-R.; Hsieh, J.; Chen, W.-T.; Chao, C.-Y. Influence of particle size on the ion effect of TiO2 nanoparticle doped nematic liquid crystal cell. Jpn. J. Appl. Phys. 2014, 53, 071701. [Google Scholar] [CrossRef]
- Li, W.; Zhu, M.; Ding, X.; Li, B.; Huang, W.; Cao, H.; Yang, Z.; Yang, H. Studies on electro-optical properties of polymer matrix/LC/SiO2 nanoparticles composites. J. Appl. Polym. Sci. 2009, 111, 1449–1453. [Google Scholar] [CrossRef]
- Rezaei Qazviniha, M.; Piri, F. Preparation, Identification, and Evaluation of the Thermal Properties of Novolac Resins Modified with TiO2, MgO, and V2O5 Oxides. Mech. Adv. Compos. Struct. 2024, 11, 1–9. [Google Scholar]
- Ota, S.; Harada, M. Filler surface adsorption of mesogenic epoxy for LC Epoxy/MgO composites with high thermal conductivity. Compos. Part C Open Access 2021, 4, 100087. [Google Scholar] [CrossRef]
- Uzay, Ç. Mechanical and thermal characterization of laminar carbon/epoxy composites modified with magnesium oxide microparticles. Polym. Compos. 2022, 43, 299–310. [Google Scholar] [CrossRef]
- Janus, M.; Kusiak-Nejman, E.; Rokicka-Konieczna, P.; Markowska-Szczupak, A.; Zając, K.; Morawski, A.W. Bacterial inactivation on concrete plates loaded with modified TiO2 photocatalysts under visible light irradiation. Molecules 2019, 24, 3026. [Google Scholar] [CrossRef] [PubMed]
- Janus, M.; Zatorska, J.; Czyżewski, A.; Bubacz, K.; Kusiak-Nejman, E.; Morawski, A.W. Self-cleaning properties of cement plates loaded with N, C-modified TiO2 photocatalysts. Appl. Surf. Sci. 2015, 330, 200–206. [Google Scholar] [CrossRef]
