Valorization of Agricultural Waste as a Chemiresistor H2S-Gas Sensor: A Composite of Biodegradable-Electroactive Polyurethane-Urea and Activated-Carbon Composite Derived from Coconut-Shell Waste
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Instrumentation
2.2. Synthesis of N, N’-bis(4′-aminophenyl)-1,4-Quinonenediimine (ACAT)
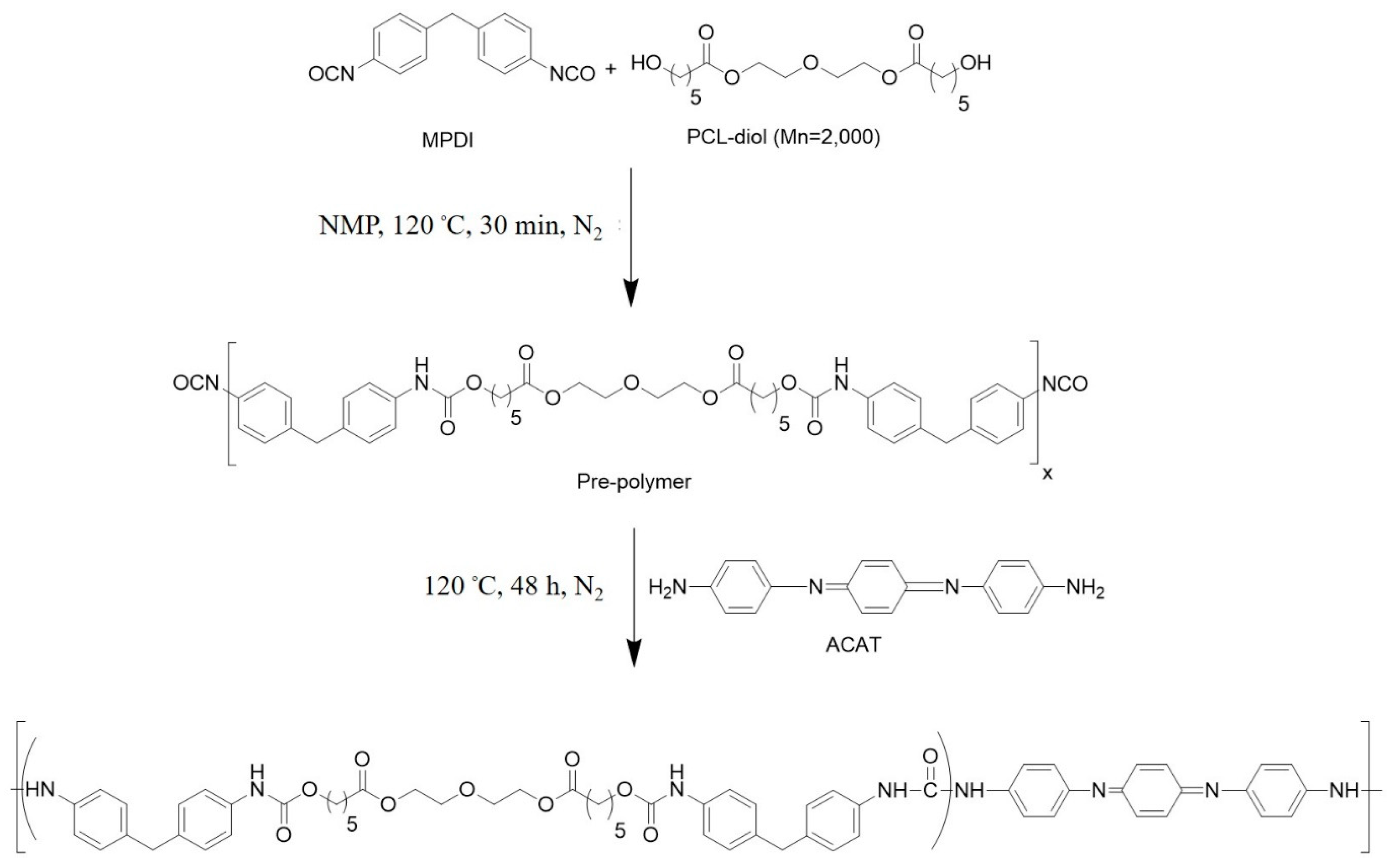
2.3. Synthesis of Polyurethane Urea (PUU-AC0)
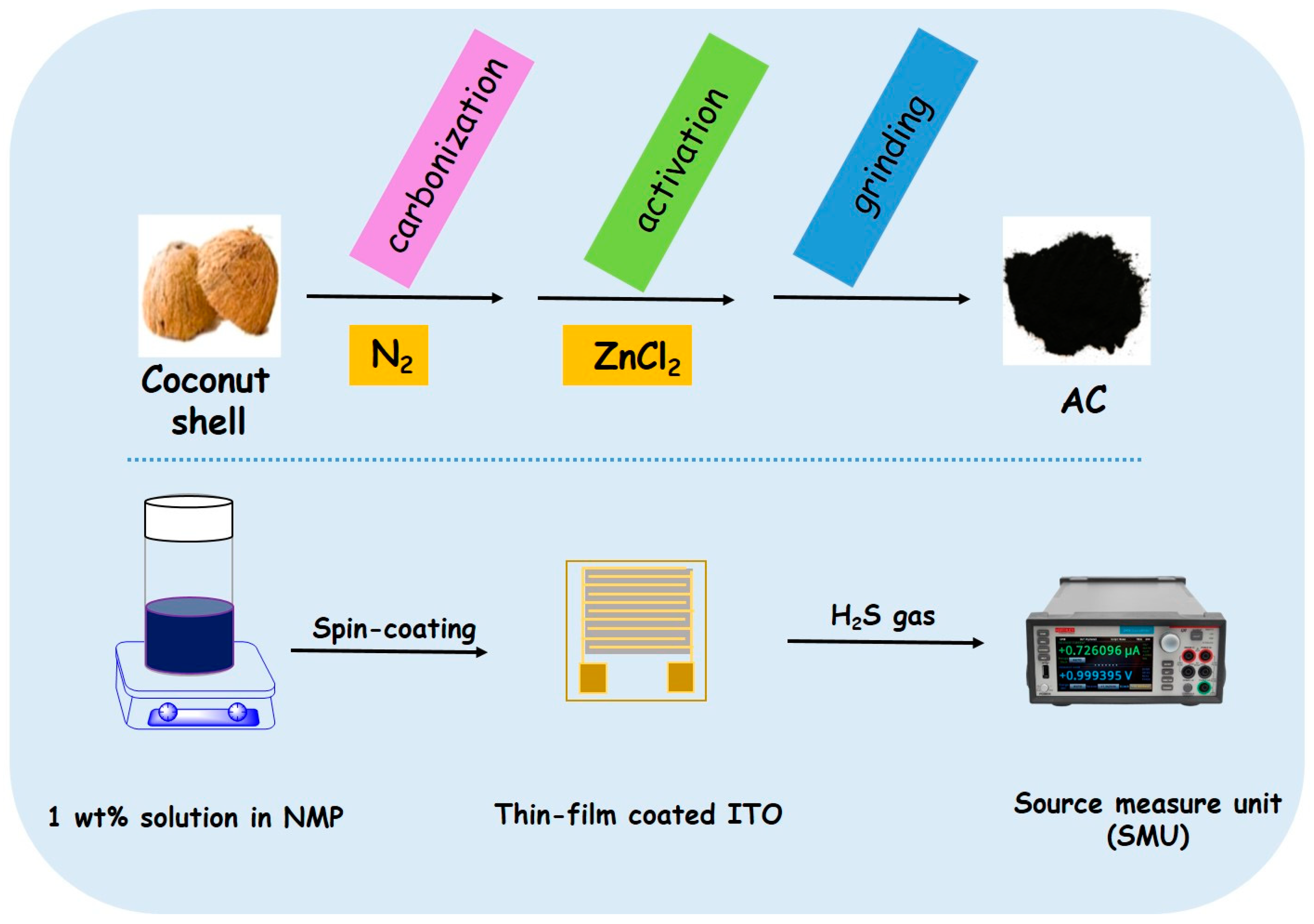
2.4. Preparation of Activated Carbon (AC) from Coconut-Shell Waste
2.5. Preparation of PUU-AC Composites
2.6. Sensor Preparation
2.7. H2S Sensing Experiment
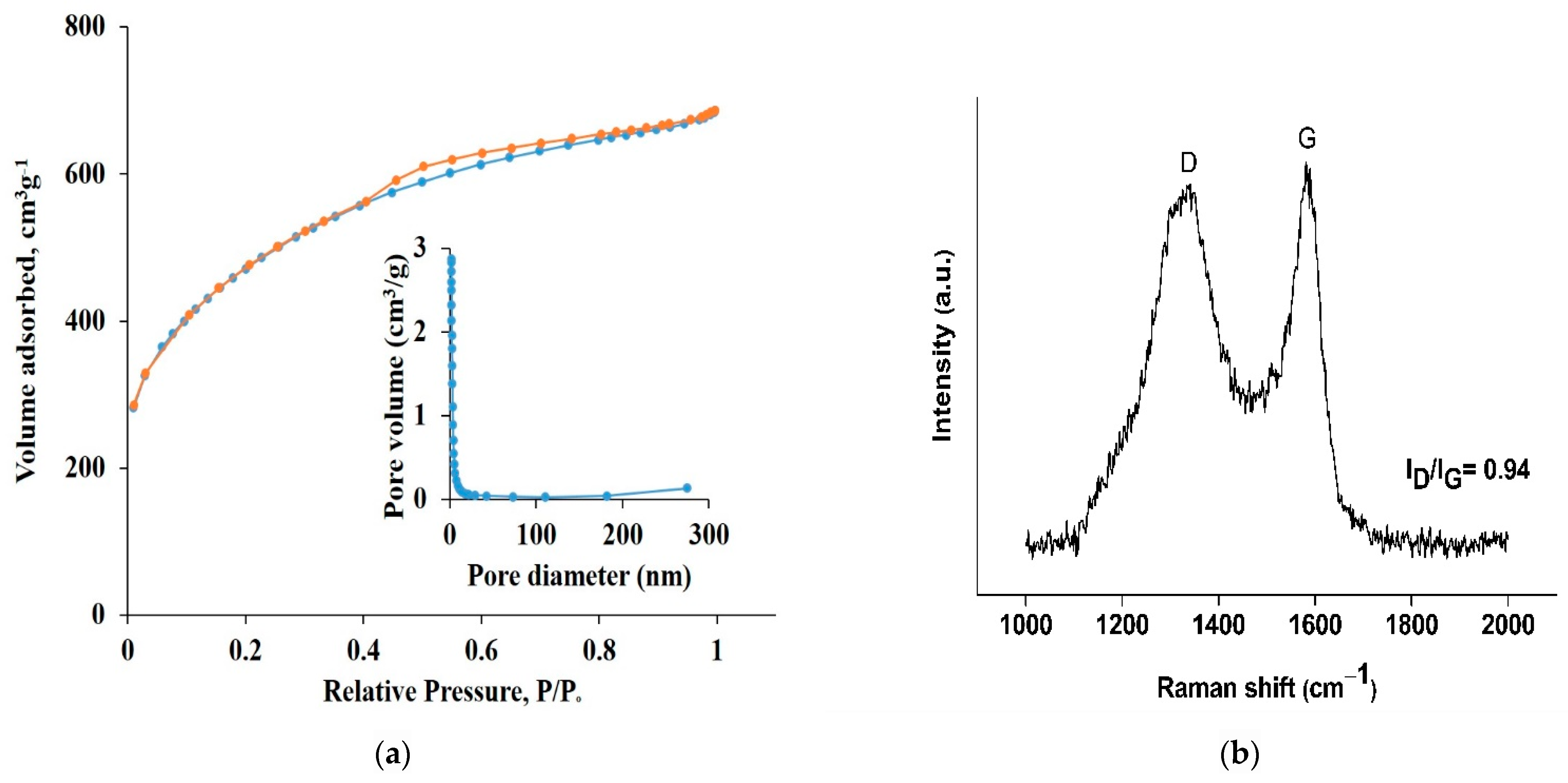
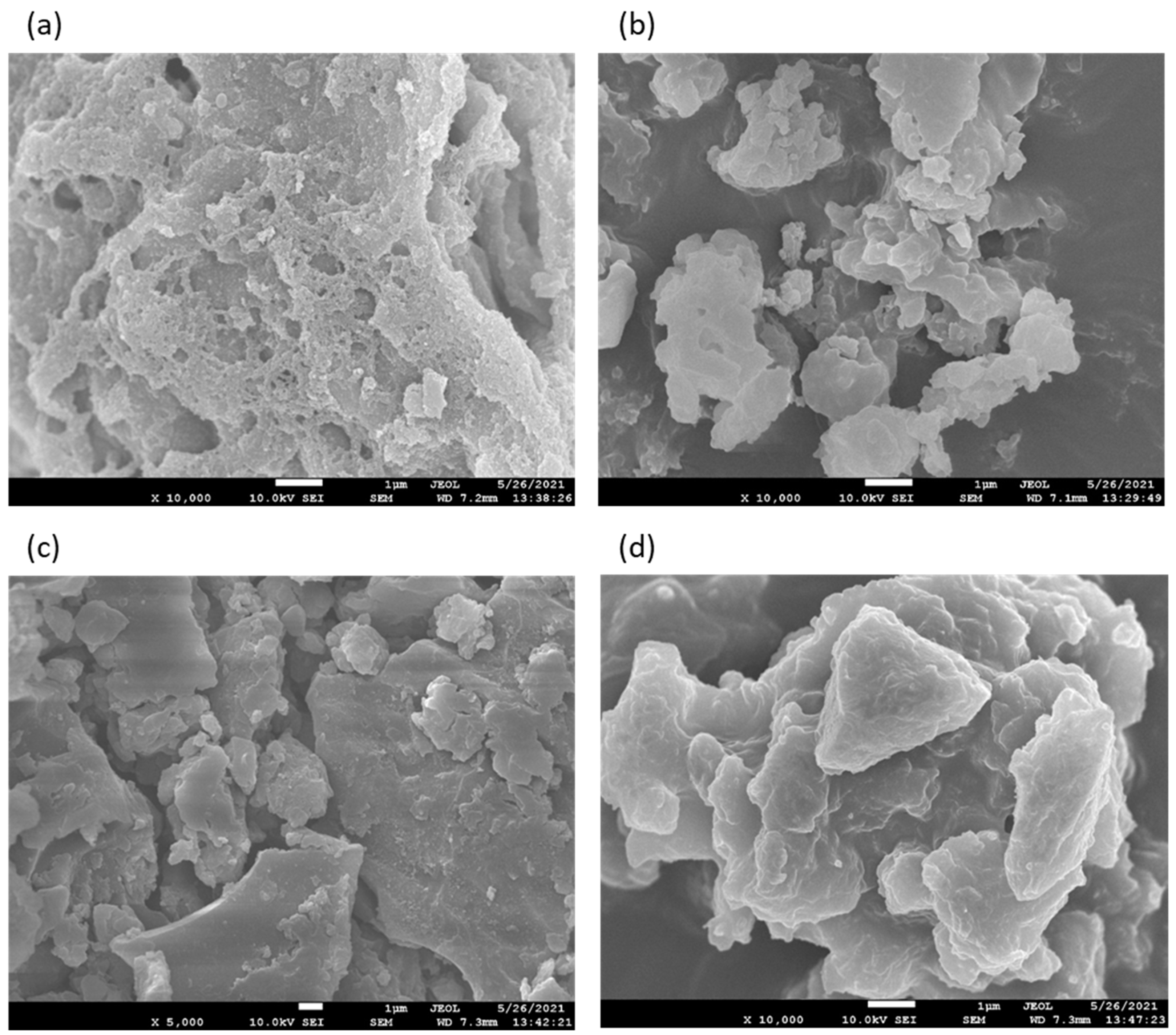
3. Results and Discussion
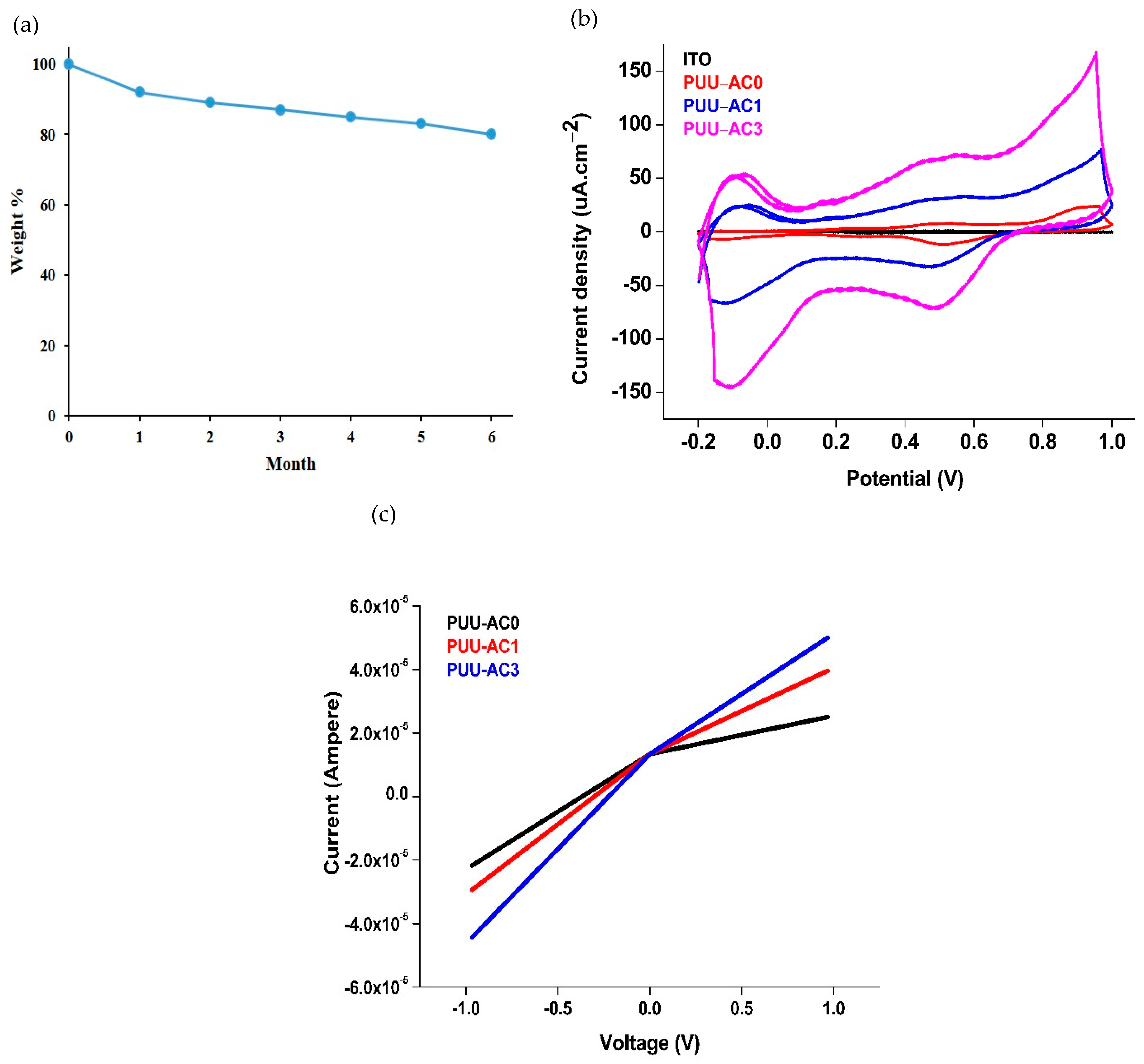
4. Biodegradability
5. Electrical Properties
6. H2S-Gas-Sensing Properties
7. Response and Sensitivity
8. Response/Recovery Time
9. Selectivity
10. Effect of Humidity
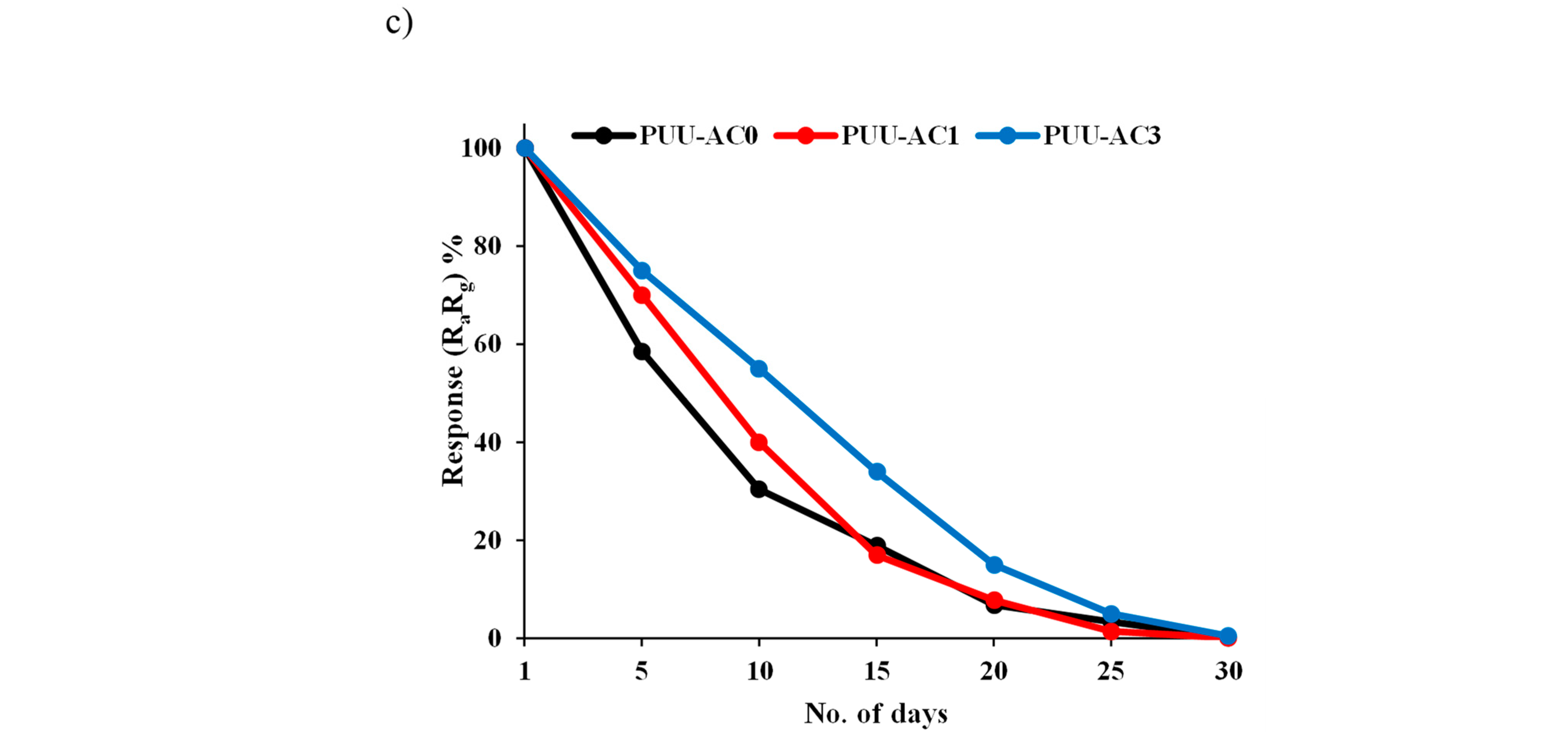
11. Stability
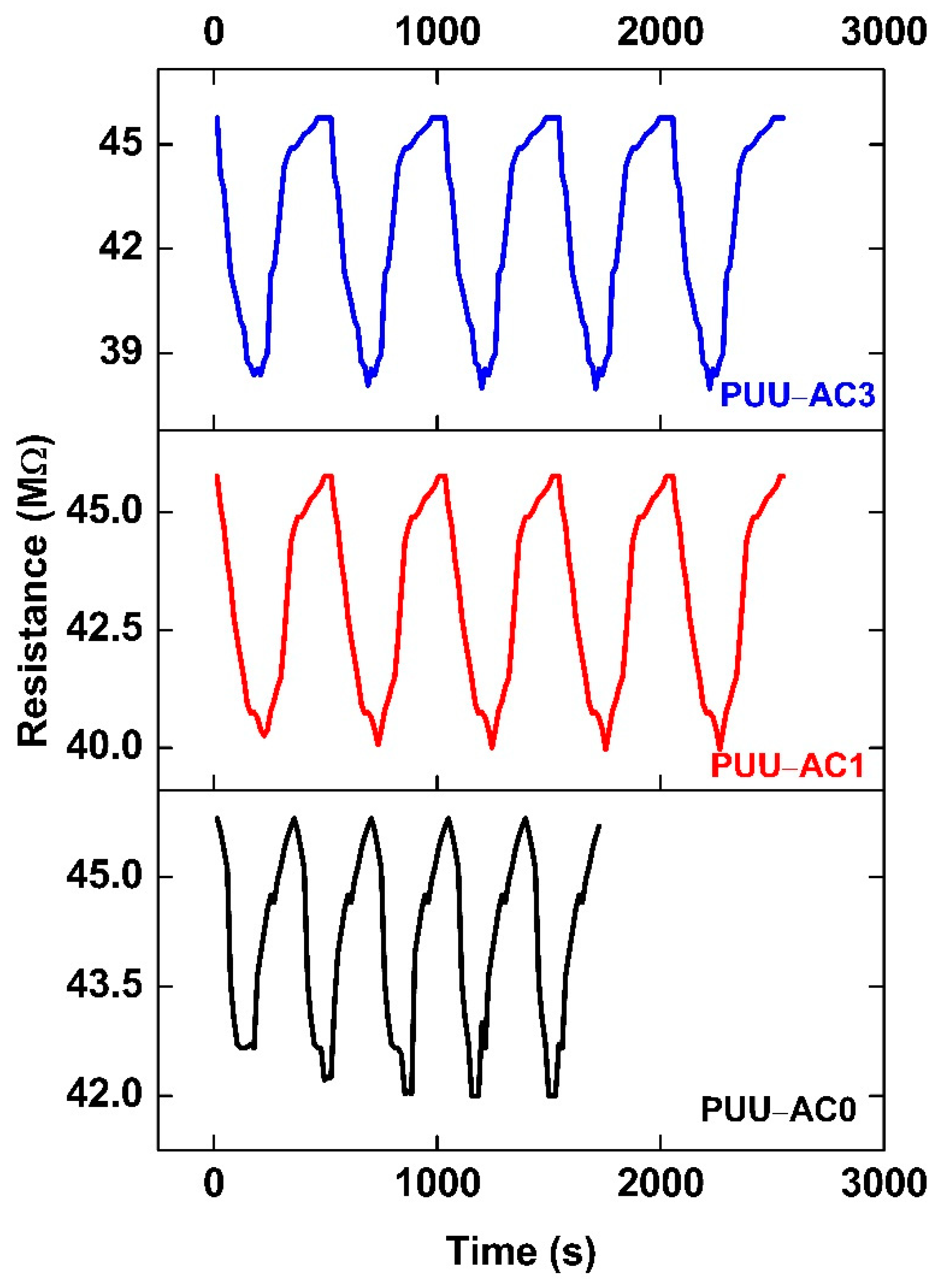
12. Repeatability
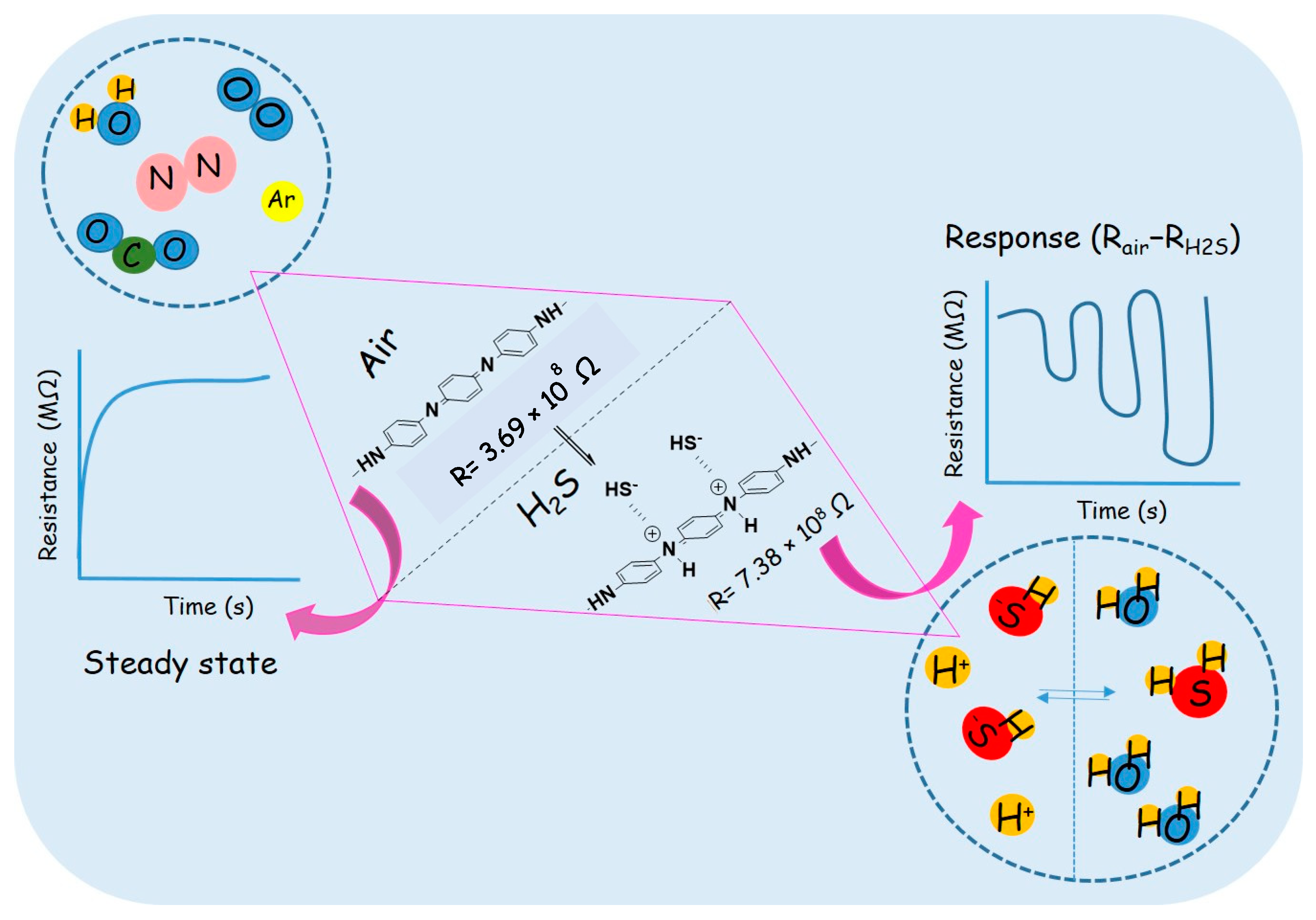
13. Gas-Sensing Mechanism
14. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verma, G.; Gupta, A. Sensing performance of room temperature operated MEMS gas sensor for ppb level detection of hydrogen sulfide: A review. J. Micromechanics Microengineering 2022, 32, 094002. [Google Scholar] [CrossRef]
- National Research Council; Committee on Acute Exposure Guideline Levels. Hydrogen Sulfide Acute Exposure Guideline Levels. In Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 9; National Academies Press: Washington, DC, USA, 1982. [Google Scholar]
- Rubright, S.L.M.; Pearce, L.L.; Peterson, J. Environmental toxicology of hydrogen sulfide. Nitric Oxide 2017, 71, 1–13. [Google Scholar] [CrossRef]
- Verma, G.; Gupta, A. Recent development in carbon nanotubes based gas sensors. J. Mater. NanoSci. 2022, 2022, 3–12. Available online: https://pubs.thesciencein.org/journal/index.php/jmns/article/view/282 (accessed on 20 January 2023).
- Tang, X.; Lahem, D.; Raskin, J.-P.; Gerard, P.; Geng, X.; Andre, N.; Debliquy, M. A Fast and Room-Temperature Operation Ammonia Sensor Based on Compound of Graphene with Polypyrrole. IEEE Sens. J. 2018, 18, 9088–9096. [Google Scholar] [CrossRef]
- Xie, L.-Q.; Zhang, Y.-H.; Gao, F.; Wu, Q.-A.; Xu, P.-Y.; Wang, S.-S.; Gao, N.-N.; Wang, Q.-X. A highly sensitive dopamine sensor based on a polyaniline/reduced graphene oxide/Nafion nanocomposite. Chin. Chem. Lett. 2017, 28, 41–48. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J.S.; Jun, J.; Kim, S.G.; Jang, J. Fabrication of water-dispersible and highly conductive PSS-doped PANI/graphene nanocomposites using a high-molecular weight PSS dopant and their application in H2S detection. Nanoscale 2014, 6, 15181–15195. [Google Scholar] [CrossRef]
