Quaternized Poly(N,N′-dimethylaminoethyl methacrylate) Star Nanostructures in the Solution and on the Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
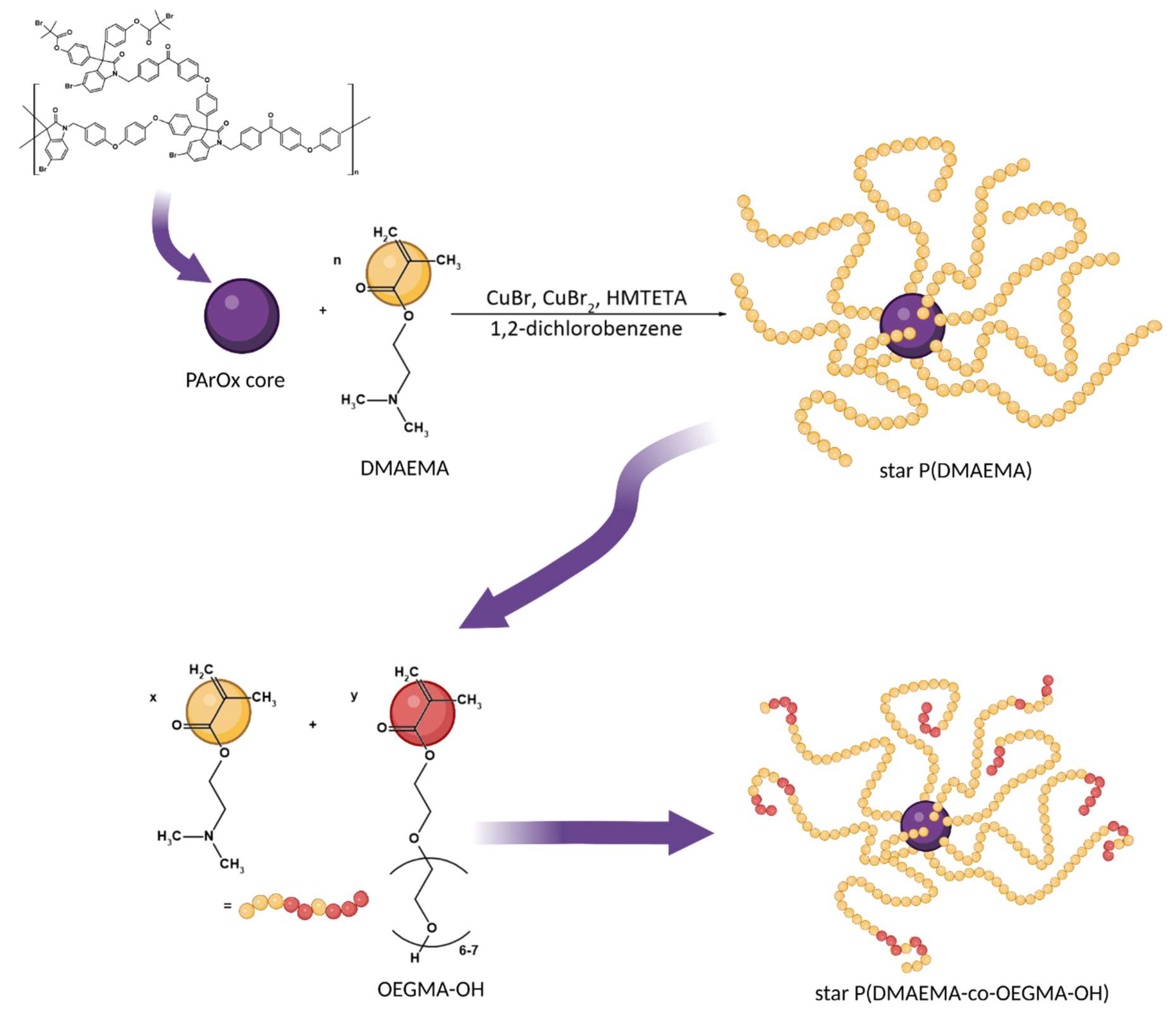
2.2. Synthesis of the Quaternized Star Polymers
2.3. Formation of Layers Composed of Quaternized Star Polymers
2.4. Evaluation of Antibacterial Activity of the Star Copolymer Nanolayers
2.5. Methods
3. Results and Discussion
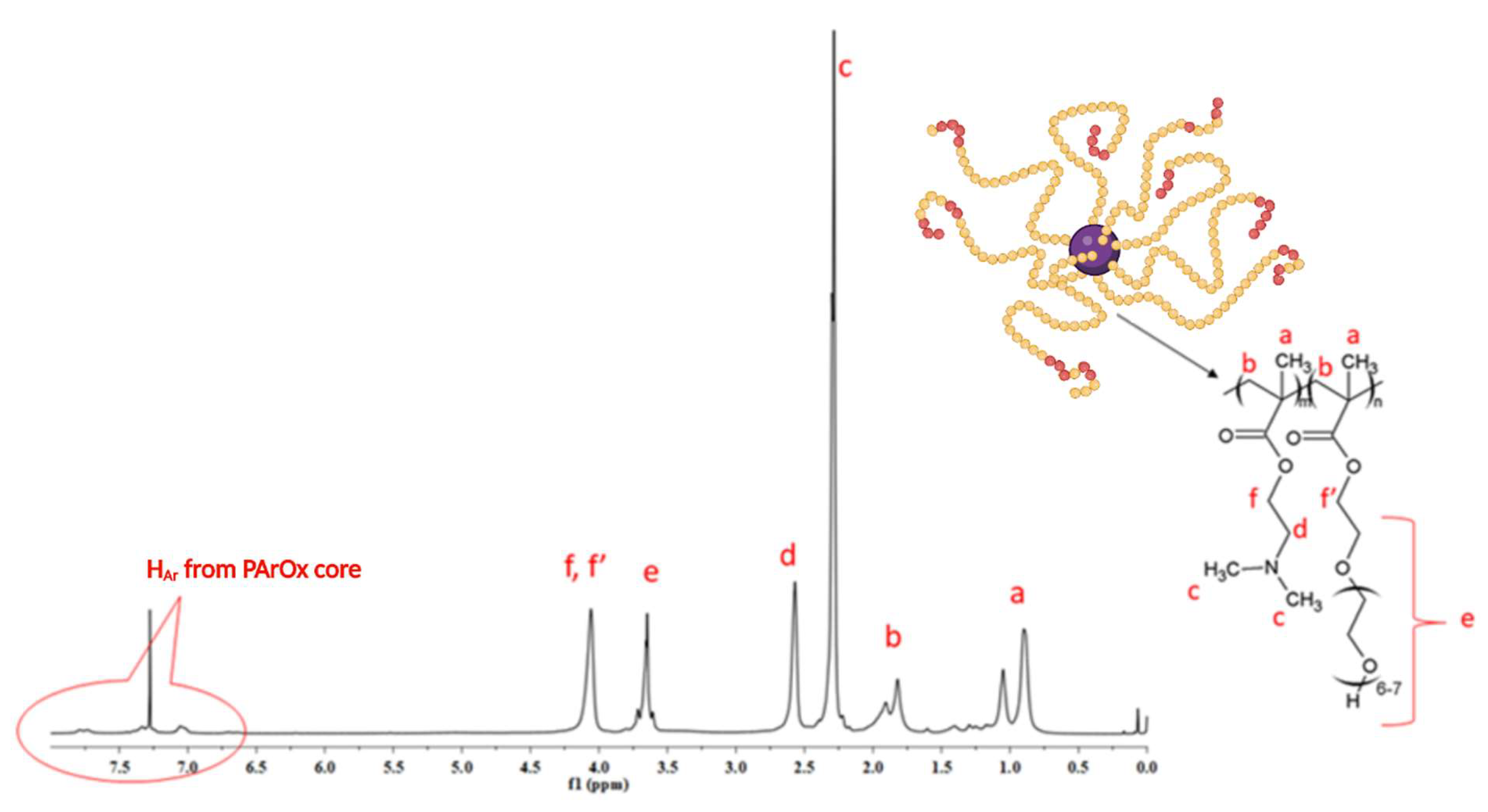
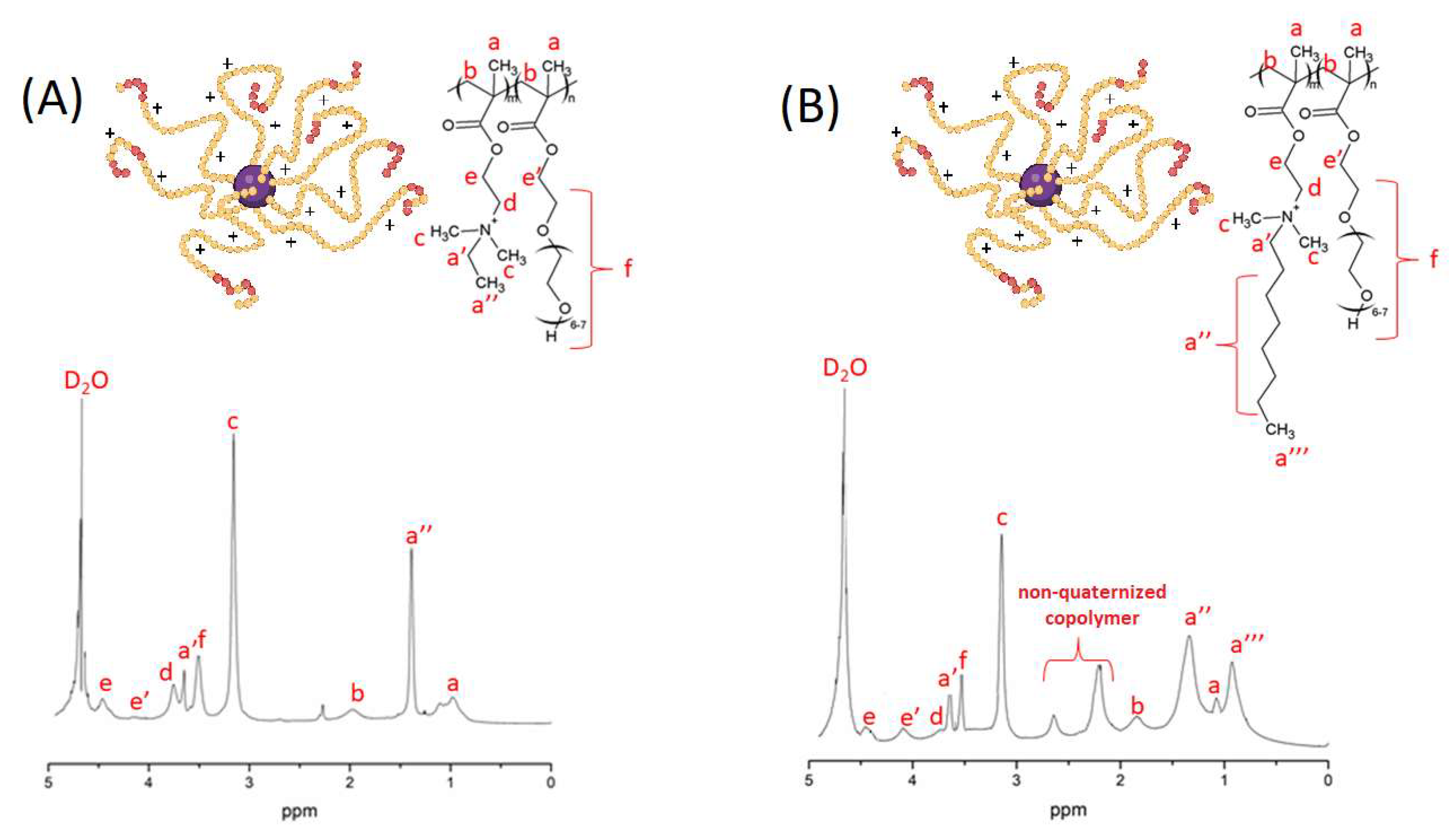
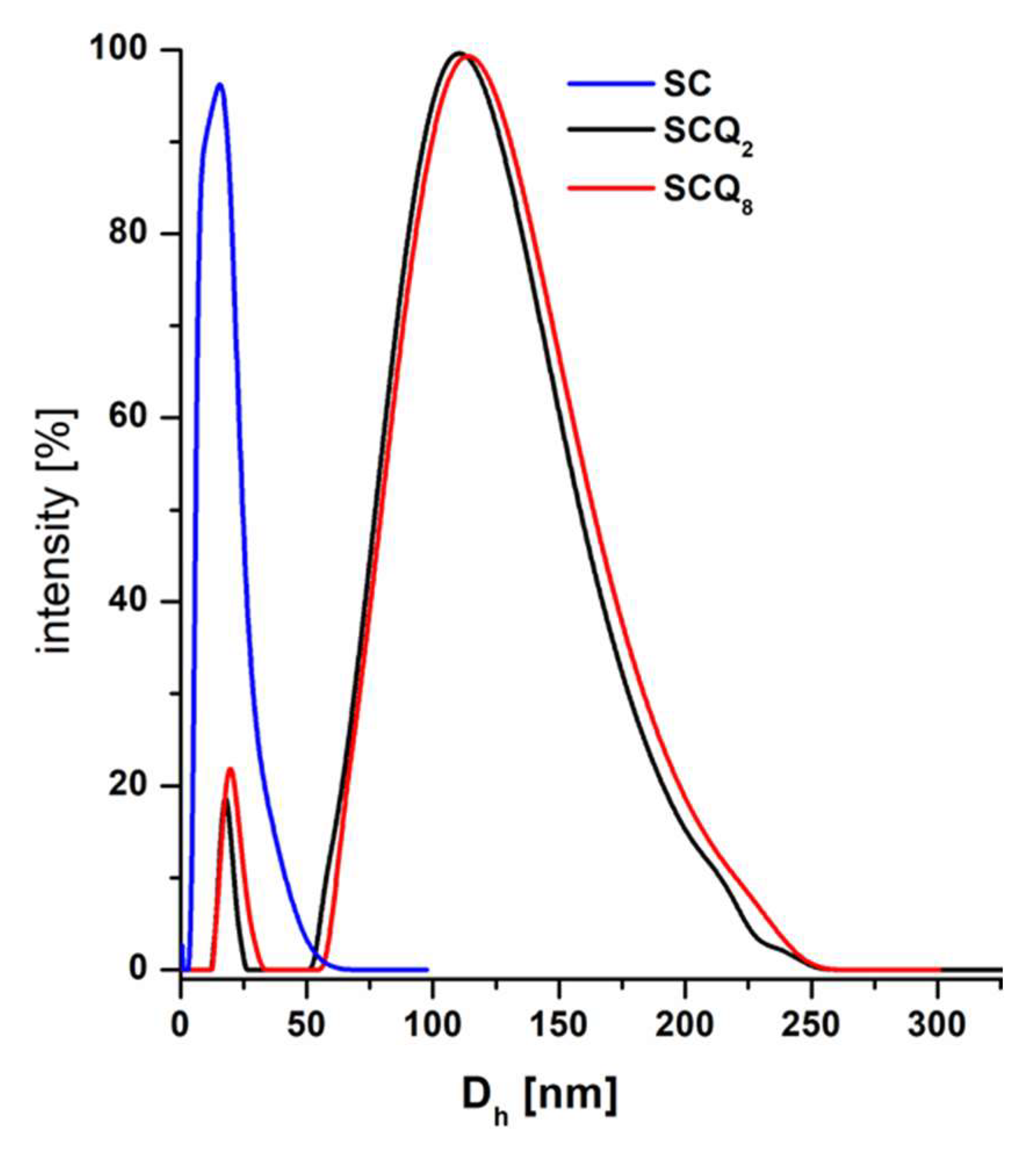
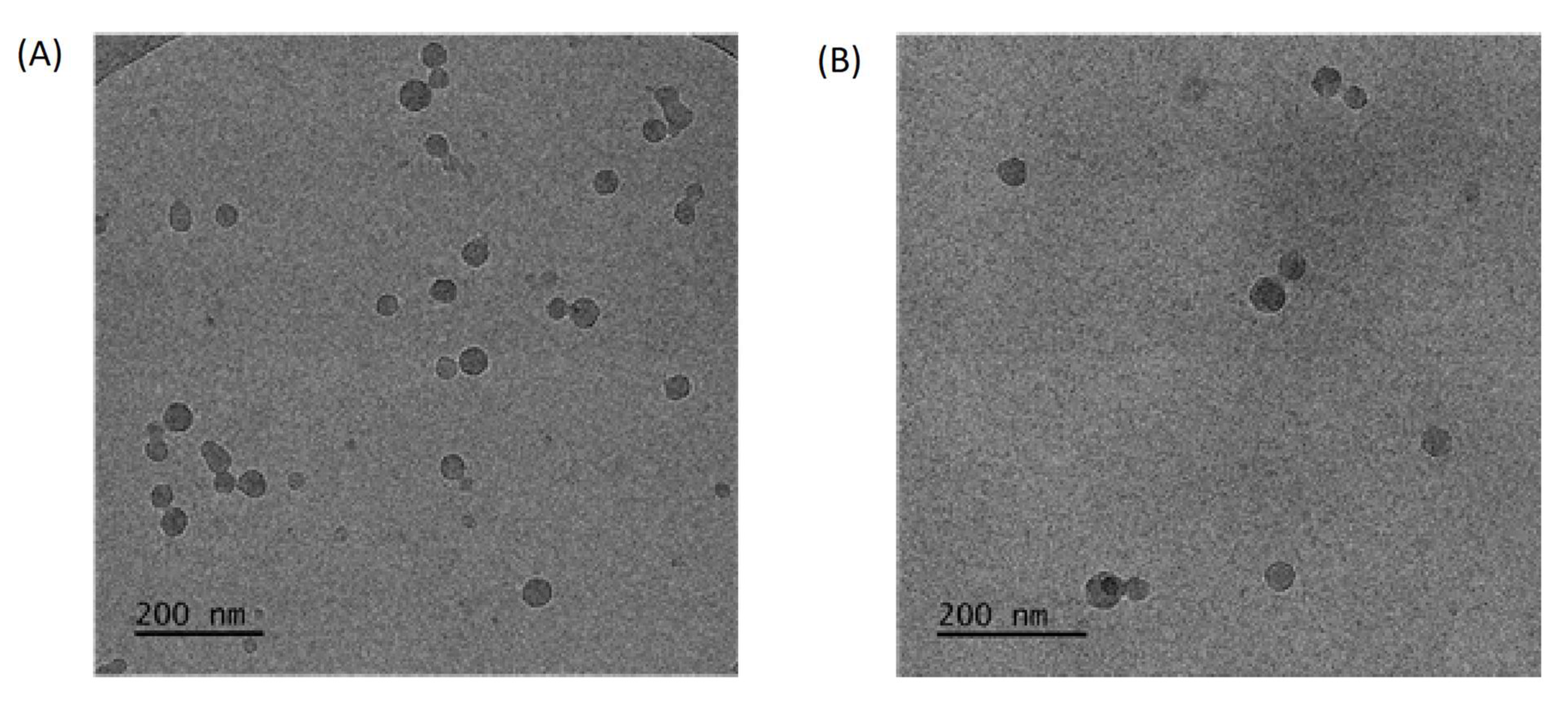
3.1. Synthesis and Solution Behavior of Quaternized Star Copolymer
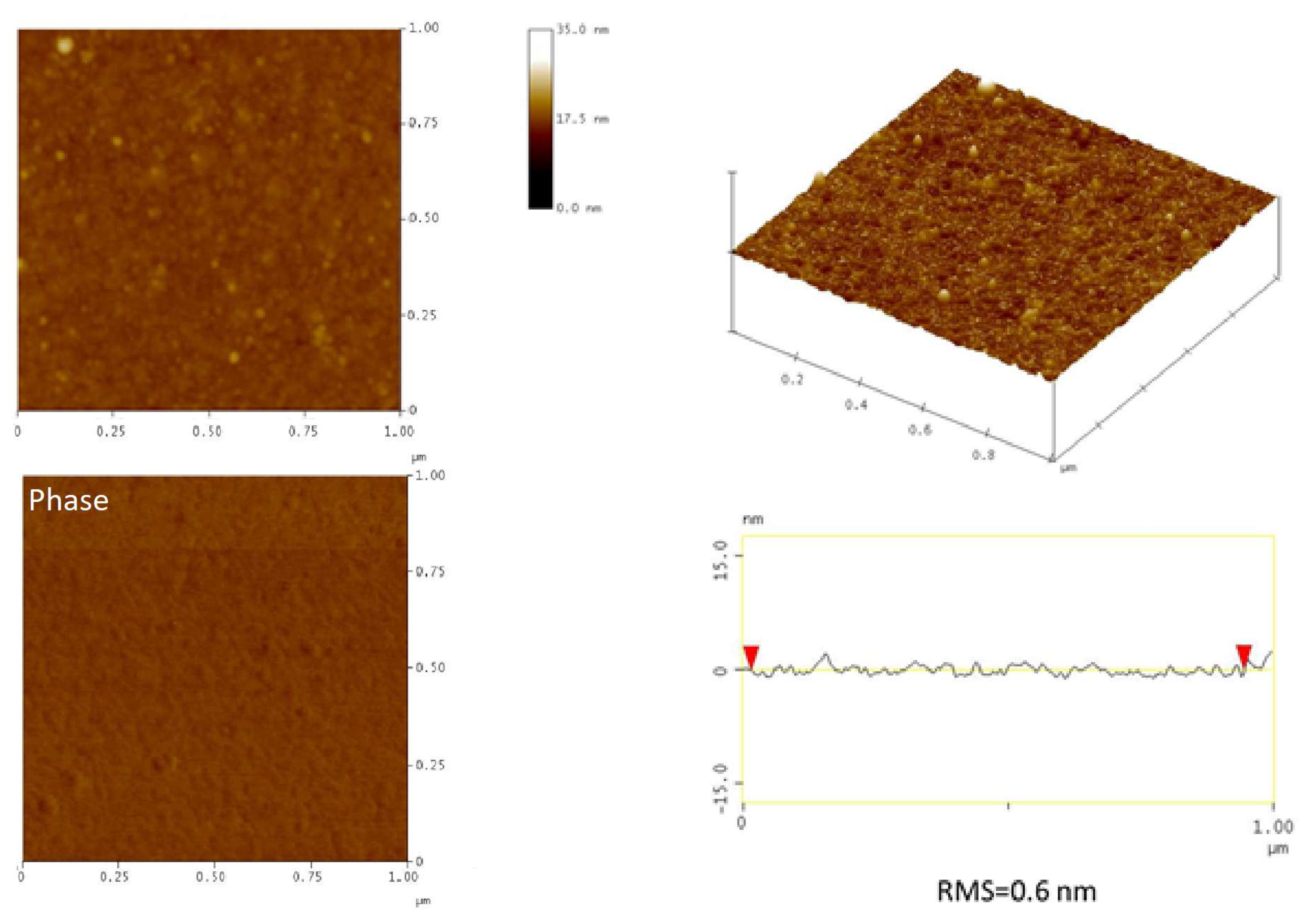
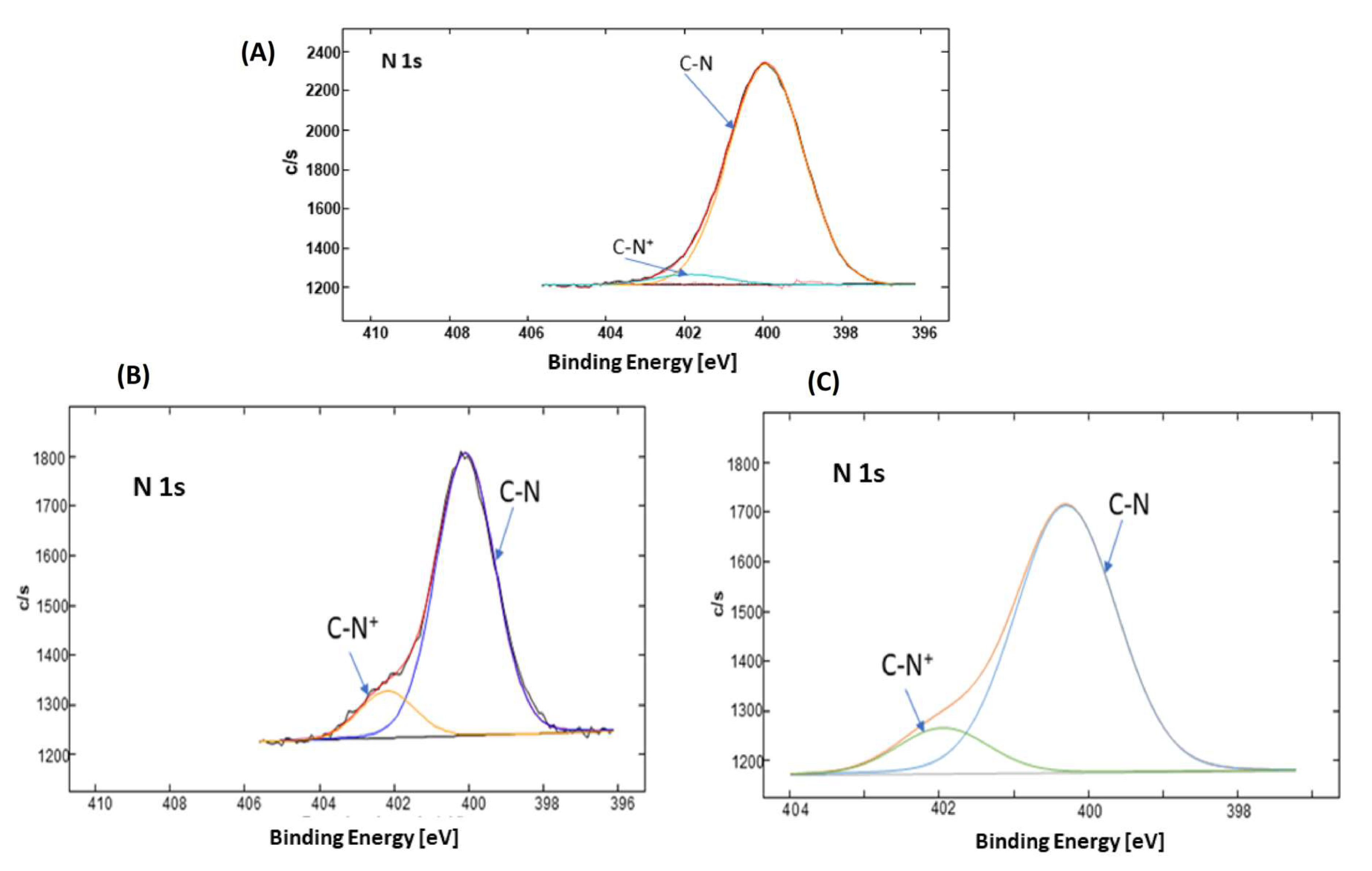
3.2. Formation and Quaternization of Star P(DMAEMA-co-OEGMA-OH) Layers
3.3. Evaluation of Antibacterial Activity of the Star Nanolayers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siedenbiedel, F.; Fuchs, A.; Moll, T.; Weide, M.; Breves, R.; Tiller, J.C. Star-Shaped Poly(Styrene)-Block -Poly(4-Vinyl-N -Methylpyridiniumiodide) for Semipermanent Antimicrobial Coatings. Macromol. Biosci. 2013, 13, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Kugler, R. Evidence of a Charge-Density Threshold for Optimum Efficiency of Biocidal Cationic Surfaces. Microbiology 2005, 151, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Murata, H.; Koepsel, R.R.; Russell, A.J.; Matyjaszewski, K. Antibacterial Polypropylene via Surface-Initiated