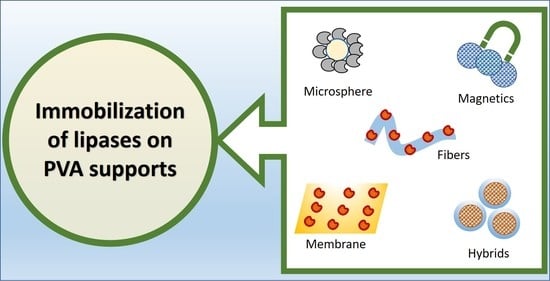
Immobilization of Lipases Using Poly(vinyl) Alcohol
Abstract
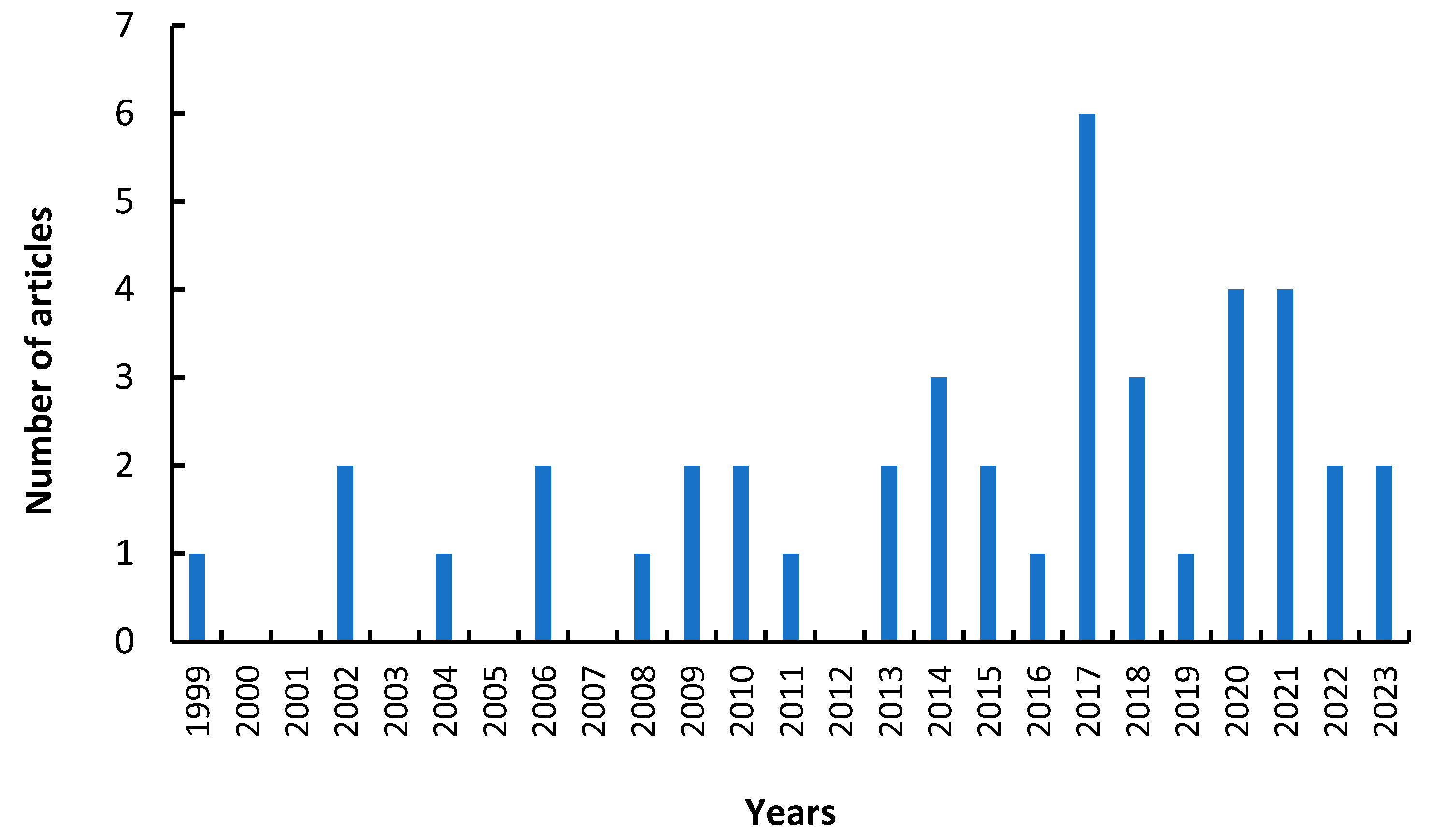
1. Introduction
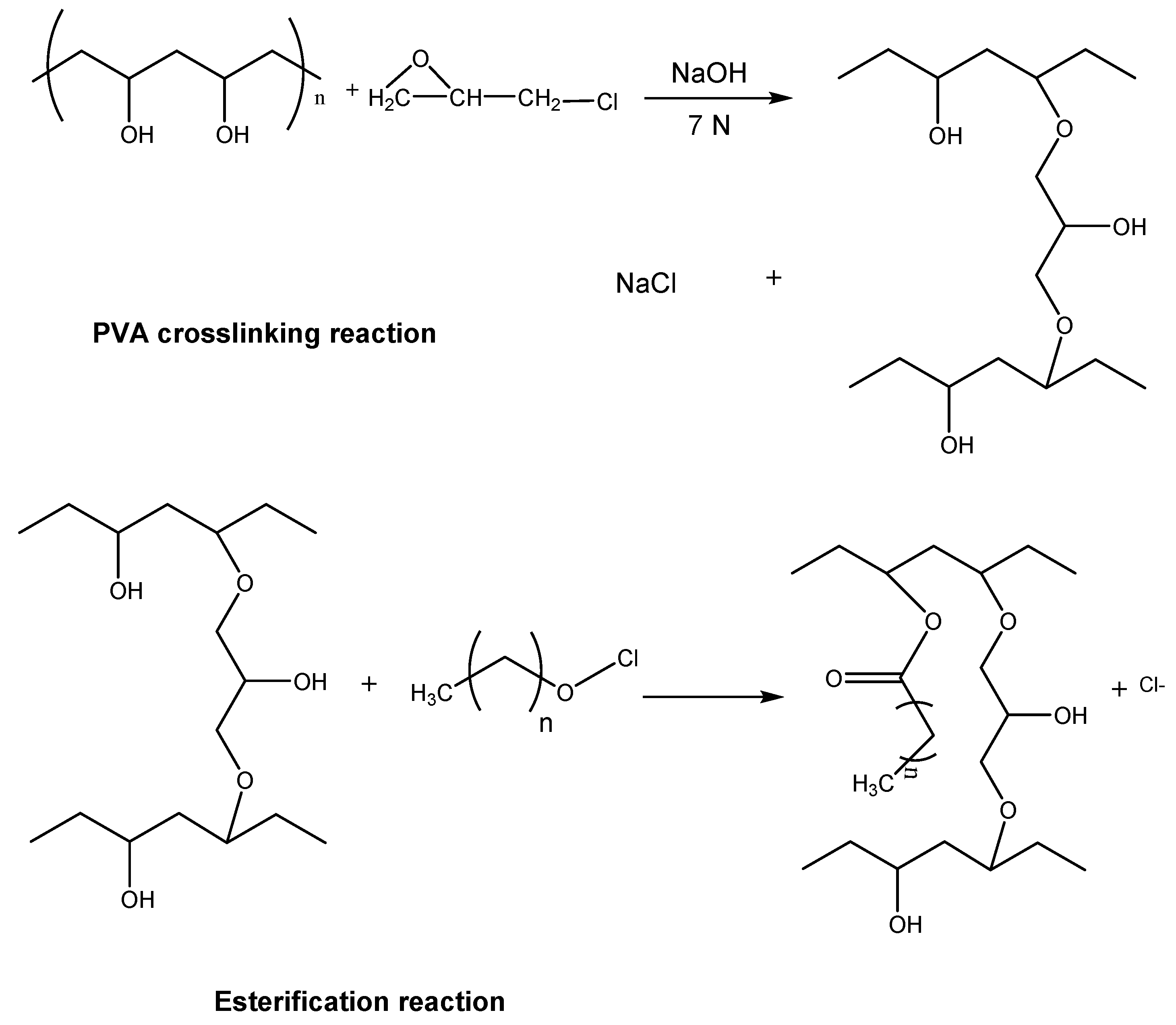
2. Immobilization of Lipases on Crosslinked PVA Matrices
3. Immobilization of Lipases on PVA Hybrid Matrices
3.1. Immobilization on PVA/Alginate
3.2. Immobilization on PVA/Chitosan
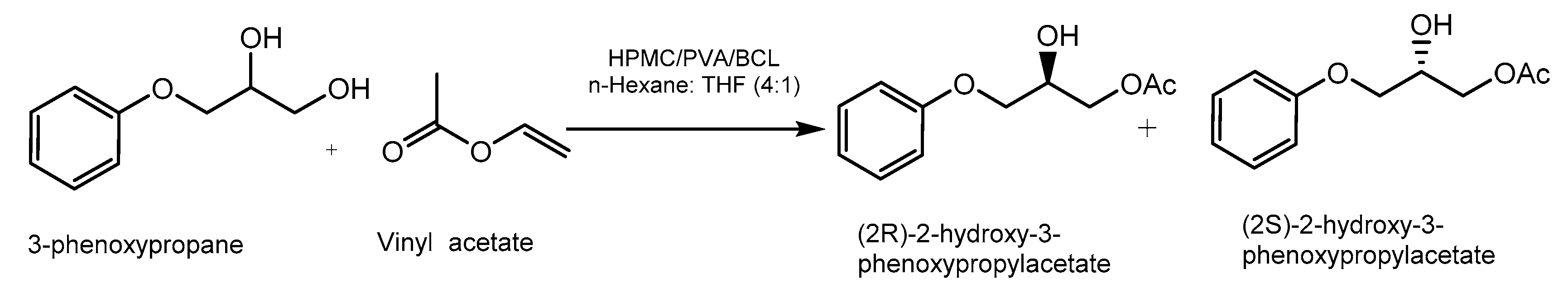
3.3. Immobilization on PVA/Hydroxypropyl Methylcellulose (HPMC)
3.4. Immobilization on Different PVA Hybrid Supports
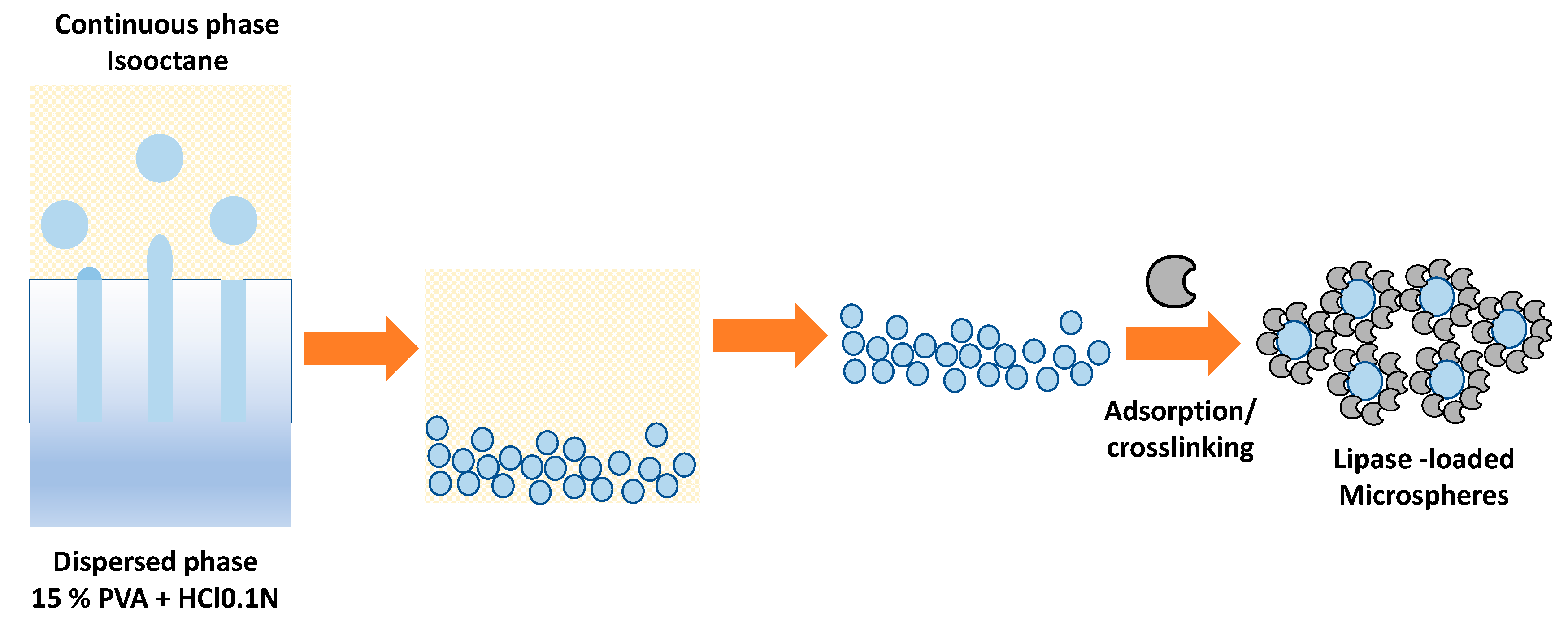
4. Immobilization of Lipases in PVA Microspheres and LentiKats®
5. Immobilization of Lipases on Magnetic PVA Supports
6. Concluding and Remarks
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cossy, J. Biocatalyts: Catalysts of the Future for Organic Synthesis and Beyond? Tetrahedron 2022, 123, 132966. [Google Scholar] [CrossRef]
- Vivek, K.; Sandhia, G.S.; Subramaniyan, S. Extremophilic Lipases for Industrial Applications: A General Review. Biotechnol. Adv. 2022, 60, 108002. [Google Scholar] [CrossRef]
- Kim, B.H.; Hwang, J.; Akoh, C.C. Liquid Microbial Lipase—Recent Applications and Expanded Use through Immobilization. Curr. Opin. Food Sci. 2023, 50, 100987. [Google Scholar] [CrossRef]
- Kralik, D.; Kovářová, A.; Vobecká, L.; Hasal, P.; Slouka, Z.; Přibyl, M. Continuous Flow Synthesis and Separation of Mandelic Acid Enantiomers in a Modular Microfluidic System. Sep. Purif. Technol. 2023, 309, 123009. [Google Scholar] [CrossRef]
- Costa, K.A.D.; Catarina, A.S.; Leal, I.C.R.; Sathler, P.C.; de Oliveira, D.; de Oliveira, A.A.S.C.; Cansian, R.L.; Dallago, R.M.; Zeni, J.; Paroul, N. Enzymatic Synthesis of Ascorbyl Oleate and Evaluation of Biological Activities. Food Res. Int. 2022, 161, 111851. [Google Scholar] [CrossRef]
- Ferrero, G.O.; Faba, E.M.S.; Eimer, G.A. Biodiesel Production from Alternative Raw Materials Using a Heterogeneous Low Ordered Biosilicified Enzyme as Biocatalyst. Biotechnol. Biofuels 2021, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Elias Guckert, F.; Sayer, C.; de Oliveira, D.; Hermes de Araújo, P.H.; Francisco Oechsler, B. Synthesis of Polybutylene Succinate via Lipase-Catalyzed Transesterification: Enzyme Stability, Reuse and PBS Properties in Bulk Polycondensations. Eur. Polym. J. 2022, 179, 111573. [Google Scholar] [CrossRef]
