1. Introduction
The chemical industry is constantly seeking opportunities to manufacture essential commodities and convert them into higher-value-added chemical-derived products [
1]. The development of these chemicals requires (i) an economically viable process; (ii) a marketing strategy that aligns with market trends; and (iii) a sustainable production route and product (see, e.g., Harmsen et al. [
2], Cussler and Moggridge [
3]). Industries involved in the transformation of pine resin, an abundant natural and non-toxic raw material, into derivatives are currently facing the challenge to keep up with market demands. This industrial sector is often referred to as the
second industry in the product value chain [
4], where the
primary industry is responsible for cleaning and separating the raw material into basic fractions (e.g., the separation of pine oleoresin into colophony and turpentine derivatives, see Zinkel and Russell [
5]) and the
third industry produces higher-value chemicals resulting from the transformation/incorporation of resin derivatives. Typically, the
second industry produces derivatives in the form of elastomers, polymers for biomedical applications, coatings, adhesives, and surfactants [
6], as well as food products and excipients for the fragrance and pharmaceutical industries [
7]. Recently, colophony derivatives were recognized as exceptional and sustainable binders that can be used in combination with other materials to improve physical properties [
8,
9] or as repellent and anti-microbial coatings [
10,
11]. Additionally, their application to the fabrication of adhesives has been successfully investigated (see Hayashi et al. [
12], Petrie [
13], Unger et al. [
14]).
Pressure-sensitive adhesives (PSAs) are a class of soft materials that adhere to different media with light pressure and short contact times, without a chemical reaction [
15]. PSAs are often used in packaging applications (tapes and labels), medical applications, and baby and feminine hygiene applications, among others. Initially, PSAs were produced from natural rubber and they have predominantly been used in medical care. However, during the 1960s, rubber-based technologies were replaced by solvent-based synthetic polymers such as polyacrylates. Natural rubber emulsion-based technologies dominated the market until the late 1970s [
16]. PSA formulations with improved thermal stability and different-molecular-weight versions of A-B-A block polymers were presented by Korpman [
17].
Environmental concerns have forced industries to look for coating techniques other than solvent-based systems. In recent decades, effective cross-linked hot-melt adhesive systems have been developed to replace those based on natural rubber [
18]. Water-based adhesive systems have become a viable alternative, and the increasing use of water emulsion-based systems ensures a greater selection of raw materials that can be incorporated into applications, meeting economic and sustainability requirements. Pine resin belongs to this group of materials and the industrial players in the market who have the ability to exploit it are encouraged to develop successful formulations.
PSAs are amorphous viscoelastic materials with a rheological behavior that is determined by the viscosity and elastic modulus. Both these properties, as well as the glass transition temperature (here represented by
), depend on the composition. The preparation of PSAs should ideally involve resin-in-water emulsions and the addition of plasticizers, diluents, emulsifiers, and stabilizers should be minimized [
19]. In addition to the environmental impact of these agents, they can also have negative effects on the functional/structural properties of PSAs, as they can migrate to the surface of adhesives and cause adhesion breakage. Kim et al. [
20] reported on the production of acrylic water emulsions, which were later used to form PSAs from water-borne commercial dispersions with various colophony esters. Resins with a lower
have practical advantages, as they are more susceptible to forming stable (easier to produce) water dispersions under normal pressure conditions [
19]. Aydin et al. [
21] introduced a method for obtaining water solvent-free dispersions of polymer and tackifier that can be used to produce colophony dispersions for incorporation into PSAs. Geoghegan and Wang [
22] described the laboratory production of resin-in-water dispersions using a resinic ester with a
of 83
. Boonstra et al. [
23] used a 4 L reactor with temperature control to prepare resin-in-water dispersions, which were further blended with acrylic latex to produce PSAs. Miller [
24] proposed a formulation where a resinic ester combined with an antioxidant—butylated hydroxytoluene—was utilized to produce the dispersion. Aarts et al. [
25] produced dispersions of tackifiers using glycerol ester. Finally, Yang et al. [
26] reported on the production of resin dispersions with tackifier in continuous mode. This rich body of knowledge, which is available in the form of patents, was crucial in setting up the experimental installation and emulsion characterization during this study.
