Effect of Feed Mass, Reactor Temperature, and Time on the Yield of Waste Polypropylene Pyrolysis Oil Produced via a Fixed-Bed Reactor
Abstract
1. Introduction
2. Materials and Methods
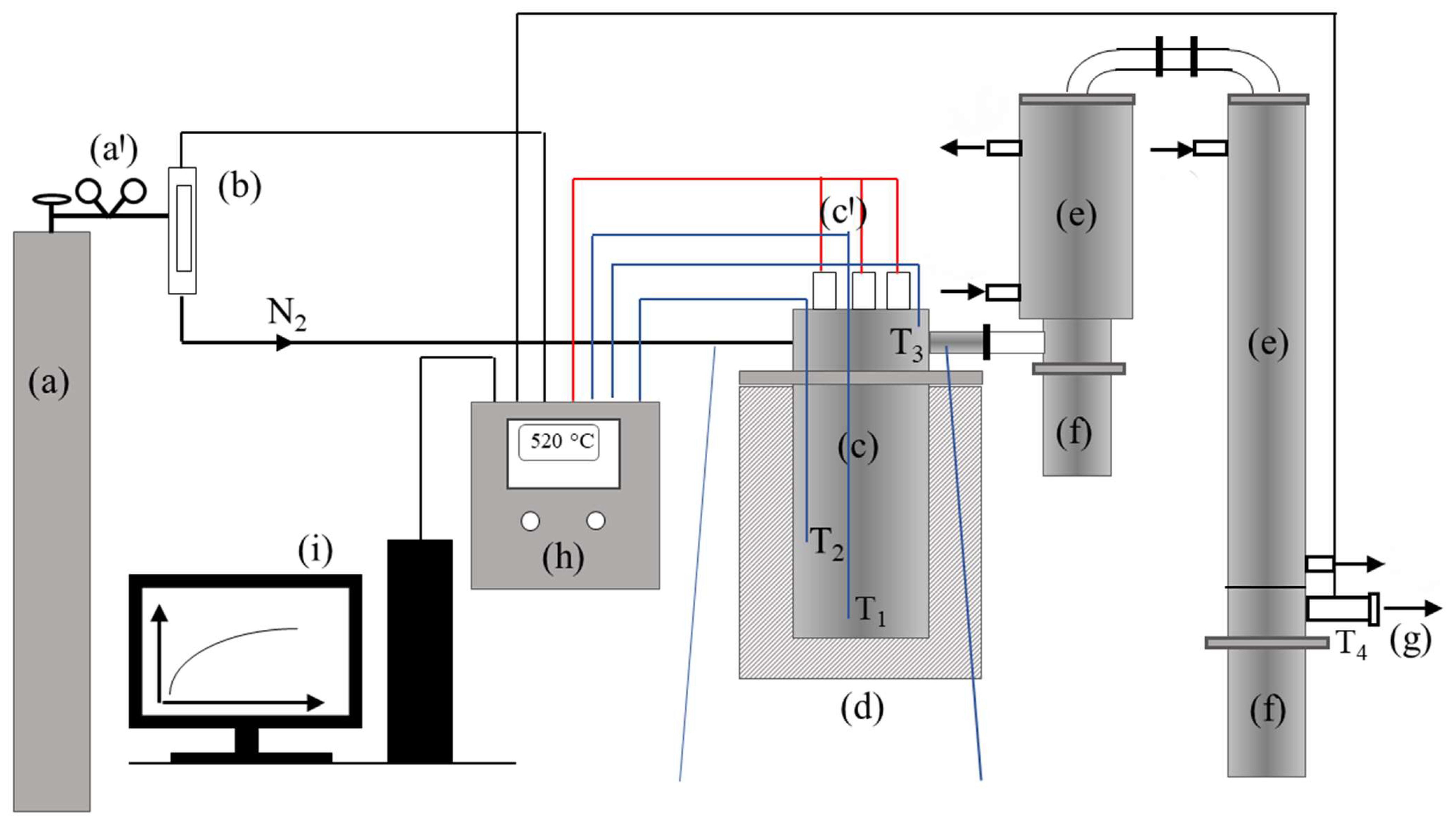
2.1. Experimental Setup
- 25% of the reactor’s effective volume for 50 g feedstock mass;
- 50% of the reactor’s effective volume for 100 g feedstock mass;
- 75% of the reactor’s effective volume for 150 g feedstock mass.
