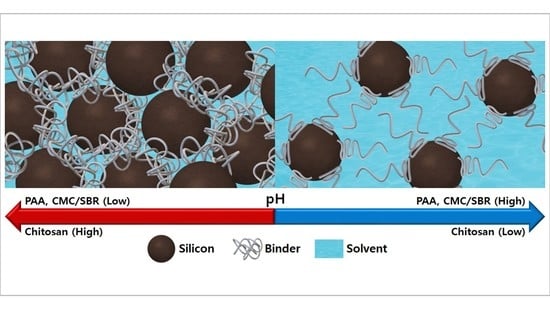
Dispersion Homogeneity of Silicon Anode Slurries with Various Binders for Li-Ion Battery Anode Coating
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Preparation of Si Anode Slurries
2.3. Characterization of Coating Efficiency
2.4. Characterization of Rheological Properties
3. Results and Discussion
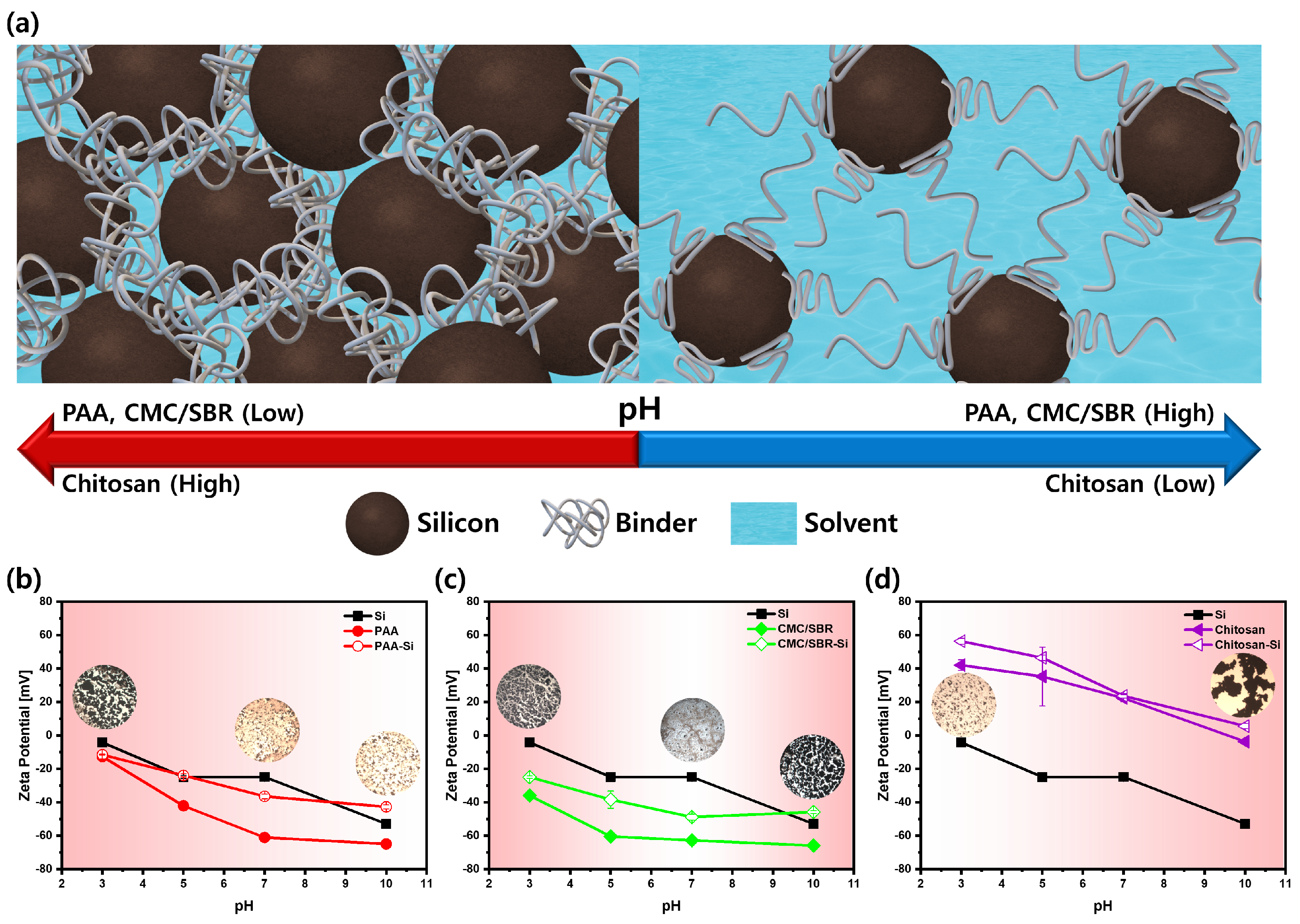
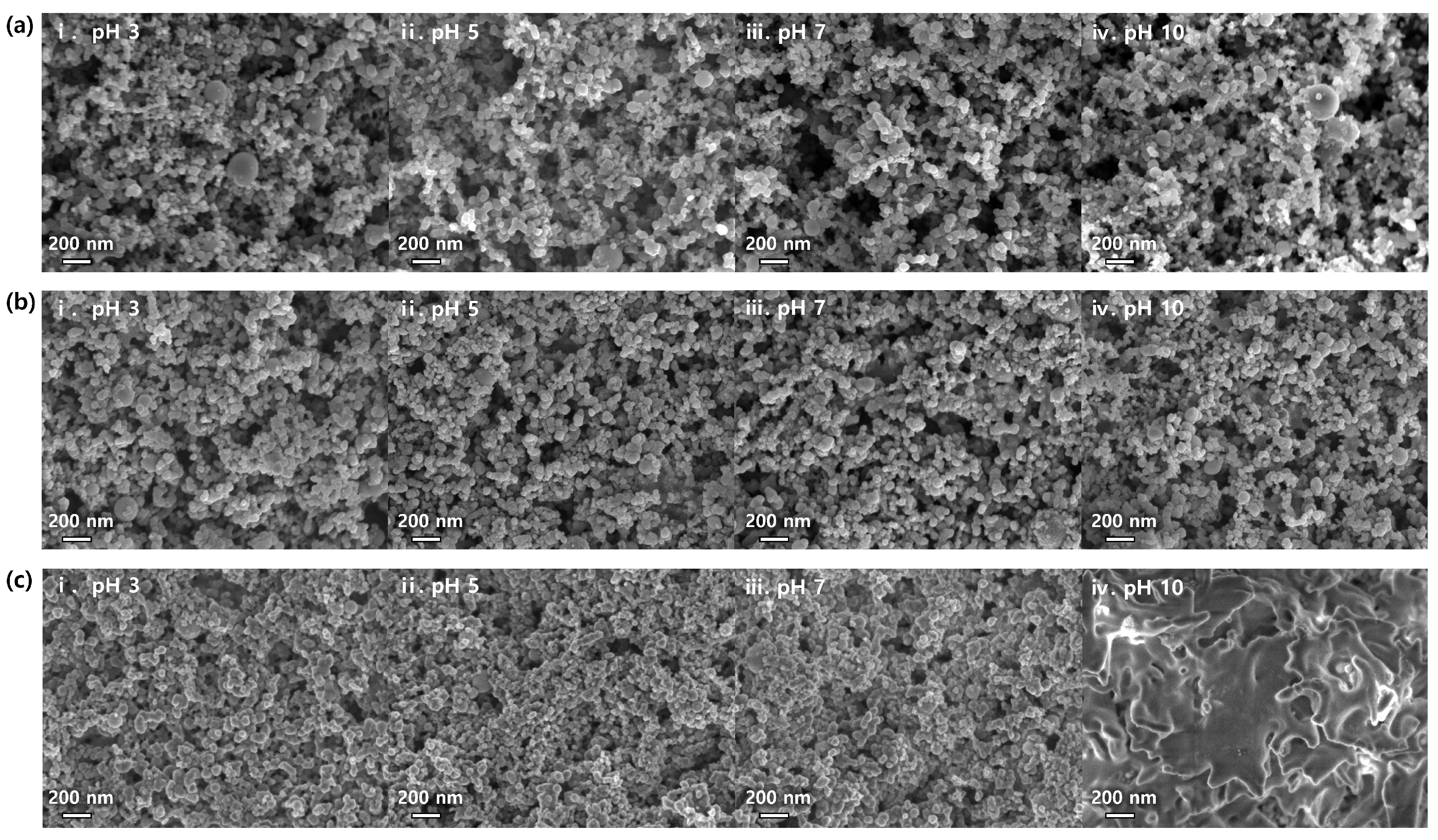
3.1. Particle Dispersity and Rheology of Si Anode Slurries
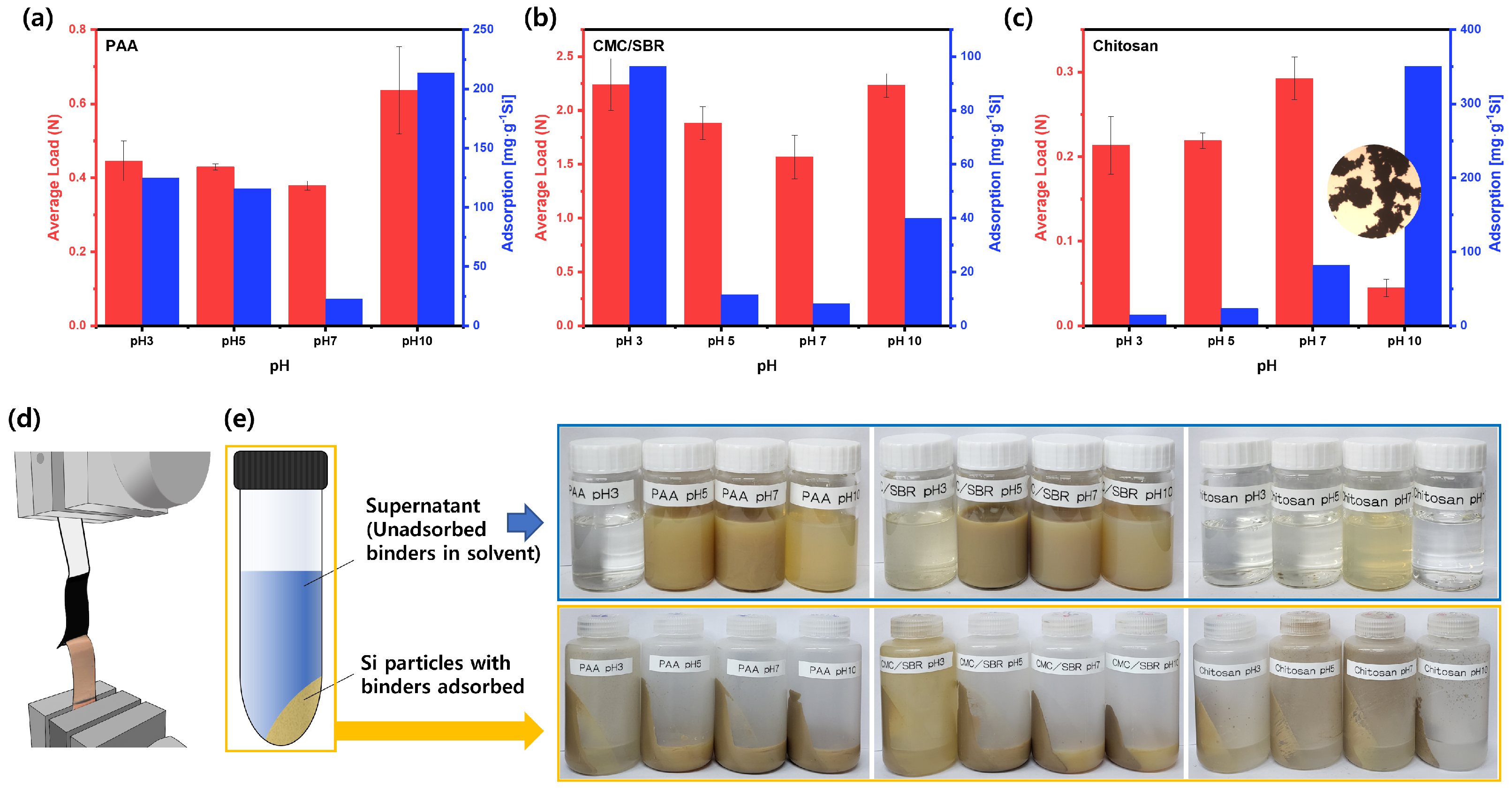
3.2. Binder Adsorptions and Adhesion Strengths
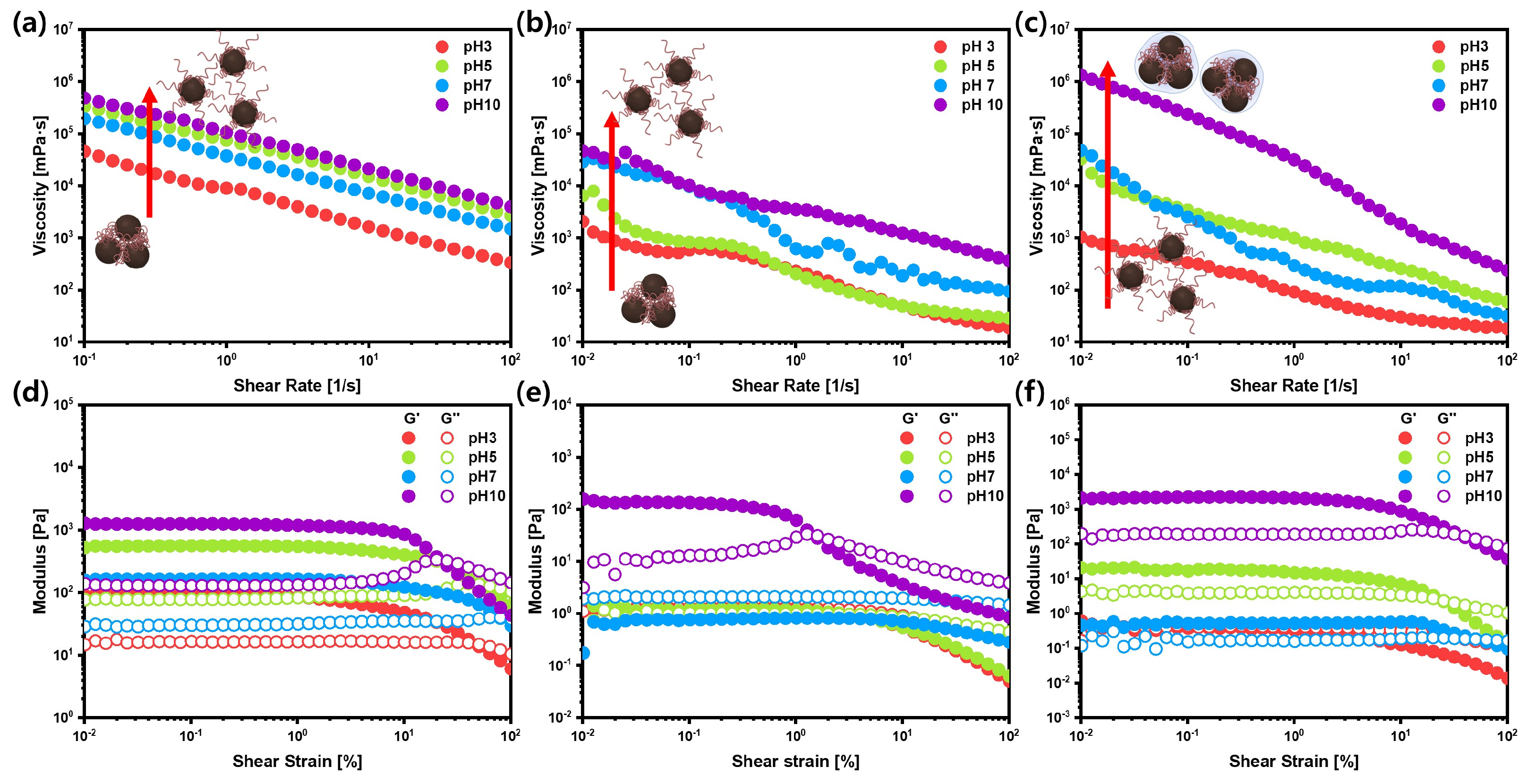
3.3. Structural Deformation and Recovery of Si Anode Slurries upon Coating Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.F.; Fuller, T.F.; Luzinov, I.; Yushin, G. Toward efficient binders for Li-ion battery Si-based anodes: Polyacrylic acid. ACS Appl. Mater. Interfaces 2010, 2, 3004–3010. [Google Scholar] [CrossRef] [PubMed]
- Szczech, J.R.; Jin, S. Nanostructured silicon for high capacity lithium battery anodes. Energy Environ. Sci. 2011, 4, 56–72. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Qiu, X.; Ma, Y.; Kong, D.; Müllen, K.; Li, X.; Zhi, L. Stable high-capacity and high-rate silicon-based lithium battery anodes upon two-dimensional covalent encapsulation. Nat. Commun. 2020, 11, 3826. [Google Scholar] [CrossRef] [PubMed]