Atom Transfer Radical Polymerization. Biomacromolecules 2007, 8, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Koepsel, R.R.; Matyjaszewski, K.; Russell, A.J. Permanent, Non-Leaching Antibacterial Surfaces—2: How High Density Cationic Surfaces Kill Bacterial Cells. Biomaterials 2007, 28, 4870–4879. [Google Scholar] [CrossRef]
- Vigliotta, G.; Mella, M.; Rega, D.; Izzo, L. Modulating Antimicrobial Activity by Synthesis: Dendritic Copolymers Based on Nonquaternized 2-(Dimethylamino)Ethyl Methacrylate by Cu-Mediated ATRP. Biomacromolecules 2012, 13, 833–841. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Tian, Z.; Sen, A.; Allcock, H.R. Preparation of Quaternized Organic–Inorganic Hybrid Brush Polyphosphazene-Co-Poly(2-(Dimethylamino)Ethyl Methacrylate) Electrospun Fibers and Their Antibacterial Properties. Polym. Chem. 2012, 3, 2082–2091. [Google Scholar] [CrossRef]
- Weng, Y.; Guo, X.; Zhao, J.; Gregory, R.L.; Xie, D. A PQAS-Containing Glass-Ionomer Cement for Improved Antibacterial Function. J. Biomed. Sci. Eng. 2010, 3, 956–963. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, Y.; Zhang, W.; Lang, M. In-Situ Formation of Silver Nanoparticles Stabilized by Amphiphilic Star-Shaped Copolymer and Their Catalytic Application. Appl. Surf. Sci. 2012, 258, 2655–2660. [Google Scholar] [CrossRef]
- Majumdar, P.; Lee, E.; Gubbins, N.; Stafslien, S.J.; Daniels, J.; Thorson, C.J.; Chisholm, B.J. Synthesis and Antimicrobial Activity of Quaternary Ammonium-Functionalized POSS (Q-POSS) and Polysiloxane Coatings Containing Q-POSS. Polymer 2009, 50, 1124–1133. [Google Scholar] [CrossRef]