2.2. Design of an Experiment and Mathematical Modeling
3. Results
3.1. Model Development
Statistical Testing
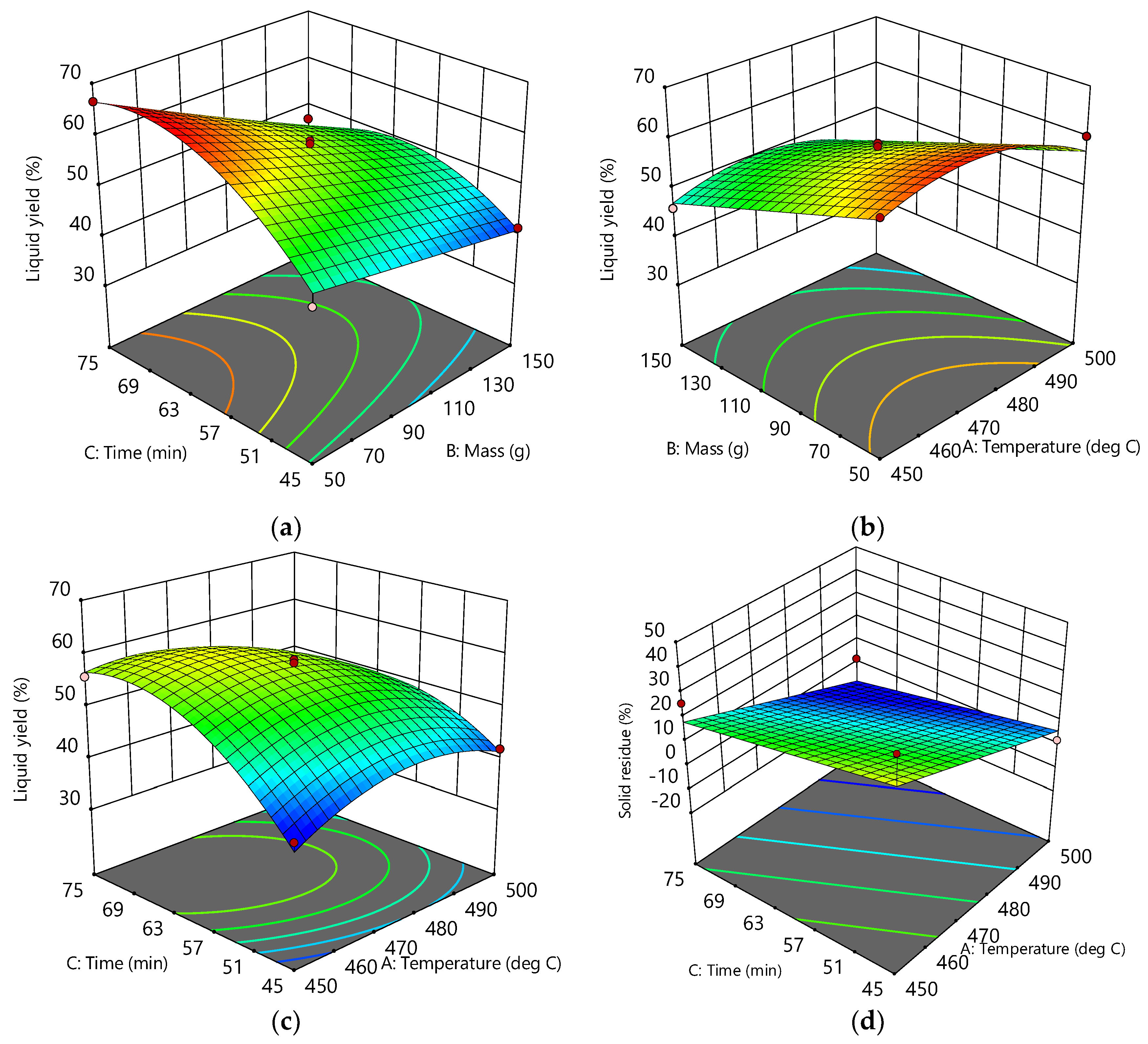
3.2. Response Surfaces and Contour Plots
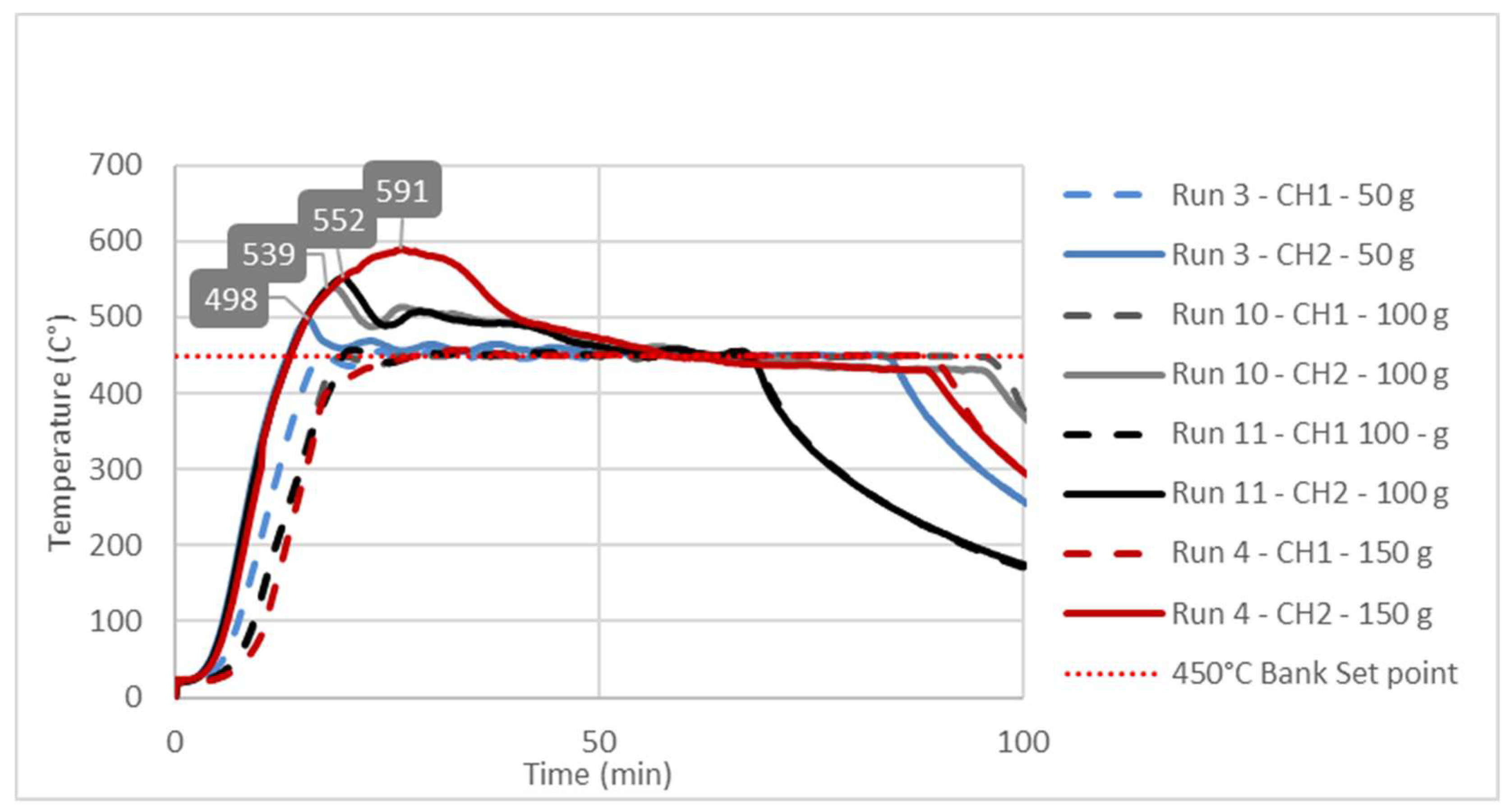
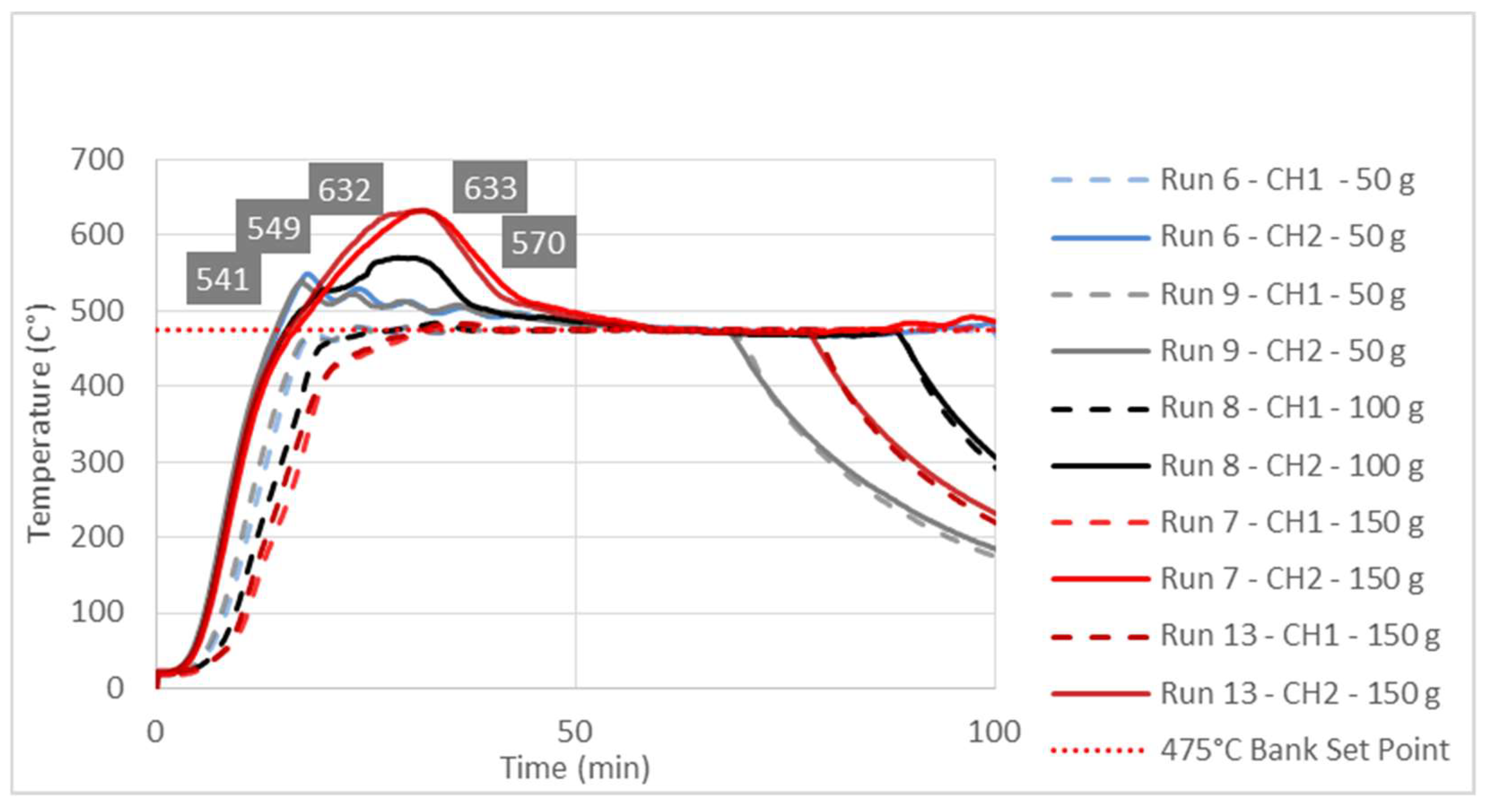
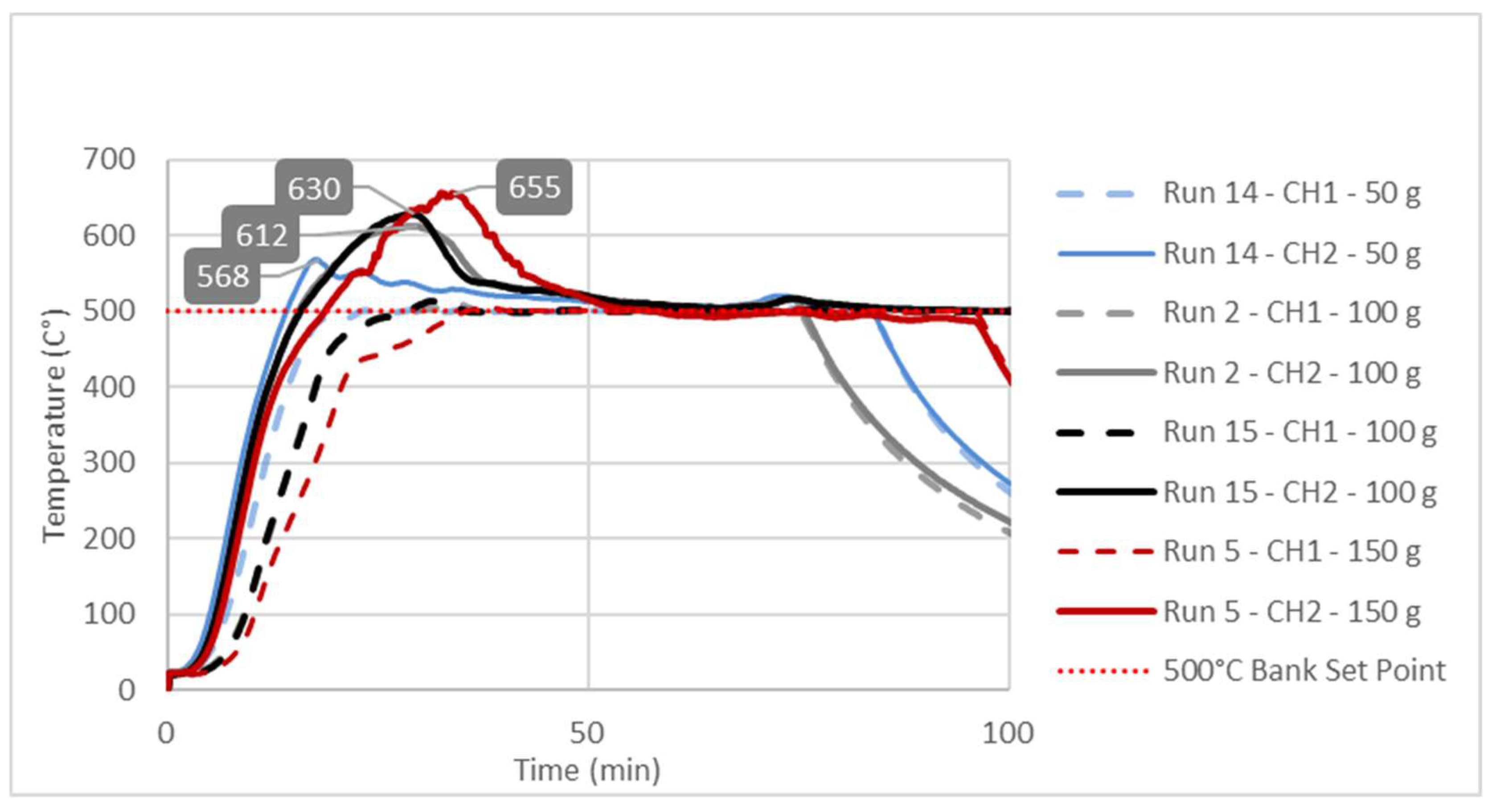
3.3. Study of Temperature Diagrams
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dai, L.; Zhou, N.; Lv, Y.; Cheng, Y.; Wang, Y.; Liu, Y.; Cobb, K.; Chen, P.; Lei, H.; Ruan, R. Pyrolysis Technology for Plastic Waste Recycling: A State-of-the-Art Review. Prog. Energy Combust. Sci. 2022, 93, 101021. [Google Scholar] [CrossRef]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.-S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef]
- Papuga, S.; Djurdjevic, M.; Ciccioli, A.; Vecchio Ciprioti, S. Catalytic Pyrolysis of Plastic Waste and Molecular Symmetry Effects: A Review. Symmetry 2023, 15, 38. [Google Scholar] [CrossRef]
- Vouvoudi, E.C.; Rousi, A.T.; Achilias, D.S. Effect of the catalyst type on pyrolysis products distribution of polymer blends simulating plastics contained in waste electric and electronic equipment. Sustain. Chem. Pharm. 2023, 34, 101145. [Google Scholar] [CrossRef]
- Gebre, S.H.; Sendeku, M.G.; Bahri, M. Recent Trends in the Pyrolysis of Non-Degradable Waste Plastics. Chem. Open 2021, 10, 1202–1226. [Google Scholar] [CrossRef] [PubMed]
- Gulab, H.; Jan, M.R.; Shah, J.; Manos, G. Plastic catalytic pyrolysis to fuels as tertiary polymer recycling method: Effect of process conditions. J. Environ. Sci. Health Part A 2010, 45, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Chen, W.; Xu, K.; Liu, Y.; Wu, J.; Zhang, F. Understanding the Structure–Activity Relationships in Catalytic Conversion of Polyolefin Plastics by Zeolite-Based Catalysts: A Critical Review. ACS Catal. 2022, 12, 14882–14901. [Google Scholar] [CrossRef]
- Mondal, B.K.; Guha, F.; Abser, M.N. Sol-gel derived Ti-doped mesoporous silica–alumina: An efficient catalyst to recover energy sources from environmental hazard waste plastics. J. Therm. Anal. Calorim. 2023, 148, 5257–5270. [Google Scholar] [CrossRef]