- Uribe, J.; Lienqueo, M.E.; Guajardo, N. Optimization and Determination of Kinetic Parameters of the Synthesis of 5-Lauryl-Hydroxymethylfurfural Catalyzed by Lipases. Catalysts 2023, 13, 19. [Google Scholar] [CrossRef]
- Kua, G.K.B.; Nguyen, G.K.T.; Li, Z. Enzyme Engineering for High-Yielding Amide Formation: Lipase-Catalyzed Synthesis of N-Acyl Glycines in Aqueous Media. Angew. Chem.-Int. Ed. 2023, 62, e202217878. [Google Scholar] [CrossRef]
- Baloch, K.A.; Singh, A.; Pudtikajorn, K.; Benjakul, S. Lipases from Different Yeast Strains: Production and Application for n-3 Fatty Acid Enrichment of Tuna Eyeball Oil. Biocatal. Agric. Biotechnol. 2023, 48, 102651. [Google Scholar] [CrossRef]
- Mahfoudhi, A.; Benmabrouk, S.; Fendri, A.; Sayari, A. Fungal Lipases as Biocatalysts: A Promising Platform in Several Industrial Biotechnology Applications. Biotechnol. Bioeng. 2022, 119, 3370–3392. [Google Scholar] [CrossRef]
- Rivas, A.G.; Vázquez, V.Á.; Flores, M.M.A.; Cerrillo-Rojas, G.V.; Correa-Aguado, H.C. Sustainable Castor Bean Biodiesel Through Ricinus communis L. Lipase Extract Catalysis. Appl. Biochem. Biotechnol. 2023, 195, 1297–1318. [Google Scholar] [CrossRef]
- Narayanan, M.; Ali, S.S.; El-Sheekh, M. A Comprehensive Review on the Potential of Microbial Enzymes in Multipollutant Bioremediation: Mechanisms, Challenges, and Future Prospects. J. Environ. Manag. 2023, 334, 117532. [Google Scholar] [CrossRef] [PubMed]
- Girelli, A.M.; Chiappini, V. Renewable, Sustainable, and Natural Lignocellulosic Carriers for Lipase Immobilization: A Review. J. Biotechnol. 2023, 365, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, C.S.; Angelotti, J.A.F.; Fernandez-Lafuente, R.; Hirata, D.B. Lipase Immobilization via Cross-Linked Enzyme Aggregates: Problems and Prospects—A Review. Int. J. Biol. Macromol. 2022, 215, 434–449. [Google Scholar] [CrossRef]
