Sustainable and Green Production of Nanostructured Cellulose by a 2-Step Mechano-Enzymatic Process
Abstract
1. Introduction
2. Methods
2.1. Reagents
2.2. Ball Milling
2.3. Enzymatic Treatments and Carbohydrate Detection Assay
2.4. Characterization of NC
2.5. Application of NC in Functional Coating
3. Results and Discussion
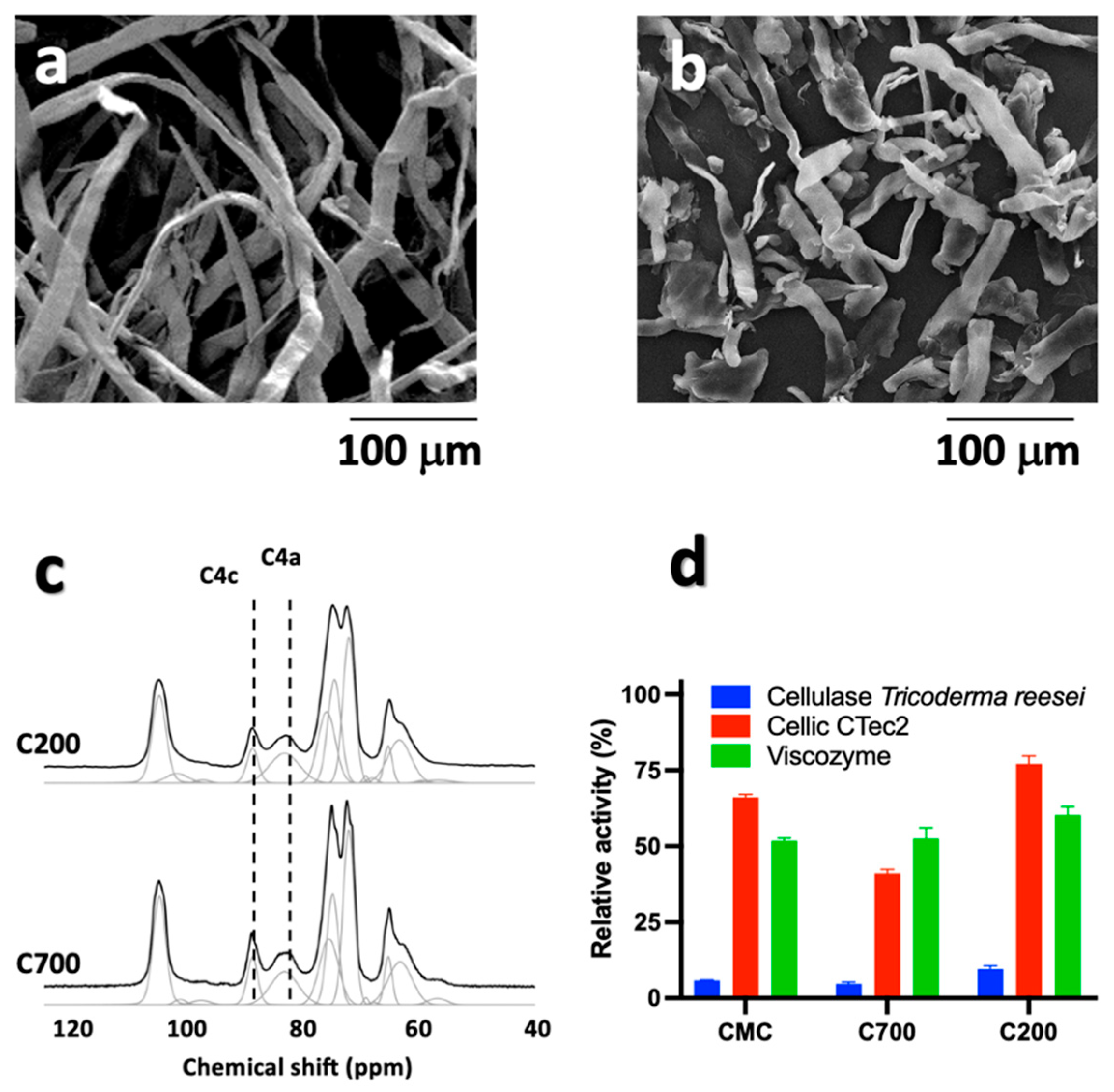
3.1. Morphological Analysis and Enzymatic Treatment of Pristine Cellulose
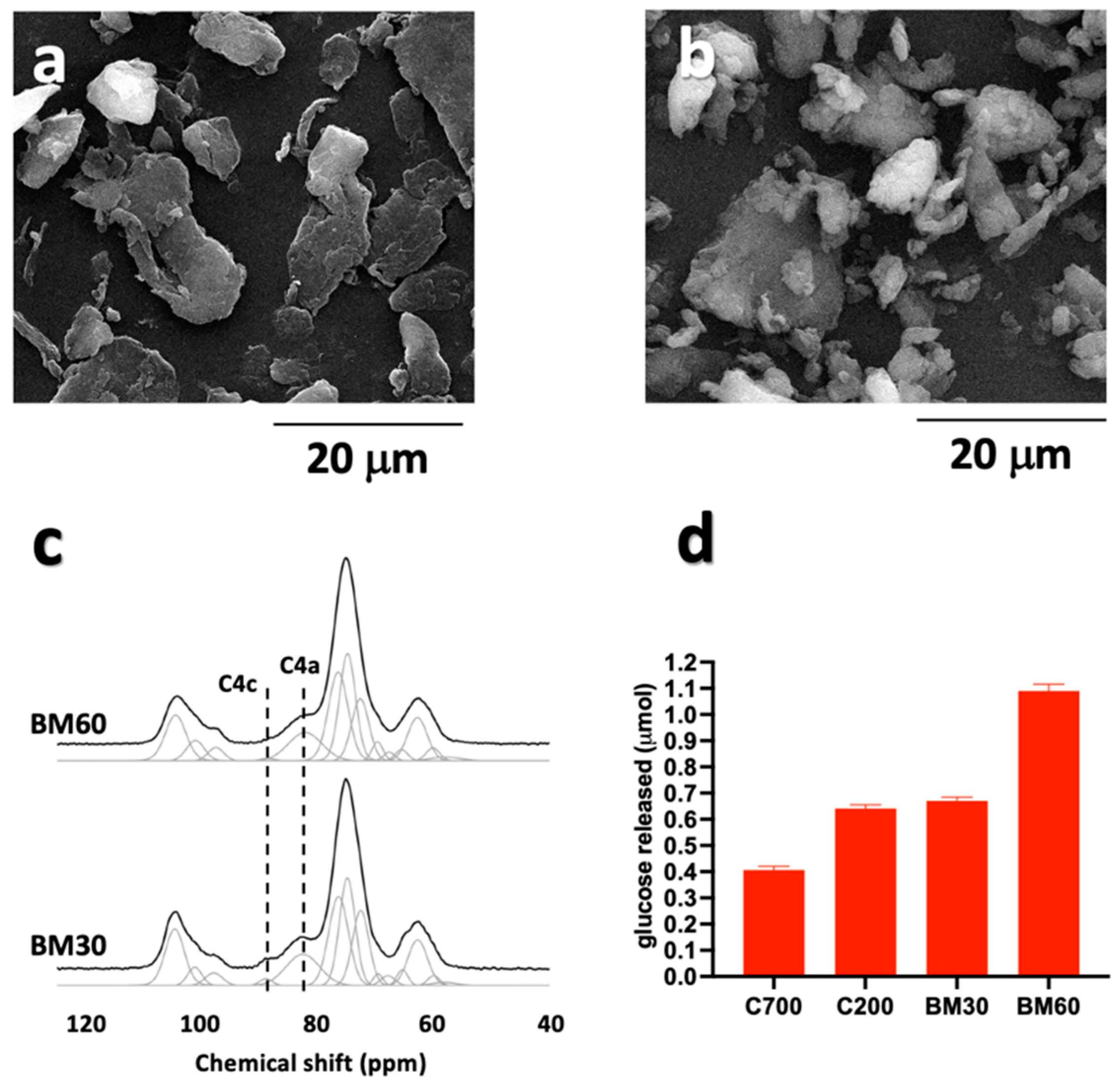
3.2. Ball Milling Treatment of Cellulose Samples
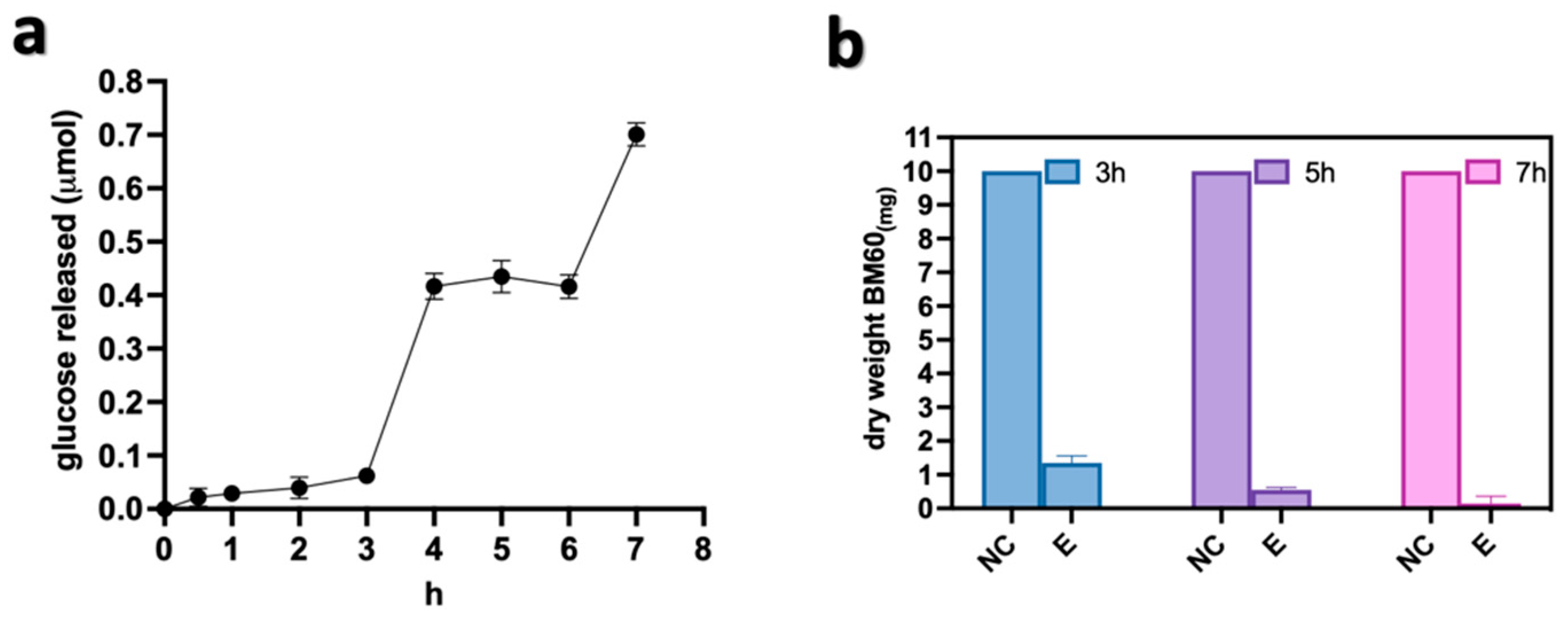
3.3. Optimization of the 2-Step Mechano-Enzymatic Process
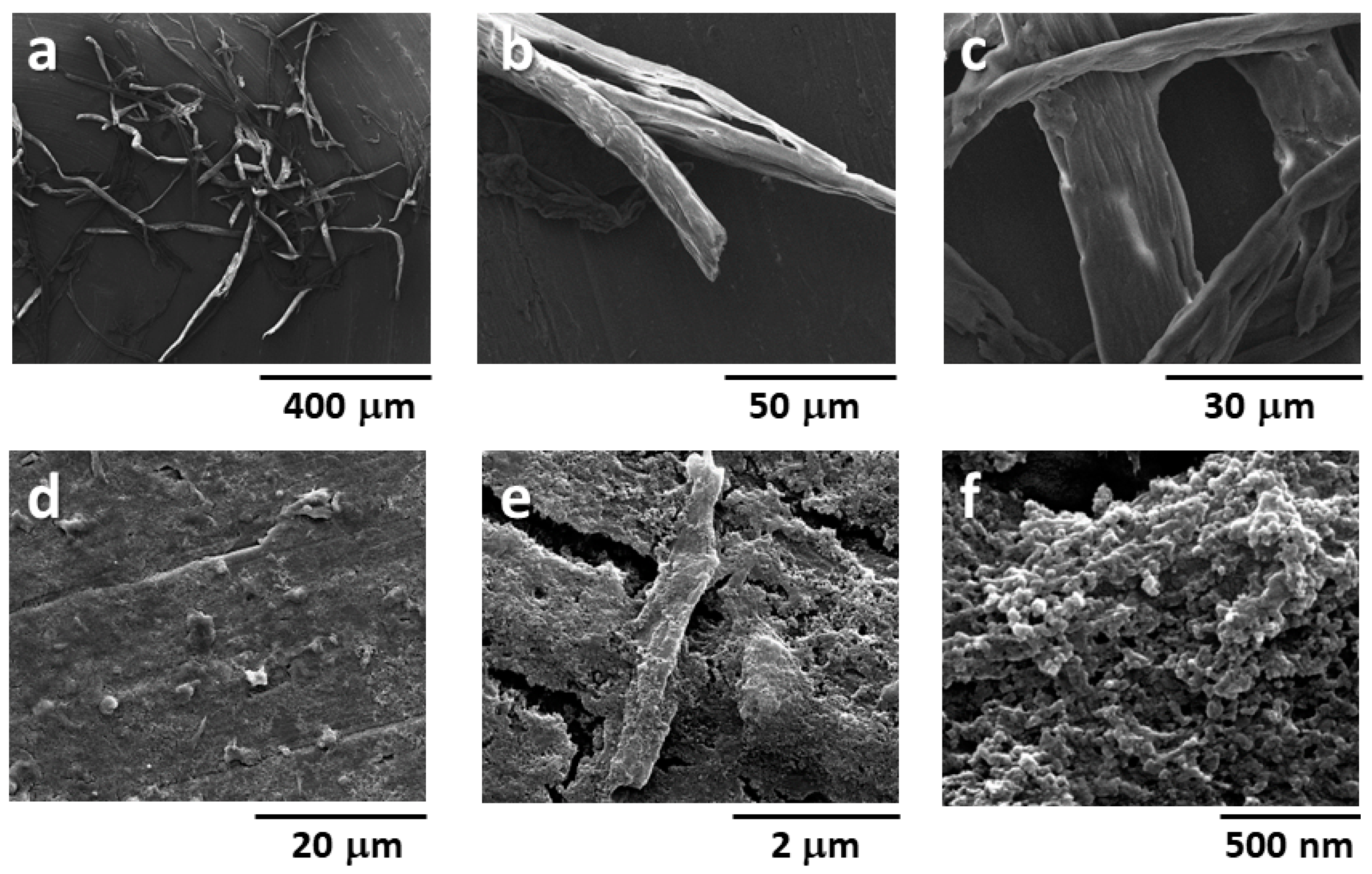
3.4. Film-Forming Property of Mechano-Enzymatic Treated Cellulose
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D. A critical review of cellulose-based nanomaterials for water purification in industrial processes. Cellulose 2019, 26, 687–701. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, N.; Munagala, M.; Rajkhowa, R.; Aallardyce, B.; Shastri, Y.; Agrawal, R. Nanocellulose: Resources, Physio-Chemical Properties, Current Uses and Future Applications. Front. Nanotechnol. 2021, 3, 747329. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of lignocellulosic biomass to nanocellulose: Structure and chemical process. Sci. World J. 2014, 2014, 631013. [Google Scholar] [CrossRef]
- Michelin, M.; Gomes, D.G.; Romaní, A.; Polizeli, M.d.L.T.M.; Teixeira, J.A. Nanocellulose production: Exploring the enzymatic route and residues of pulp and paper industry. Molecules 2020, 25, 3411. [Google Scholar] [CrossRef]
