The Use of Corn Stover-Derived Nanocellulose as a Stabilizer of Oil-in-Water Emulsion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanocellulose from Corn Stover
2.3. Conductometric Titration of TEMPO-CNF
2.4. Characterization of TEMPO-CNF
2.5. Nanocellulose-Stabilized Pickering Emulsion
2.5.1. Preparation of Nanocellulose-Stabilized Pickering Emulsion
2.5.2. Determination of Emulsion Droplet Size
2.6. Oil-in-Water Emulsion Stabilized by TEMPO-CNF and Tween 80
2.6.1. Preparation of Oil-in-Water Emulsions Stabilized by TEMPO-CNF and Tween 80
2.6.2. Thermodynamic Stability Testing of Oil-in-Water Emulsion
3. Results
3.1. Yield and Color of Corn Stover Sample after Each Treatment
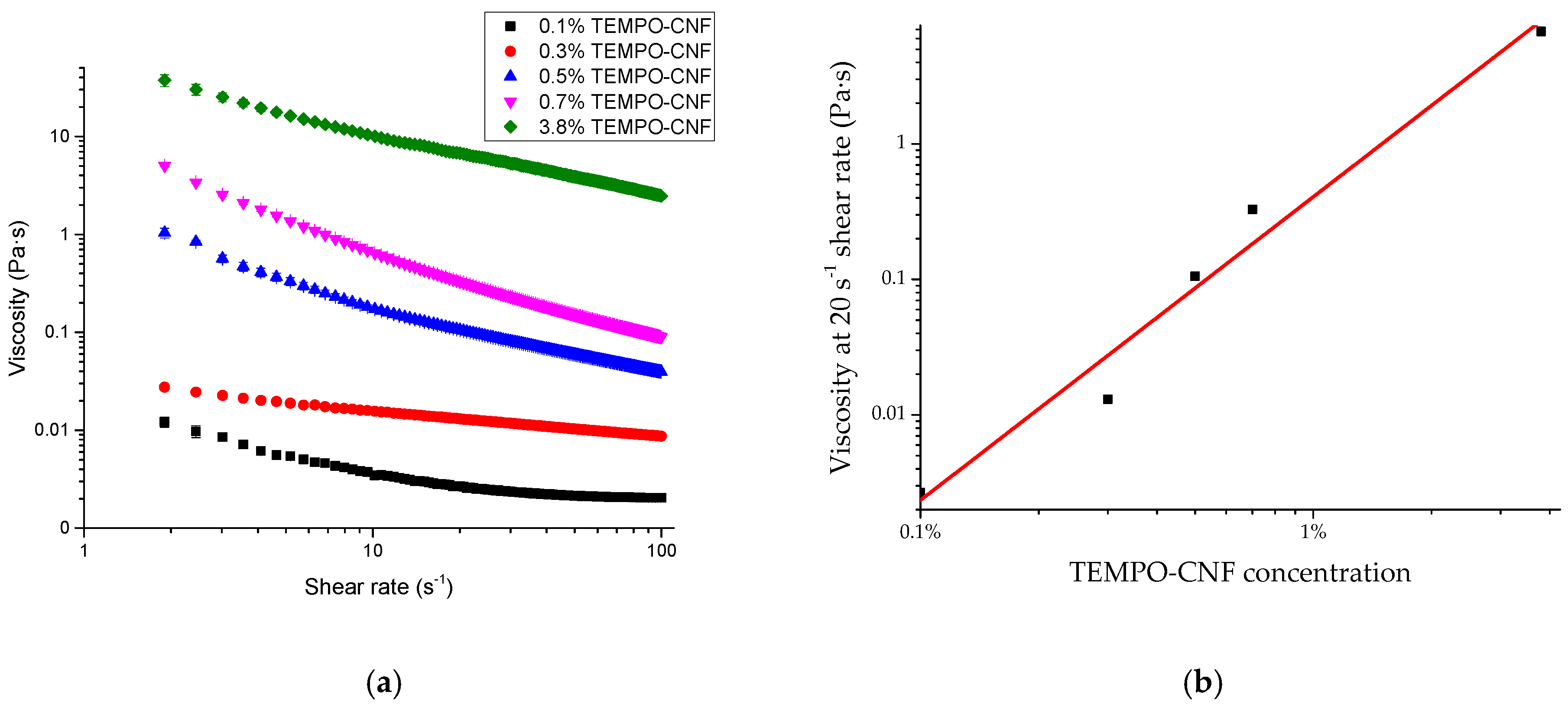
3.2. Physicochemical Properties of TEMPO-CNF
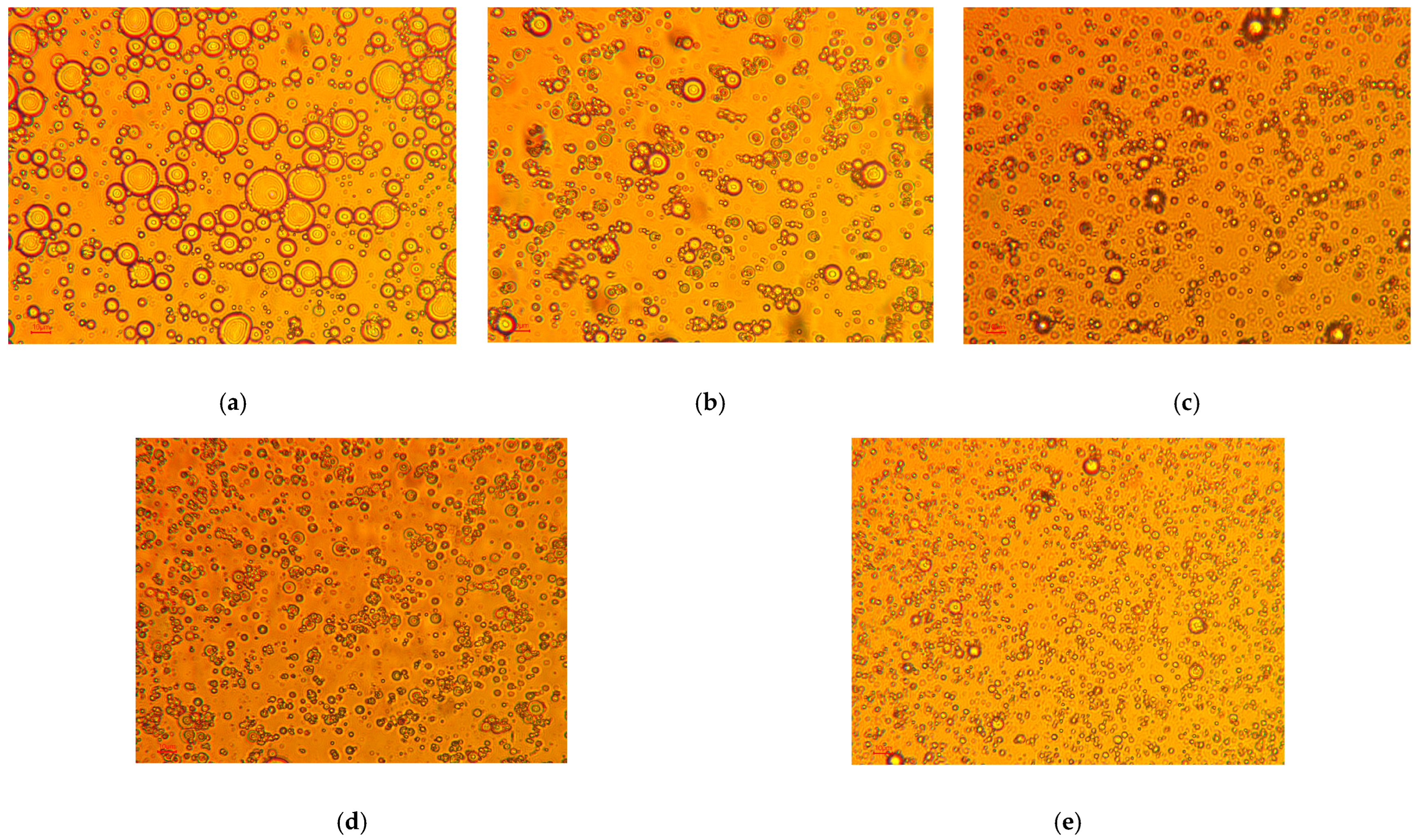
3.3. TEMPO-CNF Stabilized Pickering Emulsion
3.4. Emulsions Stabilized by TEMPO-CNF and Tween 80
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ioelovich, M.; Figovsky, O. Nano-cellulose as promising biocarrier. In Advanced Materials Research; Trans Tech Publications: Stafa-Zurich, Switzerland, 2008; Volume 47–50, pp. 1286–1289. [Google Scholar]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 41719. [Google Scholar] [CrossRef]
- Kolakovic, R.; Laaksonen, T.; Peltonen, L.; Laukkanen, A.; Hirvonen, J. Spray-dried nanofibrillar cellulose microparticles for sustained drug release. Int. J. Pharm. 2012, 430, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Minko, S.; Sharma, S.; Hardin, I.; Luzinov, I.; Daubenmire, S.W.; Zakharchenko, A.; Saremi, R.; Kim, Y.S. Textile Dyeing Using Nanocellulosic Fibers. U.S. Patent No. 9,506,187, 29 November 2016. [Google Scholar]
- Sun, X.; Wu, Q.; Zhang, X.; Ren, S.; Lei, T.; Li, W.; Xu, G.; Zhang, Q. Nanocellulose films with combined cellulose nanofibers and nanocrystals: Tailored thermal, optical and mechanical properties. Cellulose 2018, 25, 1103–1115. [Google Scholar] [CrossRef]
- Isogai, A. Cellulose nanofibers: Recent progress and future prospects. J. Fiber Sci. Technol. 2020, 76, 310–326. [Google Scholar] [CrossRef]
- Hop, T.T.T.; Mai, D.T.; Cong, T.D.; Nhi, T.T.Y.; Loi, V.D.; Huong, N.T.M.; Tung, N.T. A comprehensive study on preparation of nanocellulose from bleached wood pulps by TEMPO-mediated oxidation. Results Chem. 2022, 4, 100540. [Google Scholar]
