Self-Healing Polymer Electrolytes for Next-Generation Lithium Batteries
Abstract
1. Introduction
2. Self-Healing Mechanism
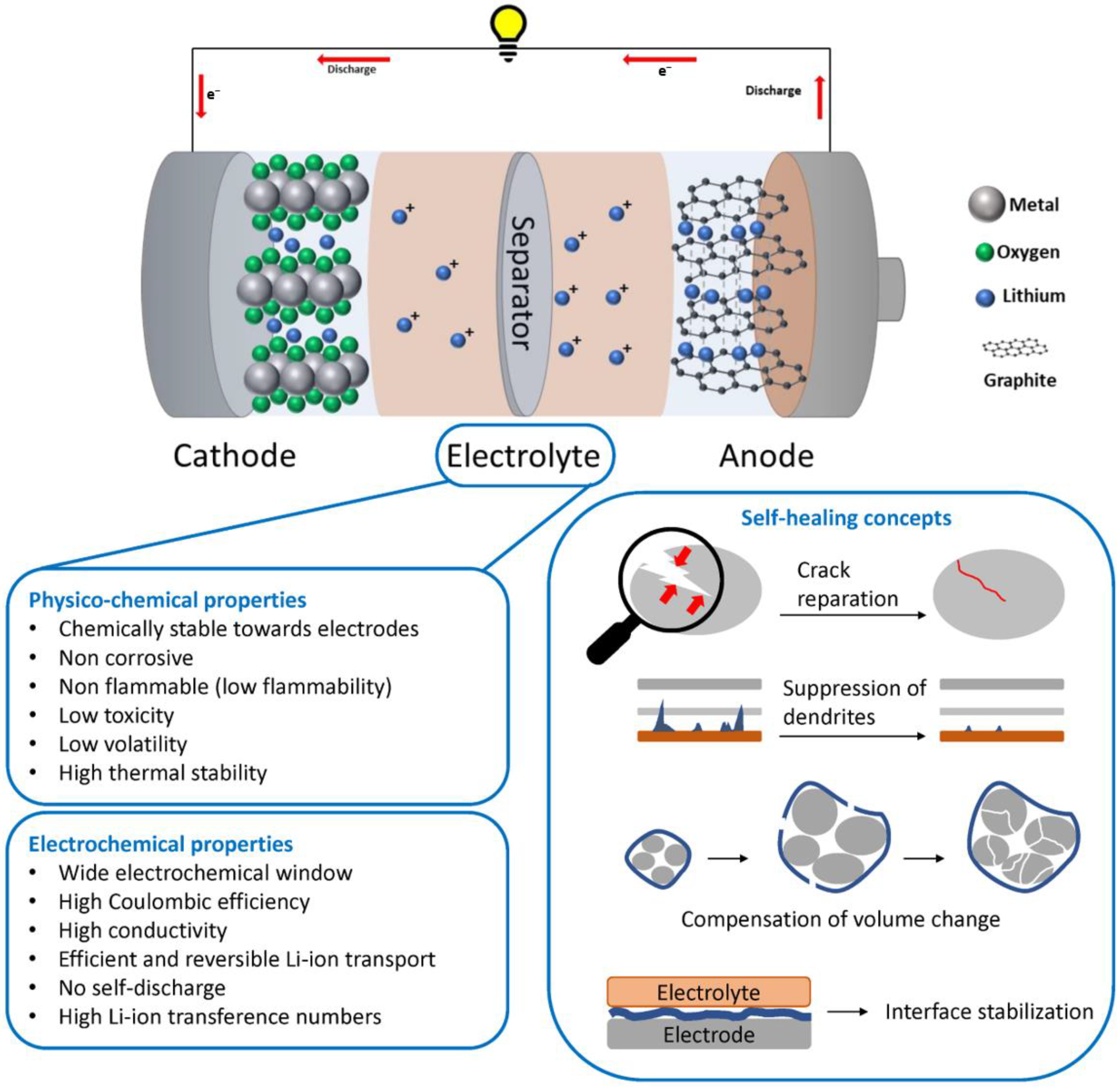
3. Self-Healing Electrolytes for Lithium Batteries
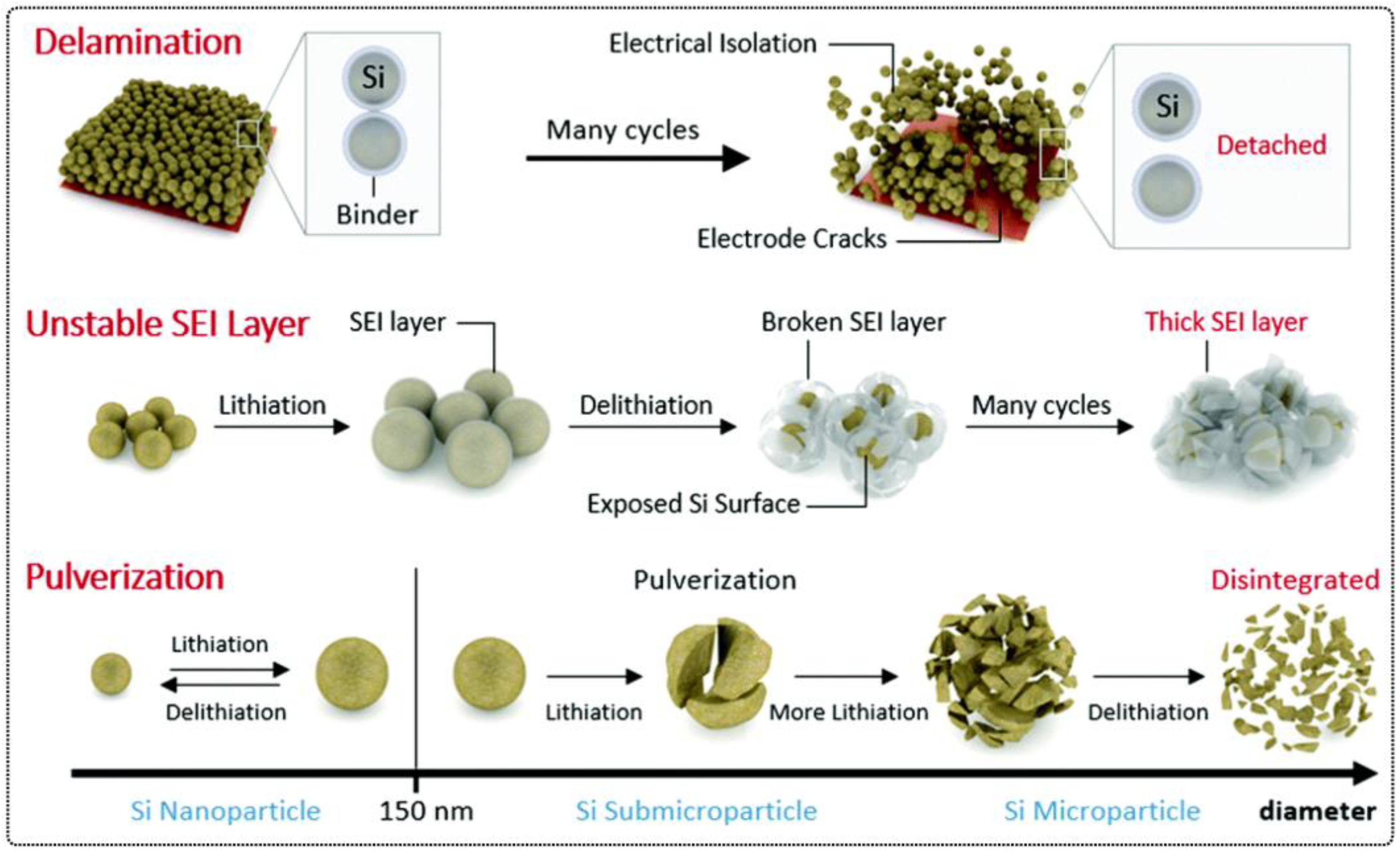
3.1. Opportunities and Challenges for Electrolytes
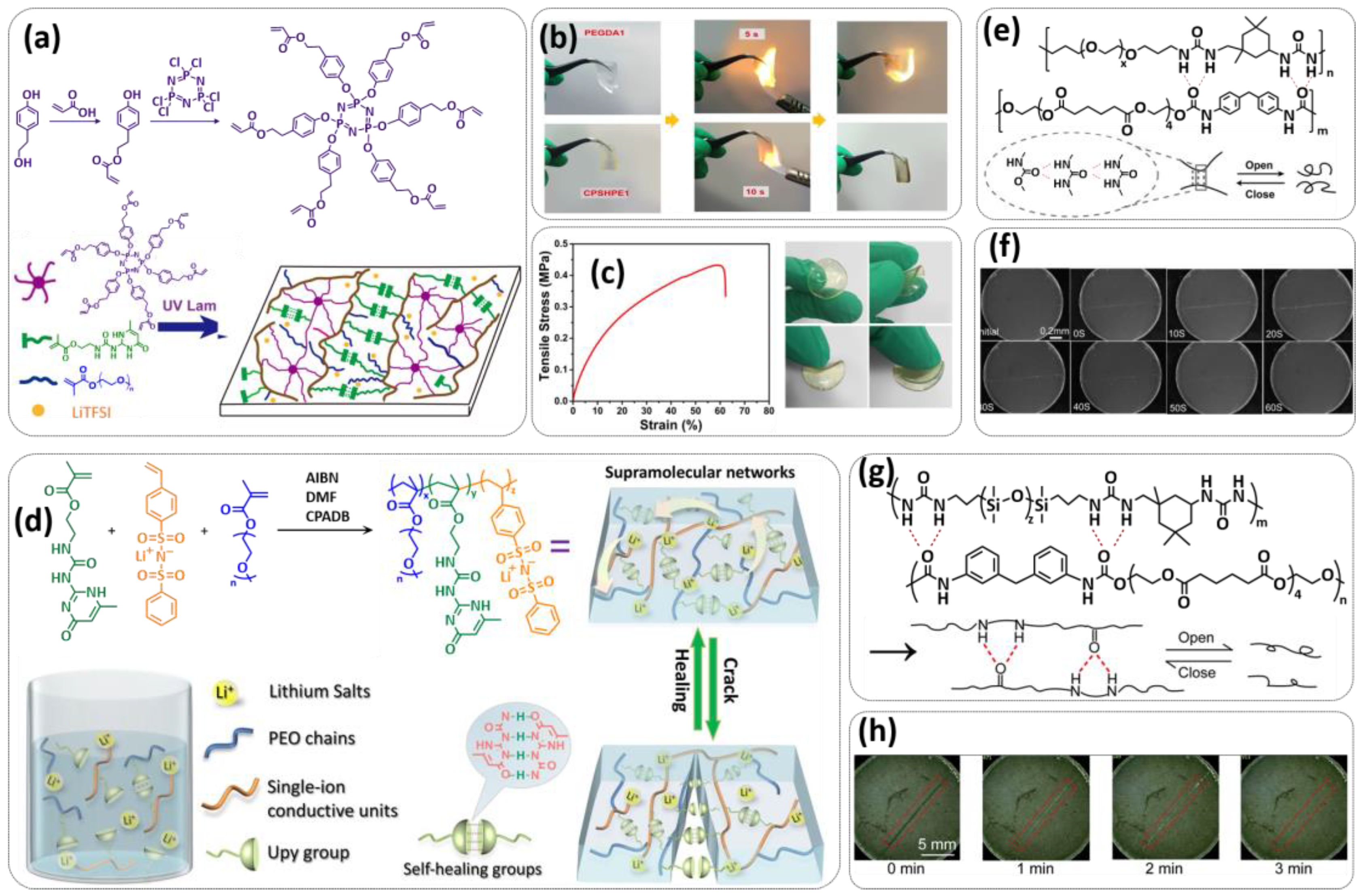
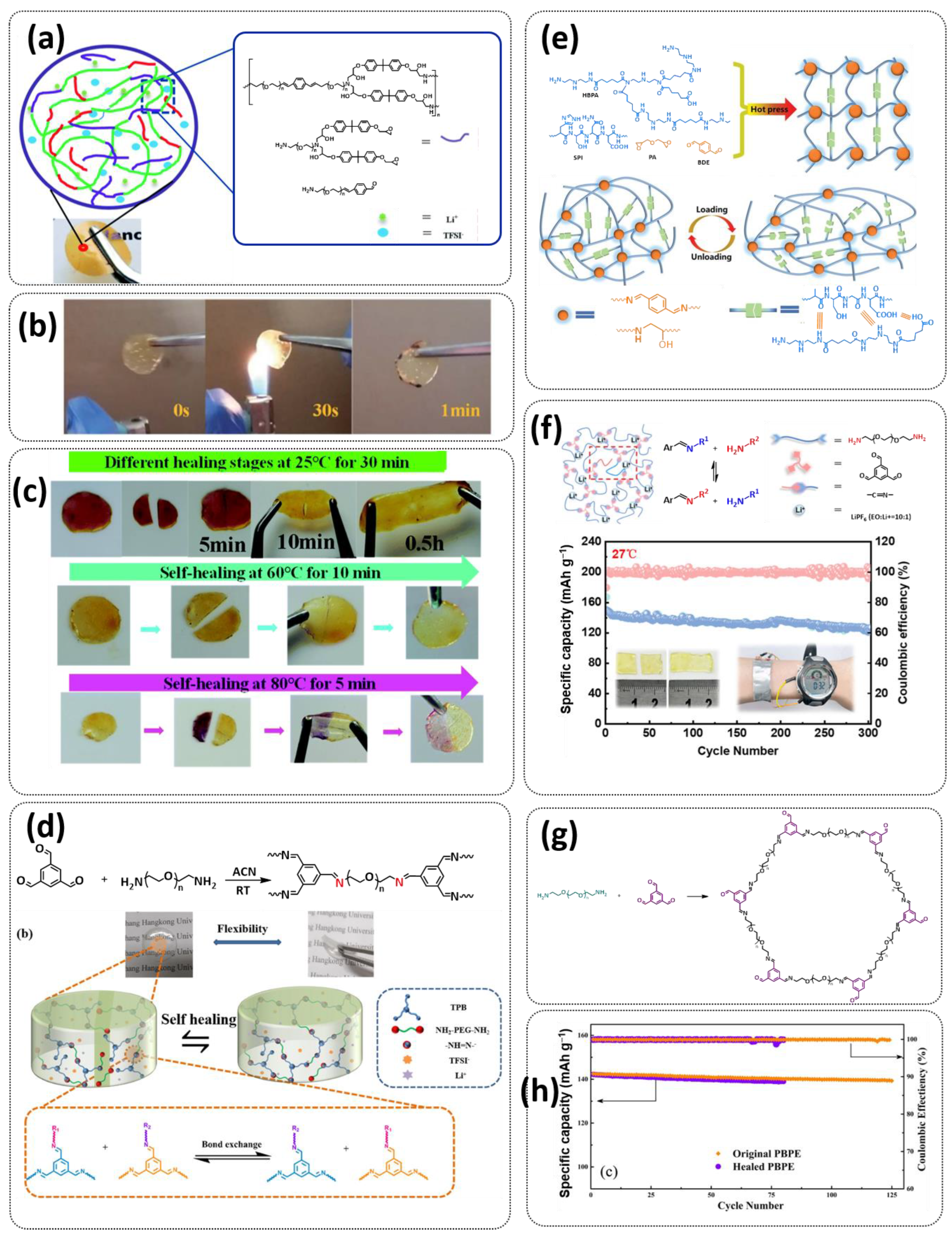
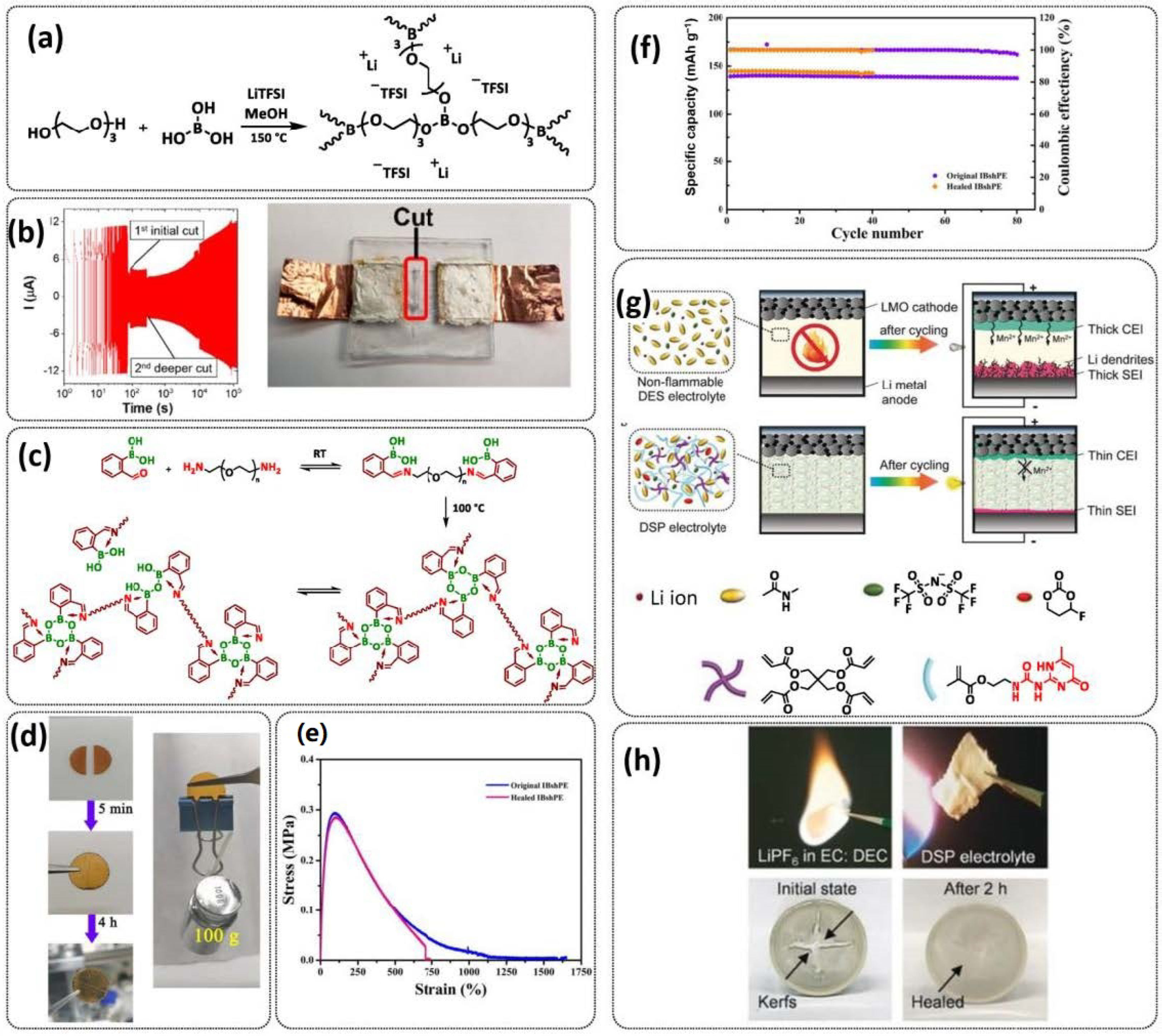
3.2. Self-Healing Solid Polymer Electrolytes (SPEs)
3.3. Self-Healing Gel Polymer Electrolytes (GPEs)
3.4. Self-Healing Composite Polymer Electrolytes (CPEs)
4. Self-Healing Binders for Lithium Batteries
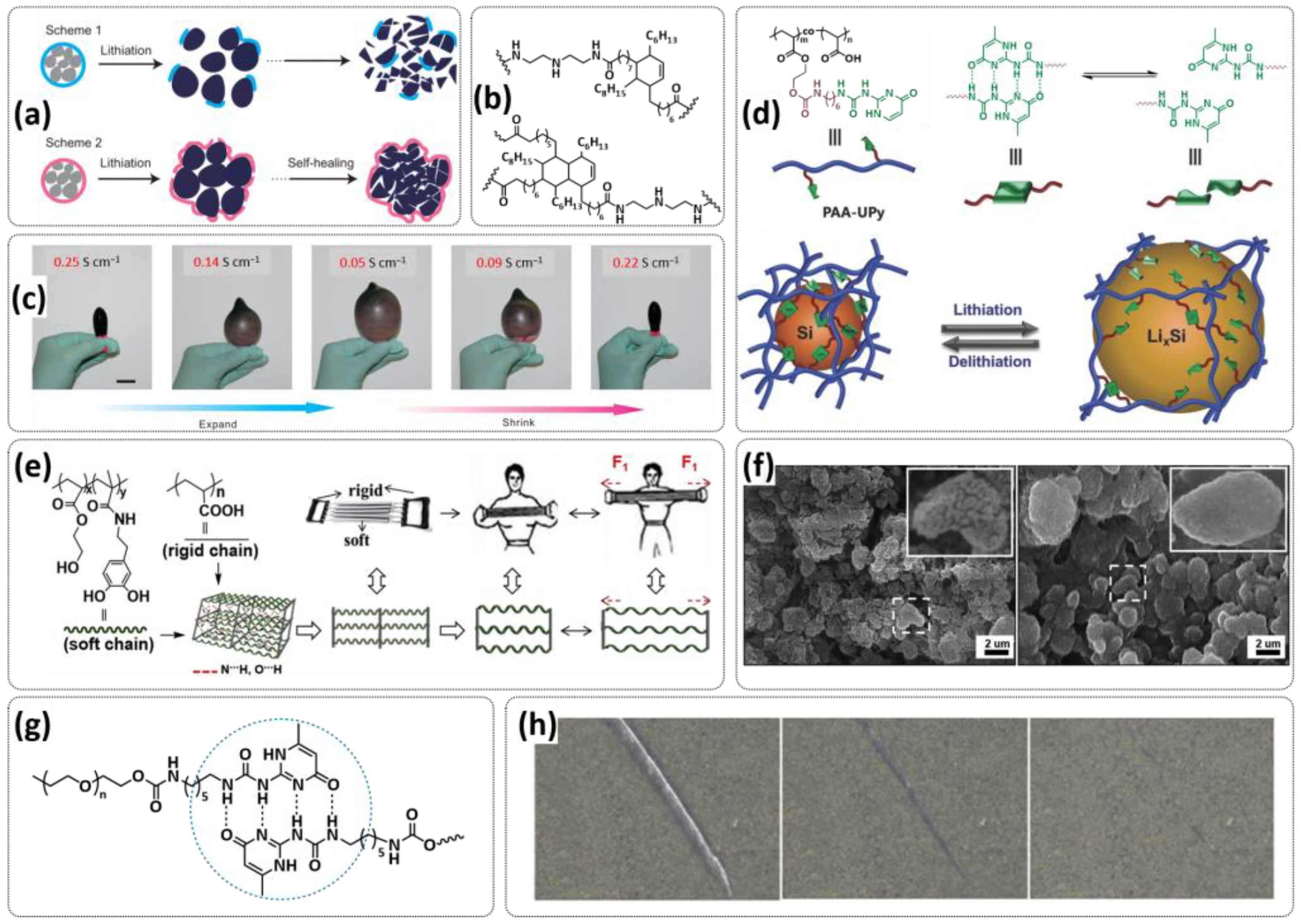
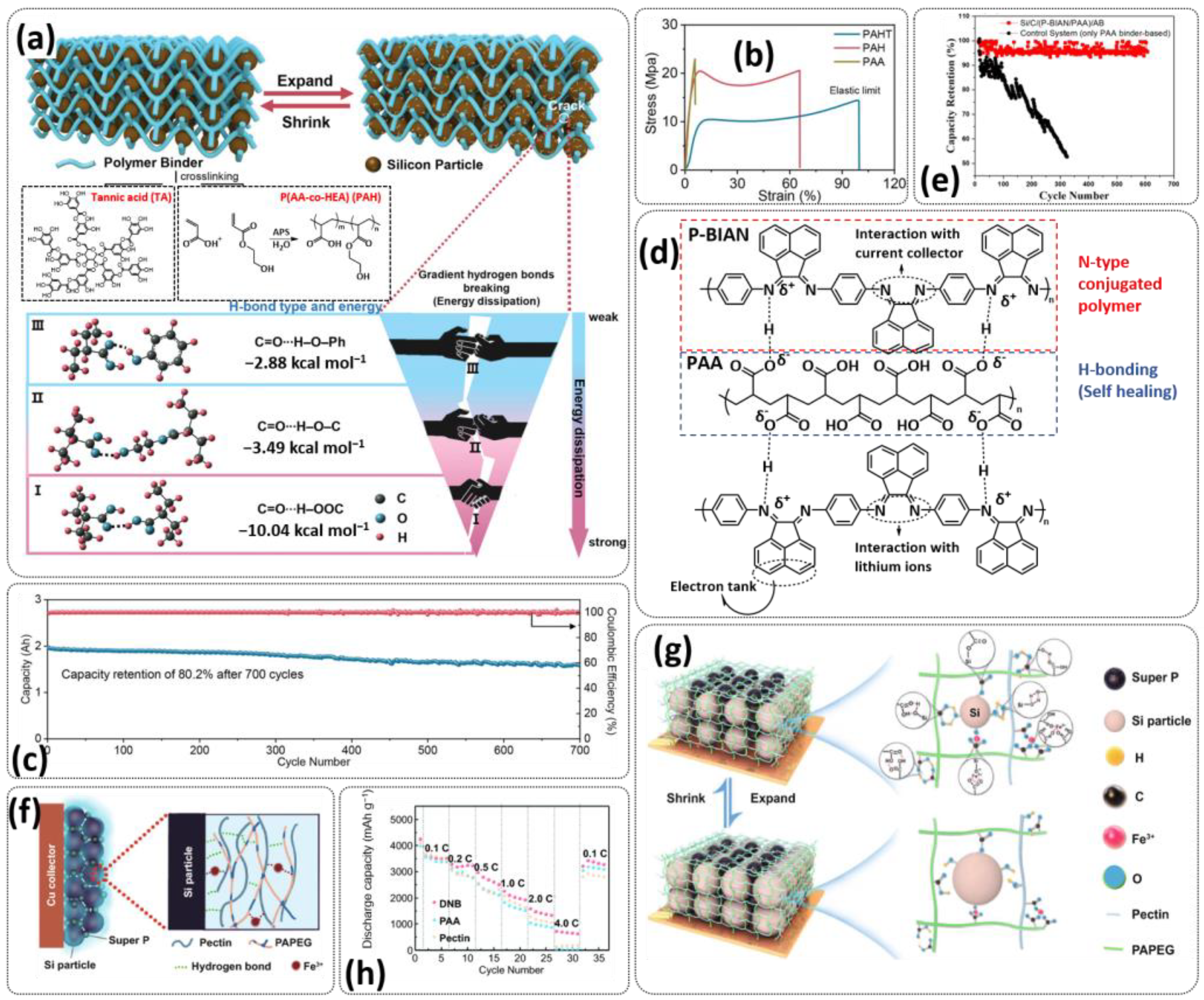
4.1. Self-Healing Binders for Si Anodes
4.2. Self-Healing Binders for Li-Metal Anodes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Lopez, J.; Mackanic, D.G.; Cui, Y.; Bao, Z. Designing polymers for advanced battery chemistries. Nat. Rev. Mater. 2019, 4, 312–330. [Google Scholar] [CrossRef]
- Fichtner, M.; Edström, K.; Ayerbe, E.; Berecibar, M.; Bhowmik, A.; Castelli, I.E.; Clark, S.; Dominko, R.; Erakca, M.; Franco, A.A.; et al. Rechargeable Batteries of the Future—The State of the Art from a BATTERY 2030+ Perspective. Adv. Energy Mater. 2022, 12, 2102904. [Google Scholar] [CrossRef]
- Amici, J.; Asinari, P.; Ayerbe, E.; Barboux, P.; Bayle-Guillemaud, P.; Behm, R.J.; Berecibar, M.; Berg, E.; Bhowmik, A.; Bodoardo, S.; et al. A Roadmap for Transforming Research to Invent the Batteries of the Future Designed within the European Large Scale Research Initiative BATTERY 2030+. Adv. Energy Mater. 2022, 12, 2102785. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Miao, Y.; Hynan, P.; von Jouanne, A.; Yokochi, A. Current Li-Ion Battery Technologies in Electric Vehicles and Opportunities for Advancements. Energies 2019, 12, 1074. [Google Scholar] [CrossRef]
- Forsyth, M.; Porcarelli, L.; Wang, X.; Goujon, N.; Mecerreyes, D. Innovative Electrolytes Based on Ionic Liquids and Polymers for Next-Generation Solid-State Batteries. Acc. Chem. Res. 2019, 52, 686–694. [Google Scholar] [CrossRef]
- Jin, S.; Mu, D.; Lu, Z.; Li, R.; Liu, Z.; Wang, Y.; Tian, S.; Dai, C. A comprehensive review on the recycling of spent lithium-ion batteries: Urgent status and technology advances. J. Clean. Prod. 2022, 340, 130535. [Google Scholar] [CrossRef]
- Abu Nayem, S.M.; Ahmad, A.; Shaheen Shah, S.; Saeed Alzahrani, A.; Saleh Ahammad, A.J.; Aziz, M.A. High Performance and Long-cycle Life Rechargeable Aluminum Ion Battery: Recent Progress, Perspectives and Challenges. Chem. Rec. 2022, 22, e202200181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Stalin, S.; Zhao, C.-Z.; Archer, L.A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 2020, 5, 229–252. [Google Scholar] [CrossRef]
- Li, G.; Huang, B.; Pan, Z.; Su, X.; Shao, Z.; An, L. Advances in three-dimensional graphene-based materials: Configurations, preparation and application in secondary metal (Li, Na, K, Mg, Al)-ion batteries. Energy Environ. Sci. 2019, 12, 2030–2053. [Google Scholar] [CrossRef]
- Abraham, K.M. How Comparable Are Sodium-Ion Batteries to Lithium-Ion Counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- Liu, F.; Wang, T.; Liu, X.; Fan, L.-Z. Challenges and Recent Progress on Key Materials for Rechargeable Magnesium Batteries. Adv. Energy Mater. 2021, 11, 2000787. [Google Scholar] [CrossRef]
- Anoopkumar, V.; Bibin, J.; Mercy, T.D. Potassium-Ion Batteries: Key to Future Large-Scale Energy Storage? ACS Appl. Energy Mater. 2020, 3, 9478–9492. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Q.; Hou, Y.; Li, L.; Tao, Z. Recent progress and strategies toward high performance zinc-organic batteries. J. Energy Chem. 2021, 63, 87–112. [Google Scholar] [CrossRef]
- Ye, T.; Li, L.; Zhang, Y. Recent Progress in Solid Electrolytes for Energy Storage Devices. Adv. Funct. Mater. 2020, 30, 2000077. [Google Scholar] [CrossRef]
- Wang, R.; Cui, W.; Chu, F.; Wu, F. Lithium metal anodes: Present and future. J. Energy Chem. 2020, 48, 145–159. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Review—Emerging Trends in the Design of Electrolytes for Lithium and Post-Lithium Batteries. J. Electrochem. Soc. 2020, 167, 050508. [Google Scholar] [CrossRef]
- Lee, W.; Muhammad, S.; Sergey, C.; Lee, H.; Yoon, J.; Kang, Y.-M.; Yoon, W.-S. Advances in the Cathode Materials for Lithium Rechargeable Batteries. Angew. Chem. Int. Ed. 2020, 59, 2578–2605. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhou, X.; Yu, G. Material and Structural Design of Novel Binder Systems for High-Energy, High-Power Lithium-Ion Batteries. Acc. Chem. Res. 2017, 50, 2642–2652. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Costa, C.M.; Lizundia, E.; Lanceros-Méndez, S. Polymers for advanced lithium-ion batteries: State of the art and future needs on polymers for the different battery components. Prog. Energy Combust. Sci. 2020, 79, 100846. [Google Scholar] [CrossRef]
- Wang, C.; Wu, H.; Chen, Z.; McDowell, M.T.; Cui, Y.; Bao, Z. Self-healing chemistry enables the stable operation of silicon microparticle anodes for high-energy lithium-ion batteries. Nat. Chem. 2013, 5, 1042. Available online: https://www.nature.com/articles/nchem.1802#supplementary-information (accessed on 15 November 2019). [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Lu, D.; Shao, Y.; Lozano, T.; Bennett, W.D.; Graff, G.L.; Polzin, B.; Zhang, J.; Engelhard, M.H.; Saenz, N.T.; Henderson, W.A.; et al. Failure Mechanism for Fast-Charged Lithium Metal Batteries with Liquid Electrolytes. Adv. Energy Mater. 2015, 5, 1400993. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, L.; Yu, Y.; Sun, J. Progress of enhancing the safety of lithium ion battery from the electrolyte aspect. Nano Energy 2019, 55, 93–114. [Google Scholar] [CrossRef]
- Mai, W.; Yu, Q.; Han, C.; Kang, F.; Li, B. Self-Healing Materials for Energy-Storage Devices. Adv. Funct. Mater. 2020, 30, 1909912. [Google Scholar] [CrossRef]
