Preparation and Characterization of a Renewable Starch-g-(MA-DETA) Copolymer and Its Adjustment for Dye Removal Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
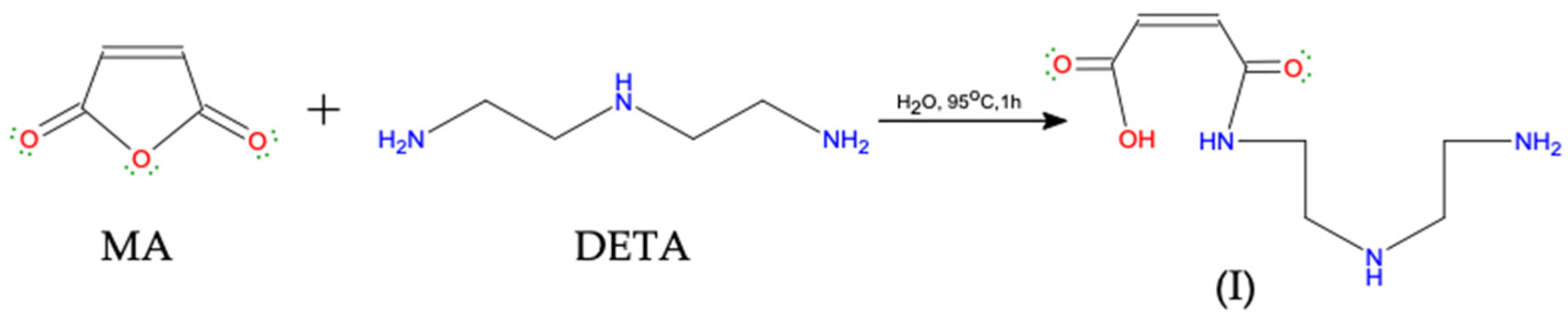
2.2.1. Preparation of Maleamic Acid Monomer
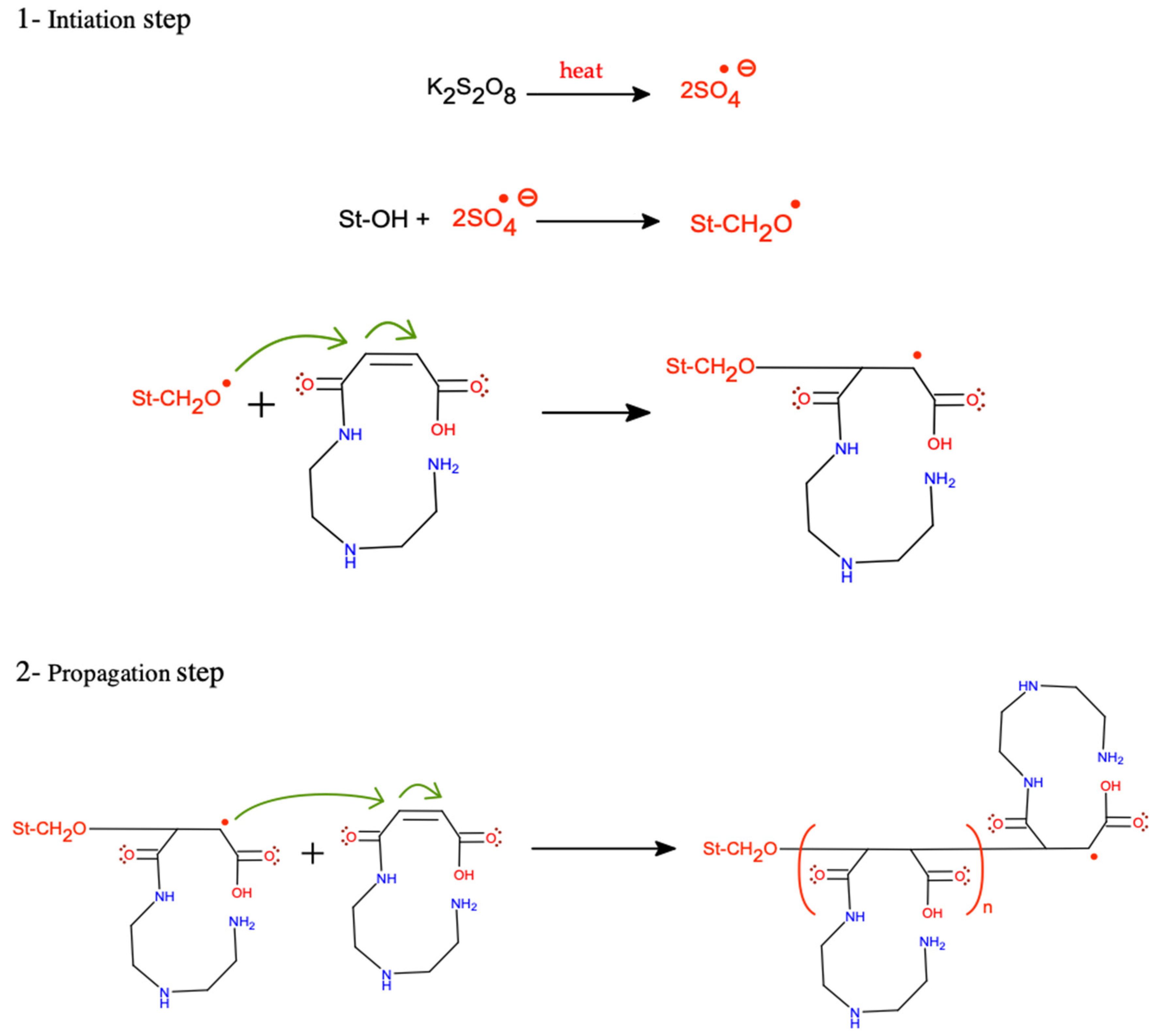
2.2.2. Preparation of St-g-(MA-DETA)
2.2.3. Calculation of Grafting Percentage and Grafting Efficiency
2.2.4. Determination of Carboxyl Content
2.2.5. Preparation of Dye Solution
2.2.6. Dye Sorption by Grafted Starch Adsorbent
2.2.7. Regeneration Experiments
2.2.8. Characterization
3. Results and Discussion
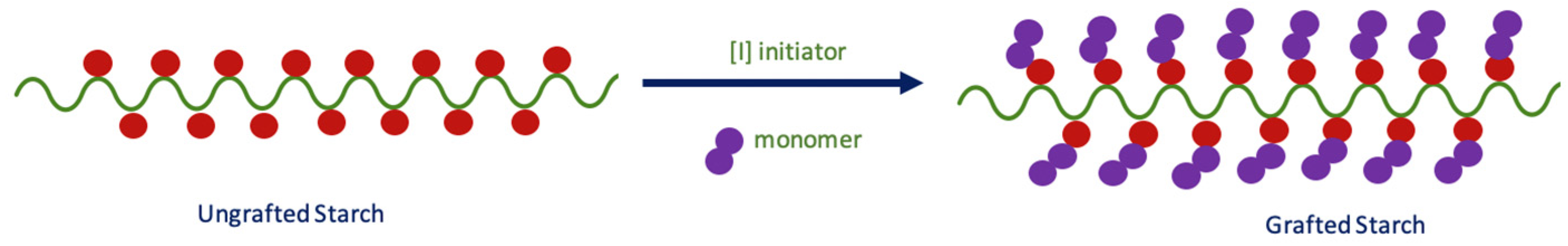
3.1. Synthesis of Grafted Starch
3.2. Characterization Results of the Samples
3.2.1. XRD Analysis
3.2.2. Fourier Transform Infrared Spectroscopy
Formation of Carboxylic Acid
Formation of an Amide Bond
Absence of Cyclic Imide
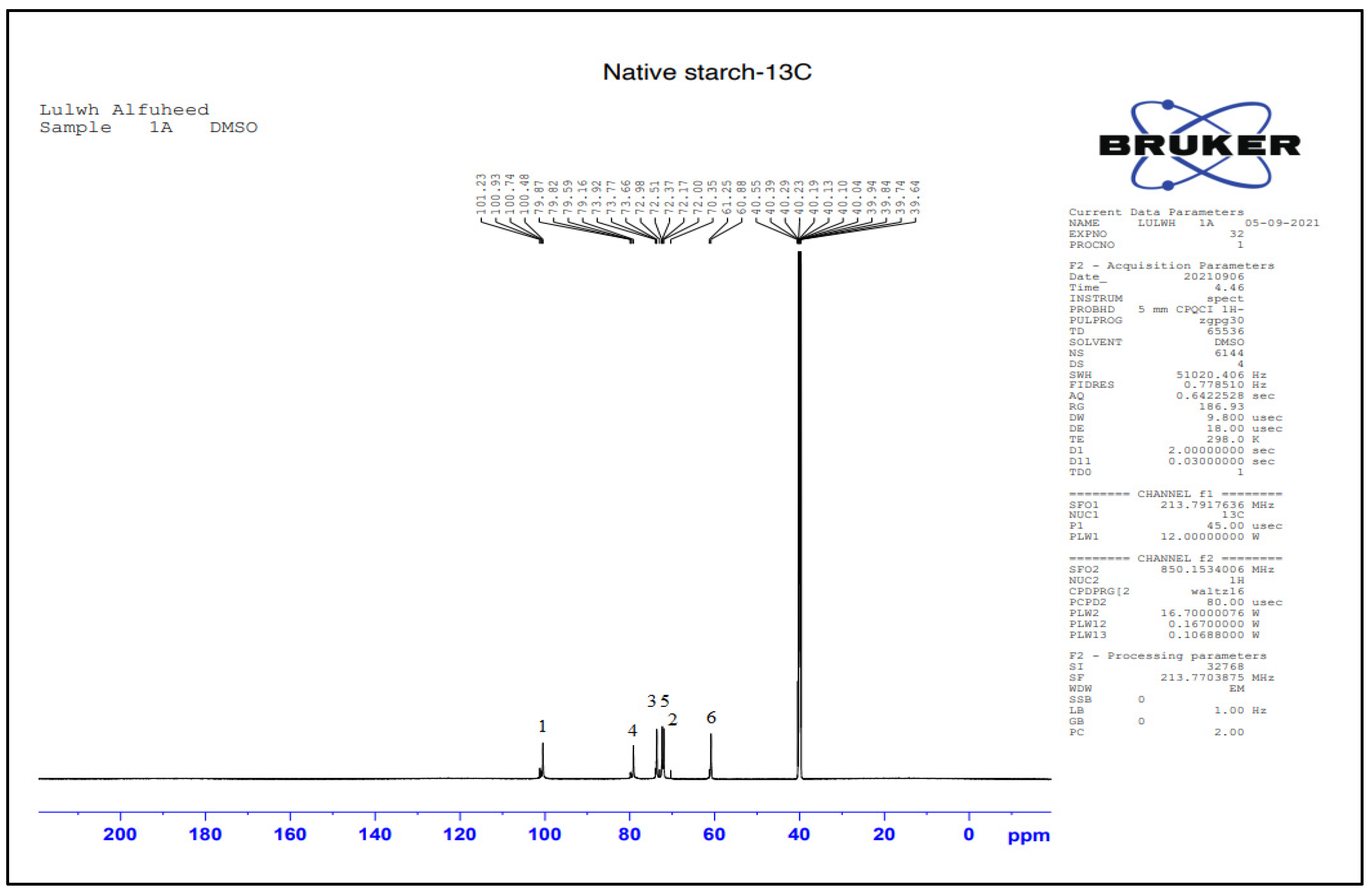
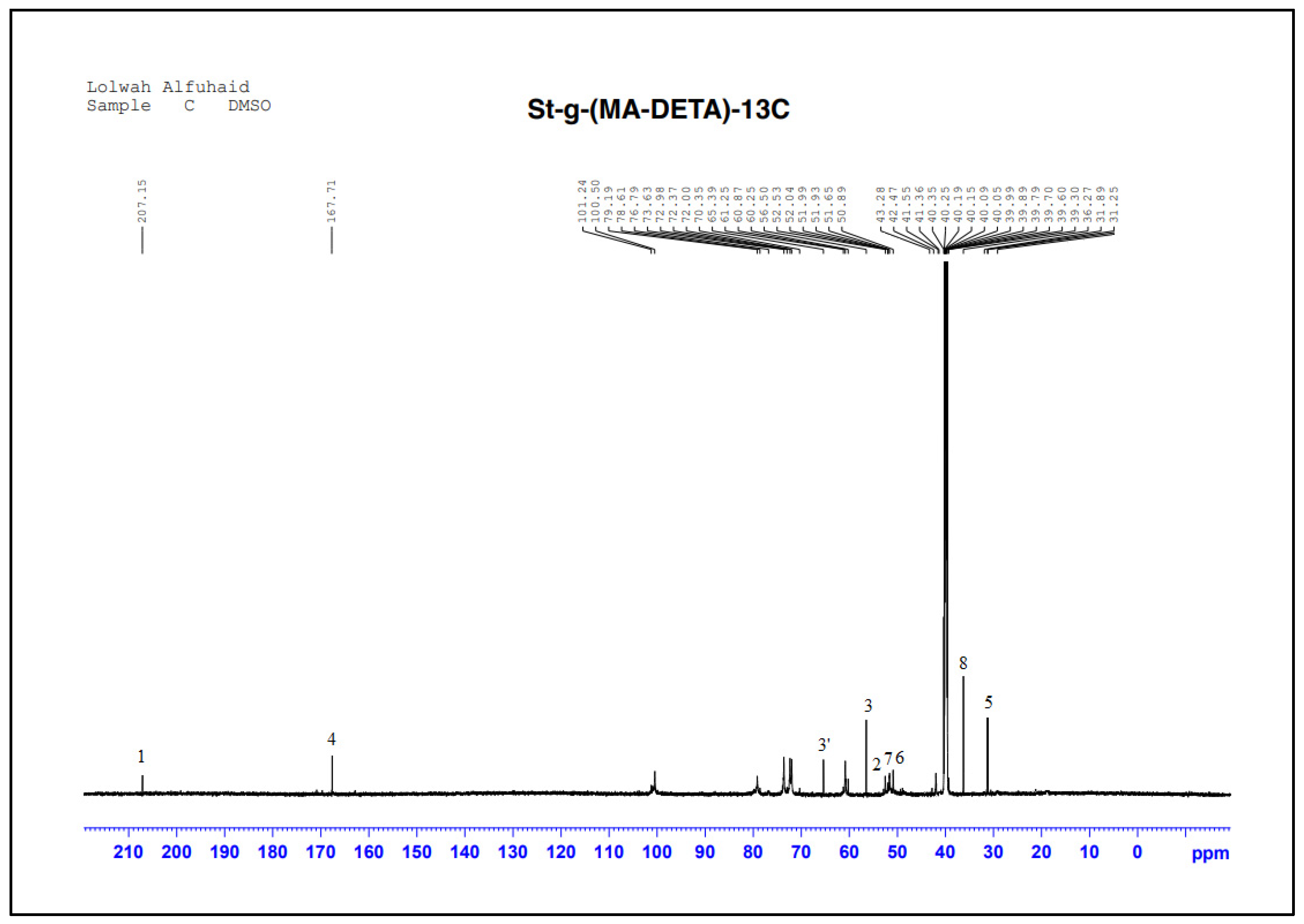
3.2.3. Structural Characterization by NMR Spectroscopy
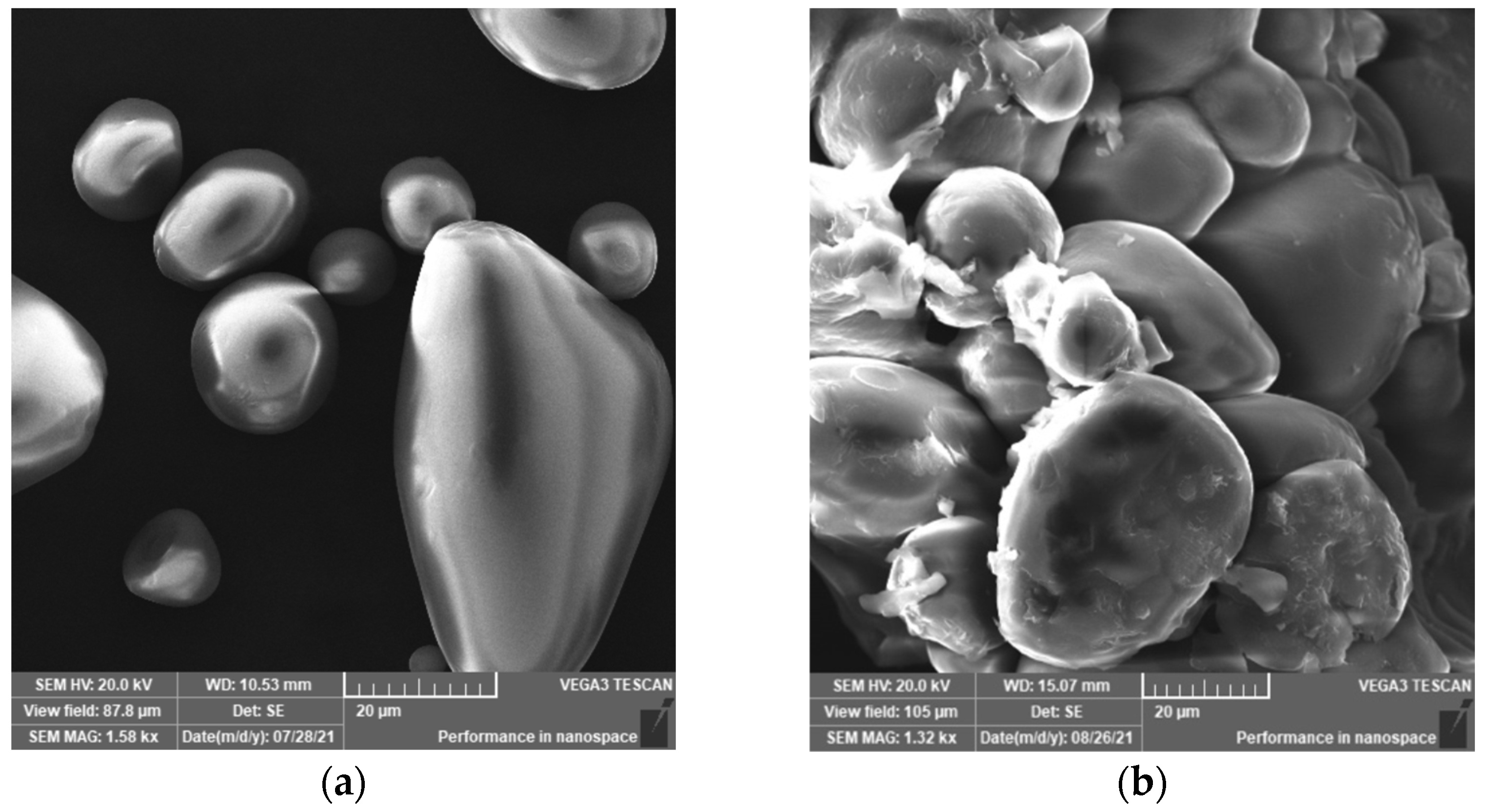
3.2.4. SEM and EDS Analysis
3.2.5. TGA
3.3. Parameters Affecting the Grafting Percentage
3.3.1. Effect of Reaction Time
3.3.2. Effect of Temperature on Graft Copolymerization
3.3.3. Effect of Initiator Concentration
3.3.4. Effect of Monomer Concentration
3.4. Determination of Acidity and the Carboxylic Acid Group
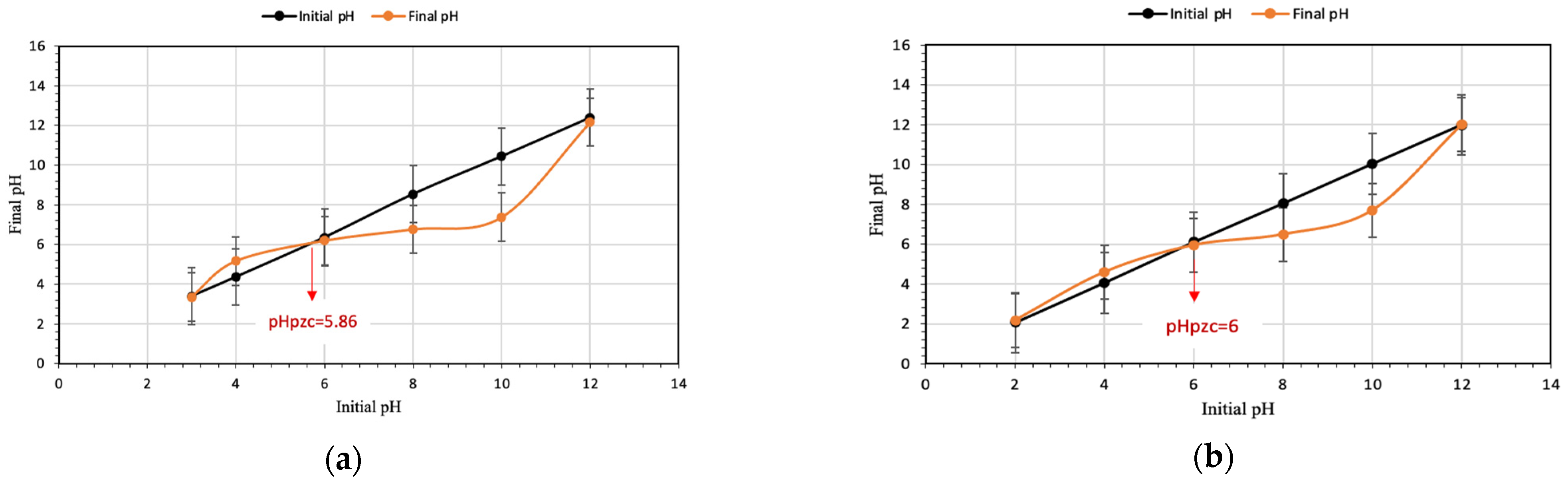
3.5. pH at Point of Zero Charge
3.6. Adsorption of Celestine Blue
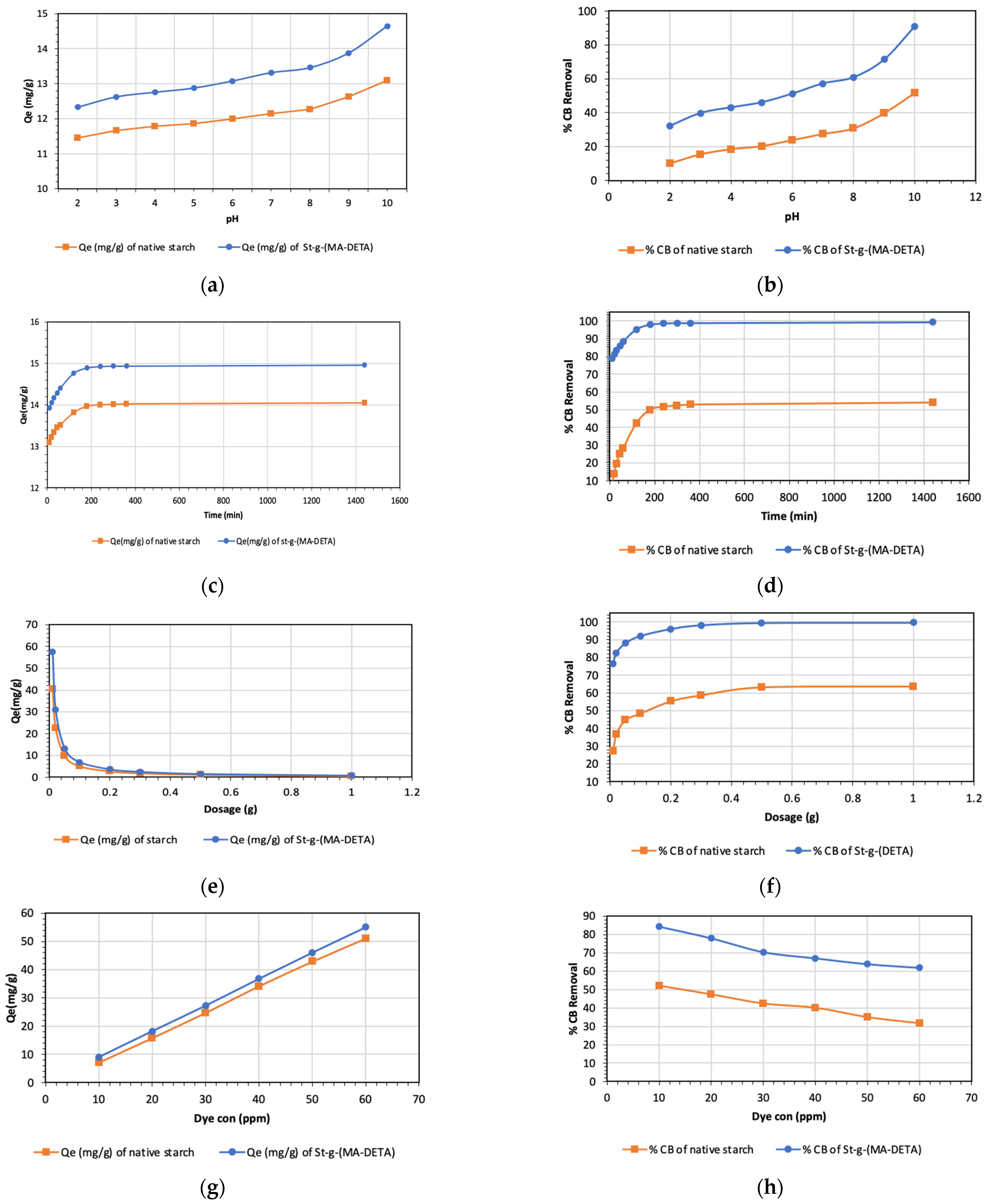
3.6.1. Effect of pH
3.6.2. Effect of Contact Time on CB Adsorption
3.6.3. Effect of Bio-Sorbent Dosage on Biosorption of CB
3.6.4. Adsorption at a Different Initial Concentration of CB Solution
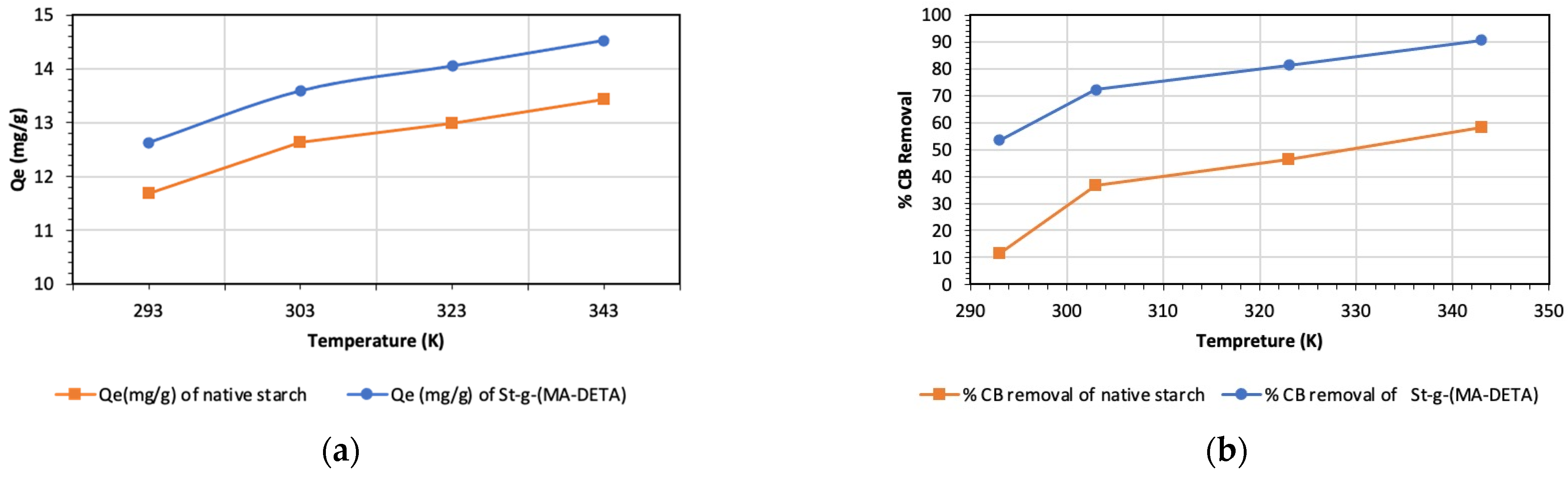
3.6.5. Adsorption at Different Temperatures
3.7. Regeneration Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, W.A.; Ali, S.; Shah, S.A. Water Pollution: Sources and Its Impact on Human Health, Control and Managing. J. Int. Coop. Dev. 2022, 5, 69. [Google Scholar] [CrossRef]
- Ani, J.U.; Akpomie, K.G.; Okoro, U.C.; Aneke, L.E.; Onukwuli, O.D.; Ujam, O.T. Potentials of activated carbon produced from biomass materials for sequestration of dyes, heavy metals, and crude oil components from aqueous environment. Appl. Water Sci. 2020, 10, 69. [Google Scholar] [CrossRef]