- Schwan, J.; Nava, G.; Mangolini, L. Critical barriers to the large scale commercialization of silicon-containing batteries. Nanoscale Adv. 2020, 2, 4368–4389. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lehto, V.P. Challenges and prospects of nanosized silicon anodes in lithium-ion batteries. Nanotechnology 2020, 32, 042002. [Google Scholar] [CrossRef] [PubMed]
- Courtel, F.M.; Niketic, S.; Duguay, D.; Abu-Lebdeh, Y.; Davidson, I.J. Water-soluble binders for MCMB carbon anodes for lithium-ion batteries. J. Power Sources 2011, 196, 2128–2134. [Google Scholar] [CrossRef]
- Yang, J.; Kim, J.; Lee, J. Effects of water-soluble polymers dissolved in an aqueous secondary fluid on the rheological properties of “capillary” suspensions. Korea-Aust. Rheol. J. 2022, 34, 159–166. [Google Scholar] [CrossRef]
- Zhu, T.; Tran, T.N.; Fang, C.; Liu, D.; Herle, S.P.; Guan, J.; Gopal, G.; Joshi, A.; Cushing, J.; Minor, A.M.; et al. Lithium substituted poly (amic acid) as a water-soluble anode binder for high-temperature pre-lithiation. J. Power Sources 2022, 521, 230889. [Google Scholar] [CrossRef]
- Vogl, U.; Das, P.; Weber, A.; Winter, M.; Kostecki, R.; Lux, S. Mechanism of interactions between CMC binder and Si single crystal facets. Langmuir 2014, 30, 10299–10307. [Google Scholar] [CrossRef]
- Casimir, A.; Zhang, H.; Ogoke, O.; Amine, J.C.; Lu, J.; Wu, G. Silicon-based anodes for lithium-ion batteries: Effectiveness of materials synthesis and electrode preparation. Nano Energy 2016, 27, 359–376. [Google Scholar] [CrossRef]