- Theivasanthi, T.; Christma, F.A.; Toyin, A.J.; Gopinath, S.C.; Ravichandran, R. Synthesis and characterization of cotton fiber-based nanocellulose. Int. J. Biol. Macromol. 2018, 109, 832–836. [Google Scholar] [CrossRef]
- Abol-Fotouh, D.; Hassan, M.A.; Shokry, H.; Roig, A.; Azab, M.S.; Kashyout, A.E.-H.B. Bacterial nanocellulose from agro-industrial wastes: Low-cost and enhanced production by Komagataeibacter saccharivorans MD1. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Costa, L.A.; Assis, D.d.J.; Gomes, G.V.; da Silva, J.B.; Fonsêca, A.F.; Druzian, J.I. Extraction and characterization of nanocellulose from corn stover. Mater. Today Proc. 2015, 2, 287–294. [Google Scholar] [CrossRef]
- Chen, Q.; Xiong, J.; Chen, G.; Tan, T. Preparation and characterization of highly transparent hydrophobic nanocellulose film using corn husks as main material. Int. J. Biol. Macromol. 2020, 158, 781–789. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.-W. Isolation of oxidized nanocellulose from rice straw using the ammonium persulfate method. Cellulose 2018, 25, 2143–2149. [Google Scholar] [CrossRef]
- Hasenhuettl, G.L.; Hartel, R.W. Food Emulsifiers and Their Applications; Springer: New York, NY, USA, 2008; Volume 19. [Google Scholar]
- Ariyaprakai, S.; Tananuwong, K. Freeze–thaw stability of edible oil-in-water emulsions stabilized by sucrose esters and Tweens. J. Food Eng. 2015, 152, 57–64. [Google Scholar] [CrossRef]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef]
- Pickering, S.U. Cxcvi.—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Salminen, H.; Bischoff, S.; Weiss, J. Formation and stability of emulsions stabilized by Quillaja saponin–egg lecithin mixtures. J. Food Sci. 2020, 85, 1213–1222. [Google Scholar] [CrossRef]
- Mikulcová, V.; Bordes, R.; Kašpárková, V. On the preparation and antibacterial activity of emulsions stabilized with nanocellulose particles. Food Hydrocoll. 2016, 61, 780–792. [Google Scholar] [CrossRef]
- Goi, Y.; Fujisawa, S.; Saito, T.; Yamane, K.; Kuroda, K.; Isogai, A. Dual functions of tempo-oxidized cellulose nanofibers in oil-in-water emulsions: A pickering emulsifier and a unique dispersion stabilizer. Langmuir 2019, 35, 10920–10926. [Google Scholar] [CrossRef] [PubMed]