- Waschinski, C.J.; Herdes, V.; Schueler, F.; Tiller, J.C. Influence of Satellite Groups on Telechelic Antimicrobial Functions of Polyoxazolines. Macromol. Biosci. 2005, 5, 149–156. [Google Scholar] [CrossRef]
- Lu, G.; Wu, D.; Fu, R. Studies on the Synthesis and Antibacterial Activities of Polymeric Quaternary Ammonium Salts from Dimethylaminoethyl Methacrylate. React. Funct. Polym. 2007, 67, 355–366. [Google Scholar] [CrossRef]
- Yancheva, E.; Paneva, D.; Maximova, V.; Mespouille, L.; Dubois, P.; Manolova, N.; Rashkov, I. Polyelectrolyte Complexes between [Cross-Linked] N-Carboxyethylchitosan and (Quaternized) Poly(2-(Dimethylamino)Ethyl Methacrylate): Preparation, Characterization, and Antibacterial Properties. Biomacromolecules 2007, 8, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bolisetty, S.; Drechsler, M.; Fang, B.; Yuan, J.; Ballauff, M.; Müller, A.H.E. PH and Salt Responsive Poly(N,N-Dimethylaminoethyl Methacrylate) Cylindrical Brushes and Their Quaternized Derivatives. Polymer 2008, 49, 3957–3964. [Google Scholar] [CrossRef]
- Roy, D.; Knapp, J.S.; Guthrie, J.T.; Perrier, S. Antibacterial Cellulose Fiber via RAFT Surface Graft Polymerization. Biomacromolecules 2007, 9, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Koepsel, R.R.; Morley, S.W.; Matyjaszewski, K.; Sun, Y.; Russell, A.J. Permanent, Nonleaching Antibacterial Surfaces. 