- Armstrong, B.L.; Hays, K.A.; Ruther, R.E.; Hawley, W.B.; Rogers, A.; Greeley, I.; Cavallaro, K.A.; Veith, G.M. Role of silicon-graphite homogeneity as promoted by low molecular weight dispersants. J. Power Sources 2022, 517, 230671. [Google Scholar] [CrossRef]
- Guo, J.; Wang, C. A polymer scaffold binder structure for high capacity silicon anode of lithium-ion battery. Chem. Commun. 2010, 46, 1428–1430. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.H.; Kim, D.H.; Kim, S.; Jeong, M.H.; Nam, J.; Ahn, K.H. Effect of neutralization of poly (acrylic acid) binder on the dispersion heterogeneity of Li-ion battery electrodes. J. Mater. Sci. 2019, 54, 13208–13220. [Google Scholar] [CrossRef]
- Sung, S.H.; Kim, S.; Park, J.H.; Park, J.D.; Ahn, K.H. Role of PVDF in rheology and microstructure of NCM cathode slurries for lithium-ion battery. Materials 2020, 13, 4544. [Google Scholar] [CrossRef]
- Park, J.S.; Jee, S.; Chun, B.; Jung, H.W. Practical operations for intermittent dual-layer slot coating processes. Korea-Aust. Rheol. J. 2022, 34, 181–186. [Google Scholar] [CrossRef]
- Ha, J.; Song, Y.; Song, N.; Yun, J.S.; Lee, D. Designing an interpenetrating network of silane-functionalized nanocomposites for enhanced particle dispersity and interfacial bonding strength. Ceram. Int. 2022, 48, 1827–1835. [Google Scholar] [CrossRef]
- Li, C.C.; Lee, J.T.; Peng, X.W. Improvements of dispersion homogeneity and cell performance of aqueous-processed LiCoO2 cathodes by using dispersant of PAA–NH4. J. Electrochem. Soc. 2006, 153, A809. [Google Scholar] [CrossRef]
