Synthesis of FeOOH-Loaded Aminated Polyacrylonitrile Fiber for Simultaneous Removal of Phenylphosphonic Acid and Phosphate from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
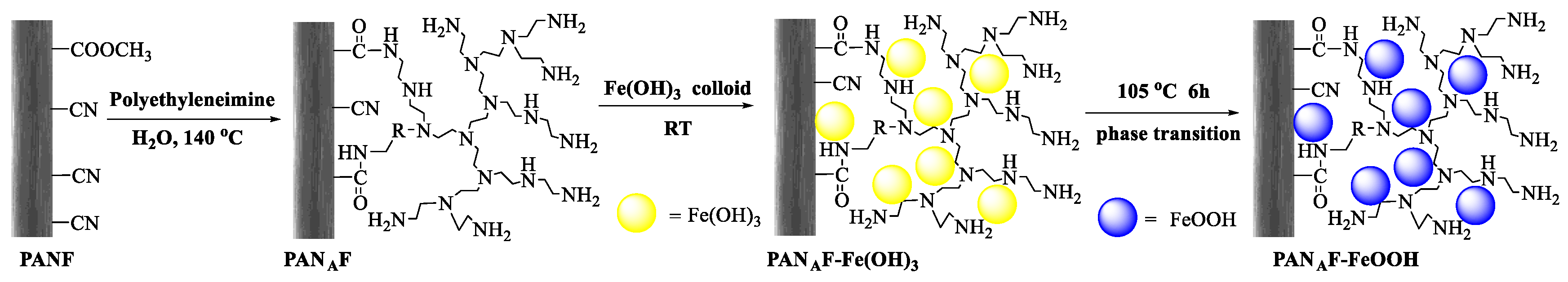
2.2. Preparation of FeOOH-Supported Polyacrylonitrile Fiber (PANAF-FeOOH)
2.3. Batch Degradation Test of PPOA
2.4. Phosphate Adsorption on PANAF-FeOOH
2.5. Analysis and Calculation Methods
3. Results and Discussion
3.1. Synthesis of PANAF-FeOOH
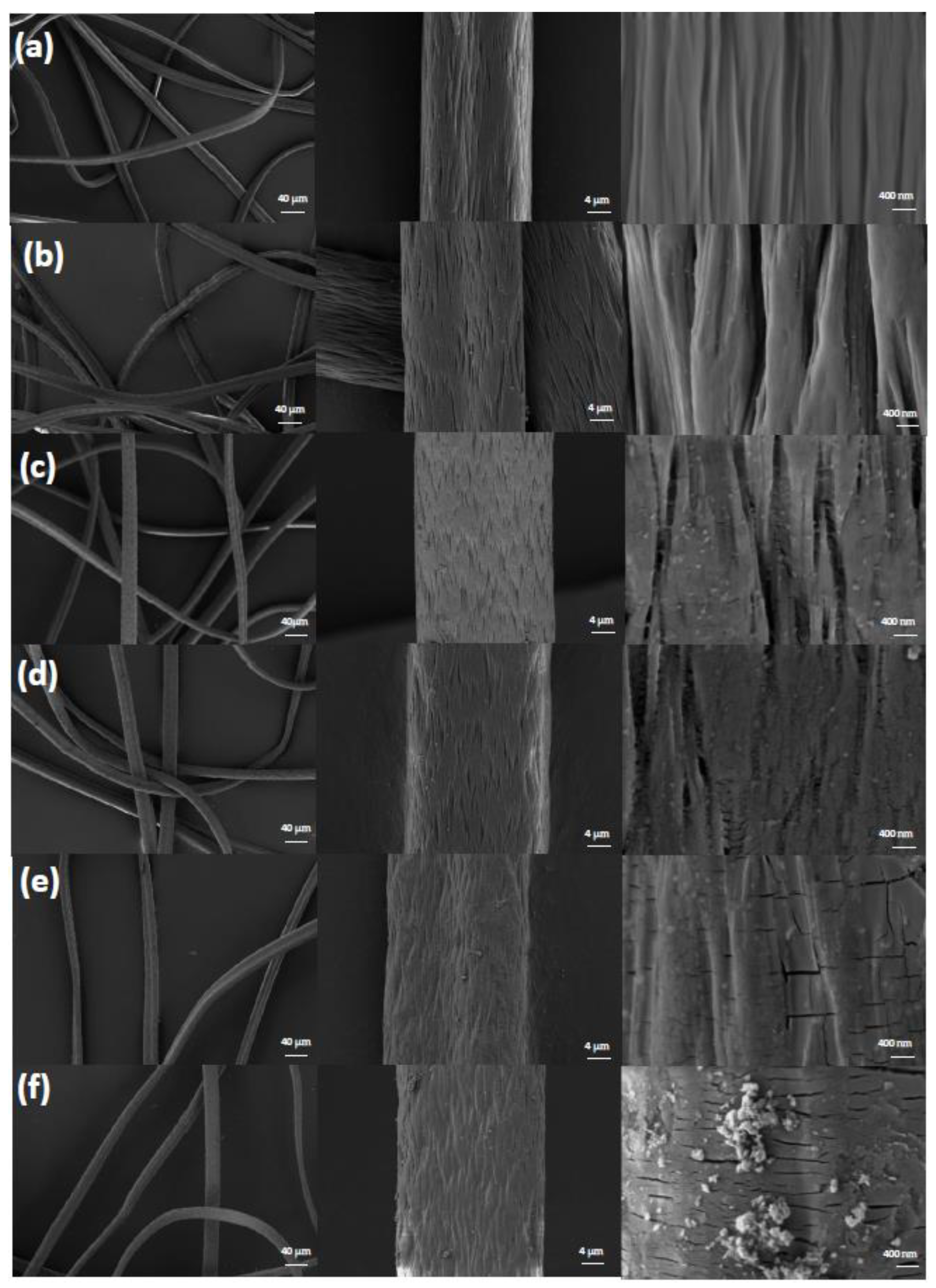
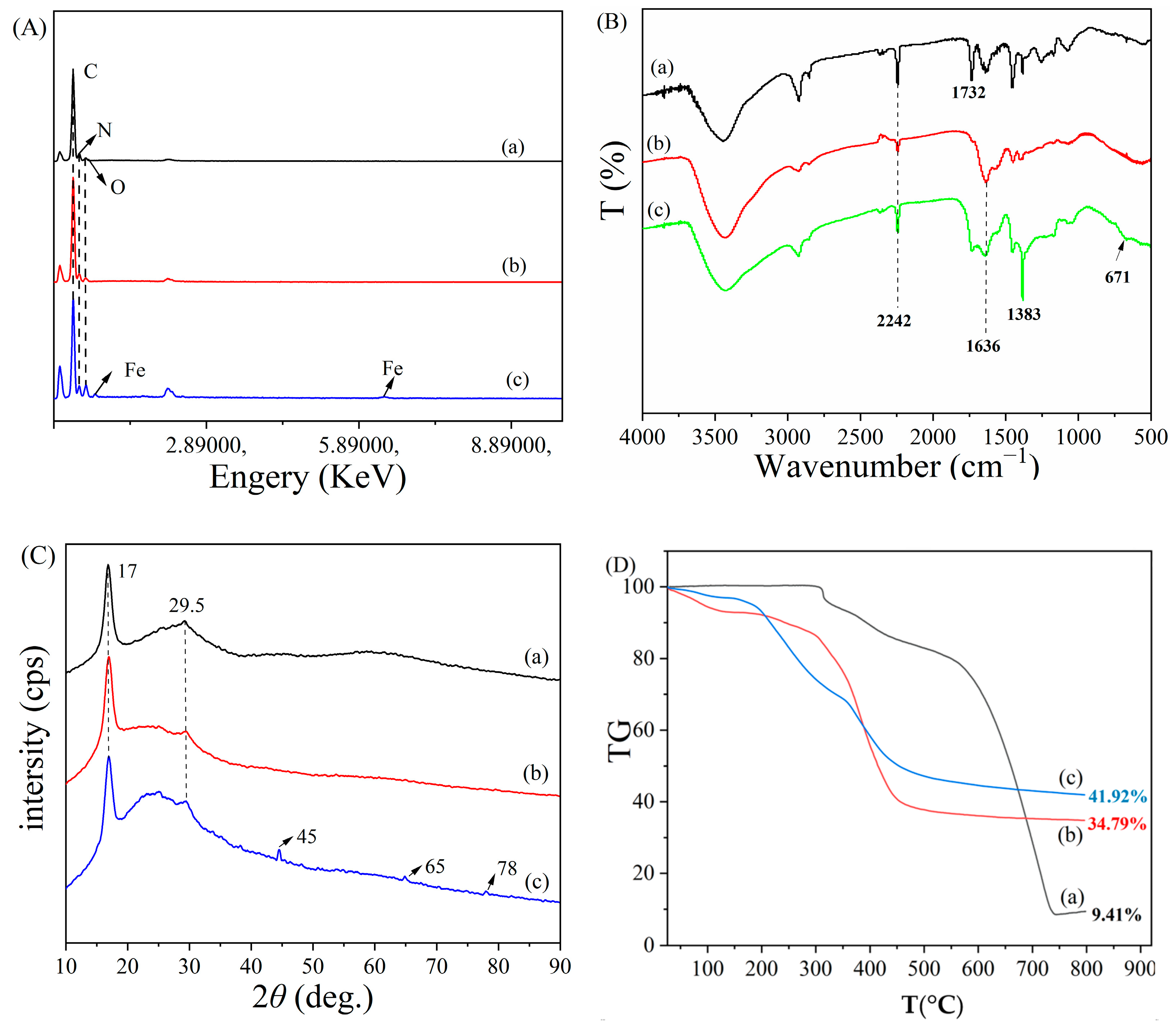
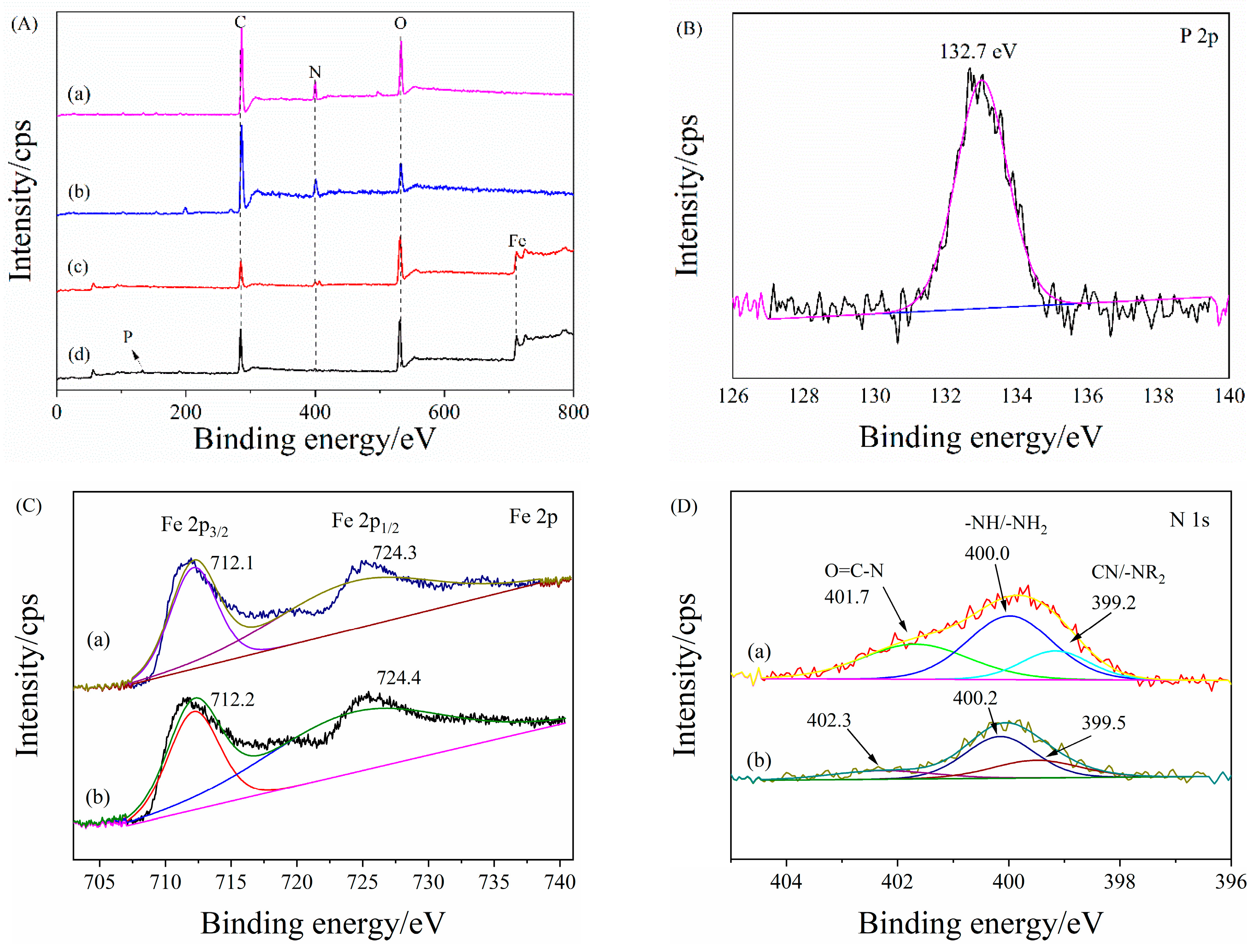
3.2. Characterization of PANAF-FeOOH
Elemental Analysis (EA)
3.3. Batch Experiment of PPOA Degradation by PANAF-FeOOH
3.3.1. Conditional Optimization of PPOA Degradation Promoted by PANAF-FeOOH
3.3.2. Influence of Coexisting Ions and Reusability of PANAF-FeOOH
3.4. Batch Experiment of Phosphate Adsorption by PANAF-FeOOH
3.4.1. Influence of pH
3.4.2. Influence of Coexisting Ions
3.4.3. Adsorption Kinetics and Thermodynamics
3.4.4. Adsorption Isotherm
3.4.5. Reusability and Continuous Flow Application of PANAF-FeOOH
3.5. Mechanisms of PPOA Degradation and Phosphate Adsorption by PANAF-FeOOH
3.6. Comparison of Different FeOOH Phosphate Adsorbents
3.7. Practical Application Capability Test of PANAF-FeOOH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Guo, L.; Shen, W.; An, Q.; Xiao, Z.; Wang, H.; Cai, W.; Zhai, S.; Li, Z. Function integrated chitosan-based beads with throughout sorption sites and inherent diffusion network for efficient phosphate removal. Carbohydr. Polym. 2020, 230, 115639. [Google Scholar] [CrossRef] [PubMed]
- Zong, E.; Huang, G.; Liu, X.; Lei, W.; Jiang, S.; Ma, Z.; Wang, J.; Song, P. A lignin-based nano-adsorbent for superfast and highly selective removal of phosphate. J. Mater. Chem. A 2018, 6, 9971–9983. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, L.; Tang, W.; Zhong, Y.; Luo, K.; Duan, M.; Xing, W.; Liang, J. Removal and recovery of phosphorus from low-strength wastewaters by flow-electrode capacitive deionization. Sep. Purif. Technol. 2020, 237, 116322. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.; Yin, H. Phosphorus internal loading and sediment diagenesis in a large eutrophic lake (Lake Chaohu, China). Environ. Pollut. 2022, 292, 118471. [Google Scholar] [CrossRef] [PubMed]
- Malone, T.C.; Newton, A. The Globalization of Cultural Eutrophication in the Coastal Ocean: Causes and Consequences. Front. Mar. Sci. 2020, 7, 670. [Google Scholar]
