Molecular Weight-Dependent Oxidation and Optoelectronic Properties of Defect-Free Macrocyclic Poly(3-hexylthiophene)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.3. Analytical Size Exclusion Chromatography (SEC)
2.4. Preparative SEC
2.5. Matrix-Assisted Laser Desorption Ionization Time-of-Flight (MALDI-TOF) Mass Spectrometry
2.6. UV–Vis and UV–Vis–NIR Spectroscopies
2.7. Raman Spectroscopy
2.8. Scanning Tunneling Microscope (STM)
2.9. Cyclic Voltammetry (CV)
2.10. Spectroelectrochemistry
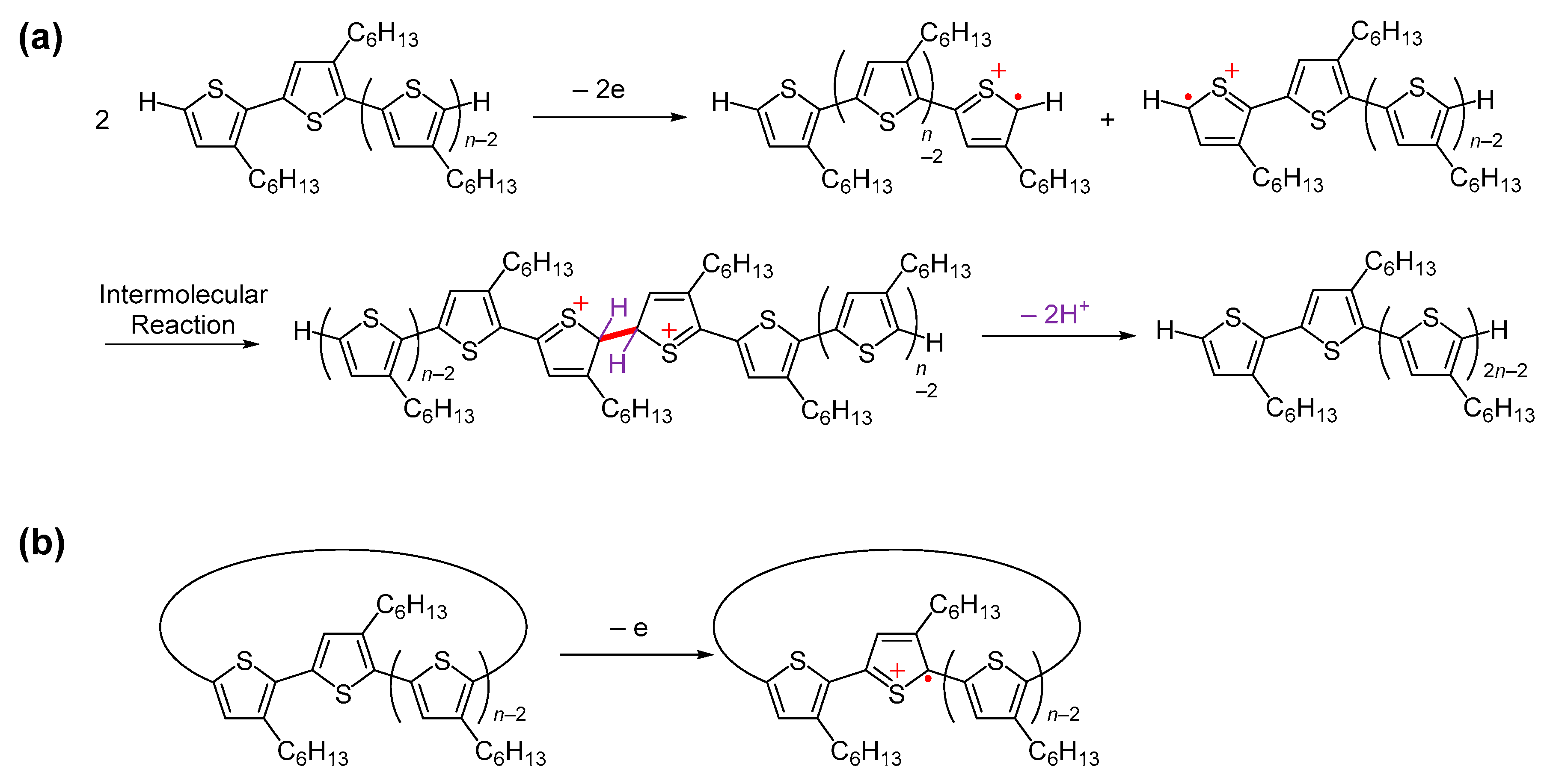
2.11. Preparation of Radical Cation (P3HT•+) and Dication (P3HT2+)
2.12. Electron Spin Resonance (ESR) Spectroscopy
2.13. Data Fitting of ESR Spectra
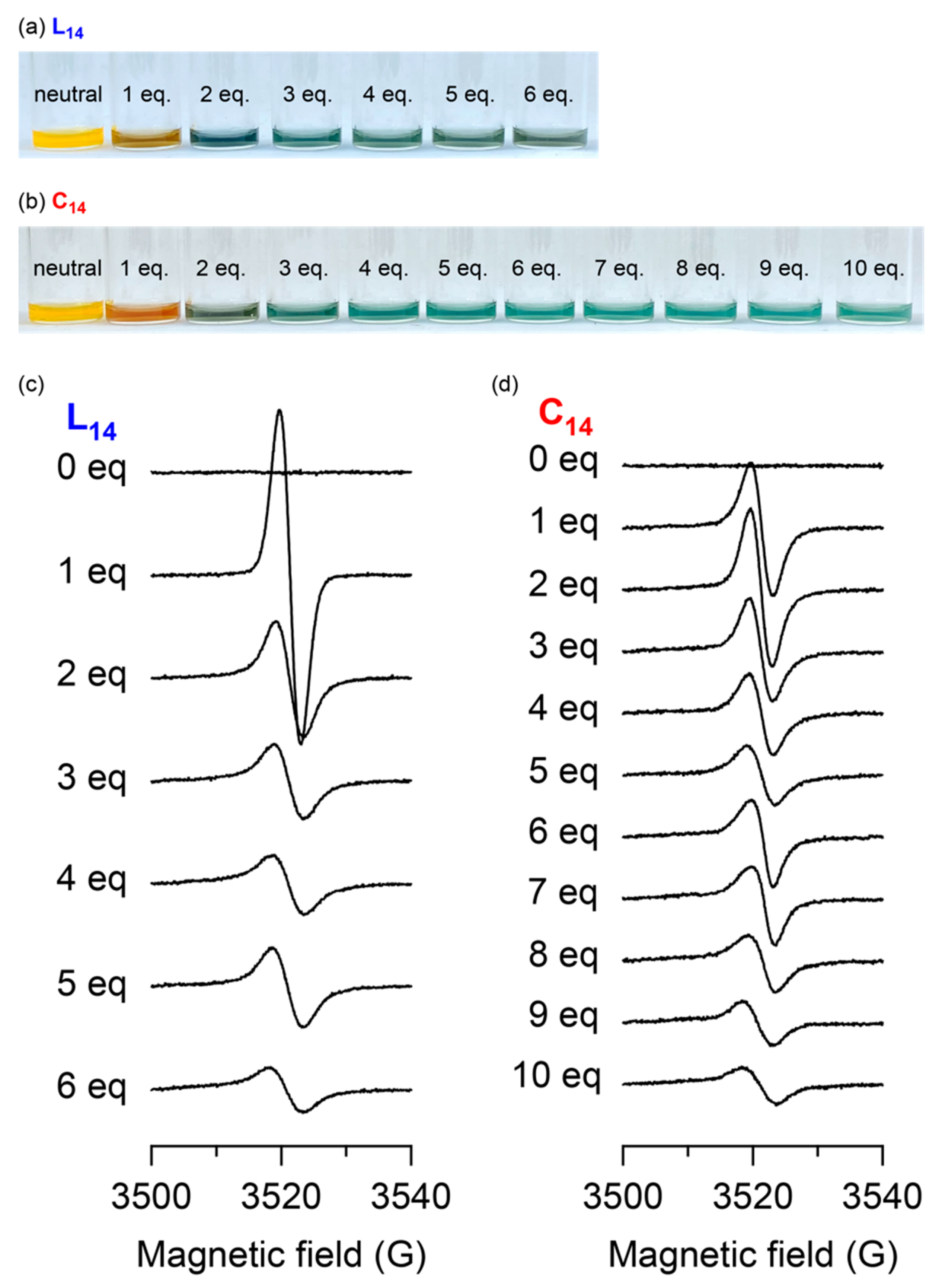
3. Results and Discussion
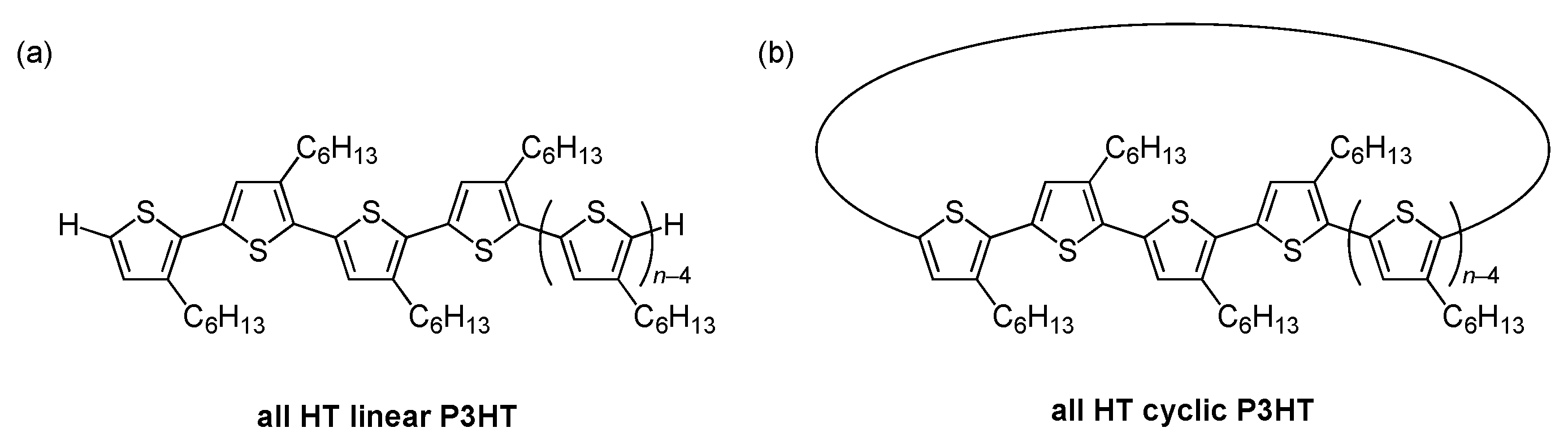
3.1. Synthesis
3.2. SEC
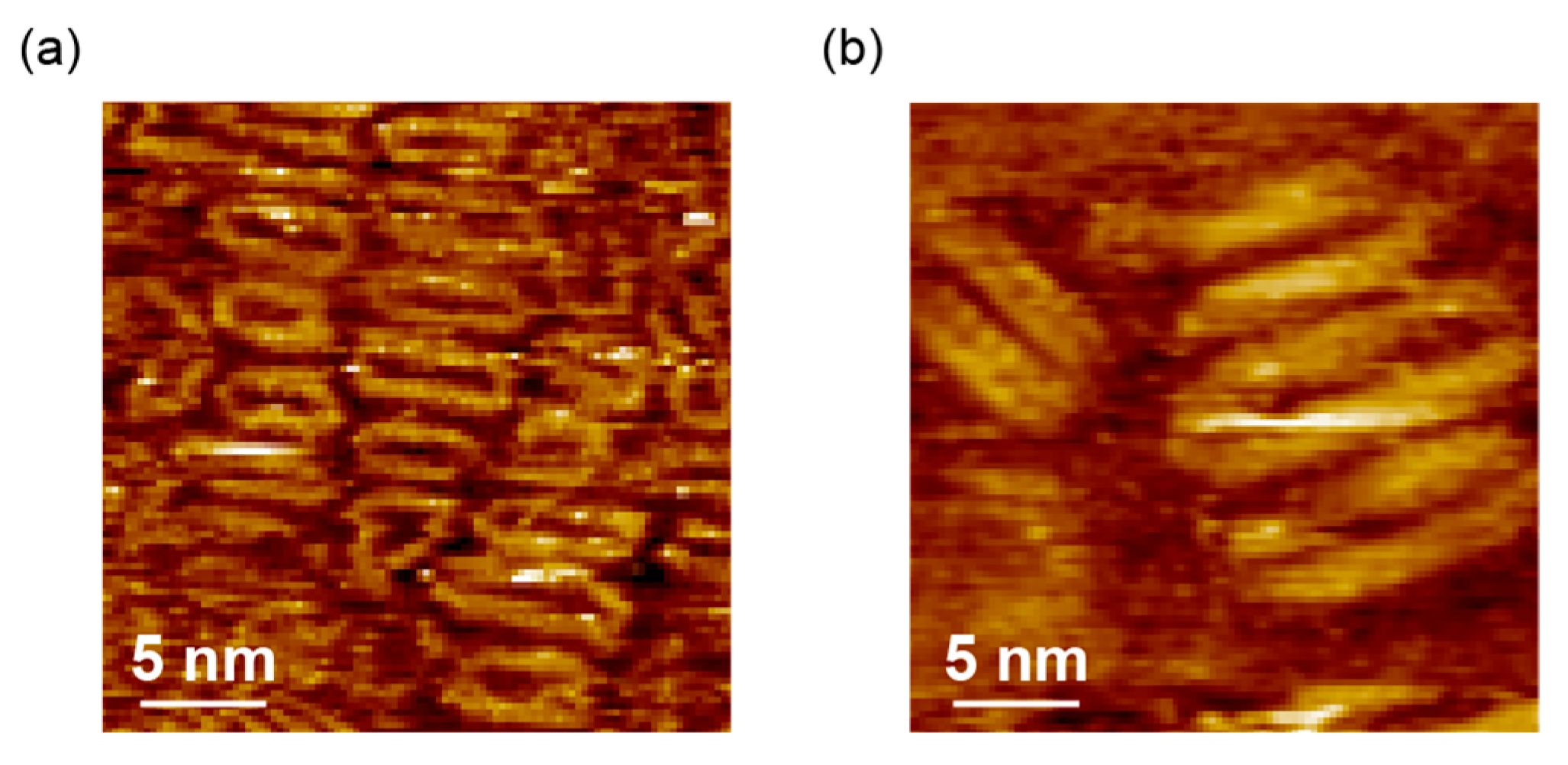
3.3. STM
3.4. Raman Spectroscopy
3.5. UV–Vis Spectroscopy
3.6. CV
3.7. Spectroelectrochemistry
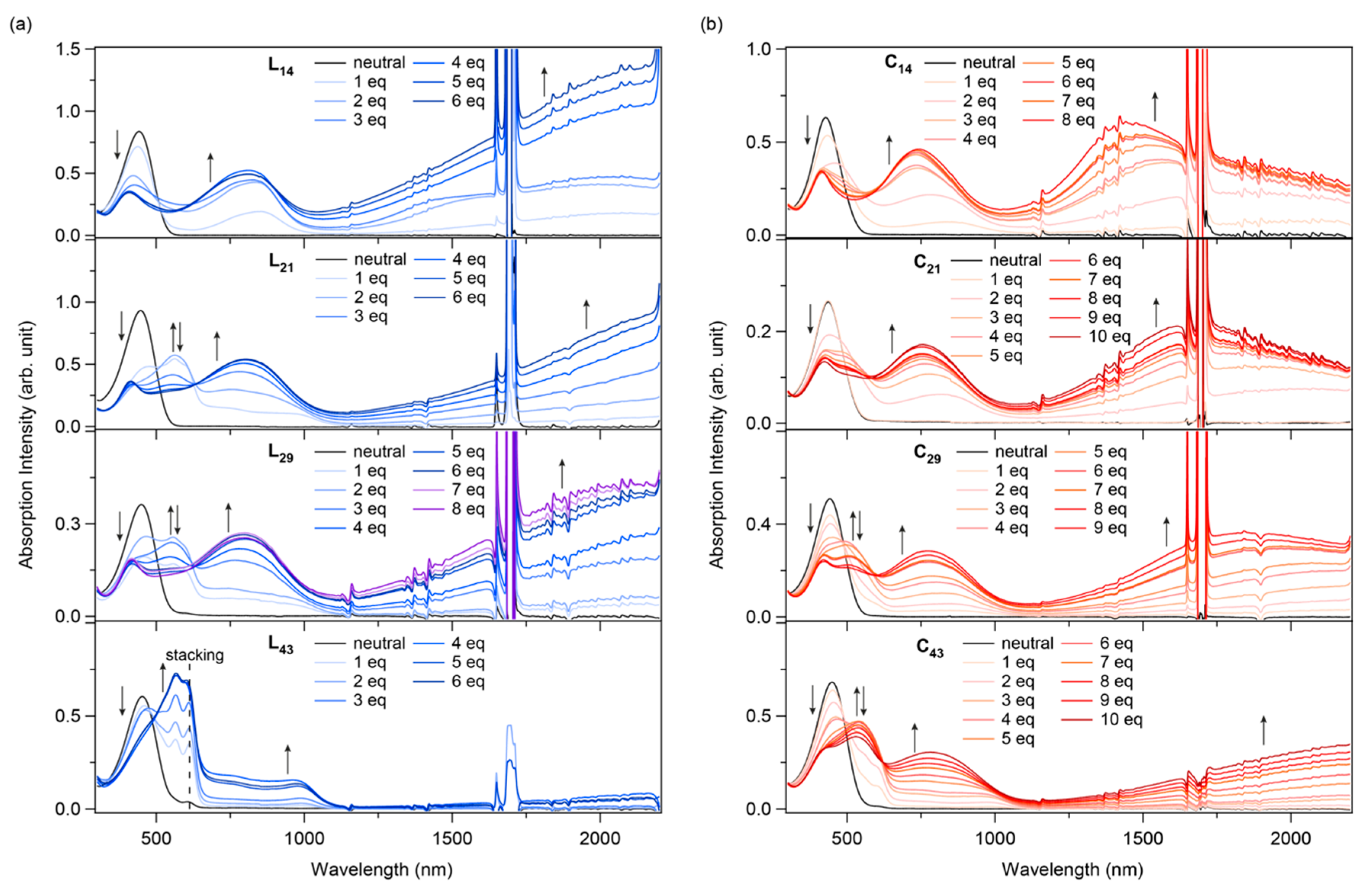
3.8. UV–Vis–NIR Spectroscopy of Chemically Oxidized P3HT
3.9. ESR Spectroscopy
3.10. VT 1H NMR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iyoda, M.; Shimizu, H. Multifunctional π-expanded oligothiophene macrocycles. Chem. Soc. Rev. 2015, 44, 6411–6424. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.E. Cycloparaphenylenes and related nanohoops. Chem. Soc. Rev. 2015, 44, 2221–2304. [Google Scholar] [CrossRef] [PubMed]