- Serra, A.C.S.; Milato, J.V.; Faillace, J.G.; Calderari, M.R.C.M. Reviewing the use of zeolites and clay based catalysts for pyrolysis of plastics and oil fractions. Braz. J. Chem. Eng. 2023, 40, 287–319. [Google Scholar] [CrossRef]
- Fadillah, G.; Fatimah, I.; Sahroni, I.; Musawwa, M.M.; Mahlia, T.M.I.; Muraza, O. Recent Progress in Low Cost Catalysts for Pyrolysis Plastic Waste to Fuels. Catalysts 2021, 11, 837. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A Review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Maqsood, T.; Dai, J.; Zhang, Y.; Guang, M.; Li, B. Pyrolysis of plastic species: A review of resources and products. J. Anal. Appl. Pyrolysis 2021, 159, 105295. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.I.; Khan, H.; Ishaq, M.; Tariq, R.; Gul, K.; Ahmad, W. Pyrolysis Study of Polypropylene and Polyethylene Into Premium Oil Products. Int. J. Green Energy 2015, 12, 663–671. [Google Scholar] [CrossRef]
- Uebe, J.; Kryzevicius, Z.; Majauskiene, R.; Dulevicius, M.; Kosychova, L.; Zukauskaite, A. Use of polypropylene pyrolysis oil in alternative fuel production. Waste Manag. Res. 2022, 40, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, Y.; Abdelouahed, L.; Hage, R.E.; Samrani, A.E.; Taouk, B. Pyrolysis of common plastics and their mixtures to produce valuable petroleum-like products. Polym. Degrad. Stab. 2022, 195, 109770. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Haghighi, M.N.; Yeganeh, H.; McDonald, A.G. Evaluation of pyrolysis process parameters on polypropylene degradation products. J. Anal. Appl. Pyrolysis 2014, 109, 272–277. [Google Scholar] [CrossRef]
- Achilias, D.S. Recycling techniques of polyolefins from plastic wastes. Glob. NEST J. 2008, 10, 114–122. [Google Scholar]
- Aisien, E.T.; Otuya, I.C.; Aisien, F.A. Thermal and Catalytic Pyrolysis of Waste Polypropylene Plastic Using Spent FCC Catalyst. Environ. Technol. Innov. 2021, 22, 101455. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Sebastian, J. Pyrolysis process to produce fuel from different types of plastic: A review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 396, 012062. [Google Scholar] [CrossRef]