- Shabaan, O.A.; Jahin, H.S.; Mohamed, G.G. Removal of anionic and cationic dyes from wastewater by adsorption using multiwall carbon nanotubes. Arab. J. Chem. 2020, 13, 4797–4810. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Qin, D.; Da, W.; Hou, B.; Hao, C.; Wu, J. Construction of magnetic lignin-based adsorbent and its adsorption properties for dyes. J. Hazard. Mater. 2019, 369, 50–61. [Google Scholar] [CrossRef]
- Shabir, M.; Yasin, M.; Hussain, M.; Shafiq, I.; Akhter, P.; Nizami, A.-S.; Jeon, B.-H.; Park, Y.-K. A review on recent advances in the treatment of dye-polluted wastewater. J. Ind. Eng. Chem. 2022, 112, 1–19. [Google Scholar] [CrossRef]
- Hussain, I.; Li, Y.; Qi, J.; Li, J.; Wang, L. Nitrogen-enriched carbon sheet for Methyl blue dye adsorption. J. Environ. Manag. 2018, 215, 123–131. [Google Scholar] [CrossRef]
- Wong, S.; Yac’Cob, N.A.N.; Ngadi, N.; Hassan, O.; Inuwa, I.M. From pollutant to solution of wastewater pollution: Synthesis of activated carbon from textile sludge for dye adsorption. Chin. J. Chem. Eng. 2018, 26, 870–878. [Google Scholar] [CrossRef]
- Kusrini, E.; Sofyan, N.; Suwartha, N.; Yesya, G.; Priadi, C.R. Chitosan-praseodymium complex for adsorption of fluoride ions from water. J. Rare Earths 2015, 33, 1104–1113. [Google Scholar] [CrossRef]
- Kusrini, E.; Alhamid, M.I.; Widiantoro, A.B.; Daud, N.Z.A.; Usman, A. Simultaneous Adsorption of Multi-Lanthanides from Aqueous Silica Sand Solution Using Pectin-Activated Carbon Composite. Arab. J. Sci. Eng. 2020, 45, 7219–7230. [Google Scholar] [CrossRef]
- Kusrini, E.; Anwar Usman, A.; Sani, F.A.; Wilson, L.D.; Abdullah, M.A. Simultaneous Adsorption of Lan-thanum and Yttrium from Aqueous Solution by Durian Rind Biosorbent. Environ. Monit. Assess. 2019, 191, 488. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Banana peel as a biosorbent for the decontamination of water pollutants. A review. Environ. Chem. Lett. 2020, 18, 1085–1112. [Google Scholar] [CrossRef]
- Suteu, D.; Blaga, A.C.; Cimpoesu, R.; Puiţel, A.C.; Tataru-Farmus, R.-E. Composites Based on Natural Polymers and Microbial Biomass for Biosorption of Brilliant Red HE-3B Reactive Dye from Aqueous Solutions. Polymers 2021, 13, 4314. [Google Scholar] [CrossRef] [PubMed]
- Justyna, S.; Jaszek, M.; Osińska-Jaroszuk, M.; Matuszewska, A.; Bancerz, R.; Janczarek, M. Natural Microbial Polysaccharides as Effective Factors for Modification of the Catalytic Properties of Fungal Cellobiose De-hydrogenase. Arch. Microbiol. 2021, 203, 4433–4448. [Google Scholar]
