Tremella fuciform Polysaccharides: Extraction, Physicochemical, and Emulsion Properties at Different pHs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction of Polysaccharides
2.3. Chemical Composition Analysis
2.4. ATR-FTIR Spectroscopic Analysis
2.5. Emulsifying Properties of TPS
2.6. Preparation of TPS Emulsions
2.7. Effect of pH on Emulsion Properties of TPS
2.7.1. Zeta Potential and Particle Size
2.7.2. Apparent Viscosity
2.7.3. Emulsion Stability
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization of TPS
3.2. ATR-FTIR Spectroscopy
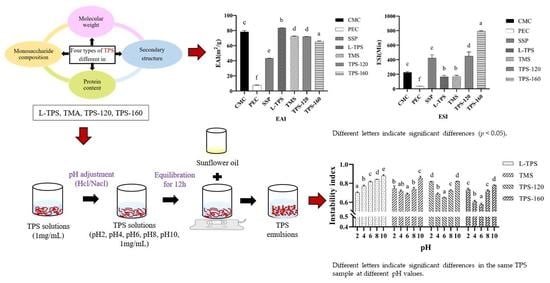
3.3. Emulsifying Properties of TPS
3.4. Effect of pH on Emulsion Properties of TPS
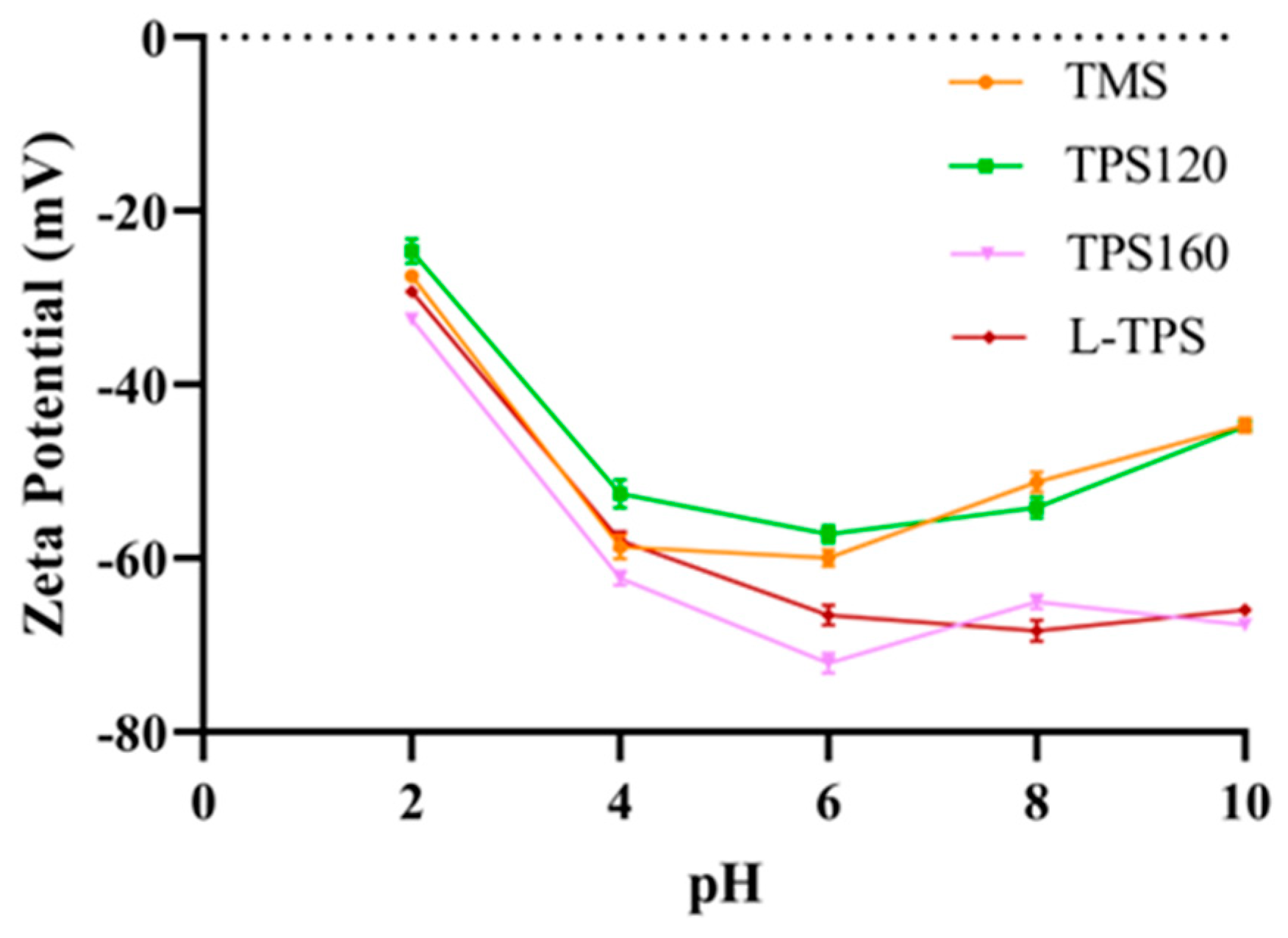
3.4.1. Zeta Potential and Particle Size
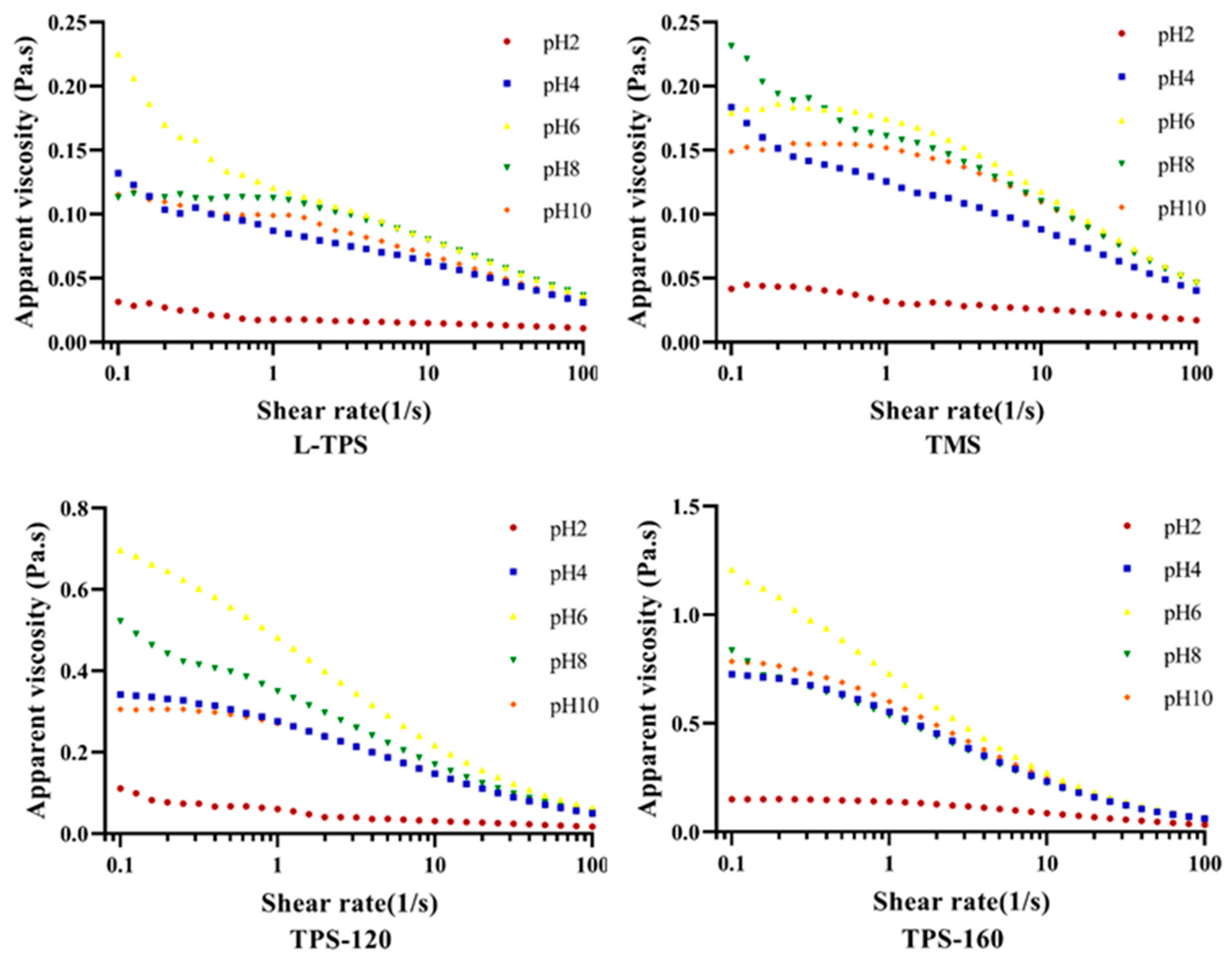
3.4.2. Apparent Viscosity
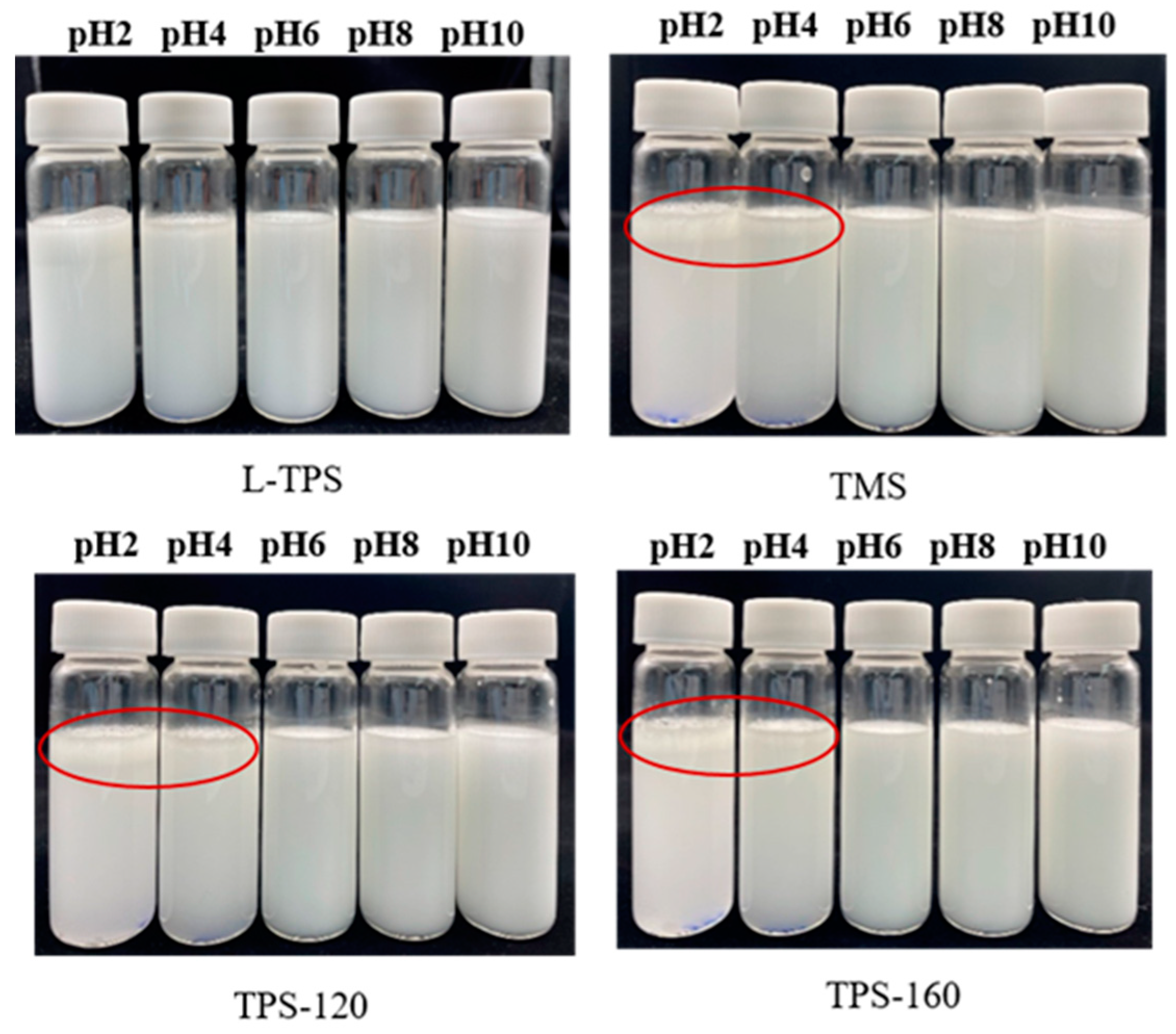
3.4.3. Emulsion Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Lal, S.N.; O’Connor, C.J.; Eyres, L. Application of emulsifiers/stabilizers in dairy products of high rheology. Adv. Colloid Interface Sci. 2006, 123, 433–437. [Google Scholar] [CrossRef]
- Ngouémazong, E.D.; Christiaens, S.; Shpigelman, A.; Van Loey, A.; Hendrickx, M. The Emulsifying and Emulsion-Stabilizing Properties of Pectin: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 705–718. [Google Scholar] [CrossRef]
- Chen, X.; Wu, X.; Zhang, K.; Sun, F.; Zhou, W.; Wu, Z.; Li, X. Purification, characterization, and emulsification stability of high- and low-molecular-weight fractions of polysaccharide conjugates extracted from green tea. Food Hydrocoll. 2022, 129, 107667. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, T.; Shi, J.; Xia, X.; Li, J.; Liu, L.; McClements, D.J.; Cao, Y.; Fu, Y.; Han, L.; et al. Physicochemical characterization, emulsifying and antioxidant properties of the polysaccharide conjugates from Chin brick tea (Camellia sinensis). Food Chem. 2022, 395, 133625. [Google Scholar] [CrossRef]
- Kumari, N.; Kumar, M.; Radha; Lorenzo, J.M.; Sharma, D.; Puri, S.; Pundir, A.; Dhumal, S.; Bhuyan, D.J.; Jayanthy, G.; et al. Onion and garlic polysaccharides: A review on extraction, characterization, bioactivity, and modifications. Int. J. Biol. Macromol. 2022, 219, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Olawuyi, I.F.; Park, J.J.; Hahn, D.; Lee, W.Y. Physicochemical and Functional Properties of Okra Leaf Polysaccharides Extracted at Different pHs. Chemistry 2022, 4, 405–418. [Google Scholar] [CrossRef]
