Exploring the Potential of Roselle Calyx and Sappan Heartwood Extracts as Natural Colorants in Poly(butylene Succinate) for Biodegradable Packaging Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Natural Colorants
2.3. Processing of Biodegradable Films
2.4. Characterizations
2.4.1. Color Parameter Index
2.4.2. Water Vapor Transmission Rate (WVTR)
2.4.3. Light Transmittance
2.4.4. Stability
2.4.5. Surface Morphology
2.4.6. Functional Groups
2.4.7. Crystallinity
2.4.8. Tensile Properties
2.4.9. Thermal Properties
2.4.10. Decomposition Temperature
3. Results and Discussion
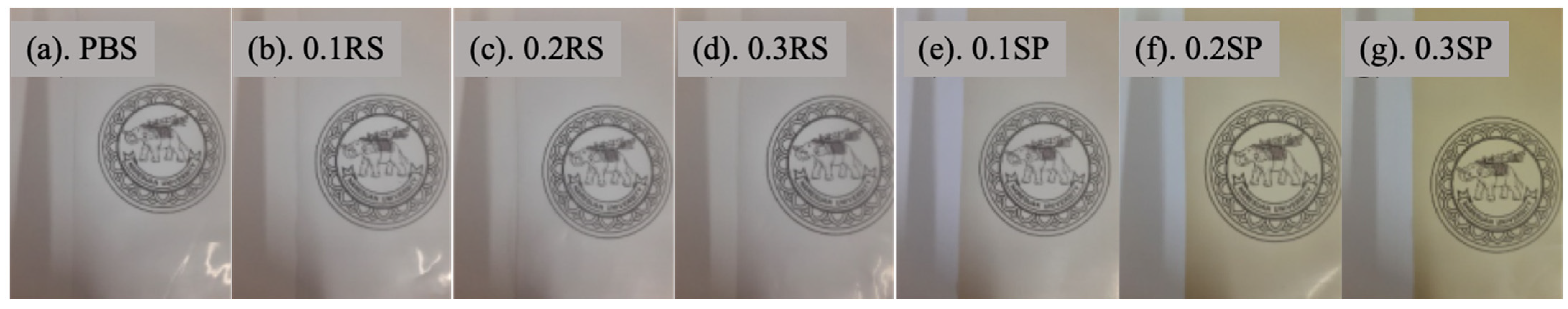
3.1. Color Parameter Index
3.2. Light Barrier Properties
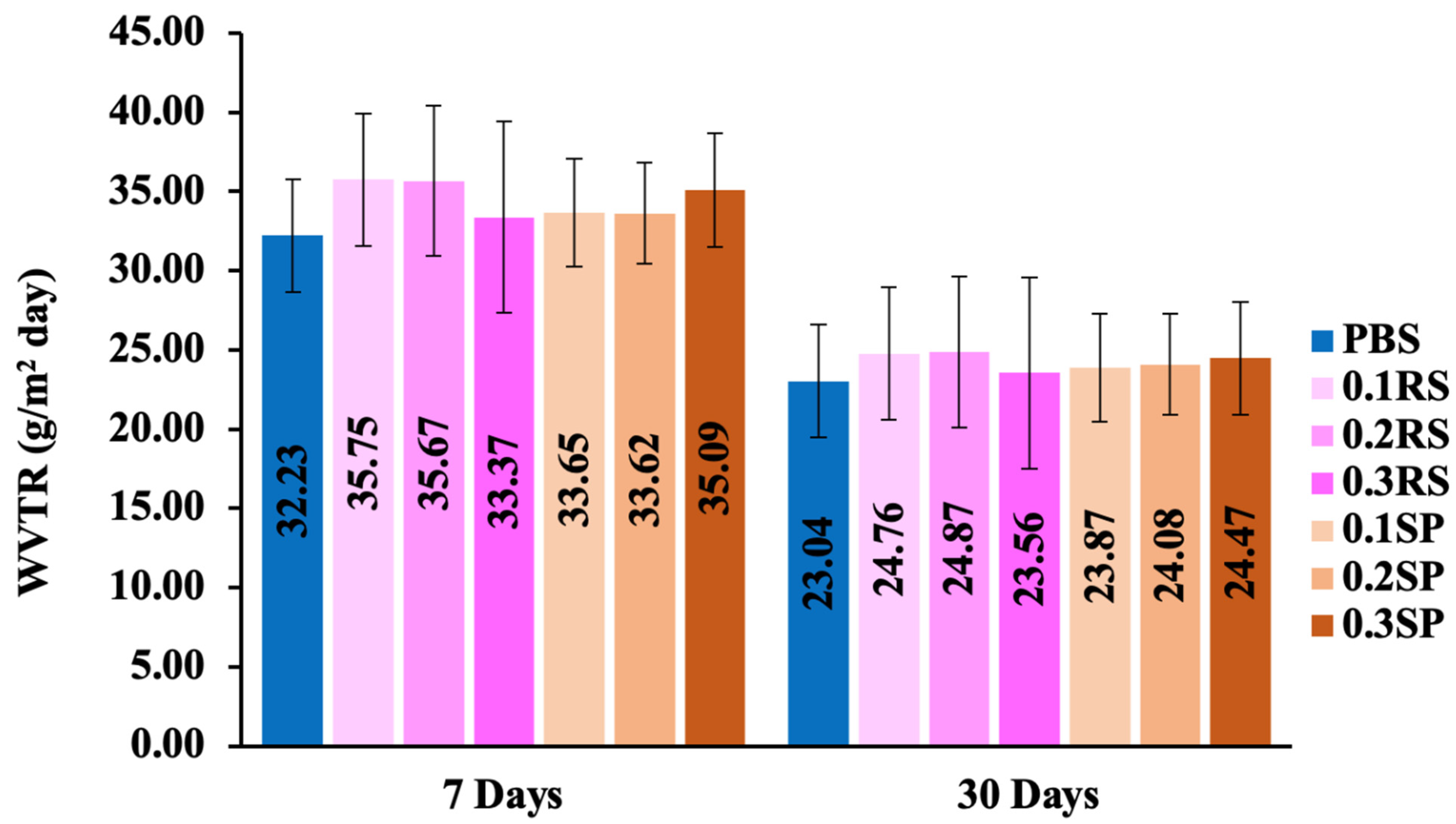
3.3. Water Vapor Transmission Rate (WVTR)
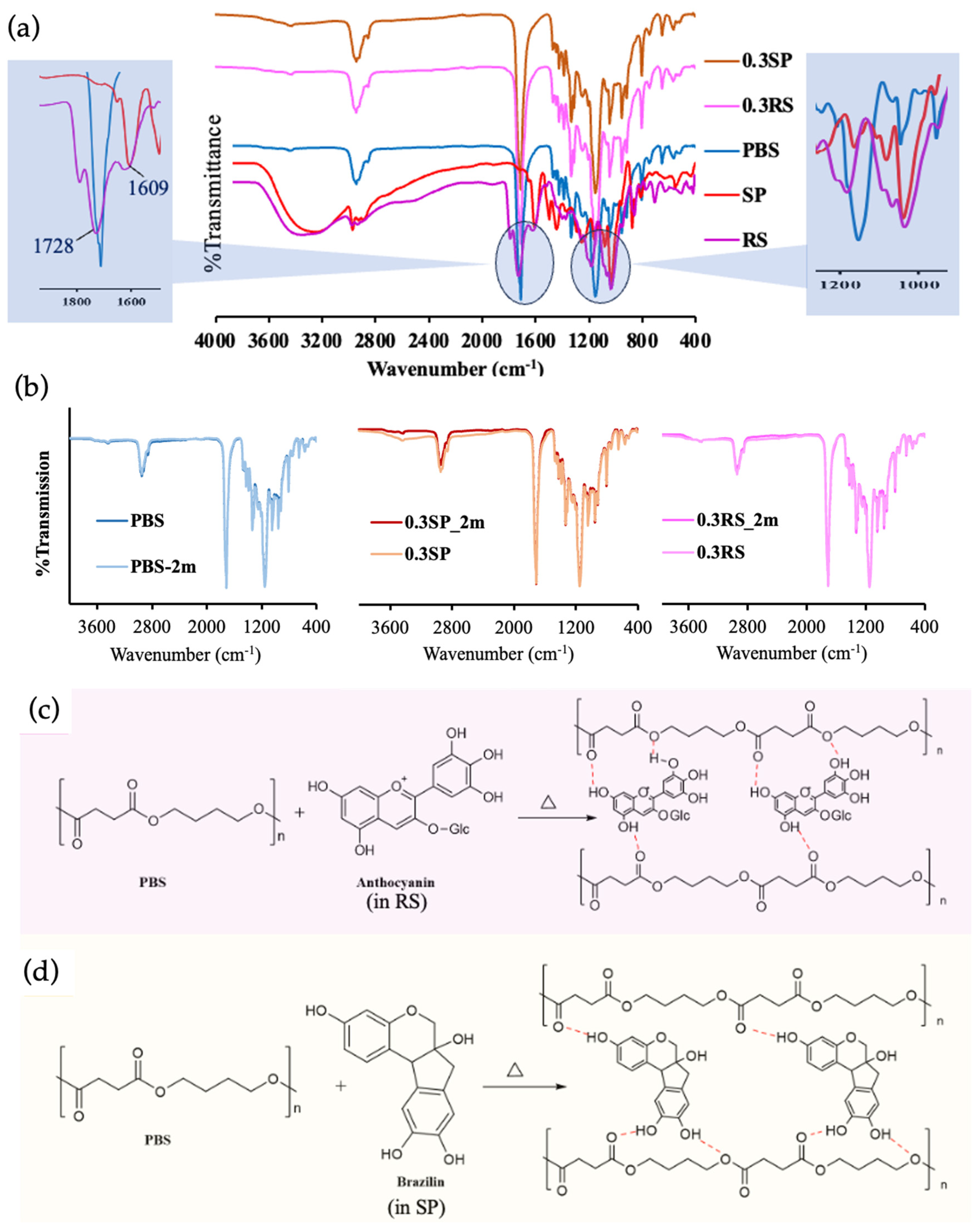
3.4. Chemical Properties
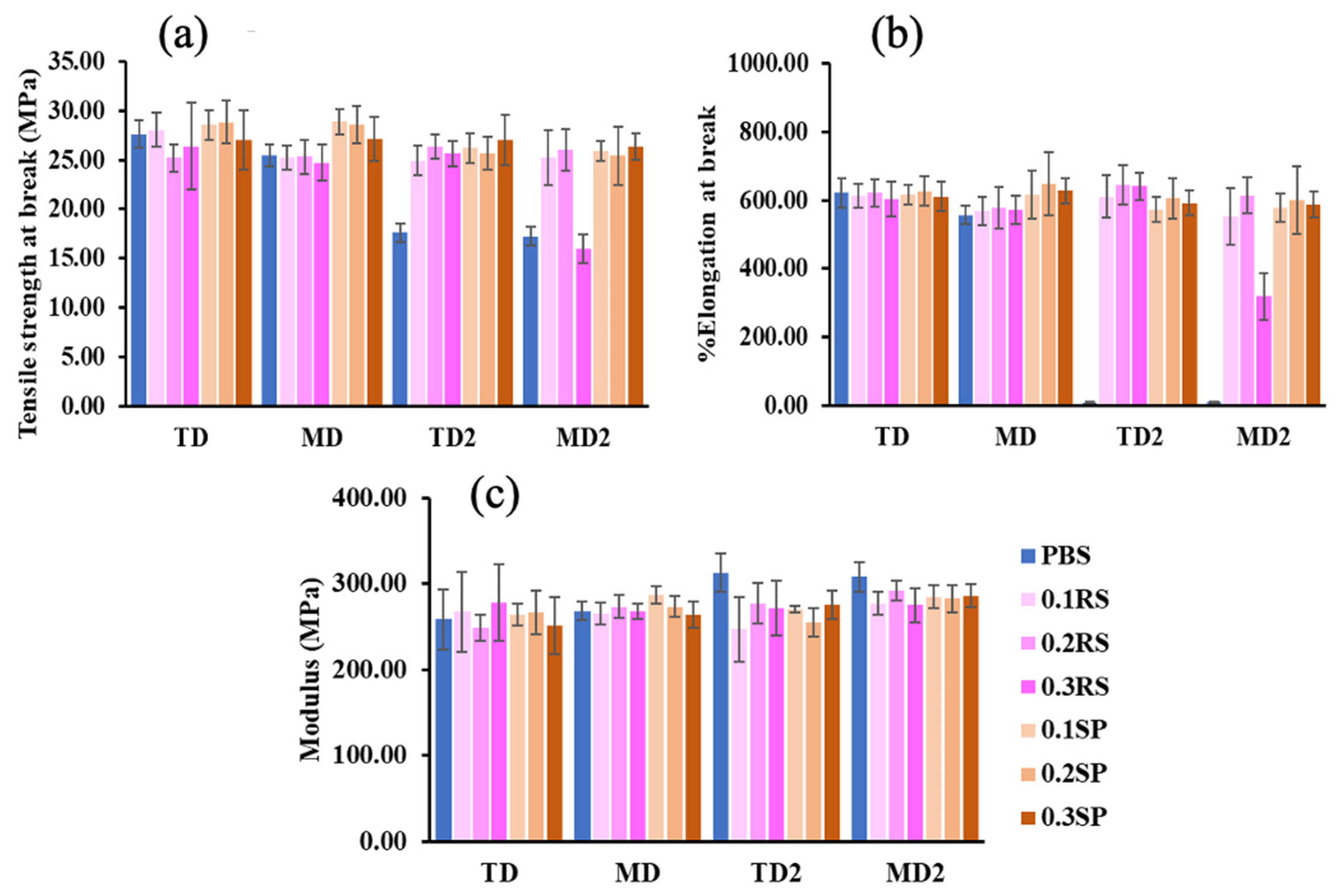
3.5. Tensile Properties
3.6. Fractured Surface Morphology
3.7. Crystallinity
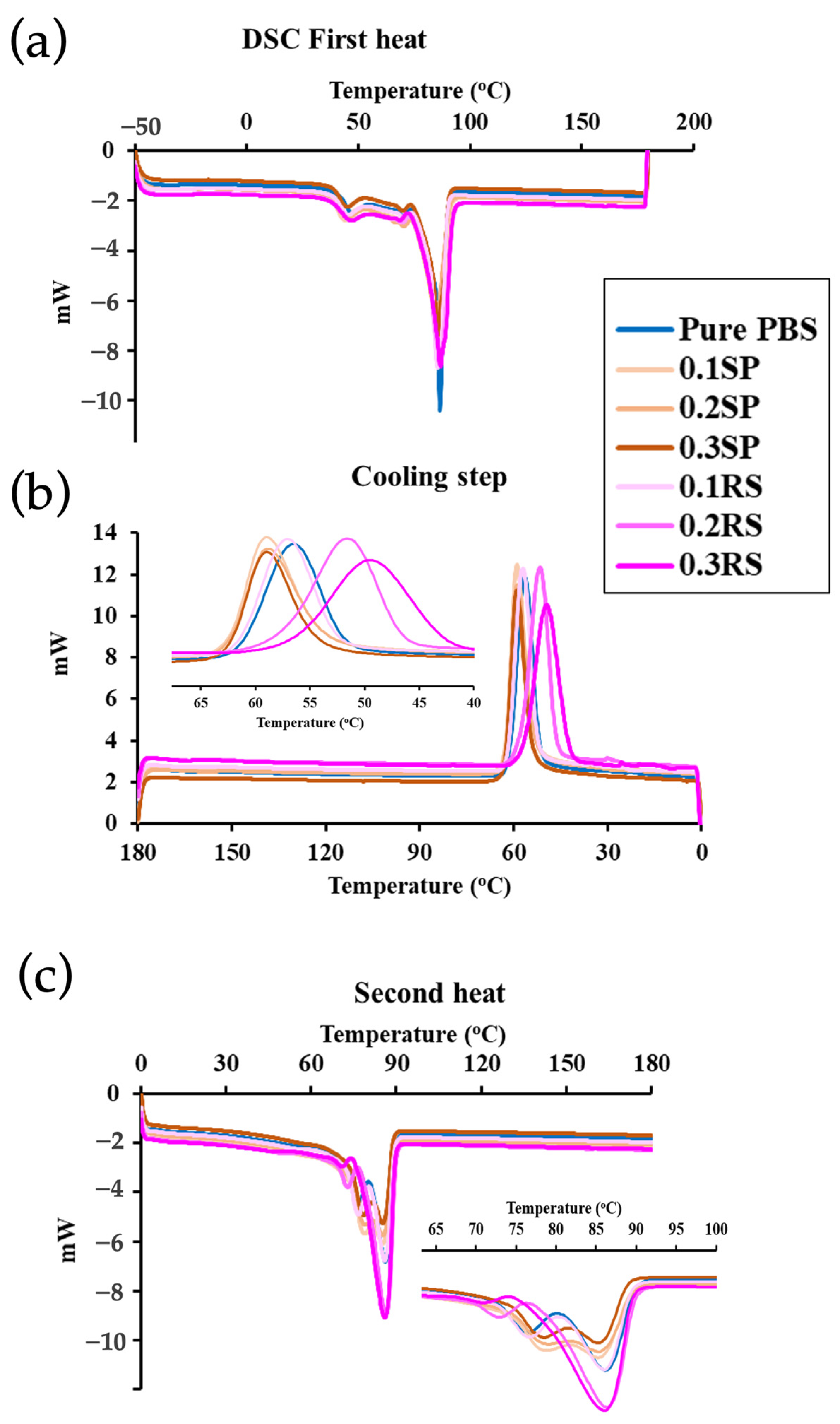
3.8. Thermal Properties
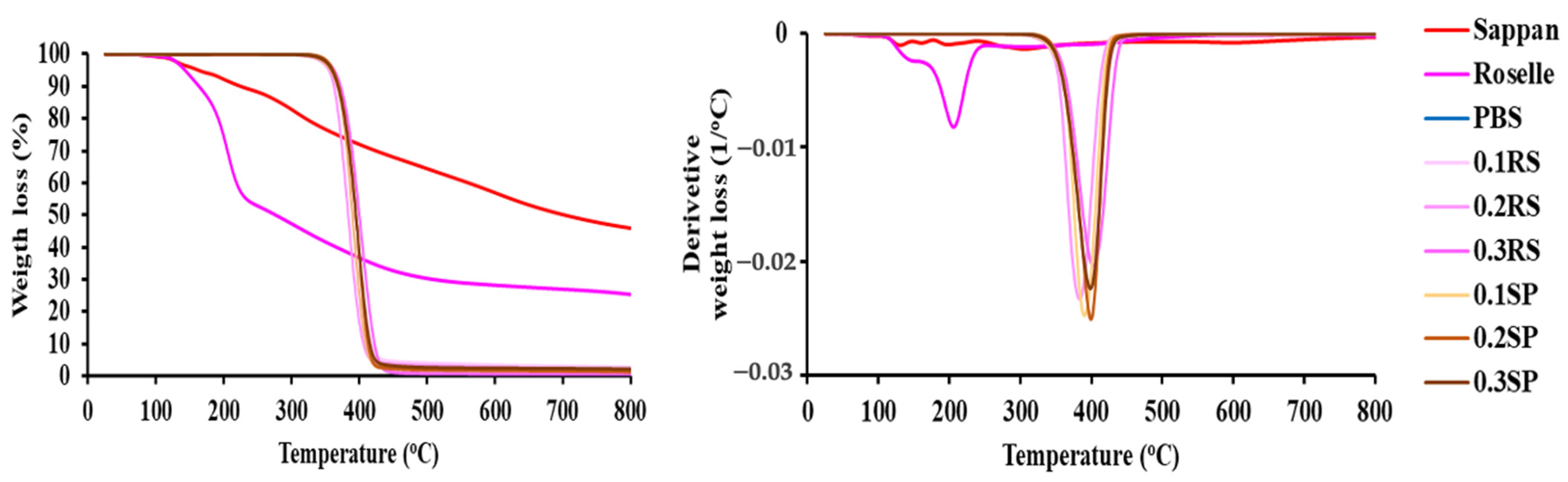
3.9. Thermal Decomposition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins, B.A.; De Albuquerque, P.B.S.; De Souz, M.P. Bio-based Films and Coatings: Sustainable Polysaccharide Packaging Alternatives for the Food Industry. J. Polym. Environ. 2022, 30, 4023–4039. [Google Scholar] [CrossRef]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of Biodegradable Polymers in Food Packaging Industry: A Comprehensive Review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. The application of natural food colorants as indicator substances in intelligent biodegradable packaging materials. Food Chem. Toxicol. 2020, 135, 110975. [Google Scholar] [CrossRef] [PubMed]
- Fisner, M.; Majer, A.; Taniguchi, S.; Bícego, M.; Turra, A.; Gorman, D. Colour spectrum and resin-type determine the concentration and composition of Polycyclic Aromatic Hydrocarbons (PAHs) in plastic pellets. Mar. Pollut. Bull. 2017, 122, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Suaduang, N.; Ross, S.; Ross, G.M.; Pratumshat, S.; Mahasaranon, S. The Physical and Mechanical Properties of Biocomposite Films Composed of Poly(lactic acid) with Spent Coffee Grounds Nattawut. Key Eng. Mater. 2019, 824, 87–93. [Google Scholar]