- Mezzomo, L.; Ferrara, C.; Brugnetti, G.; Callegari, D.; Quartarone, E.; Mustarelli, P.; Ruffo, R. Exploiting Self-Healing in Lithium Batteries: Strategies for Next-Generation Energy Storage Devices. Adv. Energy Mater. 2020, 10, 2002815. [Google Scholar] [CrossRef]
- Döhler, D.; Michael, P.; Binder, W.H. Principles of Self Healing Polymers. In Self Healing Polymers: From Principle to Application; Binder, W.H., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 7–60. [Google Scholar]
- Wang, S.; Urban, M.W. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar] [CrossRef]
- Zhao, L.; Sottos, N.R. Autonomous Strategies for Improved Performance and Reliability of Li-Ion Batteries. Adv. Energy Mater. 2021, 11, 2003139. [Google Scholar] [CrossRef]
- Ezeigwe, E.R.; Dong, L.; Manjunatha, R.; Tan, M.; Yan, W.; Zhang, J. A review of self-healing electrode and electrolyte materials and their mitigating degradation of Lithium batteries. Nano Energy 2021, 84, 105907. [Google Scholar] [CrossRef]
- Xu, J.; Ding, C.; Chen, P.; Tan, L.; Chen, C.; Fu, J. Intrinsic self-healing polymers for advanced lithium-based batteries: Advances and strategies. Appl. Phys. Rev. 2020, 7, 031304. [Google Scholar] [CrossRef]
- Liao, H.; Zhong, W.; Li, T.; Han, J.; Sun, X.; Tong, X.; Zhang, Y. A review of self-healing electrolyte and their applications in flexible/stretchable energy storage devices. Electrochim. Acta 2022, 404, 139730. [Google Scholar] [CrossRef]
- Narayan, R.; Laberty-Robert, C.; Pelta, J.; Tarascon, J.-M.; Dominko, R. Self-Healing: An Emerging Technology for Next-Generation Smart Batteries. Adv. Energy Mater. 2022, 12, 2102652. [Google Scholar] [CrossRef]
- Cho, S.; Hwang, S.Y.; Oh, D.X.; Park, J. Recent progress in self-healing polymers and hydrogels based on reversible dynamic B–O bonds: Boronic/boronate esters, borax, and benzoxaborole. J. Mater. Chem. A 2021, 9, 14630–14655. [Google Scholar] [CrossRef]
- Chan, C.Y.; Wang, Z.; Jia, H.; Ng, P.F.; Chow, L.; Fei, B. Recent advances of hydrogel electrolytes in flexible energy storage devices. J. Mater. Chem. A 2021, 9, 2043–2069. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Tang, Z.; Liu, Z.; Ruan, Z.; Ma, L.; Yang, Q.; Wang, D.; Zhi, C. Hydrogel Electrolytes for Flexible Aqueous Energy Storage Devices. Adv. Funct. Mater. 2018, 28, 1804560. [Google Scholar] [CrossRef]
- Campanella, A.; Döhler, D.; Binder, W.H. Self-Healing in Supramolecular Polymers. Macromol. Rapid Commun. 2018, 39, 1700739. [Google Scholar] [CrossRef]
- Blaiszik, B.J.; Kramer, S.L.B.; Olugebefola, S.C.; Moore, J.S.; Sottos, N.R.; White, S.R. Self-Healing Polymers and Composites. Annu. Rev. Mater. Res. 2010, 40, 179–211. [Google Scholar] [CrossRef]
- Toohey, K.S.; Sottos, N.R.; Lewis, J.A.; Moore, J.S.; White, S.R. Self-healing materials with microvascular networks. Nat. Mater. 2007, 6, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Kong, X.; Xiong, Y.; Zhang, X.; Chen, H.; Jiang, W.; Niu, Y.; Xu, W.; Ren, C. An overview of dynamic covalent bonds in polymer material and their applications. Eur. Polym. J. 2020, 141, 110094. [Google Scholar] [CrossRef]
- Guerre, M.; Taplan, C.; Winne, J.M.; Du Prez, F.E. Vitrimers: Directing chemical reactivity to control material properties. Chem. Sci. 2020, 11, 4855–4870. [Google Scholar] [CrossRef] [PubMed]
- Herbst, F.; Döhler, D.; Michael, P.; Binder, W.H. Self-healing polymers via supramolecular forces. Macromol. Rapid Commun. 2013, 34, 203–220. [Google Scholar] [CrossRef]
- Herbst, F.; Seiffert, S.; Binder, W.H. Dynamic supramolecular poly(isobutylene)s for self-healing materials. Polym. Chem. 2012, 3, 3084–3092. [Google Scholar] [CrossRef]
- Li, C.; Bhandary, R.; Marinow, A.; Ivanov, D.; Du, M.; Androsch, R.; Binder, W.H. Synthesis and Characterization of Quadrupolar-Hydrogen-Bonded Polymeric Ionic Liquids for Potential Self-Healing Electrolytes. Polymers 2022, 14, 4090. [Google Scholar] [CrossRef]
- Döhler, D.; Kang, J.; Cooper, C.B.; Tok, J.B.H.; Rupp, H.; Binder, W.H.; Bao, Z. Tuning the Self-Healing Response of Poly(dimethylsiloxane)-Based Elastomers. ACS Appl. Polym. Mater. 2020, 2, 4127–4139. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, M.; Ma, C.; Wang, Y.; Li, X.; Yu, G. A Conductive Self-Healing Hybrid Gel Enabled by Metal–Ligand Supramolecule and Nanostructured Conductive Polymer. Nano Lett. 2015, 15, 6276–6281. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, H.; Liu, X.; Sun, J. Counteranion-Mediated Intrinsic Healing of Poly(ionic liquid) Copolymers. ACS Appl. Mater. Interfaces 2018, 10, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, H.; Zhang, P.; Yu, Y. A Sunlight-Degradable Autonomous Self-Healing Supramolecular Elastomer for Flexible Electronic Devices. Chem. Mater. 2018, 30, 3752–3758. [Google Scholar] [CrossRef]
- Meurer, J.; Hniopek, J.; Ahner, J.; Schmitt, M.; Popp, J.; Zechel, S.; Peneva, K.; Hager, M.D. In-depth characterization of self-healing polymers based on pi-pi nteractions. Beilstein J. Org. Chem. 2021, 17, 2496–2504. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Wie, J.J.; Greenland, B.W.; Burattini, S.; Hayes, W.; Colquhoun, H.M.; Mackay, M.E.; Rowan, S.J. High-Strength, Healable, Supramolecular Polymer Nanocomposites. J. Am. Chem. Soc. 2012, 134, 5362–5368. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, H.; Allec, S.I.; Wong, B.M.; Nguyen, D.-S.; Wang, C. A Highly Stretchy, Transparent Elastomer with the Capability to Automatically Self-Heal Underwater. Adv. Mater. 2018, 30, 1804602. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, B.; Sanka, R.V.S.P.; Binder, W.H.; Parthasarthy, V.; Rana, S.; Karak, N. Vitrimers: Associative dynamic covalent adaptive networks in thermoset polymers. Chem. Eng. J. 2020, 385, 123820. [Google Scholar] [CrossRef]
- Reutenauer, P.; Buhler, E.; Boul, P.J.; Candau, S.J.; Lehn, J.-M. Room Temperature Dynamic Polymers Based on Diels–Alder Chemistry. Chem. A Eur. J. 2009, 15, 1893–1900. [Google Scholar] [CrossRef]