- Abdullah, N.A.; Novianti, A.; Hakim, I.I.; Putra, N.; Koestoer, R.A. Influence of Temperature on Conversion of Plastics Waste (Polystyrene) to Liquid Oil Using Pyrolysis Process. IOP Conf. Ser. Earth Environ. Sci. 2018, 105, 012033. [Google Scholar] [CrossRef]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A Review on Pyrolysis of Plastic Wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Đurđević, M.; Papuga, S.; Kolundžija, A. Analysis of the thermal behavior of a fixed bed reactor during the pyrolysis process. Hem. Ind 2024, 78, 29–40. [Google Scholar] [CrossRef]
- Maafa, I. Pyrolysis of Polystyrene Waste: A Review. Polymers 2021, 13, 225. [Google Scholar] [CrossRef]
- Papuga, S.; Gvero, P.; Vukic, L. Temperature and Time Influence on the Waste Plastics Pyrolysis in the Fixed Bed Reactor. Therm. Sci. 2016, 20, 731–741. [Google Scholar] [CrossRef]
- Esposito, L.; Cafiero, L.; De Angelis, D.; Tuffi, R.; Vecchio Ciprioti, S. Valorization of the plastic residue from a WEEE treatment plant by pyrolysis. Waste Manag. 2020, 112, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Borsella, E.; Aguado, R.; De Stefanis, A.; Olazar, M. Comparison of catalytic performance of an iron-alumina pillared montmorillonite and HZSM-5 zeolite on a spouted bed reactor. J. Anal. Appl. Pyrolysis 2018, 130, 320–331. [Google Scholar] [CrossRef]
- Cocchi, M.; Angelis, D.D.; Mazzeo, L.; Nardozi, P.; Piemonte, V.; Tuffi, R.; Vecchio Ciprioti, S. Catalytic Pyrolysis of a Residual Plastic Waste Using Zeolites Produced by Coal Fly Ash. Catalysts 2020, 10, 1113. [Google Scholar] [CrossRef]
- Khazaal, R.M.; Abdulaaima, D.A. Valuable oil recovery from plastic wastes via pressurized thermal and catalytic pyrolysis. Energy Convers. Manag. X 2023, 20, 100430. [Google Scholar] [CrossRef]
- Han, H.-Z.; Li, B.-X.; Wu, H.; Shao, W. Multi-Objective Shape Optimization of Double Pipe Heat Exchanger with Inner Corrugated Tube Using RSM Method. Int. J. Therm. Sci. 2015, 90, 173–186. [Google Scholar] [CrossRef]
- Amran, M.; Salmah, S.; Sanusi, M.; Yuhazri, M.; Mohamad, N.; Azam, M.A.; Abdullah, Z.; Mohamad, E. Surface Roughness Optimization in Drilling Process Using Response Surface Method (RSM). J. Teknol. 2014, 66, 29–35. [Google Scholar] [CrossRef]