- Schwendler, L.A.; Lansarin, M.A. Polyhydroxybutyrate-co-hydroxyvalerate/titanium dioxide composite films: Green synthesis, characterization, and photocatalytic assessment. J. Polym. Res. 2023, 30, 169. [Google Scholar] [CrossRef]
- Kremer, F.; Schönhals, A. (Eds.) Broadband Dielectric Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Rault, J. Relaxation of glasses: The Kohlrausch exponent. J. Non-Cryst. Solids 2011, 357, 339–345. [Google Scholar] [CrossRef]
- Mohomed, K. A broad spectrum analysis of the dielectric properties of poly (2-hydroxyethyl methacrylate). Polymer 2005, 46, 3847–3855. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane representation of dielectric and mechanical relaxation processes in some polymers. Polymer 1967, 8, 161–210. [Google Scholar] [CrossRef]
- Dyre, J.C. The random free-energy barrier model for ac conduction in disordered solids. J. Appl. Phys. 1988, 64, 2456–2468. [Google Scholar] [CrossRef]
- Donth, E. The Glass Transition: Relaxation Dynamics in Liquids and Disordered Materials; Springer: New York, NY, USA, 2001. [Google Scholar]
- Kovacs, A.J. La contraction isotherme du volume des polymères amorphes. J. Polym. Sci. 1958, 30, 131–147. [Google Scholar] [CrossRef]
- Kranbuehl, D.; Delos, S.; Yi, E.; Mayer, J.; Jarvie, T.; Winfree, W.; Hou, T. Dynamic dielectric analysis: Nondestructive material evaluation and cure cycle monitoring. Polym. Eng. Sci. 1986, 26, 338–345. [Google Scholar] [CrossRef]
- Senturia, S.D.; Sheppard, N.F. Epoxy Resins and Composites IV. Adv. Polym. Sci. 1986, 80, 1–47. [Google Scholar]