- Ni, Z.K.; Wang, S.R.; Zhang, B.T.; Wang, Y.M.; Li, H. Response of sediment organic phosphorus composition to lake trophic status in China. Sci. Total Environ. 2019, 652, 495–504. [Google Scholar] [CrossRef]
- Ajiboye, B.; Akinremi, O.O.; Racz, G.J. Laboratory characterization of phosphorus in fresh and oven-dried organic amendments. J. Environ. Qual. 2004, 33, 1062–1069. [Google Scholar] [CrossRef]
- Dou, Z.; Toth, J.D.; Galligan, D.T.; Ramberg, C.F.; Ferguson, J.D. Laboratory procedures for characterizing manure phosphorus. J. Environ. Qual. 2000, 29, 508–514. [Google Scholar] [CrossRef]
- Aimer, Y.; Benali, O.; Serrano, K.G. Study of the degradation of an organophosphorus pesticide using electrogenerated hydroxyl radicals or heat-activated persulfate. Sep. Purif. Technol. 2019, 208, 27–33. [Google Scholar] [CrossRef]
- Rott, E.; Steinmetz, H.; Metzger, J.W. Organophosphonates: A review on environmental relevance, biodegradability and removal in wastewater treatment plants. Sci. Total Environ. 2018, 615, 1176–1191. [Google Scholar] [CrossRef]
- Chen, C.; Pang, Y.Q.; Li, Y.X.; Li, W.; Lan, Y.Q.; Shen, W.B. Dual functional cobalt-yttrium binary oxide activation of peroxymonosulfate for degradation of phenylphosphonic acid and simultaneous adsorption of phosphate product. Chem. Eng. J. 2022, 429, 132185. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Xu, W.Q.; Han, Y.; Sun, Q. Rapid TiO2/SBA-15 synthesis from ilmenite and use in photocatalytic degradation of dimethoate under simulated solar light. Dyes Pigment. 2018, 155, 265–275. [Google Scholar] [CrossRef]
- Feng, H.; Mao, W.; Li, Y.; Wang, X.; Chen, S. Characterization of dissolved organic matter during the O3-based advanced oxidation of mature landfill leachate with and without biological pre-treatment and operating cost analysis. Chemosphere 2021, 271, 129810. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.P.; Yuan, X.Z.; Wu, Z.B.; Jiang, L.B.; Li, Y.F.; Zeng, G.M. Principle and application of hydrogen peroxide based advanced oxidation processes in activated sludge treatment: A review. Chem. Eng. J. 2018, 339, 519–530. [Google Scholar] [CrossRef]
- Lee, J.; von Gunten, U.; Kim, J.H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Lian, W.; Yi, X.; Huang, K.; Tang, T.; Wang, R.; Tao, X.; Zheng, Z.; Dang, Z.; Yin, H.; Lu, G. Degradation of tris(2-chloroethyl) phosphate (TCEP) in aqueous solution by using pyrite activating persulfate to produce radicals. Ecotoxicol. Environ. Saf. 2019, 174, 667–674. [Google Scholar] [CrossRef]
