Aryloxy Ionic Liquid-Catalyzed Homogenous Esterification of Cellulose with Low-Reactive Acyl Donors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Preparation of ILs from EmimCl [24,26,27]
2.4. Solubility of Cellulose in ILs
2.5. General Procedure for the Transesterification of Cellulose
2.6. General Procedure for the Oxidative Esterification of Cellulose
2.7. Evaluation of the DS in Cellulose Esters
2.8. General Procedure for Per-Benzoylation of the Synthesized Cellulose Ester
2.9. General Procedure for Per-Acetylation of the Synthesized Cellulose Esters
2.10. Kamlet–Taft Parameters
3. Results and Discussion
3.1. ILs Synthesis and Cellulose Dissolution in ILs
3.2. Catalytic Activity of Synthesized ILs in Transesterification of Cellulose
3.3. Optimization of Reaction Conditions and Kinetics of Cellulose Esterification
3.4. Scope of Vinyl Esters
3.5. Effect of the IL Species on Oxidative Esterification of Cellulose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Doppalapudi, S.; Jain, A.; Khan, W.; Domb, A.J. Biodegradable polymers-an overview. Polym. Adv. Technol. 2014, 25, 427–435. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Edgar, K.J.; Buchanan, C.M.; Debenham, J.S.; Rundquist, P.A.; Seiler, B.D.; Shelton, M.C.; Tindall, D. Advances in cellulose ester performance and application. Prog. Polym. Sci. 2001, 26, 1605–1688. [Google Scholar] [CrossRef]
- Hindi, S.S.Z.; Abohassan, R.A. Cellulose triacetate synthesis from cellulosic wastes by heterogeneous reactions. BioResources 2015, 10, 5030–5048. [Google Scholar] [CrossRef]
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.M.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Methylcellulose, a cellulose derivative with original physical properties and extended applications. Polymers 2015, 7, 777–803. [Google Scholar] [CrossRef]
- Varshney, V.K.; Gupta, P.K.; Naithani, S.; Khullar, R.; Bhatt, A.; Soni, P.L. Carboxymethylation of α-cellulose isolated from Lantana camara with respect to degree of substitution and rheological behavior. Carbohydr. Polym. 2006, 63, 40–45. [Google Scholar] [CrossRef]
- Mormann, W.; Demeter, J.; Wagner, T. Silylcellulose from silylation/desilylation of cellulose in ammonia. Macromol. Symp. 2001, 163, 49–58. [Google Scholar] [CrossRef]
- Mormann, W. Silylation of cellulose with hexamethyldisilazane in ammonia-activation, catalysis, mechanism, properties. Cellulose 2003, 10, 271–281. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellulose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Barthel, S.; Heinze, T. Acylation and carbanilation of cellulose in ionic liquids. Green Chem. 2006, 8, 301–306. [Google Scholar] [CrossRef]
- Huang, K.; Xia, J.; Li, M.; Lian, J.; Yang, X.; Lin, G. Homogeneous synthesis of cellulose stearates with different degrees of substitution in ionic liquid 1-butyl-3-methylimidazolium chloride. Carbohydr. Polym. 2011, 83, 1631–1635. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Cao, Y.; Sang, S.; Zhang, J.; He, J. Synthesis of cellulose benzoates under homogeneous conditions in an ionic liquid. Cellulose 2009, 16, 299–308. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Zhang, H.; He, J.; Ren, Q.; Guo, M. Homogeneous acetylation of cellulose in a new ionic liquid. Biomacromolecules 2004, 5, 266–268. [Google Scholar] [CrossRef]
- Kakuchi, R.; Yamaguchi, M.; Endo, T.; Shibata, Y.; Ninomiya, K.; Ikai, T.; Maeda, K.; Takahashi, K. Efficient and rapid direct transesterification reactions of cellulose with isopropenyl acetate in ionic liquids. RSC Adv. 2015, 5, 72071–72074. [Google Scholar] [CrossRef]
- Hinner, L.P.; Wissner, J.L.; Beurer, A.; Nebel, B.A.; Hauer, B. Homogeneous vinyl ester-based synthesis of different cellulose derivatives in 1-ethyl-3-methyl-imidazolium acetate. Green Chem. 2016, 18, 6099–6107. [Google Scholar] [CrossRef]