- Wang, S.; Qu, D.; Zhao, G.; Yang, L.; Zhu, L.; Song, H.; Liu, H. Characterization of the structure and properties of the isolating interfacial layer of oil–water emulsions stabilized by soy hull polysaccharide: Effect of pH changes. Food Chem. 2022, 370, 131029. [Google Scholar] [CrossRef]
- Gu, Y.S.; Decker, E.A.; McClements, D. Influence of pH and carrageenan type on properties of β-lactoglobulin stabilized oil-in-water emulsions. Food Hydrocoll. 2005, 19, 83–91. [Google Scholar] [CrossRef]
- Xu, X.; Luo, L.; Liu, C.; McClements, D.J. Utilization of anionic polysaccharides to improve the stability of rice glutelin emulsions: Impact of polysaccharide type, pH, salt, and temperature. Food Hydrocoll. 2017, 64, 112–122. [Google Scholar] [CrossRef]
- Hu, J.; Lin, S.; Tan, B.K.; Hamzah, S.S.; Lin, Y.; Kong, Z.; Zhang, Y.; Zheng, B.; Zeng, S. Photodynamic inactivation of Burkholderia cepacia by curcumin in combination with EDTA. Food Res. Int. 2018, 111, 265–271. [Google Scholar] [CrossRef]
- Zhu, Z.; Hu, J.; Chen, J.; Xu, W.; Wang, P.; Kong, L.; Zheng, B.; Lin, S. The promotion effect of polysaccharide isolated from fresh tremella on the growth of bifidobacteria. Adv. J. Food Sci. Technol. 2016, 12, 627–634. [Google Scholar] [CrossRef]
- Cho, E.J.; Hwang, H.J.; Kim, S.W.; Oh, J.Y.; Baek, Y.M.; Choi, J.W.; Bae, S.H.; Yun, J.W. Hypoglycemic effects of exopolysaccharides produced by mycelial cultures of two different mushrooms Tremella fuciformis and Phellinus baumii in ob/ob mice. Appl. Microbiol. Biotechnol. 2007, 75, 1257–1265. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Wei, Z.-X.; Zhang, F.-M.; Linhardt, R.J.; Sun, P.-L.; Zhang, A.-Q. Structure, bioactivities and applications of the polysaccharides from Tremella fuciformis mushroom: A review. Int. J. Biol. Macromol. 2019, 121, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.-K.; Liu, Y.; Wang, J.-H. Emulsifying Properties of Tremella Fuciformis: A Novel Promising Food Emulsifier. Int. J. Food Eng. 2019, 15, 3–4. [Google Scholar] [CrossRef]
- Hou, F.; Yang, S.; Ma, X.; Gong, Z.; Wang, Y.; Wang, W. Characterization of Physicochemical Properties of Oil-in-Water Emulsions Stabilized by Tremella fuciformis Polysaccharides. Foods 2022, 11, 3020. [Google Scholar] [CrossRef] [PubMed]
- Akyüz, A.; Ersus, S. Optimization of enzyme assisted extraction of protein from the sugar beet (Beta vulgaris L.) leaves for alternative plant protein concentrate production. Food Chem. 2021, 335, 127673. [Google Scholar] [CrossRef] [PubMed]
- Staub, A. Removeal of protein-Sevag method. Methods Carbohydr. Chem. 1965, 5, 5–6. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- IDF. Milk-Determination of nitrogen content-Part 1: Kjeldahl method. IDF 2001, 20, 2001. [Google Scholar]