- Bednarz, M.; Reineker, P.; Mena-Osteritz, E.; Bäuerle, P. Optical absorption spectra of linear and cyclic thiophenes-selection rules manifestation. J. Lumin. 2004, 110, 225–231. [Google Scholar] [CrossRef]
- Kim, W.; Sung, J.; Park, K.H.; Shimizu, H.; Imamura, M.; Han, M.; Sim, E.; Iyoda, M.; Kim, D. The role of linkers in the excited-state dynamic planarization processes of macrocyclic oligothiophene 12-mers. J. Phys. Chem. Lett. 2015, 6, 4444–4450. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Park, K.H.; Kim, W.; Tamachi, T.; Iyoda, M.; Kim, D. Relationship between dynamic planarization processes and exciton delocalization in cyclic oligothiophenes. J. Phys. Chem. Lett. 2015, 6, 451–456. [Google Scholar] [CrossRef]
- Ito, H.; Mitamura, Y.; Segawa, Y.; Itami, K. Thiophene-based, radial π-conjugation: Synthesis, structure, and photophysical properties of cyclo-1,4-phenylene-2’,5’-thienylenes. Angew. Chem. Int. Ed. 2015, 54, 159–163. [Google Scholar] [CrossRef]
- Oh, J.; Joung, H.; Kim, W.; Yang, J.; Kim, D. Impact of cyclic strain on the structural relaxation dynamics of macrocyclic thiophenes. J. Phys. Chem. C 2021, 125, 1947–1953. [Google Scholar] [CrossRef]
- Miyazawa, Y.; Wang, Z.; Matsumoto, M.; Hatano, S.; Antol, I.; Kayahara, E.; Yamago, S.; Abe, M. 1,3-Diradicals embedded in curved paraphenylene units: Singlet versus triplet state and in-plane aromaticity. J. Am. Chem. Soc. 2021, 143, 7426–7439. [Google Scholar] [CrossRef]
- Dishi, O.; Gidron, O. Macrocyclic oligofurans: A computational study. J. Org. Chem. 2018, 83, 3119–3125. [Google Scholar] [CrossRef]
