Structural Rearrangements of Polylactide/Natural Rubber Composites during Hydro- and Biotic Degradation
Abstract
1. Introduction
- the diffusion of water into the material;
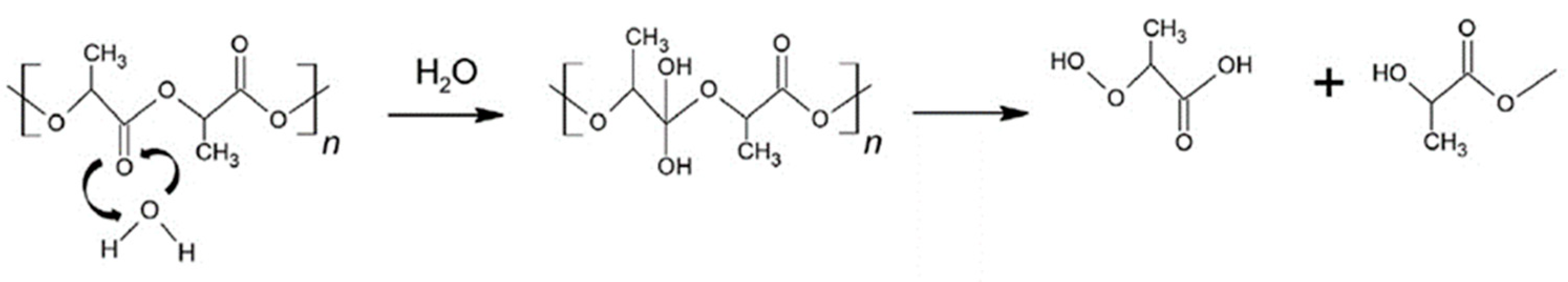
- the hydrolysis of chains in the amorphous region due to the lower resistance to water;
- the reducing of molecular weight due to the hydrolytic cleavage of side bonds and the formation of water-soluble compounds;
- the hydrolysis of the crystalline phase, which can occur by an autocatalytic mechanism due to acidic decomposition products as well as due to an increase in the concentration of carboxylic acid at the end of the chain.
2. Materials and Methods
2.1. Sample Preparation
2.2. Analysis of Crystallization
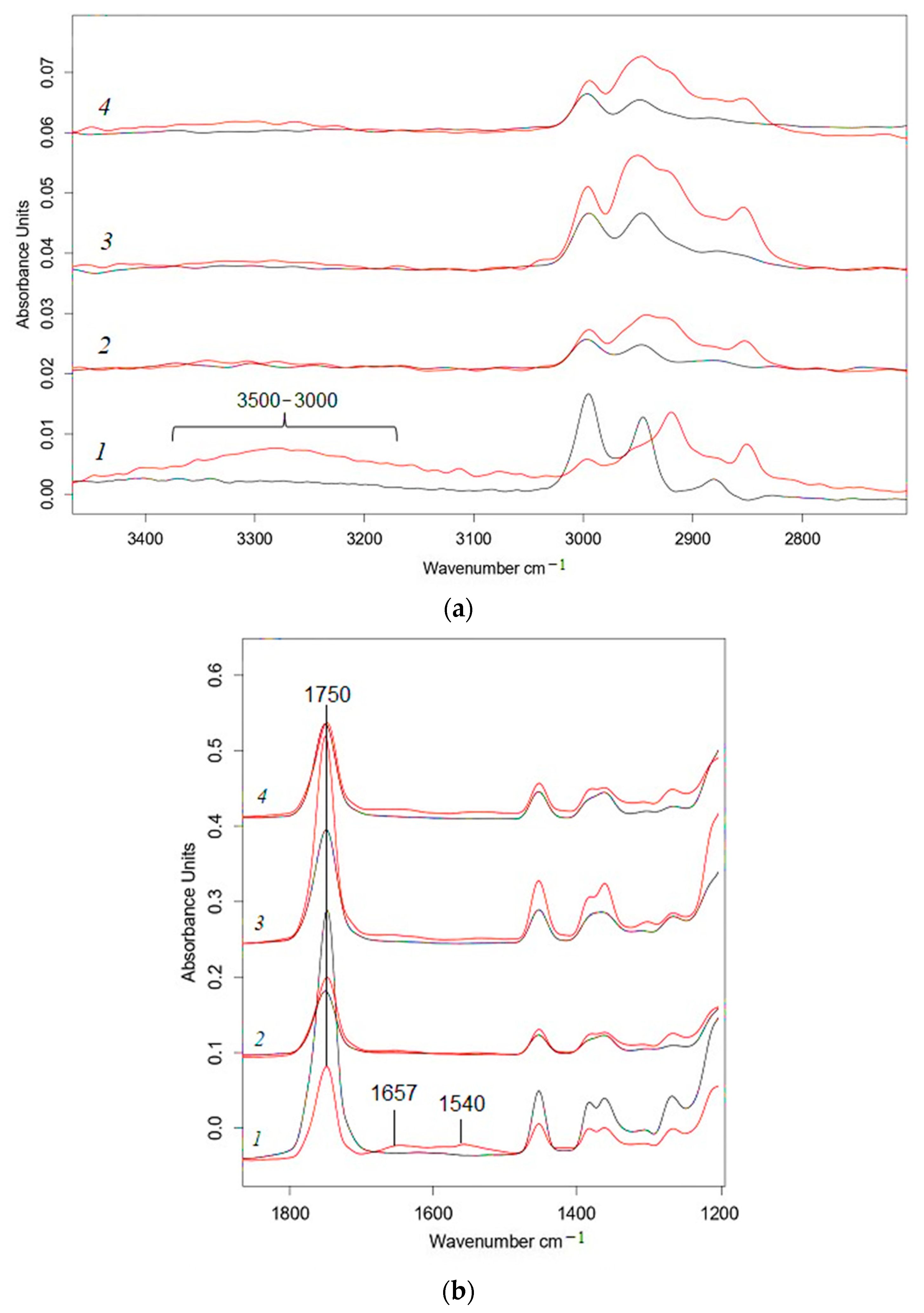
2.3. FTIR Spectroscopy
2.4. Morphology
2.5. Biotic Degradation
2.6. Water Test
2.7. X-ray Diffraction
2.8. Statistical Processing
3. Results and Discussion
4. Conclusions
- -
- As it stands, 100% PLA is well hydrolyzed in distilled water, and PLA/NR films are more actively subjected to biotic degradation compared to pure polylactide;
- -
- The influence of natural rubber on the structure and thermophysical characteristics of the PLA matrix and on the behavior of PLA/NR composites during degradation is shown;
- -
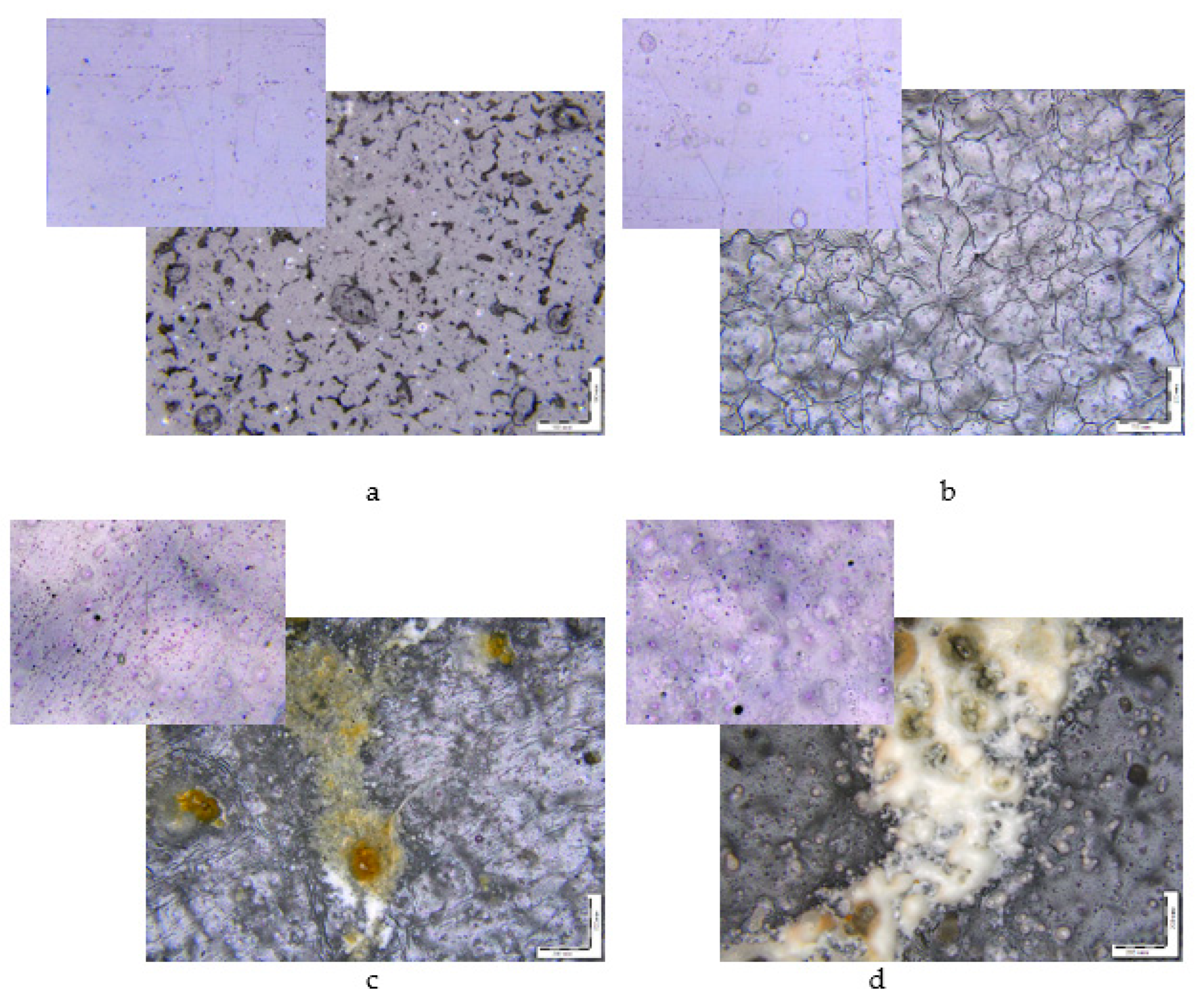
- The morphological observation indicated the surface erosion of all samples both during biotic and hydrolytic degradation;
- -
- Differences in the crystal structure of PLA in highly defective and less damaged film regions were detected by the XRD method;
- -
- FTIR-ATP spectra demonstrated a significant change in the chemical structure of PLA/NR film composites during hydro- and bio-degradation;
- -
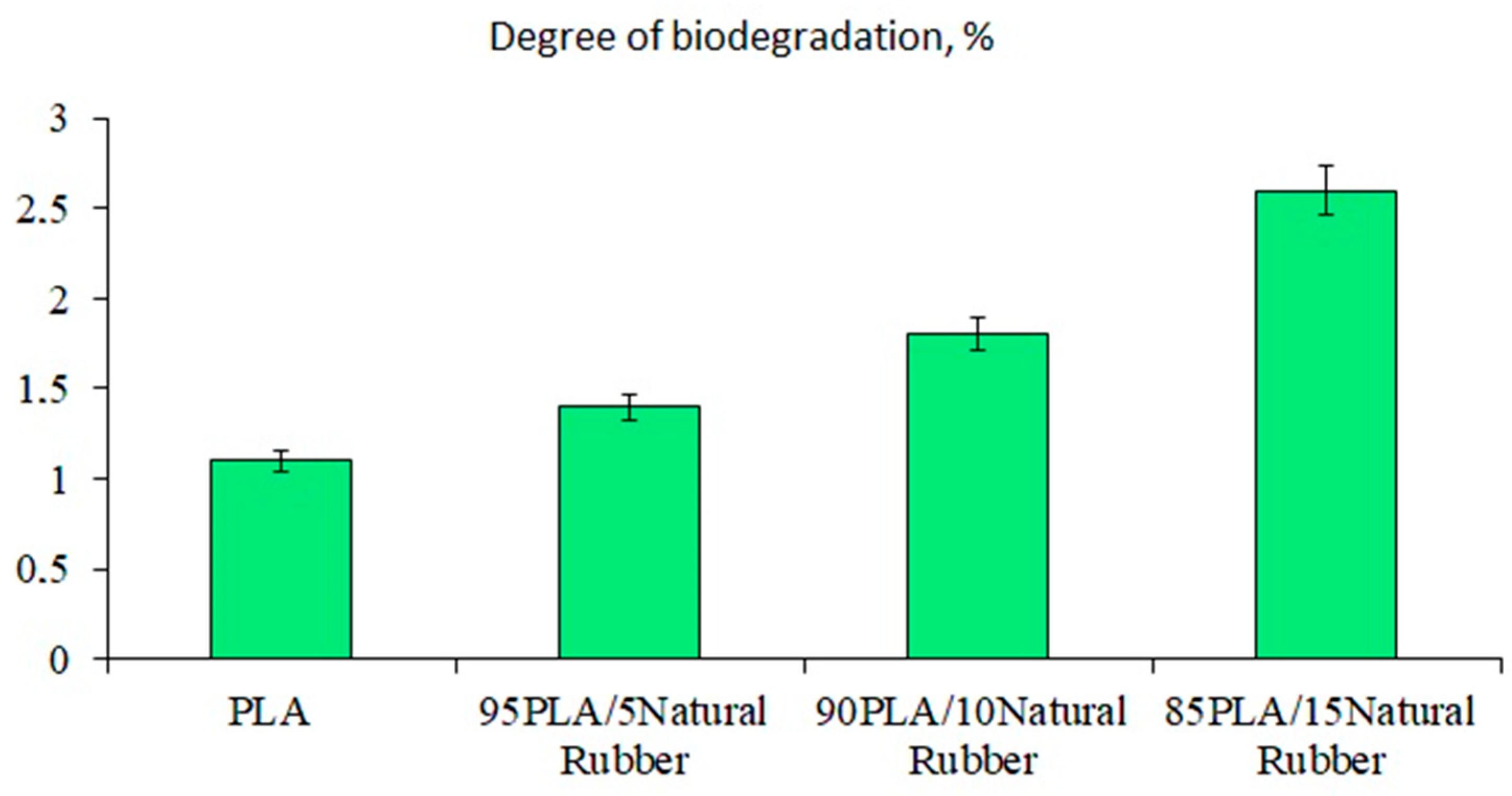