- Nansu, W.; Chaiwut, P.; Ross, S.; Ross, G.; Suphrom, N.; Mahasaranon, S. Developments of biodegradable polymer based on polylactic acid (PLA) with natural color extracts for packaging film applications. J. Met. Mater. Miner. 2021, 31, 127–133. [Google Scholar] [CrossRef]
- Suaduang, N.; Ross, S.; Ross, G.M.; Pratumshat, S.; Mahasaranon, S. Effect of spent coffee grounds filler on the physical and mechanical properties of poly(lactic acid) bio-composite films. Mater. Today Proc. 2019, 17, 2104–2110. [Google Scholar] [CrossRef]
- Kongprayoon, A.; Ross, G.M.; Limpeanchop, N.; Mahasaranon, S.; Punyodom, W.; Topham, P.D.; Ross, S. Bio-derived and biocompatible poly(lactic acid)/silk sericin nanogels and their incorporation within poly(lactide-co-glycolide) electrospun nanofibers. Polym. Chem. 2022, 13, 3343–3357. [Google Scholar] [CrossRef]
- Mlalila, N.; Hilonga, A.; Swai, H.; Devlieghere, F.; Ragaert, P. Antimicrobial packaging based on starch, poly(3-hydroxybutyrate) and poly(lactic-co-glycolide) materials and application challenges. Trends Food Sci. Technol. 2018, 74, 1–11. [Google Scholar] [CrossRef]
- Jost, V. Packaging related properties of commercially available biopolymers—An overview of the status quo. Express Polym. Lett. 2018, 12, 429–435. [Google Scholar] [CrossRef]
- Gigli, M.; Negroni, A.; Zanaroli, G.; Lotti, N.; Fava, F.; Munari, A. Environmentally friendly PBS-based copolyesters containing PEG-like subunit: Effect of block length on solid-state properties and enzymatic degradation. React. Funct. Polym. 2013, 73, 764–771. [Google Scholar] [CrossRef]
- Tuancharoensri, N.; Ross, G.M.; Kongprayoon, A.; Mahasaranon, S.; Pratumshat, S.; Viyoch, J.; Petrot, N.; Ruanthong, W.; Punyodom, W.; Topham, P.D.; et al. In situ compatibilized blends of PLA/PCL/CAB melt-blown films with high elongation: Investigation of miscibility, morphology, crystallinity and modelling. Polymers 2023, 15, 303. [Google Scholar] [CrossRef] [PubMed]
- Mirpoor, S.F.; Patanè, G.T.; Corrado, I.; Giosafatto, C.V.L.; Ginestra, G.; Nostro, A.; Foti, A.; Gucciardi, P.G.; Mandalari, G.; Barreca, D.; et al. Functionalization of Polyhydroxyalkanoates (PHA)-Based Bioplastic with Phloretin for Active Food Packaging: Characterization of Its Mechanical, Antioxidant, and Antimicrobial Activities. Int. J. Mol. Sci. 2023, 24, 11628. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.H. Bio-nanocomposite Materials for Food Packaging Applications: Types of Biopolymer and Nano-sized Filler. Agric. Agric. Sci. Procedia 2014, 2, 296–303. [Google Scholar] [CrossRef]