The design and development of products is a well-established field and industry is constantly adopting new practices to accelerate and systematize procedures. Companies recognize the potential of these practices to (i) respond successfully to market uncertainty and speed; (ii) improve knowledge and systematize creative processes; and (iii) ensure that decision-making processes are explicit and well-documented [
27]. Despite the increasing importance of this field in academia and industry, the literature on the systematic development of new water-based emulsions for PSAs remains scarce, especially because the process needs to be compatible with customers’ specifications and the extensive experimental work required.
This paper aims to fill this gap. Here, we propose a sequential approach to the systematization of procedures. The proposed strategy is aligned with current practices, specifically, those that integrate the needs of the customer into the various stages of concept (and product) design [
28]. Fundamentally, it relies on a combination of experimental work planned to maximize the obtained information and proper customer-centric decision-making tools to identify the optimal candidate concepts (in the form of emulsions or designated formulations) to develop. To identify good candidate emulsions and optimize their performance, we use the design of experiments. To systematically compare the metrics representing the product quality interpreted by customers, we use (i) engineering methods for robust design [
29], specifically the concept of the loss function introduced by Taguchi et al. [
30]; and (ii) multicriteria decision-making methods to aggregate multiple quality metrics into a single performance indicator.
This paper presents the following three novel elements: (i) a systematic customer-centered approach for developing polymer-based products; (ii) the use of robustness-centered techniques for assessing the performance of candidate emulsions, along with multi-attribute value techniques for integrating individual quality criteria into a performance metric; and (iii) a methodology based on the sequential design of experiments for optimizing formulations. The proposed approach is illustrated through the development of resin-in-water emulsion systems for use in PSA production.
In the remaining sections, boldface lowercase letters represent vectors, boldface capital letters represent continuous domains, blackboard bold capital letters represent discrete domains, and capital letters represent matrices. Finite sets containing elements are compactly represented by . The transpose operation of a matrix/vector is represented by “”.
This paper is organized as follows.
Section 2 presents (i) the materials used in the experiments; (ii) the experimental equipment; (iii) the laboratory procedure; and (iv) the equipment used for quality characterization.
Section 3 systematizes the product development procedure used herein and briefly outlines (i) the
loss function used to select the best products by considering customers’ specifications, and (ii) the linear model used to represent the multi-criteria decision-making tool utilized for ranking.
Section 4 applies the proposed approach to the design of physically viable resin-in-water emulsions. First, we select a set of promising candidate formulations. Then, we optimize them using a sequential optimal design of experiments, where the goal is to maximize the global performance metric by combining four quality characteristics. Finally, we analyze the repeatability of the production method.
Section 5 provides an overview of this work and a summary of the results.
2. Materials and Equipment Characterization
This section introduces the experimental procedure. In
Section 2.1, we characterize the materials used. In
Section 2.2, the equipment used for the experimental work is presented and the procedure is described. Finally,
Section 2.3 describes the measurement equipment used for characterizing the emulsions.
The resin industry commonly uses the softening point,
as a measure of the glass transition temperature rather than differential scan calorimetry, as it corresponds to the point at which a specific resin probe slides into polymer due to a temperature increase. A physical test is used to determine the softening temperature, where a standard probe of specific dimensions and weight is gradually heated until it starts flowing. Typically, the glass transition temperature of colophony resins is about 30–40
below the softening point, which, in turn, is highly correlated with the average molecular weight. Higher softening temperatures correspond to resins with higher average molecular weights. In our study, we used the ASTM D1525-17 standard for measuring the softening temperature [
31].
