3.1. Synthesis of Star-Shaped PCL
Three 4-arm and 6-arm PCL with different molecular weights were obtained using different monomer:initiator ratios. The temperature of 110 °C was selected as it was the optimised temperature for ROP of ε-CL with Sn(Oct)
2 as the catalyst [
27,
28]. A higher temperature promotes intermolecular and intramolecular esterification that broadens the molecular weight distributions of the synthesized polymers [
29].
The FTIR spectra show the presence of a sharp and intense band around 1720 cm
−1 for both 4-arm and 6-arm PCL, indicating the appearance of carboxylic ester (C=O) groups for the repeating units of the PCL chain (
Figures S1 and S2). The C-H band of PCL methylene group backbone appeared around 2980 cm
−1 to 2850 cm
−1 for both 4-arm and 6-arm PCL. The presence of these bands indicates the successful formation of PCL via ROP of cyclic ε-CL. The C-O stretch band for both 4-arm and 6-arm polymers appeared around 1190 cm
−1. The hydroxyl functional group band appeared around 3500 cm
−1 and 3400 cm
−1 with low intensity due to the low -OH group per PCL chain [
30].
In the
1H-NMR spectra of the synthesized 4-arm and 6-arm PCL with different molecular weights (
Figures S3 and S4), the signal for the methylene proton (a) of the pentaerythritol appeared around 4.10 ppm. The chemical shifts (b–e) appeared around 1.30 ppm to 4.10 ppm (δ 1.3 and δ 1.6, δ 2.2, δ 4.0, (
m, -CH
2-), (
t, -CH
2-), (
t, -CH
2-O-)) corresponded to the PCL backbone, indicating the successful occurrence via ROP of ε-CL. The methylene proton (f) peak was observed around 3.60 ppm (
t, -CH
2-OH), indicating the terminal group of the PCL. The core arms had a similar -OH terminal group as the active site that can initiate the ROP of the cyclic ε-caprolactone [
31].
3.2. Synthesis of Star-Shaped PCL-PEG
The coupling reaction between the star-shaped PCL and succinylated MPEG was done via the Steglich esterification reaction. The FTIR spectra (
Figures S5 and S6) show that the sharp C=O stretching band around 1725 cm
−1 was the carbonyl group of the ester linkage. The appearance of this band indicates the successful coupling reaction between PCL and PEG [
32]. Meanwhile, the sharp and intense bands that appeared around 1108 cm
−1 to 1106 cm
−1 correspond to the C-O functional group of the PEG ether polymer backbone. The C-H stretching bands for the antisymmetric and symmetric vibrations in the PEG and PCL repeating chain segments appeared around 2940 cm
−1 to 2880 cm
−1. The absence of the -OH band confirmed the coupling reaction of PCL and MPEG-COOH.
As for proton NMR for 4-arm and 6-arm PCL-PEG copolymers (
Figures S7 and S8), the chemical shifts for repeating units of the PCL backbone appeared around 1.4 ppm (
m, 2H, -CH
2-), 1.7 ppm (
m, 2H, -CH
2-), and 2.3 ppm (
t, 2H, -CH
2-). A triplet around 4.1 ppm (
t, 2H, -CH
2O-) denotes the appearance of methylene ester for the PCL polymer. Meanwhile, the chemical shifts between 3.6–3.8 ppm (
m, -OCH
2CH
2OCH
3-) and 3.4 ppm (
s, -OCH
3-) denote the repeating unit of PEG and methylene end group of MPEG, respectively. The successful conjugation of the PCL and PEG blocks was signified by the presence of a triplet around 2.8 ppm (
t, -COCH
2CH
2CO-) for the methylene group ester linkage between PCL and PEG. Apart from that, the absence of the hydroxyl end group for the homopolymer PCL peak confirmed the formation of PCL-PEG copolymer [
33].
3.3. Molecular Weight Analysis
The molecular weights of the synthesized star-shaped polymers were determined using
1H NMR and GPC analysis. The degree of polymerization for the PCL repeating chains was calculated from the integration ratio of the methylene protons for the repeating units at around 2.2 ppm and protons for the terminal unit at around 3.6 ppm based on the
1H-NMR spectrum [
34]. The M
n obtained from the synthesized polymers was close to the theoretical value (
Table 2).