- Khan, N.I.; Halder, S.; Gunjan, S.B.; Prasad, T. A review on Diels-Alder based self-healing polymer composites. IOP Conf. Ser. Mater. Sci. Eng. 2018, 377, 012007. [Google Scholar] [CrossRef]
- Kim, S.-M.; Jeon, H.; Shin, S.-H.; Park, S.-A.; Jegal, J.; Hwang, S.Y.; Oh, D.X.; Park, J. Superior Toughness and Fast Self-Healing at Room Temperature Engineered by Transparent Elastomers. Adv. Mater. 2018, 30, 1705145. [Google Scholar] [CrossRef]
- Irigoyen, M.; Fernández, A.; Ruiz, A.; Ruipérez, F.; Matxain, J.M. Diselenide Bonds as an Alternative to Outperform the Efficiency of Disulfides in Self-Healing Materials. J. Org. Chem. 2019, 84, 4200–4210. [Google Scholar] [CrossRef]
- Nishimura, Y.; Chung, J.; Muradyan, H.; Guan, Z. Silyl Ether as a Robust and Thermally Stable Dynamic Covalent Motif for Malleable Polymer Design. J. Am. Chem. Soc. 2017, 139, 14881–14884. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Negulescu, I.; Zhang, D. Dynamic Covalent Polymer Networks Based on Degenerative Imine Bond Exchange: Tuning the Malleability and Self-Healing Properties by Solvent. Macromolecules 2016, 49, 6277–6284. [Google Scholar] [CrossRef]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-Catalyzed Transesterification for Healing and Assembling of Thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Room temperature self-healable epoxy elastomer with reversible alkoxyamines as crosslinkages. Polymer 2014, 55, 3936–3943. [Google Scholar] [CrossRef]
- Chen, S.; Wen, K.; Fan, J.; Bando, Y.; Golberg, D. Progress and future prospects of high-voltage and high-safety electrolytes in advanced lithium batteries: From liquid to solid electrolytes. J. Mater. Chem. A 2018, 6, 11631–11663. [Google Scholar] [CrossRef]
- Mohtadi, R. Beyond Typical Electrolytes for Energy Dense Batteries. Molecules 2020, 25, 1791. [Google Scholar] [CrossRef]
- Osada, I.; de Vries, H.; Scrosati, B.; Passerini, S. Ionic-Liquid-Based Polymer Electrolytes for Battery Applications. Angew. Chem. Int. Ed. 2016, 55, 500–513. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Wu, N.; Shi, Y.-R.; Lang, S.-Y.; Zhou, J.-M.; Liang, J.-Y.; Wang, W.; Tan, S.-J.; Yin, Y.-X.; Wen, R.; Guo, Y.-G. Self-Healable Solid Polymeric Electrolytes for Stable and Flexible Lithium Metal Batteries. Angew. Chem. Int. Ed. 2019, 58, 18146–18149. [Google Scholar] [CrossRef]
- Zhou, B.; Zuo, C.; Xiao, Z.; Zhou, X.; He, D.; Xie, X.; Xue, Z. Self-Healing Polymer Electrolytes Formed via Dual-Networks: A New Strategy for Flexible Lithium Metal Batteries. Chem. A Eur. J. 2018, 24, 19200–19207. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, M.; Zuo, C.; Chen, G.; He, D.; Zhou, X.; Liu, C.; Xie, X.; Xue, Z. Flexible, Self-Healing, and Fire-Resistant Polymer Electrolytes Fabricated via Photopolymerization for All-Solid-State Lithium Metal Batteries. ACS Macro Lett. 2020, 9, 525–532. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, P.; Chang, C.; Guo, W.; Guo, Z.H.; Pu, X. Self-Healing Solid Polymer Electrolyte for Room-Temperature Solid-State Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2021, 13, 46794–46802. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Zhou, S.; Xu, Z.; Xiao, M.; Meng, Y. A high ion-conducting, self-healing and nonflammable polymer electrolyte with dynamic imine bonds for dendrite-free lithium metal batteries. Chem. Eng. J. 2022, 428, 131224. [Google Scholar] [CrossRef]
- Jo, Y.H.; Li, S.; Zuo, C.; Zhang, Y.; Gan, H.; Li, S.; Yu, L.; He, D.; Xie, X.; Xue, Z. Self-Healing Solid Polymer Electrolyte Facilitated by a Dynamic Cross-Linked Polymer Matrix for Lithium-Ion Batteries. Macromolecules 2020, 53, 1024–1032. [Google Scholar] [CrossRef]
- Jo, Y.H.; Zhou, B.; Jiang, K.; Li, S.; Zuo, C.; Gan, H.; He, D.; Zhou, X.; Xue, Z. Self-healing and shape-memory solid polymer electrolytes with high mechanical strength facilitated by a poly(vinyl alcohol) matrix. Polym. Chem. 2019, 10, 6561–6569. [Google Scholar] [CrossRef]
- Li, R.; Fang, Z.; Wang, C.; Zhu, X.; Fu, X.; Fu, J.; Yan, W.; Yang, Y. Six-armed and dicationic polymeric ionic liquid for highly stretchable, nonflammable and notch-insensitive intrinsic self-healing solid-state polymer electrolyte for flexible and safe lithium batteries. Chem. Eng. J. 2022, 430, 132706. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, P.; Guo, N.; Tong, Y.; Xu, Q.; Zhou, D.; Feng, Z. Self-healing solid polymer electrolyte based on imine bonds for high safety and stable lithium metal batteries. RSC Adv. 2021, 11, 2985–2994. [Google Scholar] [CrossRef]
- Zhou, B.; He, D.; Hu, J.; Ye, Y.; Peng, H.; Zhou, X.; Xie, X.; Xue, Z. A flexible, self-healing and highly stretchable polymer electrolyte via quadruple hydrogen bonding for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 11725–11733. [Google Scholar] [CrossRef]
- Gu, W.; Li, F.; Liu, T.; Gong, S.; Gao, Q.; Li, J.; Fang, Z. Recyclable, Self-Healing Solid Polymer Electrolytes by Soy Protein-Based Dynamic Network. Adv. Sci. 2022, 9, 2103623. [Google Scholar] [CrossRef]
- Guo, C.; Cao, Y.; Li, J.; Li, H.; Kumar Arumugam, S.; Oleksandr, S.; Chen, F. Solvent-free green synthesis of nonflammable and self-healing polymer film electrolytes for lithium metal batteries. Appl. Energy 2022, 323, 119571. [Google Scholar] [CrossRef]
- Sun, S.; Wu, T. Preparation and properties of self-healable solid-state polymer electrolytes based on covalent adaptive networks enabled by disulfide bond. J. Polym. Sci. 2022, 60, 2582–2590. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, J.; Yuan, H.; Lan, J.; Yu, Y.; Zhu, Y.; Yang, X. Self-healing polymer electrolyte for long-life and recyclable lithium-metal batteries. Mater. Today Energy 2022, 24, 100939. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y.; Shi, Z.; Zhou, X.; Xue, Z. Disulfide Metathesis-Assisted Lithium-Ion Conduction for PEO-Based Polymer Electrolytes. ACS Macro Lett. 2022, 11, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Cao, X.; Wan, L.; Tong, Y.; Li, T.; Xie, Y. Crosslinked network solid polymer electrolyte with self-healing ability and high stability for lithium metal battery. Polym. Int. 2022, 71, 1201–1209. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Zhou, L.; Yan, Y.; Wu, M.-X.; Wu, N. Fast self-healing solid polymer electrolyte with high ionic conductivity for lithium metal batteries. New J. Chem. 2022, 46, 4049–4051. [Google Scholar] [CrossRef]
