Development of Three-Dimensional (3D) Biodegradable Polyglycolic Acid Fiber (PGA) Preforms for Scaffold Applications: Experimental Patterning and Fiber Volume Fraction-Porosity Modeling Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Development of 3D Structures for Scaffold
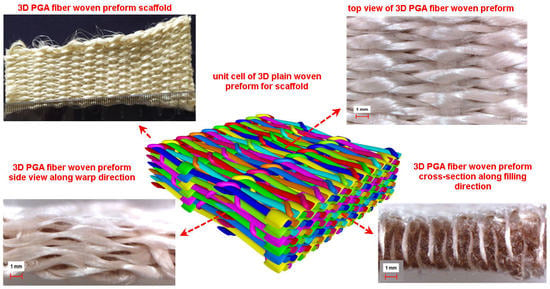
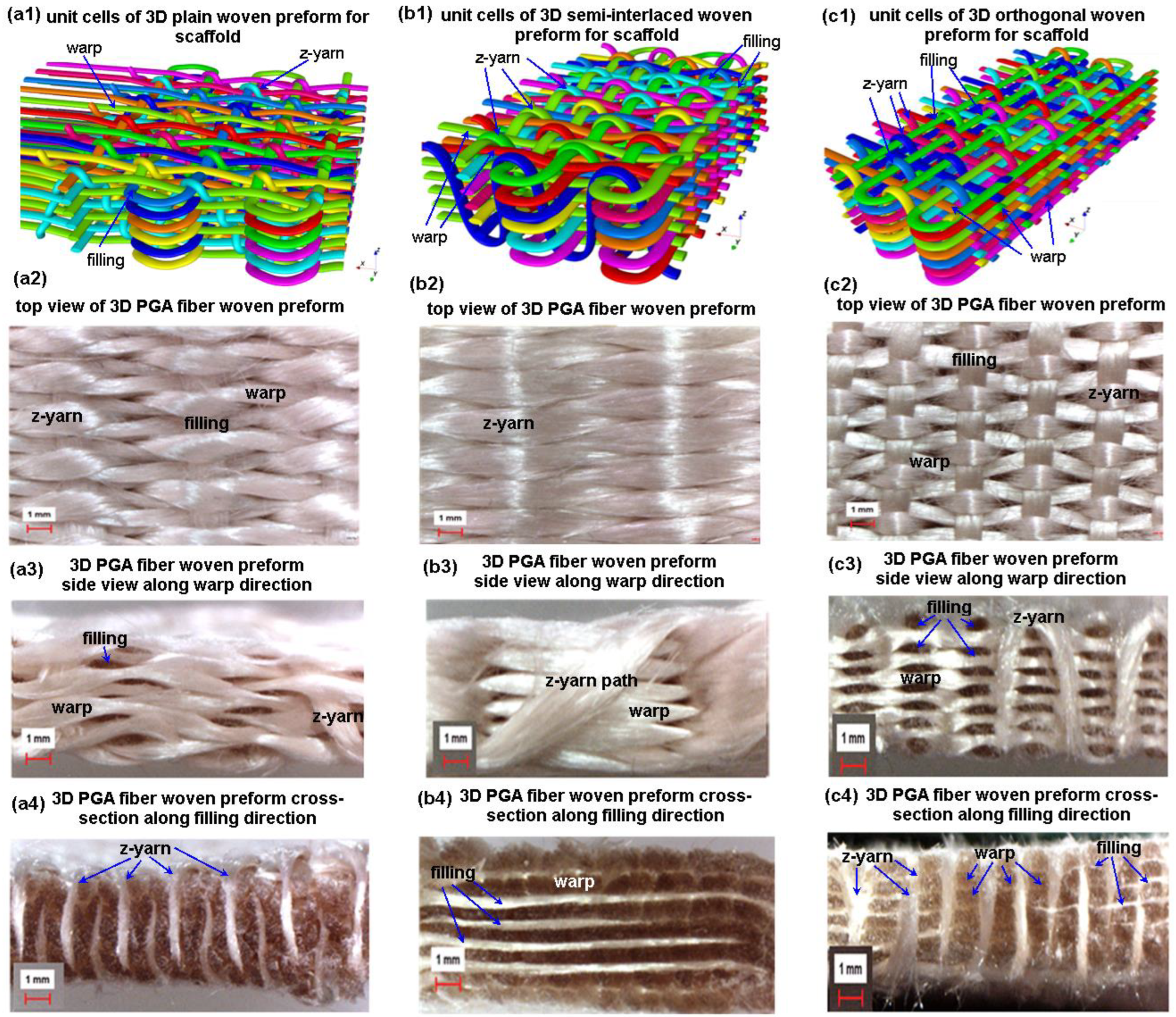
2.1.1. 3D Plain Woven Preform
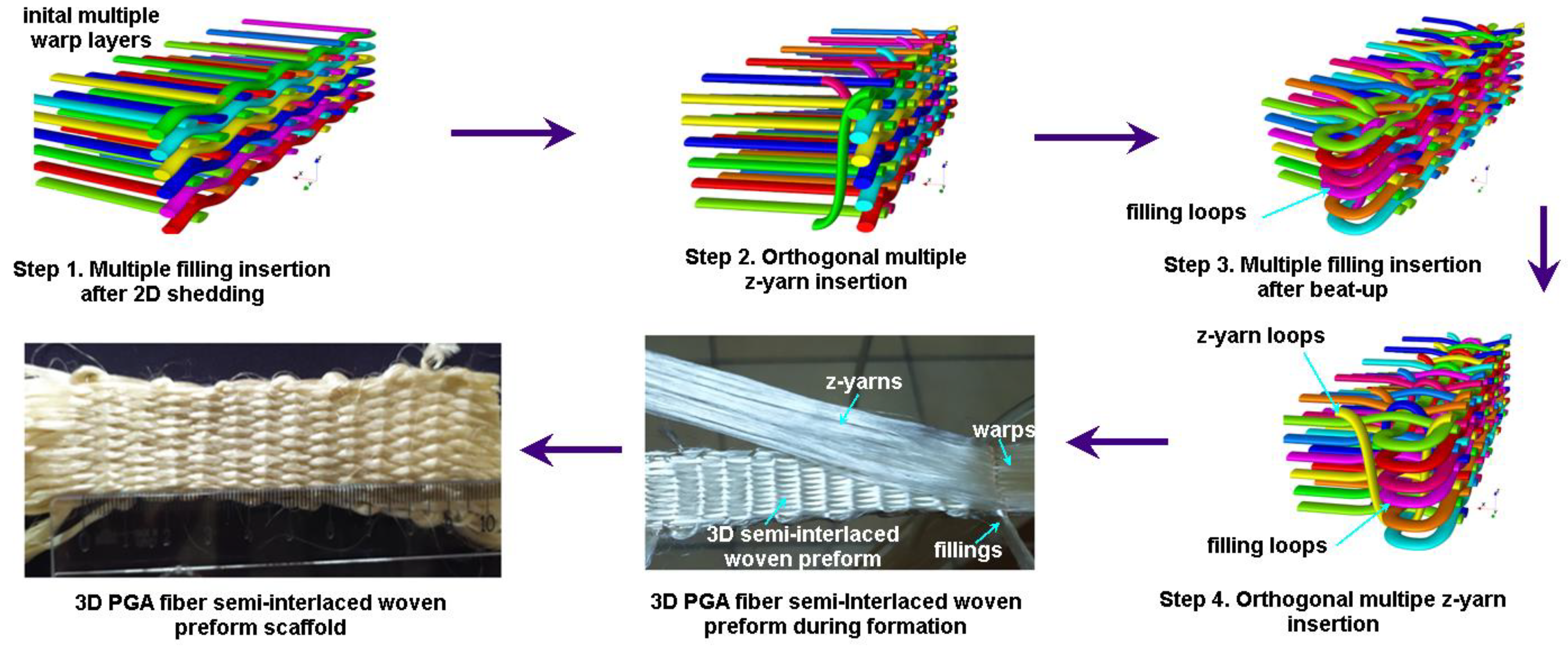
2.1.2. 3D Semi-Interlaced Woven Preforms
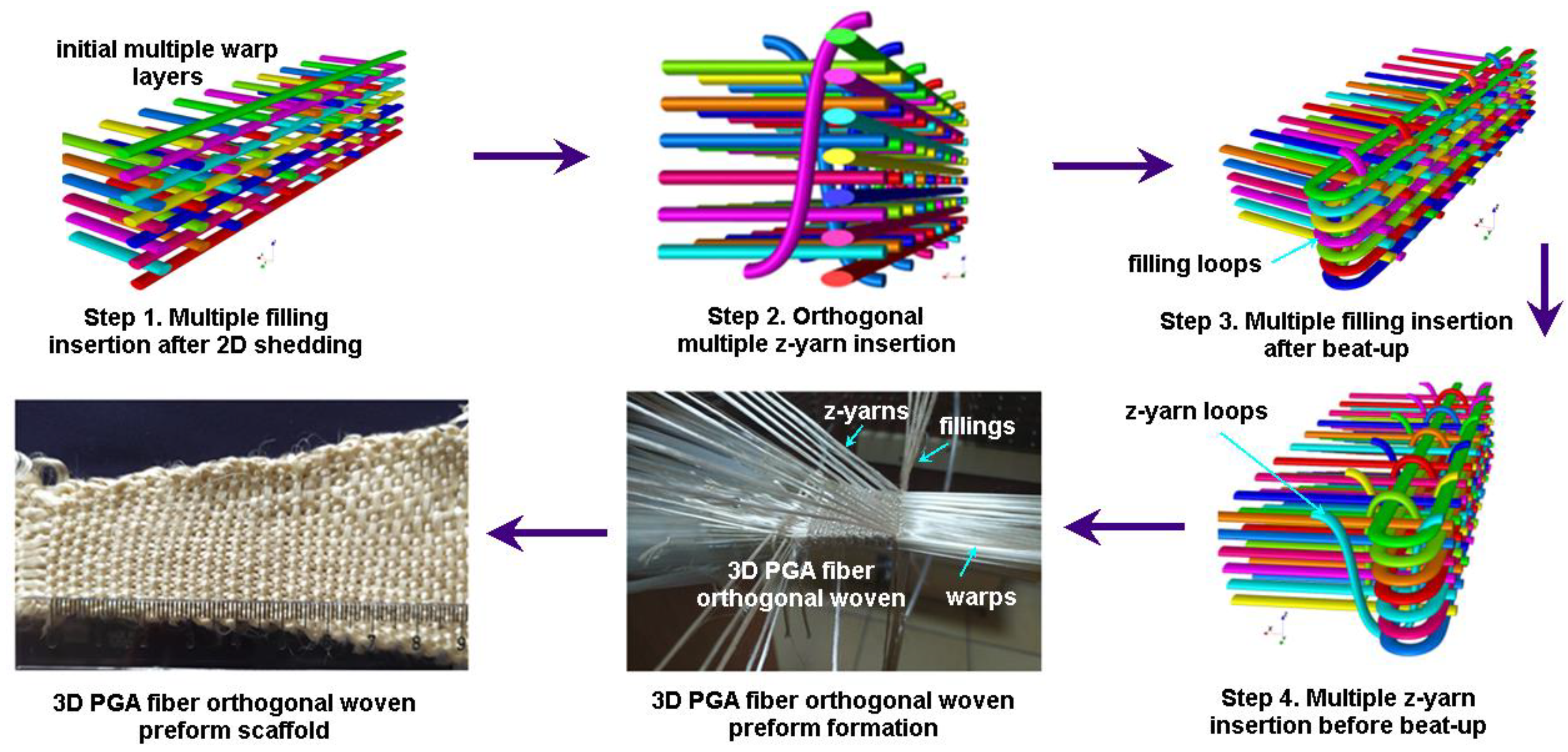
2.1.3. 3D Orthogonal Woven Preforms
2.2. Dimensions, Density, and Angle Measurements
2.2.1. Crimp Measurements
2.2.2. Fiber Volume Fraction Measurements
2.3. Geometric Modeling
2.3.1. Analytical Model on Volume Fraction and Porosity
2.3.2. Finite Element Modeling (FEM) on Volume Fraction and Porosity (TexGen)
2.4. Stiffness Calculations on 3D Preforms
3. Results and Discussion
3.1. Preform Structure for Scaffold Results
3.1.1. Preform Fiber-to-Fiber Space, Density, and Areal Density Results
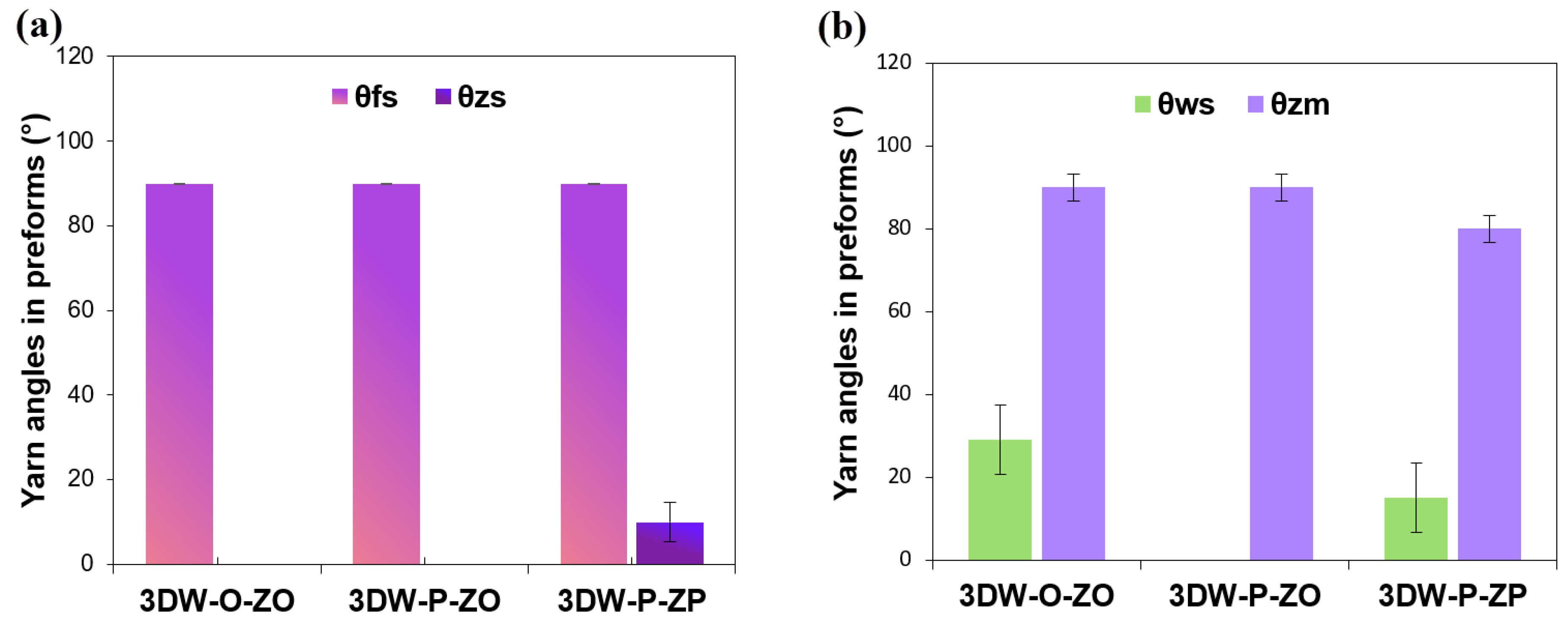
3.1.2. Preform Structure Angle Results
3.1.3. Preform Structure Crimp and Fiber Length Results
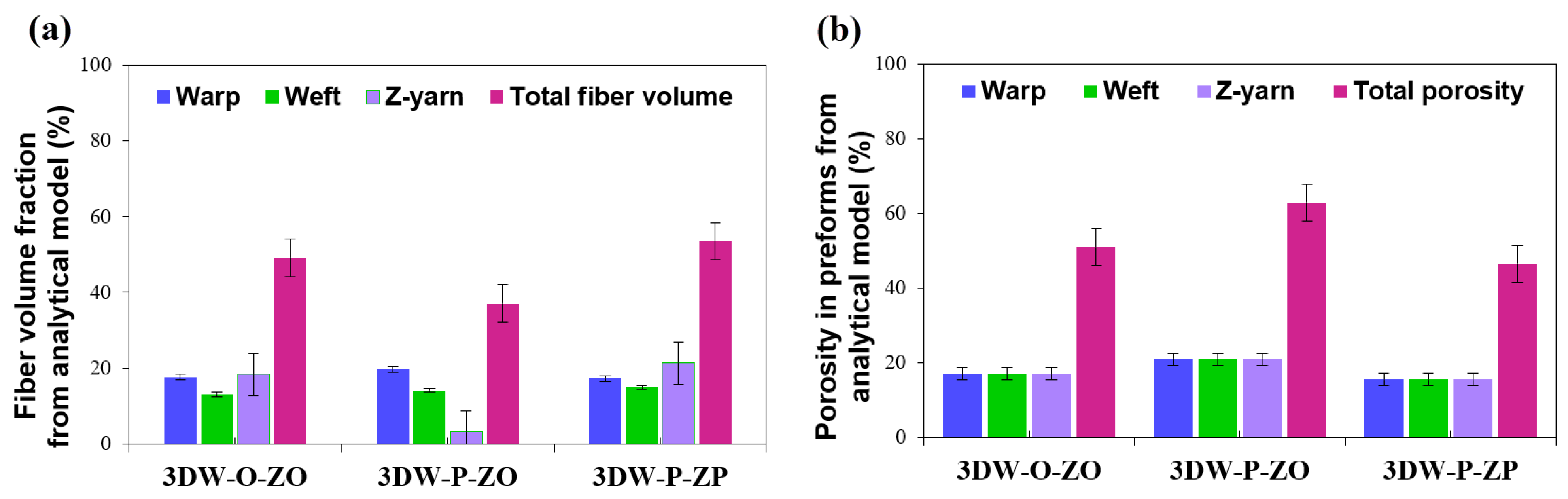
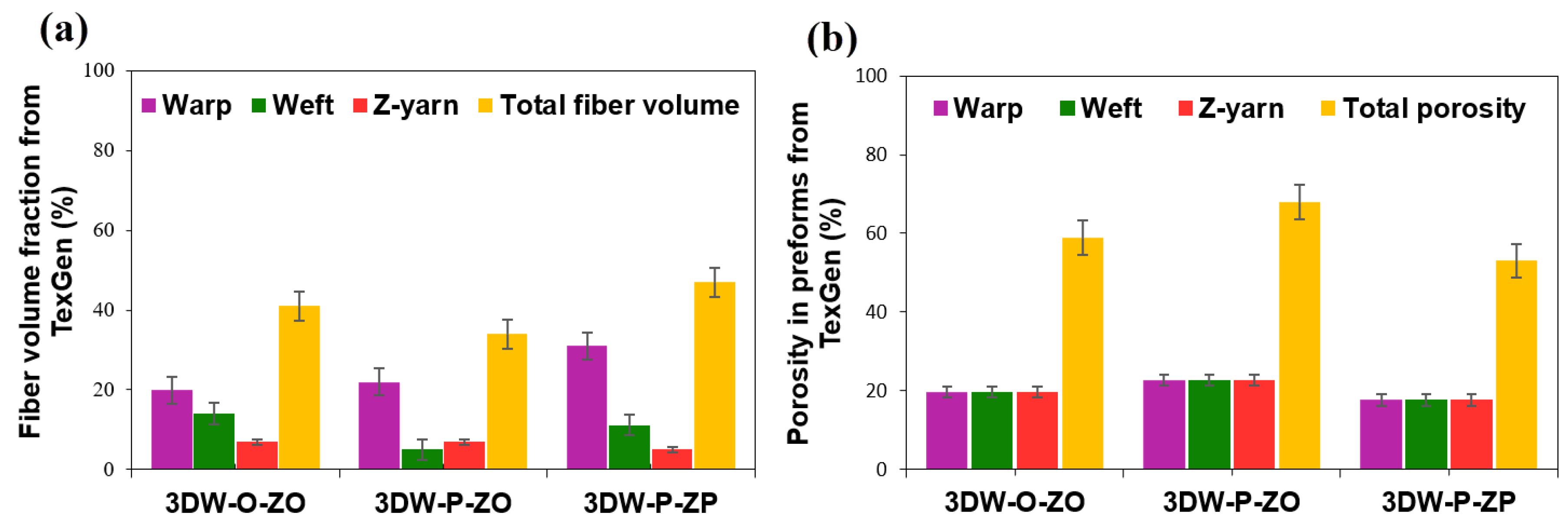
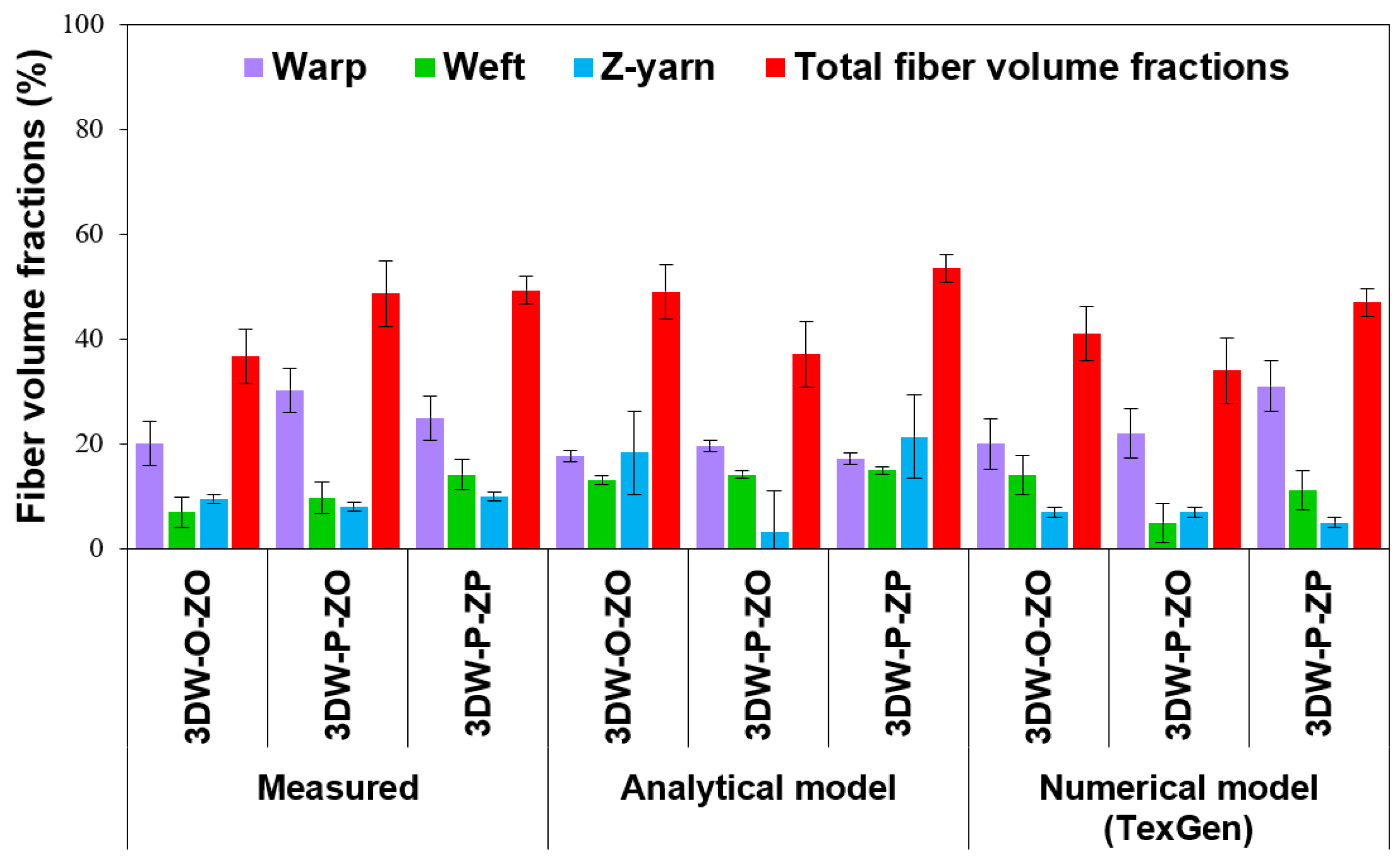
3.2. Volume Fraction and Porosity Results
Comparison of Measured Data, Analytic Model, and FE Model (TexGen)
3.3. Stiffness and Strength on 3D Preforms Results
3.4. Relevance of Possible Results of a Three-Dimensional Preform for the Scaffold of Tissue Engineering
3.5. Morphology of Preform Structure for Scaffold Results
3.5.1. 3D Plain Woven Preforms
3.5.2. 3D Semi-Interlaced Woven Preforms
3.5.3. 3D Orthogonal Woven Preforms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef]
- Bilisik, K.; Akter, M. Polymer nanocomposites based on graphite nanoplatelets (GNPs): A review on thermal-electrical conductivity, mechanical and barrier properties. J. Mater. Sci. 2022, 57, 7425–7480. [Google Scholar] [CrossRef]
- Bilisik, K.; Syduzzaman, M. Carbon nanotubes in carbon/epoxy multiscale textile preform composites: A review. Polym. Compos. 2021, 42, 1670–1697. [Google Scholar] [CrossRef]
- Azzazy, H.M.; Mansour, M.M.; Kazmierczak, S.C. From diagnostics to therapy: Prospects of quantum dots. Clin. Biochem. 2007, 40, 917–927. [Google Scholar] [CrossRef]
- Delehanty, J.B.; Mattoussi, H.; Medintz, I.L. Delivering quantum dots into cells: Strategies, progress and remaining issues. Anal. Bioanal. Chem. 2009, 393, 1091–1105. [Google Scholar] [CrossRef]
- Pelley, J.L.; Daar, A.S.; Saner, M.A. State of academic knowledge on toxicity and biological fate of quantum dots. Toxicol. Sci. 2009, 112, 276–296. [Google Scholar] [CrossRef]
- Williams, D. Benefit and risk in tissue engineering. Mater. Today 2004, 7, 24–29. [Google Scholar] [CrossRef]
- Meyer, U. The History of Tissue Engineering and Regenerative Medicine in Perspective. In Fundamentals of Tissue Engineering and Regenerative Medicine; Meyer, U., Handschel, J., Wiesmann, H., Meyer, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 5–12. [Google Scholar]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Williams, D.F. To engineer is to create: The link between engineering and regeneration. Trends Biotechnol. 2006, 24, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Griffith, L.G.; Naughton, G. Tissue engineering—Current challenges and expanding opportunities. Science 2002, 295, 1009–1014. [Google Scholar] [CrossRef]
- Rajendran, S.; Anand, S.C. Developments in medical textiles. Text. Prog. 2002, 32, 1–42. [Google Scholar] [CrossRef]
- Eriksson, S.; Sandsjö, L. Three-dimensional fabrics as medical textiles. In Advances in 3D Textiles; Xiaogang, C., Ed.; Woodhead Publishing: Soston, UK, 2015; pp. 305–340. [Google Scholar] [CrossRef]
- Khokar, N. 3D-weaving: Theory and practice. J. Text. Inst. 2001, 92, 193–207. [Google Scholar] [CrossRef]
- Bilisik, K. Three-dimensional axial braided preforms: Experimental determination of effects of structure-process parameters on unit cell. Text. Res. J. 2011, 81, 2095–2116. [Google Scholar] [CrossRef]