- Adding NR increases the degree of biodegradation of PLA/NR composites due to the formation of a heterogeneous system and an increase in the proportion of the amorphous phase in the samples.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piemonte, V.; Gironi, F. Kinetics of Hydrolytic Degradation of PLA. J. Polym. Environ. 2013, 21, 313–318. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.V.; Karpova, S.G.; Popov, A.A. Effect of Aqueous Medium on the Molecular Mobility of Polylactide. Russ. J. Phys. Chem. B 2017, 11, 531–537. [Google Scholar] [CrossRef]
- Deroiné, M.; le Duigou, A.; Corre, Y.M.; le Gac, P.Y.; Davies, P.; César, G.; Bruzaud, S. Accelerated Ageing of Polylactide in Aqueous Environments: Comparative Study between Distilled Water and Seawater. Polym. Degrad. Stab. 2014, 108, 319–329. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.; Podzorova, M.; Moskovskiy, M. Impact of Water and UV Irradiation on Nonwoven Polylactide/Natural Rubber Fiber. Polymers 2021, 13, 461. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Jia, J.; Liu, M.; Peng, S.; Zhao, Z.; Shuai, C. Degradation Mechanisms and Acceleration Strategies of Poly (Lactic Acid) Scaffold for Bone Regeneration. Mater. Des. 2021, 210, 110066. [Google Scholar] [CrossRef]
- Rosli, N.A.; Karamanlioglu, M.; Kargarzadeh, H.; Ahmad, I. Comprehensive Exploration of Natural Degradation of Poly(Lactic Acid) Blends in Various Degradation Media: A Review. Int. J. Biol. Macromol. 2021, 187, 732–741. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and Biotic Environmental Degradation of the Bioplastic Polymer Poly(Lactic Acid): A Review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar] [CrossRef]
- Kliem, S.; Kreutzbruck, M.; Bonten, C. Review on the Biological Degradation of Polymers in Various Environments. Materials 2020, 13, 4586. [Google Scholar] [CrossRef]
