A Novel Recyclable Magnetic Nano-Catalyst for Fenton-Photodegradation of Methyl Orange and Imidazole Derivatives Catalytic Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of (CDC@Fe3O4)
2.3. Catalytic Synthesis of the Tetra-Substituted Imidazole Derivatives
2.4. Apparatus
2.5. Photocatalytic Tests
3. Results and Discussion
3.1. Characterization
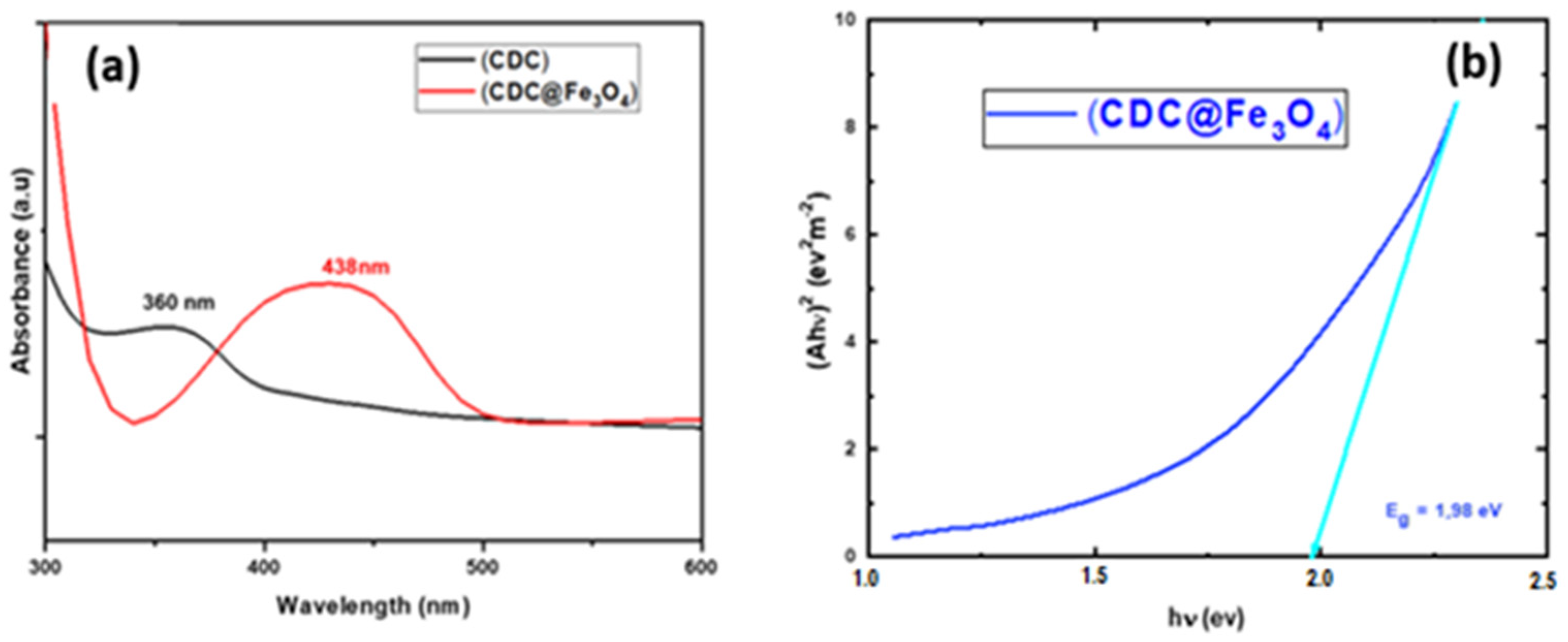
3.1.1. UV-Vis Absorption Data
3.1.2. FTIR
3.1.3. TGA
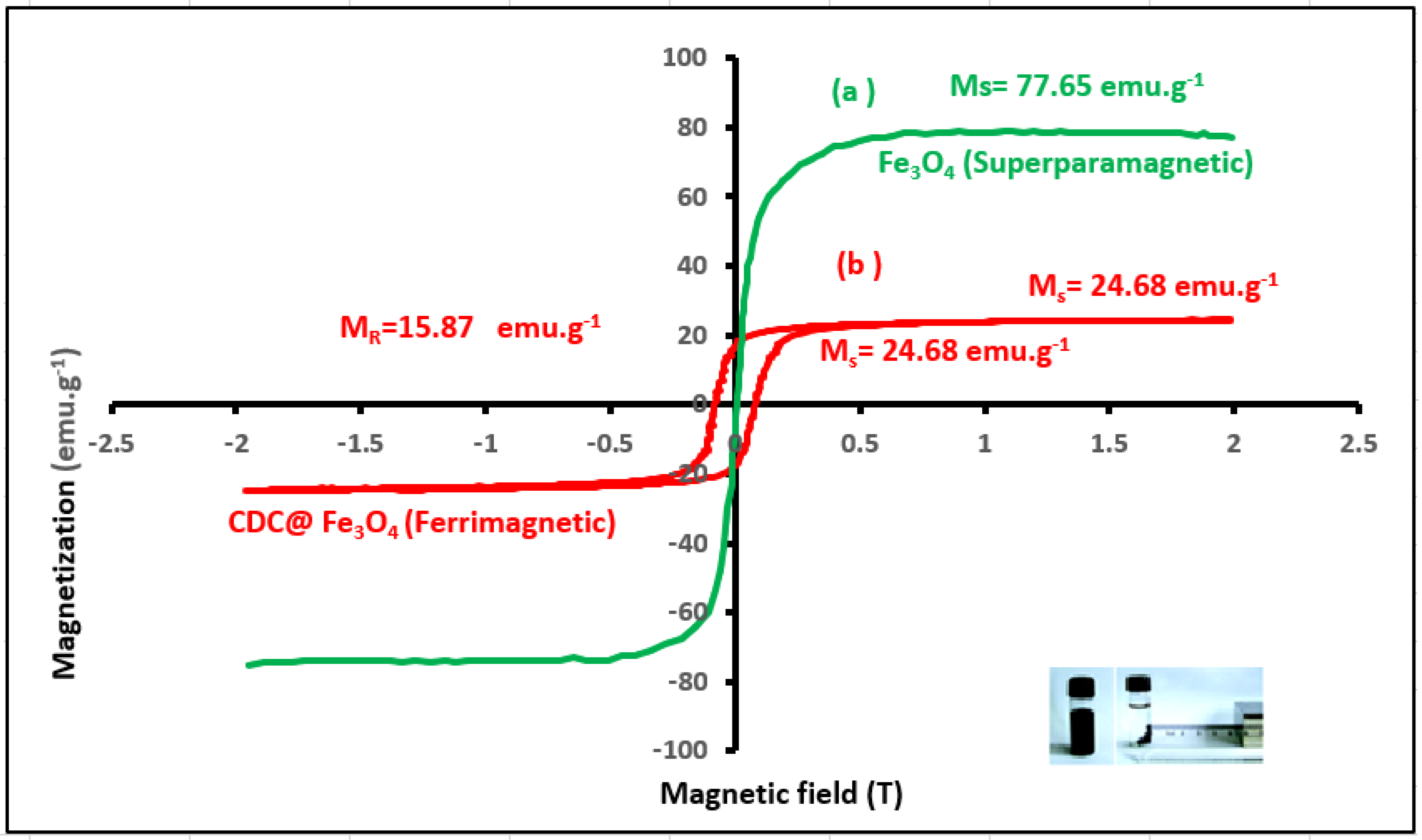
3.1.4. VSM
3.1.5. XRD
3.1.6. SEM
3.2. Optimisation of Photocatalytic Degradation Parameters
3.2.1. The Effect of the Light Source on Fenton-Photodegradation Process
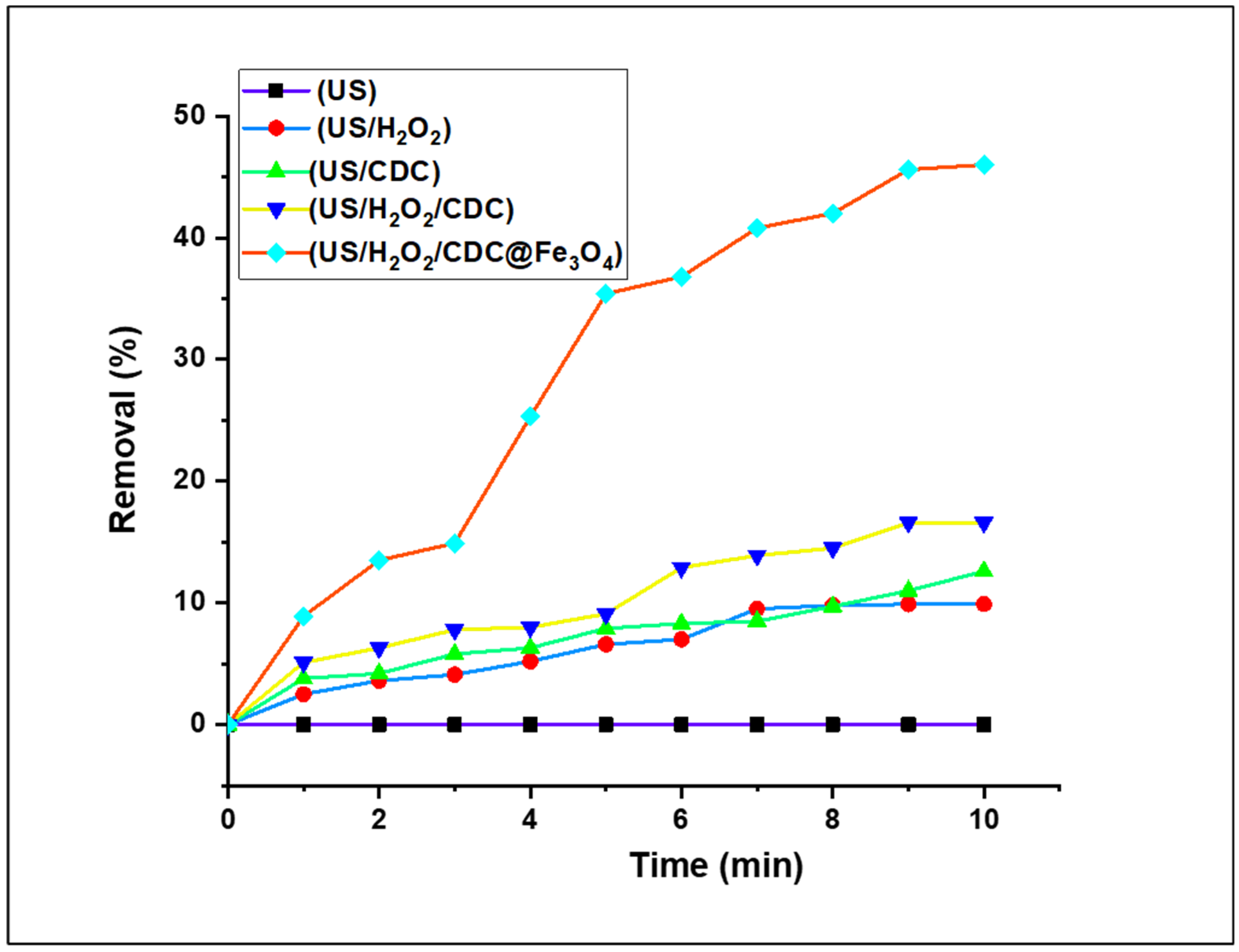
3.2.2. Preliminary Fenton-Photocatalytic Efficacity Studies of MO Reduction under Several Catalytic Systems
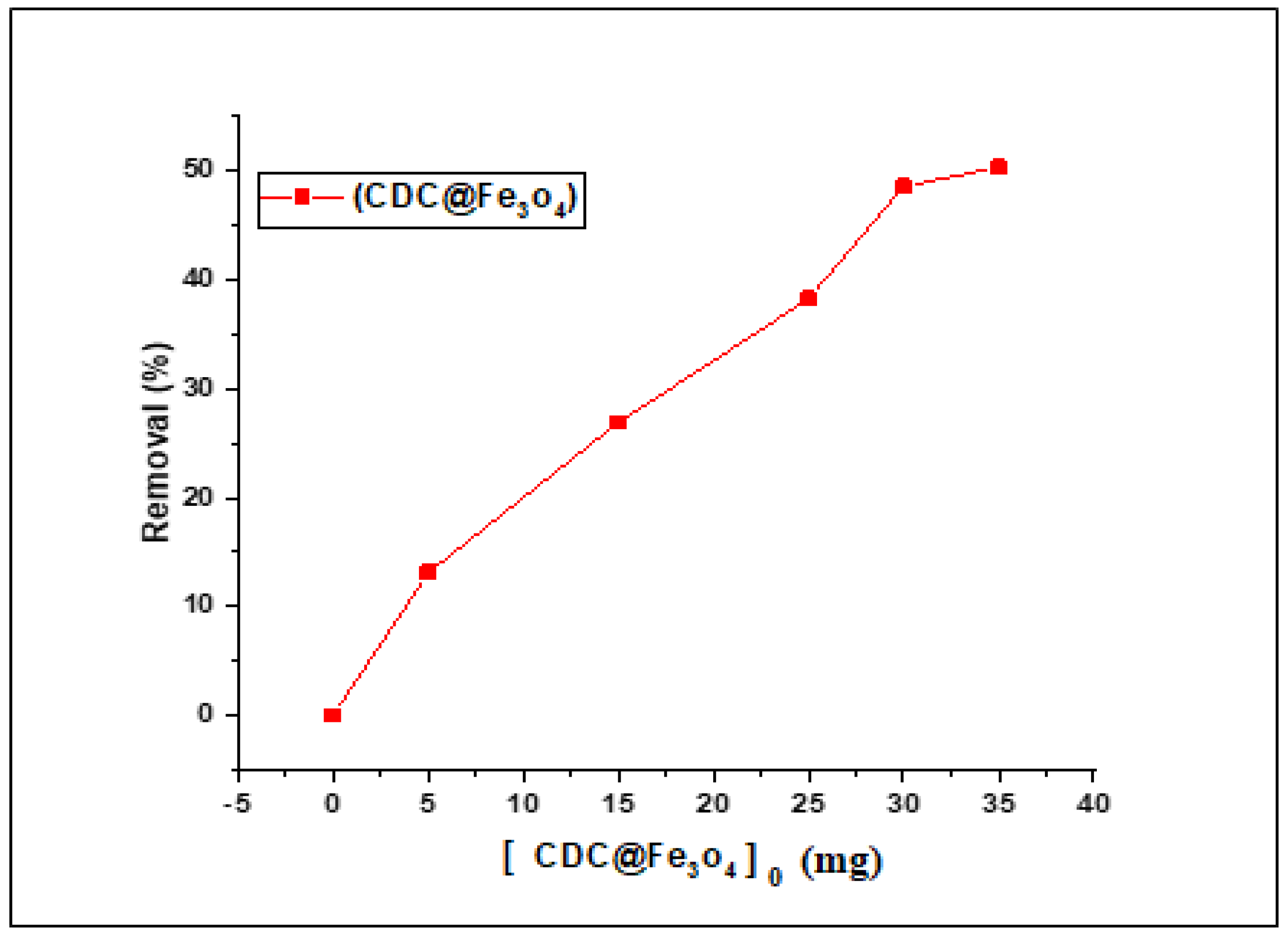
3.2.3. Effect of (CDC@Fe3O4) Load on the Fenton Photodegradation Process
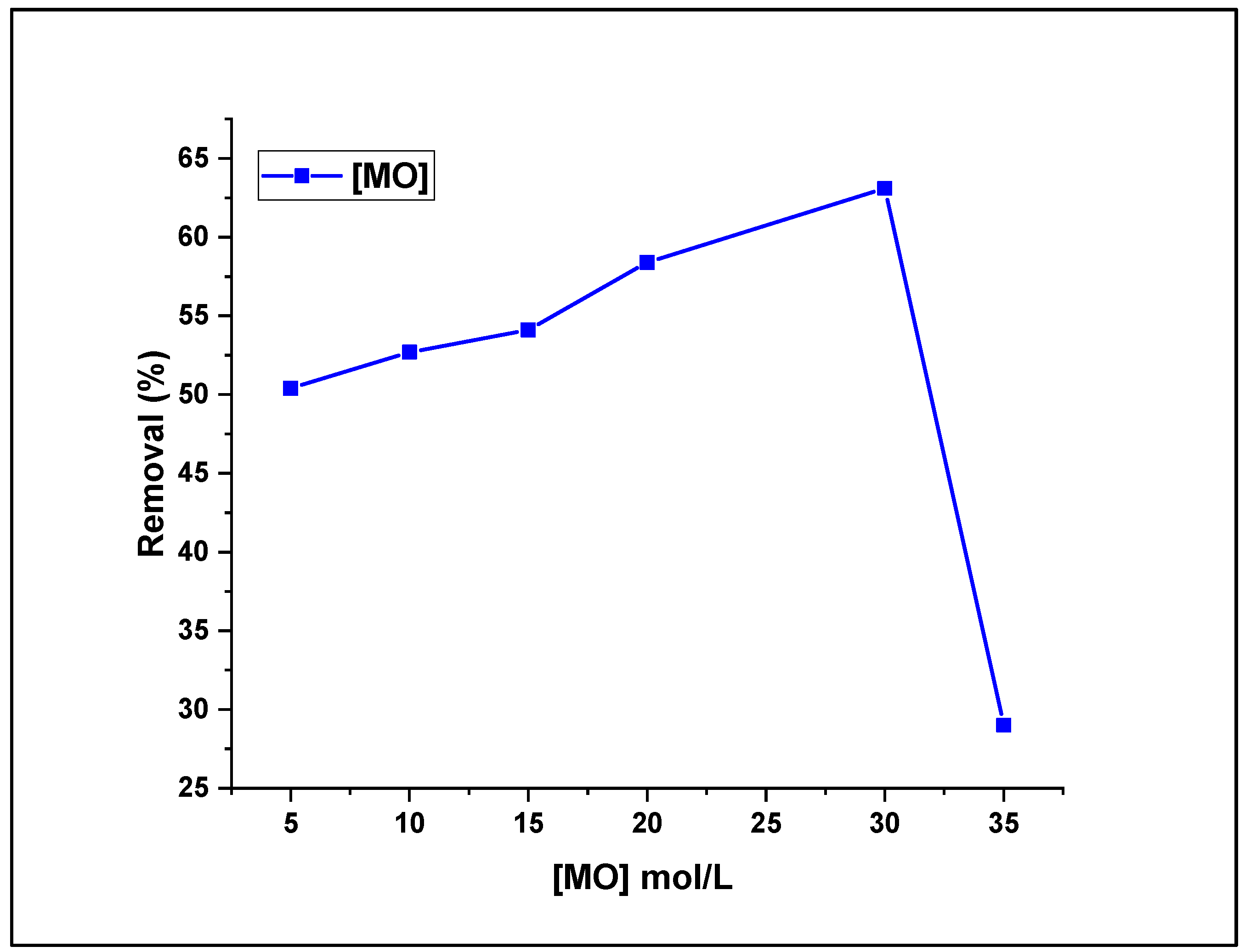
3.2.4. Effect of MO Concentration on Fenton-Photodegradation Process
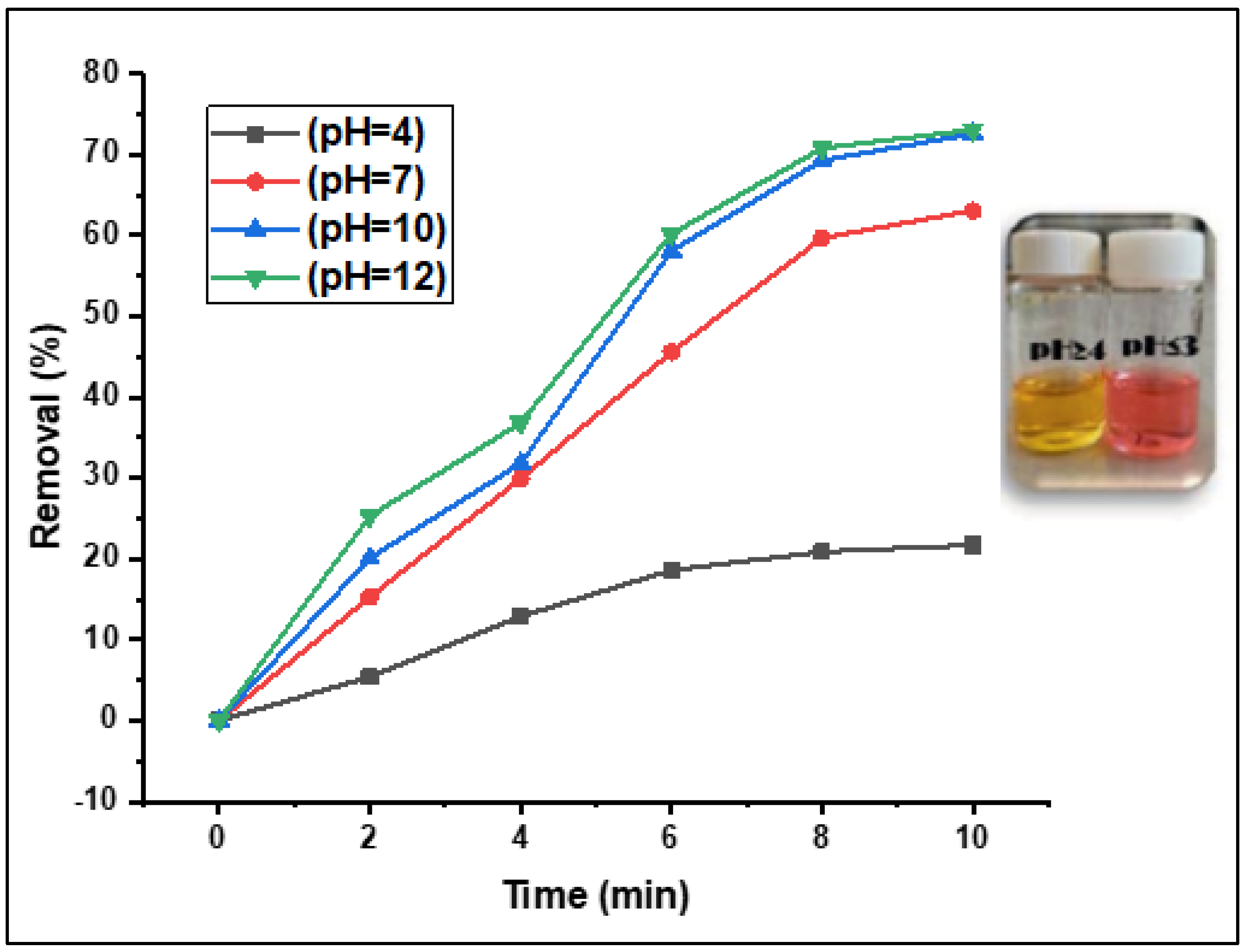
3.2.5. Effect of the Solution pH on the Fenton Photodegradation Process
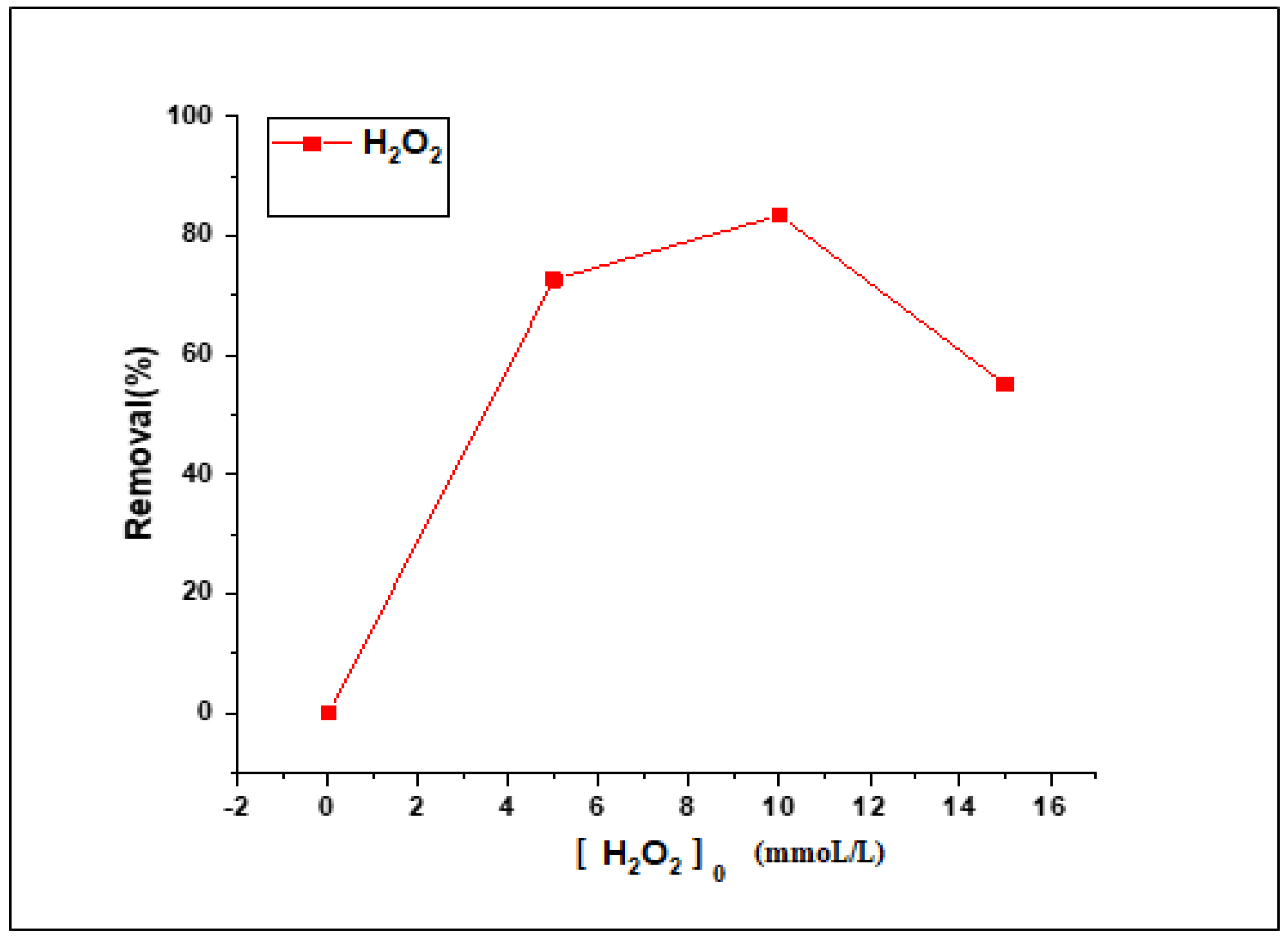
3.2.6. Effect of H2O2 Loading on the Fenton Photodegradation Process
3.2.7. Effect of Temperature on Fenton Photodegradation
3.3. Fenton-Photocatalytic Degradation of Methyl Orange: A UV-Vis Study and Proposed Fenton-Photocatalytic Mechanism
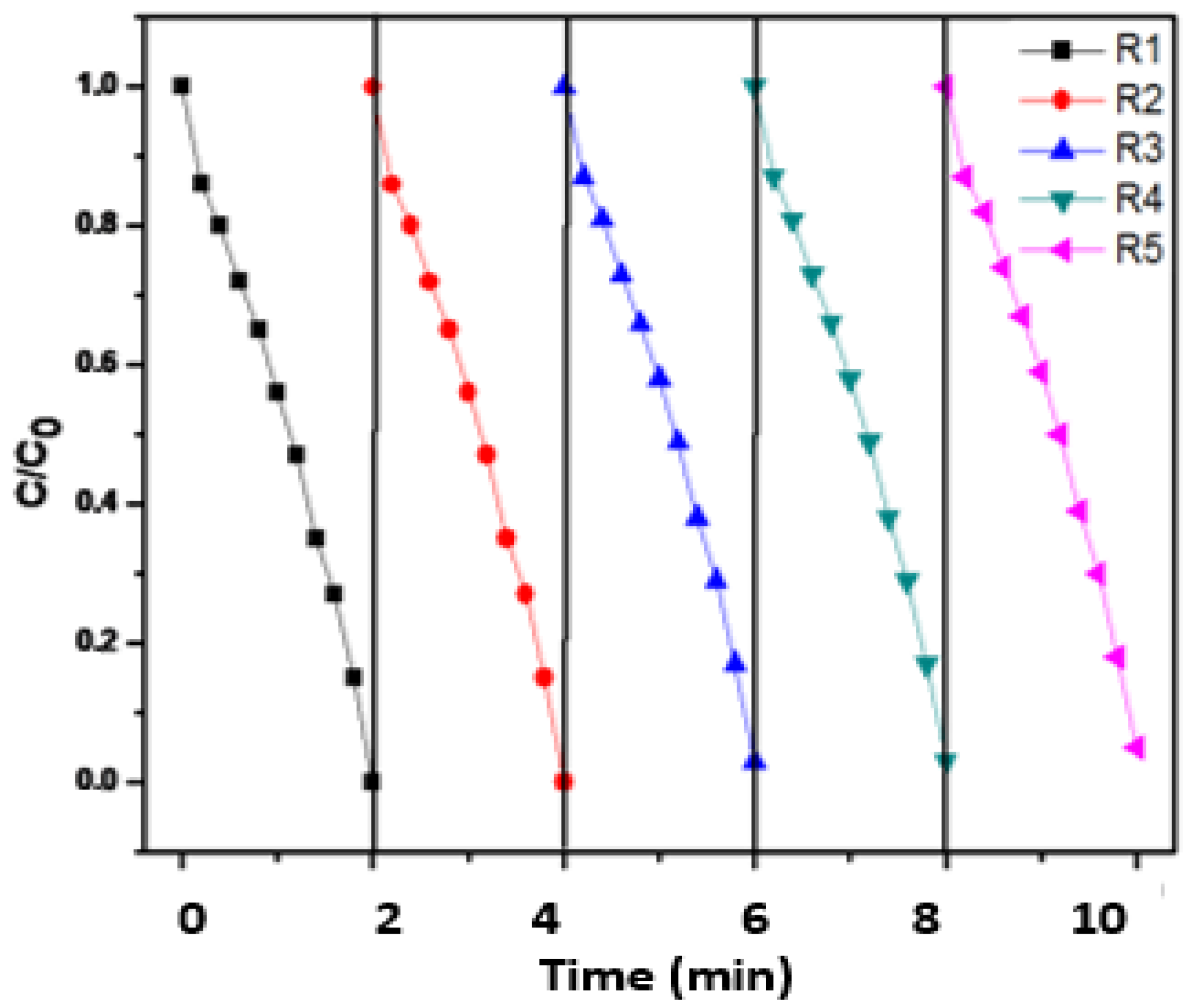
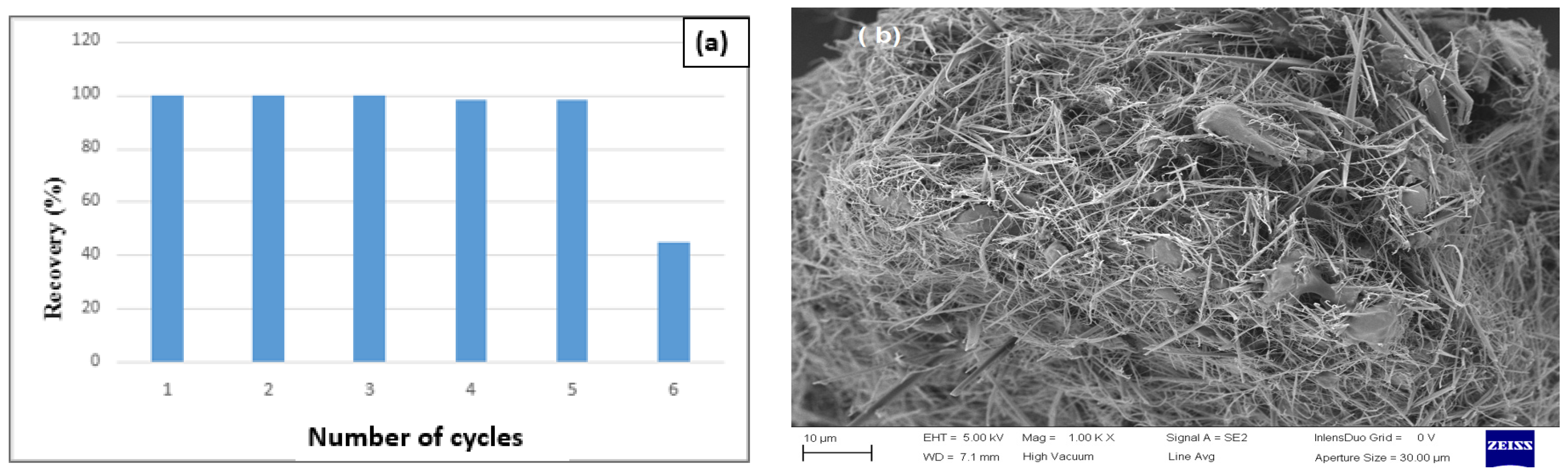
3.4. Reusability of Fenton-Photocatalytic Performance of (CDC@Fe3O4)
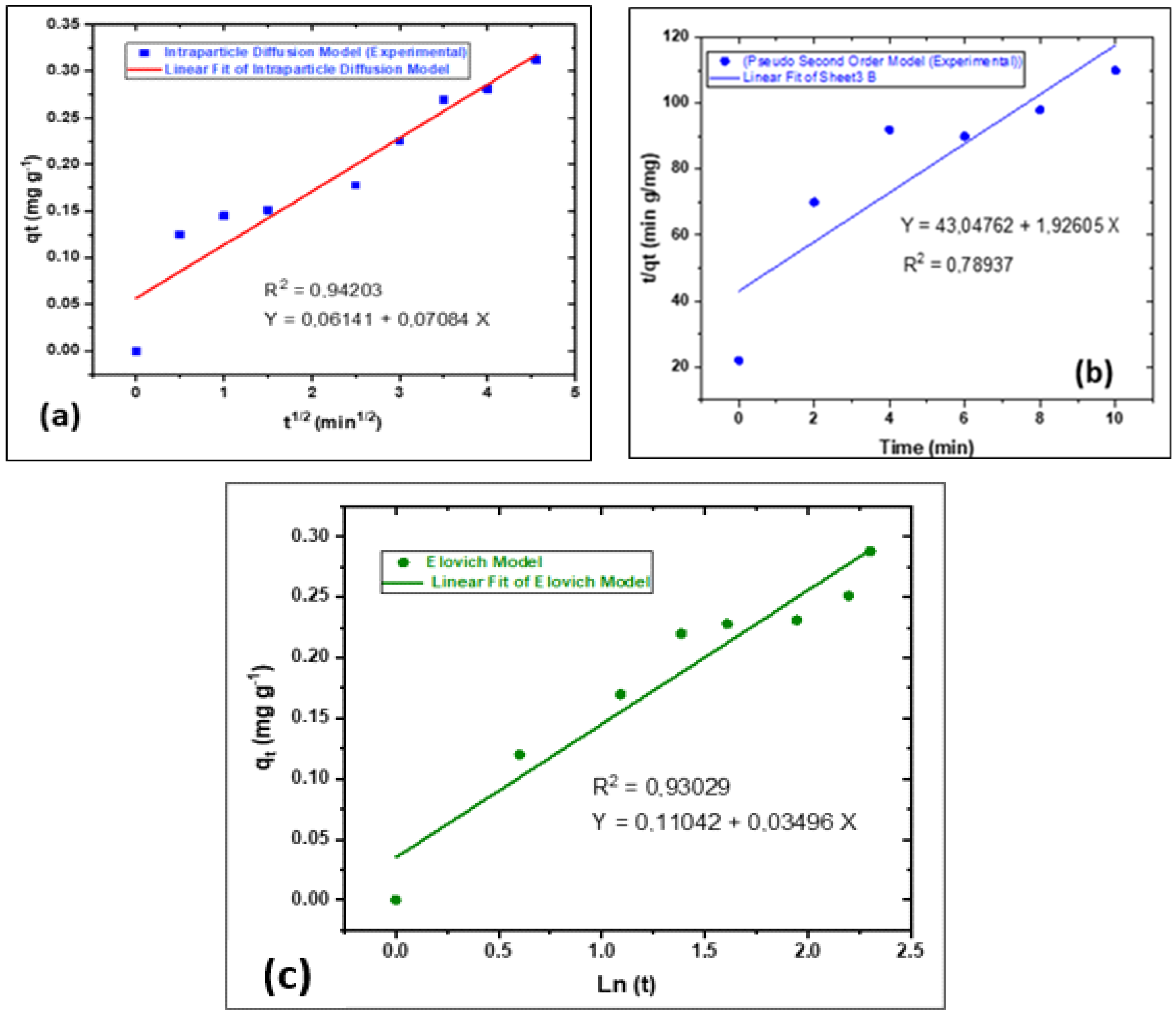
3.5. Theoretical Adsorption Kinetics Models
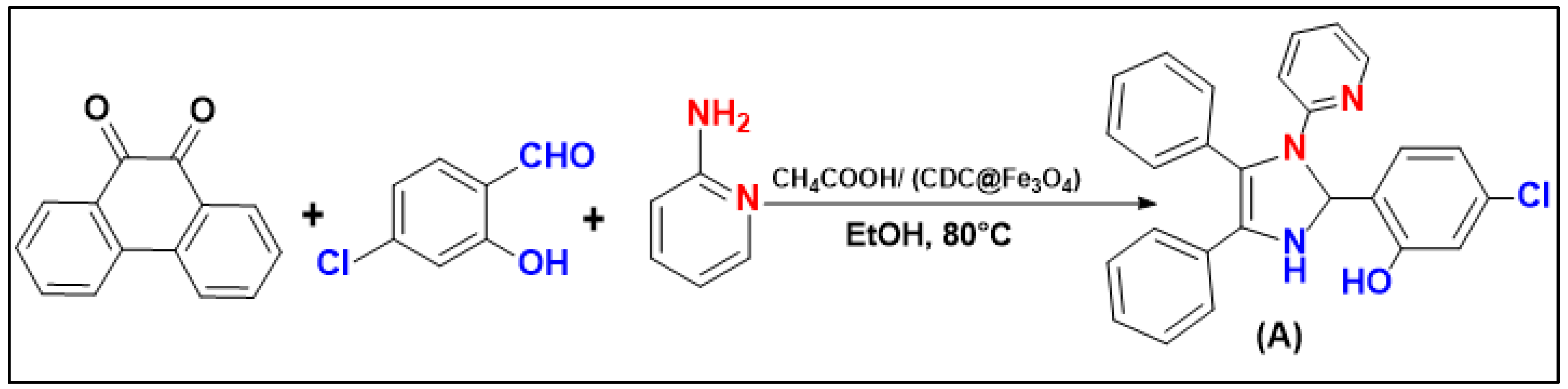
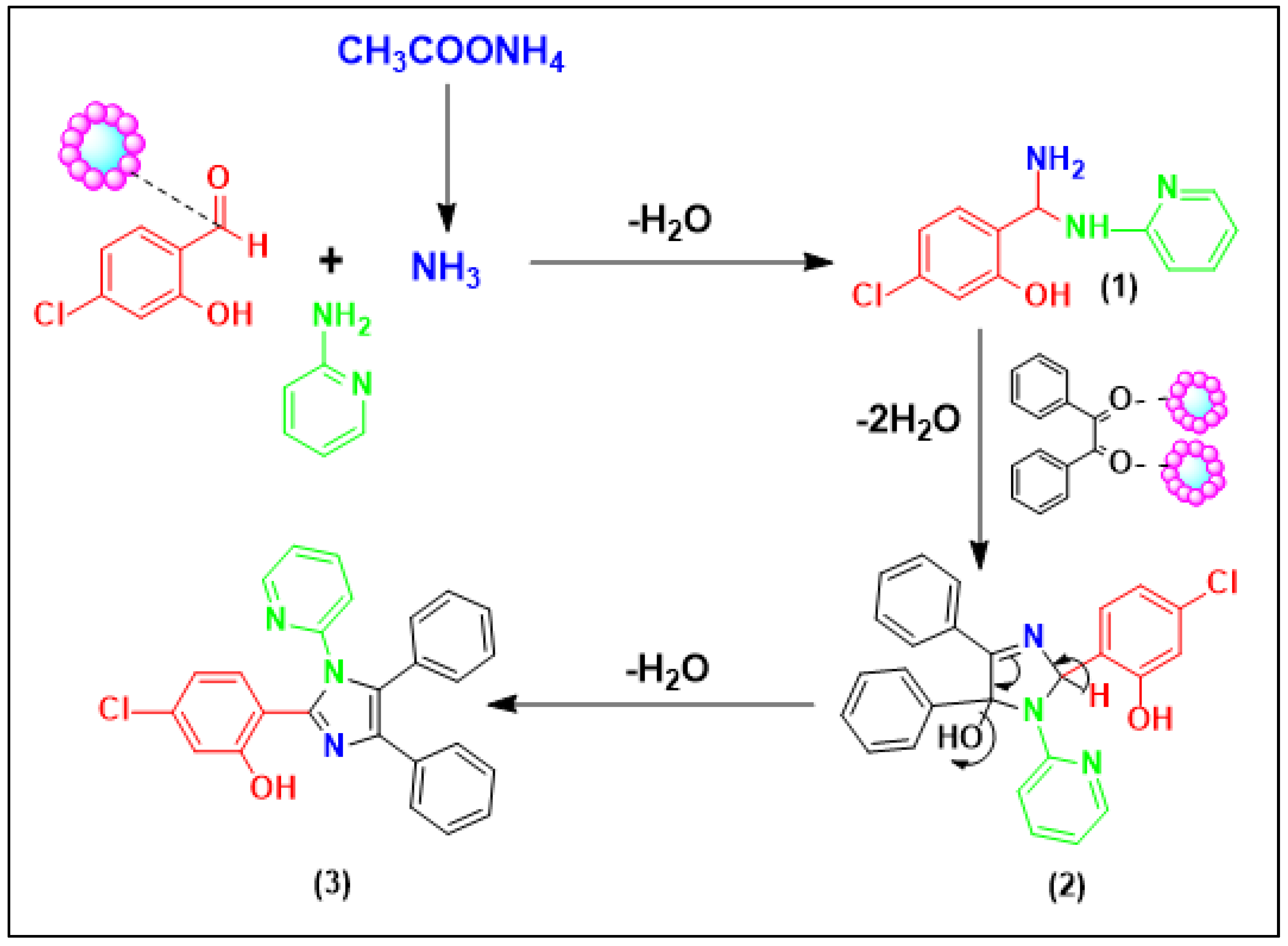
3.6. Catalytic Synthesis of the Tetra-Substituted Imidazole Derivatives
Reusability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eskikaya, O.; Isik, Z.; Arslantas, C.; Yabalak, E.; Balakrishnan, D.; Dizge, N.; Rao, K.S. Preparation of hydrochar bio-based catalyst for fenton process in dye-containing wastewater treatment. Environ. Res. 2023, 216, 114357. [Google Scholar] [CrossRef] [PubMed]
- Nieto, E.R.; Serrano, S.A.O.; Pineda, E.A.G.; Tirado, C.B.; Combariza, M.Y. Textile wastewater depuration using a green cellulose based Fe3O4 bionanocomposite. J. Environ. Chem. Eng. 2023, 11, 109516. [Google Scholar] [CrossRef]
- Repon, M.; Islam, T.; Sarwar, Z.; Rahman, M.M. Rahman, Impact of textile dyes on health and ecosystem: A review of structure, causes, and potential solutions. Environ. Sci. Pollut. Res. 2023, 30, 9207–9242. [Google Scholar]
- Aljohani, M.M.; Al-Qahtani, S.D.; Alshareef, M.; El-Desouky, M.G.; El-Bindary, A.A.; El-Metwaly, N.M.; El-Bindary, M.A. Highly efficient adsorption and removal bio-staining dye from industrial wastewater onto mesoporous Ag-MOFs. Process Saf. Environ. Prot. 2023, 172, 395–407. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Snari, R.M.; Alamrani, N.A.; Aljuhani, E.; Bayazeed, A.; Aldawsari, A.M.; El-Metwaly, N.M. Synthesis and adsorption properties of fibrous-like aerogel from acylhydrazone polyviologen: Efficient removal of reactive dyes from wastewater. J. Mater. Res. Technol. 2022, 18, 1822–1833. [Google Scholar] [CrossRef]
