Preparation of a Polyaniline-Modified Hybrid Graphene Aerogel-Like Nanocomposite for Efficient Adsorption of Heavy Metal Ions from Aquatic Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
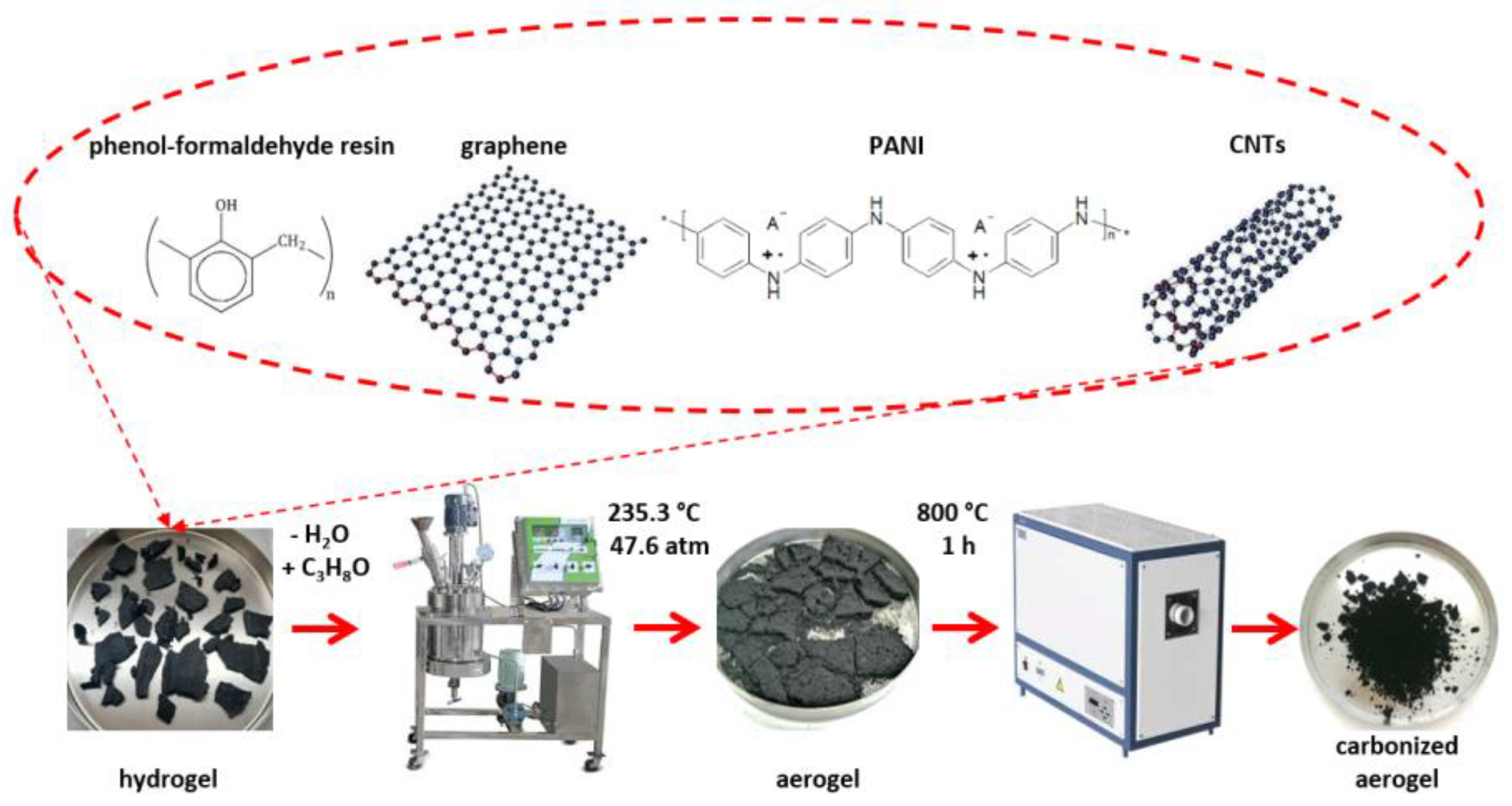
2.2. Laboratory Method for Obtaining a Hydrogel
2.3. Laboratory Method for Obtaining the Nanocomposite Material (Aerogel)
2.4. Material Characterization
2.5. pH Effect Studies
2.6. Kinetic Studies on the Carbonized Aerogel
2.7. Desorption Studies on the Carbonized Aerogel
3. Results and Discussion
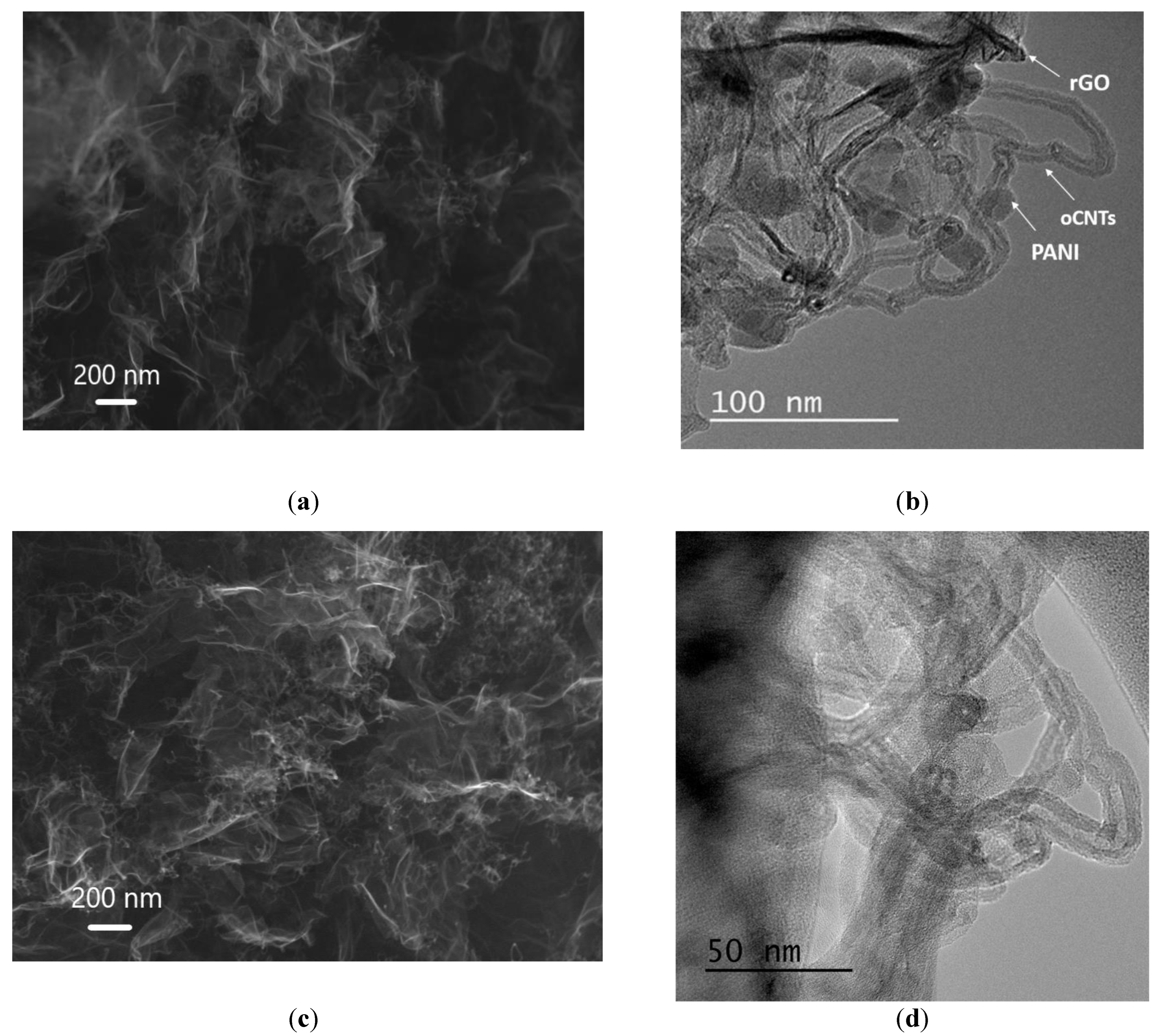
3.1. Physical-Chemical Properties of the Nanocomposites
3.2. Optimal pH Range
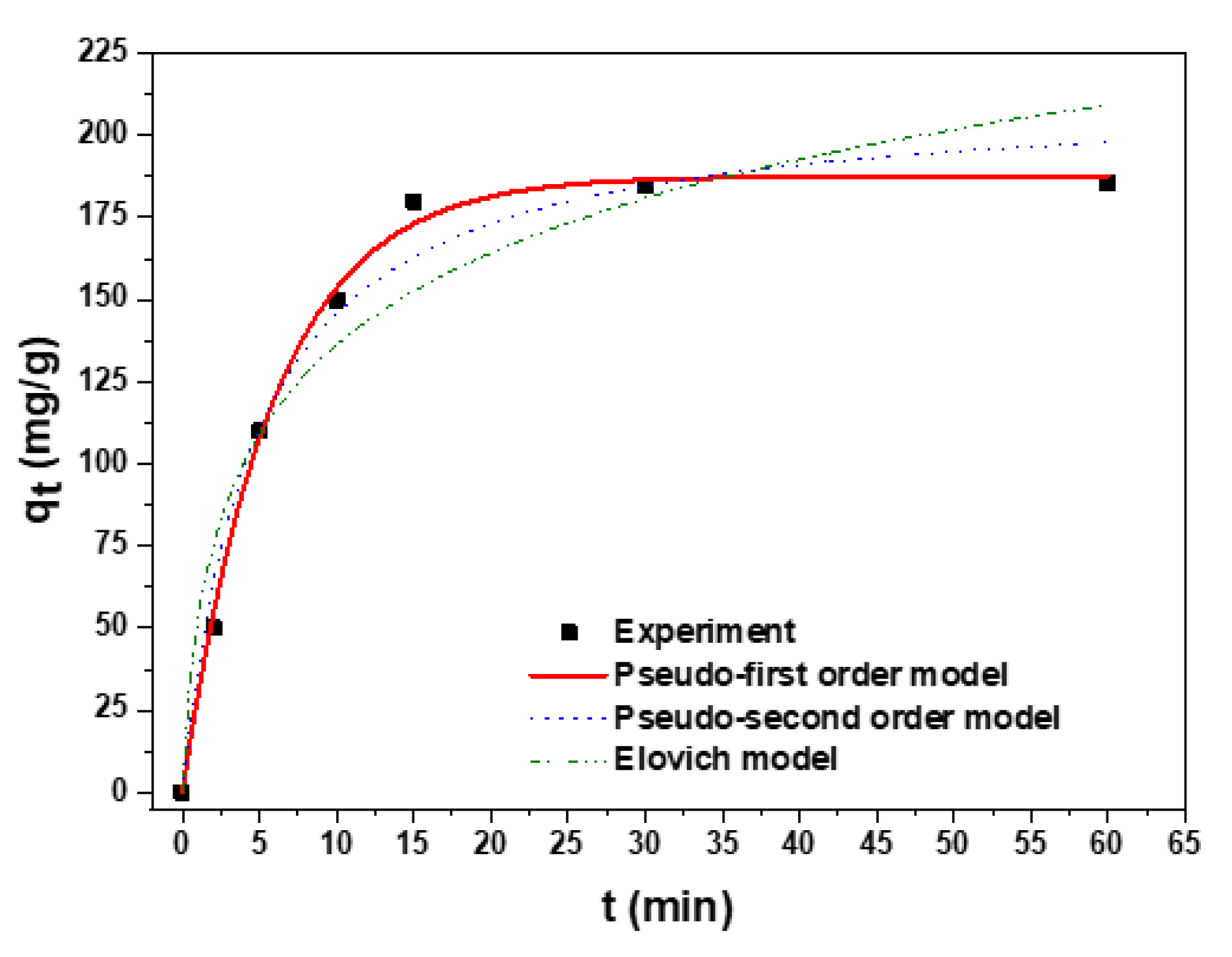
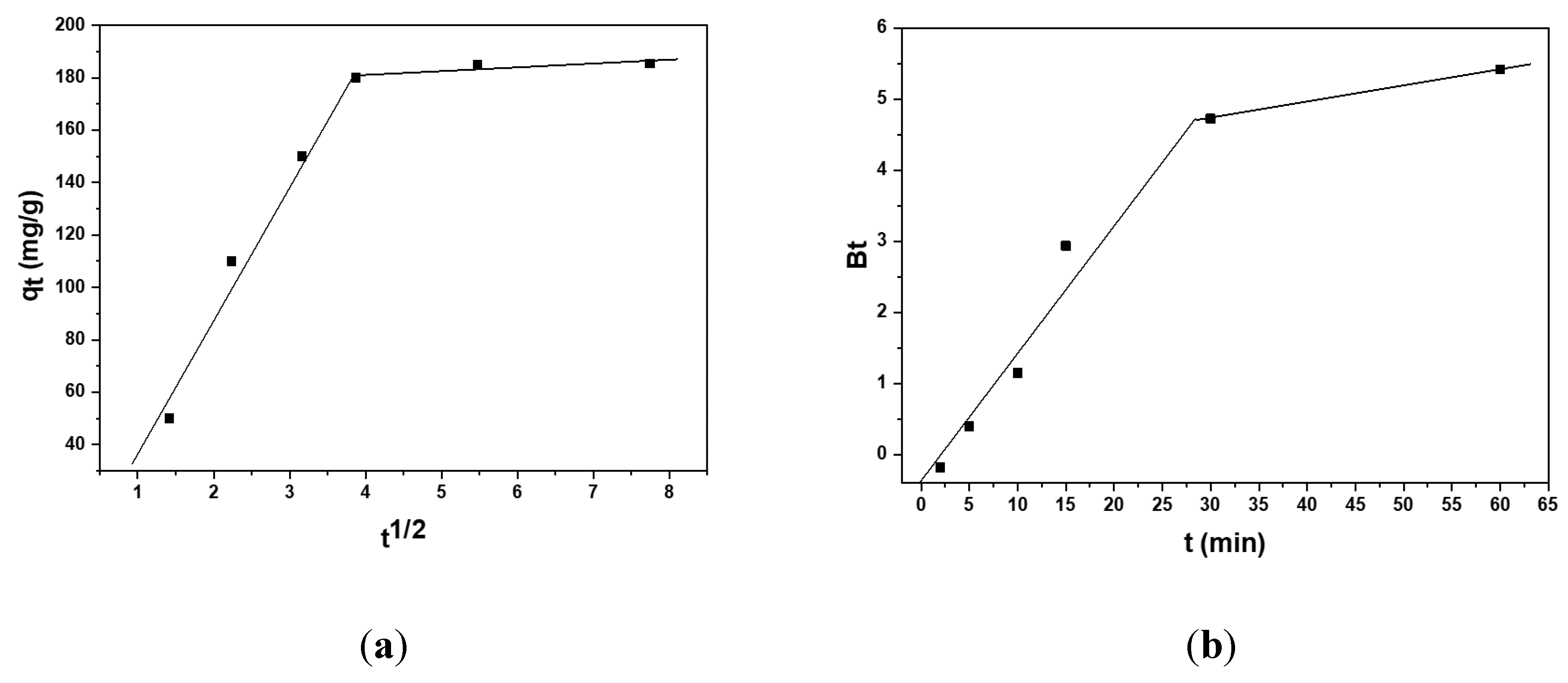
3.3. Kinetic Studies on the Adsorption Capacity of the Carbonized Aerogel
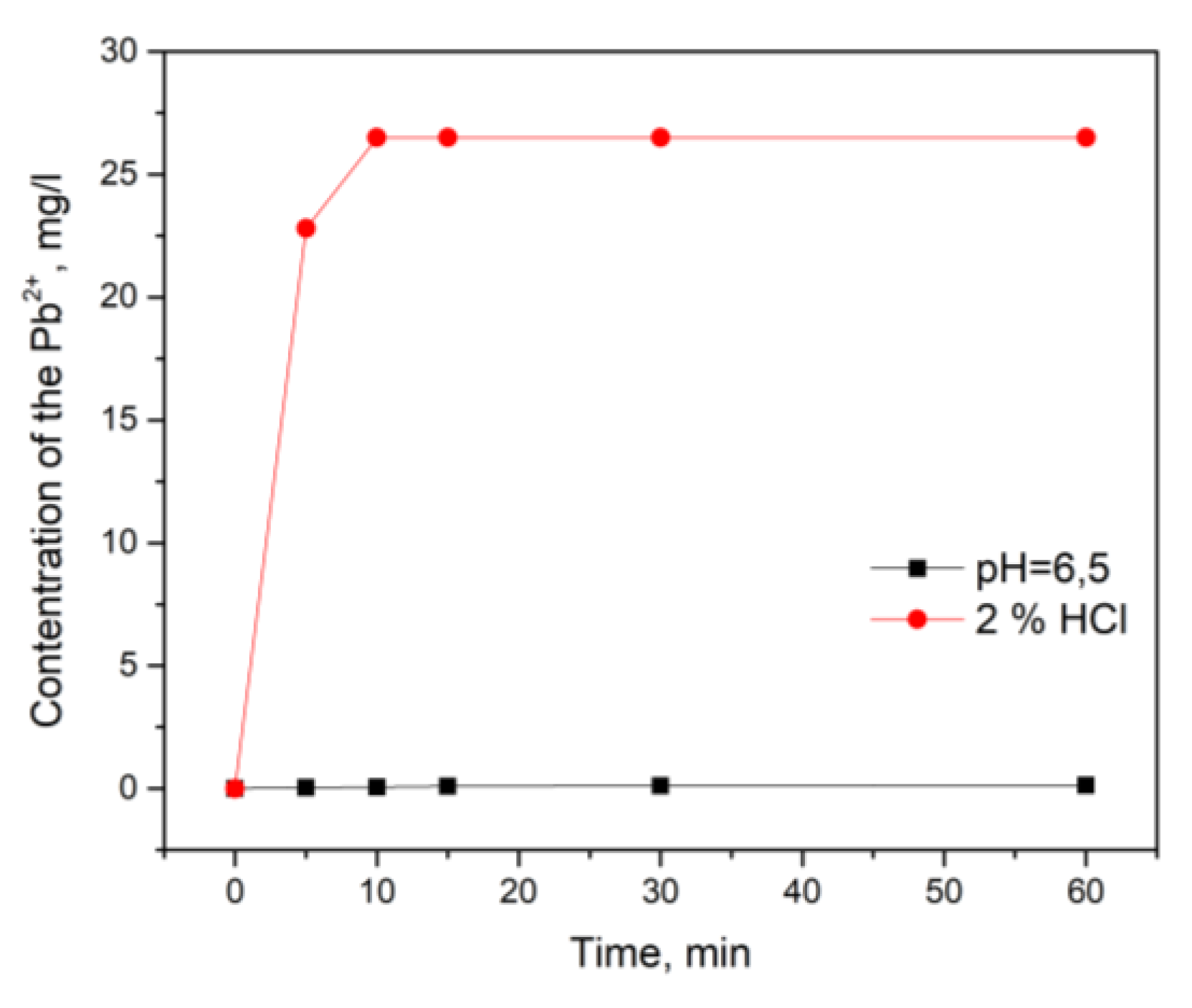
3.4. Desorption Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awual, M.R. Mesoporous composite material for efficient lead(II) detection and removal from aqueous media. J. Environ. Chem. Eng. 2019, 7, 103124. [Google Scholar] [CrossRef]
- Qian, H.; Wang, J.; Yan, L. Synthesis of lignin-poly(N-methylaniline)-reduced graphene oxide hydrogel for organic dye and lead ions removal. J. Biores Bioprod. 2020, 5, 204–210. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, J.; Wang, S.; Zhang, L. Ninhydrin-functionalized chitosan for selective removal of Pb(II) ions: Characterization and adsorption performance. Int. J. Biol. Macromol. 2021, 177, 29–39. [Google Scholar] [CrossRef]
- Mishra, S.; Verma, N. Surface ion imprinting-mediated carbon nanofiber-grafted highly porous polymeric beads: Synthesis and application towards selective removal of aqueous Pb(II). Chem. Eng. J. 2017, 313, 1142–1151. [Google Scholar] [CrossRef]
- Torres, C.E.; Quezada, T.E.; Kharissova, O.V.; Kharisov, B.I.; de la Fuente, M.I. Carbon-based aerogels and xerogels: Synthesis, properties, oil sorption capacities, and DFT simulations. J. Environ. Chem. Eng. 2021, 9, 104886. [Google Scholar] [CrossRef]