- Tanaka, S.; Iwata, T.; Iji, M. Long/short chain mixed cellulose esters: Effects of long acyl chain structures on mechanical and thermal properties. ACS Sustain. Chem. Eng. 2017, 5, 1485–1493. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, J.; Chen, W.; Zhang, M.; Yin, C.; Tian, W.; Luo, Z.; Liu, W.; He, J.; Zhang, J. Controllable synthesis of cellulose benzoates for understanding of chiral recognition mechanism and fabrication of highly efficient chiral stationary phases. Anal. Methods 2018, 10, 2844–2853. [Google Scholar] [CrossRef]
- Tang, S.; Li, X.; Wang, F.; Liu, G.; Li, Y.; Pan, F. Synthesis and hplc chiral recognition of regioselectively carbamoylated cellulose derivatives. Chirality 2012, 24, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Kakuchi, R.; Ito, R.; Nomura, S.; Abroshan, H.; Ninomiya, K.; Ikai, T.; Maeda, K.; Kim, H.J.; Takahashi, K. A mechanistic insight into the organocatalytic properties of imidazolium-based ionic liquids and a positive co-solvent effect on cellulose modification reactions in an ionic liquid. RSC Adv. 2017, 7, 9423–9430. [Google Scholar] [CrossRef]
- Köhler, S.; Liebert, T.; Schöbitz, M.; Schaller, J.; Meister, F.; Günther, W.; Heinze, T. Interactions of ionic liquids with polysaccharides 1. Unexpected acetylation of cellulose with 1-ethyl-3-methylimidazolium acetate. Macromol. Rapid Commun. 2007, 28, 2311–2317. [Google Scholar] [CrossRef]
- Hirose, D.; Wardhana Kusuma, S.B.; Nomura, S.; Yamaguchi, M.; Yasaka, Y.; Kakuchi, R.; Takahashi, K. Effect of anion in carboxylate-based ionic liquids on catalytic activity of transesterification with vinyl esters and the solubility of cellulose. RSC Adv. 2019, 9, 4048–4053. [Google Scholar] [CrossRef]
- Kusuma, S.B.W.; Hirose, D.; Takahashi, K. Transesterification reaction of cellulose with inactive esters in ionic liquids acting as both catalysts and solvents. Chem. Lett. 2019, 48, 1122–1125. [Google Scholar] [CrossRef]
- Kusuma, S.B.W.; Hirose, D.; Yoshizawa, A.; Szabó, L.; Ina, D.; Wada, N.; Takahashi, K. Direct synthesis of full-biobased cellulose esters from essential oil component α,β-unsaturated aldehydes. ACS Sustain. Chem. Eng. 2021, 9, 8450–8457. [Google Scholar] [CrossRef]
- Saptal, V.B.; Bhanage, B.M. Bifunctional ionic liquids derived from biorenewable sources as sustainable catalysts for fixation of carbon dioxide. ChemSusChem 2017, 10, 1145–1151. [Google Scholar] [CrossRef]
- Hirose, D.; Kusuma, S.B.W.; Ina, D.; Wada, N.; Takahashi, K. Direct one-step synthesis of a formally fully bio-based polymer from cellulose and cinnamon flavor. Green. Chem. 2019, 21, 4927–4931. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Taft, R.W. The solvatochromic comparison method. I. The.beta.-scale of solvent hydrogen-bond acceptor (HBA) basicities. J. Am. Chem. Soc. 1976, 98, 377–383. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.; Taft, R.W. The solvatochromic comparison method. 6. The.pi.* scale of solvent polarities. J. Am. Chem. Soc. 1977, 99, 6027–6038. [Google Scholar] [CrossRef]
- Gericke, M.; Liebert, T.; Seoud, O.A.E.; Heinze, T. Tailored media for homogeneous cellulose chemistry: Ionic liquid/co-solvent mixtures. Macromol. Mater. Eng. 2011, 296, 483–493. [Google Scholar] [CrossRef]
- Kasprzak, D.; Krystkowiak, E.; Stępniak, I.; Galiński, M. Dissolution of cellulose in novel carboxylate-based ionic liquids and dimethyl sulfoxide mixed solvents. Eur. Polym. J. 2019, 113, 89–97. [Google Scholar] [CrossRef]
- Calculated Using Advanced Chemistry Development (ACD/Laboratories) Software. Version 11.02. ACD/Laboratories: Toronto, ON, Canada, 2011.