- Alotaibi, M.T.; Mogharbel, R.T.; Alorabi, A.Q.; Alamrani, N.A.; Shahat, A.; El-Metwaly, N.M. Superior adsorption and removal of toxic industrial dyes using cubic Pm3n aluminosilica form an aqueous solution, Isotherm, Kinetic, thermodynamic and mechanism of interaction. J. Mol. Liq. 2023, 379, 121672. [Google Scholar] [CrossRef]
- Kaur, H.; Devi, N.; Siwal, S.S.; Alsanie, W.F.; Thakur, M.K.; Thakur, V.K. Metal–Organic Framework-Based Materials for Wastewater Treatment: Superior Adsorbent Materials for the Removal of Hazardous Pollutants. ACS Omega 2023, 8, 9004–9030. [Google Scholar] [CrossRef]
- Mohanty, S.; Dash, S.; Pradhan, N.; Maji, S.K. Bio-Remediation of Organic Dyes from Wastewater by Microbial Colony—A Short Review. Nano-Eng. Mater. Text. Waste Remediat. 2023, 20, 61–104. [Google Scholar]
- Quynh, H.G.; Van Thanh, H.; Phuong, N.T.T.; Duy, N.P.T.; Hung, L.H.; Van Dung, N.; Duong, N.T.H.; Long, N.Q. Long, Rapid removal of methylene blue by a heterogeneous photo-Fenton process using economical and simple-synthesized magnetite–zeolite composite. Environ. Technol. Innov. 2023, 31, 103155–103168. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Huang, W. Bismuth-Based Photocatalysts for Solar Energy Conversion. J. Mater. Chem. A 2020, 8, 24307–24352. [Google Scholar] [CrossRef]
- Qin, X.; Xu, J.; Zhu, Z.; Li, Z.; Fang, D.; Fu, H.; Zhang, S.; Zhang, H. Co78Si8B14 metallic glass: A highly efficient and ultra-sustainable Fenton-like catalyst in degrading wastewater under universal pH conditions. J. Mater. Sci. Technol. 2022, 113, 105–116. [Google Scholar] [CrossRef]
- Mehdaoui, R.; Agren, S.; Dhahri, A.; Haskouri, J.E.; Beyou, E.; Lahcini, M.; Baouab, M.H.V. New sonochemical magnetite nanoparticles functionalization approach of dithiooxamide–formaldehyde developed cellulose: From easy synthesis to recyclable 4-nitrophenol reduction. Appl. Organomet. Chem. 2021, 35, 6257–6276. [Google Scholar] [CrossRef]
- Deng, J.; Wen, X.; Wang, Q. Solvothermal in situ synthesis of Fe3O4-multi-walled carbon nanotubes with enhanced heterogeneous Fenton-like activity. Mater. Res. Bull. 2012, 47, 3369–3376. [Google Scholar] [CrossRef]
- Zubir, N.; Yacou, C.; Motuzas, J.; Zhang, X.; da Costa, J.C.D. Structural and functional investigation of graphene oxide–Fe3O4 nanocomposites for the heterogeneous Fenton-like reaction. Sci. Rep. 2014, 4, 4594. [Google Scholar] [CrossRef] [PubMed]
- Lourens, A.; Falch, A.; Malgas-Enus, R. Magnetite immobilized metal nanoparticles in the treatment and removal of pollutants from wastewater: A review. J. Mater. Sci. 2023, 58, 2951–2970. [Google Scholar] [CrossRef]