- Kayahara, E.; Kouyama, T.; Kato, T.; Takaya, H.; Yasuda, N.; Yamago, S. Isolation and characterization of the cycloparaphenylene radical cation and dication. Angew. Chem. Int. Ed. 2013, 52, 13722–13726. [Google Scholar] [CrossRef]
- Kayahara, E.; Kouyama, T.; Kato, T.; Yamago, S. Synthesis and characterization of [n] CPP (n = 5, 6, 8, 10, and 12) radical cation and dications: Size-dependent absorption, spin, and charge delocalization. J. Am. Chem. Soc. 2016, 138, 338–344. [Google Scholar] [CrossRef]
- Iyoda, M.; Tanaka, K.; Shimizu, H.; Hasegawa, M.; Nishinaga, T.; Nishiuchi, T.; Kunugi, Y.; Ishida, T.; Otani, H.; Sato, H.; et al. Multifunctional π-expanded macrocyclic oligothiophene 6-mers and related macrocyclic oligomers. J. Am. Chem. Soc. 2014, 136, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Muranaka, A.; Nishinaga, T.; Aoyagi, S.; Kobayashi, N.; Uchiyama, M.; Otani, H.; Iyoda, M. Preparation, spectroscopic characterization and theoretical study of a three-dimensional conjugated 70 π-electron thiophene 6-mer radical cation π-dimer. J. Am. Chem. Soc. 2020, 142, 5933–5937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Götz, G.; Mena-Osteritz, E.; Weil, M.; Sarkar, B.; Kaim, W.; Bäuerle, P. Molecular and electronic structure of cyclo[10]thiophene in various oxidation states: Polaron pair vs. bipolaron. Chem. Sci. 2011, 2, 781–784. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hosokawa, M.; Nakamura, M.; Sato, S.; Isono, T.; Tajima, K.; Satoh, T.; Sato, M.; Tezuka, Y.; Saeki, A.; et al. Synthesis, isolation, and properties of all head-to-tail cyclic poly(3-hexylthiophene): Fully delocalized exciton over the defect-free ring polymer. Macromolecules 2018, 51, 9284–9293. [Google Scholar] [CrossRef]
- Hestand, N.J.; Spano, F.C. The effect of chain bending on the photophysical properties of conjugated polymers. J. Phys. Chem. B 2014, 118, 8352–8363. [Google Scholar] [CrossRef]
- Koch, F.P.V.; Rivnay, J.; Foster, S.; Muller, C.; Downing, J.M.; Buchaca-Domingo, E.; Westacott, P.; Yu, L.Y.; Yuan, M.J.; Baklar, M.; et al. The impact of molecular weight on microstructure and charge transport in semicrystalline polymer semiconductors poly(3-hexylthiophene), a model study. Prog. Polym. 2013, 38, 1978–1989. [Google Scholar] [CrossRef]
- Leonat, L.; Sbarcea, G.; Branzoi, I.V. Cyclic voltammetry for energy levels estimation of organic materials. UPB Sci. Bull. 2013, 75, 111–118. [Google Scholar]
- Zade, S.S.; Bendikov, M. Cyclic oligothiophenes: Novel organic materials and models for polythiophene. A theoretical study. J. Org. Chem. 2006, 71, 2972–2981. [Google Scholar] [CrossRef]
- Zhang, F.; Götz, G.; Winkler, H.D.F.; Schalley, C.A.; Bäuerle, P. Giant cyclo[n]thiophenes with extended π conjugation. Angew. Chem. Int. Ed. 2009, 48, 6632–6635. [Google Scholar] [CrossRef] [PubMed]
- Roncali, J. Conjugated poly(thiophenes): Synthesis, functionalization, and applications. Chem. Rev. 1992, 92, 711–738. [Google Scholar] [CrossRef]