2.1. Materials
The resins used in the experiments were modulated regarding their softening point. That is, we used two resins processed under different conditions, R1 and R2, to combine new products with a given
. This part of the experimental work involved various (sequential) tests, where the compositions of the mixtures in R1 and R2 were varied until the new resins obtained (denoted here as A, B, and C (see
Table 1)) satisfied the target values of
shown in the right column in the table. Essentially, A, B, and C are the
mother resins used to produce the resin-in-water emulsions (see
Section 4). Here, our goal was to regulate the impact of resin quality, as quantified by the softening temperature, on emulsions.
We used three surfactants in the experimental work. Due to industrial property limitations imposed by the suppliers, we cannot name them. The choice of anionic surfactants for use in the emulsions was based on both prior industrial knowledge and the need to ensure compatibility with the resin systems under consideration. Here, they are denoted as S1 (surfactant of supplier 1), S2 (surfactant of supplier 2), and S3 (surfactant of supplier 3). All other reagents used, such as potassium hydroxide (KOH) and water, were of analytical grade.
2.2. Experimental Equipment and Procedure
The experimental work was conducted using a purpose-built system consisting of a jacketed reactor vessel of 500 mL with a batch-mode agitator. The temperature along the batches was automatically controlled and the agitator was designed to ensure ideal mixture conditions, with its geometry preventing the accumulation of solids and its ability to generate a vortex capable of efficiently dispersing the water phase when added to the system.
Each experiment required the configuration of five parameters that could potentially affect the quality of the resin-in-water emulsions, namely the softening temperature of the resin, the surfactant, the amount of KOH added to the system during the reaction (expressed in %wt relative to the resin mass), the agitation rate of the agitator (measured in rpm), and the amount of surfactant required (also expressed in %wt relative to the resin mass).
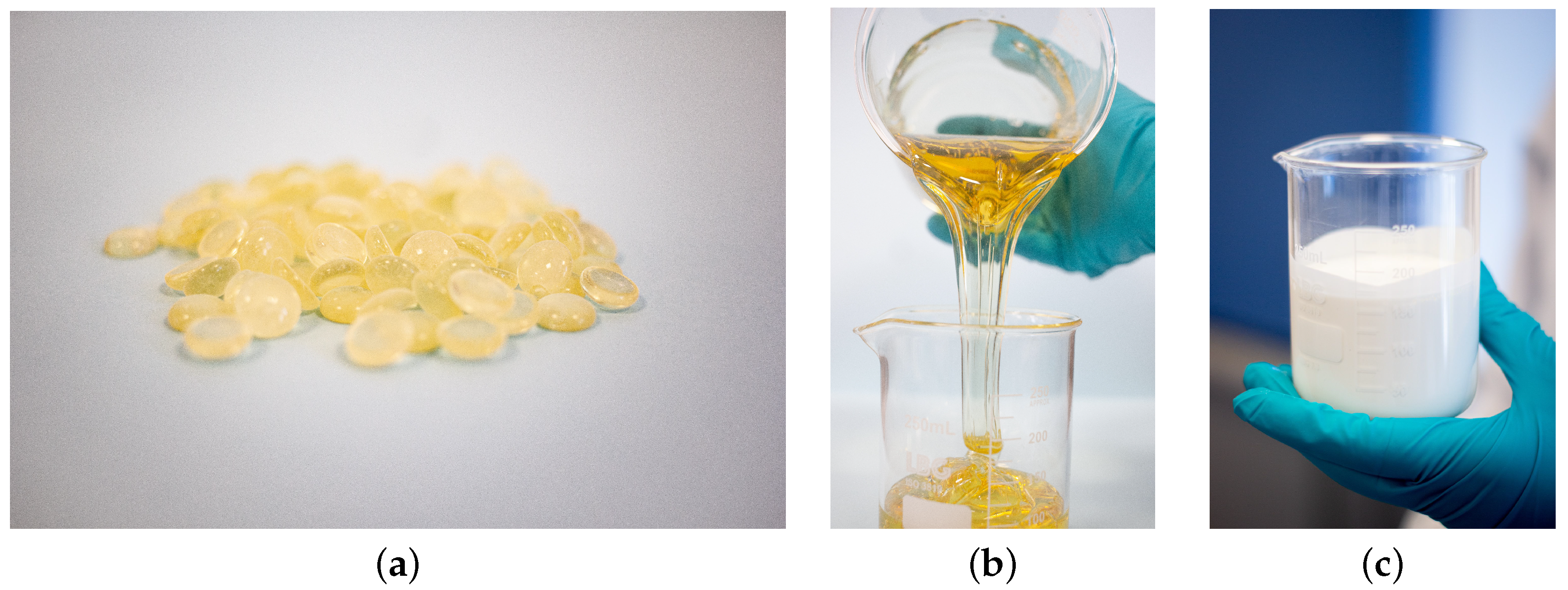
After selecting the experimental conditions, 200 g of resin was fed into the reactor (see
Figure 1a). Then, the temperature control module and heating system were switched on and the temperature at which the emulsion is prepared was set to correspond to the softening point of the resin. The agitator was also switched on and adjusted to the previously determined speed. After two hours when the resin reached a stable and nearly constant temperature and became fluid (see