- Job, N.; Théry, A.; Pirard, R.; Marien, J.; Kocon, L.; Rouzaud, J.N.; Beguin, F.; Pirard, J.-P.l. Carbon aerogels, cryogels and xerogels: Influence of the drying method on the textural properties of porous carbon materials. Carbon 2005, 43, 2481–2494. [Google Scholar] [CrossRef]
- Tabrizi, N.S.; Zamani, S. Removal of Pb(II) from aqueous solutions by graphene oxide aerogels. Water Sci. Technol. 2016, 74, 256–265. [Google Scholar] [CrossRef]
- Bloor, J.M.; Handy, R.D.; Awan, S.A.; Jenkins, D.F.L. Graphene oxide biopolymer aerogels for the removal of lead from drinking water using a novel nano-enhanced ion exchange cascade. Ecotoxicol. Environ. Saf. 2021, 208, 111422. [Google Scholar] [CrossRef]
- Han, Z.; Tang, Z.; Shen, S.; Zhao, B.; Zheng, G.; Yang, J. Strengthening of graphene aerogels with tunable density and high adsorption capacity towards Pb2+. Sci. Rep. 2014, 4, 5025. [Google Scholar] [CrossRef]
- Purnendu, S.S. Graphene-based 3D xerogel as adsorbent for removal of heavy metal ions from industrial wastewater. J. Renew. Mater. 2017, 5, 96–102. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.; Wang, Z.; Ren, Z.; Yan, S.; Duan, Y.; Zhang, J. Ultralight, superplastic, and fatigue-resistant graphene aerogel templated by graphene oxide liquid crystal stabilized air bubbles. ACS Appl. Mater. Interfaces 2019, 11, 1303–1310. [Google Scholar] [CrossRef]
- Hajjaoui, H.; Soufi, A.; Boumya, W.; Abdennouri, M.; Barka, N. Polyaniline/nanomaterial composites for the removal of heavy metals by adsorption: A Review. J. Compos. Sci. 2021, 5, 233. [Google Scholar] [CrossRef]
- Senguttuvan, S.; Senthilkumar, P.; Janaki, V.; Kamala-Kannan, S. Significance of conducting polyaniline based composites for the removal of dyes and heavy metals from aqueous solution and wastewaters—A review. Chemosphere 2021, 267, 129201. [Google Scholar] [CrossRef]