- Jabeur, I.; Pereira, E.; Barros, L.; Calhelha, R.C.; Sokovic, M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents. Food Res. Int. 2017, 100, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mu, B.; Wang, X.; Wang, A. Recent researches on natural pigments stabilized by clay minerals: A review. Dye. Pigment. 2021, 190, 109322. [Google Scholar] [CrossRef]
- Shahid, M.; Mohammad, F. Recent advancements in natural dye applications: A review. J. Clean. Prod. 2013, 53, 310–331. [Google Scholar] [CrossRef]
- Riaz, G.; Chopra, R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 2018, 102, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Vinha, A.F.; Rodrigues, F.; Nunes, M.A.; Oliveira, M.B.P.P. 11—Natural Pigments and Colorants in Foods and Beverages. In Polyphenols: Properties, Recovery, and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 363–391. [Google Scholar] [CrossRef]
- Mezni, A.; Mhadhbi, L.; Khazri, A.; Sellami, B.; Dellali, M.; Mahmoudi, E.; Beyrem, H. The Protective Effect of Hibiscus sabdariffa Calyxes Extract against Cypermethrin Induced Oxidative Stress in Mice. Pestic. Biochem. Physiol. 2020, 165, 104463. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, D.M.; Porzel, A.; Frolov, A.; El Seedi, H.R.; Wessjohann, L.A.; Farag, M.A. Comparative analysis of Hibiscus sabdariffa (roselle) hot and cold extracts in respect to their potential for α-glucosidase inhibition. Food Chem. 2018, 250, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Ngamwonglumlert, L.; Devahastin, S. Brazilein as an alternative pigment: Isolation, characterization, stability enhancement and food applications. Food Chem. 2023, 398, 133898. [Google Scholar] [CrossRef]
- Girdthep, S.; Sirirak, J.; Daranarong, D.; Daengngern, R.; Chayabutra, S. Physico-chemical characterization of natural lake pigments obtained from Caesalpinia sappan Linn. and their composite films for poly(lactic acid)-based packaging materials. Dye. Pigment. 2018, 157, 27–39. [Google Scholar] [CrossRef]