- Cao, L. Covalent Enzyme Immobilization; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germay, 2005; ISBN 9783527312320. [Google Scholar]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.S. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2022, 8, 5184–5196. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhang, H.; Wu, J.; Wang, Z.; Sun, H.; Yuan, J.; Chen, S. A Novel Zwitterionic Co-polymer with a Short Poly(Methyl Acrylic Acid) Block for Improving Both Conjugation and Separation Efficiency of a Protein without Losing Its Bioactivity. J. Mater. Chem. B 2013, 1, 2482–2488. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of Lipases on Hydrophobic Supports: Immobilization Mechanism, Advantages, Problems, and Solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of Enzymes via Immobilization: Multipoint Covalent Attachment and Other Stabilization Strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef]
- Guajardo, N.; Ahumada, K.; Domínguez de María, P. Immobilization of Pseudomonas Stutzeri Lipase through Cross-Linking Aggregates (CLEA) for Reactions in Deep Eutectic Solvents. J. Biotechnol. 2021, 337, 18–23. [Google Scholar] [CrossRef]
- Wilson, L.; Illanes, A.; Pessela, B.C.C.; Abian, O.; Fernández-Lafuente, R.; Guisán, J.M. Encapsulation of Crosslinked Penicillin G Acylase Aggregates in Lentikats: Evaluation of a Novel Biocatalyst in Organic Media. Biotechnol. Bioeng. 2004, 86, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Guajardo, N.; Ahumada, K.; Domínguez de María, P. Immobilized Lipase-CLEA Aggregates Encapsulated in Lentikats® as Robust Biocatalysts for Continuous Processes in Deep Eutectic Solvents. J. Biotechnol. 2020, 310, 97–102. [Google Scholar] [CrossRef]
- Khan, R.S.; Rather, A.H.; Wani, T.U.; Rather, S.-U.; Amna, T.; Hassan, M.S.; Sheikh, F.A. Recent Trends Using Natural Polymeric Nanofibers as Supports for Enzyme Immobilization and Catalysis. Biotechnol. Bioeng. 2023, 120, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Silveira, E.; Silva, B.; Farias, D.; Roberto, T.; Anna, S.; Junior, C.; Antonio, L.; Pinto, D.A.; Silva, P. Chitosan–Based Nanofibers for Enzyme Immobilization. Int. J. Biol. Macromol. 2021, 183, 1959–1970. [Google Scholar]