- Gimeno, M.P.; Mayoral, M.C.; Andrés, J.M. Influence of temperature on CO2 absorption rate and capacity in ionic liquids. Energy Fuels 2013, 27, 3928–3935. [Google Scholar] [CrossRef]
- Shah, S.N.; Lethesh, K.C.; Mutalib, M.I.A.; Pilus, R.B.M. Evaluation of thermophysical properties of imidazolium-based phenolate ionic liquids. Ind. Eng. Chem. Res. 2015, 54, 3697–3705. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.M.; Abraham, M.H.; Taft, R.W. Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters,. pi.*,. alpha., and. beta., and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Laurence, C.; Nicolet, P.; Dalati, M.T.; Abboud, J.-L.M.; Notario, R. The empirical treatment of solvent-solute interactions: 15 Years of .pi.*. J. Phys. Chem. 1994, 98, 5807–5816. [Google Scholar] [CrossRef]
- Hauru, L.K.J.; Hummel, M.; King, A.W.T.; Kilpeläinen, I.; Sixta, H. Role of solvent parameters in the regeneration of cellulose from ionic liquid solutions. Biomacromolecules 2012, 13, 2896–2905. [Google Scholar] [CrossRef]
- Schmeisser, M.; Illner, P.; Puchta, R.; Zahl, A.; van Eldik, R. Gutmann Donor and Acceptor Numbers for Ionic Liquids. Eur. J. Chem. 2012, 18, 10969–10982. [Google Scholar] [CrossRef]
- Schleicher, J.C.; Scurto, A.M. Kinetics and solvent effects in the synthesis of ionic liquids: Imidazolium. Green Chem. 2009, 11, 694–703. [Google Scholar] [CrossRef]