1. Synthesis by Atom Transfer Radical Polymerization. Biomacromolecules 2004, 5, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhu, X.; Shi, Z.L.; Neoh, K.G.; Kang, E.T. Polymer Microspheres with Permanent Antibacterial Surface from Surface-Initiated Atom Transfer Radical Polymerization. Ind. Eng. Chem. Res. 2005, 44, 7098–7104. [Google Scholar] [CrossRef]
- Yuan, S.J.; Pehkonen, S.O.; Ting, Y.P.; Neoh, K.G.; Kang, E.T. Antibacterial Inorganic−Organic Hybrid Coatings on Stainless Steel via Consecutive Surface-Initiated Atom Transfer Radical Polymerization for Biocorrosion Prevention. Langmuir 2009, 26, 6728–6736. [Google Scholar] [CrossRef]
- Mcgrath, J.E.; Hickner, M.A.; Höfer, R. Polymers for a Sustainable Environment and Green Energy, In Polymer Science: A Comprehensive Reference. Vol. 10; Matyjaszewski, K., Möller, M., Eds.; Elsevier, Cop: Amsterdam, The Netherlands, 2012; ISBN 9780444533494. [Google Scholar]
- Teper, P.; Chojniak-Gronek, J.; Hercog, A.; Oleszko-Torbus, N.; Płaza, G.; Kubacki, J.; Balin, K.; Kowalczuk, A.; Mendrek, B. Nanolayers of Poly[N,N′-Dimethylaminoethyl Methacrylate] with a Star Topology and Their Antibacterial Activity. Polymers 2020, 12, 230. [Google Scholar] [CrossRef]
- Gao, H. Development of Star Polymers as Unimolecular Containers for Nanomaterials. Macromol. Rapid Commun. 2012, 33, 722–734. [Google Scholar] [CrossRef]