- Bilisik, K.; Sahbaz, N. Structure-unit cell-based approach on three-dimensional representative braided preforms from four-step braiding: Experimental determination of effects of structure-process parameters on predetermined yarn path. Text. Res. J. 2012, 82, 220–241. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, C.; Liu, L.; Wang, F.; Liu, X.; Mao, J.; Wang, L. Construction and application of textile-based tissue engineering scaffolds: A review. Biomater. Sci. 2020, 8, 3574–3600. [Google Scholar] [CrossRef]
- Bilisik, K.; Karaduman, N.S.; Bilisik, N.E.; Bilisik, H.E. Three-dimensional circular various weave patterns in woven preform structures. Text. Res. J. 2014, 84, 638–654. [Google Scholar] [CrossRef]
- Bilisik, K.; Karaduman, N.S.; Bilisik, N.E.; Bilisik, H.E. Three-dimensional fully interlaced woven preforms for composites. Text. Res. J. 2013, 83, 2060–2084. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, K.; Liu, Y.; Zhang, C.; Wang, B. Application of Textile Technology in Tissue Engineering: A review. Acta Biomater. 2021, 128, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Mahadik, Y.; Hallett, S.R. Finite element modelling of tow geometry in 3D woven fabrics. Compos. Part A Appl. Sci. 2010, 41, 1192–1200. [Google Scholar] [CrossRef]
- Zeng, X.; Brown, L.P.; Endruweit, A.; Matveev, M.; Long, A.C. Geometrical modelling of 3D woven reinforcements for polymer composites: Prediction of fabric permeability and composite mechanical properties. Compos. Part A Appl. Sci. 2014, 56, 150–160. [Google Scholar] [CrossRef]
- Lin, H.; Zeng, X.; Sherburn, M.; Long, A.C.; Clifford, M.J. Automated geometric modelling of textile structures. Text. Res. J. 2012, 82, 1689–1702. [Google Scholar] [CrossRef]
- Potter, E.; Pinho, S.T.; Robinson, P.; Iannucci, L.; McMillan, A.J. Mesh generation and geometrical modelling of 3D woven composites with variable tow cross-sections. Comput. Mater. Sci. 2012, 51, 103–111. [Google Scholar] [CrossRef]
- Durville, D. Numerical simulation of entangled materials mechanical properties. J. Mater. Sci. 2005, 40, 5941–5948. [Google Scholar] [CrossRef]
- Wu, S.; Duan, B.; Qin, X.; Butcher, J.T. Living nano-micro fibrous woven fabric/hydrogel composite scaffolds for heart valve engineering. Acta Biomater. 2017, 51, 89–100. [Google Scholar] [CrossRef]
- Cao, Y.; Vacanti, J.P.; Paige, K.T.; Upton, J.; Vacanti, C.A. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast. Reconstr. Surg. 1997, 100, 297–302. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L.; Guo, B.; Ma, P.X. Interwoven aligned conductive nanofiber yarn/hydrogel composite scaffolds for engi-neered 3D cardiac anisotropy. ACS Nano 2017, 11, 5646–5659. [Google Scholar] [CrossRef]
- Little, C.J.; Bawolin, N.K.; Chen, X. Mechanical properties of natural cartilage and tissue-engineered constructs. Tissue Eng. Part B Rev. 2011, 17, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Maher, S.A.; Lowman, A.M. Hydrogels for the repair of articular cartilage defects. Tissue Eng. Part B Rev. 2011, 17, 281–299. [Google Scholar] [CrossRef]
- Gulrez, S.K.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of preparation, characterisation and applications, In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; Carpi, A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 117–150. [Google Scholar]
- Munirah, S.; Kim, S.H.; Ruszymah, B.H.; Khang, G. The use of fibrin and poly (lactic-co-glycolic acid) hybrid scaffold for articular cartilage tissue engineering: An in vivo analysis. Eur. Cell Mater. 2008, 15, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Sato, T.; Ushida, T.; Hirochika, R.; Shirasaki, Y.; Ochiai, N.; Tateishi, T. The use of a novel PLGA fiber/collagen composite web as a scaffold for engineering of articular cartilage tissue with adjustable thickness. J. Biomed. Mater. Res. Part A 2003, 67, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly (lactide-co-glycolide)(PLGA) devic-es. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Alizadeh, E.; Salehi, R.; Khalandi, B.; Davaran, S.; Akbarzadeh, A. Nanocomposite hydrogels for cartilage tissue engineering: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Poly (lactid -co-glycolide) fiber: An overview. J. Eng. Fibers Fabr. 2014, 9, 47–66. [Google Scholar]