- Gvozdenović, M.; Džunuzović, E.; Jugović, B.; Grgur, B. Polyaniline based corrosion inhibitors for conventional organic coatings. Zastita Mater. 2018, 59, 282–292. [Google Scholar] [CrossRef]
- Huang, Z.; Ji, Z.; Feng, Y.; Wang, P.; Huang, Y. Flexible and stretchable polyaniline supercapacitor with a high rate capability. Polym. Int. 2020, 70, 437–442. [Google Scholar] [CrossRef]
- Rao, H.; Chen, M.; Ge, H.; Lu, Z.; Liu, X.; Zou, P.; Wang, X.; He, H.; Zeng, X.; Wang, Y. A novel electrochemical sensor based on Au@PANI composites film modified glassy carbon electrode binding molecular imprinting technique for the determination of melamine. Biosens. Bioelectron. 2017, 87, 1029–1035. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Chemiresistive polyaniline-based gas sensors: A mini review. Sens. Actuators B Chem. 2015, 220, 534–548. [Google Scholar] [CrossRef]
- Zhang, D.; Fan, X.; Hao, X.; Dong, G. Facile Fabrication of Polyaniline Nanocapsule Modified Zinc Oxide Hexagonal Microdiscs for H2S Gas Sensing Applications. Ind. Eng. Chem. Res. 2019, 58, 1906–1913. [Google Scholar] [CrossRef]
- Raut, B.; Chougule, M.; Nalage, S.; Dalavi, D.; Mali, S.; Patil, P.; Patil, V. CSA doped polyaniline/CdS organic–inorganic nanohybrid: Physical and gas sensing properties. Ceram. Int. 2012, 38, 5501–5506. [Google Scholar] [CrossRef]
- Srivastava, S.; Sharma, S.; Kumar, S.; Agrawal, S.; Singh, M.; Vijay, Y. Characterization of gas sensing behavior of multi walled carbon nanotube polyaniline composite films. Int. J. Hydrogen Energy 2009, 34, 8444–8450. [Google Scholar] [CrossRef]
- Bairi, V.G.; Bourdo, S.E.; Sacre, N.; Nair, D.; Berry, B.C.; Biris, A.S.; Viswanathan, T. Ammonia Gas Sensing Behavior of Tanninsulfonic Acid Doped Polyaniline-TiO2 Composite. Sensors 2015, 15, 26415–26429. [Google Scholar] [CrossRef]
- Pang, Z.; Yildirim, E.; Pasquinelli, M.A.; Wei, Q. Ammonia Sensing Performance of Polyaniline-Coated Polyamide 6 Nanofibers. ACS Omega 2021, 6, 8950–8957. [Google Scholar] [CrossRef]
- Korent, A.; Soderžnik, K.; Šturm, S.; Rožman, K..; Redon, N.; Wojkiewicz, J.-L.; Duc, C. Facile Fabrication of an Ammonia-Gas Sensor Using Electrochemically Synthesised Polyaniline on Commercial Screen-Printed Three-Electrode Systems. Sensors 2020, 21, 169. [Google Scholar] [CrossRef]
- Macagnano, A.; Perri, V.; Zampetti, E.; Bearzotti, A.; De Cesare, F. Humidity effects on a novel eco-friendly chemosensor based on electrospun PANi/PHB nanofibres. Sens. Actuators B Chem. 2016, 232, 16–27. [Google Scholar] [CrossRef]