- Iozzino, V.; Askanian, H.; Leroux, F.; Verney, V.; Pantani, R. Poly(Lactic Acid)-Based Nanobiocomposites with Modu-lated Degradation Rates. Materials 2018, 11, 1943. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Robson, G.D. The Influence of Biotic and Abiotic Factors on the Rate of Degradation of Poly(Lactic) Acid (PLA) Coupons Buried in Compost and Soil. Polym. Degrad. Stab. 2013, 98, 2063–2071. [Google Scholar] [CrossRef]
- Richert, A.; Dąbrowska, G.B. Enzymatic Degradation and Biofilm Formation during Biodegradation of Polylactide and Polycaprolactone Polymers in Various Environments. Int. J Biol. Macromol. 2021, 176, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Bubpachat, T.; Sombatsompop, N.; Prapagdee, B. Isolation and role of polylactic acid-degrading bacteria on degrading enzymes productions and PLA biodegradability at mesophilic conditions. Polym. Degrad. Stab. 2018, 152, 75–85. [Google Scholar] [CrossRef]
- Decorosi, F.; Exana, M.L.; Pini, F.; Adessi, A.; Messini, A.; Giovannetti, L.; Viti, C. The Degradative Capabilities of New Amycolatopsis Isolates on Polylactic Acid. Microorganisms 2019, 7, 590. [Google Scholar] [CrossRef] [PubMed]
- Muroi, F.; Tachibana, Y.; Soulenthone, P.; Yamamoto, K.; Mizuno, T.; Sakurai, T.; Kobayashi, Y.; Kasuya, K.i. Characterization of a Poly(Butylene Adipate-Co-Terephthalate) Hydrolase from the Aerobic Mesophilic Bacterium Bacillus Pumilus. Polym. Degrad. Stab. 2017, 137, 11–22. [Google Scholar] [CrossRef]
- Odobel, C.; Dussud, C.; Philip, L.; Derippe, G.; Lauters, M.; Eyheraguibel, B.; Burgaud, G.; ter Halle, A.; Meistertzheim, A.L.; Bruzaud, S.; et al. Bacterial Abundance, Diversity and Activity During Long-Term Colonization of Non-Biodegradable and Biodegradable Plastics in Seawater. Front. Microbiol. 2021, 12, 734782. [Google Scholar] [CrossRef]
- Gu, J.D. Microbiological Deterioration and Degradation of Synthetic Polymeric Materials: Recent Research Advances. Int. Biodeterior. Biodegrad. 2003, 52, 69–91. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and Biodegradation of Poly(Lactide). Appl. Microbiol. Biotech. 2006, 72, 244–251. [Google Scholar] [CrossRef]