- Moran, J.M.; Pazzano, D.; Bonassar, L.J. Characterization of polylactic acid–polyglycolic acid composites for cartilage tissue engineering. Tissue Eng. 2003, 9, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.R.; Coutts, R.D.; Yoshioka, M.; Harwood, F.L.; Monosov, A.Z.; Amiel, D. Articular cartilage repair using allogeneic perichondrocyteseeded biodegradable porous polylactic acid (PLA): A tissue-engineering study. J. Biomed. Mater. Res. 1995, 29, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, K.A.; Agrawal, C.M.; Barber, F.A.; Burkhart, S.S. Orthopaedic applications for PLA-PGA biodegradable polymers. Arthrosc.-J. Arthrosc. Relat. Surg. 1998, 14, 726–737. [Google Scholar] [CrossRef]
- Lee, J.W.; Gardella, J.A. In vitro hydrolytic surface degradation of poly (glycolic acid): Role of the surface segregated amorphous region in the induction period of bulk erosion. Macromolecules 2001, 34, 3928–3937. [Google Scholar] [CrossRef]
- Ginde, R.M.; Gupta, R.K. In vitro chemical degradation of poly (glycolic acid) pellets and fibers. J. Appl. Polym. Sci. 1987, 33, 2411–2429. [Google Scholar] [CrossRef]
- Freed, L.E.; Vunjak-Novakovic, G.; Biron, R.J.; Eagles, D.B.; Lesnoy, D.C.; Barlow, S.K.; Langer, R. Biodegradable polymer scaffolds for tissue engineering. Biotechnology 1994, 12, 689–693. [Google Scholar] [CrossRef]
- Mooney, D.J.; Mazzoni, C.L.; Breuer, C.; McNamara, K.; Hern, D.; Vacanti, J.P.; Langer, R. Stabilized polyglycolic acid fibre-based tubes for tissue engineering. Biomaterials 1996, 17, 115–124. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, B.; Yang, G.; Gauthier, M. Poly(Lactic Acid)-Based Biomaterials: Synthesis, Modification and Applications. In Biomedical Science, Engineering and Technology; InTech: London, UK, 2012. [Google Scholar]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Xia, W.; Liu, W.; Cui, L.; Liu, Y.; Zhong, W.; Liu, D.; Cao, Y. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J. Biomed. Mater. Res.-B Appl. Biomater. 2004, 71, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Liao, S.; Chan, C.K.; Ramakrishna, S. Stem cells and biomimetic materials strategies for tissue engineering. Mater. Sci. Eng. C 2008, 28, 1189–1202. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, J.; Guo, Q.; Huang, J.; Zhang, L.; Yao, J.; Yang, F.; Wang, S.; Xu, W.; Wang, A.; et al. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials 2008, 29, 2378–2387. [Google Scholar] [CrossRef]
- Vacanti, J.P.; Vacanti, C.A. The history and scope of tissue engineering. In Principles of Tissue Engineering, 4th ed.; Elsevier Inc.: Amsterdam, The Netherlands; Imprint in Academic Press: Cambridge, MA, USA, 2014; pp. 3–8. [Google Scholar] [CrossRef]
- West, J.L.; Hubbell, J.A. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules 1999, 32, 241–244. [Google Scholar] [CrossRef]
- Stachowiak, A. Principles and Practice of Tissue Engineering; MIT OpenCourseWare, 20.109 Spring 2010; Module3, Lecture 1; Massachusetts Institute of Technology: Cambridge, MA, USA, 2010. [Google Scholar]
- Stachowiak, A.N.; Wang, Y.; Huang, Y.C.; Irvine, D.J. Homeostatic lymphoid chemokines synergize with adhesion ligands to trigger T and B lymphocyte chemokinesis. J. Immunol. 2006, 177, 2340–2348. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Yang, J.C.; Restifo, N.P. Cancer immunotherapy: Moving beyond current vaccines. Nat. Med. 2004, 10, 909–915. [Google Scholar] [CrossRef]
- Şeker, Ş.; Elçin, A.E.; Elçin, Y.M. Advances in Regenerative Medicine and Biomaterials. In Gene, Drug, and Tissue Engineering; Pereira, G.C., Ed.; Methods in Molecular Biology; Humana: New York, NY, USA, 2013; Volume 2575. [Google Scholar] [CrossRef]
- Yang, W.; Yang, F.; Wang, Y.; Both, S.K.; Jansen, J.A. In vivo bone generation via the endochondral pathway on three-dimensional electrospun fibers. Acta Biomater. 2013, 9, 4505–4512. [Google Scholar] [CrossRef]