- Virji, S.; Huang, J.; Kaner, R.B.; Weiller, B.H. Polyaniline Nanofiber Gas Sensors: Examination of Response Mechanisms. Nano Lett. 2004, 4, 491–496. [Google Scholar] [CrossRef]
- Yslas, E.I.; Ibarra, L.E.; Peralta, D.O.; Barbero, C.A.; Rivarola, V.A.; Bertuzzi, M.L. Polyaniline nanofibers: Acute toxicity and teratogenic effect on Rhinella arenarum embryos. Chemosphere 2012, 87, 1374–1380. [Google Scholar] [CrossRef]
- Bibi, A.; Rubio, Y.; Santiago, K.; Jia, H.-W.; Ahmed, M.; Lin, Y.-F.; Yeh, J.-M. H2S-Sensing Studies Using Interdigitated Electrode with Spin-Coated Carbon Aerogel-Polyaniline Composites. Polymers 2021, 13, 1457. [Google Scholar] [CrossRef]
- Zheng, J.; Ma, X.; He, X.; Gao, M.; Li, G. Praparation, characterizations, and its potential applications of PANi/ graphene oxide nanocomposite. Procedia Eng. 2012, 27, 1478–1487. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Zong, X. Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sens. Actuators B Chem. 2019, 289, 32–41. [Google Scholar] [CrossRef]
- Parmar, M.; Balamurugan, C.; Lee, D.-W. PANI and Graphene/PANI Nanocomposite Films—Comparative Toluene Gas Sensing Behavior. Sensors 2013, 13, 16611–16624. [Google Scholar] [CrossRef] [PubMed]
- Suhail, M.H.; Abdullah, O.G.; Kadhim, G.A. Hydrogen sulfide sensors based on PANI/f-SWCNT polymer nanocomposite thin films prepared by electrochemical polymerization. J. Sci. Adv. Mater. Devices 2018, 4, 143–149. [Google Scholar] [CrossRef]
- Savin, M.; Mihailescu, C.-M.; Avramescu, V.; Dinulescu, S.; Firtat, B.; Craciun, G.; Brasoveanu, C.; Pachiu, C.; Romanitan, C.; Serban, A.-B.; et al. A New Hybrid Sensitive PANI/SWCNT/Ferrocene-Based Layer for a Wearable CO Sensor. Sensors 2021, 21, 1801. [Google Scholar] [CrossRef]
- Bibi, A.; Huang, C.-H.; Lan, Y.-X.; Chen, K.-Y.; Ji, W.-F.; Yeh, J.-M.; Santiago, K.S. Effect of Surface Morphology of Electro-spun EPAA Coatings on the H2S Sensing Performance of Corresponding Interdigitated Electrodes. J. Electrochem. Soc. 2020, 167, 117510. [Google Scholar] [CrossRef]
- Zhao, B.; Sai, T.; Rahman, A.; Levon, K. Floating-Gate Ion Sensitive Field-Effect Transistor for Chemical and Biological Sensing. MRS Proc. 2004, 828. [Google Scholar] [CrossRef]
- Padua, L.M.G.; Yeh, J.-M.; Santiago, K.S. A Novel Application of Electroactive Polyimide Doped with Gold Nanoparticles: As a Chemiresistor Sensor for Hydrogen Sulfide Gas. Polymers 2019, 11, 1918. [Google Scholar] [CrossRef]
- Qazi, R.A.; Khan, M.S.; Shah, L.A.; Ullah, R.; Kausar, A.; Khattak, R. Eco-friendly electronics, based on nanocomposites of biopolyester reinforced with carbon nanotubes: A review. Polym. Technol. Mater. 2020, 59, 928–951. [Google Scholar] [CrossRef]
