Fabrication of Novel Omeprazole-Based Chitosan Coated Nanoemulgel Formulation for Potential Anti-Microbia; In Vitro and Ex Vivo Characterizations
Abstract
1. Introduction
2. Materials
2.1. Formulation and Optimization of OMP Loaded Nanoemulgel
2.1.1. Nanoemulsion Preparation
2.1.2. Nanoemulsion Stability
2.1.3. Carbopol Gelling Solution Preparation
2.1.4. Nanoemulgel Preparation
2.2. Characterization of Optimized Nanoemulsion & Nanoemulgel Formulations
2.2.1. Thermodynamic Stability Studies
2.2.2. ATR-FTIR Analysis
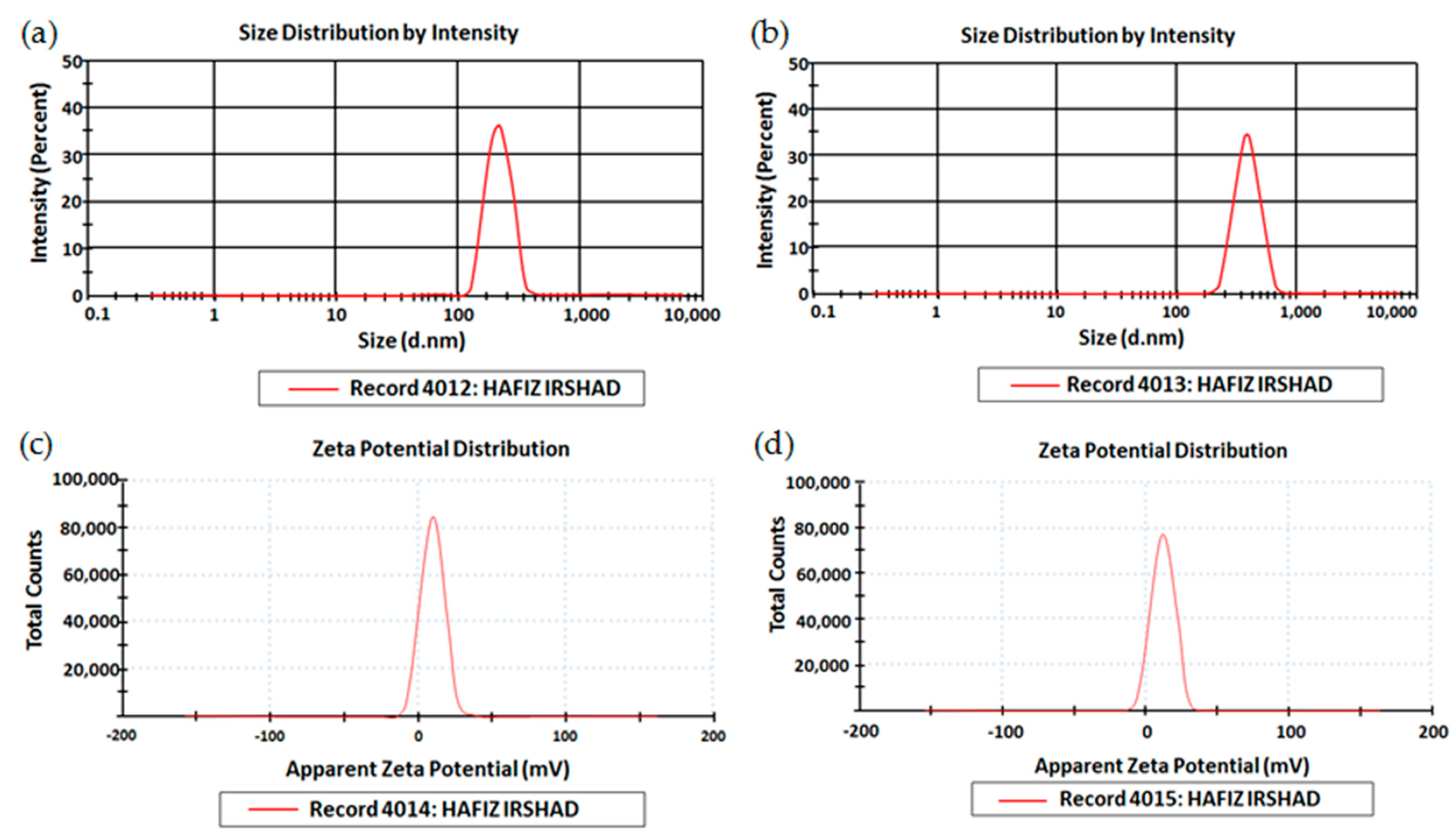
2.2.3. Size, Zeta Potential, and Polydispersity Index (PDI)
2.2.4. pH Determination
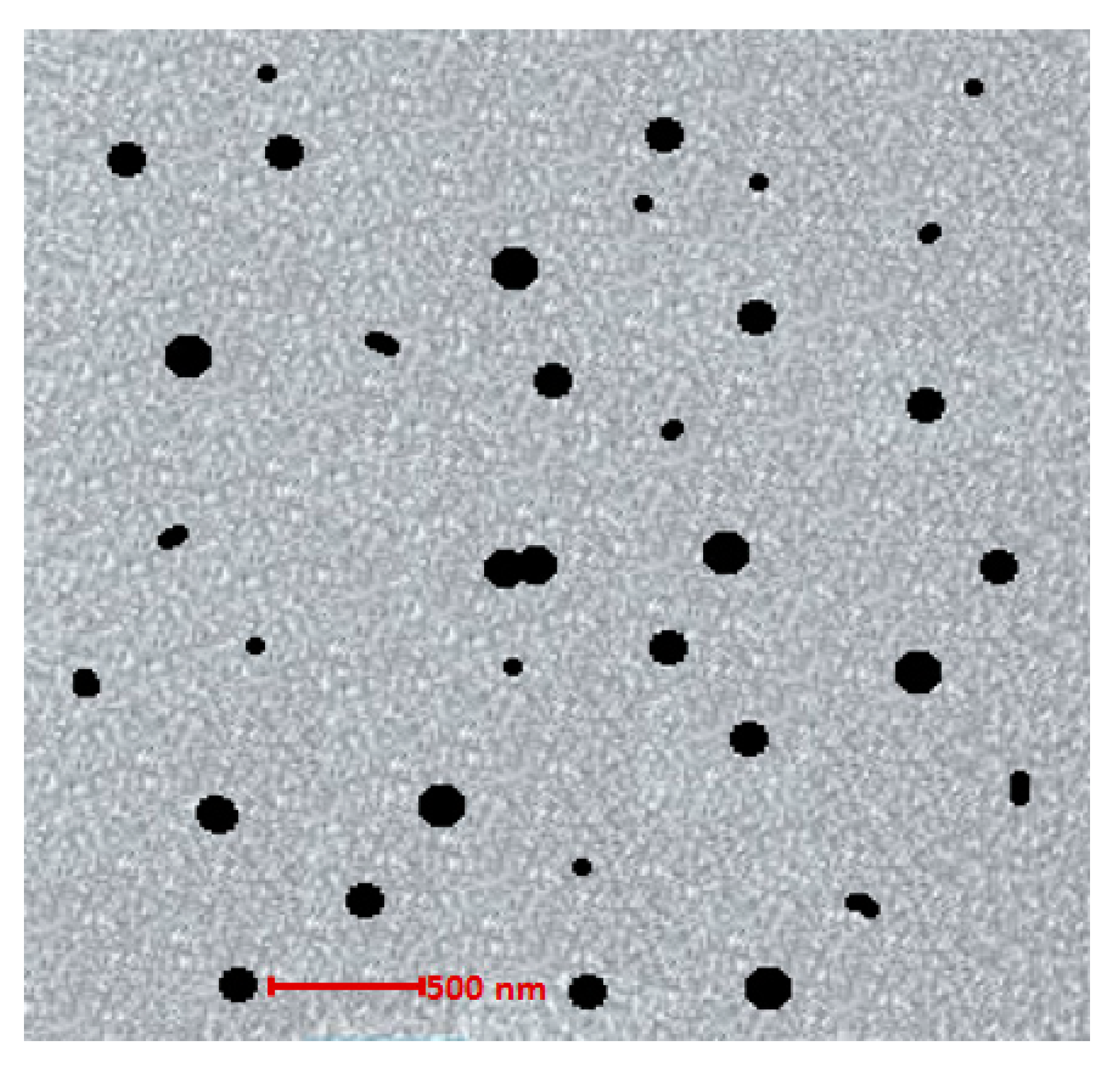
2.2.5. Surface Morphology Analysis
2.2.6. Drug Content
2.2.7. Entrapment Efficiency
2.2.8. Viscosity Determination
2.2.9. Spreadability and Extrudability Estimation
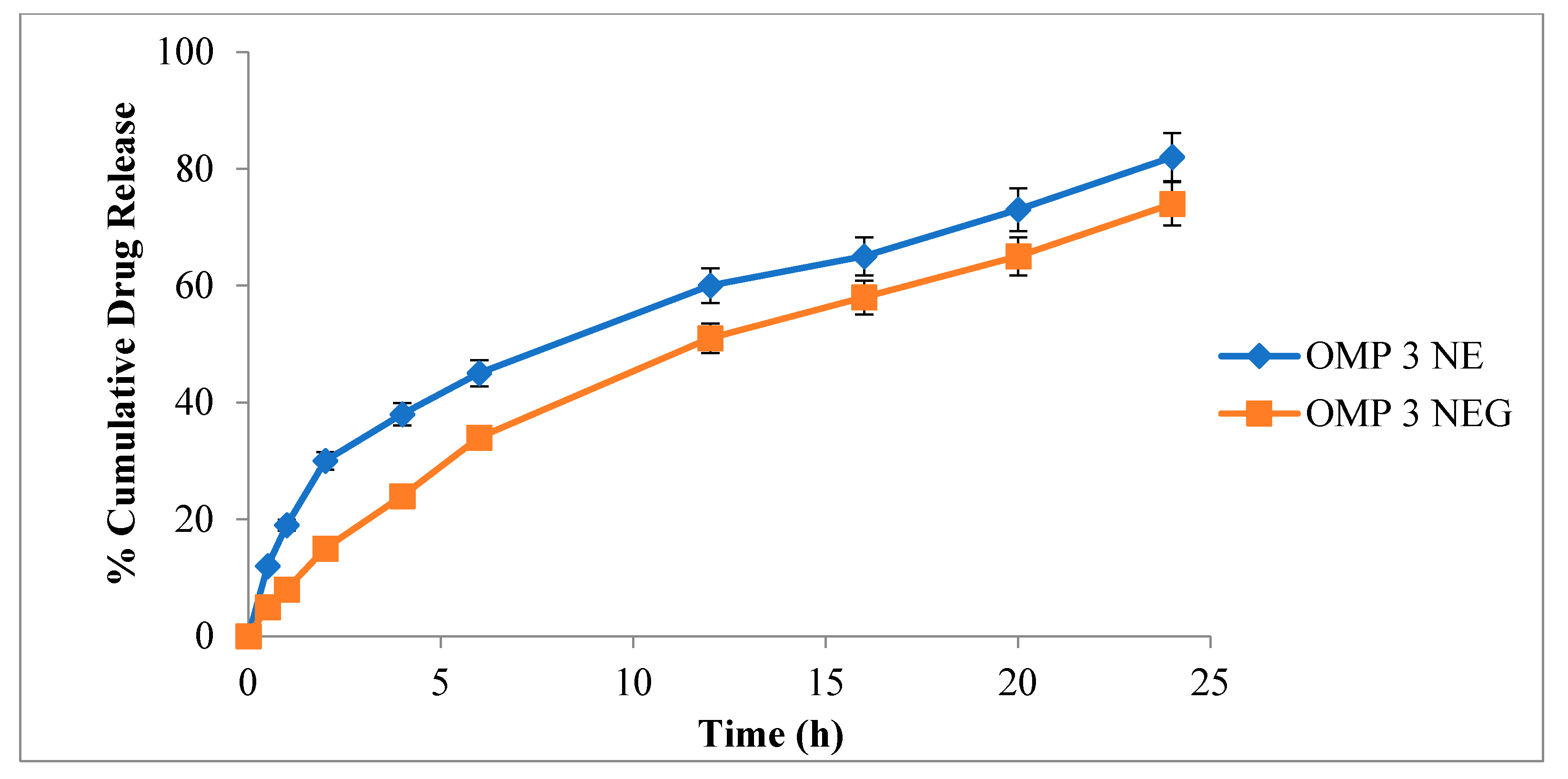
2.3. In-Vitro Drug Release Analysis
2.4. Ex-Vivo Analysis
2.4.1. Ethics
2.4.2. Rabbit Skin Preparation
2.4.3. Ex-Vivo Permeation
2.5. Anti-Microbial Assay
2.6. Stability Studies
2.7. Statistical Analysis
3. Results & Discussion
3.1. Thermodynamic Stability Studies
3.2. ATR-FTIR Analysis (Drug Excipient Compatibility)
3.3. Size, PDI, and Zeta Potential
3.4. pH Determination
3.5. Drug Content Determination
3.6. Entrapment Efficiency
3.7. Surface Morphology Analysis
3.8. Viscosity Determination
3.9. Spreadability & Extrudability Estimation
3.10. In-Vitro Drug Release Evaluation
3.11. Ex-Vivo Permeation
3.12. Microbiological Assay
3.13. Stability Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- da Rosa, T.F.; Foletto, V.S.; Serafin, M.B.; Bottega, A.; Hörner, R. Anti-infective properties of proton pump inhibitors: Perspectives. Int. Microbiol. 2022, 25, 217–222. [Google Scholar] [CrossRef]
- Sharma, V.D.; Akocak, S.; Ilies, M.A.; Fassihi, R. Solid-state interactions at the core-coat interface: Physicochemical characterization of enteric-coated omeprazole pellets without a protective sub-coat. AAPS PharmSciTech 2015, 16, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Khatibi, A.; Zahedi, P.; Ghourchian, H.; Lari, A.S. Development of microfluidic-based cellulose acetate phthalate nanoparticles containing omeprazole for antiulcer activity: In vitro and in vivo evaluations. Eur. Polym. J. 2021, 147, 110294. [Google Scholar] [CrossRef]