- Li, Y.X.; Liu, L.; Li, W.; Lan, Y.Q.; Chen, C. Simultaneously rapid degradation of phenylphosphonic acid and efficient adsorption of released phosphate in the system of peroxymonosulfate (PMS) and Co3O4-La2O2CO3/C derived from MOFs. J. Environ. Chem. Eng. 2021, 9, 106332. [Google Scholar] [CrossRef]
- Lominchar, M.A.; Rodriguez, S.; Lorenzo, D.; Santos, N.; Romero, A.; Santos, A. Phenol abatement using persulfate activated by nZVI, H2O2 and NaOH and development of a kinetic model for alkaline activation. Environ. Technol. Innov. 2018, 39, 35–43. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, C.S.; Tu, Y.J.; Huang, Y.H.; Zhang, H. Heterogeneous Degradation of Organic Pollutants by Persulfate Activated by CuO-Fe3O4: Mechanism, Stability, and Effects of pH and Bicarbonate Ions. Environ. Sci. Technol. 2015, 49, 6838–6845. [Google Scholar] [CrossRef]
- Pan, Y.W.; Zhou, M.H.; Zhang, Y.; Cai, J.J.; Li, B.; Sheng, X.J. Enhanced degradation of Rhodamine B by pre-magnetized Fe0/PS process: Parameters optimization, mechanism and interferences of ions. Sep. Purif. Technol. 2018, 203, 66–74. [Google Scholar] [CrossRef]
- Li, H.J.; Fu, Y.H.; Mei, C.G.; Wang, M. Effective degradation of Direct Red 81 using FeS-activated persulfate process. J. Environ. Manag. 2022, 308, 114616. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Bao, J.G.; Huang, Y.; Xiang, L.J.; Faheem; Ren, B.X.; Du, J.K.; Nadagouda, M.N.; Dionysiou, D.D. Efficient degradation of atrazine with porous sulfurized Fe2O3 as catalyst for peroxymonosulfate activation. Appl. Catal. B 2019, 259, 118056. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Zhao, B.B.; Lyu, H.H. Activation of peroxydisulfate by ball-milled α-FeOOH/biochar composite for phenol removal: Component contribution and internal mechanisms. Environ. Pollut. 2022, 293, 118596. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, X.L.; Sun, C.; Wan, J.Z.; He, H.; Wang, F.; Dai, Y.X.; Yang, S.G.; Lin, Y.S.; Zhan, X.H. Insights into removal of tetracycline by persulfate activation with peanut shell biochar coupled with amorphous Cu-doped FeOOH composite in aqueous solution. Environ. Sci. Pollut. Res. 2019, 26, 2820–2834. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Yang, Y.; Zhang, C.; Cheng, M.; Wang, W.; Song, B.; Luo, H.; Qin, D.; Huang, C.; Qin, F.; et al. Insight into FeOOH-mediated advanced oxidation processes for the treatment of organic polluted wastewater. Chem. Eng. J. 2023, 453, 139812. [Google Scholar] [CrossRef]