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Guna, V.K.; Gopalakrishna, K.; Kumar, Y. Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review. Carbohydr. Polym. 2016, 153, 600–618. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmed, Z.A.; Al-Hazmi, G.A.; Munshi, A.M.; Alamrani, N.A.; Al-Qahtani, S.D.; Habeebullah, T.M.; El-Metwaly, N.M. Recoverable palladium–gold nanocomposite based on microcrystalline cellulose for sono-catalytic degradation of pharmaceutical pollutants. Mater. Chem. Phys. 2023, 296, 127219. [Google Scholar] [CrossRef]
- Munshi, A.M.; Alamrani, N.A.; Alessa, H.; Aljohani, M.; Ibarhiam, S.F.; Saad, F.A.; Al-Qahtani, S.D.; El-Metwaly, N.M. Thiophene functionalized cellulose immobilized with metal organic framework for removal of heavy metals. Cellulose 2023, 30, 7235–7250. [Google Scholar] [CrossRef]
- Li, K.; Clarkson, C.M.; Wang, L.; Liu, Y.; Lamm, M.; Pang, Z.; Zhou, Y.; Qian, J.; Tajvidi, M.; Gardner, D.J.; et al. Alignment of Cellulose Nanofibers: Harnessing Nanoscale Properties to Macroscale Benefits. ACS Nano 2021, 15, 3646–3673. [Google Scholar] [CrossRef]
- Favela-Camacho, S.E.; Samaniego-Benítez, E.J.; Godínez-García, A.; Avilés-Arellano, L.M.; Pérez-Robles, J.F. How to decrease the agglomeration of magnetite nanoparticles and increase their stability using surface properties. Colloids Surf. A Physicochem. Eng. Asp. 2019, 574, 29–35. [Google Scholar] [CrossRef]
- Saeedi, S.; Rahmati, A. MNP–cellulose–OSO3H as an efficient and biodegradable heterogeneous catalyst for green synthesis of trisubstituted imidazoles. RSC Adv. 2022, 12, 11740–11749. [Google Scholar] [CrossRef] [PubMed]
- Sayin, F.; Akar, S.T.; Akar, T.; Celik, S.; Gedikbey, T. Chitosan immobilization and Fe3O4 functionalization of olive pomace: An eco–friendly and recyclable Pb2+ biosorbent. Carbohydr. Polym. 2021, 269, 118266. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jia, S.; Wu, Q.; Ran, J.; Zhang, W.; Wu, S. Studies of Fe3O4-chitosan nanoparticles prepared by co-precipitation under the magnetic field for lipase immobilization. Catal. Commun. 2011, 12, 717–720. [Google Scholar] [CrossRef]
- Wang, G.; Xiang, J.; Lin, J.; Xiang, L.; Chen, S.; Yan, B.; Fan, H.; Zhang, S.; Shi, X. Sustainable Advanced Fenton-like Catalysts Based on Mussel-Inspired Magnetic Cellulose Nanocomposites to Effectively Remove Organic Dyes and Antibiotics. ACS Appl. Mater. Interfaces 2020, 12, 51952–51959. [Google Scholar] [CrossRef] [PubMed]
- Agren, S.; Mehdaoui, R.; El Haskouri, J.; Beyou, E.; Lahcini, M.; Baouab, M.H.V. Reusable Magnetic Catalysed Synthesis of Fluorescent Imidazole Derivatives: Their Use as Chromogenic and Fluorogenic Probes for Metal Cation’s Detection. J. Mol. Struct. 2023, 1287, 135641–135656. [Google Scholar] [CrossRef]
- Ganjali, F.; Kashtiaray, A.; Zarei-Shokat, S.; Taheri-Ledari, R.; Maleki, A. Functionalized hybrid magnetic catalytic systems on micro- and nanoscale utilized in organic synthesis and degradation of dyes. Nanoscale Adv. 2022, 4, 1263–1307. [Google Scholar] [CrossRef]
- Veisi, H.; Pirhayatib, M.; Mohammadia, P.; Tamoradic, T.; Hemmatia, S.; Karmakar, B. Recent advances in the application of magnetic nanocatalysts in multicomponent reactions. RSC Adv. 2023, 13, 20530–20556. [Google Scholar] [CrossRef]