- Vilar, E.G.; Ouyang, H.; O’Sullivan, M.G.; Kerry, J.P.; Hamill, R.; O’Grady, M.N.; Mohammed, H.O.; Kilcawley, K.N. Effect of salt reduction and inclusion of 1% edible seaweeds on the chemical, sensory and volatile component profile of reformulated frankfurters. Meat Sci. 2020, 161, 108001. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, W.P.; Blais, H.N.; O’Callaghan, T.F.; Hossain, M.; Moloney, M.; Danaher, M.; O’Connor, C.; Tobin, J.T. Application of nanofiltration for the removal of chlorate from skim milk. Int. Dairy J. 2022, 128, 105321. [Google Scholar] [CrossRef]
- Belghith-Fendri, L.; Chaari, F.; Ben Jeddou, K.; Kallel, F.; Bouaziz, F.; Helbert, C.B.; Abdelkefi-Mesrati, L.; Ellouz-Chaabouni, S.; Ghribi-Aydi, D. Identification of polysaccharides extracted from pea pod by-products and evaluation of their biological and functional properties. Int. J. Biol. Macromol. 2018, 116, 947–954. [Google Scholar] [CrossRef]
- Klein, M.; Aserin, A.; Svitov, I.; Garti, N. Enhanced stabilization of cloudy emulsions with gum Arabic and whey protein isolate. Colloids Surf. B 2010, 77, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Jiang, Z.; Sun, T.; Wang, C.; Chen, Y.; Yang, Z.; Du, B.; Liu, C. Comparison of structural, antioxidant and immuno-stimulating activities of polysaccharides from Tremella fuciformis in two different regions of China. Int. J. Food Sci. 2018, 53, 1942–1953. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef]
- Liu, J.; Meng, C.-G.; Yan, Y.-H.; Shan, Y.-N.; Kan, J.; Jin, C.-H. Structure, physical property and antioxidant activity of catechin grafted Tremella fuciformis polysaccharide. Int. J. Biol. Macromol. 2016, 82, 719–724. [Google Scholar] [CrossRef]
- Fadavi, G.; Mohammadifar, M.A.; Zargarran, A.; Mortazavian, A.M.; Komeili, R. Composition and physicochemical properties of Zedo gum exudates from Amygdalus scoparia. Carbohydr. Polym. 2014, 101, 1074–1080. [Google Scholar] [CrossRef]
- Sheng, Z.; Liu, J.; Yang, B. Structure Differences of Water Soluble Polysaccharides in Astragalus membranaceus Induced by Origin and Their Bioactivity. Foods 2021, 10, 1755. [Google Scholar] [CrossRef]
- Wen, L.; Gao, Q.; Ma, C.-W.; Ge, Y.; You, L.; Liu, R.H.; Fu, X.; Liu, D. Effect of polysaccharides from Tremella fuciformis on UV-induced photoaging. J. Funct. Foods 2016, 20, 400–410. [Google Scholar] [CrossRef]
- Palacios, I.; García-Lafuente, A.; Guillamón, E.; Villares, A. Novel isolation of water-soluble polysaccharides from the fruiting bodies of Pleurotus ostreatus mushrooms. Carbohydr. Res. 2012, 358, 72–77. [Google Scholar] [CrossRef]