- Tarach, I.; Olewnik-Kruszkowska, E.; Richert, A.; Gierszewska, M.; Rudawska, A. Influence of Tea Tree Essential Oil and Poly(Ethylene Glycol) on Antibacterial and Physicochemical Properties of Polylactide-Based Films. Materials 2020, 13, 4953. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R. Hydrolysis and Biodegradation of Poly(lactic acid). In Synthesis, Structure and Properties of Poly(lactic acid); Di Lorenzo, M.L., Androsch, R., Eds.; Springer: Cham, Switzerland, 2018; pp. 119–151. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E. Influence of the Type of Buffer Solution on Thermal and Structural Properties of Polylactide-Based Composites. Polym. Degrad. Stab. 2016, 129, 87–95. [Google Scholar] [CrossRef]
- Lim, B.K.H.; Thian, E.S. Biodegradation of Polymers in Managing Plastic Waste—A Review. Sci. Total Environ. 2022, 813, 151880. [Google Scholar] [CrossRef]
- Li, S.; Girard, A.; Garreau, H.; Vert, M. Enzymatic Degradation of Polylactide Stereocopolymers with Predominant D-Lactyl Contents. Polym. Degrad. Stab. 2000, 71, 61–67. [Google Scholar] [CrossRef]
- Si, W.J.; Yuan, W.Q.; Li, Y.D.; Chen, Y.K.; Zeng, J.B. Tailoring Toughness of Fully Biobased Poly(Lactic Acid)/Natural Rubber Blends through Dynamic Vulcanization. Polym. Test. 2018, 65, 249–255. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.V.; Podzorova, M.V.; Varyan, I.A.; Tcherdyntsev, V.V.; Zadorozhnyy, M.Y.; Medvedeva, E.V. Promising Agromaterials Based on Biodegradable Polymers: Polylactide and Poly-3-Hydroxybutyrate. Polymers 2023, 15, 1029. [Google Scholar] [CrossRef]