- De Araújo, G.K.P.; De Souza, S.J.; Da Silva, M.V.; Yamashita, F.; Gonçalves, O.H.; Leimann, F.V.; Shirai, M.A. Physical, antimicrobial and antioxidant properties of starch-based film containing ethanolic propolis extract. Int. J. Food Sci. Technol. 2015, 50, 2080–2087. [Google Scholar] [CrossRef]
- Hui, S.; Wu, D.; Zhang, R.-Q.; Qiao, L.-Y.; Zhang, S.-H.; Lin, S.; Ye, J. Synthesis and Application of Amphoteric Starch Graft Polymer. Carbohydr. Polym. 2009, 78, 253–257. [Google Scholar]
- Wang, X.; Huang, L.; Zhang, C.; Deng, Y.; Xie, P.; Liu, L.; Cheng, J. Research advances in chemical modifications of starch for hydrophobicity and its applications: A review. Carbohydr. Polym. 2020, 240, 116292. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Picchioni, F. Modification of starch: A review on the application of “green” solvents and controlled functionalization. Carbohydr. Polym. 2020, 241, 116350. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Pradhan, S.S.; Tripathy, T. Poly(N,N-dimethylacrylamide-co-acrylamide) Grafted Hydroxyethyl Cellulose Hydrogel: A Useful Congo Red Dye Remover. J. Polym. Environ. 2017, 26, 2730–2747. [Google Scholar] [CrossRef]
- Meimoun, J.; Wiatz, V.; Saint-Loup, R.; Parcq, J.; Favrelle, A.; Bonnet, F.; Zinck, P. Modification of Starch by Graft Copolymerization. Starch Stärke 2017, 70, 1600351. [Google Scholar] [CrossRef]
- Zhe, Y.; Peng, H.; Wang, W.; Liu, T. Crystallization Behavior of Poly(ε-Caprolactone)/Layered Double Hydroxide Nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar]
- Guo, J.; Wang, J.; Zheng, G.; Jiang, X. Optimization of the removal of reactive golden yellow SNE dye by cross-linked cationic starch and its adsorption properties. J. Eng. Fibers Fabr. 2019, 14, 1558925019865260. [Google Scholar] [CrossRef]
- Ghorai, S.; Sarkar, A.K.; Panda, A.; Pal, S. Effective removal of Congo red dye from aqueous solution using modified xanthan gum/silica hybrid nanocomposite as adsorbent. Bioresour. Technol. 2013, 144, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Loyd, D.B.; Nigam, M.; Martinus, S.; Maloney, J.E.; Benyack, L.L.; Gainer, B. Synthesis of Substituted n-Phenylmaleimides and Use in a Diels–Alder Reaction: A Green Multi-Step Synthesis for an Undergraduate Organic Chemistry Laboratory. Green Chem. Lett. Rev. 2019, 12, 127–135. [Google Scholar]
- Singh, V.; Ali, S.Z.; Somashekar, R.; Mukherjee, P.S. Nature of Crystallinity in Native and Acid Modified Starches. Int. J. Food Prop. 2006, 9, 845–854. [Google Scholar] [CrossRef]