- Giavasis, I. Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Curr. Opin. Biotechnol. 2014, 26, 162–173. [Google Scholar] [CrossRef]
- Thakur, M.; Singh, H.K. Mushrooms, their bioactive compounds and medicinal uses: A review. Med. Plants Int. J. Phytomed. Relat. Ind. 2013, 5, 1–20. [Google Scholar] [CrossRef]
- Qun, G.; Ajun, W. Effects of molecular weight, degree of acetylation and ionic strength on surface tension of chitosan in dilute solution. Carbohydr. Polym. 2006, 64, 29–36. [Google Scholar] [CrossRef]
- Wang, D.; Wang, D.; Yan, T.; Jiang, W.; Han, X.; Yan, J.; Guo, Y. Nanostructures assembly and the property of polysaccharide extracted from Tremella Fuciformis fruiting body. Int. J. Biol. Macromol. 2019, 137, 751–760. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Yang, F.-L.; Chiu, H.-W.; Chao, H.-C.; Yang, Y.-J.; Sheu, J.-H.; Hua, K.-F.; Wu, S.-H. An Immunological Polysaccharide from Tremella fuciformis: Essential Role of Acetylation in Immunomodulation. Int. J. Mol. Sci. 2022, 23, 10392. [Google Scholar] [CrossRef]
- Singh, B.; Pal, L. Sterculia crosslinked PVA and PVA-poly (AAm) hydrogel wound dressings for slow drug delivery: Mechanical, mucoadhesive, biocompatible and permeability properties. J. Mech. Behav. Biomed. Mater. 2012, 9, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.A.G. Molecular Weight Effects in Guar Gum Adsorption and Depression of Talc. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2013. [Google Scholar]
- Singh, B.; Sharma, V.; Chauhan, D. Gastroretentive floating sterculia–alginate beads for use in antiulcer drug delivery. Chem. Eng. Res. Des. 2010, 88, 997–1012. [Google Scholar] [CrossRef]
- Yuen, S.-N.; Choi, S.-M.; Phillips, D.L.; Ma, C.-Y. Raman and FTIR spectroscopic study of carboxymethylated non-starch polysaccharides. Food Chem. 2009, 114, 1091–1098. [Google Scholar] [CrossRef]
- Chiovitti, A.; Bacic, A.; Craik, D.J.; Munro, S.L.; Kraft, G.T.; Liao, M.-L. Cell-wall polysaccharides from Australian red algae of the family Solieriaceae (Gigartinales, Rhodophyta): Novel, highly pyruvated carrageenans from the genus Callophycus. Carbohydr. Res. 1997, 299, 229–243. [Google Scholar] [CrossRef]
- De Baets, S.; Vandamme, E.J. Extracellular Tremella polysaccharides: Structure, properties and applications. Biotechnol. Lett. 2001, 23, 1361–1366. [Google Scholar] [CrossRef]
- Alba, K.; Kontogiorgos, V. Pectin at the oil-water interface: Relationship of molecular composition and structure to functionality. Food Hydrocoll. 2017, 68, 211–218. [Google Scholar] [CrossRef]