- Foo, M.L.; Ooi, C.W.; Tan, K.W.; Chew, I.M. Preparation of black cumin seed oil Pickering nanoemulsion with enhanced stability and antioxidant potential using nanocrystalline cellulose from oil palm empty fruit bunch. Chemosphere 2022, 287, 132108. [Google Scholar] [CrossRef] [PubMed]
- Gestranius, M.; Stenius, P.; Kontturi, E.; Sjöblom, J.; Tammelin, T. Phase behaviour and droplet size of oil-in-water Pickering emulsions stabilised with plant-derived nanocellulosic materials. Colloids Surf. A Physicochem. Eng. Asp. 2017, 519, 60–70. [Google Scholar] [CrossRef]
- Okita, Y.; Saito, T.; Isogai, A.J.B. Entire surface oxidation of various cellulose microfibrils by TEMPO-mediated oxidation. Biomacromolecules 2010, 11, 1696–1700. [Google Scholar] [CrossRef]
- Liu, J.; Korpinen, R.; Mikkonen, K.S.; Willför, S.; Xu, C. Nanofibrillated cellulose originated from birch sawdust after sequential extractions: A promising polymeric material from waste to films. Cellulose 2014, 21, 2587–2598. [Google Scholar] [CrossRef]
- Liu, L.; Kerr, W.L.; Kong, F. Characterization of lipid emulsions during in vitro digestion in the presence of three types of nanocellulose. J. Colloid Interface Sci. 2019, 545, 317–329. [Google Scholar] [CrossRef]
- Xu, J.; Krietemeyer, E.F.; Boddu, V.M.; Liu, S.X.; Liu, W.-C.J.C.P. Production and characterization of cellulose nanofibril (CNF) from agricultural waste corn stover. Carbohydr Polym. 2018, 192, 202–207. [Google Scholar] [CrossRef]
- Smyth, M.; García, A.; Rader, C.; Foster, E.J.; Bras, J. Extraction and process analysis of high aspect ratio cellulose nanocrystals from corn (Zea mays) agricultural residue. Ind. Crops Prod. 2017, 108, 257–266. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. J Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Hu, D.; Lin, K.-H.; Xu, Y.; Kajiyama, M.; Neves, M.A.; Ogawa, K.; Enomae, T. Microwave-assisted synthesis of fluorescent carbon dots from nanocellulose for dual-metal ion-sensor probe: Fe (III) and Mn (II). Cellulose 2021, 28, 9705–9724. [Google Scholar] [CrossRef]
- Zhou, Y.; Saito, T.; Bergström, L.; Isogai, A.J.B. Acid-free preparation of cellulose nanocrystals by TEMPO oxidation and subsequent cavitation. Biomacromolecules 2018, 19, 633–639. [Google Scholar] [CrossRef]