Figure 1b), a given volume of a solution with a 1:1 mass ratio of KOH was added to the reactor. The resulting solution was then agitated for 30 min, which corresponds to the time period during which the saponification reaction occurs. Next, the surfactant was added in small amounts. The resulting solution, now containing the surfactant, was agitated for another 30 min to ensure homogeneity. Finally, the water was added in increments in all the experiments. Essentially, the same relative amount of water was added at equal time points to minimize the effects of different mixing strategies in the emulsion characteristics. The amount of water added was calculated such that at the end of the process, the amount of solids in the water phase was about 55 %wt. Next, we describe the physical/chemical transformations that occurred during the formation of the resin-in-water emulsions.
Initially, a water-in-resin emulsion was formed. During this time, a small amount of water (in small increments) was added to the system. This dispersion was slowly stirred to yield a concentrated resin-in-water emulsion. This period was characterized by phase inversion as a result of sharp changes in viscosity and electrical conductivity. The emulsion formed was then diluted by adding more water in larger increments. The phase inversion was visually easy to identify and characterizes the point at which the dispersion water-in-resin phase forms the resin-in-water emulsion (see
Figure 1c).
2.3. Quality Characterization Equipment
To characterize the quality characteristics of the emulsions we considered four variables: (i) viscosity (expressed in cP); (ii) pH (non-dimensionally expressed); (iii) solid content (expressed in %wt); and (iv) size of the particles (represented here by the average diameter, expressed in nm). Viscosity was measured with a Brookfield DV2T spindle RV03, Brookfield, Toronto, Canada viscosimeter. pH was measured using a Mettler Toledo SevenEasyTM pH meter, Mettler Toledo, Greifensee, Switzerland. The solid content was measured using gravimetric analysis with a precision balance. The particle size was measured using a Malvern Zetasizer Nano ZS, Malvern, Worcestershire, United Kingdom system, which provided both the particle size distribution (PSD) and the cumulative distribution curves.
The stability of an emulsion depends on various parameters, with one of the most significant being the size and distribution of the particles—the smaller the dispersed particles, the stabler the system. More specifically, according to industry research on this type of emulsion, when the particle size is lower than 500 nm and has a unimodal distribution, good results in terms of stability are typically achieved. Thus, by controlling the particles’ size and the unimodality of the respective PSD, as in our experiments, the stability of the formulations, or at least the identification of formulations that may not be stable, can be indirectly ensured. We also note that during the optimization stages, visual evaluation of the emulsions was performed and some of them were reported as non-dispersed (ns), as shown in the tables of results (see
Section 4.2,
Section 4.3 and
Section 4.4). Moreover, the initially produced samples were observed after three months and no significant phase separation was observed, which is an excellent indicator of stability and demonstrates the compatibility of the resin system, including the resin–surfactant combination and the production process.