The star-shaped copolymers have a well-defined 4-arm and 6-arm structure due to the same hydroxyl active site in the homopolymer PCL [
35]. Theoretically, an MPEG segment will attach to each PCL arm; a ratio of the MPEG signal to PCL signal was used to calculate the average molecular weight (M
n) of the block copolymers. DP
arm was obtained by comparing the ratio of integration area for the peak f at around 2.3 ppm of the PCL backbone with the peak h of the PEG end group at around 3.4 ppm [
36]. The M
n of the copolymers obtained was comparable to the theoretical M
n value (
Table 3).
The M
n value obtained from the GPC analysis was comparably different from the theoretical value. These results corresponded with the study conducted by Mortazavian et al. [
37] and Yan et al. [
38], where they reported a significantly different M
n value obtained from GPC analysis compared with the the theoretical and
1H-NMR values for the synthesized star-shaped polymers. The GPC column separates samples based on the hydrodynamics size and not molecular weight. The analysis was based on the theory that a specific size in the solution correlates to a specific molecular weight given in the calibration. The retention time depends on that molecular size [
39]. In a solution, polymers with identical molecular weights but different architecture can coil up to form a sphere with the same hydrodynamic volume. However, their molecular weight can vary from one to another, although having the same volume [
40]. Moreover, the molecular weight of the star-shaped polymer in GPC analysis is expected to have a smaller hydrodynamic volume than its linear counterpart [
38,
41].
Although the molecular weight analysis using GPC is different from
1H-NMR, GPC analysis is necessary to define the molecular weight distribution of polymers. Molecular weight distribution can influence polymeric properties such as viscosity, processability, and crystallization. Minor variations in the distribution might considerably impact the polymer’s content, resulting in significant differences in the properties [