- Zhou, S.; Deng, K.; Xu, Z.; Xiao, M.; Meng, Y. Highly conductive self-healing polymer electrolytes based on synergetic dynamic bonds for highly safe lithium metal batteries. Chem. Eng. J. 2022, 442, 136083. [Google Scholar] [CrossRef]
- Jing, B.B.; Evans, C.M. Catalyst-Free Dynamic Networks for Recyclable, Self-Healing Solid Polymer Electrolytes. J. Am. Chem. Soc. 2019, 141, 18932–18937. [Google Scholar] [CrossRef]
- Zhu, X.; Fang, Z.; Deng, Q.; Zhou, Y.; Fu, X.; Wu, L.; Yan, W.; Yang, Y. Poly(ionic liquid)@PEGMA Block Polymer Initiated Microphase Separation Architecture in Poly(ethylene oxide)-Based Solid-State Polymer Electrolyte for Flexible and Self-Healing Lithium Batteries. ACS Sustain. Chem. Eng. 2022, 10, 4173–4185. [Google Scholar] [CrossRef]
- Gan, H.; Zhang, Y.; Li, S.; Yu, L.; Wang, J.; Xue, Z. Self-Healing Single-Ion Conducting Polymer Electrolyte Formed via Supramolecular Networks for Lithium Metal Batteries. ACS Appl. Energy Mater. 2021, 4, 482–491. [Google Scholar] [CrossRef]
- Jaumaux, P.; Liu, Q.; Zhou, D.; Xu, X.; Wang, T.; Wang, Y.; Kang, F.; Li, B.; Wang, G. Deep-Eutectic-Solvent-Based Self-Healing Polymer Electrolyte for Safe and Long-Life Lithium-Metal Batteries. Angew. Chem. Int. Ed. 2020, 59, 9134–9142. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Kong, W.; Zhang, Z.; Wang, L.; Hu, Y.; Zhu, G.; Chen, R.; Ma, L.; Yan, W.; Wang, Y.; et al. Ionic liquid-immobilized polymer gel electrolyte with self-healing capability, high ionic conductivity and heat resistance for dendrite-free lithium metal batteries. Nano Energy 2018, 54, 17–25. [Google Scholar] [CrossRef]
- Wang, C.; Li, R.; Chen, P.; Fu, Y.; Ma, X.; Shen, T.; Zhou, B.; Chen, K.; Fu, J.; Bao, X.; et al. Highly stretchable, non-flammable and notch-insensitive intrinsic self-healing solid-state polymer electrolyte for stable and safe flexible lithium batteries. J. Mater. Chem. A 2021, 9, 4758–4769. [Google Scholar] [CrossRef]
- Tang, Z.; Lyu, X.; Xiao, A.; Shen, Z.; Fan, X. High-Performance Double-Network Ion Gels with Fast Thermal Healing Capability via Dynamic Covalent Bonds. Chem. Mater. 2018, 30, 7752–7759. [Google Scholar] [CrossRef]
- Guo, P.; Su, A.; Wei, Y.; Liu, X.; Li, Y.; Guo, F.; Li, J.; Hu, Z.; Sun, J. Healable, Highly Conductive, Flexible, and Nonflammable Supramolecular Ionogel Electrolytes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 19413–19420. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Yang, P.; Yi, Y.; Liu, P.; Wang, T.; Shu, C.; Qu, L.; Tang, W.; Zhang, Y.; Li, M.; et al. Self-healing and high stretchable polymer electrolytes based on ionic bonds with high conductivity for lithium batteries. J. Power Sources 2020, 450, 227629. [Google Scholar] [CrossRef]
- D’Angelo, A.J.; Panzer, M.J. Design of Stretchable and Self-Healing Gel Electrolytes via Fully Zwitterionic Polymer Networks in Solvate Ionic Liquids for Li-Based Batteries. Chem. Mater. 2019, 31, 2913–2922. [Google Scholar] [CrossRef]
- Li, F.; Nguyen, G.T.M.; Vancaeyzeele, C.; Vidal, F.; Plesse, C. Healable Ionoelastomer Designed from Polymeric Ionic Liquid and Vitrimer Chemistry. ACS Appl. Polym. Mater. 2022, 5, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Cao, X.; Xue, X.; Tong, Y.; Ci, S.; Huang, H.; Zhou, D. Self-Healing and Flexible Ionic Gel Polymer Electrolyte Based on Reversible Bond for High-Performance Lithium Metal Batteries. Energy Technol. 2022, 10, 2100749. [Google Scholar] [CrossRef]
- Chen, X.; Yi, L.; Zou, C.; Liu, J.; Yu, J.; Zang, Z.; Tao, X.; Luo, Z.; Guo, X.; Chen, G.; et al. High-Performance Gel Polymer Electrolyte with Self-Healing Capability for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 5267–5276. [Google Scholar] [CrossRef]
- Xia, S.; Lopez, J.; Liang, C.; Zhang, Z.; Bao, Z.; Cui, Y.; Liu, W. High-Rate and Large-Capacity Lithium Metal Anode Enabled by Volume Conformal and Self-Healable Composite Polymer Electrolyte. Adv. Sci. 2019, 6, 1802353. [Google Scholar] [CrossRef]
- Zhou, B.; Jo, Y.H.; Wang, R.; He, D.; Zhou, X.; Xie, X.; Xue, Z. Self-healing composite polymer electrolyte formed via supramolecular networks for high-performance lithium-ion batteries. J. Mater. Chem. A 2019, 7, 10354–10362. [Google Scholar] [CrossRef]
- Whiteley, J.M.; Taynton, P.; Zhang, W.; Lee, S.-H. Ultra-thin Solid-State Li-Ion Electrolyte Membrane Facilitated by a Self-Healing Polymer Matrix. Adv. Mater. 2015, 27, 6922–6927. [Google Scholar] [CrossRef]
- Whiteley, J.M.; Hafner, S.; Zhu, C.; Zhang, W.; Lee, S.-H. Stable Lithium Deposition Using a Self-Optimizing Solid Electrolyte Composite. J. Electrochem. Soc. 2017, 164, A2962–A2966. [Google Scholar] [CrossRef]
- Katcharava, Z.; Marinow, A.; Bhandary, R.; Binder, W.H. 3D Printable Composite Polymer Electrolytes: Influence of SiO2 Nanoparticles on 3D-Printability. Nanomaterials 2022, 12, 1859. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, L.; Zhang, H.; Ji, X. Self-healing composite solid electrolytes with enhanced Li+ transport and mechanical properties for safe lithium metal batteries. Chem. Eng. J. 2022, 438, 135418. [Google Scholar] [CrossRef]
- Varzi, A.; Raccichini, R.; Passerini, S.; Scrosati, B. Challenges and prospects of the role of solid electrolytes in the revitalization of lithium metal batteries. J. Mater. Chem. A 2016, 4, 17251–17259. [Google Scholar] [CrossRef]
- Xie, Z.; Hu, B.-L.; Li, R.-W.; Zhang, Q. Hydrogen Bonding in Self-Healing Elastomers. ACS Omega 2021, 6, 9319–9333. [Google Scholar] [CrossRef]