- Dapson, R.W.; Bain, C.L. Brazilwood, sappanwood, brazilin and the red dye brazilein: From textile dyeing and folk medicine to biological staining and musical instruments. Biotech. Histochem. 2015, 90, 401–423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, X.; Zhai, X.; Huang, X.W.; Jiang, C.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Ramadhani, N.F.; Nugraha, A.P.; Rahmadhani, D.; Puspitaningrum, M.S.; Rizqianti, Y.; Kharisma, V.D.; Noor, T.N.E.B.T.A.; Ridwan, R.D.; Ernawati, D.S.; Nugraha, A.P. Anthocyanin, tartaric acid, ascorbic acid of roselle flower (Hibiscus sabdariffa L.) for immunomodulatory adjuvant therapy in oral manifestation coronavirus disease-19: An immunoinformatic approach. J. Pharm. Pharmacogn. Res. 2022, 10, 418–428. [Google Scholar] [CrossRef]
- Owoade, A.O.; Adetutu, A.; Olorunnisola, O.S. A review of chemical constituents and pharmacological properties of Hibiscus sabdariffa L. Int. J. Curr. Res. Biosci. Plant Biol. 2019, 6, 42–51. [Google Scholar] [CrossRef]
- Lema, A.A.; Mahmod, N.H.; Khandaker, M.M. Therapeutic and Economic Impacts of Roselle (Hibiscus sabdariffa L.) Anthocyanin; A Review. Biosci. Res. 2021, 18, 284–294. [Google Scholar]
- Yim, D.-G.; Seo, J.-K.; Yum, H.-W.; Zahid, M.A.; Park, J.-Y.; Parvin, R.; Go, J.; Jin, S.-K.; Koo, O.-K.; Yang, H.-S. Effects of Caesalpinia sappan L. Extract on the Color Stability, Antioxidant and Antimicrobial Activity in Cooked Pork Sausages during Cold Storage. LWT 2019, 112, 108235. [Google Scholar] [CrossRef]
- Ross, G.M.; Ross, S.; Tighe, B.J. Chapter 23—Bioplastics: New Routes, New Products. In Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Butterworth Heinemann: Oxford, UK, 2017; pp. 631–652. [Google Scholar]
- Aliotta, L.; Seggiani, M.; Lazzeri, A.; Gigante, V.; Cinelli, P. A Brief Review of Poly (Butylene Succinate) (PBS) and Its Main Copolymers: Synthesis, Blends, Composites, Biodegradability, and Applications. Polymer 2022, 14, 844. [Google Scholar] [CrossRef]
- Yan-yan, J.; Yan, L.; Ying, S.; Jinyi, Z.; Fang, D.; Yuan, S.; Ai-dong, W.A. A simple high-performance liquid chromatographic method for the determination of brazilin and its application to a pharmacokinetic study in rats. J. Ethnopharmacol. 2014, 151, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Chumsri, P.; Sirichote, A.; Itharat, A. Studies on the optimum conditions for the extraction and concentration of roselle (Hibiscus sabdariffa Linn.) extract. Songklanakarin J. Sci. Technol. 2008, 30, 133–139. [Google Scholar]
- Chatkitanan, T.; Harnkarnsujarit, N. Development of nitrite compounded starch-based films to improve color and quality of vacuum-packaged pork. Food Packag. Shelf Life 2020, 25, 100521. [Google Scholar] [CrossRef]