- Pongtanayut, K.; Thongpin, C.; Santawitee, O. The Effect of Rubber on Morphology, Thermal Properties and Mechanical Properties of PLA/NR and PLA/ENR Blends. Energy Procedia 2013, 34, 888–897. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Piorkowska, E. Mechanisms of Plastic Deformation in Biodegradable Polylactide/Poly(1,4- Cis-Isoprene) Blends. J. Appl. Polym. Sci. 2012, 124, 4579–4589. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.V.; Karpova, S.G.; Podzorova, M.V.; Khvatov, A.V.; Moskovskiy, M.N. Thermal Properties and Dynamic Characteristics of Electrospun Polylactide/Natural Rubber Fibers during Disintegration in Soil. Polymers 2022, 14, 1058. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Krivandin, A.V.; Solov’Eva, A.B.; Glagolev, N.N.; Shatalova, O.V.; Kotova, S.L. Structure Alterations of Perfluorinated Sulfocationic Membranes under the Action of Ethylene Glycol (SAXS and WAXS Studies). Polymer 2003, 44, 5789–5796. [Google Scholar] [CrossRef]
- Krivandin, A.V.; Fatkullina, L.D.; Shatalova, O.V.; Goloshchapov, A.N.; Burlakova, E.B. Small-Angle X-Ray Scattering Study of the Incorporation of ICHPHAN Antioxidant in Liposomes. Russ. J. Phys. Chem. B 2013, 7, 338–342. [Google Scholar] [CrossRef]
- Ghorpade, V.M.; Gennadios, A.; Hanna, M.A. Laboratory Composting of Extruded Poly(Lactic Acid) Sheets. Bioresour. Technol. 2001, 76, 57–61. [Google Scholar] [CrossRef]
- Longieras, A.; Tanchette, J.B.; Erre, D.; Braud, C.; Copinet, A. Compostability of Poly(Lactide): Degradation in an Inert Solid Medium. J. Polym. Environ. 2007, 15, 200–206. [Google Scholar] [CrossRef]
- Itävaara, M.; Karjomaa, S.; Selin, J.F. Biodegradation of Polylactide in Aerobic and Anaerobic Thermophilic Conditions. Chemosphere 2002, 46, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Yikmis, M.; Steinbüchel, A. Historical and Recent Achievements in the Field of Microbial Degradation of Natural and Synthetic Rubber. Appl. Environ. Microbiol. 2012, 78, 4543–4551. [Google Scholar] [CrossRef] [PubMed]