- Eskandari, E.; Kosari, M.; Davood Abadi Farahani, M.H.; Khiavi, N.D.; Saeedikhani, M.; Katal, R.; Zarinejad, M. A review on polyaniline-based materials applications in heavy metals removal and catalytic processes. Sep. Purif. Technol. 2020, 231, 115901. [Google Scholar] [CrossRef]
- Gong, J.; Chen, X.; Tang, T. Recent progress in controlled carbonization of (waste) polymers. Prog. Polym. Sci. 2019, 94, 1–32. [Google Scholar] [CrossRef]
- Ciric-Marjanovic, G. Recent advances in polyaniline research: Polymerization mechanisms, structural aspects, properties and applications. Synth. Met. 2013, 177, 1–47. [Google Scholar] [CrossRef]
- Shao, D.; Chen, C.; Wang, X. Application of polyaniline and multiwalled carbon nanotube magnetic composites for removal of Pb(II). Chem. Eng. J. 2012, 185–186, 144–150. [Google Scholar] [CrossRef]
- Yang, J.; Wu, J.-X.; Lu, Q.-F.; Lin, T.-T. Facile Preparation of lignosulfonate–graphene oxide–polyaniline ternary nanocomposite as an effective adsorbent for Pb(II) Ions. ACS Sustain. Chem. Eng. 2014, 2, 1203–1211. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Ayad, M.; Zaghlol, S. Nanostructured crosslinked polyaniline with high surface area: Synthesis, characterization and adsorption for organic dye. Chem. Eng. J. 2012, 20, 79–86. [Google Scholar] [CrossRef]
- Aung, W.M.; Marchenko, M.V.; Troshkina, I.D.; Burakova, I.V.; Gutnic, I.V.; Burakov, A.E.; Tkachev, A.G. Scandium adsorption from sulfuric-chloride solutions by PANI/CNTs nanocomposite. Adv. Mat. Technol. 2019, 16, 58–65. [Google Scholar] [CrossRef]
- Trchova, M.; Šeděnková, I.; Tobolkova, E.; Stejskal, J. FTIR spectroscopic and conductivity study of the thermal degradation of polyaniline films. Polym. Degr. Stab. 2004, 86, 179–185. [Google Scholar] [CrossRef]
- Salimikia, I.; Heydari, R.; Yazdankhah, F. Polyaniline/graphene oxide nanocomposite as a sorbent for extraction and determination of nicotine using headspace solid-phase microextraction and gas chromatography–flame ionization detector. J. Iran. Chem. Soc. 2018, 15, 1593–1601. [Google Scholar] [CrossRef]