- Magazzu, A.; Marcuello, C. Investigation of Soft Matter Nanomechanics by Atomic Force Microscopy and Optical Tweezers: A Comprehensive Review. Nanomaterials. 2023, 13, 963. [Google Scholar] [CrossRef]
- Huang, Z.; Qian, L.; Yin, Q.; Yu, N.; Liu, T.; Tian, D. Biodegradability studies of poly(butylene succinate) composites filled with sugarcane rind fiber. Polym. Test. 2018, 66, 319–326. [Google Scholar] [CrossRef]
- Hanani, Z.A.N.; Yee, F.C.; Nor-Khaizura, M.A.R. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Makris, D.P.; Yannakopoulou, K.; Kalogeropoulos, N.; Michali, I.; Karathanos, V.T. Thermal stability of anthocyanin extract of Hibiscus sabdariffa L. in the presence of β-cyclodextrin. J. Agric. Food Chem. 2008, 56, 10303–10310. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.; Chanana, H.; Khan, S.A.; Dhanalekshmi, U.M.; Ali, M.; Alghamdi, A.A.; Ahmad, A. Chemistry and Pharmacological Actions of Delphinidin, a Dietary Purple Pigment in Anthocyanidin and Anthocyanin Forms. Front. Nutr. 2022, 9, 746881. [Google Scholar] [CrossRef] [PubMed]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N.; Raghavan, G.S.V. Color and Molecular Structure Alterations of Brazilein Extracted from Caesalpinia sappan L. under Different PH and Heating Conditions. Sci. Rep. 2020, 10, 12386. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Color Difference Delta E—A Survey Colour Difference ∆ E—A Survey Faculty of Mathematics and Informatics. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Sonar, C.R.; Rasco, B.; Tang, J.; Sablani, S.S. Natural color pigments: Oxidative stability and degradation kinetics during storage in thermally pasteurized vegetable purees. J. Sci. Food Agric. 2019, 99, 5934–5945. [Google Scholar] [CrossRef]
- Liu, J.; Yong, H.; Liu, Y.; Qin, Y.; Kan, J.; Liu, J. Preparation and characterization of active and intelligent films based on fish gelatin and haskap berries (Lonicera caerulea L.) extract. Food Packag. Shelf Life 2019, 22, 100417. [Google Scholar] [CrossRef]
- Wang, H.; Hu, D.; Ma, Q.; Wang, L. Physical and antioxidant properties of flexible soy protein isolate films by incorporating chestnut (Castanea mollissima) bur extracts. LWT—Food Sci. Technol. 2016, 71, 33–39. [Google Scholar] [CrossRef]