4. Results
In this section, we utilized the approach introduced in
Section 2 and
Section 3 to the design of resin-in-water emulsions for application in PSA fabrication. In
Section 4.1, we characterize the design problem and the decision-making tool used to assess the quality performance of the formulations. In
Section 4.2, we apply the screening experimental design discussed in
Section 3.2 to find the optimal levels of the primary factors. In
Section 4.3, we used sequential experimental design to optimize the formulations by judiciously choosing the levels of the secondary factors. Finally, in
Section 4.4, we analyze the experimental repeatability of the formulations.
4.1. The Design Problem
Here, we characterize the design context and introduce the overall quality metric used to assess the formulations resulting from the experiments.
The quality characteristics were introduced in
Section 2.3, where
represented viscosity,
represented pH,
represented the solid content, and
represented the particle diameter, and they were measured using the methods described in
Section 2.3. The factors affecting the quality characteristics were listed in
Section 2.1 and
Section 2.2. The primary factors were the resin, which was characterized by the respective softening temperature and mathematically represented by
, and the surfactant,
. Both factors were discrete. The secondary factors were the amount of KOH added to the reactional system, represented by
; the agitation rate,
; and the amount of surfactant,
. All secondary factors were continuous and were used to optimize the formulations regarding the quality characteristics.
The product specifications gathered from the customers are listed in
Table 2. Normalized loss functions were constructed for all the quality characteristics by applying Equation (
2), and they are shown in the second column in
Table 3.
The weights representing the relative importance of the quality characteristics were determined using expert-based knowledge and typical customer requirements. They are shown in the third column in
Table 3. Consequently, the overall quality metric, which was obtained using Equation (
3) and used for ranking the emulsions in the remaining sections, is
Besides this metric, an additional feature emerged during the process of extraction of experience-based knowledge. The technical staff and customers recommended that for subsequent applications, the particle size distribution (PSD) should be unimodal, as it is a common indicator of the stability of the resulting emulsion. To account for this requirement, a new binary quality characteristic was introduced into the analysis,
, where
This characteristic was not explicitly included in the decision model (
8) but non-unimodal PSD emulsions (i.e.,
) were automatically discarded from the group of promising formulations.
4.2. Screening of Primary Factors
This section presents the work conducted in Stage 1 of the proposed approach (see
Figure 2), which was used for screening the primary factors.
The vector of factors included five elements, i.e., , where represented the resin composition, distinguished by the respective softening temperature; represented the surfactant, identified by the supplier; represented the amount of KOH used; represented the agitation rate; and represented the amount of surfactant. Consequently, the number of factors of the design problem, k, was 5. and represented the primary factors, that is, and . , , and represented the secondary factors, that is, and .
For the screening of the primary factors, we used a three-level full factorial design of experiments (see
Section 3.2). Specifically, the complete design consisted of
experiments, where
and
were varied in the three levels.
Table 4 shows the levels tested for each factor and the respective codes and designations (shorten to
Designat.) used in the remaining parts of the paper. In all the experiments carried out in this stage, the secondary factors were kept constant: the amount of KOH added was 2 %wt relative to the resin, the amount of surfactant was 7 %wt relative to the resin, and the agitation rate was set to 100 rpm. Furthermore, the experimental temperature was maintained at 25
in all the tests. The water was added gradually to obtain dispersions with a solid content within the range of
.
Table 5 presents the results of the experiments. The first column shows the experiment number, the second column shows the
level, the third column shows the
level, and the fourth column shows the designation of the emulsion, where the first two symbols denote the base resin used (see
Table 4), the fourth and fifth symbols denote the surfactant, and the last digit indicates the experiment number under the same conditions (i.e., for a given pair,
and
). Furthermore, columns 5 to 8 show the results of the characterization of the emulsions, and column 9 shows the average diameter, where 95% of the particles had a smaller size,
, which, in turn, is an extreme measure of the PSD obtained through image analysis (see
Section 2.3). Finally, column 10 shows the information on the unimodality of the PSD curve, and column 11 shows the overall performance metric (
8). It is important to note that, similar to the average particle diameter,
should be as small as possible.