- Leong, J.Y.; Lam, W.H.; Ho, K.W.; Voo, W.P.; Lee, M.F.X.; Lim, H.P.; Lim, S.L.; Tey, B.T.; Poncelet, D.; Chan, E.S. Advances in Fabricating Spherical Alginate Hydrogels with Controlled Particle Designs by Ionotropic Gelation as Encapsulation Systems. Particuology 2016, 24, 44–60. [Google Scholar] [CrossRef]
- Mohammadi, N.S.; Khiabani, M.S.; Ghanbarzadeh, B.; Mokarram, R.R. Enhancement of Biochemical Aspects of Lipase Adsorbed on Halloysite Nanotubes and Entrapped in a Polyvinyl Alcohol/Alginate Hydrogel: Strategies to Reuse the Most Stable Lipase. World J. Microbiol. Biotechnol. 2020, 36, 45. [Google Scholar] [CrossRef]
- Jawale, P.V.; Bhanage, B.M. Synthesis of Propyl Benzoate by Solvent-Free Immobilized Lipase-Catalyzed Transesterification: Optimization and Kinetic Modeling. Bioprocess Biosyst. Eng. 2021, 44, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Bidmanova, S.; Hrdlickova, E.; Jaros, J.; Ilkovics, L.; Hampl, A.; Damborsky, J.; Prokop, Z. Microscopic Monitoring Provides Information on Structure and Properties during Biocatalyst Immobilization. Biotechnol. J. 2014, 9, 852–860. [Google Scholar] [CrossRef]
- Dhake, K.P.; Tambade, P.J.; Qureshi, Z.S.; Singhal, R.S.; Bhanage, B.M. HPMC-PVA Film Immobilized Rhizopus Oryzae Lipase as a Biocatalyst for Transesterification Reaction. ACS Catal. 2011, 1, 316–322. [Google Scholar] [CrossRef]
- Kartal, F.; Kilinç, A. Immobilization of Pancreatic Lipase on Polyvinyl Alcohol by Cyanuric Chloride. Prep. Biochem. Biotechnol. 2006, 36, 139–151. [Google Scholar] [CrossRef]
- Krasňan, V.; Stloukal, R.; Rosenberg, M.; Rebroš, M. Immobilization of Cells and Enzymes to LentiKats®. Appl. Microbiol. Biotechnol. 2016, 100, 2535–2553. [Google Scholar] [CrossRef]
- Ak, G.; Aktuna, Y.; Kartal, F.; Kilinc, A. The Effect of Pretreatment with Substrates on the Activity of Immobilized Pancreatic Lipase. Artif. Cells Nanomed. Biotechnol. 2014, 42, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, T.K.; Kilinc, A. Immobilization of Lipase in Organic Solvent in the Presence of Fatty Acid Additives. J. Mol. Catal. B Enzym. 2010, 67, 214–218. [Google Scholar] [CrossRef]
- Telefoncu, A. Chemical Attachment of Porcine Pancreatic Lipase to Crosslinked Poly(vinyl alcohol) by Means of Adipoyldichloride. Process Biochem. 2002, 38, 641–647. [Google Scholar]