- Liu, M.; Ye, Y.; Ye, J.; Gao, T.; Wang, D.; Chen, G.; Song, Z. Recent Advances of Magnetite (Fe3O4)-Based Magnetic Materials in Catalytic Applications. Magnetochemistry 2023, 10, 110. [Google Scholar] [CrossRef]
- Pakjo, L.; Rahimpour, S.; Mofrad, R.T. Design and synthesis of ferrocene-based magnetic nanoparticle and investigation of its catalytic ability in the synthesis of novel 6-[(morpholin-4-yl)methyl] substituted pyrano [3,2-b]pyran derivatives. Appl. Organomet. Chem. 2022, 37, 6956. [Google Scholar] [CrossRef]
- He, L.; Zhou, Z.; Liu, Z.; Nan, X.; Wang, T.; Sun, X.; Bai, P. Morphology design and synthesis of magnetic microspheres as highly efficient reusable catalyst for organic dyes. Colloids Surf. A 2023, 656, 7757–7927. [Google Scholar] [CrossRef]
- Gholinejad, M.; Bagheri, H.; Zareh, F.; Sansano, J.M. Palladium supported on hydrophilic magnetic nanoparticles as a new efficient catalyst in aqueous media. J. Mol. Struct. 2023, 1288, 135804. [Google Scholar] [CrossRef]
- Li, S.R.; Tan, Y.M.; Zhang, L.; Zhou, C.H. Comprehensive Insights into Medicinal Research on Imidazole-Based Supramolecular Complexes. Pharmaceutics 2023, 15, 1348. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.D.; Tsai, W.W.; Lu, C.W. Exploring the Electroluminescent Applications of Imidazole Derivatives. Chem.-Eur. J. 2023, 23, 3040–3069. [Google Scholar] [CrossRef] [PubMed]
- Toja, E.; Selva, D.; Schiatti, P. 3-Alkyl-2-Aryl-3H-Naphth [1, 2-d] Imidazoles, a Novel Class of Nonacidic Antiinflammatory Agents. J. Med. Chem. 1984, 27, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Molinaro, A.; Ståhlman, M.; Khan, M.T.; Schmidt, C.; Mannerås-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E.; et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through MTORC1. Cell 2018, 175, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Hameed, S.; Jain, R. Ring-Substituted Imidazoles as a New Class of Anti-Tuberculosis Agents. Eur. J. Med. Chem. 2004, 39, 805–814. [Google Scholar] [CrossRef]
- Jeanmart, S.; Gagnepain, J.; Maity, P.; Lamberth, C.; Cederbaum, F.; Rajan, R.; Jacob, O.; Blum, M.; Bieri, S. Synthesis and Fungicidal Activity of Novel Imidazole-Based Ketene Dithioacetals. Bioorg. Med. Chem. 2018, 26, 2009–2016. [Google Scholar] [CrossRef]
- Keppler, B.K.; Wehe, D.; Endres, H.; Rupp, W. Synthesis, Antitumor Activity, and X-ray Structure of Bis (Imidazolium)(Imidazole) Pentachlororuthenate (III),(ImH) 2 (RuImCl5). Inorg. Chem. 1987, 26, 844–846. [Google Scholar] [CrossRef]
- James, D.A.; Koya, K.; Li, H.; Chen, S.; Xia, Z.; Ying, W.; Wu, Y.; Sun, L. Conjugated Indole-Imidazole Derivatives Displaying Cytotoxicity against Multidrug Resistant Cancer Cell Lines. Bioorg. Med. Chem. Lett. 2006, 16, 5164–5168. [Google Scholar] [CrossRef]
- Adams, C.J.; Haddow, M.F.; Hughes, R.J.; Kurawa, M.A.; Orpen, A.G. Coordination chemistry of platinum and palladium in the solid-state: Synthesis of imidazole and pyrazole complexes. Dalton Trans. 2010, 39, 3714–3724. [Google Scholar] [CrossRef]