42]. A narrow distribution with a polydispersity index (PDI) less than 1.3 in star-shaped polymers is desirable since it signifies a controlled polymerization reaction on each polymeric arm and symmetrical peaks [
43]. The PDI obtained from the GPC analysis (
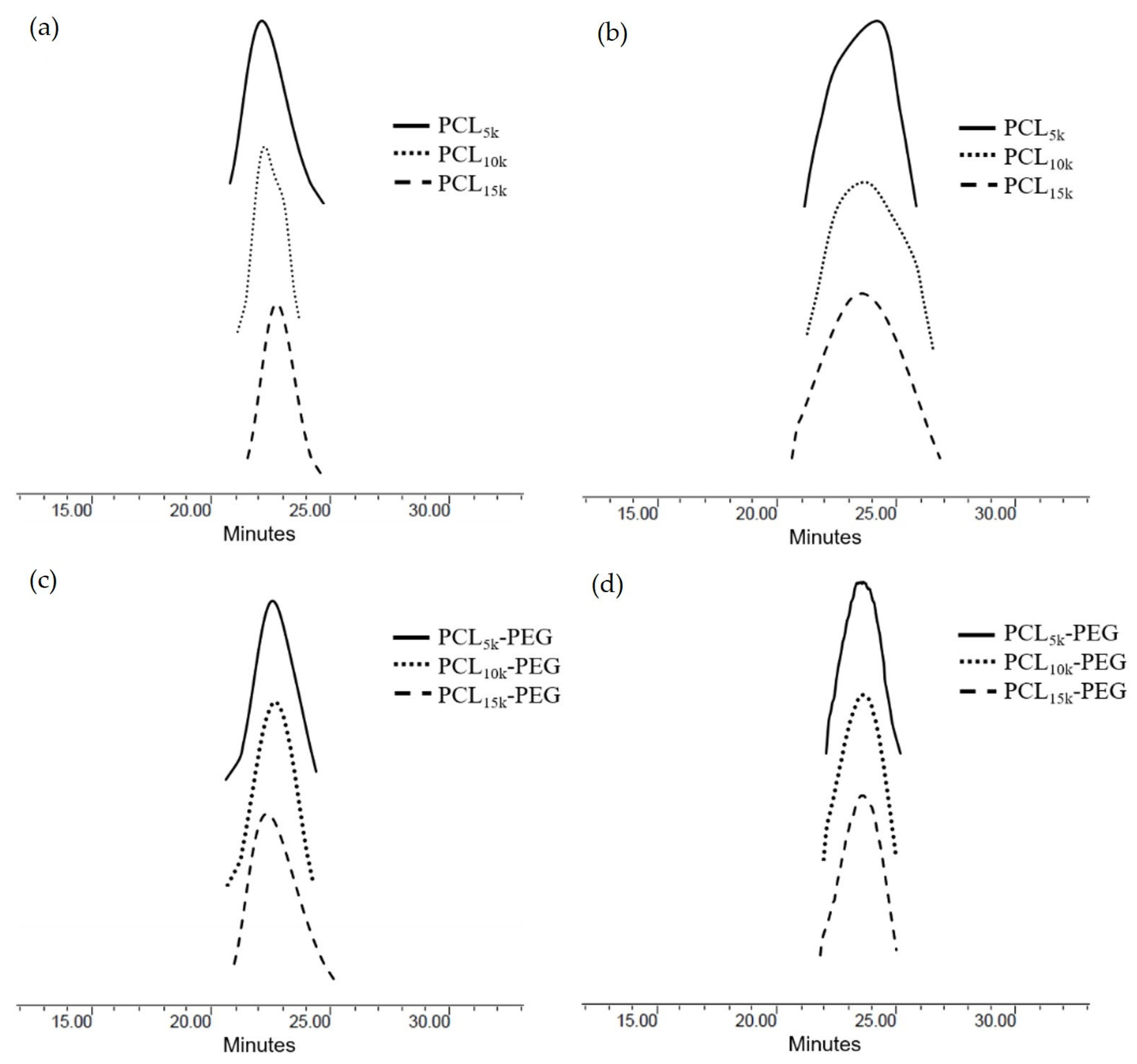
Table 3) was narrow (PDI ≈ 1.01–1.20), indicating monodispersity. Therefore, it can be said that the polymerization process of ε-CL was conducted under a controlled condition. This result is further proven by observing the GPC chromatogram (
Figure 3) for the star-shaped PCL and PCL-b-PEG, whereby the GPC curves show a unimodal peak for all polymers. The unimodal peak indicates monodispersity of the molecular weight and the absence of homopolymer PCL and MPEG monomers [
44]. This is due to the minimal transesterification or backbiting during the copolymerization reaction, which resulted in the symmetrical polymerization in each arm [
45].
3.4. Thermal Analysis
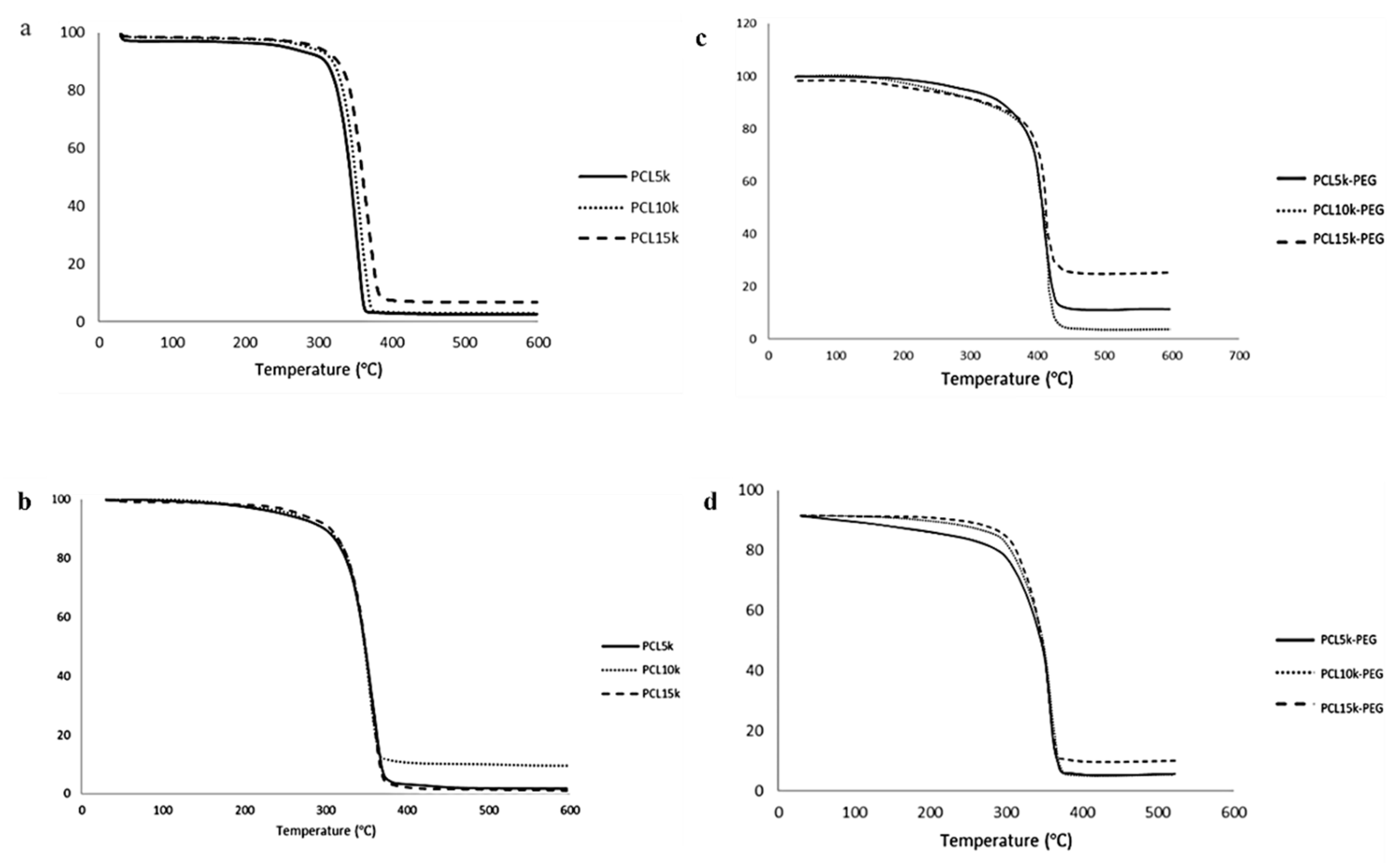
All polymers had high thermal stability based on the initial thermal degradation temperature (T
d, onset), around 343.6–374.3 °C (
Table 4 and
Table 5). The main thermal degradation temperature (T
d, max) increased with the increasing molecular weight of the homopolymer PCL. Therefore, more energy was needed to break the polymer chain [
46]. However, the overall T
d, the max temperature of 6-arm PCL, was slightly lower than its 4-arm PCL analogue. This is due to the higher branching of the 6-arm PCL. Since the thermal stability of polymers is heavily reliant on their chemical structure and molecular weight, an increase in branching decreases the polymer chain. Consequently, there is reduced cross-linking and degree of entanglement, hence less energy is required to break the ester linkage of the PCL backbone.
Meanwhile, the addition of MPEG increased the overall thermal stability of the block copolymers compared with the homopolymer PCL. The MPEG modified the -OH end group susceptible to faster thermal degradation to a more stable -OCH
3 group [