- Ali Shah, A.; Hasan, F.; Shah, Z.; Kanwal, N.; Zeb, S. Biodegradation of Natural and Synthetic Rubbers: A Review. Int. Biodeterior. Biodegrad. 2013, 83, 145–157. [Google Scholar] [CrossRef]
- Nanthini, J.; Sudesh, K. Biodegradation of Natural Rubber and Natural Rubber Products by Streptomyces Sp. Strain CFMR 7. J. Polym. Environ. 2017, 25, 606–616. [Google Scholar] [CrossRef]
- Varyan, I.; Kolesnikova, N.; Xu, H.; Tyubaeva, P.; Popov, A. Biodegradability of Polyolefin-Based Compositions: Effect of Natural Rubber. Polymers 2022, 14(3), 530. [Google Scholar] [CrossRef]
- Karpova, S.G.; Tertyshnaya, Y.V.; Podzorova, M.V.; Popov, A.A. Effect of Exposure in Aqueous Medium at Elevated Temperature on the Structure of Nonwoven Materials Based on Polylactide and Natural Rubber. Polym. Sci. Ser. A 2021, 63, 515–525. [Google Scholar] [CrossRef]
- Kuleznev, V.N.; Shershnev, V.A. Chemistry and Physics of Polymers; Lan Publishing: St. Peresburg, Russia, 2014; 368p. [Google Scholar]
- Satti, S.M.; Shah, A.A.; Auras, R.; Marsh, T.L. Isolation and Characterization of Bacteria Capable of Degrading Poly(Lactic Acid) at Ambient Temperature. Polym. Degrad. Stab. 2017, 144, 392–400. [Google Scholar] [CrossRef]
- Kale, G.; Auras, R.; Singh, S.P. Comparison of the Degradability of Poly(Lactide) Packages in Composting and Ambient Exposure Conditions. Pack. Technol. Sci. 2007, 20, 49–70. [Google Scholar] [CrossRef]
- Podzorova, M.V.; Tertyshnaya, Y.V.; Ziborov, D.M.; Poletto, M. Damage of Polymer Blends Polylactide-Polyethylene under the Effect of Ultraviolet Irradiation. AIP Conf. Proc. 2020, 2310, 020259. [Google Scholar] [CrossRef]
- Hoogsteen, W.; Postema, A.R.; Pennings, A.J.; ten Brinke, G.; Zugenmaier, P. Crystal Structure, Conformation, and Morphology of Solution-Spun Poly(L-Lactide) Fibers. Macromolecules 1990, 23, 634–642. [Google Scholar] [CrossRef]
- Yasuniwa, M.; Tsubakihara, S.; Iura, K.; Ono, Y.; Dan, Y.; Takahashi, K. Crystallization Behavior of Poly(l-Lactic Acid). Polymer 2006, 47, 7554–7563. [Google Scholar] [CrossRef]