- Cao, X.; Ding, B.; Yu, J.; Al-Deyab, S.S. Cellulose nanowhiskers extracted from TEMPO-oxidized jute fibers. Carbohydr Polym. 2012, 90, 1075–1080. [Google Scholar] [CrossRef]
- Isogai, A.; Bergström, L. Preparation of cellulose nanofibers using green and sustainable chemistry. J Curr. Opin. Green Sustain. Chem. 2018, 12, 15–21. [Google Scholar] [CrossRef]
- Isogai, A.; Zhou, Y. Diverse nanocelluloses prepared from TEMPO-oxidized wood cellulose fibers: Nanonetworks, nanofibers, and nanocrystals. Curr. Opin. Solid State Mater. Sci. 2019, 23, 101–106. [Google Scholar] [CrossRef]
- Liu, L.; Kong, F. Influence of nanocellulose on in vitro digestion of whey protein isolate. Carbohydr. Polym. 2019, 210, 399–411. [Google Scholar] [CrossRef]
- Liao, J.; Pham, K.A.; Breedveld, V. Rheological characterization and modeling of cellulose nanocrystal and TEMPO-oxidized cellulose nanofibril suspensions. Cellulose 2020, 27, 3741–3757. [Google Scholar] [CrossRef]
- Dinic, J.; Sharma, V. Power laws dominate shear and extensional rheology response and capillarity-driven pinching dynamics of entangled hydroxyethyl cellulose (HEC) solutions. Macromolecules 2020, 53, 3424–3437. [Google Scholar] [CrossRef]
- Li, C.; Sun, P.; Yang, C. Emulsion stabilized by starch nanocrystals. Starch-Stärke 2012, 64, 497–502. [Google Scholar] [CrossRef]
- Tzoumaki, M.V.; Moschakis, T.; Kiosseoglou, V.; Biliaderis, C.G. Oil-in-water emulsions stabilized by chitin nanocrystal particles. Food Hydrocoll. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Kargar, M.; Fayazmanesh, K.; Alavi, M.; Spyropoulos, F.; Norton, I.T. Investigation into the potential ability of Pickering emulsions (food-grade particles) to enhance the oxidative stability of oil-in-water emulsions. J. Colloid Interface Sci. 2012, 366, 209–215. [Google Scholar] [CrossRef]
- Yang, T.; Liu, T.-X.; Li, X.-T.; Tang, C.-H. Novel nanoparticles from insoluble soybean polysaccharides of Okara as unique Pickering stabilizers for oil-in-water emulsions. Food Hydrocoll. 2019, 94, 255–267. [Google Scholar] [CrossRef]