47]. Homopolymer MPEG has the highest thermal degradation temperature compared with homopolymer PCL and block copolymers PCL-PEG. The increase in the thermal stability demonstrated synergistic thermal stability and the compatibility of the block copolymers [
48,
49]. Compared with 4-arm PCL-PEG, 6-arm PCL-PEG has a higher T
d and onset temperature, and contains a higher PEG segment; therefore, more energy is required to initiate the degradation reaction. However, 6-arm PCL-PEG has a lower T
d and max temperature than 4-arm PCL-PEG, also due to the higher PEG segments. The 6-arm PCL-PEG have more hydrophilic regions that lead to a rapid hydrolytic degradation to produce free OH
−, which later become a catalyst for the breakage of the ester linkage and accelerate the degradation of the copolymers [
50].
All synthesized polymers showed a single step-degradation (
Figure 4). Single-step degradation indicates that the copolymers are compatible with each other as block copolymers [
51] and only one decomposition process due to the homogeneous materials [
52].
Homopolymer PCL has a T
m within the melting range of PCL, which is around 60 °C [
53] (
Figure 5). The melting point of the synthesized 4-arm and 6-arm PCL increased with M
n due to the arm’s length [
54]. The melting point of a crystal is determined by the strength of the molecule’s hydrogen bonds, the effects of molecular shape, size, and symmetry on crystal packing, and other factors such as charge transfer and dipole–dipole interactions [
55]. The increasing molecular weight of PCL (arm lengths) limits free molecular motion through crystallization and improves molecule packing in crystals, making them harder to rearrange to form lamellae regions [
56]. As a result, the higher molecular weight requires more energy absorption to melt the crystals [
57]. Apart from that, it can also be observed that the T
m of the homopolymer PCL increases with the increasing number of arms. This might be due to the increase in the compactness of the structure as polymerization of PCL occurred, which reduced the molecular surface area per atom. Consequently, it increases the stability of the polymer, making it hard to break the compact structure [
58,
59].