- Kepska, K.; Jarosz, T.; Januszkiewicz-Kaleniak, A.; Domagala, W.; Lapkowski, M.; Stolarczyk, A. Spectroelectrochemistry of poly(3-hexylthiophenes) in solution. Chem. Pap. 2018, 72, 251–259. [Google Scholar] [CrossRef]
- Dian, G.; Barbey, G.; Decroix, B. Electrochemical synthesis of polythiophenes and polyselenophenes. Synth. Met. 1986, 13, 281–289. [Google Scholar] [CrossRef]
- Trznadel, M.; Pron, A.; Zagorska, M. Effect of molecular weight on spectroscopic and spectroelectrochemical properties of regioregular poly(3-hexylthiophene). Macromolecules 1998, 31, 5051–5058. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Watanabe, Y.; Sakamoto, Y.; Suzuki, T.; Yamago, S. Selective and random syntheses of [n]cycloparaphenylenes (n = 8–13) and size dependence of their electronic properties. J. Am. Chem. Soc. 2011, 133, 8354–8361. [Google Scholar] [CrossRef] [PubMed]
- Takaba, H.; Omachi, H.; Yamamoto, Y.; Bouffard, J.; Itami, K. Selective synthesis of [12]cycloparaphenylene. Angew. Chem. Int. Ed. 2009, 48, 6112–6116. [Google Scholar] [CrossRef] [PubMed]
- Segawa, Y.; Fukazawa, A.; Matsuura, S.; Omachi, H.; Yamaguchi, S.; Irle, S.; Itami, K. Combined experimental and theoretical studies on the photophysical properties of cycloparaphenylenes. Org. Biomol. Chem. 2012, 10, 5979–5984. [Google Scholar] [CrossRef] [PubMed]