- Saxena, M.; Lochab, A.; Saxena, R. Asparagine functionalized MWCNTs for adsorptive removal of hazardous cationic dyes: Exploring kinetics, isotherm and mechanism. Surf. Interfaces 2021, 25, 101187. [Google Scholar] [CrossRef]
- Kragulj, M.; Tričković, J.; Dalmacija, B.; Kukovecz, Á.; Kónya, Z.; Molnar, J.; Rončević, S. Molecular interactions between organic compounds and functionally modified multiwalled carbon nanotubes. Chem. Eng. J. 2013, 225, 144–152. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Li, M.; Wang, H.; Zhao, M.; Wang, C. Preparation of PANI grafted at the edge of graphene oxide sheets and its adsorption of Pb(II) and methylene blue. Polym. Compos. 2018, 39, 1663–1673. [Google Scholar] [CrossRef]
- Gupta, G.K.; Mondal, M.K. Kinetics and thermodynamic analysis of maize cob pyrolysis for its bioenergy potential using thermogravimetric analyzer. J. Therm. Anal. Calorim. 2019, 137, 1431–1441. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Qi, L.; Shen, X.B.; Wang, F.C.; Lee, S.K. Microstructure and mechanical properties of bulk carbon nanotubes compacted by spark plasma sintering. Mater. Sci. Eng. A 2013, 573, 12–17. [Google Scholar] [CrossRef]
- Tan, X.L.; Chang, P.P.; Fan, Q.H.; Zhou, X.; Yu, S.M.; Wu, W.S.; Wang, X.K. Sorption of Pb(II) on Na-rectorite: Effects of pH, ionic strength, temperature, soil humic acid and fulvic acid. Colloids Surf. A Phys. Eng. Asp. 2008, 328, 8–14. [Google Scholar] [CrossRef]
- Xu, D.; Tan, X.; Chen, C.; Wang, X. Removal of Pb(II) from aqueous solution by oxidized multiwalled carbon nanotubes. J. Hazard. Mater. 2008, 154, 407–416. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Asghari, M.; Ramezanzadeh, B.; Bahlakeh, G. Fabrication of an efficient system for Zn ions removal from industrial wastewater based on graphene oxide nanosheets decorated with highly crystalline polyaniline nanofibers (GO-PANI): Experimental and ab initio quantum mechanics approaches. Chem. Eng. J. 2018, 337, 385–397. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.; McKay, G. The sorption of lead (II) ions on peat. Water Res. 1999, 33, 578–584. [Google Scholar] [CrossRef]
- McLintock, I The Elovich equation in chemisorption kinetics. Nature 1967, 216, 1204. [CrossRef]
- Weber, W.; Morris, J. Intraparticle diffusion during the sorption of surfactants onto activated carbon. J. Sanit. Eng. Div. 1963, 89, 53–61. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S., Jr. The exchange adsorption of ions from aqueous solutions by organic zeolites, II: Kinetics. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef]
- Jaroniec, M.; Gadkaree, K.P.; Choma, J. Relation between adsorption potential distribution and pore volume distribution for microporous carbons. Colloids Surf. A Phys. Chim. Eng. Aspec. 1996, 118, 203–210. [Google Scholar] [CrossRef]
- Solic, M.; Maletic, S.; Kragulj Isakovski, M.; Nikic, J.; Watson, M.; Konya, Z.; Roncevic, S. Removing low levels of Cd(II) and Pb(II) by adsorption on two types of oxidized multiwalled carbon nanotubes. J. Environ. Chem. Eng. 2021, 9, 105402. [Google Scholar] [CrossRef]
- Govarthanan, M.; Chang-Hyun, J.; Woong, K. Synthesis and characterization of lanthanum-based metal organic framework decorated polyaniline for effective adsorption of lead ions from aqueous solutions. Environ. Pollut. 2022, 303, 119049. [Google Scholar] [CrossRef]
- Bhaumika, M.; Maityb, A.; Brink, H.G. Zero valent nickel nanoparticles decorated polyaniline nanotubes for the efficient removal of Pb(II) from aqueous solution: Synthesis, characterization and mechanism investigation. Chem. Eng. J. 2020, 417, 127910. [Google Scholar] [CrossRef]