- Li, W.-J.; Shanti, R.M.; Tuan, R.S. Electrospinning Technology for Nanofibrous Scaffolds in Tissue Engineering. In Nanotechnologies for the Life Sciences; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; p. 9. [Google Scholar]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Bagher, Z.; Sene, A.A.; Aflatoonian, R.; Mehdizadeh, M.; Milan, P.B.; Ghazizadeh, L.; Ashrafi, M.; Amjadi, F. Evaluation of polyglycolic acid as an animal-free biomaterial for three-dimensional culture of human endometrial cells. Clin. Exp. Reprod. Med. 2022, 49, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Pandita, V.; Kumar, A.; Parihar, D.S.; Puranik, N.; Bajpai, T.; Khan, R. 3D printing: Advancement in biogenerative engineering to combat shortage of organs and bioapplicable materials. Regen. Eng. Transl. Med. 2021, 8, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J.K.; Riley, S.L.; Palazzolo, R.; Brown, S.C.; Monkhouse, D.C.; Coates, M.; Griffith, L.G.; Landeen, L.K.; Ratcliffe, A. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials 2002, 23, 4739–4751. [Google Scholar] [CrossRef]
- Vellayappan, M.V.; Jaganathan, S.K.; Supriyanto, E. Review: Unraveling the less explored flocking technology for tissue engineering scaffolds. RSC Adv. 2015, 5, 73225–73240. [Google Scholar] [CrossRef]
- Nuernberger, S.; Cyran, N.; Albrecht, C.; Redl, H.; Vécsei, V.; Marlovits, S. The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials 2011, 32, 1032–1040. [Google Scholar] [CrossRef]
- Simon, J.L.; Roy, T.D.; Parsons, J.R.; Rekow, E.D.; Thompson, V.P.; Kemnitzer, J.; Ricci, J.L. Engineered cellular response to scaffold architecture in a rabbit trephine defect. J. Biomed. Mater. Res. Part A 2003, 66, 275–282. [Google Scholar] [CrossRef]
- Woodfield, T.B.F.; Blitterswijk, C.V.; Wijn, J.D.; Sims, T.J.; Hollander, A.P.; Riesle, J. Polymer scaffolds fabricated with pore-size gradients as a model for studying the zonal organization within tissue-engineered cartilage constructs. Tissue Eng. 2005, 11, 1297–1311. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials 2009, 30, 2956–2965. [Google Scholar] [CrossRef]
- Kim, U.J.; Park, J.; Kim, H.J.; Wada, M.; Kaplan, D.L. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials 2005, 26, 2775–2785. [Google Scholar] [CrossRef]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused deposition modeling of novel scaffold architectures for tissue engi-neering applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Kock, L.; van Donkelaar, C.C.; Ito, K. Tissue engineering of functional articular cartilage: The current status. Cell Tissue Res. 2012, 347, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef]
- Kim, B.S.; Mooney, D.J. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998, 16, 224–230. [Google Scholar] [CrossRef]
- Hannes Welsch, G.; Mamisch, T.C.; Zak, L.; Blanke, M.; Olk, A.; Marlovits, S.; Trattnig, S. Evaluation of cartilage repair tissue after matrix-associated autologous chondrocyte transplantation using a hyaluronic-based or a collagen-based scaffold with morphological mocart scoring and biochemical T2 mapping: Preliminary results. Am. J. Sports Med. 2010, 38, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Stenhamre, H.; Nannmark, U.; Lindahl, A.; Gatenholm, P.; Brittberg, M. Influence of pore size on the redifferentiation potential of human articular chondrocytes in poly (urethane urea) scaffolds. J. Tissue Eng. Regen. Med. 2011, 5, 578–588. [Google Scholar] [CrossRef]
- Mygind, T.; Stiehler, M.; Baatrup, A.; Li, H.; Zou, X.; Flyvbjerg, A.; Kassem, M.; Bünger, C. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials 2007, 28, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.M.; Ko, L.Y.; Huang, T.J. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009, 5, 670–679. [Google Scholar] [CrossRef]
- Kelly, D.J.; Prendergast, P.J. Prediction of the optimal mechanical properties for a scaffold used in osteochondral defect repair. Tissue Eng. 2006, 12, 2509–2519. [Google Scholar] [CrossRef]
- Genes, N.G.; Rowley, J.A.; Mooney, D.J.; Bonassar, L.J. Effect of substrate mechanics on chondrocyte adhesion to modified alginate surfaces. Arch. Biochem. Biophys. 2004, 422, 161–167. [Google Scholar] [CrossRef]