- Forgham, H.; Zhu, J.; Qiao, R.; Davis, T.P. Star Polymer Nanomedicines─Challenges and Future Perspectives. ACS Appl. Polym. Mater. 2022, 4, 6784–6796. [Google Scholar] [CrossRef]
- Teper, P.; Oleszko-Torbus, N.; Bochenek, M.; Hajduk, B.; Kubacki, J.; Jałowiecki, Ł.; Płaza, G.; Kowalczuk, A.; Mendrek, B. Hybrid Nanolayers of Star Polymers and Silver Nanoparticles with Antibacterial Activity. Colloids Surf. B Biointerfaces 2022, 213, 112404. [Google Scholar] [CrossRef] [PubMed]
- Mendrek, B.; Fus-Kujawa, A.; Teper, P.; Botor, M.; Kubacki, J.; Sieroń, A.L.; Kowalczuk, A. Star Polymer-Based Nanolayers with Immobilized Complexes of Polycationic Stars and DNA for Deposition Gene Delivery and Recovery of Intact Transfected Cells. Int. J. Pharm. 2020, 589, 119823. [Google Scholar] [CrossRef] [PubMed]
- Gasteier, P.; Reska, A.; Schulte, P.; Salber, J.; Offenhäusser, A.; Moeller, M.; Groll, J. Surface Grafting of PEO-Based Star-Shaped Molecules for Bioanalytical and Biomedical Applications. Macromol. Biosci. 2007, 7, 1010–1023. [Google Scholar] [CrossRef] [PubMed]
- Plamper, F.A.; Schmalz, A.; Penott-Chang, E.; Drechsler, M.; Jusufi, A.; Ballauff, M.; Müller, A.H.E. Synthesis and Characterization of Star-Shaped Poly(N,N-Dimethylaminoethyl Methacrylate) and Its Quaternized Ammonium Salts. Macromolecules 2007, 40, 5689–5697. [Google Scholar] [CrossRef]
- Teper, P.; Sotirova, A.; Mitova, V.; Oleszko-Torbus, N.; Utrata-Wesołek, A.; Koseva, N.; Kowalczuk, A.; Mendrek, B. Antimicrobial Activity of Hybrid Nanomaterials Based on Star and Linear Polymers of N,N′-Dimethylaminoethyl Methacrylate with in Situ Produced Silver Nanoparticles. Materials 2020, 13, 3037. [Google Scholar] [CrossRef]