- Cano, A.; Cháfer, M.; Chiralt, A.; González-martínez, C. Development and characterization of active films based on starch-PVA, containing silver nanoparticles. Food Packag. Shelf Life 2016, 10, 16–24. [Google Scholar] [CrossRef]
- Escobar-Ortiz, A.; Castaño-Tostado, E.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Reynoso-Camacho, R. Anthocyanins extraction from Hibiscus sabdariffa and identification of phenolic compounds associated with their stability. J. Sci. Food Agric. 2021, 101, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Settharaksa, S.; Monton, C.; Charoenchai, L. Optimization of Caesalpinia sappan L. heartwood extraction procedure to obtain the highest content of brazilin and greatest antibacterial activity. J. Integr. Med. 2019, 17, 351–358. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H. GC–MS and FTIR analyses of oils from Hibiscus sabdariffa, Stigma maydis and Chromolaena odorata leaf obtained from Malaysia: Potential sources of fatty acids. Chem. Data Collect. 2019, 20, 100200. [Google Scholar] [CrossRef]
- Yang, C.; Tang, H.; Wang, Y.; Liu, Y.; Wang, J.; Shi, W.; Li, L. Development of PLA-PBSA Based Biodegradable Active Film and Its Application to Salmon Slices. Food Packag. Shelf Life 2019, 22, 100393. [Google Scholar] [CrossRef]
- Phua, Y.J.; Chow, W.S.; Mohd Ishak, Z.A. The Hydrolytic Effect of Moisture and Hygrothermal Aging on Poly(Butylene Succinate)/Organo-Montmorillonite Nanocomposites. Polym. Degrad. Stab. 2011, 96, 1194–1203. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, X.; Qin, Y.-R.; Zhang, Y.-H.; Wang, X.-P.; Wang, J.-Y.; Ning, Z.-X.; Ruan, Q.-J.; Zhang, Y.-S. Preparation and Characterization of a Novel Colorimetric Indicator Film Based on Gelatin/Polyvinyl Alcohol Incorporating Mulberry Anthocyanin Extracts for Monitoring Fish Freshness. Food Res. Int. 2019, 126, 108604. [Google Scholar] [CrossRef] [PubMed]
- Messin, T.; Marais, S.; Follain, N.; Guinault, A.; Gaucher, V.; Delpouve, N.; Sollogoub, C. Biodegradable PLA/PBS Multinanolayer Membrane with Enhanced Barrier Performances. J. Memb. Sci. 2020, 598, 117777. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X. Polybutylene succinate/cellulose nanocrystals: Role of phthalic anhydride in squeeze oriented bionanocomposites. Carbohydr. Polym. 