- Carbone, K.; Casarci, M.; Varrone, M. Crosslinked Poly(Vinyl Alcohol) Supports for the Immobilization of a Lipolytic Enzyme. J. Appl. Polym. Sci. 1999, 74, 1881–1889. [Google Scholar] [CrossRef]
- Nunes, M.A.P.; Vila-real, H. Immobilization of Naringinase in PVA—Alginate Matrix Using an Innovative Technique. Appl. Biochem. Biotechnol. 2010, 160, 2129–2147. [Google Scholar] [CrossRef]
- Bonine, B.M.; Polizelli, P.P.; Bonilla-Rodriguez, G.O. Immobilization of a Plant Lipase from Pachira Aquatica in Alginate and Alginate/PVA Beads. Enzym. Res. 2014, 2014, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Ortiz, B.R.; Ríos-González, L.J. Influence of the Reaction Parameters on Biodiesel Production Catalyzed by a Lipase from Thermomyces Lanuginosus Immobilized in PVA-Alginate Beads. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 2127–2132. [Google Scholar] [CrossRef]
- Dave, R.; Madamwar, D. Esterification in Organic Solvents by Lipase Immobilized in Polymer of PVA-Alginate-Boric Acid. Process Biochem. 2006, 41, 951–955. [Google Scholar] [CrossRef]
- İspirli Doğaç, Y.; Deveci, İ.; Mercimek, B.; Teke, M. A Comparative Study for Lipase Immobilization onto Alginate Based Composite Electrospun Nanofibers with Effective and Enhanced Stability. Int. J. Biol. Macromol. 2017, 96, 302–311. [Google Scholar] [CrossRef]
- Mohammadi, N.S.; Khiabani, M.S.; Ghanbarzadeh, B.; Mokarram, R.R. Improvement of Lipase Biochemical Properties via a Two-Step Immobilization Method: Adsorption onto Silicon Dioxide Nanoparticles and Entrapment in a Polyvinyl Alcohol/Alginate Hydrogel. J. Biotechnol. 2020, 323, 189–202. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Bhanage, B.M. Immobilization of Lipase on Biocompatible Co-Polymer of Polyvinyl Alcohol and Chitosan for Synthesis of Laurate Compounds in Supercritical Carbon Dioxide Using Response Surface Methodology. Process Biochem. 2015, 50, 1224–1236. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Bhanage, B.M. Investigation of Deactivation Thermodynamics of Lipase Immobilized on Polymeric Carrier. Bioprocess Biosyst. Eng. 2017, 40, 741–757. [Google Scholar] [CrossRef]