- Li, C.C.; Lee, J.T.; Lo, C.Y.; Wu, M.S. Effects of PAA-NH4 addition on the dispersion property of aqueous LiCoO2 slurries and the cell performance of as-prepared LiCoO2 cathodes. Electrochem. Solid-State Lett. 2005, 8, A509. [Google Scholar] [CrossRef]
- Li, L.C.; Tian, Y. Zeta potential. Encycl. Pharm. Technol. 1997, 6, 4117–4128. [Google Scholar]
- Hanaor, D.; Michelazzi, M.; Leonelli, C.; Sorrell, C.C. The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. J. Eur. Ceram. Soc. 2012, 32, 235–244. [Google Scholar] [CrossRef]
- Huang, L.H.; Chen, D.; Li, C.C.; Chang, Y.L.; Lee, J.T. Dispersion homogeneity and electrochemical performance of Si anodes with the addition of various water-based binders. J. Electrochem. Soc. 2018, 165, A2239. [Google Scholar] [CrossRef]
- Wu, C.C.; Li, C.C. Distribution uniformity of water-based binders in si anodes and the distribution effects on cell performance. ACS Sustain. Chem. Eng. 2020, 8, 6868–6876. [Google Scholar] [CrossRef]
- Liu, H.; Ojha, B.; Morris, C.; Jiang, M.; Wojcikiewicz, E.P.; Rao, P.P.; Du, D. Positively charged chitosan and N-trimethyl chitosan inhibit Aβ40 fibrillogenesis. Biomacromolecules 2015, 16, 2363–2373. [Google Scholar] [CrossRef] [PubMed]