- Pirich, C.L.; Picheth, G.F.; Fontes, A.M.; Delgado-Aguilar, M.; Ramos, L.P. Disruptive enzyme-based strategies to isolate nanocelluloses: A review. Cellulose 2020, 27, 5457–5475. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Squinca, P.; Bilatto, S.; Badino, A.C.; Farinas, C.S. Nanocellulose Production in Future Biorefineries: An Integrated Approach Using Tailor-Made Enzymes. ACS Sustain. Chem. Eng. 2020, 8, 2277–2286. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose nanocrystals vs. Cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Derradji, M.; Bessa, W.; Belgacemi, R. Chapter 5. Cellulose Nanoparticles: Extractions; Royal Society of Chemistry: London, UK, 2021; pp. 113–148. [Google Scholar] [CrossRef]
- Kimura, Y.; Tsuchida, A.; Katsuraya, K. High-Performance and Specialty Fibers: Concepts, Technology and Modern Applications of Man-Made Fibers for the Future; Springer: Tokyo, Japan, 2016; ISBN 9784431552031. [Google Scholar] [CrossRef]
- Hoeng, F.; Denneulin, A.; Bras, J. Use of nanocellulose in printed electronics: A review. Nanoscale 2016, 8, 13131–13154. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Reports 2019, 21, e00316. [Google Scholar] [CrossRef] [PubMed]
- Sehaqui, H.; de Larraya, U.P.; Liu, P.; Pfenninger, N.; Mathew, A.P.; Zimmermann, T.; Tingaut, P. Enhancing adsorption of heavy metal ions onto biobased nanofibers from waste pulp residues for application in wastewater treatment. Cellulose 2014, 21, 2831–2844. [Google Scholar] [CrossRef]