- Tan, T.; Wang, F.; Zhang, H. Preparation of PVA/Chitosan Lipase Membrane Reactor and Its Application in Synthesis of Monoglyceride. J. Mol. Catal. B Enzym. 2002, 18, 325–331. [Google Scholar] [CrossRef]
- Batista, K.A.; Lopes, F.M.; Yamashita, F.; Fernandes, K.F. Lipase Entrapment in PVA/Chitosan Biodegradable Film for Reactor Coatings. Mater. Sci. Eng. C 2013, 33, 1696–1701. [Google Scholar] [CrossRef]
- Mathpati, A.C.; Vyas, V.K.; Bhanage, B.M. Kinetic Resolution of 1,2-Diols Using Immobilized Burkholderia Cepacia Lipase: A Combined Experimental and Molecular Dynamics Investigation. J. Biotechnol. 2017, 262, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mathpati1, A.; Kalghatgi, S.; Mathpati, C.; Bhanage, B. Immobilized Lipase Catalyzed Synthesis of N-Amyl Acetate: Parameter Optimization, Heterogeneous Kinetics, Continuous Flow Operation and Reactor Modeling. J. Organ. Behav. 2018, 93, 2906–2916. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Bhanage, B.M. Solvent Stability Study with Thermodynamic Analysis and Superior Biocatalytic Activity of Burkholderia Cepacia Lipase Immobilized on Biocompatible Hybrid Matrix of Poly(Vinyl Alcohol) and Hypromellose. J. Phys. Chem. B 2014, 118, 14808–14819. [Google Scholar] [CrossRef]
- Martin, L.S.; Ceron, A.; Oliveira, P.C.; Zanin, G.M.; de Castro, H.F. Different Organic Components on Silica Hybrid Matrices Modulate the Lipase Inhibition by the Glycerol Formed in Continuous Transesterification Reactions. J. Ind. Eng. Chem. 2018, 62, 462–470. [Google Scholar] [CrossRef]
- Vilas Bôas, R.N.; Lima, R.; Silva, M.V.C.; Freitas, L.; Aguiar, L.G.; de Castro, H.F. Continuous Production of Monoacylglycerol via Glycerolysis of Babassu Oil by Immobilized Burkholderia cepacia Lipase in a Packed Bed Reactor. Bioprocess Biosyst. Eng. 2021, 44, 2205–2215. [Google Scholar] [CrossRef]
- Paula, A.V.; Urioste, D.; Santos, J.C.; de Castro, H.F. Porcine Pancreatic Lipase Immobilized on Polysiloxane–Polyvinyl Alcohol Hybrid Matrix: Catalytic Properties and Feasibility to Mediate Synthesis of Surfactants and Biodiesel. J. Chem. Technol. Biotechnol. 2008, 83, 1163–1169. [Google Scholar] [CrossRef]