- Ali, M.M.; Stöver, H.D.H. Well-Defined Amphiphilic Thermosensitive Copolymers Based on Poly(Ethylene Glycol Monomethacrylate) and Methyl Methacrylate Prepared by Atom Transfer Radical Polymerization. Macromolecules 2004, 37, 5219–5227. [Google Scholar] [CrossRef]
- Mendrek, B.; Fus, A.; Klarzyńska, K.; Sieroń, A.L.; Smet, M.; Kowalczuk, A.; Dworak, A. Synthesis, Characterization and Cytotoxicity of Novel Thermoresponsive Star Copolymers of N,N′-Dimethylaminoethyl Methacrylate and Hydroxyl-Bearing Oligo(Ethylene Glycol) Methacrylate. Polymers 2018, 10, 1255. [Google Scholar] [CrossRef]
- Tiller, J.C.; Liao, C.-J.; Lewis, K.; Klibanov, A.M. Designing Surfaces That Kill Bacteria on Contact. Proc. Natl. Acad. Sci. USA 2001, 98, 5981–5985. [Google Scholar] [CrossRef]
- Huang, J.; Koepsel, R.R.; Murata, H.; Wu, W.; Lee, S.B.; Kowalewski, T.; Russell, A.J.; Matyjaszewski, K. Nonleaching Antibacterial Glass Surfaces via “Grafting Onto”: The Effect of the Number of Quaternary Ammonium Groups on Biocidal Activity. Langmuir 2008, 24, 6785–6795. [Google Scholar] [CrossRef]
- Venkataraman, S.; Zhang, Y.; Liu, L.; Yang, Y.-Y. Design, Syntheses and Evaluation of Hemocompatible Pegylated-Antimicrobial Polymers with Well-Controlled Molecular Structures. Biomaterials 2010, 31, 1751–1756. [Google Scholar] [CrossRef]