- Cházaro-Ruiz, L.F.; Kellenberger, A.; Dunsch, L. In situ ESR/UV-vis-NIR and ATR-FTIR spectroelectrochemical studies on the p-doping of copolymers of 3-methylthiophene and 3-hexylthiophene. J. Phys. Chem. B 2009, 113, 2310–2316. [Google Scholar] [CrossRef]
- Park, Y.D.; Lee, H.S.; Choi, Y.J.; Kwak, D.; Cho, J.H.; Lee, S.; Cho, K. Solubility-induced ordered polythiophene precursors for high-performance organic thin-film transistors. Adv. Funct. Mater. 2009, 19, 1200–1206. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Sahalianov, I.; Hynynen, J.; Barlow, S.; Marder, S.R.; Müller, C.; Zozoulenko, I. UV-to-IR absorption of molecularly p-doped polythiophenes with alkyl and oligoether side chains: Experiment and interpretation based on density functional theory. J. Phys. Chem. B 2020, 124, 11280–11293. [Google Scholar] [CrossRef]
- Tourillon, G.; Garnier, F. Effect of dopant on the physicochemical and electrical properties of organic conducting polymers. J. Phys. Chem. 1983, 87, 2289–2292. [Google Scholar] [CrossRef]
- Heimel, G. The optical signature of charges in conjugated polymers. ACS. Cent. Sci. 2016, 2, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Fichou, D.; Horowitz, G.; Xu, B.; Garnier, F. Stoichiometric control of the successive generation of the radical cation and dication of extended a-conjugated oligothiophenes—A quantitative model for doped polythiophene. Synth. Met. 1990, 39, 243–259. [Google Scholar] [CrossRef]
- Sato, M.A.; Hiroi, M. Oxidized states of soluble oligothiophenes and polythiophenes. Polymer 1996, 37, 1685–1689. [Google Scholar] [CrossRef]