- Li, X.; Shao, H.; Ma, Q.; Yu, W.; Dong, X. Compressible metal-organic framework-nanofibrous reinforced chitosan aerogel for efficient removal of Pb(II) ions. Mater. Today Commun. 2022, 33, 104917. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, W.; Zou, B.; Wang, X.; Zhou, L.; Ye, Z.; Zhao, Q. Efficient adsorption of Cu(II), Pb(II) and Ni(II) from waste water by PANI@APTS-magnetic attapulgite composites. Appl. Clay Sci. 2021, 209, 106151. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Chen, J.; Cheng, R.; Chen, F.; Li, X.; Zhang, X.; Zeng, T.; Hou, H. A new strategy for efficient removal of Cd(II) and Pb(II) by porous and high-capacity N-doped carbon aerogels microspheres. J. Mol. Liq. 2021, 341, 117354. [Google Scholar] [CrossRef]
- Cheraghipour, E.; Pakshir, M. Process optimization and modeling of Pb(II) ions adsorption on chitosan-conjugated magnetite nano-biocomposite using response surface methodology. Chemosphere 2020, 260, 127560. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shang, H.; Sun, X.; Hou, L.; Wen, M.; Qiao, Y. Preparation of thermo-sensitive surface ion-imprinted polymers based on multi-walled carbon nanotube composites for selective adsorption of lead(II) ion. Coll. Surf. A Physicochem. Eng. Aspects. 2020, 585, 124139. [Google Scholar] [CrossRef]
- Waly, S.M.; El-Wakil, A.M.; Abou El-Maaty, W.M.; Awad, F.S. Efficient removal of Pb(II) and Hg(II) ions from aqueous solution by amine and thiol modified activated carbon. J. Saudi Chem. Soc. 2021, 25, 101296. [Google Scholar] [CrossRef]
- Pelalak, R.; Heidari, Z.; Khatami, S.M.; Kurniawan, T.A.; Marjani, A.; Shirazian, S. Oak wood ash/GO/Fe3O4 adsorption efficiencies for cadmium and lead removal from aqueous solution. Kinetics, equilibrium and thermodynamic evaluation. Arab. J. Chem. 2021, 14, 102991. [Google Scholar] [CrossRef]
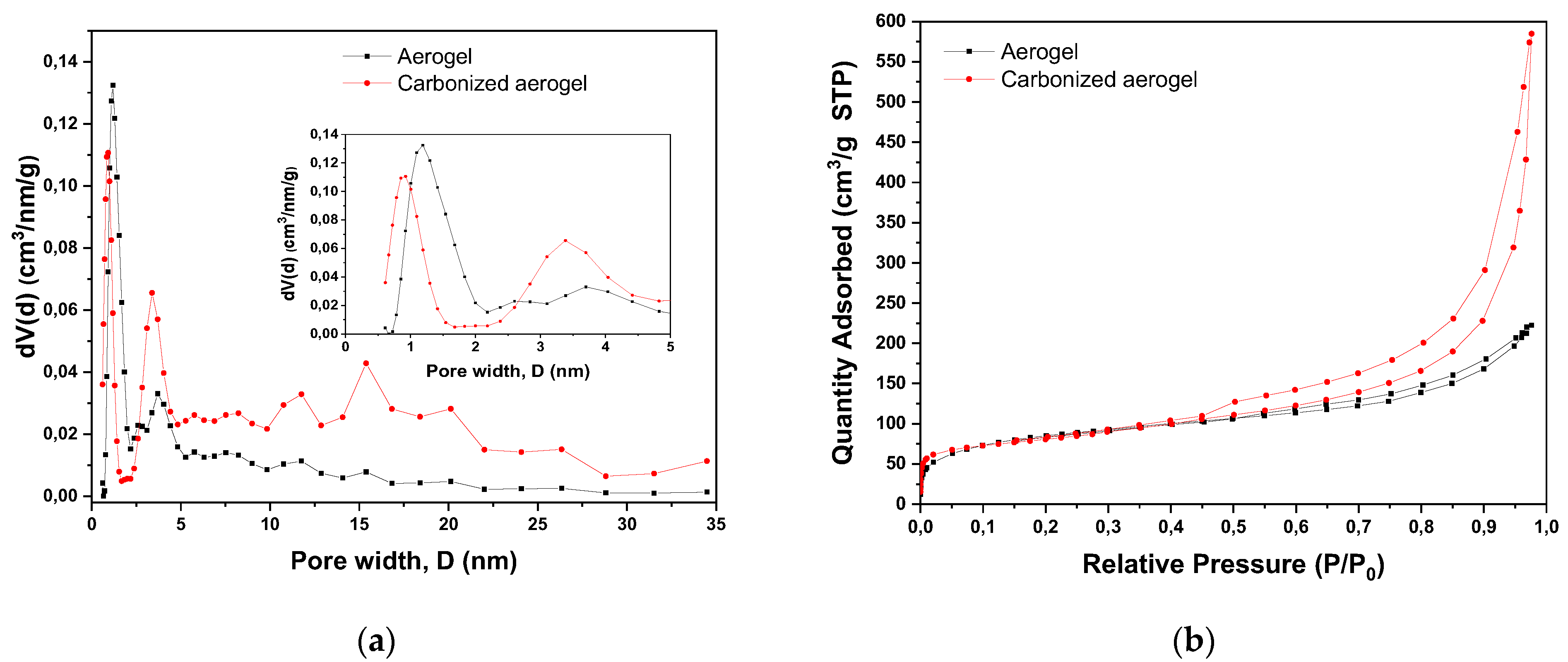
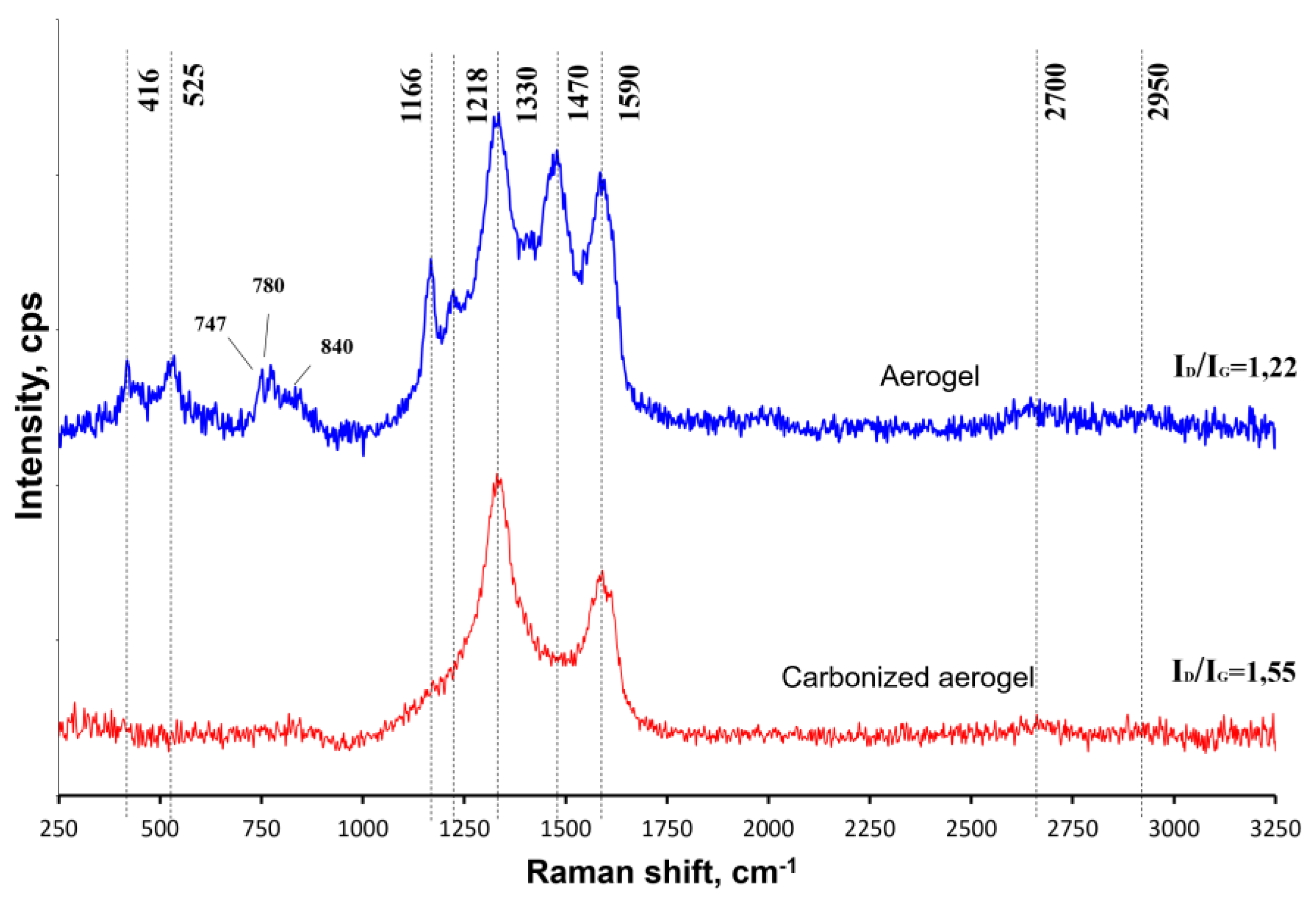
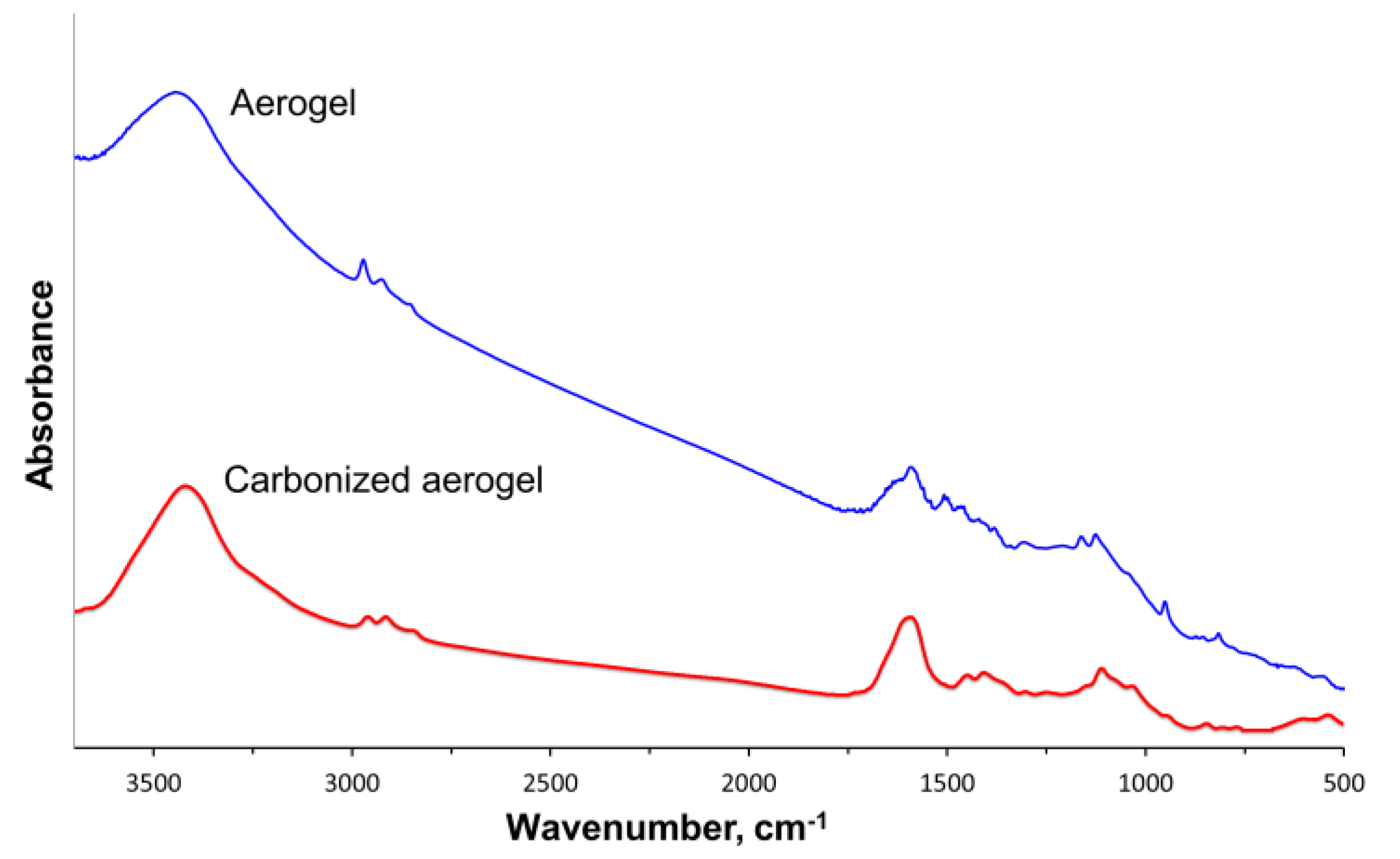
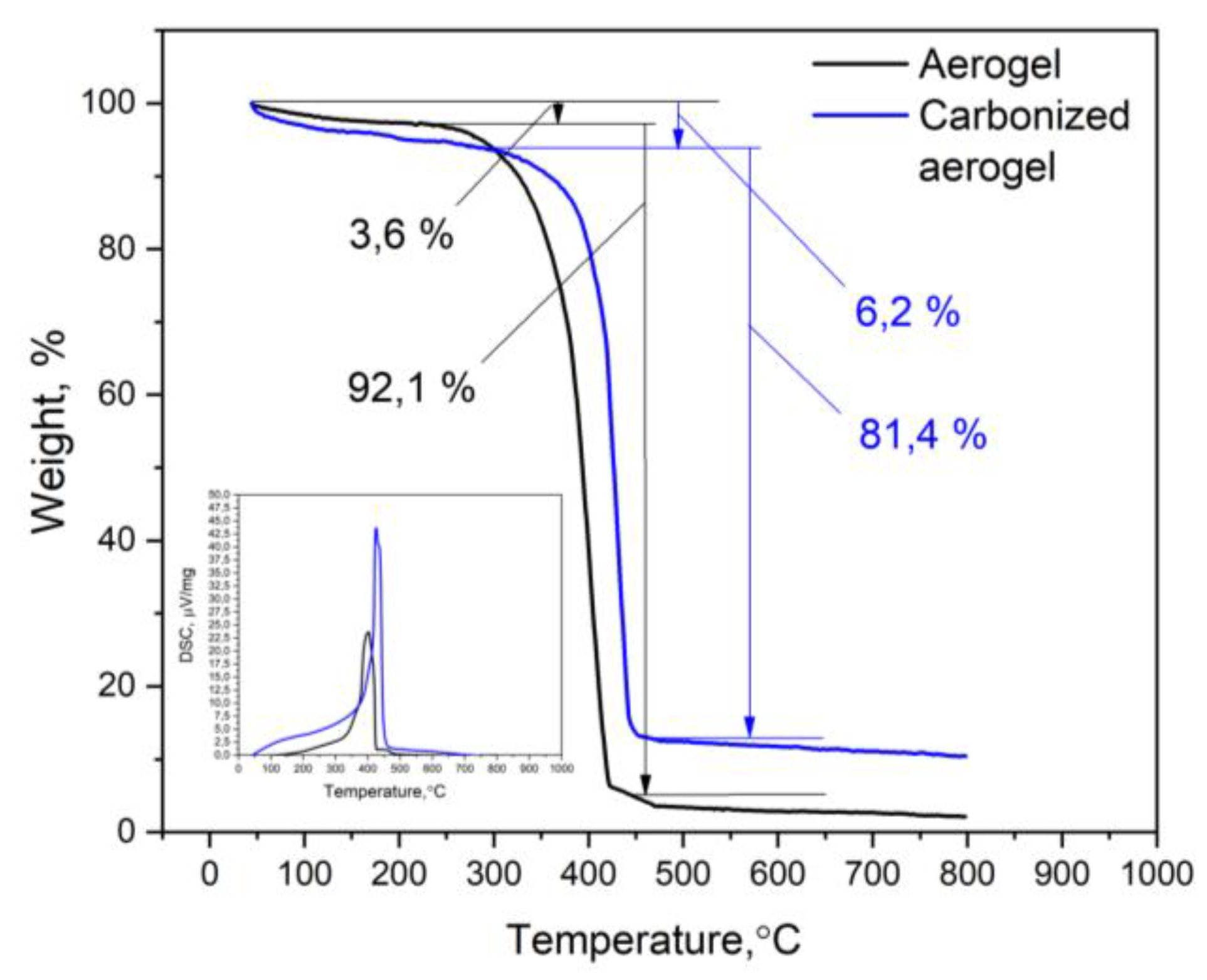