- Bashir, M.J.K.; Abu Amr, S.S.; Aziz, S.Q.; Aun, N.C.; Sethupathi, S. Wastewater Treatment Processes Optimization Using Response Surface Methodology (RSM) Compared with Conventional Methods: Review and Comparative Study. Middle-East J. Sci. Res. 2015, 23, 244–252. [Google Scholar] [CrossRef]
- Said, K.A.M.; Amin, M.A.M. Overview on the Response Surface Methodology (RSM) in Extraction Processes. J. Appl. Sci. Process. Eng. 2016, 2, 8–17. [Google Scholar] [CrossRef]
- Dutta, N.; Mondal, P.; Gupta, A. Optimization of Process Parameters Using Response Surface Methodology for Maximum Liquid Yield during Thermal Pyrolysis of Blend of Virgin and Waste High-Density Polyethylene. J. Mater. Cycles Waste Manag. 2022, 24, 1182–1193. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.K. Optimization of Process Parameters by Response Surface Methodology (RSM) for Catalytic Pyrolysis of Waste High-Density Polyethylene to Liquid Fuel. J. Environ. Chem. Eng. 2014, 2, 115–122. [Google Scholar] [CrossRef]
- Mo, Y.; Zhao, L.; Wang, Z.; Chen, C.-L.; Tan, G.-Y.A.; Wang, J.-Y. Enhanced Styrene Recovery from Waste Polystyrene Pyrolysis Using Response Surface Methodology Coupled with Box–Behnken Design. Waste Manag. 2014, 34, 763–769. [Google Scholar] [CrossRef]
- Pinto, F.; Paradela, F.; Gulyurtlu, I.; Ramos, A.M. Prediction of Liquid Yields from the Pyrolysis of Waste Mixtures Using Response Surface Methodology. Fuel Process. Technol. 2013, 116, 271–283. [Google Scholar] [CrossRef]
- Selvaganapathy, T.; Muthuvelayudham, R.; Jayakumar, M. Process Parameter Optimization Study on Thermolytic Polystyrene Liquid Fuel Using Response Surface Methodology (RSM). Mater. Today Proc. 2020, 26, 2729–2739. [Google Scholar] [CrossRef]
- Faisal, F.; Rasul, M.G.; Chowdhury, A.A.; Jahirul, M.I. Optimisation of Process Parameters to Maximise the Oil Yield from Pyrolysis of Mixed Waste Plastics. Sustainability 2024, 16, 2619. [Google Scholar] [CrossRef]
- Ore, O.T.; Adebiyi, F.M. Process modelling of waste tyre pyrolysis for gas production using response surface methodology. Unconv. Resour. 2024, 4, 100080. [Google Scholar] [CrossRef]