- Whang, K.; Healy, K.E.; Elenz, D.R.; Nam, E.K.; Tsai, D.C.; Thomas, C.H.; Nuber, G.W.; Glorieux, F.H.; Travers, R.; Sprague, S.M. Engineering bone regeneration with bioabsorbable scaffolds with novel microarchitecture. Tissue Eng. 1999, 5, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.F.; Chua, S.C.; Sudarmadji, N.; Yeong, W.Y. Engineering functionally graded tissue engineering scaffolds. J. Mech. Behav. Biomed. Mater. 2008, 1, 140–152. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Muschler, G.F.; Nakamoto, C.; Griffith, L.G. Engineering principles of clinical cell-based tissue engineering. J. Bone Jt. Surg. 2004, 86, 1541–1558. [Google Scholar] [CrossRef] [PubMed]
- Tsuruga, E.; Takita, H.; Itoh, H.; Wakisaka, Y.; Kuboki, Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J. Biochem. 1997, 121, 317–324. [Google Scholar] [CrossRef]
- Singh, H.; Teoh, S.H.; Low, H.T.; Hutmacher, D.W. Flow modelling within a scaffold under the influence of uni-axial and bi-axial bioreactor rotation. J. Biotechnol. 2005, 119, 181–196. [Google Scholar] [CrossRef]
- Zeltinger, J.; Sherwood, J.K.; Graham, D.A.; Müeller, R.; Griffith, L.G. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001, 7, 557–572. [Google Scholar] [CrossRef]
- Olivares, A.L.; Marsal, È.; Planell, J.A.; Lacroix, D. Finite element study of scaffold architecture design and culture conditions for tissue engineering. Biomaterials 2009, 30, 6142–6149. [Google Scholar] [CrossRef]
- Jeong, C.G.; Hollister, S.J. Mechanical and biochemical assessments of three-dimensional poly (1, 8-octanediol-co-citrate) scaffold pore shape and permeability effects on in vitro chondrogenesis using primary chondrocytes. Tissue Eng. Part A 2010, 16, 3759–3768. [Google Scholar] [CrossRef]
- Moutos, F.T.; Freed, L.E.; Guilak, F. A biomimetic three-dimensional woven composite scaffold for functional tissue engi-neering of cartilage. Nat. Mater. 2007, 6, 162–167. [Google Scholar] [CrossRef]
- Bilisik, K. Multiaxis 3D woven preform and properties of multiaxis 3D woven and 3D orthogonal woven carbon/epoxy composites. J. Reinf. Plast. Compos. 2010, 29, 1173–1186. [Google Scholar] [CrossRef]
- Ansar, M.; Wang, X.; Zhou, C. Modeling strategies of 3D woven composites: A review. Compos. Struct. 2011, 93, 1947–1963. [Google Scholar] [CrossRef]
- Labanieh, A.R.; Legrand, X.; Koncar, V.; Soulat, D. Novel optimization method to estimate the geometrical properties of a multiaxial 3D woven preform. J. Reinf. Plast. Compos. 2013, 32, 700–712. [Google Scholar] [CrossRef]
- Midani, M.; Seyam, A.F.; Saleh, M.N.; Pankow, M. The effect of the through-thickness yarn component on the in-and out-of-plane properties of composites from 3D orthogonal woven preforms. J. Text. Inst. 2019, 110, 317–327. [Google Scholar] [CrossRef]
- Green, S.D.; Matveev, M.Y.; Long, A.C.; Ivanov, D.; Hallett, S.R. Mechanical modelling of 3D woven composites considering realistic unit cell geometry. Compos. Struct. 2014, 118, 284–293. [Google Scholar] [CrossRef]
- Lu, L.; Mikos, A.G. The importance of new processing techniques in tissue engineering. MRS Bull. 1996, 21, 28–32. [Google Scholar] [CrossRef]
- Suture Material & Biomaterial. Available online: https://www.meta-biomed.com/product/suture/overview.php (accessed on 17 December 2022).
- Van de Velde, K.; Kiekens, P. Biopolymers: Overview of several properties and consequences on their applica-tions. Polym. Test. 2002, 21, 433–442. [Google Scholar] [CrossRef]
- Yildirim, H.K. Development of Biodegradable Three Dimensional (3D) Woven Preform Structures for Artificial Organs. Ph.D. Thesis, Graduate School of Natural and Applied Sciences, Erciyes University, Talas-Kayseri, Turkey, 2022. (In Turkish, Abstract: English). [Google Scholar]
- ASTM Standards. D3171-99; Standard Test Methods for Constituent Content of Composite Materials. ASTM International: West Conshohocken, PA, USA, 1991.
- ASTM Standards. D2734-91; Standard Test Methods for Void Content of Reinforced Plastics. ASTM International: West Conshohocken, PA, USA, 1991.