- Diefenthaeler, H.S.; Bianchin, M.D.; Marques, M.S.; Nonnenmacher, J.L.; Bender, E.T.; Bender, J.G.; Nery, S.F.; Cichota, L.C.; Külkamp-Guerreiro, I.C. Omeprazole nanoparticles suspension: Development of a stable liquid formulation with a view to pediatric administration. Int. J. Pharm. 2020, 589, 119818. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, J.-P.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug repositioning: A brief overview. J. Pharm. Pharmacol. 2020, 72, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Toshner, M.; Spiekerkoetter, E.; Bogaard, H.; Hansmann, G.; Nikkho, S.; Prins, K.W. Repurposing of medications for pulmonary arterial hypertension. Pulm. Circ. 2020, 10, 2045894020941494. [Google Scholar] [CrossRef]
- Riahi, M.M.; Sahebkar, A.; Sadri, K.; Nikoofal-Sahlabadi, S.; Jaafari, M. Stable and sustained release liposomal formulations of celecoxib: In vitro and in vivo anti-tumor evaluation. Int. J. Pharm. 2018, 540, 89–97. [Google Scholar] [CrossRef]
- Naito, Y.; Terukina, T.; Galli, S.; Kozai, Y.; Vandeweghe, S.; Tagami, T.; Ozeki, T.; Ichikawa, T.; Coelho, P.G.; Jimbo, R. The effect of simvastatin-loaded polymeric microspheres in a critical size bone defect in the rabbit calvaria. Int. J. Pharm. 2014, 461, 157–162. [Google Scholar] [CrossRef]
- Mohammad Sadeghi, H.; Adeli, I.; Mousavi, T.; Daniali, M.; Nikfar, S.; Abdollahi, M. Drug Repurposing for the Management of Depression: Where Do We Stand Currently? Life 2021, 11, 774. [Google Scholar] [CrossRef]
- Travi, B.L. Current status of antihistamine drugs repurposing for infectious diseases. Med. Drug Discov. 2022, 15, 100140. [Google Scholar] [CrossRef]
- MA, S. Repurposing Old Drugs: Substituted Benzodiazepines as New Antibacterial Agents. Masters’s Thesis, Durham University, Durham, UK, 2017. [Google Scholar]
- Spillier, Q.; Vertommen, D.; Ravez, S.; Marteau, R.; Thémans, Q.; Corbet, C.; Feron, O.; Wouters, J.; Frédérick, R. Anti-alcohol abuse drug disulfiram inhibits human PHGDH via disruption of its active tetrameric form through a specific cysteine oxidation. Sci. Rep. 2019, 9, 4737. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Ruokolainen, L.; Suomalainen, A.; Sinkko, H.; Karisola, P.; Lehtimäki, J.; Lehto, M.; Hanski, I.; Alenius, H.; Fyhrquist, N. Soil exposure modifies the gut microbiota and supports immune tolerance in a mouse model. J. Allergy Clin. Immunol. 2019, 143, 1198–1206.e12. [Google Scholar] [CrossRef] [PubMed]