The same trend is observed on the block copolymers PCL-PEG. The addition of PEG increases the crystalline melting temperature of the copolymers due to the interaction between the melting of PCL and PEG blocks [
36]. The temperature is close to the melting point of PEG because PEG has a higher T
m than PCL. Apart from that, 6-arm PCL-PEG has a higher crystalline melting point compared with 4-arm PCL-PEG. The different number of arms results in different PEG added to each PCL arm. The addition of PEG increases the polymer chain mobility that allows chain rearrangement [
60,
61]. PEG promotes easier rearrangement to form a more stable crystalline structure. Hence, more energy is needed to melt the crystalline structure and free the polymer chains of the 6-arm PCL-PEG [
60,
62].
The monomodal exotherm peak indicates that the PCL homopolymers can crystallize completely and give crystals when quenched from the melt. Meanwhile, the monomodal peaks of the PCL-PEG copolymers might come from the crystalline domain of the dominant PEG segment, making the melting temperature of PCL blocks impossible to measure individually because of inadequate phase separation and improved phase compatibility between PCL and PEG blocks. A certain amount of PEG can stimulate the crystallization of block copolymers as it can move freely compared with the rigid PCL core [
63]. This shows that the block copolymers are comprised of semicrystalline PEG segments and PCL blocks scattered within the crystalline PEG phase [
64].
3.5. Hydrogel Formulations
Twelve hydrogel formulations were prepared by varying the composition of the 4-arm and 6-arm PCL-PEG to observe the effect of different polymer compositions on the properties of the formulations. Amphiphilic PCL-PEG was chosen to ensure homogenous hydrogel formation [
65]. The PCL as the inner segment encapsulates hydrophobic ciprofloxacin model drugs. Meanwhile, the PEG as the outer segment increased the polymer’s solubility in hydrogel formulation to form a homogeneous formulation. Carbopol 940 was selected as the gelling agent due to its simplicity to be developed at room temperature and its large viscosity range, which made it easier to modify the physical properties of the gel [
66]. Carbopol also possesses high thixotropy, a rheological characteristic referring to changes in viscosity with the time of application, which was desirable for topical gel [
67]. Compared with other gelling agents such as Carbopol 934 and Ultrez 10, Carbopol 940 was found to have superior flow-modifying properties for topical delivery and a greater effect in extending drug release from hydrogel formulations compared with Carbopol 934 [
68,
69]. Additionally, Carbopol 940 was reported to show no obvious toxicity to the target organs and tissues during long-term repeated treatment for skin wound healing [
70]. The amount of Carbopol 940 used was kept to 0.5%
w/
w to get excellent skin feel for topical application [
71]. Ciprofloxacin was selected as a hydrophobic model drug with antimicrobial properties. The ciprofloxacin model drug used was limited to a minimum amount that demonstrated its efficacy [
72,
73].
After complete dispersion of the hydrogel components, TEA was added to the homogenous formulation to alter the pH value and thicken the hydrogel. Carbopol was then able to absorb and retain water, making the crosslinking chains to hydrate. As a result, the chains partially uncoiled due to the electrostatic repulsion to form a gel network with stronger bonds [
74,
75]. The formulations obtained had an opaque white appearance with good homogeneity, indicating good hydrogel characteristics (
Table 6) [
76]. After three weeks of studies, observation on the hydrogel formulations showed no physical changes in the colour and homogeneity, indicating the stability of the formulations.
The viscosity of the hydrogel formulations was high, with a value above 10,000 cP. Previous studies showed that gels with high viscosity have high consistency and good adherence to the skin [
67]. One of the essential physical characterizations of the hydrogel formulations is viscosity because it contributes to the properties of the formulations, such as spreadability, pourability, and consistency [
77]. Higher gel viscosity also prolonged the release of the drug, improving patient compliance with treatment due to a reduced number of applications [
78].
The overall trend for the viscosity shows that Formulation B has a higher viscosity than A for all formulations. The viscosity depends on the concentration (%) of the polymer in the solution and the molecular weight. Hence, a greater polymer concentration resulted in a higher viscosity of the solution [
79]. Meanwhile, the same increasing trend was found when comparing the viscosity between formulations with 4-arm PCL-PEG (F1–F3) and formulations with 6-arm PCL-PEG (F4–F6). The increase in the viscosity was associated with the increase in the overall molecular weight of the star-shaped polymers [
65]. First, the higher viscosity of the formulations was due to the higher molecular weight of PCL. Second, as the number of arms increased, the amount of PEG segments attached to each arm also increased. Therefore, even though 4-arm and 6-arm homopolymer PCL have the same molecular weight, the addition of the PEG segment to each arm makes the overall molecular weight of 6-arm PCL-PEG higher than that of 4-arm PCL-PEG. Bigger polymer molecules possess more intermolecular interaction, and hence increase the viscosity [
80].