- Pignataro, L.; Papalia, T.; Slawin, A.M.Z.; Goldup, S.M. Unusual mechanistic course of some NHC-mediated transesterifications. Org. Lett. 2009, 11, 1643–1646. [Google Scholar] [CrossRef]
- Cindradewi, A.W.; Bandi, R.; Park, C.-W.; Park, J.-S.; Lee, E.-A.; Kim, J.-K.; Kwon, G.-J.; Han, S.-Y.; Lee, S.-H. Preparation and characterization of cellulose acetate film reinforced with cellulose nanofibril. Polymers 2021, 13, 2990. [Google Scholar] [CrossRef] [PubMed]
- Omollo, E.; Zhang, C.; Mwasiagi, J.I.; Ncube, S. Electrospinning cellulose acetate nanofibers and a study of their possible use in high-efficiency filtration. J. Ind. Text. 2014, 45, 716–729. [Google Scholar] [CrossRef]
- Yu, Y.; Hua, L.; Zhu, W.; Shi, Y.; Cao, T.; Qiao, Y.; Hou, Z. Ionic liquid–catalyzed internal redox esterification reaction. Synth. Commun. 2013, 43, 1287–1298. [Google Scholar] [CrossRef]
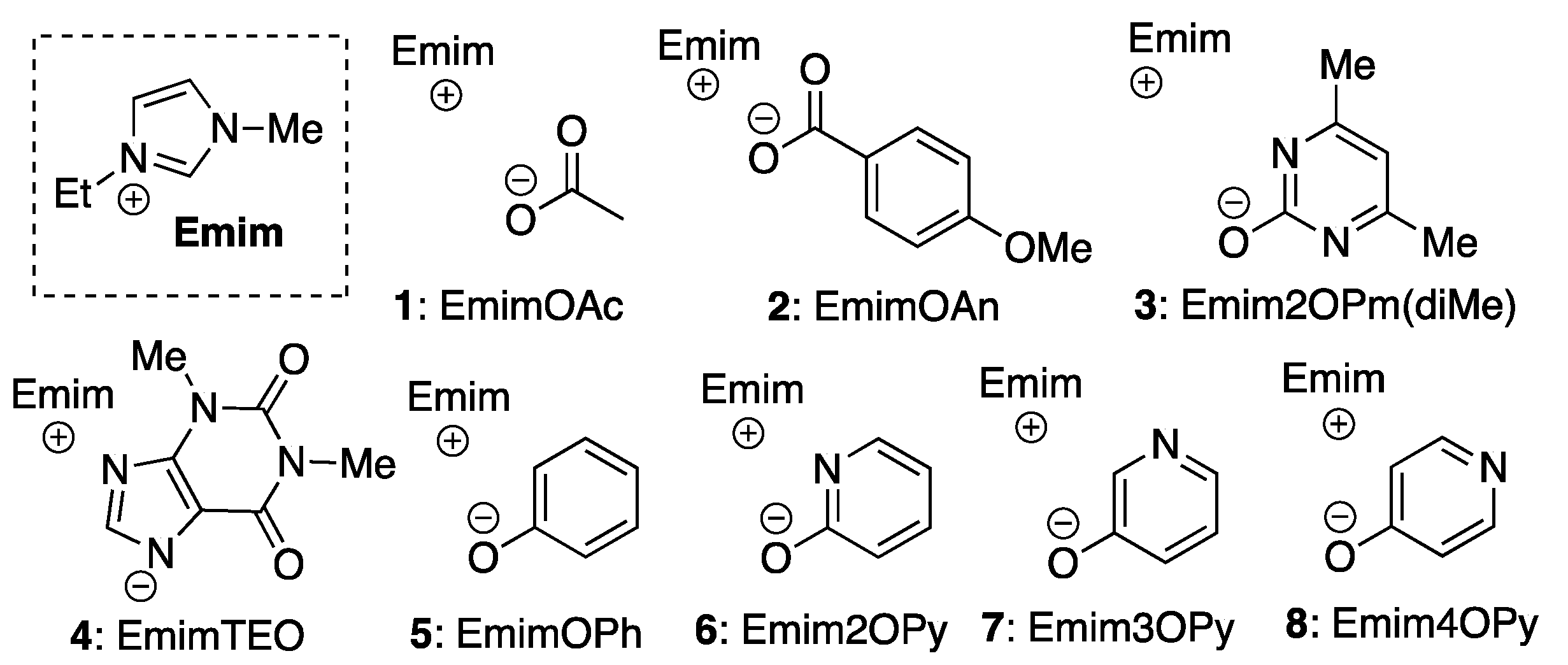

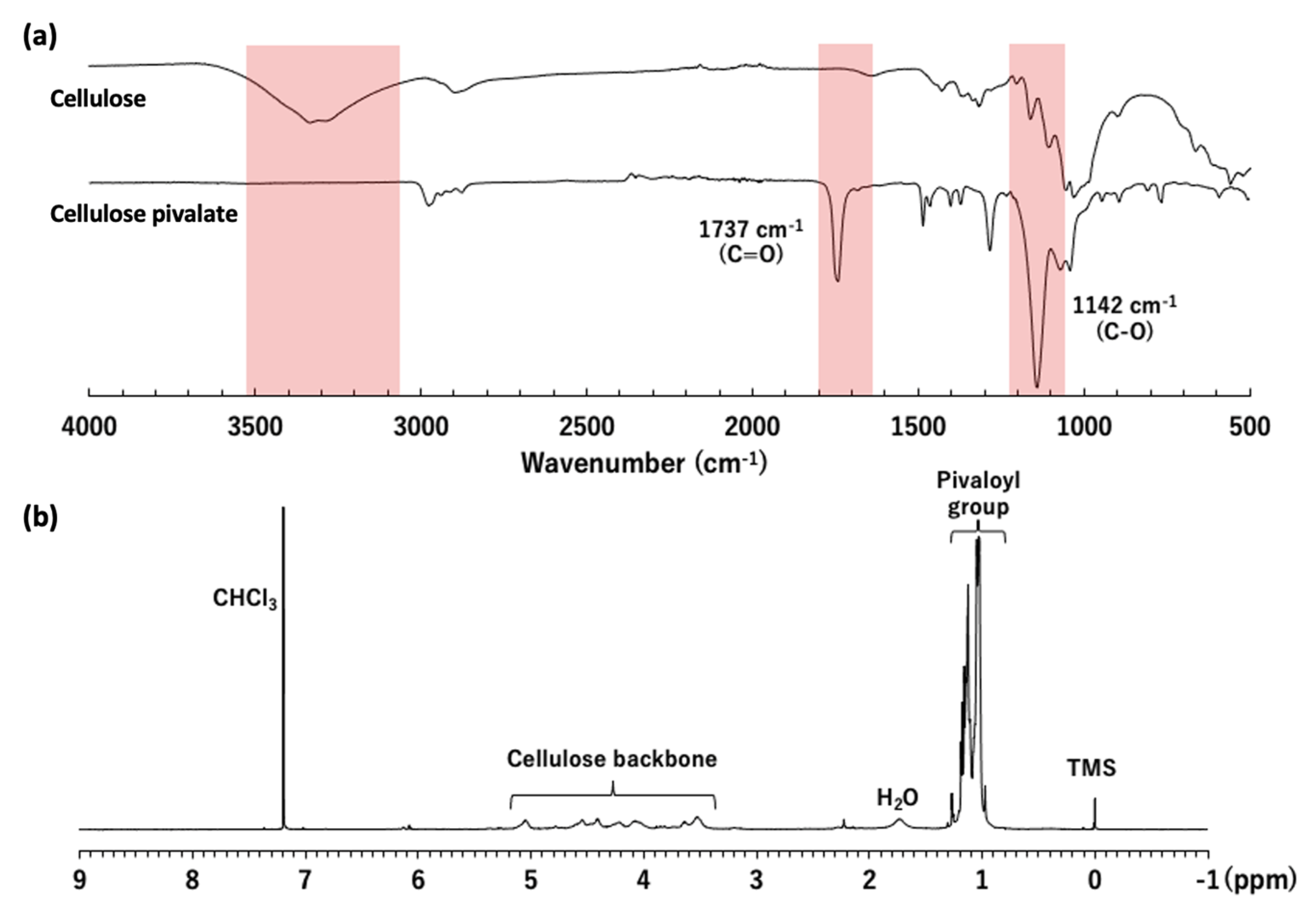



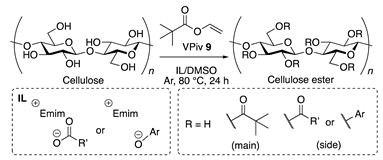
| Entry | IL | pKa b | Solubility c | DSmain d | DSside d | Selectivity (%) |
|---|---|---|---|---|---|---|
| 1 | 1 | 4.79 | <75 | 2.43 | 0.54 | 82 |
| 2 | 2 | 4.47 | <45 | 2.78 | 0.03 | 99 |
| 3 | 3 | 10.3 | <15 | 2.85 | n.d. | >99 |
| 4 | 4 | 8.60 | <15 | 2.73 | n.d. | >99 |
| 5 | 6 | 12.0 | <75 | 2.99 | n.d. | >99 |
| 6 | 7 | 9.15 | <60 | 2.85 | n.d. | >99 |
| 7 | 8 | 5.19 | <30 | 2.74 | n.d. | >99 |
| Entry | Solvents | α | β |
|---|---|---|---|
| 1 | Emim2OPy/DMSO (1/20) | 0.20 | 1.04 |
| 2 | Emim4OPy/DMSO (1/20) | 0.26 | 0.93 |