- Mirmoghtadaie, L.; Aliabadi, S.S.; Hosseini, S.M. Recent approaches in physical modification of protein functionality. Food Chem. 2016, 199, 619–627. [Google Scholar] [CrossRef]
- Kontogiorgos, V. Polysaccharides at fluid interfaces of food systems. Adv. Colloid Interface Sci. 2019, 270, 28–37. [Google Scholar] [CrossRef]
- Xu, Q.-X.; Shi, J.-J.; Zhang, J.-G.; Li, L.; Jiang, L.; Wei, Z.-J. Thermal, emulsifying and rheological properties of polysaccharides sequentially extracted from Vaccinium bracteatum Thunb leaves. Int. J. Biol. Macromol. 2016, 93, 1240–1252. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-J.; Zhang, J.-G.; Sun, Y.-H.; Qu, J.; Li, L.; Prasad, C.; Wei, Z.-J. Physicochemical properties and antioxidant activities of polysaccharides sequentially extracted from peony seed dreg. Int. J. Biol. Macromol. 2016, 91, 23–30. [Google Scholar] [CrossRef]
- Costa, A.L.R.; Gomes, A.; Furtado, G.D.F.; Tibolla, H.; Menegalli, F.C.; Cunha, R.L. Modulating in vitro digestibility of Pickering emulsions stabilized by food-grade polysaccharides particles. Carbohydr. Polym. 2020, 227, 115344. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; Hogan, S.A.; López-Rubio, A.; Brodkorb, A. Nano- and microstructural evolution of alginate beads in simulated gastrointestinal fluids. Impact of M/G ratio, molecular weight and pH. Carbohydr. Polym. 2019, 223, 115121. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Tan, C.P.; Hamid, N.S.A.; Yusof, S. Effect of arabic gum, xanthan gum and orange oil contents on ζ-potential, conductivity, stability, size index and pH of orange beverage emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 47–56. [Google Scholar] [CrossRef]
- Han, Y.; Cheng, Z.; Zhang, Y.; Zhang, N.; Zhu, X.; Chen, X.; Shao, Y.; Cheng, Y.; Wang, C.; Luo, Y.; et al. Effect of metal ions and pH on the emulsifying properties of polysaccharide conjugates prepared from low-grade green tea. Food Hydrocoll. 2020, 102, 105624. [Google Scholar] [CrossRef]
- Qu, D.; Wang, S.; Zhao, H.; Liu, H.; Zhu, D.; Jiang, L. Structure and interfacial adsorption behavior of soy hull polysaccharide at the oil/water interface as influenced by pH. Food Hydrocoll. 2021, 116, 106638. [Google Scholar] [CrossRef]
- Behrouzain, F.; Razavi, S.M.; Joyner, H. Mechanisms of whey protein isolate interaction with basil seed gum: Influence of pH and protein-polysaccharide ratio. Carbohydr. Polym. 2020, 232, 115775. [Google Scholar] [CrossRef]
- Osano, J.P.; Hosseini-Parvar, S.H.; Matia-Merino, L.; Golding, M. Emulsifying properties of a novel polysaccharide extracted from basil seed (Ocimum bacilicum L.): Effect of polysaccharide and protein content. Food Hydrocoll. 2014, 37, 40–48. [Google Scholar] [CrossRef]