- Huang, X.; Nakagawa, S.; Houjou, H.; Yoshie, N. Insights into the Role of Hydrogen Bonds on the Mechanical Properties of Polymer Networks. Macromolecules 2021, 54, 4070–4080. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Chen, R.; Zhang, H.; Shi, D.; Wang, Y.; Dong, W.; Ma, P.; Zhao, Y. Use of Quadruple Hydrogen Bonding as the Switching Phase in Thermo- and Light-Responsive Shape Memory Hydrogel. ACS Appl. Polym. Mater. 2021, 3, 2884–2888. [Google Scholar] [CrossRef]
- Lugger, S.J.D.; Houben, S.J.A.; Foelen, Y.; Debije, M.G.; Schenning, A.P.H.J.; Mulder, D.J. Hydrogen-Bonded Supramolecular Liquid Crystal Polymers: Smart Materials with Stimuli-Responsive, Self-Healing, and Recyclable Properties. Chem. Rev. 2022, 122, 4946–4975. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Mahmood, N.; Beiner, M.; Binder, W.H. Self-Healing Materials from V- and H-Shaped Supramolecular Architectures. Angew. Chem. Int. Ed. 2015, 54, 10188–10192. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef]
- Galiński, M.; Lewandowski, A.; Stępniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Gao, Y.-R.; Cao, J.-F.; Shu, Y.; Wang, J.-H. Research progress of ionic liquids-based gels in energy storage, sensors and antibacterial. Green Chem. Eng. 2021, 2, 368–383. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Chang, Z.; Wu, W.; Wu, G.; Wu, R.; Li, J. Recent achievements in self-healing materials based on ionic liquids: A review. J. Mater. Sci. 2020, 55, 13543–13558. [Google Scholar] [CrossRef]
- Yang, G.; Song, Y.; Wang, Q.; Zhang, L.; Deng, L. Review of ionic liquids containing, polymer/inorganic hybrid electrolytes for lithium metal batteries. Mater. Des. 2020, 190, 108563. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Mecerreyes, D.; Forsyth, M.; Zhang, H.; Armand, M. Polymeric ionic liquids for lithium-based rechargeable batteries. Mol. Syst. Des. Eng. 2019, 4, 294–309. [Google Scholar] [CrossRef]
- McGrath, L.M.; Rohan, J.F. Pyrrolidinium Containing Ionic Liquid Electrolytes for Li-Based Batteries. Molecules 2020, 25, 6002. [Google Scholar] [CrossRef]
- Baskoro, F.; Wong, H.Q.; Yen, H.-J. Strategic Structural Design of a Gel Polymer Electrolyte toward a High Efficiency Lithium-Ion Battery. ACS Appl. Energy Mater. 2019, 2, 3937–3971. [Google Scholar] [CrossRef]
- Liang, S.; Yan, W.; Wu, X.; Zhang, Y.; Zhu, Y.; Wang, H.; Wu, Y. Gel polymer electrolytes for lithium ion batteries: Fabrication, characterization and performance. Solid State Ion. 2018, 318, 2–18. [Google Scholar] [CrossRef]
- Tamate, R.; Watanabe, M. Recent progress in self-healable ion gels. Sci. Technol. Adv. Mater. 2020, 21, 388–401. [Google Scholar] [CrossRef]
- Boaretto, N.; Meabe, L.; Martinez-Ibañez, M.; Armand, M.; Zhang, H. Review—Polymer Electrolytes for Rechargeable Batteries: From Nanocomposite to Nanohybrid. J. Electrochem. Soc. 2020, 167, 070524. [Google Scholar] [CrossRef]
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on Polymer-Based Composite Electrolytes for Lithium Batteries. Front. Chem. 2019, 7, 522. [Google Scholar] [CrossRef]
- Zhang, L.; Al-Mamun, M. Investigating ionic liquids for optimizing lithium metal anode. Green Energy Environ. 2022, 7, 173–175. [Google Scholar] [CrossRef]
- Rupp, H.; Bhandary, R.; Kulkarni, A.; Binder, W. Printable Electrolytes: Tuning 3D-Printing by Multiple Hydrogen Bonds and Added Inorganic Lithium-Salts. Adv. Mater. Technol. 2022, 7, 2200088. [Google Scholar] [CrossRef]
- Rupp, H.; Binder, W.H. 3D Printing of Solvent-Free Supramolecular Polymers. Front. Chem. 2021, 9, 771974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, J.; Yue, L.; Wang, Q.; Chai, J.; Liu, Z.; Zhou, X.; Li, H.; Guo, Y.; Cui, G.; et al. Safety-Reinforced Poly(Propylene Carbonate)-Based All-Solid-State Polymer Electrolyte for Ambient-Temperature Solid Polymer Lithium Batteries. Adv. Energy Mater. 2015, 5, 1501082. [Google Scholar] [CrossRef]
- Fan, X.; Chen, L.; Ji, X.; Deng, T.; Hou, S.; Chen, J.; Zheng, J.; Wang, F.; Jiang, J.; Xu, K.; et al. Highly Fluorinated Interphases Enable High-Voltage Li-Metal Batteries. Chem 2018, 4, 174–185. [Google Scholar] [CrossRef]
- Liu, K.; Pei, A.; Lee, H.R.; Kong, B.; Liu, N.; Lin, D.; Liu, Y.; Liu, C.; Hsu, P.-c.; Bao, Z.; et al. Lithium Metal Anodes with an Adaptive “Solid-Liquid” Interfacial Protective Layer. J. Am. Chem. Soc. 2017, 139, 4815–4820. [Google Scholar] [CrossRef] [PubMed]
- Li, N.W.; Shi, Y.; Yin, Y.X.; Zeng, X.X.; Li, J.Y.; Li, C.J.; Wan, L.J.; Wen, R.; Guo, Y.G. A Flexible Solid Electrolyte Interphase Layer for Long-Life Lithium Metal Anodes. Angew. Chem. Int. Ed. 2018, 57, 1505–1509. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, X.; Yao, P.; Li, J.; Zhu, J. Towards high energy density lithium battery anodes: Silicon and lithium. Chem. Sci. 2019, 10, 7132–7148. [Google Scholar] [CrossRef]
- Krauskopf, T.; Richter, F.H.; Zeier, W.G.; Janek, J. Physicochemical Concepts of the Lithium Metal Anode in Solid-State Batteries. Chem. Rev. 2020, 120, 7745–7794. [Google Scholar] [CrossRef]
- Hu, Z.; Li, J.; Zhang, X.; Zhu, Y. Strategies to Improve the Performance of Li Metal Anode for Rechargeable Batteries. Front. Chem. 2020, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Higgins, T.M.; Park, S.-H.; King, P.J.; Zhang, C.; McEvoy, N.; Berner, N.C.; Daly, D.; Shmeliov, A.; Khan, U.; Duesberg, G.; et al. A Commercial Conducting Polymer as Both Binder and Conductive Additive for Silicon Nanoparticle-Based Lithium-Ion Battery Negative Electrodes. ACS Nano 2016, 10, 3702–3713. [Google Scholar] [CrossRef] [PubMed]
- Casimir, A.; Zhang, H.; Ogoke, O.; Amine, J.C.; Lu, J.; Wu, G. Silicon-based anodes for lithium-ion batteries: Effectiveness of materials synthesis and electrode preparation. Nano Energy 2016, 27, 359–376. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Lopez, J.; Lu, Z.; Cui, Y.; Bao, Z. High-Areal-Capacity Silicon Electrodes with Low-Cost Silicon Particles Based on Spatial Control of Self-Healing Binder. Adv. Energy Mater. 2015, 5, 1401826. [Google Scholar] [CrossRef]