- Morteza-Semnani, K.; Saeedi, M.; Akbari, J.; Eghbali, M.; Babaei, A.; Hashemi, S.M.H.; Nokhodchi, A. Development of a novel nanoemulgel formulation containing cumin essential oil as skin permeation enhancer. Drug Deliv. Transl. Res. 2022, 12, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, D.K.; Sharma, A.; Kaur, N.; Gupta, G.D.; Narang, R.K.; Rai, V.K. Nanoemulgel for efficient topical delivery of finasteride against androgenic alopecia. J. Pharm. Innov. 2021, 16, 735–746. [Google Scholar] [CrossRef]
- Sjöström, J.; Fryklund, J.; Kühler, T.; Larsson, H. In vitro antibacterial activity of omeprazole and its selectivity for Helicobacter spp. are dependent on incubation conditions. Antimicrob. Agents Chemother. 1996, 40, 621–626. [Google Scholar] [CrossRef]
- Iber, B.T.; Kasan, N.A.; Torsabo, D.; Omuwa, J.W. A review of various sources of chitin and chitosan in nature. J. Renew. Mater. 2022, 10, 1097. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Ezhilarasi, P.; Kondamareddy, K.K.; Rajan, D.K.; Sathishkumar, P.; Rajarajeswaran, J.; Conterno, L. Green and eco-friendly approaches for the extraction of chitin and chitosan: A review. Carbohydr. Polym. 2022, 119349. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N.A. Production, properties, and some new applications of chitin and its derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef]
- Wattjes, J.; Niehues, A.; Cord-Landwehr, S.; Hoßbach, J.; David, L.; Delair, T.; Moerschbacher, B.M. Enzymatic production and enzymatic-mass spectrometric fingerprinting analysis of chitosan polymers with different nonrandom patterns of acetylation. J. Am. Chem. Soc. 2019, 141, 3137–3145. [Google Scholar] [CrossRef]
- Negi, A.; Kesari, K.K. Chitosan Nanoparticle Encapsulation of Antibacterial Essential Oils. Micromachines 2022, 13, 1265. [Google Scholar] [CrossRef]