- Alison, L.; Ruhs, P.A.; Tervoort, E.; Teleki, A.; Zanini, M.; Isa, L.; Studart, A.R. Pickering and network stabilization of biocompatible emulsions using chitosan-modified silica nanoparticles. Langmuir 2016, 32, 13446–13457. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, D.G.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current trends in Pickering emulsions: Particle morphology and applications. Engineering 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Alison, L.; Demirörs, A.F.; Tervoort, E.; Teleki, A.; Vermant, J.; Studart, A.R. Emulsions stabilized by chitosan-modified silica nanoparticles: PH control of structure–Property relations. Langmuir 2018, 34, 6147–6160. [Google Scholar] [CrossRef]
- Xiong, J.; Dupré, N.; Mazouzi, D.; Guyomard, D.; Roué, L.; Lestriez, B. Influence of the polyacrylic acid binder neutralization degree on the initial electrochemical behavior of a silicon/graphite electrode. ACS Appl. Mater. Interfaces 2021, 13, 28304–28323. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, H.M. Crosslinked carboxymethylchitosan-g-poly (acrylic acid) copolymer as a novel superabsorbent polymer. Carbohydr. Res. 2006, 341, 887–896. [Google Scholar] [CrossRef]
- Komaba, S.; Okushi, K.; Ozeki, T.; Yui, H.; Katayama, Y.; Miura, T.; Saito, T.; Groult, H. Polyacrylate modifier for graphite anode of lithium-ion batteries. Electrochem. Solid-State Lett. 2009, 12, A107. [Google Scholar] [CrossRef]