- Shi, T.S.; Jiang, F.; Wang, P.; Yue, T.; Sun, W. Deep purification of As(V) in drinking water by silica gel loaded with FeOOH and MnO2. J. Cent. South Univ. 2021, 28, 1692–1706. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.L. MOF-derived Co3O4-C@FeOOH as an efficient catalyst for catalytic ozonation of norfloxacin. J. Hazard. Mater. 2021, 403, 123697. [Google Scholar] [CrossRef]
- Ding, S.; Wan, J.Q.; Ma, Y.W.; Wang, Y.; Pu, M.J.; Li, X.T.; Sun, J. Water stable SiO2-coated Fe-MOF-74 for aqueous dimethyl phthalate degradation in PS activated medium. J. Hazard. Mater. 2021, 411, 125194. [Google Scholar] [CrossRef]
- Ai, H.; Li, X.; Chen, C.; Xu, L.; Fu, M.-L.; Sun, W.; Yuan, B. Immobilization of β-FeOOH nanomaterials on the basalt fiber as a novel porous composite to effectively remove phosphate from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127815. [Google Scholar] [CrossRef]
- Zhao, L.J.; Ma, N.; Tao, M.L.; Zhang, W.Q. Covalently functionalized polyacrylonitrile fibers for heterogeneous catalysis and pollutant adsorption: A review. Mater. Today Chem. 2022, 26, 101206. [Google Scholar] [CrossRef]
- Xu, G.; Wang, L.; Li, M.; Tao, M.; Zhang, W. Phosphorous acid functionalized polyacrylonitrile fibers with a polarity tunable surface micro-environment for one-pot C–C and C–N bond formation reactions. Green Chem. 2017, 19, 5818–5830. [Google Scholar] [CrossRef]
- Xu, G.; Jin, M.; Kalkhajeh, Y.K.; Wang, L.; Tao, M.; Zhang, W. Proton sponge functionalized polyacrylonitrile fibers as an efficient and recyclable superbasic catalyst for Knoevenagel condensation in Water. J. Clean. Prod. 2019, 231, 77–86. [Google Scholar] [CrossRef]
- Xu, G.; Wang, L.; Xie, Y.J.; Tao, M.L.; Zhang, W.Q. Highly selective and efficient adsorption of Hg2+ by a recyclable aminophosphonic acid functionalized polyacrylonitrile fiber. J. Hazard. Mater. 2018, 344, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Xie, Y.J.; Cao, J.; Tao, M.L.; Zhang, W.Q. Highly selective and efficient chelating fiber functionalized by bis(2-pyridylmethyl)amino group for heavy metal ions. Polym. Chem. 2016, 7, 3874–3883. [Google Scholar] [CrossRef]
- Xu, W.S.; Zheng, W.J.; Wang, F.J.; Xiong, Q.Z.; Shi, X.L.; Kalkhajeh, Y.K.; Xu, G.; Gao, H.J. Using iron ion-loaded aminated polyacrylonitrile fiber to efficiently remove wastewater phosphate. Chem. Eng. J. 2021, 403, 126349. [Google Scholar] [CrossRef]