- Varghese, A.G.; Paul, S.A.; Latha, M.S. Remediation of heavy metals and dyes from wastewater using cellulose-based adsorbents. Environ. Chem. Lett. 2019, 17, 867–877. [Google Scholar] [CrossRef]
- Tong, W.Y.; bin Abdullah, A.Y.K.; binti Rozman, N.A.S.; bin Wahid, M.I.A.; Hossain, M.S.; Ring, L.C.; Lazim, Y.; Tan, W.N. Antimicrobial wound dressing film utilizing cellulose nanocrystal as drug delivery system for curcumin. Cellulose 2018, 25, 631–638. [Google Scholar] [CrossRef]
- Yook, S.; Park, H.; Park, H.; Lee, S.Y.; Kwon, J.; Youn, H.J. Barrier coatings with various types of cellulose nanofibrils and their barrier properties. Cellulose 2020, 27, 4509–4523. [Google Scholar] [CrossRef]
- Yi, T.; Zhao, H.; Mo, Q.; Pan, D.; Liu, Y.; Huang, L.; Xu, H.; Hu, B.; Song, H. From cellulose to cellulose nanofibrils—A comprehensive review of the preparation and modification of cellulose nanofibrils. Materials 2020, 13, 5062. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Johar, N.; Ahmad, I. Starch biocomposite film reinforced by multiscale rice husk fiber. Compos. Sci. Technol. 2017, 151, 147–155. [Google Scholar] [CrossRef]
- Kaffashsaie, E.; Yousefi, H.; Nishino, T.; Matsumoto, T.; Mashkour, M.; Madhoushi, M.; Kawaguchi, H. Direct conversion of raw wood to TEMPO-oxidized cellulose nanofibers. Carbohydr. Polym. 2021, 262, 117938. [Google Scholar] [CrossRef]
- Arantes, V.; Dias, I.K.R.; Berto, G.L.; Pereira, B.; Marotti, B.S.; Nogueira, C.F.O. The current status of the enzyme-mediated isolation and functionalization of nanocelluloses: Production, properties, techno-economics, and opportunities. Cellulose 2020, 27, 10571–10630. [Google Scholar] [CrossRef]