- Mannan, A.; Kazmia, K.R.; Khan, I.H.; Khan, M.S. A Method for the Determination of Relative Crystallinity of Minerals by X-Ray Diffraction. Pak. J. Sci. Ind. Res. 2006, 49, 72–76. [Google Scholar]
- Li, P.; He, X.; Zuo, Y.; Li, X.; Wu, Y. Synthesis and characterization of lactic acid esterified starch by an in-situ solid phase method. Int. J. Biol. Macromol. 2019, 156, 1316–1322. [Google Scholar] [CrossRef]
- Zuo, Y.; He, X.; Li, P.; Li, W.; Wu, Y. Preparation and Characterization of Hydrophobically Grafted Starches by In Situ Solid Phase Polymerization. Polymers 2019, 11, 72. [Google Scholar] [CrossRef]
- Keirudin, A.A.; Zainuddin, N.; Yusof, N.A. Crosslinked Carboxymethyl Sago Starch/Citric Acid Hydrogel for Sorption of Pb2+, Cu2+, Ni2+ and Zn2+ from Aqueous Solution. Polymers 2020, 12, 2465. [Google Scholar] [CrossRef]
- Lai, J.C.; Rahman, W.A.W.A.; Toh, W.Y. Characterisation of sago pith waste and its composites. Ind. Crop. Prod. 2013, 45, 319–326. [Google Scholar] [CrossRef]
- Balgude, D.; Sabnis, A.; Ghosh, S.K. Investigation of cardanol-based reactive polyamide as a crosslinker in epoxy zinc-rich primer. J. Coat. Technol. Res. 2017, 14, 583–595. [Google Scholar] [CrossRef]
- Saeb, M.R.; Najafi, F.; Bakhshandeh, E.; Khonakdar, H.A.; Mostafaiyan, M.; Simon, F.; Scheffler, C.; Mäder, E. Highly Curable Epoxy/Mwcnts Nanocomposites: An Effective Approach to Function-alization of Carbon Nanotubes. Chem. Eng. J. 2015, 259, 117–125. [Google Scholar] [CrossRef]
- Schmidt, P.; Eschig, S. An Industrial Applicable Method for the Synthesis of N-alkylated Maleimides Based on Fatty Amines. Eur. J. Lipid Sci. Technol. 2018, 121, 1800320. [Google Scholar] [CrossRef]
- Zhicheng, J.; Du, L.; Zhang, C.; Sugiyama, Y.; Wang, W.; Palui, G.; Wang, S.; Mattoussi, H. Modification of Poly(Maleic Anhydride)-Based Polymers with H2N-R Nucleophiles: Addition Or Substitution Reaction? Bioconjugate Chem. 2019, 30, 871. [Google Scholar]
- Pant, B.R.; Jeon, H.-J.; Song, H.H. Radiation cross-linked carboxymethylated starch and iron removal capacity in aqueous solution. Macromol. Res. 2011, 19, 307–312. [Google Scholar] [CrossRef]
- Shen, W.; Chen, S.; Shi, S.; Li, X.; Zhang, X.; Hu, W.; Wang, H. Adsorption of Cu(II) and Pb(II) onto diethylenetriamine-bacterial cellulose. Carbohydr. Polym. 2009, 75, 110–114. [Google Scholar] [CrossRef]