- Zheng, W.J.; Wu, Q.W.; Xu, W.S.; Xiong, Q.Z.; Kalkhajeh, Y.K.; Zhang, C.C.; Xu, G.; Zhang, W.F.; Ye, X.X.; Gao, H.J. Efficient capture of phosphate from wastewater by a recyclable ionic liquid functionalized polyacrylonitrile fiber: A typical “release and catch” mechanism. Environ. Sci. Water Res. Technol. 2022, 8, 607–618. [Google Scholar] [CrossRef]
- Xu, G.; Xu, W.S.; Tian, S.; Zheng, W.J.; Yang, T.; Wu, Y.X.; Xiong, Q.Z.; Kalkhajeh, Y.K.; Gao, H.J. Enhanced phosphate removal from wastewater by recyclable fiber supported quaternary ammonium salts: Highlighting the role of surface polarity. Chem. Eng. J. 2021, 416, 127889. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, X.; Liu, J.; Zhang, L. Phosphorus removal from wastewater using nano-particulates of hydrated ferric oxide doped activated carbon fiber prepared by Sol–Gel method. Chem. Eng. J. 2012, 200–202, 619–626. [Google Scholar] [CrossRef]
- Xu, G.; Cao, J.; Zhao, Y.L.; Zheng, L.S.; Tao, M.L.; Zhang, W.Q. Phosphorylated Polyacrylonitrile Fibers as an Efficient and Greener Acetalization Catalyst. Chem. Asian J. 2017, 12, 2565–2575. [Google Scholar] [CrossRef]
- Zheng, L.S.; Li, P.Y.; Tao, M.L.; Zhang, W.Q. Regulation of polar microenvironment on the surface of tertiary amines functionalized polyacrylonitrile fiber and its effect on catalytic activity in Knoevenagel condensation. Catal. Commun. 2019, 118, 19–24. [Google Scholar] [CrossRef]
- Grzybek, J.; Gil, B.; Roth, W.J.; Skoczek, M.; Kowalczyk, A.; Chmielarz, L. Characterization of Co and Fe-MCM-56 catalysts for NH3-SCR and N2O decomposition: An in situ FTIR study. Spectrochim. Acta Part A 2018, 196, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Fan, H.M.; Zhou, R.Y.; Yin, W.Y.; Wang, R.B.; Yang, M.; Xue, Z.Y.; Yang, Y.S.; Yu, J.X. Decorating UiO-66-NH2 crystals on recyclable fiber bearing polyamine and amidoxime bifunctional groups via cross-linking method with good stability for highly efficient capture of U(VI) from aqueous solution. J. Hazard. Mater. 2022, 424, 127273. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Yang, J.; Jia, X.H.; Li, Y.; Song, H.J. Polydopamine/FeOOH-modified interface in carbon cloth/polyimide composites for improved mechanical/tribological properties. Mater. Chem. Phys. 2020, 243, 122677. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, G.; Du, H.M.; Zhang, C.L.; Ma, N.; Zhang, W.Q. Prolinamide functionalized polyacrylonitrile fiber with tunable linker length and surface microenvironment as efficient catalyst for Knoevenagel condensation and related multicomponent tandem reactions. J. Catal. 2019, 374, 217–229. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, J.; Chen, Y.; Wen, X.; Chen, W.; Wang, Y.; Su, L.; Cao, J. Synthesis of nZVI-BC composite for persulfate activation to degrade pyrene: Performance, correlative mechanisms and degradation pathways. Process Saf. Environ. 2022, 162, 733–745. [Google Scholar] [CrossRef]
- Meddah, S.; El Hadi Samar, M.; Bououdina, M.; Khezami, L. Outstanding performance of electro-Fenton/ultra-violet/ultra-sound assisted-persulfate process for the complete degradation of hazardous pollutants in contaminated water. Process Saf. Environ. 2022, 165, 739–753. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Yan, Y.; Yan, J.; Pan, Y.; Zhang, Y.; Lai, B. Enhanced sulfamethoxazole degradation by peroxymonosulfate activation with sulfide-modified microscale zero-valent iron (S-mFe0): Performance, mechanisms, and the role of sulfur species. Chem. Eng. J. 2019, 376, 121302. [Google Scholar] [CrossRef]
- Wang, B.; Li, Q.; Lv, Y.; Fu, H.; Liu, D.; Feng, Y.; Xie, H.; Qu, H. Insights into the mechanism of peroxydisulfate activated by magnetic spinel CuFe2O4/SBC as a heterogeneous catalyst for bisphenol S degradation. Chem. Eng. J. 2021, 416, 129162. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.L.; Luo, W.; Sun, J.; Xu, Q.X.; Chen, F.; Zhao, J.W.; Wang, S.N.; Yao, F.B.; Wang, D.B.; et al. Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge. Bioresour. Technol. 2018, 247, 537–544. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Q.; Sun, Y.F.; Yao, C.X.; Yang, Z.; Yang, W.B. Improved utilization of active sites for phosphorus adsorption in FeOOH/anion exchanger nanocomposites via a glycol-solvothermal synthesis strategy. J. Environ. Sci. 2022, 111, 313–323. [Google Scholar] [CrossRef]
- Sun, H.M.; Zhou, Q.; Zhao, L.; Wu, W.Z. Enhanced simultaneous removal of nitrate and phosphate using novel solid carbon source/zero-valent iron composite. J. Clean. Prod. 2021, 289, 125757. [Google Scholar] [CrossRef]
- Liu, F.L.; Zuo, J.E.; Chi, T.; Wang, P.; Yang, B. Removing phosphorus from aqueous solutions by using iron-modified corn straw biochar. Front. Environ. Sci. Eng. 2015, 9, 1066–1075. [Google Scholar] [CrossRef]