- Filson, P.B.; Dawson-Andoh, B.E.; Schwegler-Berry, D. Enzymatic-mediated production of cellulose nanocrystals from recycled pulp. Green Chem. 2009, 11, 1808–1814. [Google Scholar] [CrossRef]
- Levasseur, A.; Drula, E.; Lombard, V.; Coutinho, P.M.; Henrissat, B. Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol. Biofuels 2013, 6, 41. [Google Scholar] [CrossRef]
- Kocherbitov, V.; Ulvenlund, S.; Kober, M.; Jarring, K.; Arnebran, T. Hydration of microcrystalline cellulose and milled cellulose studied by sorption calorimetry. J. Phys. Chem. B 2008, 112, 3728–3734. [Google Scholar] [CrossRef] [PubMed]
- Avolio, R.; Bonadies, I.; Capitani, D.; Errico, M.E.; Gentile, G.; Avella, M. A multitechnique approach to assess the effect of ball milling on cellulose. Carbohydr. Polym. 2012, 87, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Avolio, R.; Graziano, V.; Pereira, Y.D.F.; Cocca, M.; Gentile, G.; Errico, M.E.; Ambrogi, V.; Avella, M. Effect of cellulose structure and morphology on the properties of poly(butylene succinate-co-butylene adipate) biocomposites. Carbohydr. Polym. 2015, 133, 408–420. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Han, L.; Wang, B.; Xu, H.; Zhong, Y.; Zhang, L.; Mao, Z.; Sui, X. Biodegradable regenerated cellulose-dispersed composites with improved properties via a pickering emulsion process. Carbohydr. Polym. 2018, 179, 86–92. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. NPJ Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Dadwal, A.; Sharma, S.; Satyanarayana, T. Thermostable cellulose saccharifying microbial enzymes: Characteristics, recent advances and biotechnological applications. Int. J. Biol. Macromol. 2021, 188, 226–244. [Google Scholar] [CrossRef]
- Ing, N.; Deng, K.; Chen, Y.; Aulitto, M.; Gin, J.W.; Pham, T.L.M.; Petzold, C.J.; Singer, S.W.; Bowen, B.; Sale, K.L.; et al. A multiplexed nanostructure-initiator mass spectrometry (NIMS) assay for simultaneously detecting glycosyl hydrolase and lignin modifying enzyme activities. Sci. Rep. 2021, 11, 11803. [Google Scholar] [CrossRef]
- Prato, S.; Vitale, R.M.; Contursi, P.; Lipps, G.; Saviano, M.; Rossi, M.; Bartolucci, S. Molecular modeling and functional characterization of the monomeric primase-polymerase domain from the Sulfolobus solfataricus plasmid pIT3. FEBS J. 2008, 275, 4389–4402. [Google Scholar] [CrossRef]
- Blumer-Schuette, S.E.; Kataeva, I.; Westpheling, J.; Adams, M.W.; Kelly, R.M. Extremely thermophilic microorganisms for biomass conversion: Status and prospects. Curr. Opin. Biotechnol. 2008, 19, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Aulitto, M.; Fusco, F.A.; Fiorentino, G.; Bartolucci, S.; Contursi, P.; Limauro, D. A thermophilic enzymatic cocktail for galactomannans degradation. Enzyme Microb. Technol. 2018, 111, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Aulitto, M.; Martinez-Alvarez, L.; Fusco, S.; She, Q.; Bartolucci, S.; Peng, X.; Contursi, P. Genomics, Transcriptomics, and Proteomics of SSV1 and Related Fusellovirus: A Minireview. Viruses 2022, 14, 2082. [Google Scholar] [CrossRef]