- Poole, C.P.; Farach, H.A. Line shapes in electron spin resonance. Bull. Magn. Resn. 1979, 1, 162–194. [Google Scholar]
- Enengl, C.; Enengl, S.; Pluczyk, S.; Havlicek, M.; Lapkowski, M.; Neugebauer, H.; Ehrenfreund, E. Doping-induced absorption bands in P3HT: Polarons and bipolarons. ChemPhysChem 2016, 17, 3836–3844. [Google Scholar] [CrossRef]




| Mn,SEC (g·mol–1) a | Mp,SEC (g·mol–1) a | Mw/Mn | Mp,C/Mp,L | |
|---|---|---|---|---|
| L14 | 2300 | 2400 | 1.07 | 0.92 |
| C14 | 2200 | 2200 | 1.03 | |
| L21 | 3500 | 3500 | 1.05 | 0.83 |
| C21 | 2900 | 2900 | 1.04 | |
| L26 | 4300 | 4100 | 1.08 | 0.76 |
| C26 | 3100 | 3100 | 1.05 | |
| L29 | 5100 | 5300 | 1.17 | 0.68 |
| C29 | 3900 | 3600 | 1.08 | |
| L43 | 7200 | 8700 | 1.13 | 0.71 |
| C43 | 5700 | 6200 | 1.19 |
| Abs λmax (nm) a | Abs λonset (nm) a,b | (eV) b | (V) c | HOMO (eV) d | |
|---|---|---|---|---|---|
| L14 | 438 | 527 | 2.35 | 0.14 | –4.94 |
| C14 | 426 | 527 | 2.35 | 0.06 | –4.86 |
| L21 | 444 | 531 | 2.33 | 0.12 | –4.92 |
| C21 | 433 | 528 | 2.35 | 0.09 | –4.89 |
| L26 | 446 | 534 | 2.32 | 0.10 | –4.90 |
| C26 | 438 | 530 | 2.34 | 0.10 | –4.90 |
| L29 | 448 | 534 | 2.32 | 0.10 | –4.90 |
| C29 | 441 | 533 | 2.33 | 0.11 | –4.91 |
| L43 | 452 | 537 | 2.31 | 0.07 | –4.91 |
| C43 | 448 | 538 | 2.30 | 0.12 | –4.89 |
| Transition (nm) | ||||
|---|---|---|---|---|
| TN a | TC b | TP/B + C c | TP/B d | |
| L14 | 442 | - | 815 | >2200 |
| C14 | 428 | - | 742 | 1440 |
| L21 | 447 | 562 | 766 | >2200 |