- Hanurry, E.Y.; Hsu, W.-H.; Darge, H.F.; Birhan, Y.S.; Mekonnen, T.W.; Andrgie, A.T.; Chou, H.-Y.; Cheng, C.-C.; Lai, J.-Y.; Tsai, H.-C. In Vitro Sirna Delivery via Diethy-lenetriamine- and Tetraethylenepentamine-Modified Carboxyl Group-Terminated Poly(Amido)Amine Generation 4.5 Den-drimers. Mater. Sci. Eng. C 2020, 106, 110245. [Google Scholar] [CrossRef]
- Belskaya, O.B.; Mironenko, R.; Talsi, V.; Rodionov, V.; Sysolyatin, S.; Likholobov, V. A Study of Pd/C Catalysts in the Liquid-Phase Hydrogenation of 1,3,5-Trinitrobenzene and 2,4,6-Trinitrobenzoic Acid. Selection of Hydrogenation Conditions for Selective Production of 1,3,5-Triaminobenzene. Procedia Eng. 2016, 152, 110–115. [Google Scholar] [CrossRef]
- Morrison, J.J.; Brandt, V.K.; Yeates, S.G. Comment on: Synthesis of New Azo Compounds Based on N-(4-Hydroxyphenyl)maleimide and N-(4-Methylphenyl)maleimide. Molecules 2010, 15, 7498–7508. Molecules 2019, 24, 2319. [Google Scholar] [CrossRef]
- Li, M.; Liang, Y.-D.; Wu, Y.-X.; Li, K.-S. Synergistic effect of complexes of ethylenediamine double maleamic acid radical and lanthanum (III) with pentaerythritol on the thermal stability of poly(vinyl chloride). Polym. Degrad. Stab. 2017, 140, 176–193. [Google Scholar] [CrossRef]
- Ge, M.; Du, M.; Zheng, L.; Wang, B.; Zhou, X.; Jia, Z.; Hu, G.; Alam, S.M.J. A Maleic Anhydride Grafted Sugarcane Bagasse Adsorbent and Its Performance on the Removal of Methylene Blue from Related Wastewater. Mater. Chem. Phys. 2017, 192, 147–155. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Xu, S.-A. Effects of amylose/amylopectin starch on starch-based superabsorbent polymers prepared by γ-radiation. Starch 2016, 69, 1500294. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, K.; Wang, P. Study on structure and molecular dynamics of starch/poly(sodium acrylate)-grafted superabsorbent by 13C solid state NMR. Fibers Polym. 2008, 9, 271–275. [Google Scholar] [CrossRef]
- Pineda, R.; Maria, L.; de Muñoz-Prieto, E.; Rius-Alonso, C.A.; Palacios-Alquisira, J. Preparation and Characterization of Potato Starch Nanoparticles with Acrylamide by Microwave Radiation. Cienc. Desarro. 2018, 9, 149–159. [Google Scholar] [CrossRef]
- Lanthong, P.; Nuisin, R.; Kiatkamjornwong, S. Graft copolymerization, characterization, and degradation of cassava starch-g-acrylamide/itaconic acid superabsorbents. Carbohydr. Polym. 2006, 66, 229–245. [Google Scholar] [CrossRef]