- Cao, Y.; E Uhrich, K. Biodegradable and biocompatible polymers for electronic applications: A review. J. Bioact. Compat. Polym. 2018, 34, 3–15. [Google Scholar] [CrossRef]
- Gaidajis, G.; Angelakoglou, K.; Aktsoglou, D. E-waste: Environmental Problems and Current Management Engineering Science and Technology Review. J. Eng. Sci. Technol. Rev. 2010, 3, 193–199. Available online: www.jestr.org (accessed on 20 January 2023). [CrossRef]
- Tan, M.J.; Owh, C.; Chee, P.L.; Kyaw, A.K.K.; Kai, D.; Loh, X.J. Biodegradable electronics: Cornerstone for sustainable electronics and transient applications. J. Mater. Chem. C 2016, 4, 5531–5558. [Google Scholar] [CrossRef]
- Kausar, A. Eco-polymer and Carbon Nanotube Composite: Safe Technology. Handb. Ecomater. 2019, 4, 2827–2842. [Google Scholar] [CrossRef]
- Lei, T.; Guan, M.; Liu, J.; Lin, H.-C.; Pfattner, R.; Shaw, L.; McGuire, A.F.; Huang, T.-C.; Shao, L.; Cheng, K.-T.; et al. Biocompatible and totally disintegrable semiconducting polymer for ultrathin and ultralightweight transient electronics. Proc. Natl. Acad. Sci. USA 2017, 114, 5107–5112. [Google Scholar] [CrossRef]
- Irimia-Vladu, M. “Green” electronics: Biodegradable and biocompatible materials and devices for sustainable future. Chem. Soc. Rev. 2013, 43, 588–610. [Google Scholar] [CrossRef]
- Mühl, S.; Beyer, B. Bio-Organic Electronics—Overview and Prospects for the Future. Electronics 2014, 3, 444–461. [Google Scholar] [CrossRef]
- Liu, H.; Jian, R.; Chen, H.; Tian, X.; Sun, C.; Zhu, J.; Yang, Z.; Sun, J.; Wang, C. Application of Biodegradable and Biocompatible Nanocomposites in Electronics: Current Status and Future Directions. Nanomaterials 2019, 9, 950. [Google Scholar] [CrossRef]
- Li, R.; Wang, L.; Kong, D.; Yin, L. Recent progress on biodegradable materials and transient electronics. Bioact. Mater. 2017, 3, 322–333. [Google Scholar] [CrossRef]
- Feig, V.R.; Tran, H.; Bao, Z. Biodegradable Polymeric Materials in Degradable Electronic Devices. ACS Cent. Sci. 2018, 4, 337–348. [Google Scholar] [CrossRef]
- Kaur, T.; Kulanthaivel, S.; Thirugnanam, A.; Banerjee, I.; Pramanik, K. Biological and mechanical evaluation of poly(lactic-co-glycolic acid)-based composites reinforced with 1D, 2D and 3D carbon biomaterials for bone tissue regeneration. Biomed. Mater. 2017, 12, 025012. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Y.; Wang, T.; Ye, Z.; Zhang, H.; Dong, B.; Li, C.Y. A biodegradable, all-polymer micromotor for gas sensing applications. J. Mater. Chem. C 2016, 4, 5945–5952. [Google Scholar] [CrossRef]