- Alaboalirat, M.; Qi, L.; Arrington, K.J.; Qian, S.; Keum, J.K.; Mei, H.; Littrell, K.C.; Sumpter, B.G.; Carrillo, J.-M.Y.; Verduzco, R.; et al. Amphiphilic Bottlebrush Block Copolymers: Analysis of Aqueous Self-Assembly by Small-Angle Neutron Scattering and Surface Tension Measurements. Macromolecules 2018, 52, 465–476. [Google Scholar] [CrossRef]
- Khanna, K.; Varshney, S.; Kakkar, A. Miktoarm Star Polymers: Advances in Synthesis, Self-Assembly, and Applications. Polym. Chem. 2010, 1, 1171. [Google Scholar] [CrossRef]
- Lian, X.; Wu, D.; Song, X.; Zhao, H. Synthesis and Self-Assembly of Amphiphilic Asymmetric Macromolecular Brushes. Macromolecules 2010, 43, 7434–7445. [Google Scholar] [CrossRef]
- Hiemenz, P.C.; Lodge, T. Polymer Chemistry, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9781574447798. [Google Scholar] [CrossRef]
- Vlassi, E.; Papagiannopoulos, A.; Pispas, S. Star Polyelectrolytes with Mixed Arms of PDMAEMA and POEGMA: Self-Assembly and Coassembly with Insulin. Macromol. Chem. Phys. 2022, 223, 2200008. [Google Scholar] [CrossRef]
| Layer | Mass of Fluorescein Sodium Salt Eluted from 1 cm2 of Surface [g] | Quantity of Ammonium Groups on cm2 of the Surface |
|---|---|---|
| SC-LQ2 | 2.79∙10−4 | 4.47∙1017 |
| SC-LQ8 | 2.22∙10−4 | 3.55∙1017 |
| Strain | Sample | Number of Bacteria [CFU/mL] 1 h | Growth Inhibition * [%] 1 h | Number of Bacteria [CFU/mL] 24 h | Growth Inhibition * [%] 24 h |
|---|---|---|---|---|---|
| E. coli | SC-L | 2.0∙105 | 41 | 1.4∙104 | 95 |