- Santiago, J.S.J.; Salvia-Trujillo, L.; Palomo, A.; Niroula, A.; Xu, F.; Van Loey, A.M.; Hendrickx, M.E. Process-induced water-soluble biopolymers from broccoli and tomato purées: Their molecular structure in relation to their emulsion stabilizing capacity. Food Hydrocoll. 2018, 81, 312–327. [Google Scholar] [CrossRef]
- Krägel, J.; Derkatch, S.; Miller, R. Interfacial shear rheology of protein–surfactant layers. Adv. Colloid Interface Sci. 2008, 144, 38–53. [Google Scholar] [CrossRef]
- Bourbon, A.; Pinheiro, A.; Ribeiro, C.; Miranda, C.; Maia, J.; Teixeira, J.; Vicente, A. Characterization of galactomannans extracted from seeds of Gleditsia triacanthos and Sophora japonica through shear and extensional rheology: Comparison with guar gum and locust bean gum. Food Hydrocoll. 2010, 24, 184–192. [Google Scholar] [CrossRef]
- Carneiro-Da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.; Teixeira, J.A.; Vicente, A.A. Influence of concentration, ionic strength and pH on zeta potential and mean hydrodynamic diameter of edible polysaccharide solutions envisaged for multinanolayered films production. Carbohydr. Polym. 2011, 85, 522–528. [Google Scholar] [CrossRef]
- Sriprablom, J.; Luangpituksa, P.; Wongkongkatep, J.; Pongtharangkul, T.; Suphantharika, M. Influence of pH and ionic strength on the physical and rheological properties and stability of whey protein stabilized o/w emulsions containing xanthan gum. J. Food Eng. 2019, 242, 141–152. [Google Scholar] [CrossRef]
- Nakauma, M.; Funami, T.; Noda, S.; Ishihara, S.; Al-Assaf, S.; Nishinari, K.; Phillips, G.O. Comparison of sugar beet pectin, soybean soluble polysaccharide, and gum arabic as food emulsifiers. 1. Effect of concentration, pH, and salts on the emulsifying properties. Food Hydrocoll. 2008, 22, 1254–1267. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.; Boskou, D.; Kiosseoglou, V. Stabilization of olive oil-lemon juice emulsion with polysaccharides. Food Chem. 2005, 90, 627–634. [Google Scholar] [CrossRef]
- Albano, K.M.; Cavallieri, L.F.; Nicoletti, V.R. Electrostatic interaction between proteins and polysaccharides: Physicochemical aspects and applications in emulsion stabilization. Food Rev. Int. 2019, 35, 54–89. [Google Scholar] [CrossRef]
- Tavernier, I.; Patel, A.R.; Van der Meeren, P.; Dewettinck, K. Emulsion-templated liquid oil structuring with soy protein and soy protein: κ-carrageenan complexes. Food Hydrocoll. 2017, 65, 107–120. [Google Scholar] [CrossRef]