- Shao, Y.; Tang, C.-H. Characteristics and oxidative stability of soy protein-stabilized oil-in-water emulsions: Influence of ionic strength and heat pretreatment. Food Hydrocoll. 2014, 37, 149–158. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, X.; Xiao, Y.; Hu, L.; Eggersdorfer, M.; Chen, D.; Yang, Z.; Weitz, D.A. Pickering emulsions stabilized by colloidal surfactants: Role of solid particles. Particuology 2022, 64, 153–163. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New Pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir 2011, 27, 7471–7479. [Google Scholar] [CrossRef]
- Chen, Q.-H.; Zheng, J.; Xu, Y.-T.; Yin, S.-W.; Liu, F.; Tang, C.-H. Surface modification improves fabrication of pickering high internal phase emulsions stabilized by cellulose nanocrystals. Food Hydrocoll. 2018, 75, 125–130. [Google Scholar] [CrossRef]
- Jiménez Saelices, C.; Capron, I. Design of Pickering micro-and nanoemulsions based on the structural characteristics of nanocelluloses. Biomacromolecules 2018, 19, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Marefati, A.; Rayner, M.; Timgren, A.; Dejmek, P.; Sjöö, M. Freezing and freeze-drying of Pickering emulsions stabilized by starch granules. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 512–520. [Google Scholar] [CrossRef]
- Qu, Z.; Guo, S.; Sproncken, C.C.; Surís-Valls, R.; Yu, Q.; Voets, I.K. Enhancing the Freeze–thaw durability of concrete through ice recrystallization inhibition by poly (vinyl alcohol). ACS Omega 2020, 5, 12825–12831. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhao, Y.; Zhong, Q.; Wu, T. Inhibiting ice recrystallization by nanocelluloses. Biomacromolecules 2019, 20, 1667–1674. [Google Scholar] [CrossRef]
- Pelegrini, B.; Fernandes, F.; Fernandes, T.; de Oliveira, J.; Rosseto, H.; Junior, A.; Reis, A.; Castelani, E.; Sobral, F.; Shirabayashi, W. Novel green strategy to improve the hydrophobicity of cellulose nanocrystals and the interfacial elasticity of Pickering emulsions. Cellulose 2021, 28, 6201–6238. [Google Scholar]
- Mendes, J.; Norcino, L.; Martins, H.; Manrich, A.; Otoni, C.; Carvalho, E.; Piccoli, R.; Oliveira, J.; Pinheiro, A.; Mattoso, L. Correlating emulsion characteristics with the properties of active starch films loaded with lemongrass essential oil. Food Hydrocoll. 2020, 100, 105428. [Google Scholar] [CrossRef]
- Dickinson, E. Biopolymer interactions in emulsion systems: Influences on creaming, flocculation, and rheology. In Macromolecular Interactions in Food Technology; American Chemical Society: Washington, DC, USA, 1996; Volume 650, pp. 197–207. [Google Scholar]