- ISO Standarts. ISO 139; Textiles-Standard Atmospheres for Conditioning and Testing. International Organization for Standardization: Geneva, Switzerland, 2005.
- Grosberg, P. The Tensile Properties of Woven Fabric. In Structural Mechanism of Fiber, Yarns and Fabrics; Hearl, J.W.S., Grosberg, P., Backer, S., Eds.; Wiley-Interscience: Hoboken, NJ, USA, 1969. [Google Scholar]
- Sherburn, M. Geometric and Mechanical Modelling of Textiles. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2007. [Google Scholar]
- Lin, H.; Brown, L.P.; Long, A.C. Modelling and Simulating Textile Structures Using TexGen. Adv. Mater. Res. 2011, 331, 44–47. [Google Scholar] [CrossRef]
- Long, A.; Brown, L. Modelling the geometry of textile reinforcements for composites: Texgen. In Composite Reinforcements for Optimum Performance; Woodhead Publishing Ltd.: Cambridge, UK, 2011; pp. 239–264. [Google Scholar]
- Brown, L.P.; Endruweit, A.; Long, A.; Jones, I.A. Characterisation and modelling of complex textile geometries using TexGen. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Milan, Italy, 17–19 September 2018; Volume 406, p. 012024. [Google Scholar]
- Brown, L.; Matveev, M.; Spackman, G. Louisepb/TexGen: TexGen v3.12.1 (Version v3.12.1). Zenodo. Available online: https://zenodo.org/record/4537855 (accessed on 12 February 2021).
- Jones, R.M. (Ed.) Micromechanical behavior of a lamina. In Mechanics of Composite Materials, 2nd ed.; Taylor & Francis: Boca Raton, FL, USA, 1999; pp. 121–135. [Google Scholar]
- Tavafoghi, M.; Sheikhi, A.; Tutar, R.; Jahangiry, J.; Baidya, A.; Haghniaz, R.; Khademhosseini, A. Engineering tough, injectable, naturally derived, bioadhesive composite hydrogels. Adv. Health Mater. 2020, 9, 1901722. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.M.; Guilak, F.; Vail, T.P.; Wang, J.Y.; Setton, L.A. Tensile properties of articular cartilage are altered by meniscectomy in a canine model of osteoarthritis. J. Orthop. Res. 1999, 17, 503–508. [Google Scholar] [CrossRef]
- Takigawa, T.; Morino, Y.; Urayama, K.; Masuda, T. Poisson’s ratio of polyacrylamide (PAAm) gels. Polym. Gels Netw. 1996, 4, 1–5. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Zhu, W.; Mow, V.C.; Koob, T.J.; Eyre, D.R. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. J. Orthop. Res. 1993, 11, 771–781. [Google Scholar] [CrossRef] [PubMed]







| Fiber Type | Fiber Diameter (μm) | Fiber Density (g/cm3) | Tensile Strength (MPa) | Tensile Modulus (GPa) | Elongation at Break (%) | Yarn Linear Density (Tex, Denier) | Yarn Dimension (Width × Thickness) (50 Ends, mm) | Degradation Time (Month) |
|---|---|---|---|---|---|---|---|---|
| Polyglycolic acid (PGA, Meta Biomed Inc., Cheongju, Ch‘ungch‘ong-bukto, Republic of Korea) | 13.70 [97] | 1.50 [97] | 60–99.70 [98] | 6–7 [98] | 1.50–20 [98] | 6.22 (56) [97] | 1.50 × 0.60 | 6–12 [97] |
| Volume Fraction Formulae for 3D Plain Preform | Equation Number | Explanation | |
|---|---|---|---|
| (8) | Warp volume percent in preform | ||
| (9) | Filling volume percent in preform | ||
| (10) | Z-fiber volume percent in preform | ||
| (11) | Preform volume percent | ||
| (12) | Porosity percent in preform | ||
| Volume Fraction Formulae for 3D Semi-Interlaced Preform | Equation Number | Explanation | |
|---|---|---|---|
| (13) | Warp volume percent in preform | ||
| (14) | Filling volume percent in preform | ||
| (15) | Z-fiber volume percent in preform | ||
| Volume Fraction Formulae for 3D Orthogonal Preform | Equation Number | Explanation | |
|---|---|---|---|
| (16) | Warp volume percent in preform | ||
| (17) | Filling volume percent in preform | ||
| (18) | Z-fiber volume percent in preform | ||
| Yarn-to-Yarn Space | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Preform Structure | Layer Number | Total Yarn Number | Structure | Yarn–Yarn Distance (Top × Side × Cross-Section) | |||||||
| Warp (N × M) | Filling (N + 1, N) | Z-Yarn (M + 1) | Width × Thickness (Sw × St, mm) | Warp-Warp (mm) | Filling-Filling (mm) | Z-Yarn-Z-Yarn (mm) | |||||
| 3DW-O-ZO | 6 | 6 × 20 | 7 × 1 | 1 × 21 | 28.00 × 5.66 | 1 × 0.5 × 1 | 3 × 7 × 0.3 | 1 × 6 × 1 | |||
| 3DW-P-ZO | 6 × 20 | 6 × 1 | 1 × 21 | 20.30 × 3.99 | 1 × 1 × 1 | 8 × 8 × 1 | 1 × 10 × 1 | ||||
| 3DW-P-ZP | 6 × 20 | 6 × 1 | 1 × 20 | 27.66 × 4.00 | 1 × 0.8 × 1 | 5 × 5 × 0.3 | 1 × 11 × 1 | ||||