| Material | SBET, m2/g | SDFT, m2/g | VDFT, cm3/g | Vmicro, cm3/g | Vmeso, cm3/g |
|---|---|---|---|---|---|
| Aerogel | 289 | 228 | 0.316 | 0.062 | 0.258 |
| Carbonized aerogel | 315 | 275 | 0.747 | 0.215 | 0.535 |
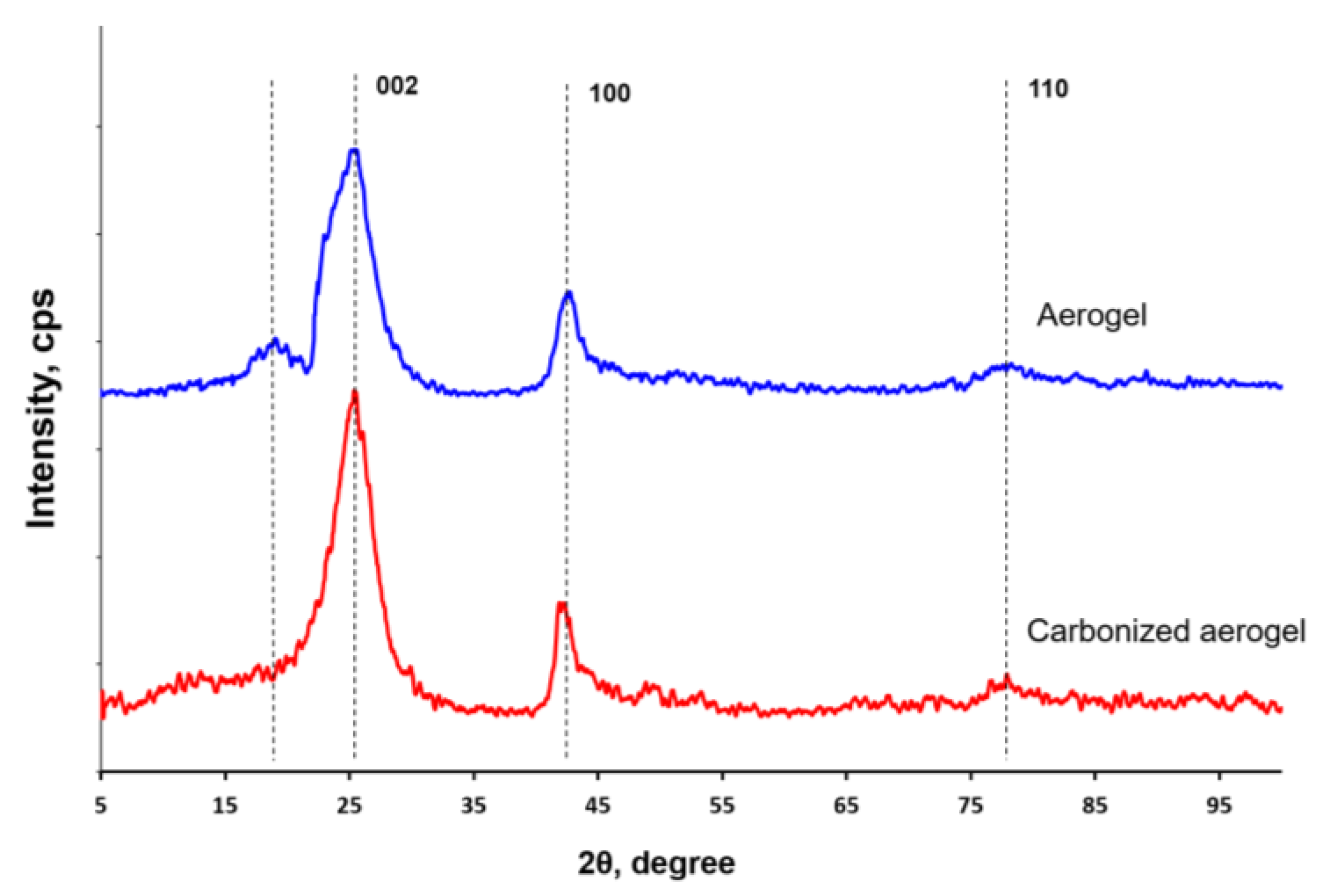
| Material | Interplanar Distance (d) for 2θ Angle, Å | |||
|---|---|---|---|---|
| at ~19° | at ~25° | at ~42° | at ~78° | |
| Aerogel | 4.63 | 3.49 | 2.12 | 1.22 |
| Carbonized aerogel | 3.51 | 2.13 | 1.22 | |
| Model | Equation (Integral Form) | |
|---|---|---|
| Pseudo-first-order | (2) | |
| Pseudo-second-order | (3) | |
| Elovich | (4) | |
| Weber–Morris (intraparticle diffusion) | (5) | |
| Boyd (external (film) diffusion) | (6) |
| Experiment | Boyd | Weber–Morris | ||||
|---|---|---|---|---|---|---|
| R2 | R2 | |||||
| ~185 | 0.9647 | 51.86 | 16.047 | 0.9808 | ||
| Pseudo-first-order | Pseudo-second-order | |||||
| R2 | R2 | |||||
| 0.1708 | 187.56 | 0.9972 | 0.0010 | 213.17 | 0.9793 | |
| Elovich | ||||||
| β | α | R2 | ||||
| 0.0241 | 433 | 0.9331 | ||||
| Material Type | Experimental Conditions | ||||
|---|---|---|---|---|---|
| pH | Ce, mg/L | m, g | t, min | qe, mg/g | |
| Oxidized multiwalled carbon nanotubes (oxMWCNTs6h) [38] | 5.0 | 1 | 5 mg | 20 | 4.8 |
| Lanthanum-based metal organic framework decorated polyaniline (La-MOF@x%PANI) composite [39] | 6.0 | 140 | 1 g/L | 1140 | 185 |
| Zero-valent nickel nanoparticle decorated polyaniline (PANI-NSA@Ni0) composite nanotubes [40] | 5.0 | 100 | 0.5 g/L | 5 | 170 |
| Compressible metal organic framework nanofibrous reinforced chitosan aerogel (UiO-66-NH2/PANNs/CS) [41] | 5.0 | 100 | 3 mg | 240 | 84 |
| PANI@APTS-magnetic attapulgite composites (PANI@APTS-Fe3O4/ATP-0.7) [42] | 5.0 | 100 | 20 mg | 15 | 130 |
| N-doped carbon aerogel (NCA) microspheres [43] | 6.0 | 250 | 10 mg | 20 | 209 |
| Chitosan-conjugated magnetite (CH-MNP-CA) nanoparticles [44] | 6.1 | 30 | 0.5 g/L | 60 | 114 |