- Bashir, S.M.; Ahmed Rather, G.; Patrício, A.; Haq, Z.; Sheikh, A.A.; Shah, M.Z.u.H.; Singh, H.; Khan, A.A.; Imtiyaz, S.; Ahmad, S.B. Chitosan Nanoparticles: A Versatile Platform for Biomedical Applications. Materials 2022, 15, 6521. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Feng, J.; You, H.; Zhou, S.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. The microstructure, antibacterial and antitumor activities of chitosan oligosaccharides and derivatives. Mar. Drugs 2022, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Gulbake, A.; Shilpi, S.; Jain, A.; Hurkat, P.; Jain, S.K. A new horizon in modifications of chitosan: Syntheses and applications. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 90. [Google Scholar] [CrossRef]
- Khan, A.; Alamry, K.A. Recent advances of emerging green chitosan-based biomaterials with potential biomedical applications: A review. Carbohydr. Res. 2021, 506, 108368. [Google Scholar] [CrossRef]
- Raafat, D.; Von Bargen, K.; Haas, A.; Sahl, H.-G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef]
- Pasanphan, W.; Buettner, G.R.; Chirachanchai, S. Chitosan gallate as a novel potential polysaccharide antioxidant: An EPR study. Carbohydr. Res. 2010, 345, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Gulati, N.; Dua, K.; Dureja, H. Role of chitosan based nanomedicines in the treatment of chronic respiratory diseases. Int. J. Biol. Macromol. 2021, 185, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Sen, K.K.; Gandhi, A.; Jana, S.; Roy, C. Drug Delivery Applications of Chitosan. In Industrial Applications of Marine Biopolymers; CRC Press: Boca Raton, FL, USA, 2017; pp. 305–349. [Google Scholar]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Uluata, S.; Decker, E.A.; McClements, D.J. Optimization of nanoemulsion fabrication using microfluidization: Role of surfactant concentration on formation and stability. Food Biophys. 2016, 11, 52–59. [Google Scholar] [CrossRef]
- Sakeena, M.H.F.; Muyhanna, F.A.; Ghassan, Z.A.; Kanakal, M.M.; Elrashid, S.M.; Elrashid, S.M.; Munavvar, A.S.; Azmin, M.N. Formulation and in vitro Evaluation of Ketoprofen in Palm Oil Esters Nanoemulsion for Topical Delivery. J. Oleo Sci. 2010, 59, 223–228. [Google Scholar] [CrossRef]
- Nnamani, P.O.; Ugwu, A.A.; Nnadi, O.H.; Kenechukwu, F.C.; Ofokansi, K.C.; Attama, A.A.; Lehr, C.M. Formulation and evalu-ation of transdermal nanogel for delivery of artemether. Drug Deliv. Transl. Res. 2021, 11, 1655–1674. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Dudhe, R.; Sharma, P. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Shaker, D.S.; Ishak, R.A.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A review on mechanisms for the transdermal delivery of hydrophobic and hydrophilic drugs. Sci. Pharm. 2019, 87, 17. [Google Scholar] [CrossRef]