- Zou, W.; Liu, X.; Yu, L.; Qiao, D.; Chen, L.; Liu, H.; Zhang, N. Synthesis and Characteri-zation of Biodegradable Starch-Polyacrylamide Graft Copolymers Using Starches with Different Microstructures. J. Polym. Environ. 2012, 21, 359–365. [Google Scholar] [CrossRef]
- Joshi, J.M.; Sinha, V.K. Ceric ammonium nitrate induced grafting of polyacrylamide onto carboxymethyl chitosan. Carbohydr. Polym. 2007, 67, 427–435. [Google Scholar] [CrossRef]
- Tridib, T.; Singh, R.P. High Performance Flocculating Agent Based on Partially Hydrolysed Sodium Alginate-G-Polyacrylamide. Eur. Polym. J. 2000, 36, 1471–1476. [Google Scholar]
- Mishra, D.K.; Tripathy, J.; Srivastava, A.; Mishra, M.M.; Behari, K. Graft copolymer (chitosan-g-N-vinyl formamide): Synthesis and study of its properties like swelling, metal ion uptake and flocculation. Carbohydr. Polym. 2008, 74, 632–639. [Google Scholar] [CrossRef]
- Adelaida, Á.; Bierbrauer, K.; Pucci, G.; López-González, M.; Strumia, M. Study of Optimization of the Synthesis and Properties of Biocomposite Films Based on Grafted Chitosan. J. Food Eng. 2012, 109, 752–761. [Google Scholar]
- Ranjbar-Mohammadi, M.; Arami, M.; Bahrami, H.; Mazaheri, F.; Mahmoodi, N.M. Grafting of chitosan as a biopolymer onto wool fabric using anhydride bridge and its antibacterial property. Colloids Surf. B Biointerfaces 2010, 76, 397–403. [Google Scholar] [CrossRef]
- Angellier, H.; Molina-Boisseau, S.; Belgacem, M.N.; Dufresne, A. Surface Chemical Modification of Waxy Maize Starch Nanocrystals. Langmuir 2005, 21, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Wim, T.; Belgacem, N.M.; Dufresne, A. Starch Nanocrystals with Large Chain Surface Modifi-cations. Langmuir 2006, 22, 4804–4810. [Google Scholar]
- Benxi, W.; Hu, X.; Zhang, B.; Li, H.; Xu, X.; Jin, Z.; Tian, Y. Effect of Defatting on Acid Hy-drolysis Rate of Maize Starch with Different Amylose Contents. Int. J. Biol. Macromol. 2013, 62, 652–656. [Google Scholar]
- Soumi, D.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent Advances on the Removal of Dyes from Wastewater Using Various Adsorbents: A Critical Review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar]
- Kuang, Y.; Zhang, X.; Zhou, S. Adsorption of Methylene Blue in Water onto Activated Carbon by Surfactant Modification. Water 2020, 12, 587. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Biosorption and regeneration potentials of magnetite nanoparticle loaded Solanum tuberosum peel for celestine blue dye. Int. J. Phytoremediat. 2020, 23, 347–361. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Y.; Shen, L.; Wang, X.; Chen, J.; Ma, A.; Jiang, W. Lead(II) and methylene blue removal using a fully biodegradable hydrogel based on starch immobilized humic acid. Chem. Eng. J. 2015, 268, 348–355. [Google Scholar] [CrossRef]