- Thonglhueng, N.; Sirisangsawang, R.; Sukpancharoen, S.; Phetyim, N. Optimization of iodine number of carbon black obtained from waste tire pyrolysis plant via response surface methodology. Heliyon 2022, 8, e11971. [Google Scholar] [CrossRef]
- Quesada, L.; Pérez, A.; Godoy, V.; Peula, F.J.; Calero, M.; Blázquez, G. Optimization of the pyrolysis process of a plastic waste to obtain a liquid fuel using different mathematical models. Energy Conv. Manag. 2019, 15, 19–26. [Google Scholar] [CrossRef]
- Wirawan, R.; Farizal, P. Plastic waste pyrolysis optimization to produce fuel grade using factorial design. In Proceedings of the 4th International Conference on Energy, Environment, Epidemiology and Information System (ICENIS 2019), E3S Web Conference, Semarang, Indonesia, 28 October 2019. [Google Scholar] [CrossRef]
- Gonzalez-Aguilar, A.M.; Cabrera-Madera, V.P.; Vera-Rozo, J.R.; Riesco-Ávila, J.M. Effects of Heating Rate and Temperature on the Thermal Pyrolysis of Expanded Polystyrene Post-Industrial Waste. Polymers 2022, 14, 4957. [Google Scholar] [CrossRef]
- Irfan, M.; Nabi, R.A.U.; Hussain, H.; Naz, M.Y.; Shukrullah, S.; Khawaja, H.A.; Rahman, S.; Ghanim, A.A.J.; Kruszelnicka, I.; Ginter-Kramarczyk, D.; et al. Response Surface Methodology Analysis of Pyrolysis Reaction Rate Constants for Predicting Efficient Conversion of Bulk Plastic Waste into Oil and Gaseous Fuel. Energies 2020, 15, 9594. [Google Scholar] [CrossRef]
- Xie, S.; Kumagai, S.; Kameda, T.; Saito, Y.; Yoshioka, T. Prediction of pyrolyzate yields by response surface methodology: A case study of cellulose and polyethylene co-pyrolysis. Bioresour. Technol. 2021, 337, 5435. [Google Scholar] [CrossRef]
- Papuga, S.; Djurdjevic, M.; Tomović, G.; Vecchio Ciprioti, S. Pyrolysis of Tyre Waste in a Fixed-Bed Reactor. Symmetry 2023, 15, 2146. [Google Scholar] [CrossRef]
- Kremer, I.; Tomić, T.; Katančić, Z.; Erceg, M.; Papuga, S.; Vuković, J.P.; Schneider, D.R. Catalytic Pyrolysis of Mechanically Non-Recyclable Waste Plastics Mixture: Kinetics and Pyrolysis in Laboratory-Scale Reactor. J. Environ. Manag. 2021, 296, 113145. [Google Scholar] [CrossRef]
- Hartulistiyoso, E.; Sigiro, F.; Yulianto, M. Temperature Distribution of the Plastics Pyrolysis Process to Produce Fuel at 450 °C. Procedia Env. Sci. 2015, 28, 234–241. [Google Scholar] [CrossRef]