- Carvalho, V.F.; Salata, G.C.; de Matos, J.K.; Costa-Fernandez, S.; Chorilli, M.; Steiner, A.A.; de Araujo, G.L.; Silveira, E.R.; Costa-Lotufo, L.V.; Lopes, L.B. Optimization of composition and obtainment parameters of biocompatible nanoemulsions intended for intraductal administration of piplartine (piperlongumine) and mammary tissue targeting. Int. J. Pharm. 2019, 567, 118460. [Google Scholar] [CrossRef]
- Leonyza, A.; Surini, S. Optimization of sodium deoxycholate-based transfersomes for percutaneous delivery of peptides and proteins. Int. J. Appl. Pharm. 2019, 11, 329–332. [Google Scholar] [CrossRef]
- Rashid, S.A.; Bashir, S.; Ullah, H.; Khan, D.H.; Shah, P.A.; Danish, M.Z.; Khan, M.H.; Mahmood, S.; Sohaib, M.; Irfan, M.M. Development, characterization and optimization of methotrexate-olive oil nano-emulsion for topical application. Pak. J. Pharm. Sci. 2021, 34, 205–215. [Google Scholar]
- Tirmiara, N.; Reveny, J.; Silalahi, J. Formulation and Evaluation of Moringa Seed Oil Nanoemulsion Gel. Asian J. Pharm. Res. Dev. 2019, 7, 1–5. [Google Scholar] [CrossRef]
- da Silva, T.N.; Reynaud, F.; de Souza Picciani, P.H.; e Silva, K.G.d.H.; Barradas, T.N. Chitosan-based films containing nanoemulsions of methyl salicylate: Formulation development, physical-chemical and in vitro drug release characterization. Int. J. Biol. Macromol. 2020, 164, 2558–2568. [Google Scholar] [CrossRef]
- Saraogi, G.K.; Tholiya, S.; Mishra, Y.; Mishra, V.; Albutti, A.; Nayak, P.; Tambuwala, M.M. Formulation development and evaluation of pravastatin-loaded nanogel for hyperlipidemia management. Gels 2022, 8, 81. [Google Scholar] [CrossRef]
- Rajitha, P.; Shammika, P.; Aiswarya, S.; Gopikrishnan, A.; Jayakumar, R.; Sabitha, M. Chaulmoogra oil based methotrexate loaded topical nanoemulsion for the treatment of psoriasis. J. Drug Deliv. Sci. Technol. 2019, 49, 463–476. [Google Scholar] [CrossRef]