| Samples | Peak Molecular Mass (× 106 g/mol) | Total Carbohydrate (%) | Moisture Content (%) | Ash Content (%) | Protein Content (%) |
|---|---|---|---|---|---|
| L-TPS | 17.54 ± 0.23 d | 72.67 ± 2.55 b | 12.22 ± 0.12 b | 6.25 ± 0.05 a | 2.33 ± 0.19 b |
| TMS | 13.38 ± 1.39 c | 80.99 ± 2.07 a | 7.69 ± 0.14 a | 5.79 ± 0.08 a | 0.73 ± 0.02 a |
| TPS-120 | 29.94 ± 3.30 b | 78.86 ± 1.06 a | 8.16 ± 0.03 a | 6.86 ± 0.07 a | 0.87 ± 0.01a |
| TPS-160 | 41.68 ± 5.12 a | 79.02 ± 0.95 a | 7.80 ± 0.03 a | 5.84 ± 0.02 a | 0.80 ± 0.002 a |
| Sample | Fuc | Gal | Glc | Xyl | Man | Gal-A | Glc-A |
|---|---|---|---|---|---|---|---|
| L-TPS | 13.17 ± 1.17 b | 0.96 ± 0.12 b | 14.98 ± 3.33 b | 14.92 ± 3.89 a | 32.07 ± 3.71 b | 0.13 ± 0.01 b | 25.38 ± 2.98 b |
| TMS | 19.26 ± 2.71 a | 0.55 ± 0.13 a | 4.29 ± 0.64 a | 16.51 ± 3.19 a | 44.83 ± 5.94 a | 0.13 ± 0.01 b | 14.44 ± 1.22 a |
| TPS120 | 20.00 ± 1.44 a | 0.44 ± 0.20 a | 3.31 ± 0.88 a | 18.08 ± 2.99 a | 44.91 ±3.12 a | 0.41 ± 0.19 a | 12.85 ± 1.34 a |
| TPS160 | 19.15 ± 1.65 a | 1.00 ± 0.14 b | 5.49 ± 1.73 a | 16.74 ± 1.02 a | 43.70 ± 4.29 a | 0.22 ± 0.02 a | 13.70 ± 2.04 a |
| Sample | pH 2 | pH 4 | pH 6 | pH 8 | pH 10 | |
|---|---|---|---|---|---|---|
| L-TPS | d3,2 | 8.92 ± 0.03 a | 9.90 ± 0.16 b | 4.65 ± 0.03 c | 4.71 ± 0.03 c | 3.84 ± 0.02 d |
| d4,3 | 23.40 ± 0.40 a | 20.70 ± 0.06 b | 20.30 ± 0.06 d | 17.70 ± 2.00 c | 10.90 ± 0.06 d | |
| TMS | d3,2 | 22.20 ± 0.53 a | 15.10 ± 0.00 b | 5.45 ± 0.01 c | 4.41 ± 0.04 d | 4.27 ± 0.02 d |
| d4,3 | 125.00 ± 7.0 a | 37.20 ± 0.00 b | 11.70 ±0.06 c | 11.70 ± 0.06 c | 11.60 ± 0.06 c | |
| TPS-120 | d3,2 | 58.70 ± 0.06 a | 13.20 ± 0.06 b | 4.41 ± 0.06 c | 3.50 ± 0.01 d | 3.63 ± 0.00 e |
| d4,3 | 78.60 ± 0.06 a | 32.50 ± 0.06 b | 11.30 ± 0.00 c | 10.30 ± 0.00 d | 10.10 ± 0.00 e | |
| TPS-160 | d3,2 | 30.10 ± 0.44 a | 13.80 ± 0.26 b | 5.57 ± 0.06 c | 4.29 ± 0.05 d | 4.62 ± 0.01 d |
| d4,3 | 90.40 ± 2.26 a | 32.80 ± 2.83 b | 12.00 ± 0.06 c | 14.90 ± 2.01 c | 11.80 ± 0.00 c |
| Sample | pH 2 | pH 4 | pH 6 | pH 8 | pH 10 | |
|---|---|---|---|---|---|---|
| L-TPS | K (Pa·sn) | 0.031 ± 0.009 a | 0.244 ± 0.002 b | 0.318 ± 0.012 c | 0.305 ± 0.004 c | 0.256 ± 0.051 b |
| n | 0.851 ± 0.046 b | 0.695 ± 0.013 a | 0.642 ± 0.021 a | 0.667 ± 0.006 a | 0.680 ± 0.016 a | |
| R2 | 0.999 | 0.998 | 0.998 | 0.997 | 0.998 | |
| TMS | K (Pa·sn) | 0.077 ± 0.012 a | 0.356 ± 0.032 b | 0.439 ± 0.010 d | 0.392 ± 0.014 c | 0.379 ± 0.018 bc |
| n | 0.799 ± 0.018 b | 0.658 ± 0.024 a | 0.625 ± 0.031 a | 0.638 ± 0.065 a | 0.634 ± 0.030 a | |
| R2 | 0.998 | 0.997 | 0.997 | 0.997 | 0.996 | |