- Fournier, J.; Williams, G.; Duch, C.; Aldridge, G.A. Changes in molecular dynamics during bulk polymerization of an epoxide− amine system as studied by dielectric relaxation spectroscopy. Macromolecules 1996, 29, 7097–7107. [Google Scholar] [CrossRef]
- Włodarska, M. Curing Reaction and Dielectric Properties of Rigid and Elastic Liquid Crystal Epoxy Networks Modified with Nanofillers. Int. J. Polym. Sci. 2018, 2018, 9578654. [Google Scholar] [CrossRef]
- Włodarska, M.; Mossety-Leszczak, B.; Kisiel, M.; Zając, W.; Okrasa, L. Changes in molecular relaxations and network properties of a triaromatic liquid crystal epoxy resin with nonterminal functional groups. J. Polym. Sci. 2023, 61, 3244–3255. [Google Scholar] [CrossRef]
- Li, J.; Aung, H.H.; Du, B. Curing regime-modulating insulation performance of anhydride-cured epoxy resin: A review. Molecules 2023, 28, 547. [Google Scholar] [CrossRef] [PubMed]


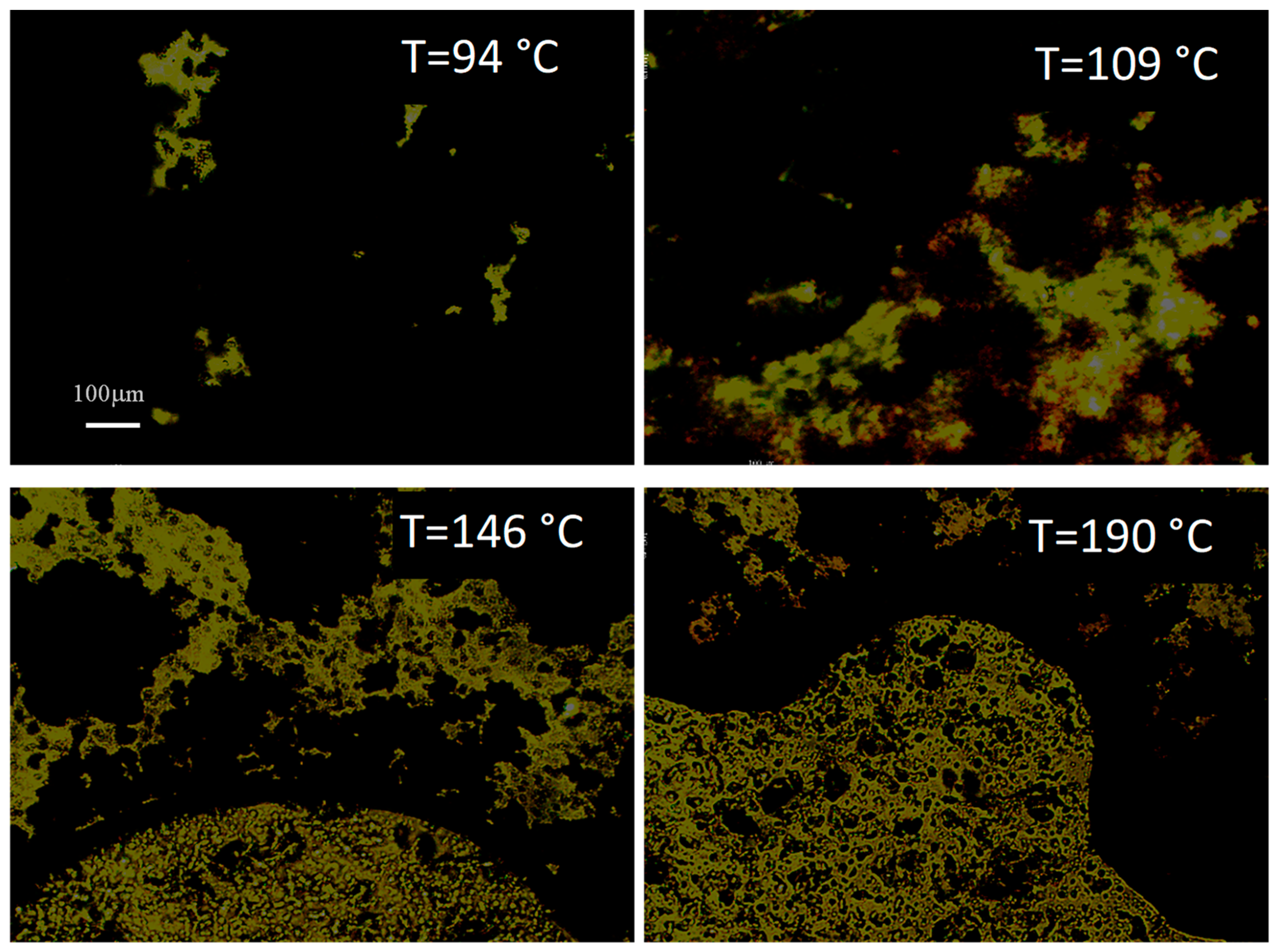



| Phase Transition | Temperature [°C] | Stage |
|---|---|---|
| Solid (S) to Nematic (N) Nematic (N) to Isotropic liquid (I) | 117 142 | heating |
| Isotropic liquid (I) to Nematic (N) Nematic (N) to Solid (S) | 140 113 | cooling |
| Mixture | Acronym | Percentage of Oxides in the Mixture |
|---|---|---|
| NTEM/DDM | LCEN | 0 |
| NTEM/DDM + SiO2 | LCEN/SiO2 | 8.6% |
| NTEM/DDM + TiO2 | LCEN/TiO2 | 9.7% |
| Sample Acronym | Curing Temperature [°C] | Curing Time [h] |
|---|---|---|
| LCEN | 185 | 3 |
| LCEN/SiO2 | 195 | 3 |
| LCEN/TiO2 | 190 | 2 |
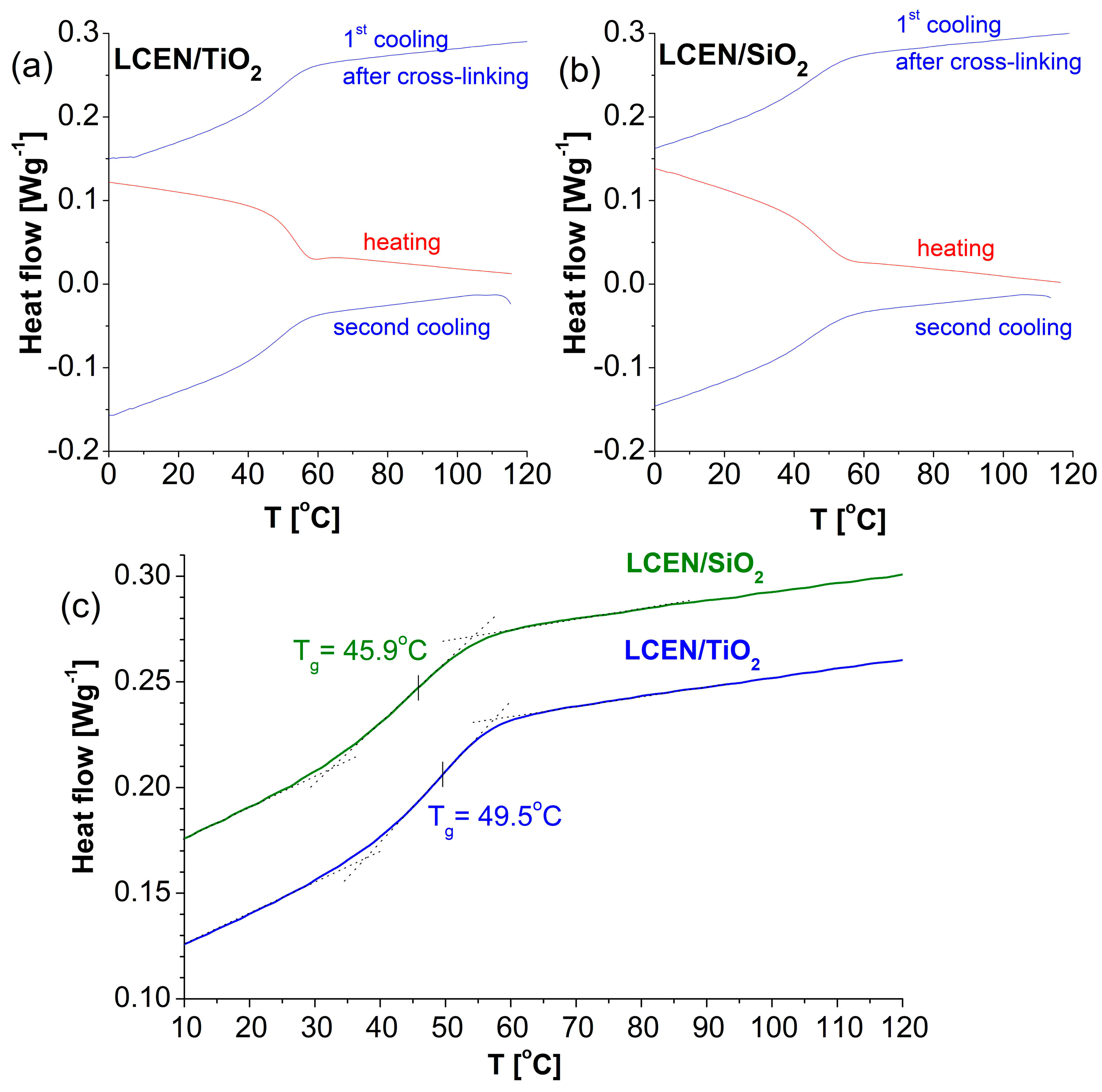