- Gurpreet, K.; Singh, S. Review of nanoemulsion formulation and characterization techniques. Indian J. Pharm. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Khan, R.U.; Shah, S.U.; Rashid, S.A.; Naseem, F.; Shah, K.U.; Farid, A.; Hakeem, K.R.; Kamli, M.R.; Althubaiti, E.H.; Alamoudi, S.A. Lornoxicam-Loaded Chitosan-Decorated Nanoemulsion: Preparation and In Vitro Evaluation for Enhanced Transdermal Delivery. Polymers 2022, 14, 1922. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, N.A.; Fernandes, A.V.; Volfová, G.; Pydi, C.R.; Kumar, L.; Verma, R.; Marques, S.M.; Shirodkar, R.K. Development of cream to enhance the antifungal activity and reduce the side effects of fluconazole for the treatment of Candida albicans. Tenside Surfactants Deterg. 2022, 59, 231–239. [Google Scholar] [CrossRef]
- Thamer, A.K.; Abood, A.N. Preparation and In vitro Characterization of Aceclofenac Nanosuspension (ACNS) for Enhancement of Percutaneous Absorption using Hydrogel Dosage Form. Iraqi J. Pharm. Sci. 2021, 30, 86–98. [Google Scholar] [CrossRef]
- Mohammed, M.; Rozyanty, R.; Mohammed, A.M.; Osman, A.F.; Adam, T.; Dahham, O.S.; Hashim, U.; Noriman, N.Z.; Betar, B.O. Fabrication and characterization of zinc oxide nanoparticle-treated kenaf polymer composites for weather resistance based on a solar UV radiation. BioResources 2018, 13, 6480–6496. [Google Scholar] [CrossRef]
- Miastkowska, M.; Śliwa, P. Influence of terpene type on the release from an O/W nanoemulsion: Experimental and theoretical studies. Molecules 2020, 25, 2747. [Google Scholar] [CrossRef]
- Sharma, S.K.; Al-Badi, A.H.; Govindaluri, S.M.; Al-Kharusi, M.H. Predicting motivators of cloud computing adoption: A developing country perspective. Comput. Hum. Behav. 2016, 62, 61–69. [Google Scholar] [CrossRef]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef]
- Akhtar, N.; Singh, V.; Yusuf, M.; Khan, R.A. Non-invasive drug delivery technology: Development and current status of transdermal drug delivery devices, techniques and biomedical applications. Biomed. Eng./Biomed. Tech. 2020, 65, 243–272. [Google Scholar] [CrossRef]
- Bolla, P.K.; Clark, B.A.; Juluri, A.; Cheruvu, H.S.; Renukuntla, J. Evaluation of formulation parameters on permeation of ibuprofen from topical formulations using Strat-M® membrane. Pharmaceutics 2020, 12, 151. [Google Scholar] [CrossRef]
- Bakshi, P.; Jiang, Y.; Nakata, T.; Akaki, J.; Matsuoka, N.; Banga, A.K. Formulation development and characterization of nanoemulsion-based formulation for topical delivery of heparinoid. J. Pharm. Sci. 2018, 107, 2883–2890. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment strategies for infected wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed]