- Arayaphan, J.; Maijan, P.; Boonsuk, P.; Chantarak, S. Synthesis of photodegradable cassava starch-based double network hydrogel with high mechanical stability for effective removal of methylene blue. Int. J. Biol. Macromol. 2020, 168, 875–886. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Jabeen, A.; Iqbal, M.; Noreen, S.; Naseem, Z. Adsorptive behavior of rice bran-based composites for malachite green dye: Isotherm, kinetic and thermodynamic studies. J. Mol. Liq. 2017, 237, 322–333. [Google Scholar] [CrossRef]
- Aniagor, C.O.; Afifi, M.A.; Hashem, A. Heavy metal adsorptive application of hydrolyzed corn starch. J. Polym. Res. 2021, 28, 405. [Google Scholar] [CrossRef]
- Sarmah, D.; Karak, N. Double network hydrophobic starch based amphoteric hydrogel as an effective adsorbent for both cationic and anionic dyes. Carbohydr. Polym. 2020, 242, 116320. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, M.; Saravanakumar, M.P. Ultrahigh Adsorption Capacity of Starch Derived Zinc Based Carbon Foam for Ad-sorption of Toxic Dyes and Its Preliminary Investigation on Oil-Water Separation. J. Clean. Prod. 2018, 197, 511–524. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.-C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Huayu, H.; Lin, C.; Zhang, Y.; Cai, X.; Huang, Z.; Chen, C.; Qin, Y.; Liang, J. Preparation of a Stable Nanoscale Manganese Residue-Derived Fes@Starch-Derived Carbon Composite for the Adsorption of Safranine T. Nanomaterials 2019, 9, 839. [Google Scholar] [CrossRef]


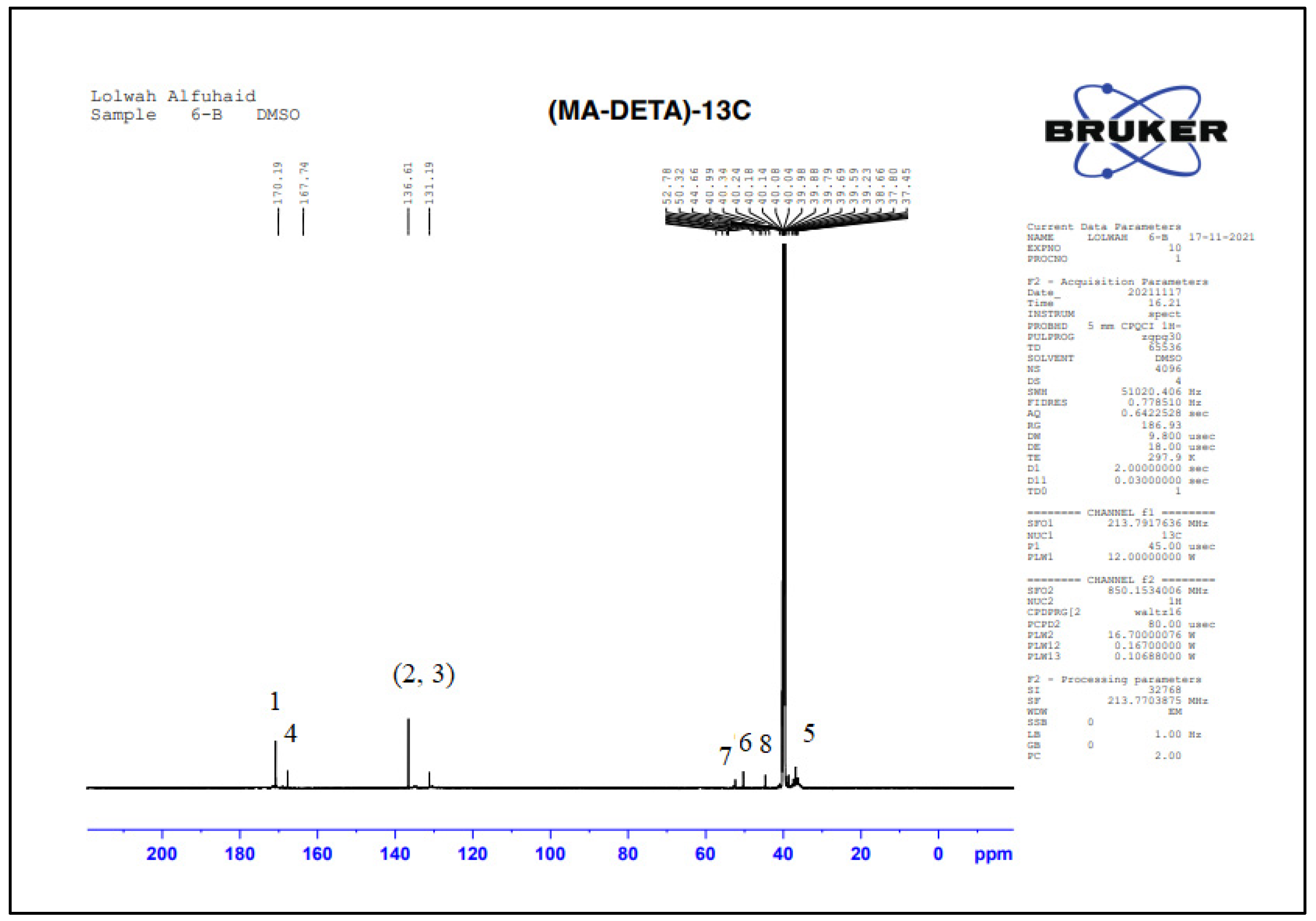





| Suggested Structure | Carbon Atoms | Chemical Shift (ppm) |
|---|---|---|
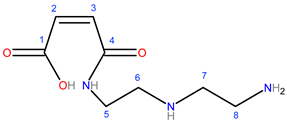 | 1 | 170.19 |
| 2, 3 | 136.61–131.19 | |
| 4 | 167.74 | |
| 5 | 40.99 | |
| 6 | 50.32 | |
| 7 | 52.78 | |
| 8 | 44.66 |
| Suggested Structure | Carbon Atoms | Chemical Shift (ppm) |
|---|---|---|
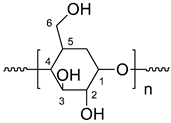 | 1 | 100 |
| 2 | 72.3 | |
| 3 | 73.3 | |
| 4 | 79.8 | |
| 5 | 72.9 | |
| 6 | 60.8 |
| Suggested Structure | Carbon Atoms | Chemical Shift (ppm) |
|---|---|---|
 | 1 | 207.15 |
| 2 | 52.53 | |
| 3 | 56.50 and 65.39 | |
| 4 | 167.71 | |
| 5 | 31.89 | |
| 6 | 50.89 | |
| 7 | 51.93 | |
| 8 | 36.27 |