- Ranoo, S.; Lahiri, B.; Nandy, M.; Philip, J. Enhanced magnetic heating efficiency at acidic pH for magnetic nanoemulsions stabilized with a weak polyelectrolyte. J. Colloid Interface Sci. 2020, 579, 582–597. [Google Scholar] [CrossRef] [PubMed]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.; Hoemann, C.; Leroux, J.; Atkinson, B.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Afzal, S.; Maswal, M.; Dar, A.A. Rheological behavior of pH responsive composite hydrogels of chitosan and alginate: Characterization and its use in encapsulation of citral. Colloids Surfaces B Biointerfaces 2018, 169, 99–106. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Lee, J.H.; Paik, U.; Hackley, V.A.; Choi, Y.M. Effect of poly (acrylic acid) on adhesion strength and electrochemical performance of natural graphite negative electrode for lithium-ion batteries. J. Power Sources 2006, 161, 612–616. [Google Scholar] [CrossRef]
- Chen, L.; Xie, X.; Xie, J.; Wang, K.; Yang, J. Binder effect on cycling performance of silicon/carbon composite anodes for lithium ion batteries. J. Appl. Electrochem. 2006, 36, 1099–1104. [Google Scholar] [CrossRef]
- Liu, W.R.; Yang, M.H.; Wu, H.C.; Chiao, S.; Wu, N.L. Enhanced cycle life of Si anode for Li-ion batteries by using modified elastomeric binder. Electrochem. Solid-State Lett. 2004, 8, A100. [Google Scholar] [CrossRef]
- Biggs, S.; Healy, T.W. Electrosteric stabilisation of colloidal zirconia with low-molecular-weight polyacrylic acid. An atomic force microscopy study. J. Chem. Soc. Faraday Trans. 1994, 90, 3415–3421. [Google Scholar] [CrossRef]