- de Castro, M.D.C.; Garcia, P.S.; Andrade, M.M.; Grossmann, M.V.E.; Simões, B.M.; Samulewski, R.B.; Baron, A.M. Lipase Immobilization on Biodegradable Film with Sericin. Biotechnol. Appl. Biochem. 2022, 69, 660–667. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, Y.; Singh, K.; Bhattacharya, A. Lipase Immobilized on Poly(Vinyl Alcohol) Modified Polysulfone Membrane: Application in Hydrolytic Activities for Olive Oil. Polym. Bull. 2010, 64, 141–158. [Google Scholar] [CrossRef]
- Kartal, F.; Akkaya, A.; Kilinc, A. Immobilization of Porcine Pancreatic Lipase on Glycidyl Methacrylate Grafted Poly Vinyl Alcohol. J. Mol. Catal. B Enzym. 2009, 57, 55–61. [Google Scholar] [CrossRef]
- Pandey, G.; Munguambe, D.M.; Tharmavaram, M.; Rawtani, D.; Agrawal, Y.K. Manuscript Title: Halloysite Nanotubes—An Efficient ‘Nano-Support’ for the immobilization of α-Amylase. Appl. Clay Sci. 2017, 136, 184–191. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Wang, S.; Tao, S.; Ai, N.; Wang, Y. Fabrication of Enzyme-Immobilized Halloysite Nanotubes for Affinity Enrichment of Lipase Inhibitors from Complex Mixtures. J. Chromatogr. A 2015, 1392, 20–27. [Google Scholar] [CrossRef]
- Bergamasco, J.; de Araujo, M.V.; de Vasconcellos, A.; Luizon Filho, R.A.; Hatanaka, R.R.; Giotto, M.V.; Aranda, D.A.G.; Nery, J.G. Enzymatic Transesterification of Soybean Oil with Ethanol Using Lipases Immobilized on Highly Crystalline PVA Microspheres. Biomass Bioenergy 2013, 59, 218–233. [Google Scholar] [CrossRef]
- Piacentini, E.; Yan, M.; Giorno, L. Development of Enzyme-Loaded PVA Microspheres by Membrane Emulsification. J. Membr. Sci. 2017, 524, 79–86. [Google Scholar] [CrossRef]
- Bilal, M.; Rashid, E.U.; Munawar, J.; Iqbal, H.M.N.; Cui, J.; Zdarta, J.; Ashraf, S.S.; Jesionowski, T. Magnetic Metal-Organic Frameworks Immobilized Enzyme-Based Nano-Biocatalytic Systems for Sustainable Biotechnology—A Review. Int. J. Biol. Macromol. 2023, 237, 123968. [Google Scholar] [CrossRef]
- Manikandan, S.K.; Giannakoudakis, D.A.; Prekodravac, J.R.; Nair, V.; Colmenares, J.C. Role of Catalyst Supports in Biocatalysis. J. Chem. Technol. Biotechnol. 2023, 98, 7–21. [Google Scholar] [CrossRef]