2018, 196, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Liminana, P.; Garcia-Sanoguera, D.; Quiles-Carrillo, L.; Balart, R.; Montanes, N. Development and characterization of environmentally friendly composites from poly(butylene succinate) (PBS) and almond shell flour with different compatibilizers. Compos. Part. B Eng. 2018, 144, 153–162. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, S.; Fang, X.; Zhou, X.; Wang, J.; Bai, F.; Peng, S. Renewable and Flexible UV-Blocking Film from Poly(Butylene Succinate) and Lignin. Eur. Polym. J. 2019, 116, 265–274. [Google Scholar] [CrossRef]
- Mehaya, F.M.; Mohammad, A.A. Thermostability of bioactive compounds during roasting process of coffee beans. Heliyon 2020, 6, e05508. [Google Scholar] [CrossRef]


| Sample | Origin | 2 Months | ||||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ∆E* | L* | a* | b* | ∆E* | |
| (a). PBS | 89.28 | −0.86 | −4.18 | Ref | 89.44 | −1.06 | −4.50 | 0.41 |
| ±0.08 | ±0.05 | ±0.13 | ±0.21 | ±0.05 | ±0.07 | |||
| (b). 0.1 RS | 87.54 | −0.20 | −4.72 | 1.94 | 89.14 | −1.00 | −3.72 | 0.50 |
| ±0.29 | ±0.00 | ±0.15 | ±0.26 | ±0.00 | ±0.19 | |||
| (c). 0.2 RS | 87.26 | 0.46 | −5.10 | 2.58 | 89.06 | −0.90 | −3.52 | 0.70 |
| ±0.09 | ±0.05 | ±0.07 | ±0.15 | ±0.00 | ±0.16 | |||
| (d). 0.3 RS | 85.58 | 1.86 | −5.20 | 4.70 | 88.70 | −0.70 | −2.90 | 1.41 |
| ±0.45 | ±0.15 | ±0.10 | ±0.37 | ±0.00 | ±0.12 | |||
| (e). 0.1 SP | 84.20 | 0.36 | 16.54 | 21.37 | 84.32 | 1.16 | 12.66 | 3.96 |
| ±0.16 | ±0.05 | ±0.42 | ±0.38 | ±0.18 | ±0.71 | |||
| (f). 0.2 SP | 82.68 | 0.46 | 26.58 | 31.49 | 81.32 | 2.04 | 21.86 | 5.16 |
| ±0.22 | ±0.09 | ±0.69 | ±1.37 | ±0.63 | ±2.40 | |||
| (g). 0.3 SP | 82.92 | −0.24 | 29.90 | 34.67 | 80.22 | 2.20 | 26.60 | 4.91 |
| ±0.29 | ±0.11 | ±1.12 | ±1.23 | ±0.55 | ±2.51 | |||
| Samples | %T at Origin | %T at 2 Months | ||||||
|---|---|---|---|---|---|---|---|---|
| 280 nm (UV-C) | 315 nm (UV-B) | 400 nm (UV-A) | 700 nm (Visible) | 280 nm (UV-C) | 315 nm (UV-B) | 400 nm (UV-A) | 700 nm (Visible) | |
| PBS | 4.33 ± 1.15 | 11.00 ± 1.73 | 28.00 ± 2.65 | 65.33 ± 2.08 | 5.00 ± 1.00 | 11.67 ± 1.53 | 29.67 ± 2.08 | 66.33 ± 1.53 |
| 0.1 RS | 4.33 ± 0.58 | 11.00 ± 1.00 | 29.67 ± 1.15 | 66.33 ± 0.58 | 5.67 ± 1.53 | 13.00 ± 1.73 | 30.67 ± 3.21 | 66.33 ± 1.53 |
| 0.2 RS | 2.67 ± 0.58 | 8.00 ± 1.00 | 24.00 ± 1.00 | 61.67 ± 1.15 | 4.33 ± 1.15 | 9.67 ± 2.31 | 26.33 ± 2.89 | 62.33 ± 2.08 |
| 0.3 RS | 2.33 ± 0.58 | 7.00 ± 1.00 | 21.67 ± 2.08 | 59.33 ± 1.53 | 3.33 ± 1.15 | 8.33 ± 2.89 | 25.00 ± 4.36 | 61.67 ± 3.21 |