| Control | 3.4∙105 | – | 3.2∙105 | – | |
| SC-LQ2 | 1.4∙108 | 30 | 0 | 100 | |
| Control | 2.0∙108 | – | 2.0∙108 | – | |
| SC-LQ8 | 2.6∙104 | 92 | 6.8∙104 | 79 | |
| Control | 3.4∙105 | – | 3.2∙105 | – | |
| B. subtilis | SC-L | 2∙104 | 90 | 3.3∙105 | 51 |
| Control | 2∙105 | – | 6.7∙105 | – | |
| SC-LQ2 | 2.67∙103 | 11 | 0 | 100 | |
| Control | 3.0∙103 | – | 2.42∙103 | – | |
| SC-LQ8 | 2.2∙104 | 89 | 3.2∙105 | 52 | |
| Control | 2∙105 | – | 6.7∙105 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teper, P.; Celny, A.; Kowalczuk, A.; Mendrek, B. Quaternized Poly(N,N′-dimethylaminoethyl methacrylate) Star Nanostructures in the Solution and on the Surface. Polymers 2023, 15, 1260. https://doi.org/10.3390/polym15051260
Teper P, Celny A, Kowalczuk A, Mendrek B. Quaternized Poly(N,N′-dimethylaminoethyl methacrylate) Star Nanostructures in the Solution and on the Surface. Polymers. 2023; 15(5):1260. https://doi.org/10.3390/polym15051260
Chicago/Turabian StyleTeper, Paulina, Anna Celny, Agnieszka Kowalczuk, and Barbara Mendrek. 2023. "Quaternized Poly(N,N′-dimethylaminoethyl methacrylate) Star Nanostructures in the Solution and on the Surface" Polymers 15, no. 5: 1260. https://doi.org/10.3390/polym15051260
APA StyleTeper, P., Celny, A., Kowalczuk, A., & Mendrek, B. (2023). Quaternized Poly(N,N′-dimethylaminoethyl methacrylate) Star Nanostructures in the Solution and on the Surface. Polymers, 15(5), 1260. https://doi.org/10.3390/polym15051260