The analysis of the results showed that (i) three of the formulations did not produce stable emulsions (i.e., formulations A2:S3, A3:S1, and A3:S3), which may indicate that resins with lower (lower average molecular weight) have better emulsification properties in water; (ii) the PSD curves obtained for three of the formulations were not unimodal (i.e., formulations A1:S3, A2:S1, and A3:S2), which may indicate that they were physically unstable); (iii) the most promising formulation obtained from the resin with was A1:S2, which was optimized in Stage 2; and (iv) the most promising emulsion obtained from resin A2 was A2:S2, which was also optimized in Stage 2.
To avoid an overly strict limitation of the candidate alternatives, which is common in product design, formulation A1:S1 was also considered for optimization despite its sub-optimal performance (see the value
O in the first line in
Table 5). This was because (i) the concepts resulting from the subsequent stages were widened if at least two surfactants were included in the set of formulations to optimize, and (ii) the deficiency of formulation A1:S1 was related to a single quality characteristic—the viscosity (see the value
in the first line in
Table 5)— indicating good performance in all other criteria. In summary, formulations A1:S1, A1:S2, and A2:S2 were optimized, which is discussed in
Section 4.3.
and
were fixed to
or 0 and none of the formulations that were optimized were based on the resin with a higher
.
4.3. Optimization of Secondary Factors
Now, we optimize the promising formulations identified in Stage 1. The secondary factors used in the optimization were the amount of KOH used relative to the resin weight,
; the agitation rate,
; and the amount of surfactant relative to the resin weight,
. The sequence of the experiments followed the procedure described in
Section 2.2. In contrast to Stage 1, here, we did not use the previously identified factorial plan but followed the sequential optimal design-based approach introduced in
Section 3.2 for each emulsion. The results are shown in
Table 6, which can be interpreted similarly to
Table 5.
The accumulation of experiments followed a sequential order, where the next experiment was prescribed based on the updated model(s) relating the factors to the quality characteristics. It should be noted that the optimization procedure required a different number of experiments for each emulsion (seven experiments for A1:S1 and four for the other two formulations). This was due to the fact that the models fitted with the previously obtained observations had a lower prediction ability, as they relied on a small and sometimes dispersed sample of points. The experiments from Stage 1 were considered the reference conditions and were incorporated into the sequence as the initial condition. The convergence was achieved with an error of 0.053 for A1:S1 and less than 0.03 for the other two formulations, values considered within the specified tolerance. The trajectory to the optimum passed through conditions where no emulsion was formed (see Experiment 11) or the PSD was not unimodal (see Experiment 14), both indicating unstable formulations.
Table 7 presents a summary of the most promising formulations identified in this phase, which are analyzed regarding their experimental repeatability in
Section 4.4. It is important to note that the optimal conditions for emulsions A2:S1 and A2:S2 were identical, so the final decision about the most promising formulation requires an accurate economical analysis.
4.4. Formulations’ Repeatability
Here, we assess the repeatability of the experiments conducted to produce the optimal formulations shown in
Table 7. In our context, repeatability refers to the ability to produce formulations with similar performance quality characteristics under the same experimental conditions. This measure is crucial in the industrialization of a concept, as it represents the inherent variation in the production process (i.e., precision). Processes with lower precision may require more accurate control systems to ensure that the customers’ specifications are systematically achieved. The repeatability analysis followed the guidelines described in
Section 3.2 and the coefficient of variation was determined using Equation (
7).
We replicated the optimal formulations by conducting three additional experiments for each formulation. The optimization experiments from Stage 2 (see
Table 7) were also included in the data set used for analysis. The complete set of observations is shown in
Table 8. The results showed that the coefficient of variation was below 10% for all the quality characteristics, with the exception of viscosity and the particles’
for A1:S1. Additional experiments for this formulation may be necessary to further assess its repeatability. Overall, the results demonstrated the repeatability of the production procedure and the resin–surfactant combinations.