- Khalid, N.; Kalsoom, U.; Ahsan, Z.; Bilal, M. Non-Magnetic and Magnetically Responsive Support Materials Immobilized Peroxidases for Biocatalytic Degradation of Emerging Dye Pollutants—A Review. Int. J. Biol. Macromol. 2022, 207, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Mijone, P.D.; Bôas, R.N.V.; Bento, H.B.S.; Reis, C.E.R.; de Castro, H.F. Coating and Incorporation of Iron Oxides into a Magnetic-Polymer Composite to Be Used as Lipase Support for Ester Syntheses. Renew. Energy 2020, 149, 1167–1173. [Google Scholar] [CrossRef]
- Silva, M.V.C.; Aguiar, L.G.; de Castro, H.F.; Freitas, L. Optimization of the Parameters That Affect the Synthesis of Magnetic Co-polymer Styrene-Divinilbezene to Be Used as Efficient Matrix for Immobilizing Lipases. World J. Microbiol. Biotechnol. 2018, 34, 169. [Google Scholar] [CrossRef] [PubMed]




| Support Types or Immobilization Types | Advantages | Disadvantages | Binding |
|---|---|---|---|
| Crosslinking enzyme aggregates (CLEA) | High stability and high activity | Low mechanical resistance | Covalent |
| Immobead 150 P (Co-polymer of methacrylate) | High mechanical resistance, high stability, scalability | Low activity | Covalent |
| Accurel MP 1000 | High activity, scalability | Leaching | Adsorption |
| Lewatit VP OC 1600 | High activity, scalability | Leaching | Adsorption |
| Alginate | Avoids agglomeration, biodegradable | Low mechanical resistance | Entrapment |
| Poly(vinyl) alcohol | High mechanical resistance, scalability | Mass transfer problems | Entrapment |
| Chitosan | High degree of functionalization, Biodegradable, high stability | Mass transfer problems, low activity | Entrapment, covalent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guajardo, N. Immobilization of Lipases Using Poly(vinyl) Alcohol. Polymers 2023, 15, 2021. https://doi.org/10.3390/polym15092021
Guajardo N. Immobilization of Lipases Using Poly(vinyl) Alcohol. Polymers. 2023; 15(9):2021. https://doi.org/10.3390/polym15092021
Chicago/Turabian StyleGuajardo, Nadia. 2023. "Immobilization of Lipases Using Poly(vinyl) Alcohol" Polymers 15, no. 9: 2021. https://doi.org/10.3390/polym15092021
APA StyleGuajardo, N. (2023). Immobilization of Lipases Using Poly(vinyl) Alcohol. Polymers, 15(9), 2021. https://doi.org/10.3390/polym15092021