- Low, K.; Chartuprayoon, N.; Echeverria, C.; Li, C.; Bosze, W.; Myung, N.V.; Nam, J. Polyaniline/poly(ε-caprolactone) composite electrospun nanofiber-based gas sensors: Optimization of sensing properties by dopants and doping concentration. Nanotechnology 2014, 25, 115501. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Guo, B.; Ma, P.X. In situ forming biodegradable electroactive hydrogels. Polym. Chem. 2013, 5, 2880–2890. [Google Scholar] [CrossRef]
- Cui, H.; Wang, Y.; Cui, L.; Zhang, P.; Wang, X.; Wei, Y.; Chen, X. In Vitro Studies on Regulation of Osteogenic Activities by Electrical Stimulus on Biodegradable Electroactive Polyelectrolyte Multilayers. Biomacromolecules 2014, 15, 3146–3157. [Google Scholar] [CrossRef]
- Hardy, J.G.; Mouser, D.J.; Arroyo-Currás, N.; Geissler, S.; Chow, J.K.; Nguy, L.; Kim, J.M.; Schmidt, C.E. Biodegradable electroactive polymers for electrochemically-triggered drug delivery. J. Mater. Chem. B 2014, 2, 6809–6822. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Zhuang, X.; Zhang, P.; Wei, Y.; Wang, X.; Chen, X. Synthesis and Characterization of Novel Biodegradable and Electroactive Hydrogel Based on Aniline Oligomer and Gelatin. Macromol. Biosci. 2011, 12, 241–250. [Google Scholar] [CrossRef]
- Ramesh, S.; Rivero, I.; Yan, J.; Downey, A.; Laflamme, S.; Zellner, E. Solventless fabrication of biodegradable sensors for measuring soft tissue deformation. IISE Annu. Conf. Expo 2018, 2018, 2062–2067. [Google Scholar]
- O’Bryan, G.; Wong, B.; McElhanon, J.R. Stress Sensing in Polycaprolactone Films via an Embedded Photochromic Compound. ACS Appl. Mater. Interfaces 2010, 2, 1594–1600. [Google Scholar] [CrossRef]
- Vanblarcom, D.S.; Peppas, N.A. Microcantilever sensing arrays from biodegradable, pH-responsive hydrogels. Biomed. Microdevices 2011, 13, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, Z.; Hu, H.; Kong, Z.; Chen, C.; Tian, Y.; Zhang, W.; Bin Ying, W.; Zhang, R.; Zhu, J. A polyurethane integrating self-healing, anti-aging and controlled degradation for durable and eco-friendly E-skin. Chem. Eng. J. 2021, 410, 128363. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Mohd Razali, N.A.; Lin, W.-C.; Yu, Z.-W.; Istiqomah, D.; Kotsuchibashi, Y.; Su, H.-H. Development of Thermo-Responsive Polycaprolactone–Polydimethylsiloxane Shrinkable Nanofibre Mesh. Nanomaterials 2020, 10, 1427. [Google Scholar] [CrossRef] [PubMed]
- Molina, B.G.; Bendrea, A.D.; Cianga, L.; Armelin, E.; del Valle, L.J.; Cianga, I.; Alemán, C. The biocompatible polythiophene-g-polycaprolactone copolymer as an efficient dopamine sensor platform. Polym. Chem. 2017, 8, 6112–6122. [Google Scholar] [CrossRef]