| 3 | EmimOAc | 0.40 | 0.95–1.09 [38,39] |
| 4 | DMSO | −0.11 | 0.72 [40] |
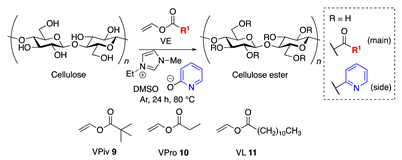
| Entry | VE | DSmain b | DSside b | Selectivity (%) |
|---|---|---|---|---|
| 1 | 9 | 2.96 | n.d. | >99 |
| 2 | 9 | 2.43 c | 0.54 c | 82 c |
| 3 | 10 | 2.85 | n.d. | >99 |
| 4 | 11 | 2.96 | n.d. | >99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshizawa, A.; Maruyama, C.; Kusuma, S.B.W.; Wada, N.; Kuroda, K.; Hirose, D.; Takahashi, K. Aryloxy Ionic Liquid-Catalyzed Homogenous Esterification of Cellulose with Low-Reactive Acyl Donors. Polymers 2023, 15, 419. https://doi.org/10.3390/polym15020419
Yoshizawa A, Maruyama C, Kusuma SBW, Wada N, Kuroda K, Hirose D, Takahashi K. Aryloxy Ionic Liquid-Catalyzed Homogenous Esterification of Cellulose with Low-Reactive Acyl Donors. Polymers. 2023; 15(2):419. https://doi.org/10.3390/polym15020419
Chicago/Turabian StyleYoshizawa, Akina, Chie Maruyama, Samuel Budi Wardhana Kusuma, Naoki Wada, Kosuke Kuroda, Daisuke Hirose, and Kenji Takahashi. 2023. "Aryloxy Ionic Liquid-Catalyzed Homogenous Esterification of Cellulose with Low-Reactive Acyl Donors" Polymers 15, no. 2: 419. https://doi.org/10.3390/polym15020419
APA StyleYoshizawa, A., Maruyama, C., Kusuma, S. B. W., Wada, N., Kuroda, K., Hirose, D., & Takahashi, K. (2023). Aryloxy Ionic Liquid-Catalyzed Homogenous Esterification of Cellulose with Low-Reactive Acyl Donors. Polymers, 15(2), 419. https://doi.org/10.3390/polym15020419