- Luo, Y.B.; Wang, X.L.; Wang, Y.Z. Effect of TiO2 Nanoparticles on the Long-Term Hydrolytic Degradation Behavior of PLA. Polym. Degrad. Stab. 2012, 97, 721–728. [Google Scholar] [CrossRef]
- Noor, H.; Satti, S.M.; ud Din, S.; Farman, M.; Hasan, F.; Khan, S.; Badshah, M.; Shah, A.A. Insight on Esterase from Pseudomonas Aeruginosa Strain S3 That Depolymerize Poly(Lactic Acid) (PLA) at Ambient Temperature. Polym. Degrad. Stab. 2020, 174, 109096. [Google Scholar] [CrossRef]
- Mistry, A.N.; Kachenchart, B.; Wongthanaroj, A.; Somwangthanaroj, A.; Luepromchai, E. Rapid Biodegradation of High Molecular Weight Semi-Crystalline Polylactic Acid at Ambient Temperature via Enzymatic and Alkaline Hydrolysis by a Defined Bacterial Consortium. Polym. Degrad. Stab. 2022, 202, 110051. [Google Scholar] [CrossRef]
- Ndazi, B.S.; Karlsson, S. Characterization of Hydrolytic Degradation of Polylactic Acid/Rice Hulls Composites in Water at Different Temperatures. Express Polym. Lett. 2011, 5, 119–131. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Rayón, E.; Jiménez, A. Disintegrability under Composting Conditions of Plasticized PLA–PHB Blends. Polym. Degrad. Stab. 2014, 108, 307–318. [Google Scholar] [CrossRef]
- Copinet, A.; Bertrand, C.; Govindin, S.; Coma, V.; Couturier, Y. Effects of Ultraviolet Light (315 Nm), Temperature and Relative Humidity on the Degradation of Polylactic Acid Plastic Films. Chemosphere 2004, 55, 763–773. [Google Scholar] [CrossRef]
- Maquelin, K.; Kirschner, C.; Choo-Smith, L.-P.; van den Braak, N.; Endtz, H.P.; Naumann, D.; Puppels, G.J. Identification of Medically Relevant Microorganisms by Vibrational Spectroscopy. J. Microbiol. Methods 2002, 51, 255–271. [Google Scholar] [CrossRef]
- Schmitt, J.; Flemming, H.C. FTIR-Spectroscopy in Microbial and Material Analysis. Int. Biodeterior. Biodegrad. 1998, 41, 1–11. [Google Scholar] [CrossRef]