Apart from viscosity, pH value also plays a crucial role in the properties of the gel. Alkaline formulations with a pH >8 will agglomerate and become too concentrated, affecting the spreadability on human skin, while acidic formulations with pH <5 dilutes the formulation and irritates the skin [
81]. The optimum viscosity of Carbopol for good spreadability is typically achieved at a pH range of 6.5–7.5 [
82]. Therefore, the pH value of the formulation was adjusted to the range between 7.0–7.4, which was in the optimum range and suitable to be used on human skin [
26,
83]. There were no significant changes in the pH of the formulation after several weeks of studies, indicating good stability and long lifespan of the formulations [
84].
3.6. Drug Loading (DL) and Entrapment Efficiency (EE)
The drug was incorporated into the hydrogels via in situ loading. The polymer precursor solution was mixed with a drug-loaded polymer, and the hydrogel network formulation and drug entrapment were achieved simultaneously [
85]. Based on calculated values, both formulations showed high drug entrapment efficiency (EE) and drug loading (DL) with a value >99%. This proves the role of the PCL core as a drug cargo to enhance the capability to load hydrophobic drugs, which was due to the hydrophobic–hydrophobic interaction between ciprofloxacin and the PCL block [
86]. This result was consistent with the literature, where PCL-PEG micelles favored the hydrophobic atorvastatin drug than the hydrophilic rosuvastatin drugs [
87].
There are no significant differences in the EE and DL of all the formulations, signifying the amount of the copolymers used, and the number of polymeric arms did not significantly enhance the loading capacity. However, the overall trend for the drug loading and entrapment efficiency is that Formulation B has a slightly higher DL and EE than Formulation A. A higher concentration of star-shaped PCL-PEG increased the opportunity for a higher amount of polymer for drug entrapment [
88]. Apart from that, the polymer concentration enhances the viscosity of the gel, increasing the diffusional resistance of the drug molecules. Consequently, more drug is entrapped in the cargo as the drug diffusion out of the polymeric cargo is reduced due to a longer diffusional path [
89]. The same observation has been reported in the study conducted by Huang et al. [
90].
The DL and EE of the formulations are also affected by the molecular weight of the polymers, where higher molecular weight polymers have a slightly higher DL and EE. As the molecular weight of the polymer increased due to the longer PCL segment, the hydrophobic-hydrophobic interaction between ciprofloxacin and the PCL also improved, thus allowing higher drug loading in the formulation. Meanwhile, the attachment of the PEG segment increases the overall molecular weight of the star-shaped PCL-PEG, giving the polymer a higher degree of entanglement. This reduces the drug’s molecular diffusion area and permeation across the matrix gel [
91].
Star-shaped PCL-PEG copolymers offer additional drug-conjugated sites and looser space for drug loading. The high number of arms affects the drug loading and entrapment capability [
92], as it enhances the DL and EE due to the larger sites that can act as a drug reservoir. Therefore, compared to 4-arm PCL-PEG, 6-arm PCL-PEG with more hydrophobic arms in the polymers, led to bigger cores that easily encapsulated the hydrophobic drugs.
3.7. Drug Release Analysis
The release of the drug loaded via in situ loading is determined by diffusion, hydrogel swelling, reversible-polymer interaction, or the degradation of labile covalent bonds [
93]. Due to the prolonged degradation rate of PCL, the formulations containing 4-arm and 6-arm PCL-PEG in this study were expected to exhibit a controlled release behavior via diffusion-controlled release [
94]. The drug began to release from the surface of the sphere and then continued to release from the inner layers of the sphere [
95]. Since the ciprofloxacin was encapsulated in the PCL inner segment of the star-shaped PCL-PEG instead on the surface of the system, this resulted in slower drug release with no initial burst rate.
The drug release of Formulation B is higher compared with that of Formulation A (
Table S1) for the hydrogel containing 4-arm PCL-PEG (F1–F3). Higher DL and EE in Formulation B than A leads to a higher amount of drug released per unit area exposed to the surface of the polymer matrix [
96]. Additionally, increasing the molecular weight of the polymers also enhanced the DL and EE of the formulations, hence the higher the drug release. This result corresponded with the study done by Younis et al. [
97].