| Sample | Tg [K] from DSC | Tg [K] from BDS | Tv [K] | D | m | Ea′ (Tg) [kJ mol−1] |
|---|---|---|---|---|---|---|
| LCEN | 283 [35] | 289 ± 10 | 182 ± 9 | 23.9 ± 4.5 | 47.7 | 264 |
| LCEN/TiO2 | 322.7 | 323 ± 8 | 239 ± 7 | 14.6 ± 1.9 | 69.4 | 429 |
| LCEN/SiO2 | 319.1 | 326 ± 5 | 271 ± 4 | 7.1 ± 0.8 | 90.1 | 562 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okrasa, L.; Włodarska, M.; Kisiel, M.; Mossety-Leszczak, B. Modification of the Dielectric and Thermal Properties of Organic Frameworks Based on Nonterminal Epoxy Liquid Crystal with Silicon Dioxide and Titanium Dioxide. Polymers 2024, 16, 1320. https://doi.org/10.3390/polym16101320
Okrasa L, Włodarska M, Kisiel M, Mossety-Leszczak B. Modification of the Dielectric and Thermal Properties of Organic Frameworks Based on Nonterminal Epoxy Liquid Crystal with Silicon Dioxide and Titanium Dioxide. Polymers. 2024; 16(10):1320. https://doi.org/10.3390/polym16101320
Chicago/Turabian StyleOkrasa, Lidia, Magdalena Włodarska, Maciej Kisiel, and Beata Mossety-Leszczak. 2024. "Modification of the Dielectric and Thermal Properties of Organic Frameworks Based on Nonterminal Epoxy Liquid Crystal with Silicon Dioxide and Titanium Dioxide" Polymers 16, no. 10: 1320. https://doi.org/10.3390/polym16101320
APA StyleOkrasa, L., Włodarska, M., Kisiel, M., & Mossety-Leszczak, B. (2024). Modification of the Dielectric and Thermal Properties of Organic Frameworks Based on Nonterminal Epoxy Liquid Crystal with Silicon Dioxide and Titanium Dioxide. Polymers, 16(10), 1320. https://doi.org/10.3390/polym16101320