- Lopez, J.; Chen, Z.; Wang, C.; Andrews, S.C.; Cui, Y.; Bao, Z. The Effects of Cross-Linking in a Supramolecular Binder on Cycle Life in Silicon Microparticle Anodes. ACS Appl. Mater. Interfaces 2016, 8, 2318–2324. [Google Scholar] [CrossRef]
- Munaoka, T.; Yan, X.; Lopez, J.; To, J.W.F.; Park, J.; Tok, J.B.-H.; Cui, Y.; Bao, Z. Ionically Conductive Self-Healing Binder for Low Cost Si Microparticles Anodes in Li-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703138. [Google Scholar] [CrossRef]
- Kim, D.; Hyun, S.; Han, S.M. Freestanding silicon microparticle and self-healing polymer composite design for effective lithiation stress relaxation. J. Mater. Chem. A 2018, 6, 11353–11361. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Y.; Chen, Y.; Huang, J.; Zhang, T.; Zeng, H.; Wang, C.; Liu, G.; Deng, Y. A Quadruple-Hydrogen-Bonded Supramolecular Binder for High-Performance Silicon Anodes in Lithium-Ion Batteries. Small 2018, 14, 1801189. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, J.; Zhang, T.; Nuli, Y.; Wang, J.; Hirano, S.-i. Silicon Microparticle Anodes with Self-Healing Multiple Network Binder. Joule 2018, 2, 950–961. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Zhang, T.; Wang, X.; Gao, Y.; Fang, Q. Self-healing strategy for Si nanoparticles towards practical application as anode materials for Li-ion batteries. Electrochem. Commun. 2018, 87, 22–26. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, S.; Kim, J.; Park, S.; Lee, S.; Yoo, S.; Kim, J.; Choi, N.-S.; Ryu, J.-H.; Park, S. Room-Temperature Crosslinkable Natural Polymer Binder for High-Rate and Stable Silicon Anodes. Adv. Funct. Mater. 2020, 30, 1908433. [Google Scholar] [CrossRef]
- Jeong, Y.K.; Choi, J.W. Mussel-Inspired Self-Healing Metallopolymers for Silicon Nanoparticle Anodes. ACS Nano 2019, 13, 8364–8373. [Google Scholar] [CrossRef]
- Gupta, A.; Badam, R.; Matsumi, N. Heavy-Duty Performance from Silicon Anodes Using Poly(BIAN)/Poly(acrylic acid)-Based Self-Healing Composite Binder in Lithium-Ion Secondary Batteries. ACS Appl. Energy Mater. 2022, 5, 7977–7987. [Google Scholar] [CrossRef]
- Jiang, M.; Mu, P.; Zhang, H.; Dong, T.; Tang, B.; Qiu, H.; Chen, Z.; Cui, G. An Endotenon Sheath-Inspired Double-Network Binder Enables Superior Cycling Performance of Silicon Electrodes. Nano-Micro Lett. 2022, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Jang, W.; Rajeev, K.K.; Lee, J.-H.; Kim, Y.; Kim, T.-H. Ion-conductive self-healing polymer network based on reversible imine bonding for Si electrodes. J. Power Sources 2021, 499, 229968. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, X.; Zhao, P.; Fan, H.; Zhang, Z.; Deng, J.; Ungar, G.; Song, J. Gradient H-Bonding Binder Enables Stable High-Areal-Capacity Si-Based Anodes in Pouch Cells. Adv. Mater. 2021, 33, 2104416. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Kim, E.; K.K, R.; Kim, Y.; Kim, T.-H. A conductive self healing polymeric binder using hydrogen bonding for Si anodes in lithium ion batteries. Sci. Rep. 2020, 10, 14966. [Google Scholar] [CrossRef]
- K.K, R.; Nam, J.; Kim, E.; Kim, Y.; Kim, T.-H. A self-healable polymer binder for Si anodes based on reversible Diels–Alder chemistry. Electrochim. Acta 2020, 364, 137311. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, C.; Pei, A.; Lopez, J.; Shi, F.; Chen, Z.; Sendek, A.D.; Lee, H.-W.; Lu, Z.; Schneider, H.; et al. High-Performance Lithium Metal Negative Electrode with a Soft and Flowable Polymer Coating. ACS Energy Lett. 2016, 1, 1247–1255. [Google Scholar] [CrossRef]
- Cui, X.; Chu, Y.; Qin, L.; Pan, Q. Stabilizing Li Metal Anodes through a Novel Self-Healing Strategy. ACS Sustain. Chem. Eng. 2018, 6, 11097–11104. [Google Scholar] [CrossRef]
- Yu, Z.; Mackanic, D.G.; Michaels, W.; Lee, M.; Pei, A.; Feng, D.; Zhang, Q.; Tsao, Y.; Amanchukwu, C.V.; Yan, X.; et al. A Dynamic, Electrolyte-Blocking, and Single-Ion-Conductive Network for Stable Lithium-Metal Anodes. Joule 2019, 3, 2761–2776. [Google Scholar] [CrossRef]
- Wang, G.; Chen, C.; Chen, Y.; Kang, X.; Yang, C.; Wang, F.; Liu, Y.; Xiong, X. Self-Stabilized and Strongly Adhesive Supramolecular Polymer Protective Layer Enables Ultrahigh-Rate and Large-Capacity Lithium-Metal Anode. Angew. Chem. Int. Ed. 2020, 59, 2055–2060. [Google Scholar] [CrossRef]
- Cui, X.; Chu, Y.; Wang, X.; Zhang, X.; Li, Y.; Pan, Q. Stabilizing Lithium Metal Anodes by a Self-Healable and Li-Regulating Interlayer. ACS Appl. Mater. Interfaces 2021, 13, 44983–44990. [Google Scholar] [CrossRef]
- Beaulieu, L.Y.; Eberman, K.W.; Turner, R.L.; Krause, L.J.; Dahn, J.R. Colossal Reversible Volume Changes in Lithium Alloys. Electrochem. Solid-State Lett. 2001, 4, A137–A140. [Google Scholar] [CrossRef]
- Liu, N.; Lu, Z.; Zhao, J.; McDowell, M.T.; Lee, H.-W.; Zhao, W.; Cui, Y. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 2014, 9, 187–192. [Google Scholar] [CrossRef]
- Kwon, T.-w.; Choi, J.W.; Coskun, A. The emerging era of supramolecular polymeric binders in silicon anodes. Chem. Soc. Rev. 2018, 47, 2145–2164. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-Dependent Fracture of Silicon Nanoparticles During Lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, X.; Wang, Y.; Lu, C. Effects of size and concentration on diffusion-induced stress in lithium-ion batteries. J. Appl. Phys. 2016, 120, 025302. [Google Scholar] [CrossRef]
- Zhou, X.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G. Self-Assembled Nanocomposite of Silicon Nanoparticles Encapsulated in Graphene through Electrostatic Attraction for Lithium-Ion Batteries. Adv. Energy Mater. 2012, 2, 1086–1090. [Google Scholar] [CrossRef]
- Li, H.; Huang, X.; Chen, L.; Zhou, G.; Zhang, Z.; Yu, D.; Jun Mo, Y.; Pei, N. The crystal structural evolution of nano-Si anode caused by lithium insertion and extraction at room temperature. Solid State Ion. 2000, 135, 181–191. [Google Scholar] [CrossRef]