However, as the polymer concentration in the formulations increases, the viscosity of the polymer gel is enhanced. Subsequently, the density of the polymer matrix and diffusion path length that the drug molecules have to cross becomes greater. Therefore, the drug diffusion coefficient across the hydrogel matrix is decreased [
89,
96]. This can be seen in the drug release pattern of formulations containing 6-arm PCL-PEG (F4–F6), where Formulation A has higher drug release than Formulation B due to its lower viscosity. Apart from that, formulations containing 4-arm PCL-PEG have a higher release rate than those containing 6-arm PCL-PEG. This might be due to its lower molecular weight, which makes them possess a high elastic modulus. Thus, the matrix becomes more deformable, causing the pores to expand due to osmotic pressure [
98]. In addition, the blending of hydrophilic PEG into the hydrophobic PCL can increase pore formation and increase the rate of polymer degradation, leading to faster drug release [
99].
The percentage of drug release from the formulations was low across the 7 h of the study (
Figure 6). At 7 h, the highest drug release was only about 25% of the total encapsulated drug. This is related to the presence of PCL, which influenced the hydrophobic–hydrophobic interaction between the model drug and PCL. The low rate occurred because the release behavior is not severely affected by the degradation of PCL blocks, as the PCL degradation rate is very slow in an aqueous medium due to crystalline and hydrophobic properties. Hence, the release of ciprofloxacin was facilitated by the penetration of water into the amorphous region of the polymer matrix that diffuses out of the matrix together with the drug [
100]. The same trend was reported in the study conducted by Kheiri Manjili et al. [
101] and Zamani et al. [
102].
3.8. Drug Release Kinetic Study
The development of mathematical modelling requires knowledge of all phenomena influencing drug release kinetics which has a significant value in the formulation optimization [
103]. Various mathematical kinetic models (Zero-order, First-order, Higuchi, Hixson–Crowell, and Korsmeyer–Peppas) were used to examine the most suitable dissolution profile for the polymeric hydrogel formulations, which is the main fitting model for hydrogel-based drug delivery systems [
104].
The zero-order model describes drug release that is independent of the drug concentration. Conversely, the first-order model corresponds to a drug release that is dependent on the drug concentration. The Higuchi model explains the diffusion process for the release of low-solubility drugs distributed within an insoluble and swellable porous matrix based on Fick’s diffusion law. The Hixson–Crowell equation defines drug release from dosage forms with variable diameter and surface area. Meanwhile, Korsmeyer–Peppas explains a mixed release mechanism that is linked to a number of factors such as polymer swelling, polymer erosion, matrix porosity, and drug diffusion rates for drugs in swelling systems [
104,
105]. The best linearity for all formulations was obtained from the values in the Korsmeyer–Peppas model (
Table 7). Hence, Korsmeyer–Peppas is the best-fitted mathematical modell for polymeric hydrogel formulations. This result is similar to those of previous studies that also reported the same drug release kinetics for the polymeric hydrogel drug release systems [
105,
106].
The data from the in vitro drug release studies were plotted as cumulative drug release (%) versus the log of time. Then, the
n value was obtained from the slope of the graph to characterize different release mechanisms for the matrices [
107]. Based on the results, the
n value of the formulations was higher than 0.89 (
n > 0.89), indicating a Case II transport mechanism [
108]. This mechanism showed that the drug release is by both diffusion and relaxation of the polymer chain, which might be due to the chain disentanglement and swelling of the hydrophilic polymer, such as Carbopol, that can initiate water diffusion. Apart from that, the higher number of hydrophilic PEG segments in each arm of the star-shaped PCL-PEG can also facilitate water diffusion into the formulation compared with the PCL segment [
99]. This proved that the drug release of ciprofloxacin from the PCL-PEG hydrogel was indeed facilitated by the penetration of water into the polymer matrix. The mechanism also describes the effect of polymer hydration and swelling behavior on drug release for polymeric and swellable systems. The drugs probably diffuse out of an outer gel layer that erodes, releasing the polymers containing the drugs into the aqueous medium [
104].
3.9. Statistical Analysis
Statistical analysis was carried out to analyze whether significant differences can be reported in the drug release of all the formulations. The treatment (between columns) effect measures the average difference between subjects given a particular treatment and the overall mean [
109]. The formulations were subjected to 7 h of dissolution study. The results showed that the percentage of drug release at each time point using ANOVA was statistically significant with a
p value < 0.05. This indicates that the drug release profile depends on the drugs’ dissolution behavior and release pattern from the hydrogel. In this study, drug release is affected by the diffusion and relaxation of the hydrophilic polymer chain. Therefore, drug release may be enhanced in a time-dependent way by tailoring the composition of the hydrophilic polymers in the hydrogel formulations [
110].