- Zuliani, L.; Serpico, A.; De Simone, M.; Frison, N.; Fusco, S. Biorefinery gets hot: Thermophilic enzymes and microorganisms for second-generation bioethanol production. Processes 2021, 9, 1583. [Google Scholar] [CrossRef]
- Aulitto, M.; Fusco, S.; Franzén, C.J.; Strazzulli, A.; Moracci, M.; Bartolucci, S.; Contursi, P. Draft Genome Sequence of Bacillus coagulans MA-13, a Thermophilic Lactic Acid Producer from Lignocellulose. Microbiol. Resour. Announc. 2019, 8, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Szentner, K.; Waśkiewicz, A.; Borysiak, S. Production of nanocellulose by enzymatic treatment for application in polymer composites. Materials 2021, 14, 2124. [Google Scholar] [CrossRef]
- Adney, B.; Baker, J. Measurement of Cellulase Activities. NREL Lab. Anal. Proced. 2008. Available online: https://www.nrel.gov/docs/gen/fy08/42628.pdf (accessed on 19 February 2023).
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Li, Y.; Tian, J.; Yang, C.; Hsiao, B.S. Nanocomposite film containing fibrous cellulose scaffold and Ag/TiO2 nanoparticles and its antibacterial activity. Polymers 2018, 10, 1052. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 2009, 10, 162–165. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Ferrer, A.; Tyagi, P.; Yin, Y.; Salas, C.; Pal, L.; Rojas, O.J. Nanocellulose in thin films, coatings, and plies for packaging applications: A review. BioResources 2017, 12, 2143–2233. [Google Scholar] [CrossRef]