- Brazão Farinha, C.; de Brito, J.; Veiga, R. Incorporation of high contents of textile, acrylic and glass waste fibres in cement-based mortars. Influence on mortars’ fresh, mechanical and deformability behaviour. Constr. Build. Mater. 2021, 303, 124424. [Google Scholar] [CrossRef]
- Liu, H.; Bruton, T.A.; Doyle, F.M.; Sedlak, D.L. In Situ Chemical Oxidation of Contaminated Groundwater by Persulfate: Decomposition by Fe(III)- and Mn(IV)-Containing Oxides and Aquifer Materials. Environ. Sci. Technol. 2014, 48, 10330–10336. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Prulho, R.; Brigante, M.; Dong, W.; Hanna, K.; Mailhot, G. Activation of persulfate by Fe(III) species: Implications for 4-tert-butylphenol degradation. J. Hazard. Mater. 2017, 322 Pt B, 380–386. [Google Scholar] [CrossRef]
- Ma, X.; Yuan, H.; Qiao, Q.; Zhang, S.; Tao, H. Enhanced catalysis for degradation of rhodamine B by amino-functionalized Fe-MOFs with high adsorption capacity. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131099. [Google Scholar] [CrossRef]
- Yang, T.-S.; Zhang, Y.; Cao, X.-Q.; Zhang, J.; Kan, Y.-J.; Wei, B.; Zhang, Y.-Z.; Wang, Z.-Z.; Jiao, Z.-Y.; Zhang, X.-X.; et al. Water caltrop-based carbon catalysts for cooperative adsorption and heterogeneous activation of peroxymonosulfate for tetracycline oxidation via electron transfer and non-radical pathway. Appl. Surf. Sci. 2022, 606, 154823. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, N.; Feng, C.; Zhang, Z. Adsorption for phosphate by crosslinked/non-crosslinked-chitosan-Fe(III) complex sorbents: Characteristic and mechanism. Chem. Eng. J. 2018, 353, 361–372. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Ding, J.; Zhang, Z.; Gao, C.; Halimi, M.; Demey, H.; Yang, Z.; Yang, W. High phosphate removal using La(OH)3 loaded chitosan based composites and mechanistic study. J. Environ. Sci. 2021, 106, 105–115. [Google Scholar] [CrossRef]
- Li, X.; Elgarhy, A.H.; Hassan, M.E.; Chen, Y.; Liu, G.; Elkorashey, R.M. Removal of inorganic and organic phosphorus compounds from aqueous solution by ferrihydrite decoration onto graphene. Environ. Monit. Assess. 2020, 192, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, H.; Lei, X.; Lian, Q.; Roy, A.; Doucet, D.; Yan, H.; Zappi, M.E.; Gang, D.D. A comparative study for phosphate adsorption on amorphous FeOOH and goethite (α-FeOOH): An investigation of relationship between the surface chemistry and structure. Environ. Res. 2021, 199, 111223. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Qu, M.; Zhang, S.; Quan, F.; Zhang, M.; Shen, W.; Mei, Y. Preparation of FeOOH supported by melamine sponge and its application for efficient phosphate removal. J. Environ. Chem. Eng. 2022, 10. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Zhao, H.; Hu, X.; Yuan, B.; Fu, M.-L. Tunable porous ferric composite for effective removal of phosphate in water. Colloids Surfaces A: Physicochem. Eng. Asp. 2019, 582, 123854. [Google Scholar] [CrossRef]
- Harijan, D.K.; Chandra, V. Akaganeite nanorods decorated graphene oxide sheets for removal and recovery of aqueous phosphate. J. Water Process. Eng. 2017, 19, 120–125. [Google Scholar] [CrossRef]
- Zhang, X.; Gang, D.D.; Sun, P.; Lian, Q.; Yao, H. Goethite dispersed corn straw-derived biochar for phosphate recovery from synthetic urine and its potential as a slow-release fertilizer. Chemosphere 2020, 262, 127861. [Google Scholar] [CrossRef]



| Entry | Sample | C (%) | N (%) | H (%) |
|---|---|---|---|---|
| 1 | PANF | 66.37 | 24.86 | 5.18 |
| 2 | PANAF | 55.14 | 20.15 | 6.57 |
| 3 | PANAF-FeOOH | 47.32 | 18.33 | 4.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, R.; Xu, W.; Liu, P.; Zhao, S.; Xu, G.; Xiong, Q.; Zhang, W.; Zhang, C.; Ye, X. Synthesis of FeOOH-Loaded Aminated Polyacrylonitrile Fiber for Simultaneous Removal of Phenylphosphonic Acid and Phosphate from Aqueous Solution. Polymers 2023, 15, 1918. https://doi.org/10.3390/polym15081918
Zhou R, Xu W, Liu P, Zhao S, Xu G, Xiong Q, Zhang W, Zhang C, Ye X. Synthesis of FeOOH-Loaded Aminated Polyacrylonitrile Fiber for Simultaneous Removal of Phenylphosphonic Acid and Phosphate from Aqueous Solution. Polymers. 2023; 15(8):1918. https://doi.org/10.3390/polym15081918
Chicago/Turabian StyleZhou, Rui, Wusong Xu, Peisen Liu, Shangyuan Zhao, Gang Xu, Qizhong Xiong, Weifeng Zhang, Chaochun Zhang, and Xinxin Ye. 2023. "Synthesis of FeOOH-Loaded Aminated Polyacrylonitrile Fiber for Simultaneous Removal of Phenylphosphonic Acid and Phosphate from Aqueous Solution" Polymers 15, no. 8: 1918. https://doi.org/10.3390/polym15081918
APA StyleZhou, R., Xu, W., Liu, P., Zhao, S., Xu, G., Xiong, Q., Zhang, W., Zhang, C., & Ye, X. (2023). Synthesis of FeOOH-Loaded Aminated Polyacrylonitrile Fiber for Simultaneous Removal of Phenylphosphonic Acid and Phosphate from Aqueous Solution. Polymers, 15(8), 1918. https://doi.org/10.3390/polym15081918