| Density | |||||||||||
| Preform Structure | Layer Number | Total Yarn Number | Structure | Preform Density (Top × Side × Cross-Section) | Preform Areal Density (g/cm3) | ||||||
| Warp (N × M) | Filling (N + 1, N) | z-yarn (M + 1) | Length × Width × Thickness (Sl × Sw × St, mm) | Warp (ends/10 mm) | Filling (ends/10 mm) | Z-yarn (ends/10 mm) | |||||
| 3DW-O-ZO | 6 | 6 × 20 | 7 × 1 | 1 × 21 | 100 × 28.00 × 5.66 | 8 × – × 8 | 4 × 4 × – | 6 × 2 × 6 | 0.55 ± 0.10 | ||
| 3DW-P-ZO | 6 × 20 | 6 × 1 | 1 × 21 | 125 × 20.30 × 3.99 | 9 × – × 9 | 2 × 2 × – | 9 × 1 × 9 | 0.73 ± 0.10 | |||
| 3DW-P-ZP | 6 × 20 | 6 × 1 | 1 × 20 | 100 × 27.66 × 4.00 | 9 × – × – | 2 × 2 × – | 8 × 1 × 8 | 0.74 ± 0.10 | |||
| Angles | |||||||||||
| Preform Structure | Layer Number | Total Yarn Number | Warp Angle | Filling Angle | Z-Yarn Angle | ||||||
| Warp | Filling | z-yarn | (θwc°)–(θwm°) | (θws°) | (θfc°) | (θfm°)–(θfs°) | (θzc°) | (θzm°) | (θzs°) | ||
| 3DW-O-ZO | 6 | 6 × 20 | 7 × 1 | 1 × 21 | 0–0 | 29 ± 14.50 | 90 | 0–90 | 70 | 90 ± 5.77 | 0 |
| 3DW-P-ZO | 6 × 20 | 6 × 1 | 1 × 21 | 0–0 | 0 | 90 | 0–90 | 40 | 90 ± 5.77 | 0 | |
| 3DW-P-ZP | 6 × 20 | 6 × 1 | 1 × 20 | * −75 | 15 ± 14.50 | 13 | 0–90 | 40 | 80 ± 5.77 | 10 ± 5.77 | |
| Crimp | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preform Structure | Layer Number | Uncrimp Yarn Length | Structure | Crimp | ||||||
| Warp (lw, mm) | Filling (lf, mm) | Z-Yarn (lzt, mm) | Length (Sl, mm) | Width (Sw, mm) | Thickness (St, mm) | Warp (Cw, %) | Filling (Cf, %) | Z-Yarn (Cz, %) | ||
| 3DW-O-ZO | 6 | 51 | 30 | 7 | 50 | 28 | 6.36 | 2.00 ± 1.75 | 7.14 ± 3.57 | 10.06 ± 20.06 |
| 3DW-P-ZO | 30 | 20 | 8 | 29 | 18 | 6.00 | 3.44 ± 1.75 | 11.11 ± 3.57 | 33.33 ± 20.06 | |
| 3DW-P-ZP | 38 | 24 | 15 | 36 | 23 | 10.00 | 5.55 ± 1.75 | 14.28 ± 3.57 | 50.00 ± 20.06 | |
| Yarn Length | ||||||||||
| Preform Structure | Layer Number | Total Yarn Number | Uncrimp Warp Length (lw,mm) | Uncrimp Filling Length (lf, mm) | Uncrimp Z-Yarn Length | |||||
| Warp | Filling | Z-Yarn | Z-Yarn Arc Length (lza, mm) | Z-Yarn Length (lz, mm) | Total Z-Yarn Length (lzt, mm) | |||||
| 3DW-O-ZO | 6 | 6 × 20 | 7 × 1 | 1 × 21 | 151 | 46.34 | 6 | 6.36 | 12.36 | |
| 3DW-P-ZO | 6 × 20 | 6 × 1 | 1 × 21 | 140 | 40.30 | 10 | 3.99 | 13.99 | ||
| 3DW-P-ZP | 6 × 20 | 6 × 1 | 1 × 20 | 114 | 47.66 | 5 | 12.50 | 17.50 | ||
| Fiber Volume Fraction (%) | 3D PGA Fiber Woven Preform Structures | ||
|---|---|---|---|
| 3DW-O-ZO | 3DW-P-ZO | 3DW-P-ZP | |
| Measured values | |||
| Warp directional fiber volume fraction (Vfw) | 20.06 ± 4.15 | 30.24 ± 4.15 | 24.87 ± 4.15 |
| Weft directional fiber volume fraction (Vff) | 7.03 ± 2.93 | 9.71 ± 2.93 | 14.15 ± 2.93 |
| Z-directional fiber volume fraction (Vfz) | 9.55 ± 0.83 | 8.03 ± 0.83 | 9.96 ± 0.83 |
| Warp directional porosity (Vwpr) | 21.11 ± 2.37 | 17.11 ± 2.37 | 16.89 ± 2.37 |
| Weft directional porosity (Vfpr) | 21.11 ± 2.37 | 17.11 ± 2.37 | 16.89 ± 2.37 |
| Z-directional porosity (Vzpr) | 21.11 ± 2.37 | 17.11 ± 2.37 | 16.89 ± 2.37 |
| Total preform scaffold fiber volume fraction (Vfp) | 36.66 ± 7.12 | 48.66 ± 7.12 | 49.33 ± 7.12 |
| Total preform scaffold porosity fraction (Vtpr) | 63.34 ± 7.12 | 51.34 ± 7.12 | 50.67 ± 7.12 |
| Analytical model | |||
| Warp directional fiber volume fraction (Vfw) | 17.67 ± 1.32 | 19.72 ± 1.32 | 17.23 ± 1.32 |
| Weft directional fiber volume fraction (Vff) | 13.09 ± 0.93 | 14.17 ± 0.93 | 14.96 ± 0.93 |
| Z-directional fiber volume fraction (Vfz) | 18.32 ± 9.72 | 3.19 ± 9.72 | 21.33 ± 9.72 |
| Warp directional porosity (Vwpr) | 16.97 ± 2.83 | 20.97 ± 2.83 | 15.49 ± 2.83 |
| Weft directional porosity (Vfpr) | 16.97 ± 2.83 | 20.97 ± 2.83 | 15.49 ± 2.83 |
| Z-directional porosity (Vzpr) | 16.97 ± 2.83 | 20.97 ± 2.83 | 15.49 ± 2.83 |
| Total preform scaffold fiber volume fraction (Vfp) | 49.08 ± 8.50 | 37.08 ± 8.50 | 53.52 ± 8.50 |
| Total preform scaffold porosity (Vtpr) | 50.92 ± 8.50 | 62.92 ± 8.50 | 46.48 ± 8.50 |
| TexGen model | |||
| Warp directional fiber volume fraction (Vfw) | 20.00 ± 5.85 | 22.00 ± 5.85 | 31.00 ± 5.85 |
| Weft directional fiber volume fraction (Vff) | 14.00 ± 4.60 | 5.00 ± 4.60 | 11.20 ± 4.60 |
| Z-directional fiber volume fraction (Vfz) | 7.00 ± 1.15 | 7.00 ± 1.15 | 5.00 ± 1.15 |
| Warp directional porosity (Vwpr) | 19.66 ± 2.51 | 22.66 ± 2.51 | 17.66 ± 2.51 |