| 0.1 SP | 3.33 ± 1.15 | 10.00 ± 1.73 | 25.00 ± 2.65 | 63.67 ± 1.53 | 2.67 ± 1.15 | 8.00 ± 1.73 | 22.67 ± 2.89 | 60.67 ± 2.08 |
| 0.2 SP | 2.33 ± 1.15 | 8.67 ± 2.31 | 22.67 ± 3.21 | 62.33 ± 2.08 | 1.67 ± 0.58 | 6.67 ± 0.58 | 20.33 ± 1.15 | 61.00 ± 1.00 |
| 0.3 SP | 1.33 ± 0.58 | 6.33 ± 1.53 | 18.00 ± 1.73 | 60.00 ± 1.00 | 1.67 ± 0.58 | 7.67 ± 1.53 | 21.00 ± 2.65 | 61.00 ± 1.00 |
| Profile at Peak | ||||||
|---|---|---|---|---|---|---|
| Samples | Tg (°C) | Tc (°C) | ∆Hc (Jg−1) | Tm (°C) | ∆Hm (Jg−1) | %Xc |
| PBS | 47.10 | 56.51 | 45.08 | 86.27 | 16.61 | 15.06 ± 4.1 |
| 0.1 RS | 45.27 | 57.19 | 44.43 | 86.11 | 39.78 | 36.07 ± 3.2 |
| 0.2 RS | 46.26 | 51.68 | 47.32 | 86.37 | 31.08 | 28.18 ± 6.0 |
| 0.3 RS | 46.09 | 49.62 | 49.32 | 86.27 | 37.28 | 33.80 ± 4.4 |
| 0.1 SP | 43.76 | 59.03 | 41.09 | 85.30 | 47.47 | 43.04 ± 3.1 |
| 0.2 SP | 44.93 | 58.83 | 49.32 | 86.03 | 37.28 | 33.80 ± 5.4 |
| 0.3 SP | 44.77 | 58.99 | 41.35 | 85.16 | 46.04 | 41.74 ± 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nansu, W.; Ross, S.; Waisarikit, A.; Ross, G.M.; Charoensit, P.; Suphrom, N.; Mahasaranon, S. Exploring the Potential of Roselle Calyx and Sappan Heartwood Extracts as Natural Colorants in Poly(butylene Succinate) for Biodegradable Packaging Films. Polymers 2023, 15, 4193. https://doi.org/10.3390/polym15204193
Nansu W, Ross S, Waisarikit A, Ross GM, Charoensit P, Suphrom N, Mahasaranon S. Exploring the Potential of Roselle Calyx and Sappan Heartwood Extracts as Natural Colorants in Poly(butylene Succinate) for Biodegradable Packaging Films. Polymers. 2023; 15(20):4193. https://doi.org/10.3390/polym15204193
Chicago/Turabian StyleNansu, Wordpools, Sukunya Ross, Amonrut Waisarikit, Gareth M. Ross, Pensri Charoensit, Nungruthai Suphrom, and Sararat Mahasaranon. 2023. "Exploring the Potential of Roselle Calyx and Sappan Heartwood Extracts as Natural Colorants in Poly(butylene Succinate) for Biodegradable Packaging Films" Polymers 15, no. 20: 4193. https://doi.org/10.3390/polym15204193
APA StyleNansu, W., Ross, S., Waisarikit, A., Ross, G. M., Charoensit, P., Suphrom, N., & Mahasaranon, S. (2023). Exploring the Potential of Roselle Calyx and Sappan Heartwood Extracts as Natural Colorants in Poly(butylene Succinate) for Biodegradable Packaging Films. Polymers, 15(20), 4193. https://doi.org/10.3390/polym15204193