- Ruan, Y.; Chen, Y.; Gu, L.; Luo, Y.; He, L. Preparation of Imidazole Derivatives via Bisfunctionalization of Alkynes Catalyzed by Ruthenium Carbonyl. Synthesis 2019, 51, 3520–3528. [Google Scholar] [CrossRef]
- Tashiro, T.; Shimura, Y. Removal of mercuric ions by systems based on cellulose derivatives. J. Appl. Polym. Sci. 1982, 27, 747–756. [Google Scholar] [CrossRef]
- Ghali, E.; Baouabb, M.H.V.; Roudesli, M.S. Preparation, characterization and application of a [copper (II)/ethylenediamine–cotton] complex for the removal of AB25 from aqueous solution in a laboratory scale column. Chem. Eng. J. 2011, 174, 18–26. [Google Scholar] [CrossRef]
- Radoń, A.; Drygała, A.; Hawełek, Ł.; Łukowiec, D. Structure and optical properties of Fe3O4 nanoparticles synthesized by co-precipitation method with different organic modifiers. Mater. Charact. 2017, 131, 148–156. [Google Scholar] [CrossRef]
- Saragi, T.; Depi, B.L.; Butarbutar, S.; Permana, B. The impact of synthesis temperature on magnetite nanoparticles size synthesized by co-precipitation method. Lop Conf. Ser. J. Phys. Conf. Ser. 2018, 1013, 012190. [Google Scholar] [CrossRef]
- Deotale, A.J.; Nandedkar, R.V. Correlation between Particle Size, Strain and Band Gap of Iron Oxide Nanoparticles. Mater. Today Proc. 2016, 3, 2069–2076. [Google Scholar] [CrossRef]
- Zanata, L.; Tofanello, A.; Martinho, H.S.; Souza, J.A.; Rosa, D.S. Iron oxide nanoparticles–cellulose: A comprehensive insight on nanoclusters formation. J. Mater. Sci. 2022, 57, 324–335. [Google Scholar] [CrossRef]
- Chirita, E.; Marius, M.C. Highly crystalline porous magnetite and vacancy-ordered maghemite microcrystals of rhombohedral habit. J. Cryst. Growth 2013, 380, 182–186. [Google Scholar]
- Yazdani, F.; Seddigh, M. Magnetite nanoparticles synthesized by co-precipitation method: The effects of various iron anions on specifications. Mater. Chem. Phys. 2016, 184, 318–323. [Google Scholar] [CrossRef]
- Manikandan, A.; Vijaya, J.J.; Mary, J.A.; Kennedy, L.J.; Dinesh, A. Structural, optical and magnetic properties of Fe3O4 nanoparticles prepared by a facile microwave combustion method. J. Ind. Eng. Chem. 2014, 20, 2077–2085. [Google Scholar] [CrossRef]
- da Silva Filho, E.; Santana, S.; Melo, J.; Oliveira, F.; Airoldi, C. X-ray diffraction and thermogravimetry data of cellulose, chlorodeoxycellulose and aminodeoxycellulose. J. Therm. Anal. Calorim. 2010, 100, 315–321. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. Engl. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.S.; Lee, Y.H.; Musa, A.; Salmah, A.A.; Zamri, I. Use of Fe3O4 nanoparticles for enhancement of biosensor response to the herbicide 2,4-dichlorophenoxyacetic acid. Sensors 2008, 8, 5775. [Google Scholar] [CrossRef] [PubMed]