- Lee, C.G.; Cho, Y.J.; Song, P.S.; Kang, Y.; Kim, J.S.; Choi, M.J. Effects of temperature distribution on the catalytic pyrolysis of polystyrene waste in a swirling fluidized-bed reactor. Catal Today 2003, 79–80, 453–464. [Google Scholar] [CrossRef]
- Cahyono, M.S.; Fenti, U.I. Influence of Heating Rate and Temperature on the Yield and Properties of Pyrolysis Oil Obtained from Waste Plastic Bag. Conserve J. Energy Environ. Stud. 2017, 1, 1–8. [Google Scholar] [CrossRef]
| Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 | |
|---|---|---|---|---|---|
| Run | A: Temperature | B: Mass | C: Time | Liquid Yield | Solid Residue |
| °C | g | Min | % | % | |
| 1 | 475 | 100 | 60 | 56.43 | 6.88 |
| 2 | 500 | 100 | 45 | 41.85 | 1.93 |
| 3 | 450 | 50 | 60 | 62.82 | 23.26 |
| 4 | 450 | 150 | 60 | 46.02 | 20.68 |
| 5 | 500 | 150 | 60 | 40.73 | 1.96 |
| 6 | 475 | 50 | 75 | 66.54 | 3.76 |
| 7 | 475 | 150 | 75 | 47.17 | 1.81 |
| 8 | 475 | 100 | 60 | 58.95 | 6.09 |
| 9 | 475 | 50 | 45 | 46.8 | 36.28 |
| 10 | 450 | 100 | 75 | 55.72 | 25.94 |
| 11 | 450 | 100 | 45 | 39.75 | 44.89 |
| 12 | 475 | 100 | 60 | 58.34 | 5.94 |
| 13 | 475 | 150 | 45 | 41.49 | 8.11 |
| 14 | 500 | 50 | 60 | 60.18 | 1.42 |
| 15 | 500 | 100 | 75 | 41.15 | 1.6 |
| 16 | 475 | 100 | 60 | 56.31 | 6.58 |
| Response | Source | Sequential p-Value | Lack-of-Fit p-Value | Adjusted R2 | Predicted R2 | |
|---|---|---|---|---|---|---|
| 1 Liquid yield | Linear | 0.0109 | 0.0085 | 0.4898 | 0.2655 | |
| 2FI | 0.4536 | 0.0073 | 0.4843 | −0.0773 | ||
| Quadratic | 0.0021 | 0.0877 | 0.9231 | 0.5707 | Suggested | |
| 2 Solid residue | Linear | 8.15 | 0.7156 | 0.6445 | 0.4479 | Suggested |
| 2FI | 7.72 | 0.8087 | 0.6812 | 0.2079 | ||
| Quadratic | 6.27 | 0.9157 | 0.7893 | −0.3458 |
| Factor | Name | Units | Minimum | Maximum | Coded Low | Coded High | Mean |
|---|---|---|---|---|---|---|---|
| A | Temperature | deg C | 450.00 | 500.00 | −1 ↔ 450.00 | +1 ↔ 500.00 | 475.00 |
| B | Mass | g | 50.00 | 150.00 | −1 ↔ 50.00 | +1 ↔ 150.00 | 100.00 |
| C | Time | min | 45.00 | 75.00 | −1 ↔ 45.00 | +1 ↔ 75.00 | 60.00 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Response 1 Liquid yield | Model | 1181.74 | 7 | 168.82 | 33.79 | <0.0001 |