Then, t-test analysis was used to compare the significant difference between Formulation A and Formulation B for F1–F6. Based on the result, Formulation A was significantly different from Formulation B for F1–F6 (p value < 0.05), indicating the effect of different polymers’ concentrations (%) on drug release. Apart from that, F3 and F6, which possessed the highest drug release percentages in the formulations containing 4-arm and 6-arm PCL-PEG, respectively, were also compared to observe whether they were statistically different from each other. Based on the result, F3 and F6 were significantly different in the effect of the formulations’ compositions towards drug release with a p value < 0.05. As such, F3 and F6 were chosen for further analysis in this study.
3.10. Antimicrobial Activity
The antimicrobial activity was performed on four formulations with the highest drug release rate. Since the release rate is significantly affected by the polymers’ composition, the formulations with the highest polymer composition (F3 and F6) were selected to represent both 4-arm and 6-arm PCL-PEG, respectively. The in vitro study measured the diameter of zones of inhibition for each formulation and compared them to the control. Staphylococcus aureus was chosen as the Gram-positive bacteria (
Figure S9), and Escherichia coli was selected as the Gram-negative bacteria (
Figure S10). These bacteria were selected as they were among the most frequently isolated pathogens from wound infections [
111,
112].
Based on the antimicrobial study (
Table S2), the formulations containing ciprofloxacin model drugs (F3 and F6) showed activity against both Gram-positive and Gram-negative bacteria. Meanwhile, no inhibition zones were observed for STDF3 and STDF6 since both formulations did not contain ciprofloxacin to inhibit the bacteria. As such, no antibacterial activity was observed, indicating that the inhibition zone occurred due to the release of the ciprofloxacin model drug from the PCL-PEG hydrogel formulations.
There were no inhibition zones observed during the first hour for both F3 and F6, showing no initial burst of the drug released from the drug cargo. After 12 h of observation, F3 had higher antibacterial activity than F6. Meanwhile, after 24 h, it could be seen that the inhibition zone for both formulations doubled compared to the 12 h incubation zone, indicating a controlled ciprofloxacin drug release from the polymeric hydrogel formulations. Apart from that, the inhibition zone for F3 was higher compared with that of F6 for both bacteria, due to its higher drug release rate. The highest inhibition zone for both formulations after 24 h of incubation was
E. coli. This result was expected since ciprofloxacin was highly effective against Gram-negative bacteria, especially Enterobacteriaceae such as
E. coli, compared to Gram-positive bacteria [
113]. Additionally,
E. coli possessed an abundance of outer membrane proteins called porins that increased the sensitivity of
E. coli towards ciprofloxacin [
114]. These results correspond with past studies that reported the sensitivity of these bacterial strains against ciprofloxacin [
114,
115]. These results indicate the capability of the PCL-PEG polymeric hydrogel blends to transport hydrophobic drugs. Modifications to the water-based hydrogel enabled the hydrophobic–hydrophobic interaction within the system for effective delivery towards wound healing applications.
3.11. Morphological Analysis
The F3 and F6 formulations were subjected to SEM analysis to study morphology and its possible effect on the release rate of the polymeric hydrogels. The analysis shows that the formulations possessed an entangled irregular crosslinking network (
Figure 7). A porous structure was observed on all formulations, indicating a high degree of swelling of the developed gels. This is a desirable structure as it affects the amount of water penetration into the hydrogel network [
116]. In addition, pore structures affect the properties of hydrogels, such as swelling rate, mechanical strength, and degree of sensitivity [
117].
Based on SEM analysis, F6 has smaller pores compared with F3 due to the higher PEG composition and viscosity, making the gel more compact. The hydrogels with denser and tighter networks hinder the polymer chain’s mobility and minimize the water uptake capacity, lowering the swelling of the hydrogels [
118]. Consequently, it reduces the drug release rate of the formulations as the release mechanism depends on the water diffusion and swelling behavior.
Additionally, Formulation A has larger pores compared with Formulation B, which increases the release rate of the formulations. Therefore, F6 (A) has a higher release rate than F6 (B). However, the data show that F3 (B) has a higher release rate than F3 (A). Since ciprofloxacin is already loaded into the PCL-PEG during preparation, this causes in the underutilization of the total pore volume. As a result, the release rate depends on the drug loading capacity [
119]. These results corresponded with the drug release and antimicrobial study of the hydrogels.