- Rehman, H.U.; Chen, Y.; Hedenqvist, M.S.; Pathan, R.; Liu, H.; Wang, H.; Chen, T.; Zhang, X.; Li, H. High-cycle-life and high-loading copolymer network with potential application as a soft actuator. Mater. Des. 2019, 182, 108010. [Google Scholar] [CrossRef]
- Travlou, N.A.; Seredych, M.; Rodríguez-Castellón, E.; Bandosz, T.J. Activated carbon-based gas sensors: Effects of surface features on the sensing mechanism. J. Mater. Chem. A 2014, 3, 3821–3831. [Google Scholar] [CrossRef]
- Travlou, N.A.; Ushay, C.; Seredych, M.; Rodríguez-Castellón, E.; Bandosz, T.J. Nitrogen-Doped Activated Carbon-Based Ammonia Sensors: Effect of Specific Surface Functional Groups on Carbon Electronic Properties. ACS Sens. 2016, 1, 591–599. [Google Scholar] [CrossRef]
- Park, M.-S.; Lee, S.; Jung, M.-J.; Kim, H.G.; Lee, Y.-S. NO gas sensing ability of activated carbon fibers modified by an electron beam for improvement in the surface functional group. Carbon Lett. 2016, 20, 19–25. [Google Scholar] [CrossRef]
- Travlou, N.A.; Rodríguez-Castellón, E.; Bandosz, T.J. Sensing of NH3 on heterogeneous nanoporous carbons in the presence of humidity. Carbon 2016, 100, 64–73. [Google Scholar] [CrossRef]
- Khaleed, A.A.; Bello, A.; Dangbegnon, J.K.; Momodu, D.Y.; Madito, M.J.; Ugbo, F.U.; Manyala, N. Effect of activated carbon on the enhancement of CO sensing performance of NiO. J. Alloys Compd. 2017, 694, 155–162. [Google Scholar] [CrossRef]
- Gratuito, M.; Panyathanmaporn, T.; Chumnanklang, R.-A.; Sirinuntawittaya, N.; Dutta, A. Production of activated carbon from coconut shell: Optimization using response surface methodology. Bioresour. Technol. 2008, 99, 4887–4895. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-Y.; Jhuo, Y.-S.; Wu, P.-S.; Lin, C.-H.; Yu, Y.-H.; Yeh, J.-M. Electrochemical studies for the electroactivity of amine-capped aniline trimer on the anticorrosion effect of as-prepared polyimide coatings. Eur. Polym. J. 2009, 45, 485–493. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Shi, M.; Zhang, H.; Ma, P.X.; Guo, B. Electroactive anti-oxidant polyurethane elastomers with shape memory property as non-adherent wound dressing to enhance wound healing. Chem. Eng. J. 2019, 375, 121999. [Google Scholar] [CrossRef]
- Chaudhary, R.; Maji, S.; Shrestha, R.; Shrestha, R.; Shrestha, T.; Ariga, K.; Shrestha, L. Jackfruit Seed-Derived Nanoporous Carbons as the Electrode Material for Supercapacitors. C 2020, 6, 73. [Google Scholar] [CrossRef]
- Ho, Z.H.; Adnan, L.A. Phenol Removal from Aqueous Solution by Adsorption Technique Using Coconut Shell Activated Carbon. Trop. Aquat. Soil Pollut. 2021, 1, 98–107. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, L.-Y.; Liang, Y.; Chen, Q.; Li, Z.-S.; Li, C.-H.; Wang, Z.-H.; Li, W. Coconut-based activated carbon fibers for efficient adsorption of various organic dyes. RSC Adv. 2018, 8, 42280–42291. [Google Scholar] [CrossRef]