- Cordier, P.; Tournilhac, F.; Soulié-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]




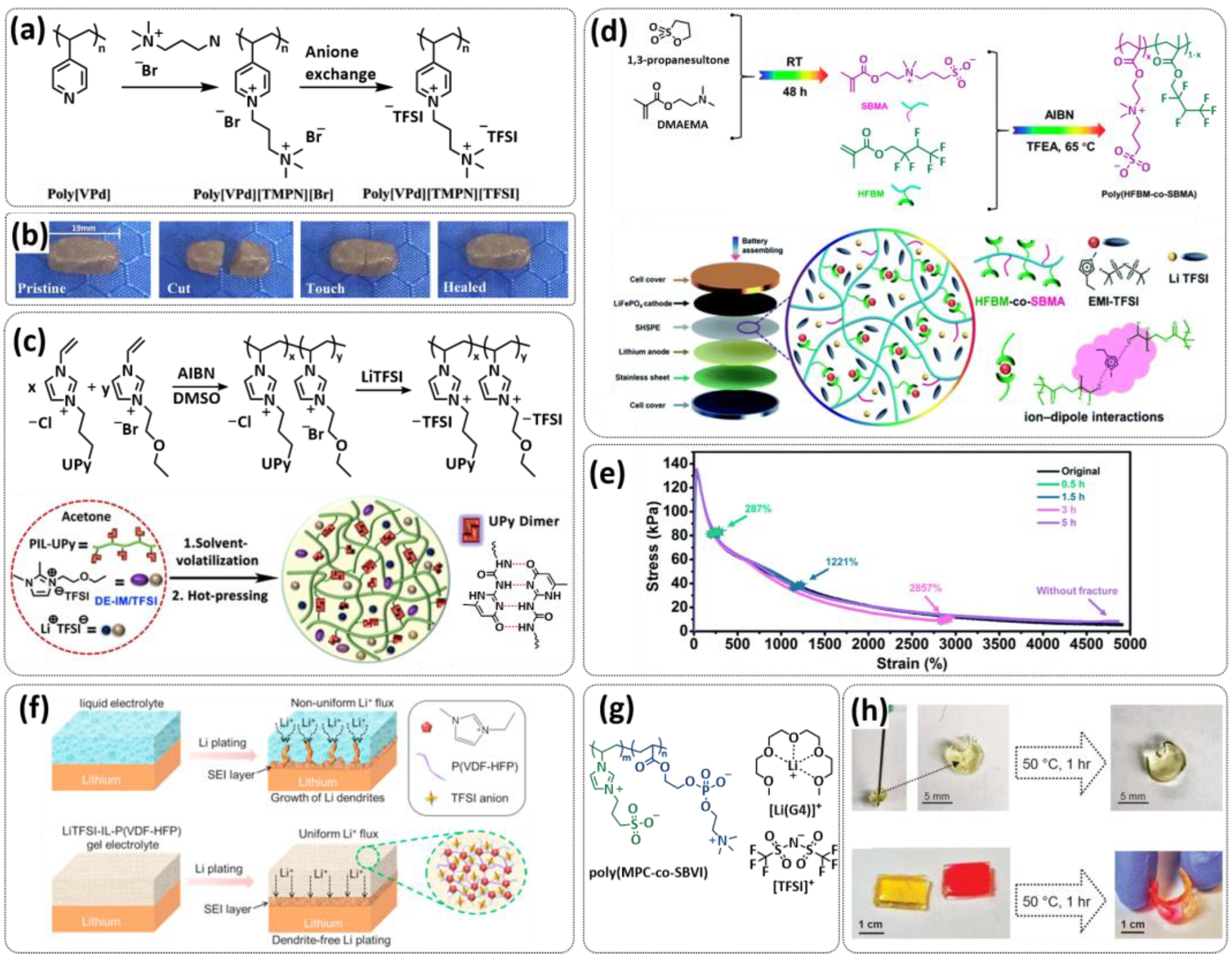





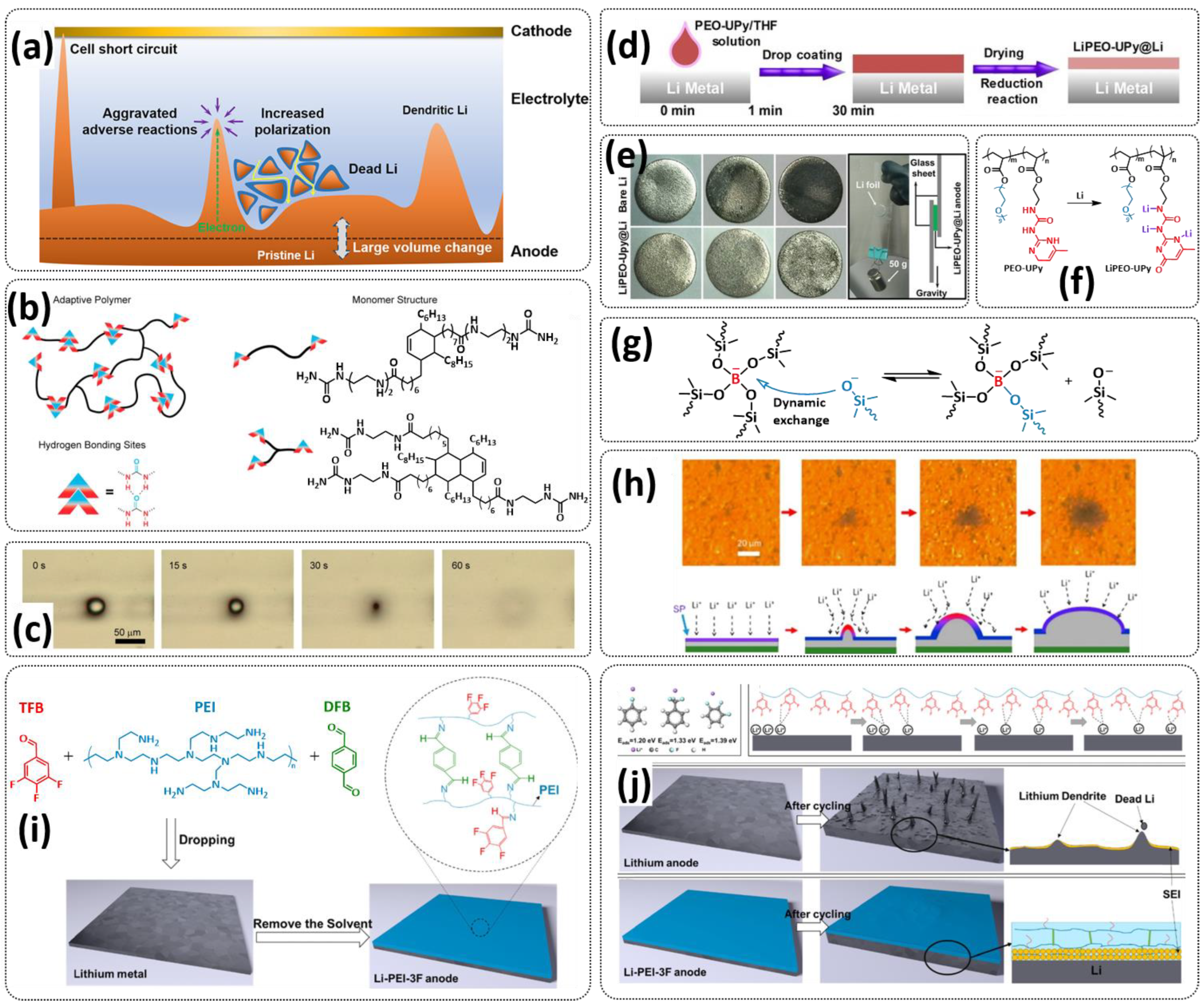

| Entry | Self-Healing Component/Electrolyte | Type | Healing Mechanism | Battery/Additives | Healing Conditions | Performance/Ionic Conductivity | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 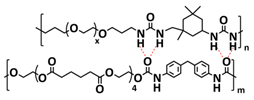 | SPE | H-bonds | LMB, Li/SHSPE/NCM, 2 M LiClO4 (or LiTFSI or LiBF4) in DEC/DMC/EC | RT, 60 s | 170.6 mA h g−1 at 0.1C, σ (RT) = 1.72 × 10−5 S cm−1 | [70] |
| 2 | 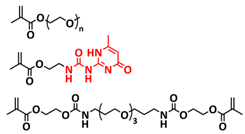 | SPE | H-bonds | LMB, LiFePO4/DN-SHPE/Li, LITFSI (EO/Li+ molar ratio 16:1) | 60 °C, 2 h | 137.9 mA h g−1 at 0.1C, σ (30 °C) = 1.72 × 10−5 S cm−1 | [71] |
| 3 | 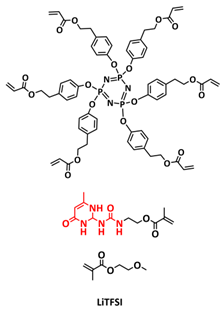 | SPE | H-bonds | LMB, Li/CPSHPE1/LFP, LiTFSI (EO/Li+ molar ratio 16:1) | 60 °C, 3 h | 130 mA h g−1 at 0.1C, σ (30 °C) = 8.9 × 10−5 S cm−1 | [72] |
| 4 |  | SPE | Imine dynamic covalent bonds | LMB, Li|SHSPE| LiFePO4, LiPF6 (EO/Li+ molar ratio 10:1) | RT, 24 h | 156 mA h g−1 at 0.1C, σ (RT) = 7.48 × 10−4 S cm−1 | [73] |
| 5 | 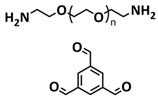 | SPE | Imine dynamic covalent bonds | LMB, LFP/PBPE/Li, 0.8 M LiTFSI + 0.3 M LiDFOB + 0.0.2 M LiPF6 in EC/FEC/EPC (75:25:5 vol) | 60 °C, 0.5 h | 158 mA h g−1 at 0.1C, σ (RT) = 4.79 × 10−3 S cm−1 | [74] |
| 6 |  | SPE | H-bonds, ionic interactions | NA | 40 °C, 1 h | NA, σ (30 °C) = 4.4 × 10−5 S cm−1 | [49] |
| 7 | 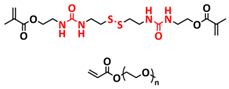 | SPE | H-bonds, disulfide dynamic covalent bonds | LIB and LMB, Li|3PEG-SSH| LFP, LiTFSI (EO/Li+ molar ratio 16:1) | RT, 30 min | 140 mA h g−1 at 0.1C, σ (RT) = 7.28 × 10−6 S cm−1 | [75] |
| 8 | 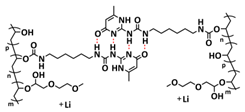 | SPE | H-bonds | LMB, Li/PVA-UPy-PEG750/LFP, LiClO4 (EO/Li+ molar ratio 11:1) | 60 °C, 1 h | 145 mA h g−1 at 0.1C, σ (60 °C) = 1.5 × 10−4 S cm−1 | [76] |
| 9 | 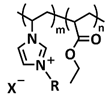 | SPE | Ionic interactions | NA | 55 °C, 7.5 h | NA, σ (RT) = 1.6 × 10−7 S cm−1 | [52] |
| 10 | 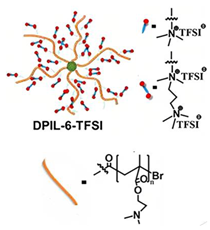 | SPE | Ionic interactions | LIB, LFP/SPE/Li, EMIMTFSI, LiTFSI | RT, <2 h | 153 mA h g−1 at 0.1C, σ (RT) = 5.5 × 10−5 S cm−1 | [77] |
| 11 |  | SPE | Imine dynamic covalent bonds | LMB, Li|ShSPE-3|LiFePO4, LiTFSI (EO/Li+ molar ratio 8/16/24) | RT, 30 min | 141 mA h g−1 at 0.1C, σ (60 °C) = 1.7 × 10−4 S cm−1 | [78] |
| 12 | 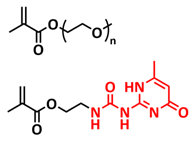 | SPE | H-bonds | LIB, LFP/shPE/Li, LiTFSI (EO/Li+ molar ratio 20:1) | RT, 2 h | 155 mA h g−1 at 0.1C, σ (60 °C) = 1.1 × 10−4 S cm−1 | [79] |
| 13 | 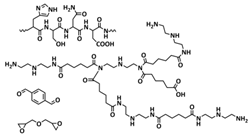 | SPE | Imine dynamic covalent bonds, H-bonds | LIB and LMB, Li|SPI-3Li|LiFePO4, LiTFSI | 100 °C or hot water | 32.6 mA h g−1 at 0.2C, σ (30 °C) = 3.3 × 10−4 S cm−1 | [80] |
| 14 | 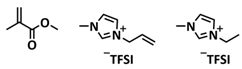 | SPE | Ionic interactions | LMB, Li/EMIMTFSI/P (MMA-co-AMIMTFSI)-40/LFP, LiTFSI | RT, 5 min | 135 mA h g−1 at 0.1C, σ (RT) = 1.9 × 10−4 S cm−1 | [81] |
| 15 | 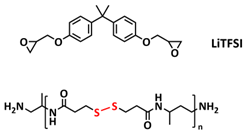 | SPE | Disulfide dynamic covalent bonds, ionic interactions | NA, LiTFSI 10–20 wt%. | 100 °C, 1 h | NA, σ (80 °C) = 3.3 × 10−6 S cm−1 | [82] |
| 16 | 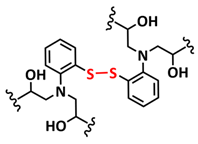 | SPE | Disulfide dynamic covalent bonds, H-bonds | LMB, LiFePO4/RFSPE-3/Li | RT, 1 h | 96 mA h g−1 at 0.2C, σ (RT) = 2.1 × 10−3 S cm−1 | [83] |
| 17 | 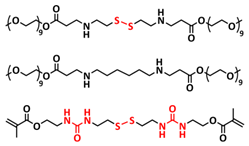 | SPE | Disulfide dynamic covalent bonds, H-bonds | LMB, Li/PESS20/LiFePO4, LiClO4 (EO/Li+ molar ratio 16:1) | 80 °C, 2 h | 138.2 mA h g−1 at 0.1C, σ (30 °C) = 1.2 × 10−4 S cm−1 | [84] |
| 18 |  | SPE | Imine dynamic covalent bonds | LMB, Li|shCLSPE-3400|LiFePO4, LiTFSI (EO/Li+ molar ratio 20:1) | RT, 10 min | 141.6 mA h g−1 at 0.1C, σ (RT) = 4.8 × 10−4 S cm−1 | [85] |
| 19 |  | SPE | H-bonds | LMB, 2 M LiClO4 (or LiTFSI or LiBF4) | RT, 3 min | NA, σ (RT) = 2.5 × 10−4 S cm−1 | [86] |
| 20 | 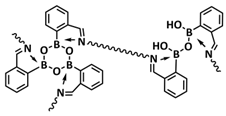 | SPE | Borate and imine dynamic covalent bonds | LMB, LFP/IBshPE/Li, 0.02 M LiPF6 + 0.3 M LiDFOB + 0.8 M LiFSI in FEC/EC (30:70 vol) | RT, 4 h | 130.5 mA h g−1 at 2C, σ (30 °C) = 5 × 10−3 S cm−1 | [87] |
| 21 |  | SPE | Borate dynamic covalent bonds | NA, LITFSI | 60 °C, 34 h | NA, σ (90 °C) = 3.5 × 10−4 S cm−1 | [88] |
| 22 | 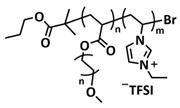 + PEO + LITFSI + PEO + LITFSI | SPE | Ionic interactions | LIB and LMB, Li/PEO@ BPIL-3-SPE/LiFePO4, LiTFSI 10%wt. | 60 °C, <30 min | 163 mA h g−1 at 0.2C, σ (30 °C) = 1 × 10−5 S cm−1 | [89] |
| 23 |  | SIPE | H-bonds, ionic interactions | LMB, Li/SIGPE-5 membrane/LFP, PC | 60 °C, 12 h | 129 mA h g−1 at 0.1C, σ (60 °C) = 1.4 × 10−5 S cm−1 | [90] |
| 24 | 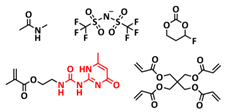 | DSP | H-bonds | LMB, Li/DSP/LiMn2O4, LiTFSI, FEC, NMA | RT, 1 h | 117 mA h g−1 at 0.1C, σ (RT) = 1.79 × 10−3 S cm−1 | [91] |
| 25 | 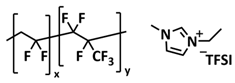 | GPE | Ion–dipole interaction | LMB, LiFePO4/LiTFSI-IL-P (VDF-HFP)/Li, 1 M LiTFSI in DME/DOL | RT, 12 h | 145 mA h g−1 at 0.2C, σ (RT) = 8.8 × 10−4 S cm−1 | [92] |
| 26 | 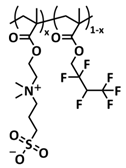 | GPE | Ion–dipole interaction | LIB, Li/SHSPE3/LiFePO4, EMIMTFSI 25 wt.%, LiTFSI | 60 °C, 10 min | 145 mA h g−1 at 0.2C, σ (60 °C) = 5 × 10−4 S cm−1 | [93] |
| 27 |  | GPE | Diels–Adler reaction, ion–dipole interaction | LIB, EMIMTFSI | 100 °C, 20 min | NA, σ (RT) = 3.3 × 10−3 S cm−1 | [94] |
| 28 |  | GPE | H-bonds, ionic interactions | LIB, Li|ionogel|LiFePO4, DE-IM/TFSI, LiTFSI | 55 °C, 1 h | 147.5 mA h g−1 at 0.2C, σ (RT) = 1.5 × 10−3 S cm−1 | [95] |
| 29 | 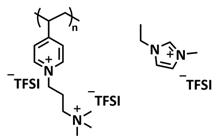 | GPE | Ionic interactions | LMB, Li/PVT-45%EMIMTFSI/LiFePO4, LiTFSI | RT, <1 h | 145 mA h g−1 at 0.1C, σ (RT) = 1.3 × 10−4 S cm−1 | [96] |
| 30 |  | GPE | Ionic interactions | LIB and LMB, graphite|f-ZI solvate ionogel|NCM | 55 °C, 1 h | 140 mA h g−1 at 0.1C, σ (RT) = 6 × 10−4 S cm−1 | [97] |