| Weft directional porosity (Vfpr) | 19.66 ± 2.51 | 22.66 ± 2.51 | 17.66 ± 2.51 |
| Z-directional porosity (Vzpr) | 19.66 ± 2.51 | 22.66 ± 2.51 | 17.66 ± 2.51 |
| Total preform scaffold fiber volume fraction (Vfp) | 41.00 ± 6.50 | 34.00 ± 6.50 | 47.00 ± 6.50 |
| Total preform scaffold porosity (Vtpr) | 59.00 ± 6.50 | 68.00 ± 6.50 | 53.00 ± 6.50 |
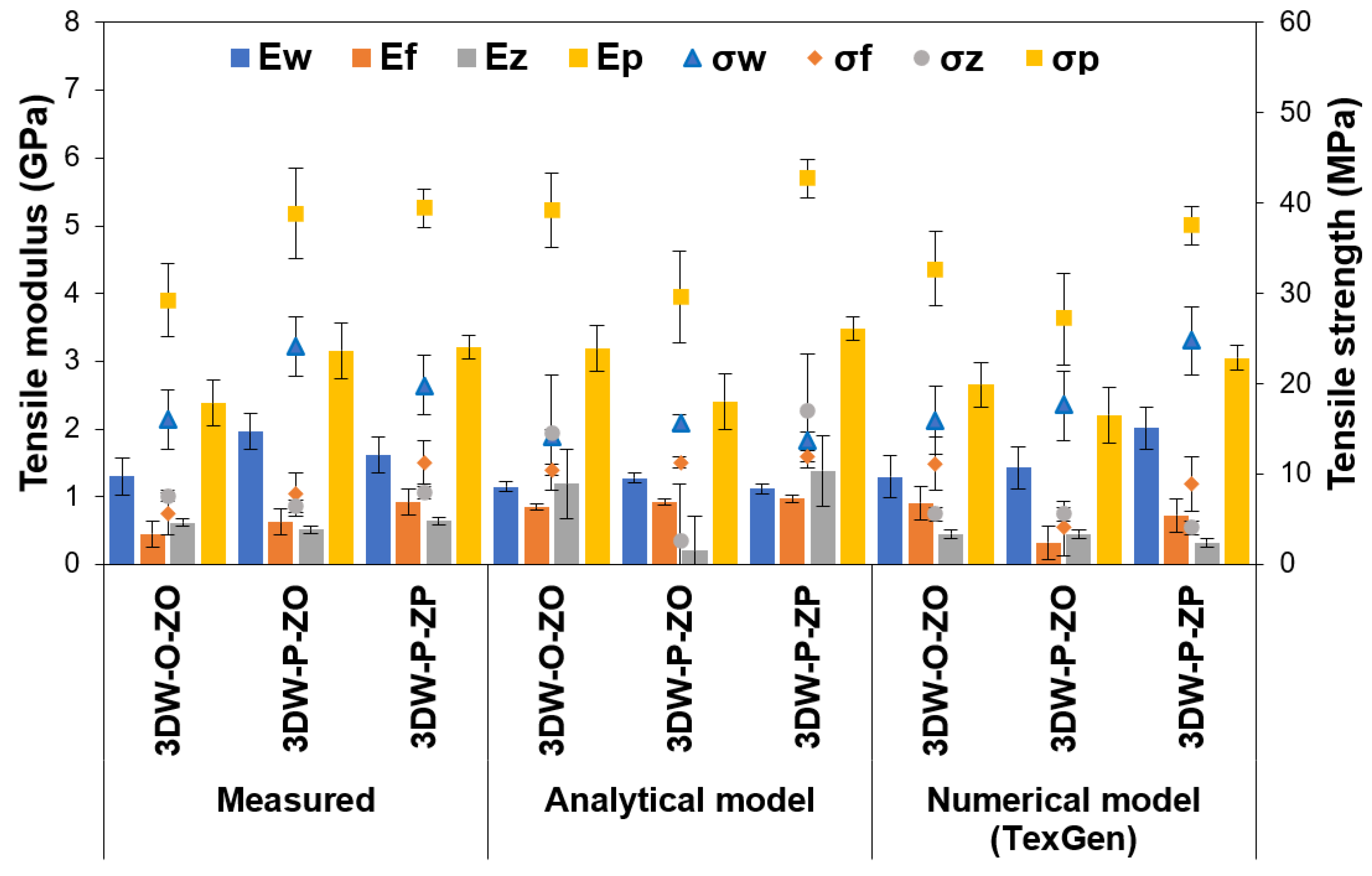
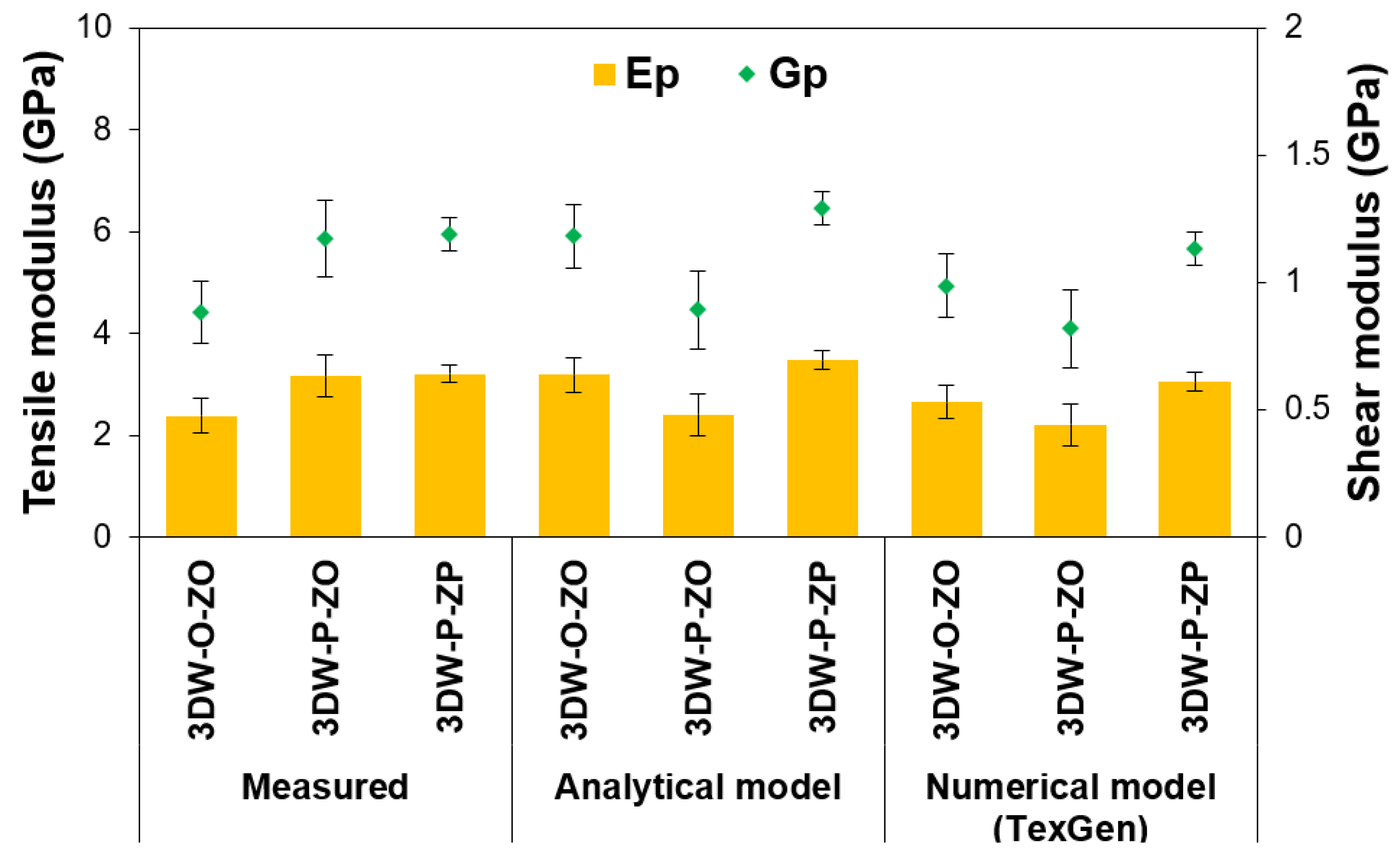
| Fiber Volume Fractions | Preform Structure | Tensile Modulus (GPa) | Shear Modulus (GPa) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ewarp (E11) | Efilling (E22) | Ez-yarn (E33) | Ep | Gwarp-filling (G12) | Gfilling-z-yarn (G23) | Gz-yarn-warp (G13) | Gp | ||
| Measured | 3DW-O-ZO | 1.30 ± 0.27 | 0.46 ± 0.19 | 0.62 ± 0.05 | 2.38 ± 0.34 | 0.48 | 0.17 | 0.23 | 0.88 ± 0.12 |
| 3DW-P-ZO | 1.97 ± 0.27 | 0.63 ± 0.19 | 0.52 ± 0.05 | 3.16 ± 0.41 | 0.73 | 0.23 | 0.19 | 1.17 ± 0.15 | |
| 3DW-P-ZP | 1.62 ± 0.27 | 0.92 ± 0.19 | 0.65 ± 0.05 | 3.21 ± 0.18 | 0.60 | 0.34 | 0.24 | 1.19 ± 0.07 | |
| Analytical model | 3DW-O-ZO | 1.15 ± 0.07 | 0.85 ± 0.05 | 1.19 ± 0.52 | 3.19 ± 0.34 | 0.43 | 0.32 | 0.44 | 1.18 ± 0.12 |
| 3DW-P-ZO | 1.28 ± 0.07 | 0.92 ± 0.05 | 0.21 ± 0.52 | 2.41 ± 0.41 | 0.48 | 0.34 | 0.10 | 0.89 ± 0.15 | |
| 3DW-P-ZP | 1.12 ± 0.07 | 0.97 ± 0.05 | 1.39 ± 0.52 | 3.48 ± 0.18 | 0.42 | 0.36 | 0.51 | 1.29 ± 0.07 | |
| FE model (TexGen) | 3DW-O-ZO | 1.30 ± 0.31 | 0.91 ± 0.24 | 0.46 ± 0.06 | 2.66 ± 0.34 | 0.48 | 0.34 | 0.17 | 0.99 ± 0.12 |
| 3DW-P-ZO | 1.43 ± 0.31 | 0.33 ± 0.24 | 0.46 ± 0.06 | 2.21 ± 0.41 | 0.53 | 0.12 | 0.17 | 0.82 ± 0.15 | |
| 3DW-P-ZP | 2.02 ± 0.31 | 0.73 ± 0.24 | 0.33 ± 0.06 | 3.06 ± 0.18 | 0.75 | 0.27 | 0.12 | 1.13 ± 0.07 | |
| Fiber Volume Fractions | Preform Structure | Poisson Ratios | Tensile Strengths (MPa) | ||||||
| νwarp-filling ν12 | νfilling-z-yarn ν23 | νz-yarn-warp ν13 | νp | σwarp σ11 | σfilling σ22 | σz-yarn σ33 | σps | ||
| Measured | 3DW-O-ZO | 0.44 | 0.45 | 0.45 | 0.42 | 16.04 ± 3.32 | 5.64 ± 2.34 | 7.65 ± 0.66 | 29.29 ± 4.11 |
| 3DW-P-ZO | 0.43 | 0.45 | 0.45 | 0.41 | 24.16 ± 3.32 | 7.78 ± 2.34 | 6.44 ± 0.66 | 38.87 ± 5.04 | |
| 3DW-P-ZP | 0.43 | 0.44 | 0.45 | 0.40 | 19.88 ± 3.32 | 11.32 ± 2.34 | 7.98 ± 0.66 | 39.40 ± 2.15 | |
| Analytical model | 3DW-O-ZO | 0.44 | 0.44 | 0.44 | 0.40 | 14.13 ± 0.87 | 10.47 ± 0.61 | 14.65 ± 6.34 | 39.20 ± 4.11 |
| 3DW-P-ZO | 0.44 | 0.44 | 0.45 | 0.42 | 15.77 ± 0.87 | 11.34 ± 0.61 | 2.57 ± 6.34 | 29.62 ± 5.04 | |
| 3DW-P-ZP | 0.44 | 0.44 | 0.43 | 0.40 | 13.78 ± 0.87 | 11.97 ± 0.61 | 17.05 ± 6.34 | 42.75 ± 2.15 | |
| FE model (TexGen) | 3DW-O-ZO | 0.44 | 0.44 | 0.45 | 0.41 | 15.99 ± 3.82 | 11.20 ± 3.00 | 5.61 ± 0.75 | 32.75 ± 4.11 |
| 3DW-P-ZO | 0.43 | 0.45 | 0.45 | 0.42 | 17.59 ± 3.82 | 4.02 ± 3.00 | 5.61 ± 0.75 | 27.17 ± 5.04 | |
| 3DW-P-ZP | 0.42 | 0.45 | 0.45 | 0.41 | 24.77 ± 3.82 | 8.97 ± 3.00 | 4.02 ± 0.75 | 37.54 ± 2.15 | |
| Articular cartilage | |||||||||
| Tensile modulus (MPa, 10% ε) | Complex shear modulus (MPa) | Ultimate tensile strengths (MPa) | |||||||
| 5.00–25.50 [90] | 0.20–2.00 [90] | 15–35 [90] | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kundak, H.; Bilisik, K. Development of Three-Dimensional (3D) Biodegradable Polyglycolic Acid Fiber (PGA) Preforms for Scaffold Applications: Experimental Patterning and Fiber Volume Fraction-Porosity Modeling Study. Polymers 2023, 15, 2083. https://doi.org/10.3390/polym15092083
Kundak H, Bilisik K. Development of Three-Dimensional (3D) Biodegradable Polyglycolic Acid Fiber (PGA) Preforms for Scaffold Applications: Experimental Patterning and Fiber Volume Fraction-Porosity Modeling Study. Polymers. 2023; 15(9):2083. https://doi.org/10.3390/polym15092083
Chicago/Turabian StyleKundak, Hikmet, and Kadir Bilisik. 2023. "Development of Three-Dimensional (3D) Biodegradable Polyglycolic Acid Fiber (PGA) Preforms for Scaffold Applications: Experimental Patterning and Fiber Volume Fraction-Porosity Modeling Study" Polymers 15, no. 9: 2083. https://doi.org/10.3390/polym15092083
APA StyleKundak, H., & Bilisik, K. (2023). Development of Three-Dimensional (3D) Biodegradable Polyglycolic Acid Fiber (PGA) Preforms for Scaffold Applications: Experimental Patterning and Fiber Volume Fraction-Porosity Modeling Study. Polymers, 15(9), 2083. https://doi.org/10.3390/polym15092083