- Bakti, A.I.; Gareso, P.L. Characterization of Active Carbon Prepared from Coconuts Shells using FTIR, XRD and SEM Techniques. J. Ilm. Pendidik. Fis. Al-Biruni 2018, 7, 33–39. [Google Scholar] [CrossRef]
- Shrestha, R.; Chaudhary, R.; Shrestha, T.; Tamrakar, B.; Shrestha, R.; Maji, S.; Hill, J.; Ariga, K.; Shrestha, L. Nanoarchitectonics of Lotus Seed Derived Nanoporous Carbon Materials for Supercapacitor Applications. Materials 2020, 13, 5434. [Google Scholar] [CrossRef]
- Song, C.; Wu, S.; Cheng, M.; Tao, P.; Shao, M.; Gao, G. Adsorption Studies of Coconut Shell Carbons Prepared by KOH Activation for Removal of Lead(II) From Aqueous Solutions. Sustainability 2013, 6, 86–98. [Google Scholar] [CrossRef]
- Baheiraei, N.; Yeganeh, H.; Ai, J.; Gharibi, R.; Azami, M.; Faghihi, F. Synthesis, characterization and antioxidant activity of a novel electroactive and biodegradable polyurethane for cardiac tissue engineering application. Mater. Sci. Eng. C 2014, 44, 24–37. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Yang, Y.; Yu, H.; Dong, X. Highly sensitive H2S sensors based on metal-organic framework driven γ-Fe2O3 on reduced graphene oxide composites at room temperature. Sens. Actuators B Chem. 2020, 325, 8804. [Google Scholar] [CrossRef]
- Bibi, A.; Rubio, Y.R.M.; Xian-Lun, L.; Sathishkumar, N.; Chen, C.-Y.; Santiago, K.S.; Chen, H.-T.; Lin, Y.-F.; Yeh, J.-M. Detection of hydrogen sulfide using polyaniline incorporated with graphene oxide aerogel. Synth. Met. 2021, 282, 116934. [Google Scholar] [CrossRef]
- Ali, I.M.; Shano, A.M.; Bakr, N.A. H2S gas sensitivity of PAni nano fibers synthesized by hydrothermal method. J. Mater. Sci. Mater. Electron. 2018, 29, 11208–11214. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, X.; Wu, X.; Cui, H.; Chen, D. Investigation of Gas-Sensing Property of Acid-Deposited Polyaniline Thin-Film Sensors for Detecting H2S and SO2. Sensors 2016, 16, 1889. [Google Scholar] [CrossRef]
- Raut, B.; Godse, P.; Pawar, S.; Chougule, M.; Bandgar, D.; Patil, V. Novel method for fabrication of polyaniline–CdS sensor for H2S gas detection. Measurement 2012, 45, 94–100. [Google Scholar] [CrossRef]
- Mousavi, S.; Kang, K.; Park, J.; Park, I. A room temperature hydrogen sulfide gas sensor based on electrospun polyaniline–polyethylene oxide nanofibers directly written on flexible substrates. RSC Adv. 2016, 6, 104131–104138. [Google Scholar] [CrossRef]