| A-Temperature | 52.02 | 1 | 52.02 | 10.41 | 0.0121 | |
| B-Mass | 464.06 | 1 | 464.06 | 92.88 | <0.0001 | |
| C-Time | 206.96 | 1 | 206.96 | 41.42 | 0.0002 | |
| AC | 69.47 | 1 | 69.47 | 13.90 | 0.0058 | |
| BC | 49.42 | 1 | 49.42 | 9.89 | 0.0137 | |
| A2 | 119.96 | 1 | 119.96 | 24.01 | 0.0012 | |
| C2 | 219.85 | 1 | 219.85 | 44.00 | 0.0002 | |
| Residual | 39.97 | 8 | 5.00 | |||
| Lack of Fit | 34.60 | 5 | 6.92 | 3.87 | 0.1474 | |
| Response 2 Solid residue | Model | 1876.17 | 2 | 938.09 | 13.17 | 0.0007 |
| A-Temperature | 1454.22 | 1 | 1454.22 | 20.41 | 0.0006 | |
| C-Time | 421.95 | 1 | 421.95 | 5.92 | 0.0301 | |
| Residual | 926.32 | 13 | 71.26 |
| Response 1 Liquid yield | Std. Dev. | 2.24 | R2 | 0.9673 |
| Mean | 51.27 | Adjusted R2 | 0.9387 | |
| C.V. % | 4.36 | Predicted R2 | 0.7716 | |
| Adeq Precision | 17.9664 | |||
| Response 2 Solid residue | Std. Dev. | 8.44 | R2 | 0.6695 |
| Mean | 12.32 | Adjusted R2 | 0.6186 | |
| C.V. % | 68.51 | Predicted R2 | 0.4514 | |
| Adeq Precision | 11.3510 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papuga, S.; Savković, J.; Djurdjevic, M.; Ciprioti, S.V. Effect of Feed Mass, Reactor Temperature, and Time on the Yield of Waste Polypropylene Pyrolysis Oil Produced via a Fixed-Bed Reactor. Polymers 2024, 16, 1302. https://doi.org/10.3390/polym16101302
Papuga S, Savković J, Djurdjevic M, Ciprioti SV. Effect of Feed Mass, Reactor Temperature, and Time on the Yield of Waste Polypropylene Pyrolysis Oil Produced via a Fixed-Bed Reactor. Polymers. 2024; 16(10):1302. https://doi.org/10.3390/polym16101302
Chicago/Turabian StylePapuga, Saša, Jelena Savković, Milica Djurdjevic, and Stefano Vecchio Ciprioti. 2024. "Effect of Feed Mass, Reactor Temperature, and Time on the Yield of Waste Polypropylene Pyrolysis Oil Produced via a Fixed-Bed Reactor" Polymers 16, no. 10: 1302. https://doi.org/10.3390/polym16101302
APA StylePapuga, S., Savković, J., Djurdjevic, M., & Ciprioti, S. V. (2024). Effect of Feed Mass, Reactor Temperature, and Time on the Yield of Waste Polypropylene Pyrolysis Oil Produced via a Fixed-Bed Reactor. Polymers, 16(10), 1302. https://doi.org/10.3390/polym16101302