- Kharazi, P.; Rahimi, R.; Rabbani, M. Study on porphyrin/ZnFe2O4@ polythiophene nanocomposite as a novel adsorbent and visible light driven photocatalyst for the removal of methylene blue and methyl orange. Mater. Res. Bull. 2018, 103, 133–141. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Y.; Bu, X.; Wu, Q.; Hang, Z.; Dong, Z.; Wu, X. Facile one-step synthesis of TiO2/Ag/SnO2 ternary heterostructures with enhanced visible light photocatalytic activity. Sci. Rep. 2018, 8, 10532. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, G.; Lu, Z.; Sun, J.; Zhuang, J.; Yang, W. Effect of ultrasonic treatment on dispersibility of Fe3O4nanoparticles and synthesis of multi-core Fe3O4/SiO2 core/shell nanoparticles. J. Mater. Chem. 2005, 15, 4252–4257. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment II: Hybrid methods. Adv. Environ. Res. 2004, 8, 553–597. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Tabrizi, S.B.; Molanee, S. Ultrasonic degradation of Rhodamine B in aqueous solution: Influence of operational parameters. J. Hazard. Mater. 2008, 152, 381–386. [Google Scholar] [CrossRef]
- Ince, N.H.; Tezcanli-Güyer, G. Impacts of pH and molecular structure on ultrasonic degradation of azo dyes. Ultrasonics 2004, 42, 591–596. [Google Scholar] [CrossRef]
- Mehdaoui, R.; Agren, S.; Haskouri, J.E.; Beyou, E.; Lahcini, M.; Baouab, M.H.V. An optimized sono-heterogeneous Fenton degradation of olive-oil mill wastewater organic matter by new magnetic glutarlaldehyde-crosslinked developed cellulose. Environ. Sci. Pollut. Res. 2022, 5, 2–19. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Fu, C.C.; Juang, R.S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Farzana, M.H.; Meenakshi, S. Synergistic effect of chitosan and titanium dioxide on the removal of toxic dyes by the photodegradation technique. Ind. Eng. Chem. Res. 2014, 53, 55–63. [Google Scholar] [CrossRef]
- Shubha, P.; Savitha, H.S.; Adil, S.F.; Khan, M.; Hatshan, M.R.; Kavalli, K.; Shaik, B. Straightforward Synthesis of Mn3O4/ZnO/Eu2O3-Based Ternary Heterostructure Nano-Photocatalyst and Its Application for the Photodegradation of Methyl Orange and Methylene Blue Dyes. Molecules 2021, 26, 4661. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, N.; Gao, Y.; Feng, C. Photocatalytic degradation of methylene blue by magnetically recoverable Fe3O4/Ag6Si2O7 under simulated visible light. Powder Technol. 2018, 326, 247–254. [Google Scholar] [CrossRef]
- Sanad, M.M.S.; Farahat, M.M.; El-Hout, S.I.; El-Sheikh, S.M. Preparation and characterization of magnetic photocatalyst from the banded iron formation for effective photodegradation of methylene blue under UV and visible illumination. J. Environ. Chem. Eng. 2021, 9, 105127–105141. [Google Scholar] [CrossRef]
- Golshan, M.; Zare, M.; Goudarzi, G.; Abtahi, M.; Babaei, A.A. Fe3O4@HAP-enhanced photocatalytic degradation of Acid Red73 in aqueous suspension: Optimization, kinetic, and mechanism studies. Mater. Res. Bull. 2017, 91, 59–67. [Google Scholar] [CrossRef]
- Zhao, X.; Baharinikoo, L.; Mahdizadeh, B.; Farizhandi, A.A.K. Experimental modelling studies on the removal of dyes and heavy metal ions using ZnFe2O4 nanoparticles. Sci. Rep. 2022, 12, 5987. [Google Scholar] [CrossRef]
- Oyewo, A.; Adeniyi, A.; Sithole, B.B.; Onyango, M.S. Sawdust-based cellulose nanocrystals incorporated with ZnO nanoparticles as efficient adsorption media in the removal of methylene blue dye. ACS Omega 2020, 30, 18798–18807. [Google Scholar] [CrossRef]