- Cheng, Z.X.; Ren, X.H.; Xu, J.Q.; Pan, Q.Y. Mesoporous In2O3: Effect of Material Structure on the Gas Sensing. J. Nanomater. 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Agbor, N.; Petty, M.; Monkman, A. Polyaniline thin films for gas sensing. Sens. Actuators B Chem. 1995, 28, 173–179. [Google Scholar] [CrossRef]





| Conductivity (S/cm) | ||||
|---|---|---|---|---|
| Substrate | Thickness (mm) | Dopant | Un-Doped Doped | |
| PUU-AC0 | 0.194 | HCl | 6.95 × 10−7 | 1.60 × 10−6 |
| PUU-AC1 | 0.124 | HCl | 8.46 × 10−7 | 3.04 × 10−6 |
| PUU-AC3 | 0.134 | HCl | 9.94 × 10−7 | 3.7 × 10−6 |
| PUU-AC0 | 0.005 | H2S gas (20 ppm) | 1.22 × 10−5 | 6.22 × 10−5 |
| PUU-AC1 | 0.005 | H2S gas (20 ppm) | 7.92 × 10−5 | 7.1 × 10−4 |
| PUU-AC3 | 0.008 | H2S gas (20 ppm) | 3.6 × 10−4 | 3.80 × 10−3 |
| Glass (reference) | 2.09 × 10−6 | |||
| Material | Operating Temperature (°C) | Response Time (s) | Recovery Time (s) | Sensitivity (ppm−1) | Concentration (ppm) | Reference |
|---|---|---|---|---|---|---|
| PANI-NFs | 30 | 0.824 | 0.839 | 3.2 % | 25 | [73] |
| PANI-SSA | 5.6% | 25 µL/L | [74] | |||
| PANI-HCl/SSA | 34.5% | 25 µL/L | [74] | |||
| PANI-CdS | RT | 71 | 345 | 15% | 20 | [75] |
| PANI-PEO NFs | RT | 120 | 250 | 25% | 10 | [76] |
| PANI/ZnO | RT | 63 | 40.5% | 50 | [12] | |
| SnO2/rGO/PANI | RT | 80 | 88 | 80% | 5 | [23] |
| PUU-AC3 | RT | 32 | 315 | 0.2724 | 20 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bibi, A.; Santiago, K.S.; Yeh, J.-M.; Chen, H.-H. Valorization of Agricultural Waste as a Chemiresistor H2S-Gas Sensor: A Composite of Biodegradable-Electroactive Polyurethane-Urea and Activated-Carbon Composite Derived from Coconut-Shell Waste. Polymers 2023, 15, 685. https://doi.org/10.3390/polym15030685
Bibi A, Santiago KS, Yeh J-M, Chen H-H. Valorization of Agricultural Waste as a Chemiresistor H2S-Gas Sensor: A Composite of Biodegradable-Electroactive Polyurethane-Urea and Activated-Carbon Composite Derived from Coconut-Shell Waste. Polymers. 2023; 15(3):685. https://doi.org/10.3390/polym15030685
Chicago/Turabian StyleBibi, Aamna, Karen S. Santiago, Jui-Ming Yeh, and Hsiu-Hui Chen. 2023. "Valorization of Agricultural Waste as a Chemiresistor H2S-Gas Sensor: A Composite of Biodegradable-Electroactive Polyurethane-Urea and Activated-Carbon Composite Derived from Coconut-Shell Waste" Polymers 15, no. 3: 685. https://doi.org/10.3390/polym15030685
APA StyleBibi, A., Santiago, K. S., Yeh, J.-M., & Chen, H.-H. (2023). Valorization of Agricultural Waste as a Chemiresistor H2S-Gas Sensor: A Composite of Biodegradable-Electroactive Polyurethane-Urea and Activated-Carbon Composite Derived from Coconut-Shell Waste. Polymers, 15(3), 685. https://doi.org/10.3390/polym15030685