- Sunderkötter, C.; Becker, K. Frequent bacterial skin and soft tissue infections: Diagnostic signs and treatment. JDDG J. Dtsch. Dermatol. Ges. 2015, 13, 501–526. [Google Scholar] [CrossRef]
- Kamel, R.; El-Wakil, N.A.; Abdelkhalek, A.A.; Elkasabgy, N.A. Topical cellulose nanocrystals-stabilized nanoemulgel loaded with ciprofloxacin HCl with enhanced antibacterial activity and tissue regenerative properties. J. Drug Deliv. Sci. Technol. 2021, 64, 102553. [Google Scholar] [CrossRef]
- Moradi, M.; Hasanvandian, F.; Isari, A.A.; Hayati, F.; Kakavandi, B.; Setayesh, S.R. CuO and ZnO co-anchored on g-C3N4 nanosheets as an affordable double Z-scheme nanocomposite for photocatalytic decontamination of amoxicillin. Appl. Catal. B Environ. 2021, 285, 119838. [Google Scholar] [CrossRef]
- Latif, M.S.; Al-Harbi, F.F.; Nawaz, A.; Rashid, S.A.; Farid, A.; Mohaini, M.A.; Alsalman, A.J.; Hawaj, M.A.A.; Alhashem, Y.N. Formulation and Evaluation of Hydrophilic Polymer Based Methotrexate Patches: In Vitro and In Vivo Characterization. Polymers 2022, 14, 1310. [Google Scholar] [CrossRef]



| F. Codes | Omeprazole | Olive Oil | Tween 80 | Span 80 | Distilled Water |
|---|---|---|---|---|---|
| Blank NE | - | 10 | 15 | 3.5 | 71.5 |
| OMP1NE | 0.3 | 7.5 | 10 | 6.5 | 75.7 |
| OMP2NE | 0.3 | 12.5 | 13 | 10 | 64.2 |
| OMP3NE | 0.3 | 10 | 15 | 3.5 | 71.2 |
| Formulations | Temperature (°C) | Odor Change | Appearance | Phase Separation | Centrifugation Stability | Thermodynamic Test |
|---|---|---|---|---|---|---|
| Blank NE | 8 | No | Light brown | No | ** | *** |
| 25 | No | Light brown | No | ** | *** | |
| 40 | No | Light brown | No | ** | *** | |
| OMP3NE | 8 | No | Light brown | No | ** | *** |
| 25 | No | Light brown | No | ** | *** | |
| 40 | No | Light brown | No | ** | *** | |
| OMP3NEG | 8 | No | Light brown | No | ** | *** |
| 25 | No | Light brown | No | ** | *** | |
| 40 | No | Light brown | No | ** | *** |
| Day | Viscosity (mPa s) | |||||
|---|---|---|---|---|---|---|
| 8 °C | 25 °C | 40 °C | ||||
| OMP3NE | OMP3NEG | OMP3NE | OMP3NEG | OMPNE | OMP3NEG | |
| 0 | 5889 ± 8.62 | 12,455 ± 14.53 | 5889 ± 8.62 | 12,455 ± 14.53 | 5889 ± 8.62 | 12,455 ± 14.53 |
| 1 | 5793 ± 7.26 | 12,299 ± 13.63 | 5801 ± 8.23 | 12,366 ± 12.58 | 5843 ± 7.89 | 12,378 ± 13.54 |