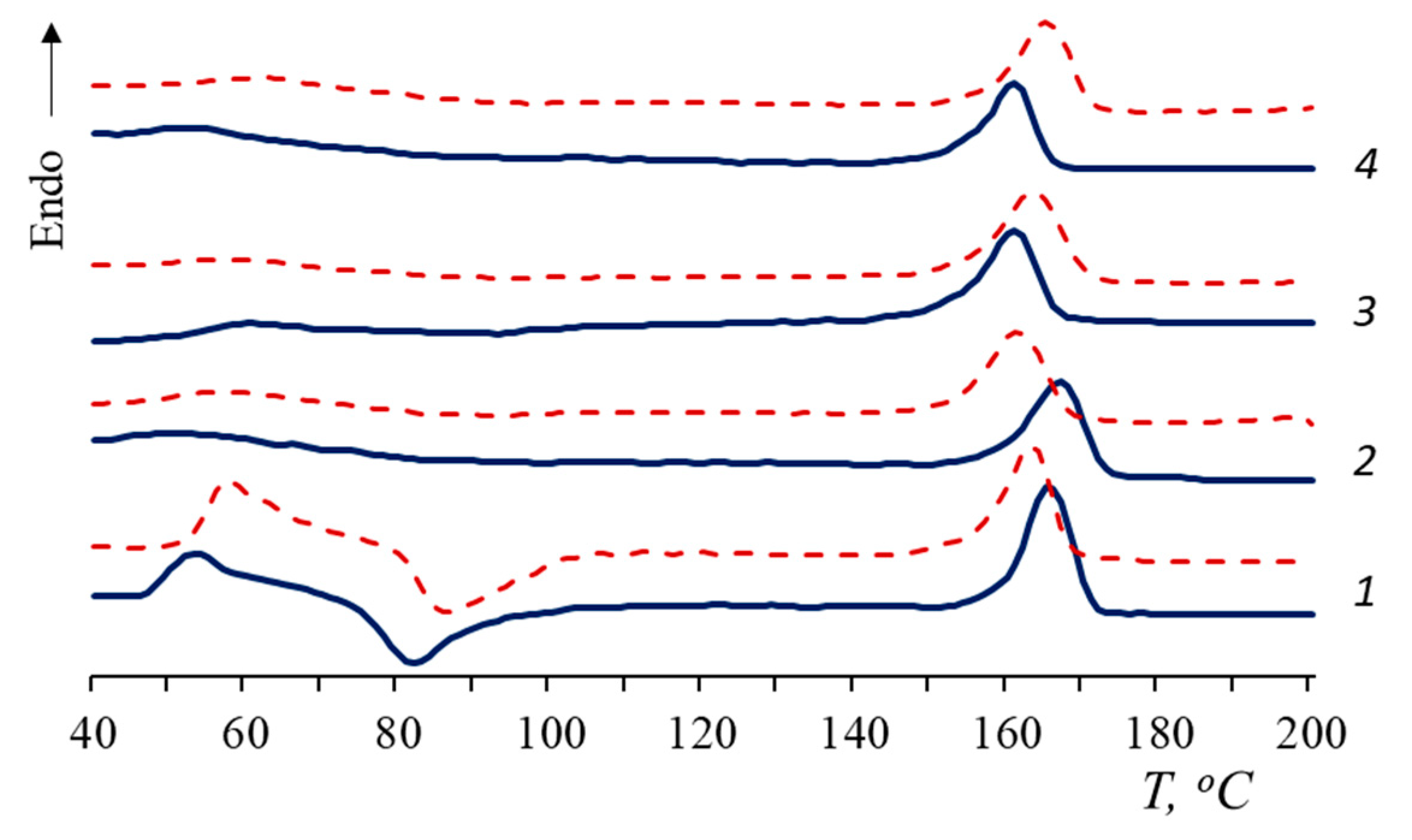
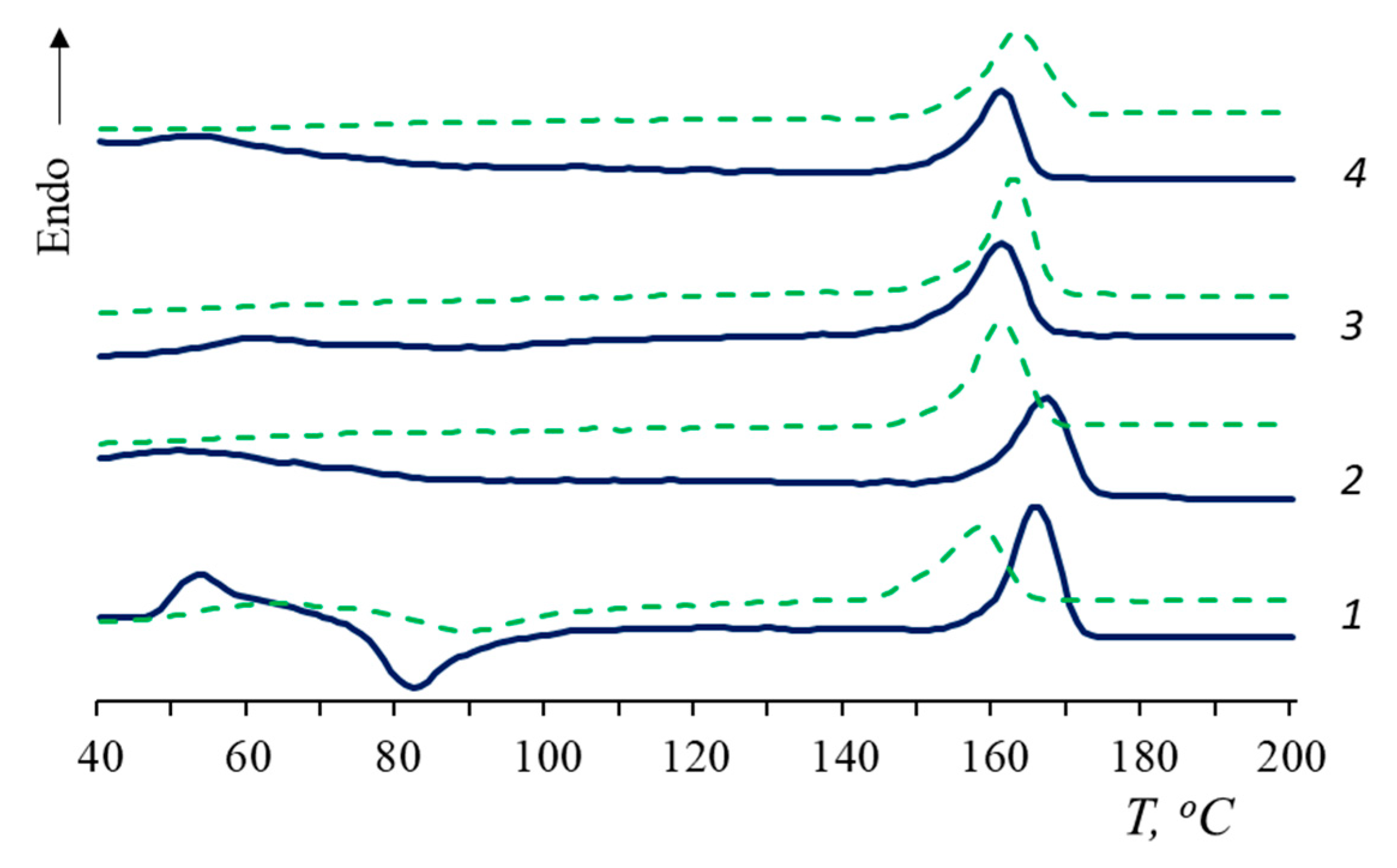


| NR Content, wt.% | Tg, °C (Δ ± 0.5 °C) | Tg, °C (Δ ± 0.5 °C) Sturm | Tg, °C (Δ ± 0.5 °C) Water | Tcc, °C (Δ ± 0.5 °C) | Tcc, °C (Δ ± 0.5 °C) Sturm | Tcc, °C (Δ ± 0.5 °C) Water | Tm, °C (Δ ± 0.5 °C) | Tm, °C (Δ ± 0.5 °C) Sturm | Tm, °C (Δ ± 0.5 °C) Water | χ, % (Δ ± 0.5%) | χ, % (Δ ± 0.5%) Sturm | χ, % (Δ ± 0.5%) Water |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 48 | 52 | - | 81 | 85 | 89 | 165 | 163 | 158 | 22 | 20 | 23 |
| 5 | (48) | - | - | - | - | - | 165 | 162 | 161 | 31 | 29 | 31 |
| 10 | (50) | - | - | - | - | - | 161 | 163 | 163 | 30 | 28 | 32 |
| 15 | (51) | - | - | - | - | - | 161 | 164 | 164 | 30 | 26 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tertyshnaya, Y.V.; Podzorova, M.V.; Khramkova, A.V.; Ovchinnikov, V.A.; Krivandin, A.V. Structural Rearrangements of Polylactide/Natural Rubber Composites during Hydro- and Biotic Degradation. Polymers 2023, 15, 1930. https://doi.org/10.3390/polym15081930
Tertyshnaya YV, Podzorova MV, Khramkova AV, Ovchinnikov VA, Krivandin AV. Structural Rearrangements of Polylactide/Natural Rubber Composites during Hydro- and Biotic Degradation. Polymers. 2023; 15(8):1930. https://doi.org/10.3390/polym15081930
Chicago/Turabian StyleTertyshnaya, Yulia V., Maria V. Podzorova, Anastasia V. Khramkova, Vasily A. Ovchinnikov, and Aleksey V. Krivandin. 2023. "Structural Rearrangements of Polylactide/Natural Rubber Composites during Hydro- and Biotic Degradation" Polymers 15, no. 8: 1930. https://doi.org/10.3390/polym15081930
APA StyleTertyshnaya, Y. V., Podzorova, M. V., Khramkova, A. V., Ovchinnikov, V. A., & Krivandin, A. V. (2023). Structural Rearrangements of Polylactide/Natural Rubber Composites during Hydro- and Biotic Degradation. Polymers, 15(8), 1930. https://doi.org/10.3390/polym15081930