| Hermo-sensitive surface ion-imprinted polymers based on multiwalled carbon nanotube composites [45] | 6.0 | 100 | 20 mg | 15 | 83 |
| Chemical modification of activated carbon (AT-MAC) [46] | 5.5 | 250 | 0.01 | 100 | 250 |
| Magnetic oak wood ash/graphene oxide (Ash/GO/Fe3O4) [47] | 6.0 | 10 | 1 g/L | 60 | 99.7% |
| Carbonized r-GO/o-CNT/PANI aerogel (this work) | 6.0 | 100 | 0.005 | 15 | 185 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, T.S.; Burakov, A.E.; Burakova, I.V.; Pasko, T.V.; Dyachkova, T.P.; Mkrtchyan, E.S.; Memetova, A.E.; Ananyeva, O.A.; Shigabaeva, G.N.; Galunin, E.V. Preparation of a Polyaniline-Modified Hybrid Graphene Aerogel-Like Nanocomposite for Efficient Adsorption of Heavy Metal Ions from Aquatic Media. Polymers 2023, 15, 1101. https://doi.org/10.3390/polym15051101
Kuznetsova TS, Burakov AE, Burakova IV, Pasko TV, Dyachkova TP, Mkrtchyan ES, Memetova AE, Ananyeva OA, Shigabaeva GN, Galunin EV. Preparation of a Polyaniline-Modified Hybrid Graphene Aerogel-Like Nanocomposite for Efficient Adsorption of Heavy Metal Ions from Aquatic Media. Polymers. 2023; 15(5):1101. https://doi.org/10.3390/polym15051101
Chicago/Turabian StyleKuznetsova, Tatiana S., Alexander E. Burakov, Irina V. Burakova, Tatiana V. Pasko, Tatiana P. Dyachkova, Elina S. Mkrtchyan, Anastasia E. Memetova, Oksana A. Ananyeva, Gulnara N. Shigabaeva, and Evgeny V. Galunin. 2023. "Preparation of a Polyaniline-Modified Hybrid Graphene Aerogel-Like Nanocomposite for Efficient Adsorption of Heavy Metal Ions from Aquatic Media" Polymers 15, no. 5: 1101. https://doi.org/10.3390/polym15051101
APA StyleKuznetsova, T. S., Burakov, A. E., Burakova, I. V., Pasko, T. V., Dyachkova, T. P., Mkrtchyan, E. S., Memetova, A. E., Ananyeva, O. A., Shigabaeva, G. N., & Galunin, E. V. (2023). Preparation of a Polyaniline-Modified Hybrid Graphene Aerogel-Like Nanocomposite for Efficient Adsorption of Heavy Metal Ions from Aquatic Media. Polymers, 15(5), 1101. https://doi.org/10.3390/polym15051101