- Ferrer, A.; Pal, L.; Hubbe, M. Nanocellulose in packaging: Advances in barrier layer technologies. Ind. Crops Prod. 2017, 95, 574–582. [Google Scholar] [CrossRef]
- Lu, P.; Guo, M.; Xu, Z.; Wu, M. Application of nanofibrillated cellulose on BOPP/LDPE film as oxygen barrier and antimicrobial coating based on cold plasma treatment. Coatings 2018, 8, 207. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Athira, B.K.; Rubiyah, H.M.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aulitto, M.; Castaldo, R.; Avolio, R.; Errico, M.E.; Xu, Y.-Q.; Gentile, G.; Contursi, P. Sustainable and Green Production of Nanostructured Cellulose by a 2-Step Mechano-Enzymatic Process. Polymers 2023, 15, 1115. https://doi.org/10.3390/polym15051115
Aulitto M, Castaldo R, Avolio R, Errico ME, Xu Y-Q, Gentile G, Contursi P. Sustainable and Green Production of Nanostructured Cellulose by a 2-Step Mechano-Enzymatic Process. Polymers. 2023; 15(5):1115. https://doi.org/10.3390/polym15051115
Chicago/Turabian StyleAulitto, Martina, Rachele Castaldo, Roberto Avolio, Maria Emanuela Errico, Yong-Quan Xu, Gennaro Gentile, and Patrizia Contursi. 2023. "Sustainable and Green Production of Nanostructured Cellulose by a 2-Step Mechano-Enzymatic Process" Polymers 15, no. 5: 1115. https://doi.org/10.3390/polym15051115
APA StyleAulitto, M., Castaldo, R., Avolio, R., Errico, M. E., Xu, Y.-Q., Gentile, G., & Contursi, P. (2023). Sustainable and Green Production of Nanostructured Cellulose by a 2-Step Mechano-Enzymatic Process. Polymers, 15(5), 1115. https://doi.org/10.3390/polym15051115