| 2 | 5710 ± 7.77 | 12,150 ± 14.01 | 5745 ± 9.65 | 12,108 ± 11.47 | 5796 ± 7.62 | 12,198 ± 12.49 |
| 7 | 5655 ± 8.11 | 11,988 ± 13.31 | 5547 ± 7.62 | 11,785 ± 11.23 | 5579 ± 8.41 | 11,847 ± 12.69 |
| 14 | 5449 ± 8.91 | 11,929 ± 13.92 | 5481 ± 8.22 | 11,547 ± 12.36 | 5436 ± 9.55 | 11,785 ± 11.23 |
| 28 | 5385 ± 7.72 | 11,560 ± 15.21 | 5300 ± 7.56 | 11,498 ± 12.84 | 5317 ± 7.62 | 11,542 ± 12.82 |
| Formulations | Spreadability | ||
|---|---|---|---|
| 8 °C | 25 °C | 40 °C | |
| OMP3NE | 18.37 ± 1.09 | 22.61 ± 1.53 | 27.33 ± 1.78 |
| OMP3NEG | 13.89 ± 1.32 | 17.31 ± 1.41 | 21.64 ± 1.59 |
| Parameters | Temperature | |
|---|---|---|
| 4 ± 2 °C | 40 ± 2 °C | |
| Particle Size [4] | 365.3 ± 6.32 | 367.1 ± 6.12 |
| PDI | 0.313 ± 0.31 | 0.311 ± 0.13 |
| Zeta Potential (mV) | −15.7 ± 6.1 | −15.1 ± 6.5 |
| pH | 6.21 ± 0.21 | 6.12 ± 0.42 |
| Phase Separation | Nil | Nil |
| Clarity | Transparent and Clear | Transparent and Clear |
| Drug Content (%) ± SD | 90.89 ± 0.12 | 90.78 ± 0.10 |
| Color Change | No Change | No Change |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, I.; Alhodaib, A.; Naz, I.; Ahmad, W.; Ullah, H.; Amin, A.; Nawaz, A. Fabrication of Novel Omeprazole-Based Chitosan Coated Nanoemulgel Formulation for Potential Anti-Microbia; In Vitro and Ex Vivo Characterizations. Polymers 2023, 15, 1298. https://doi.org/10.3390/polym15051298
Ullah I, Alhodaib A, Naz I, Ahmad W, Ullah H, Amin A, Nawaz A. Fabrication of Novel Omeprazole-Based Chitosan Coated Nanoemulgel Formulation for Potential Anti-Microbia; In Vitro and Ex Vivo Characterizations. Polymers. 2023; 15(5):1298. https://doi.org/10.3390/polym15051298
Chicago/Turabian StyleUllah, Irshad, Aiyeshah Alhodaib, Iffat Naz, Waqar Ahmad, Hidayat Ullah, Adnan Amin, and Asif Nawaz. 2023. "Fabrication of Novel Omeprazole-Based Chitosan Coated Nanoemulgel Formulation for Potential Anti-Microbia; In Vitro and Ex Vivo Characterizations" Polymers 15, no. 5: 1298. https://doi.org/10.3390/polym15051298
APA StyleUllah, I., Alhodaib, A., Naz, I., Ahmad, W., Ullah, H., Amin, A., & Nawaz, A. (2023). Fabrication of Novel Omeprazole-Based Chitosan Coated Nanoemulgel Formulation for Potential Anti-Microbia; In Vitro and Ex Vivo Characterizations. Polymers, 15(5), 1298. https://doi.org/10.3390/polym15051298