- Panda, N.; Sahoo, H.; Mohapatra, S. Decolourization of methyl orange using Fenton-like mesoporous Fe2O3–SiO2 composite. J. Hazard. Mater. 2011, 185, 359–365. [Google Scholar] [CrossRef]
- Patel, J.; Singh, A.K.; Carabineiro, S.A.C. Assessing the photocatalytic degradation of fluoroquinolone norfloxacin by Mn:ZnS quantum dots: Kinetic study, degradation pathway and influencing factors. Nanomat 2020, 10, 964. [Google Scholar] [CrossRef]
- Montañez, J.P.; Heredia, C.L.; Sham, E.L.; Torres, E.M.F. Photodegradation of herbicide Metsulfuronmethyl with TiO2 supported on magnetite particles coated with SiO2. J. Environ. Chem. Eng. 2018, 6, 7402–7410. [Google Scholar] [CrossRef]
- Jamoussi, B.; Chakroun, R.; Timoumi, A.; Essalah, K. Synthesis and Characterization of New Imidazole Phthalocyanine for Photodegradation of Micro-Organic Pollutants from Sea Water. Catalysts 2020, 10, 906. [Google Scholar] [CrossRef]
- Zhu, Y.; Xue, J.; Xu, T.; He, G.; Chen, H. Enhanced photocatalytic activity of magnetic core–shell Fe3O4@Bi2O3–RGO heterojunctions for quinolone antibiotics degradation under visible light. J. Mater. Sci. Mater. Electron. 2017, 28, 8519–8528. [Google Scholar] [CrossRef]
- Ruíz-Baltazar, Á.D.J.; Méndez-Lozano, N.; Larrañaga-Ordáz, D.; Reyes-López, S.Y.; Antuñano, M.A.Z.; Campos, R.P. Magnetic Nanoparticles of Fe3O4 Biosynthesized by Cnicus benedictus Extract: Photocatalytic Study of Organic Dye Degradation and Antibacterial Behavior. Processes 2020, 8, 946–977. [Google Scholar] [CrossRef]
- Jain, A.; Hashmi, S.; Sanwaria, A.K.; Singh, M.A.B.H.; Susan, S.A.C. Carabineiro, Catalytic Properties of Graphene Oxide Synthesized by a “Green” Process for Efficient Abatement of Auramine-O Cationic Dye. Anal. Chem. Lett. 2020, 10, 21–32. [Google Scholar] [CrossRef]
- Jain, B.; Hashmi, A.; Sanwaria, S.; Singh, A.K.; Susan, A.B.H.; Carabineiro, S.A. Intensified elimination of aqueous heavy metal ions using chicken feathers chemically modified by a batch method. J. Mol. Liq. 2020, 312, 113475–113498. [Google Scholar]
- Vanhecke, D.; Crippa, F.; Lattuada, M.; Balog, S.; Rothen-Rutishauser, B.; Petri-Fink, A. Characterization of the shape anisotropy of superparamagnetic iron oxide nanoparticles during thermal decomposition. Materials 2020, 13, 2018. [Google Scholar] [CrossRef]
- Du, T.; Zhou, L.F.; Zhang, Q.; Liu, L.Y.; Li, G.; Luo, W.B.; Liu, H.K. Mesoporous structured aluminaosilicate with excellent adsorption performances for water purification. Sustain. Mater. Technol. 2018, 18, 80–111. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Le, N.P.T.; Tran, P.H. An efficient multicomponent synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles catalyzed by a magnetic nanoparticle supported Lewis acidic deep eutectic solvent. Tran RSC Adv. 2019, 9, 38148–38153. [Google Scholar] [CrossRef]










| Cycle Number | Removal Rate |
|---|---|
| Cycle 1 | 97.9% |
| Cycle 2 | 93.8% |
| Cycle 3 | 90.3% |
| Cycle 4 | 85.4% |
| Cycle 5 | 82.0% |