- Szilagyi, I.; Trefalt, G.; Tiraferri, A.; Maroni, P.; Borkovec, M. Polyelectrolyte adsorption, interparticle forces, and colloidal aggregation. Soft Matter 2014, 10, 2479–2502. [Google Scholar] [CrossRef]
- Eom, Y.; Kim, F.; Yang, S.E.; Son, J.S.; Chae, H.G. Rheological design of 3D printable all-inorganic inks using BiSbTe-based thermoelectric materials. J. Rheol. 2019, 63, 291–304. [Google Scholar] [CrossRef]
- Özkan, M.; Dimic-Misic, K.; Karakoc, A.; Hashmi, S.G.; Lund, P.; Maloney, T.; Paltakari, J. Rheological characterization of liquid electrolytes for drop-on-demand inkjet printing. Org. Electron. 2016, 38, 307–315. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Vatansever, C. Three interval thixotropy test to determine structural regeneration of a glucomannan based hydrocolloid film at air/water interface: Interfacial, molecular, thermal and surface characterization. Food Hydrocoll. 2016, 61, 458–468. [Google Scholar] [CrossRef]
- Ju, Y.; Ha, J.; Song, Y.; Yun, J.S.; Lee, D. Optimizing the printability and dispersibility of functionalized zirconium oxide/acrylate composites with various nano-to micro-particle ratios. Ceram. Int. 2020, 46, 26903–26910. [Google Scholar] [CrossRef]
- Ju, Y.; Ha, J.; Song, Y.; Lee, D. Revealing the enhanced structural recovery and gelation mechanisms of cation-induced cellulose nanofibrils composite hydrogels. Carbohydr. Polym. 2021, 272, 118515. [Google Scholar] [CrossRef] [PubMed]
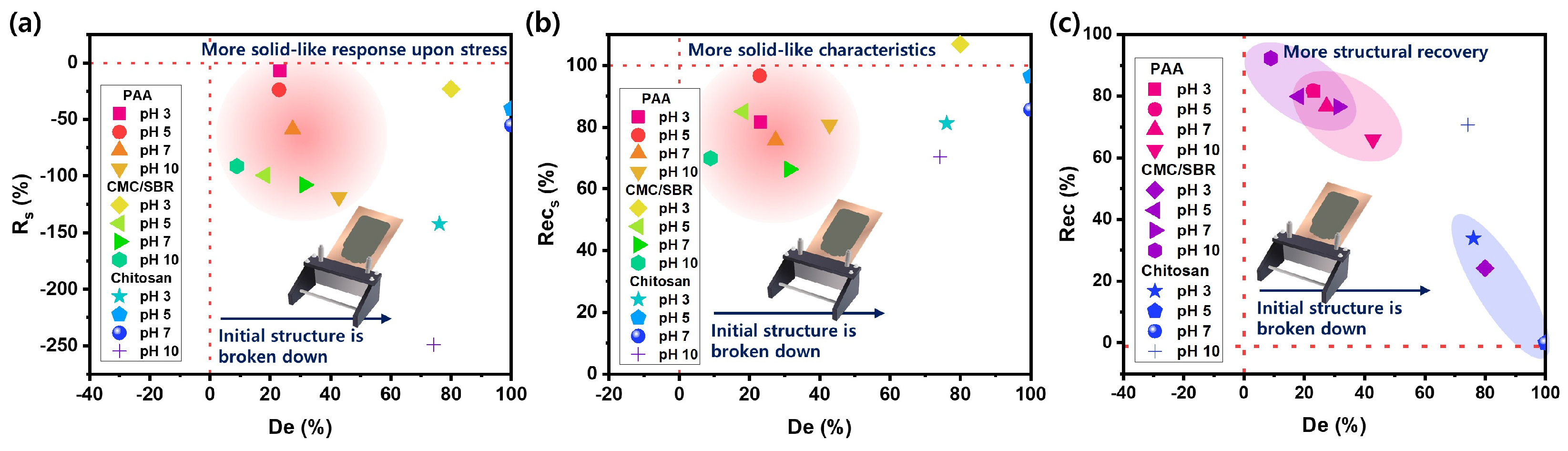
| Structural Deformation Parameters | Equation | Definition |