| 31 | 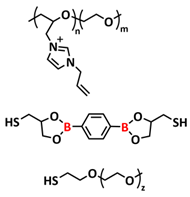 | GPE | Borate dynamic covalent bonds, ionic interactions | NA | 120 °C, 2 h | NA, σ (30 °C) = 1.6 × 10−5 S cm−1 | [98] |
| 32 | 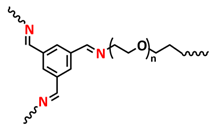 | GPE | Imine dynamic covalent bonds | LMB, LiFePO4/IGPE-50/Li, 0.1 M LiTFSI in BMImTFSI | RT, 10 s | 155 mA h g−1 at 0.1C, σ (5 °C) = 3.1 × 10−4 S cm−1 | [99] |
| 33 | 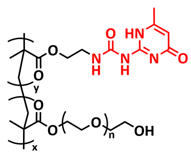 | GPE | H-bonds | LIB, Li/GPE-20/LFP, EMIMTFSI | RT, 15 h | 161.5 mA h g−1 at 0.05C, σ (RT) = 7.5 × 10−4 S cm−1 | [100] |
| 34 | 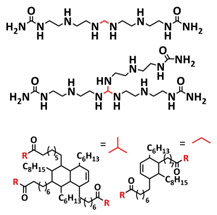 | CPE | H-bonds | LIB, Li/CPE/Li4Ti5O12, 1 M LiPF6 in EC/DEC | RT, 1 h | 157 mA h g−1 at 0.2 C; NA | [101] |
| 35 | 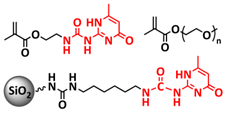 + LiTFSI + LiTFSI | CPE | H-bonds | LIB, Li|SHCPE-10|LFP, LiTFSI (EO/Li+ molar ratio 16:1) | RT, 1 h | 145 mA h g−1 at 0.2C, σ (30 °C) = 8 × 10−4 S cm−1 | [102] |
| 36 | 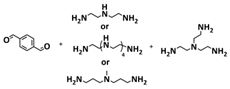 + Li2S-P2S5 + Li2S-P2S5 | CPM | Imine dynamic covalent bonds | LIB, FeS2/CPM/Li | NA | 450 mA h g−1 at 0.2C, σ (30 °C) = 1 × 10−4 S cm−1 | [103,104] |
| 37 | 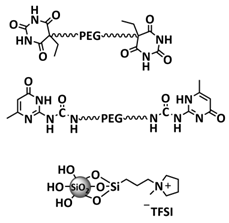 | CPE | H-bonds | LIB, LITFSI (EO/Li+ molar ratio 5:1) | NA | NA, σ (80 °C) = 1 × 10−3 S cm−1 | [105] |
| 38 | 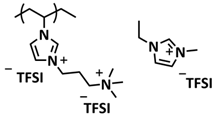 + BN nanosheets + LiTFSI + BN nanosheets + LiTFSI | CPE | Ionic interactions | LMB, LFP/PolyIL-5/Li, LiTFSI | RT, 1 h | 152 mA h g−1 at 0.1C, σ (RT) = 1.6 × 10−4 S cm−1 | [106] |
| Entry | Self-Healing Binder | Healing Mechanism | Battery | Healing Conditions | Electrode/Electrolyte | Performance | Reference |
|---|---|---|---|---|---|---|---|
| 1 |  | H-bonds | LIB | RT | Si a/1 M LiPF6 in EC/DEC/FEC | Particle size 3–8 µm, 2617 mA h g−1 at 0.4 C; 80% after 90 cycles | [24] |
| 2 | 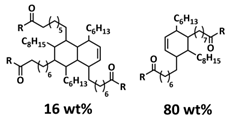 | H-bonds | LIB | RT | Si a/1 M LiPF6 in EC/DEC/VC/FEC | Particle size 500 nm-1.5 µm, 2620 mA h g−1 at 0.1 C; 80% after 500 cycles | [137] |
| 3 |  | H-bonds | LIB | - | Si a/1 M LiPF6 in EC/DEC/VC/FEC | Particle size 800 nm, 1700 mA h g−1 at 0.1 C; 80% after 178 cycles | [138] |
| 4 |  | H-bonds | LIB | RT, 3 h | Si a/1 M LiPF6 in EC/DEC/FEC | Particle size 800 nm, 1300 mA h g−1 at 0.5 C; 80% after 150 cycles | [139] |
| 5 | 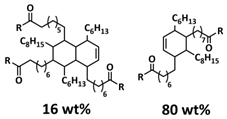 | H-bonds | LIB | RT | Si b/1 M LiPF6 in EC/FEC | Particle size 1–5 µm, 2212 mA h g−1 at 0.1 C; 92% after 100 cycles | [140] |
| 6 |  PAA -UPY PAA -UPY | H-bonds | LIB | RT | Si c | Particle size 70 nm, 4194 mA h g−1 at 0.5 C; 63% after 110 cycles | [141] |
| 7 | 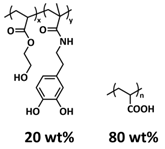 PAA-P(HEA-co-DMA) PAA-P(HEA-co-DMA) | H-bonds | LIB | RT | Si c/1 M LiPF6 in DMC/EC/FEC | Particle size 2–6 µm, 2850 mA h g−1 at 0.4 C; 99.7% after 110 cycles | [142] |
| 8 |  UPy-PEG-UPy UPy-PEG-UPy | H-Bonds | LIB | RT | Si c/1 M LiPF6 in DMC/EC | Particle size 50–70 nm, 1847 mA h g−1 at 0.4 C; 79% after 400 cycles | [143] |
| 9 | 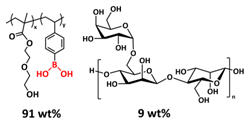 | H-bonds, borate ester bonds | LIB | RT, 3 h | Si c/1.3 M LiPF6 in DEC/EC | Particle size 50 nm, 2750 mA h g−1 at 0.2 C; 87% after 100 cycles | [144] |
| 10 |  Poly(DMA-BA-PEGda) = PDBP Poly(DMA-BA-PEGda) = PDBP | Metal coordination | LIB | RT, 24 h | Si c/1 M LiPF6 in DMC/EC | Particle size 50 nm; 82% after 350 cycles at 1 C | [145] |
| 11 |  | H-bonds | LIB | RT | Si c/1 M LiPF6 in EC/DEC | 2100 mA h g−1 at 500 mA g−1; 95% after 600 cycles | [146] |
| 12 |  | H-bonds, metal coordination | LIB | RT | Si c/1 M LiPF6 in EC/DEC | 1115 mA h g−1 at 1 C; 86% after 50 cycles | [147] |
| 13 | 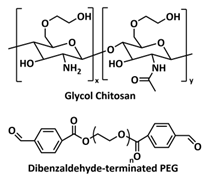 | Amine dynamic covalent bonds | LIB | RT | Si c/1 M LiPF6 in EC/DMC/FEC | 2141 mA h g−1 at 0.5 C; 65% after 150 cycles | [148] |
| 14 | 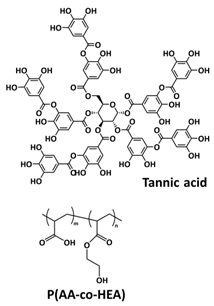 | H-bonds | LIB | RT | Si c/1 M LiPF6 in EC/DEC | 1976 mA h at 1 C; 80% after 700 cycles | [149] |
| 15 |  PAA-g-PEG PAA-g-PEG | H-bonds | LIB | RT | Si c/1 M LiPF6 in EC/EMC/FEC | 1450 mA h g−1 at 0.5 C; 99% after 350 cycles | [150] |
| 16 |  | Diels–Adler reaction | LIB | 65 °C, 1 h | Si c/1 M LiPF6 in EC/EMC/FEC | 1076 mA h g−1 at 0.5 C; 99.7% after 200 cycles | [151] |
| 17 |  | H-bonds | LMB | RT, 60 s | Li d/1 M LiTFSI in DOL/DME/LiNO3 | 2617 mA h g−1 at 0.4 A g−1; 80% after 90 cycles | [152] |
| 18 | 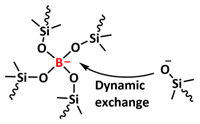 | Borate dynamic covalent bonds | LMB | RT, 1 h | Li e/1 M LiTFSI in DOL/DME | 1 mA h cm−2 at 0.5 mA cm−2 for 120 cycles | [130] |
| 19 | 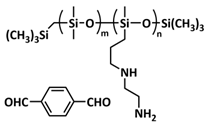 PDMS-DFB PDMS-DFB | Imine dynamic covalent bonds | LMB | RT, 12 h | Li f/1 M LiTFSI in DOL/DME/LiNO3 | 1 mA h cm−2 at 0.5 mA cm−2 for 120 cycles | [153] |
| 20 | 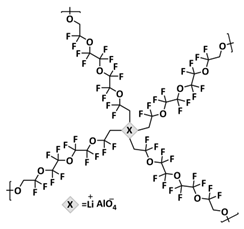 | Al-O dynamic covalent bonds | LMB | RT, 12 h | Li g/1 M LiPF6 in EC/DEC/FEC | 1 mA h cm−2 at 1 mA cm−2 for 250 cycles | [154] |
| 21 |  PEO-UPy PEO-UPy | H-bonds | LMB | RT | Li h/1 M LiPF6 in EC/FEC | 10 mA h cm−2 at 5 mA cm−2 for 1000 cycles | [155] |
| 22 | 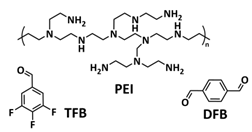 | Amine dynamic covalent bonds | LMB | RT | Li h/1 M LiTFSI in DOL/DME | 1 mA h cm−2 at 1 mA cm−2 for 250 cycles | [156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinow, A.; Katcharava, Z.; Binder, W.H. Self-Healing Polymer Electrolytes for Next-Generation Lithium Batteries. Polymers 2023, 15, 1145. https://doi.org/10.3390/polym15051145
Marinow A, Katcharava Z, Binder WH. Self-Healing Polymer Electrolytes for Next-Generation Lithium Batteries. Polymers. 2023; 15(5):1145. https://doi.org/10.3390/polym15051145
Chicago/Turabian StyleMarinow, Anja, Zviadi Katcharava, and Wolfgang H. Binder. 2023. "Self-Healing Polymer Electrolytes for Next-Generation Lithium Batteries" Polymers 15, no. 5: 1145. https://doi.org/10.3390/polym15051145
APA StyleMarinow, A., Katcharava, Z., & Binder, W. H. (2023). Self-Healing Polymer Electrolytes for Next-Generation Lithium Batteries. Polymers, 15(5), 1145. https://doi.org/10.3390/polym15051145