| TPS-120 | K (Pa·sn) | 0.271 ± 0.039 a | 0.513 ± 0.070 b | 0.772 ± 0.029 d | 0.652 ± 0.045 c | 0.599 ± 0.045 bc |
| n | 0.680 ± 0.026 b | 0.547 ± 0.023 a | 0.553 ± 0.041 a | 0.523 ± 0.011 a | 0.532 ± 0.013 a | |
| R2 | 0.998 | 0.995 | 0.994 | 0.995 | 0.994 | |
| TPS-160 | K (Pa·sn) | 0.365 ± 0.039 a | 0.830 ± 0.039 b | 0.973 ± 0.039 c | 0.850 ± 0.039 b | 0.856 ± 0.039 b |
| n | 0.606 ± 0.016 b | 0.461 ± 0.011 a | 0.451 ± 0.009 a | 0.470 ± 0.020 a | 0.453 ± 0.021 a | |
| R2 | 0.996 | 0.991 | 0.991 | 0.991 | 0.990 | |
| L-TPS emulsion | K (Pa·sn) | 0.005 ± 0.002 a | 0.055 ± 0.006 b | 0.039 ± 0.002 c | 0.038 ± 0.005 c | 0.040 ± 0.003 c |
| n | 0.920 ± 0.020 b | 0.838 ± 0.007 a | 0.844 ± 0.029 a | 0.844 ± 0.011 a | 0.839 ± 0.012 a | |
| R2 | 0.994 | 0.987 | 0.999 | 0.999 | 0.999 | |
| TMS emulsion | K (Pa·sn) | 0.172 ± 0.010 a | 0.023 ± 0.010 b | 0.053 ± 0.004 d | 0.041 ± 0.001 c | 0.037 ± 0.003 c |
| n | 0.617 ± 0.021 a | 0.878 ± 0.011 d | 0.835 ± 0.008 c | 0.836 ± 0.015 c | 0.778 ± 0.017 b | |
| R2 | 0.730 | 0.989 | 0.999 | 0.999 | 0.999 | |
| TPS-120 emulsion | K (Pa·sn) | 0.589 ± 0.033 d | 0.067 ± 0.012 a | 0.182 ± 0.007 c | 0.165 ± 0.003 b | 0.188 ± 0.003 c |
| n | 0.423 ± 0.025 a | 0.815 ± 0.014 c | 0.686 ± 0.015 b | 0.693 ± 0.005 b | 0.680 ± 0.010 b | |
| R2 | 0.998 | 0.980 | 0.998 | 0.998 | 0.998 | |
| TPS-160 emulsion | K (Pa·sn) | 1.136 ± 0.075 c | 0.338 ± 0.003 b | 0.270 ± 0.017 a | 0.242 ± 0.027 a | 0.262 ± 0.003 a |
| n | 0.359 ± 0.025 a | 0.582 ± 0.016 b | 0.630 ± 0.001 c | 0.665 ± 0.005 d | 0.636 ± 0.013 c | |
| R2 | 0.997 | 0.994 | 0.996 | 0.997 | 0.997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; Roos, Y.H.; Gómez-Mascaraque, L.G.; Lu, X.; Miao, S. Tremella fuciform Polysaccharides: Extraction, Physicochemical, and Emulsion Properties at Different pHs. Polymers 2023, 15, 1771. https://doi.org/10.3390/polym15071771
Tian L, Roos YH, Gómez-Mascaraque LG, Lu X, Miao S. Tremella fuciform Polysaccharides: Extraction, Physicochemical, and Emulsion Properties at Different pHs. Polymers. 2023; 15(7):1771. https://doi.org/10.3390/polym15071771
Chicago/Turabian StyleTian, Lili, Yrjö H. Roos, Laura G. Gómez-Mascaraque, Xu Lu, and Song Miao. 2023. "Tremella fuciform Polysaccharides: Extraction, Physicochemical, and Emulsion Properties at Different pHs" Polymers 15, no. 7: 1771. https://doi.org/10.3390/polym15071771
APA StyleTian, L., Roos, Y. H., Gómez-Mascaraque, L. G., Lu, X., & Miao, S. (2023). Tremella fuciform Polysaccharides: Extraction, Physicochemical, and Emulsion Properties at Different pHs. Polymers, 15(7), 1771. https://doi.org/10.3390/polym15071771