| C21 | 437 | about 500 | 755 | about 1700 |
| L29 | 450 | 551 | 797 | >2200 |
| C29 | 442 | 508 | 773 | 1850 |
| L43 | 453 | 566 | - | - |
| C43 | 449 | 532 | 782 | >2200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, R.; Utagawa, A.; Fushimi, K.; Li, F.; Isono, T.; Tajima, K.; Satoh, T.; Sato, S.-i.; Hirata, H.; Kikkawa, Y.; et al. Molecular Weight-Dependent Oxidation and Optoelectronic Properties of Defect-Free Macrocyclic Poly(3-hexylthiophene). Polymers 2023, 15, 666. https://doi.org/10.3390/polym15030666
Sato R, Utagawa A, Fushimi K, Li F, Isono T, Tajima K, Satoh T, Sato S-i, Hirata H, Kikkawa Y, et al. Molecular Weight-Dependent Oxidation and Optoelectronic Properties of Defect-Free Macrocyclic Poly(3-hexylthiophene). Polymers. 2023; 15(3):666. https://doi.org/10.3390/polym15030666
Chicago/Turabian StyleSato, Ryohei, Atsuo Utagawa, Koji Fushimi, Feng Li, Takuya Isono, Kenji Tajima, Toshifumi Satoh, Shin-ichiro Sato, Hiroshi Hirata, Yoshihiro Kikkawa, and et al. 2023. "Molecular Weight-Dependent Oxidation and Optoelectronic Properties of Defect-Free Macrocyclic Poly(3-hexylthiophene)" Polymers 15, no. 3: 666. https://doi.org/10.3390/polym15030666
APA StyleSato, R., Utagawa, A., Fushimi, K., Li, F., Isono, T., Tajima, K., Satoh, T., Sato, S.-i., Hirata, H., Kikkawa, Y., & Yamamoto, T. (2023). Molecular Weight-Dependent Oxidation and Optoelectronic Properties of Defect-Free Macrocyclic Poly(3-hexylthiophene). Polymers, 15(3), 666. https://doi.org/10.3390/polym15030666