| Washing | Bleaching Treatment | Alkaline Treatment | Oxidation Treatment | |
|---|---|---|---|---|
| Step yield (%) | 87.4 ± 9.0 | 55.1 ± 1.7 | 66.3 ± 4.8 | 86.9 ± 11.2 |
| Overall yield (%) | 87.4 ± 9.0 | 48.2 ± 5.2 | 31.9 ± 4.1 | 27.7 ± 5.1 |
| L* | 24.3 ± 3.2 | 34.0 ± 1.0 | 34.6 ± 4.8 | 35.4 ± 1.5 |
| a* | 4.4 ± 0.3 | 3.1 ± 0.1 | 3.3 ± 0.7 | 2.1 ± 0.1 |
| b* | 12.3 ± 0.06 | 9.8 ± 0.2 | 7.0 ± 0.3 | 4.4 ± 0.4 |
| Physicochemical Properties | Value |
|---|---|
| Length (nm) | 353 ± 116 |
| Width (nm) | 4.0 ± 0.6 |
| Length/width ratio | 88 ± 32 |
| Zeta potential (mV) | −65 ± 3 |
| Concentrated gel solid content | 3.80% ± 0.08% |
| Light transmittance at 600 nm (0.1 wt% gel suspension) | 100.0% ± 0.2% |
| Carboxyl group (mmol/g) | 1.48 ± 0.09 |
| TEMPO-CNF Concentration (%) | Creaming Layer Percentage 1 (%) |
|---|---|
| 0 | N/A 2 |
| 0.1 | 10.9 ± 0.2 |
| 0.3 | 9.4 ± 1.4 |
| 0.5 | 0 |
| 0.7 | 0 |
| Lemongrass Essential Oil Concentration (w/w) | TEMPO-CNF Concentration (w/w) | After Centrifugation | After 2 Freeze-Thaw Cycles |
|---|---|---|---|
| 1% | 0 | No phase separation | Phase separation |
| 2.5% | 0 | No phase separation | Phase separation |
| 5% | 0 | No phase separation | Phase separation |
| 1% | 0.3% | No phase separation | No phase separation |
| 2.5% | 0.3% | No phase separation | No phase separation |
| 5% | 0.3% | No phase separation | No phase separation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Gerard, G.; Peng, Z.; Yu, Z. The Use of Corn Stover-Derived Nanocellulose as a Stabilizer of Oil-in-Water Emulsion. Polymers 2023, 15, 757. https://doi.org/10.3390/polym15030757
Liu L, Gerard G, Peng Z, Yu Z. The Use of Corn Stover-Derived Nanocellulose as a Stabilizer of Oil-in-Water Emulsion. Polymers. 2023; 15(3):757. https://doi.org/10.3390/polym15030757
Chicago/Turabian StyleLiu, Lingling, Gina Gerard, Zimeng Peng, and Zhile Yu. 2023. "The Use of Corn Stover-Derived Nanocellulose as a Stabilizer of Oil-in-Water Emulsion" Polymers 15, no. 3: 757. https://doi.org/10.3390/polym15030757
APA StyleLiu, L., Gerard, G., Peng, Z., & Yu, Z. (2023). The Use of Corn Stover-Derived Nanocellulose as a Stabilizer of Oil-in-Water Emulsion. Polymers, 15(3), 757. https://doi.org/10.3390/polym15030757