| Sample Name | Grafting Percentage (%) | To (°C) | Total Weight Loss (%) at 800 °C |
|---|---|---|---|
| Native starch | 0 | 248.81 | 91.53 |
| St-g-(MA-DETA) | 15 | 262.43 | 83.30 |
| 19.06 | 267.48 | 82.59 | |
| 29.17 | 314.83 | 76.76 |
| Grafting Percentage | Grafting Efficiency | Conversion Percentage | |
|---|---|---|---|
| Time | |||
| 3 | 14.95 | 88.97 | 3.91 |
| 6 | 19.09 | 98.28 | 4.05 |
| 9 | 27.44 | 116.63 | 4.34 |
| 12 | 21.09 | 102.79 | 4.12 |
| 15 | 8.63 | 74.24 | 3.69 |
| Temperature °C | |||
| 30 | 12.34 | 10.98 | 3.82 |
| 40 | 13.25 | 11.69 | 3.85 |
| 50 | 15.32 | 13.28 | 3.92 |
| 60 | 27.44 | 21.53 | 4.34 |
| 70 | 20.42 | 16.96 | 4.09 |
| 90 | 7.13 | 6.65 | 3.64 |
| Initiator [I] mol/L | |||
| 0.05 | 2.54 | 2.478 | 3.49 |
| 0.3 | 27.44 | 21.53 | 4.34 |
| 0.6 | 29.17 | 22.58 | 4.39 |
| 0.8 | 14.56 | 12.71 | 3.89 |
| 1 | 6.19 | 5.83 | 3.61 |
| Monomer [M] mol/L | |||
| 0.15 | 7.42 | 6.91 | 68.12 |
| 0.23 | 10.16 | 9.23 | 76.43 |
| 0.45 | 27.44 | 21.53 | 93.04 |
| 0.90 | 4.89 | 4.66 | 8.54 |
| 1 | 4.74 | 4.52 | 5.76 |
| Samples | Native Starch | St-g-(MA-DETA) |
|---|---|---|
| Grafting percentage | 0 | 29.17 |
| Meq of acidity/100 g of starch | 2 | 30 |
| Carboxyl content (%) | 0.09 | 1.35 |
| Degree of substitution (DS%) | 0.32 | 5.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfuhaid, L.; Al-Abbad, E.; Alshammari, S.; Alotaibi, A.; Malek, N.; Al-Ghamdi, A. Preparation and Characterization of a Renewable Starch-g-(MA-DETA) Copolymer and Its Adjustment for Dye Removal Applications. Polymers 2023, 15, 1197. https://doi.org/10.3390/polym15051197
Alfuhaid L, Al-Abbad E, Alshammari S, Alotaibi A, Malek N, Al-Ghamdi A. Preparation and Characterization of a Renewable Starch-g-(MA-DETA) Copolymer and Its Adjustment for Dye Removal Applications. Polymers. 2023; 15(5):1197. https://doi.org/10.3390/polym15051197
Chicago/Turabian StyleAlfuhaid, Lolwah, Eman Al-Abbad, Shouq Alshammari, Aljawharah Alotaibi, Naved Malek, and Azza Al-Ghamdi. 2023. "Preparation and Characterization of a Renewable Starch-g-(MA-DETA) Copolymer and Its Adjustment for Dye Removal Applications" Polymers 15, no. 5: 1197. https://doi.org/10.3390/polym15051197
APA StyleAlfuhaid, L., Al-Abbad, E., Alshammari, S., Alotaibi, A., Malek, N., & Al-Ghamdi, A. (2023). Preparation and Characterization of a Renewable Starch-g-(MA-DETA) Copolymer and Its Adjustment for Dye Removal Applications. Polymers, 15(5), 1197. https://doi.org/10.3390/polym15051197