|---|---|---|
| (%) | Degree of structural deformation (+) : initial structure is broken down (−) : further structure is newly developed | |
| (%) | Degree of structural recovery < 100%: structure is partially recovered > 100%: new structure is generated | |
| (%) | Relative solidity (+) (%): enhanced solid-like character by stress (−) (%): enhanced liquid-like character by stress | |
| (%) | Degree of recovery for relative solidity (%) < 100%: solid character is partially recovered (%) > 100%: more solid-like character |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.; Song, Y.; Youn, B.; Lee, D. Dispersion Homogeneity of Silicon Anode Slurries with Various Binders for Li-Ion Battery Anode Coating. Polymers 2023, 15, 1152. https://doi.org/10.3390/polym15051152
Kim B, Song Y, Youn B, Lee D. Dispersion Homogeneity of Silicon Anode Slurries with Various Binders for Li-Ion Battery Anode Coating. Polymers. 2023; 15(5):1152. https://doi.org/10.3390/polym15051152
Chicago/Turabian StyleKim, Bogyoung, Yeeun Song, Byungwook Youn, and Doojin Lee. 2023. "Dispersion Homogeneity of Silicon Anode Slurries with Various Binders for Li-Ion Battery Anode Coating" Polymers 15, no. 5: 1152. https://doi.org/10.3390/polym15051152
APA StyleKim, B., Song, Y., Youn, B., & Lee, D. (2023). Dispersion Homogeneity of Silicon Anode Slurries with Various Binders for Li-Ion Battery Anode Coating. Polymers, 15(5), 1152. https://doi.org/10.3390/polym15051152