| Entry | Catalyst Load (mg) | Energy Output | Time | Solvent | Yield (%) |
|---|---|---|---|---|---|
| 1 | 1 | 80 °C | 12 h | Acetic Acid | 21% |
| 2 | 2 | 80 °C | 12 h | Acetic Acid | 25% |
| 3 | 3 | 80 °C | 12 h | Acetic Acid | 46% |
| 4 | 4 | 80 °C | 12 h | Acetic Acid | 50% |
| 5 | 5 | 80 °C | 12 h | Acetic Acid | 63% |
| 6 | 6 | 80 °C | 12 h | Acetic Acid | 63% |
| 7 | 7 | 80 °C | 12 h | Acetic Acid | 63% |
| 8 | 5 | 80 °C | 12 h | Ethanol | 75% |
| 9 | 5 | 80 °C | 12 h | Water | 71% |
| 10 | 5 | 120 °C | 15 h | Acetic Acid | 63% |
| 11 | 5 | US | 30 min | Ethanol | 95% |
| 12 | 5 | US/50 °C | 30 min | Ethanol | 95% |
| Entry | Benzyl Derivative | Aldehyde | Primary Amine | Product | Yield (%) |
|---|---|---|---|---|---|
| A |  | 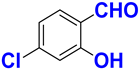 | 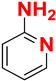 |  | 95% |
| B |  | 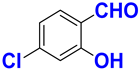 | 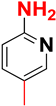 |  | 91% |
| C |  | 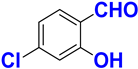 | 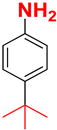 | 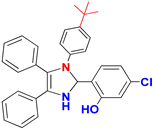 | 93% |
| D |  | 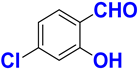 |  |  | 90% |
| E |  | 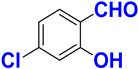 |  |  | 97% |
| F | 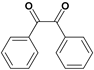 | 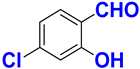 |  | 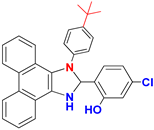 | 95% |
| G |  | 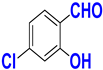 | 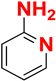 | 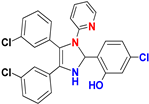 | 93% |
| H | 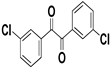 |  |  | 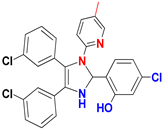 | 94% |
| I | 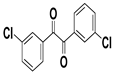 | 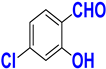 |  |  | 93% |
| (CDC@Fe3O4) a | TON b | TOF (h−1) c |
|---|---|---|
| Run 1 | 418.27 | 836.54 |
| Run 2 | 398.75 | 797.50 |
| Run 3 | 390.38 | 780.76 |
| Run 4 | 384.81 | 769.62 |
| Run 5 | 376.44 | 734.88 |
| Run 6 | 223.08 | 446.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albalawi, M.A.; Hajri, A.K.; Jamoussi, B.; Albalawi, O.A. A Novel Recyclable Magnetic Nano-Catalyst for Fenton-Photodegradation of Methyl Orange and Imidazole Derivatives Catalytic Synthesis. Polymers 2024, 16, 140. https://doi.org/10.3390/polym16010140
Albalawi MA, Hajri AK, Jamoussi B, Albalawi OA. A Novel Recyclable Magnetic Nano-Catalyst for Fenton-Photodegradation of Methyl Orange and Imidazole Derivatives Catalytic Synthesis. Polymers. 2024; 16(1):140. https://doi.org/10.3390/polym16010140
Chicago/Turabian StyleAlbalawi, Marzough A., Amira K. Hajri, Bassem Jamoussi, and Omnia A. Albalawi. 2024. "A Novel Recyclable Magnetic Nano-Catalyst for Fenton-Photodegradation of Methyl Orange and Imidazole Derivatives Catalytic Synthesis" Polymers 16, no. 1: 140. https://doi.org/10.3390/polym16010140
APA StyleAlbalawi, M. A., Hajri, A. K., Jamoussi, B., & Albalawi, O. A. (2024). A Novel Recyclable Magnetic Nano-Catalyst for Fenton-Photodegradation of Methyl Orange and Imidazole Derivatives Catalytic Synthesis. Polymers, 16(1), 140. https://doi.org/10.3390/polym16010140







