Type-A Gelatin-Based Hydrogel Infiltration and Degradation in Titanium Foams as a Potential Method for Localised Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of the Type-A Gelatin-Based Hydrogel
2.2. Fabrication and Characterization of the Titanium Foams
2.3. Infiltration-Degradation for the Gelatin-Based Hydrogels into the Ti30 and Ti60 Foams
3. Results
3.1. Synthesis and Characterization of the Gelatin-Based Hydrogels
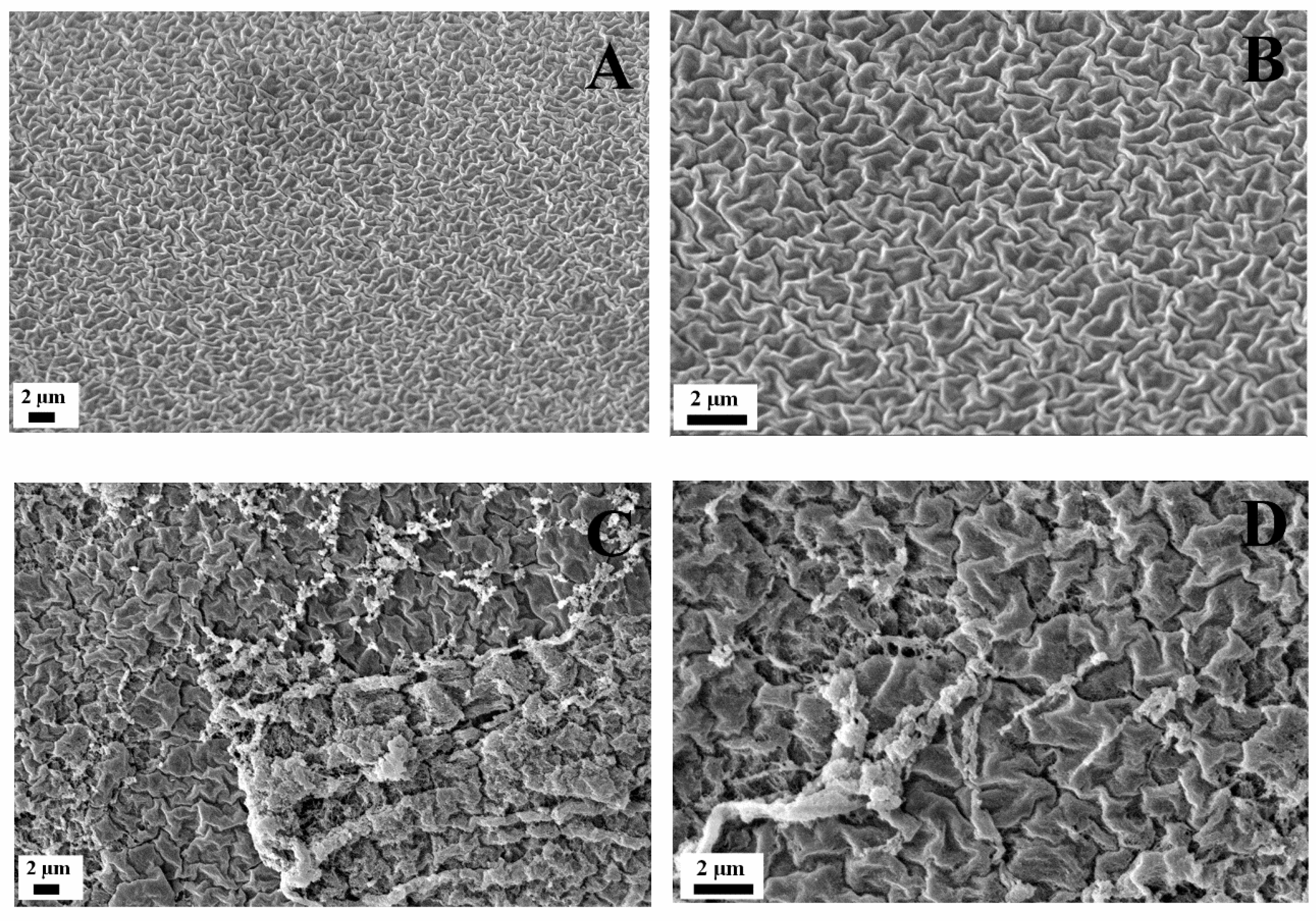
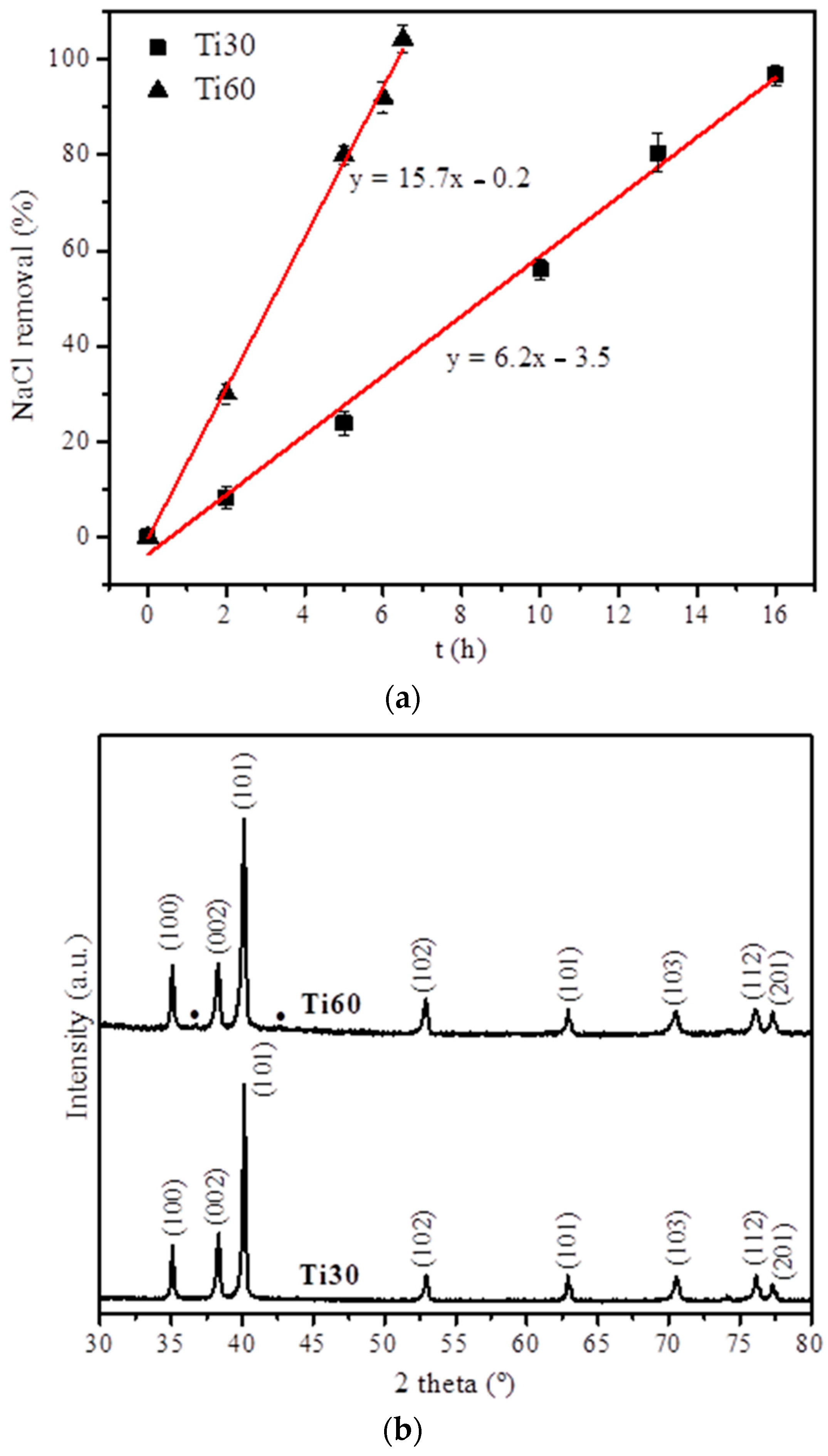
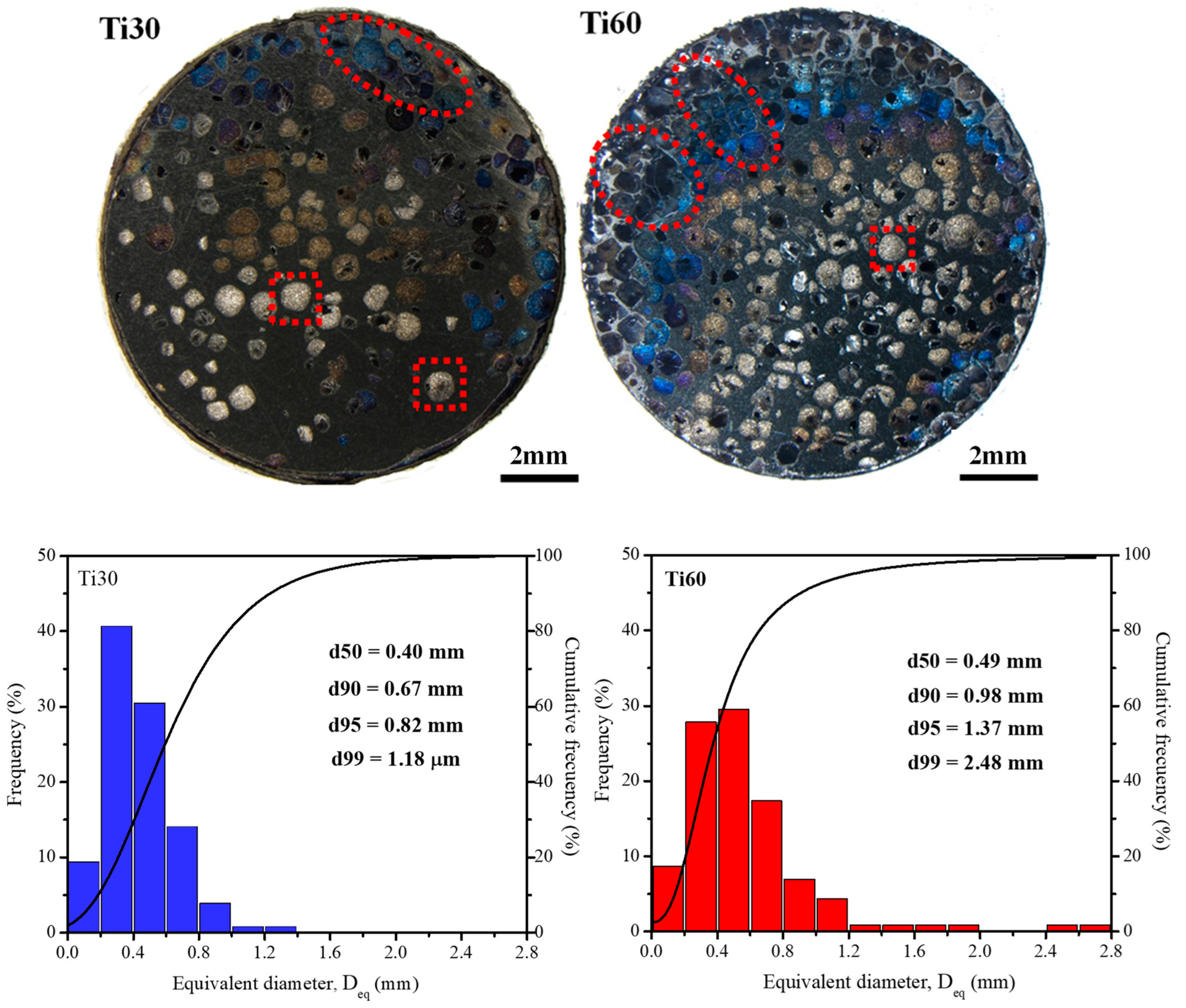
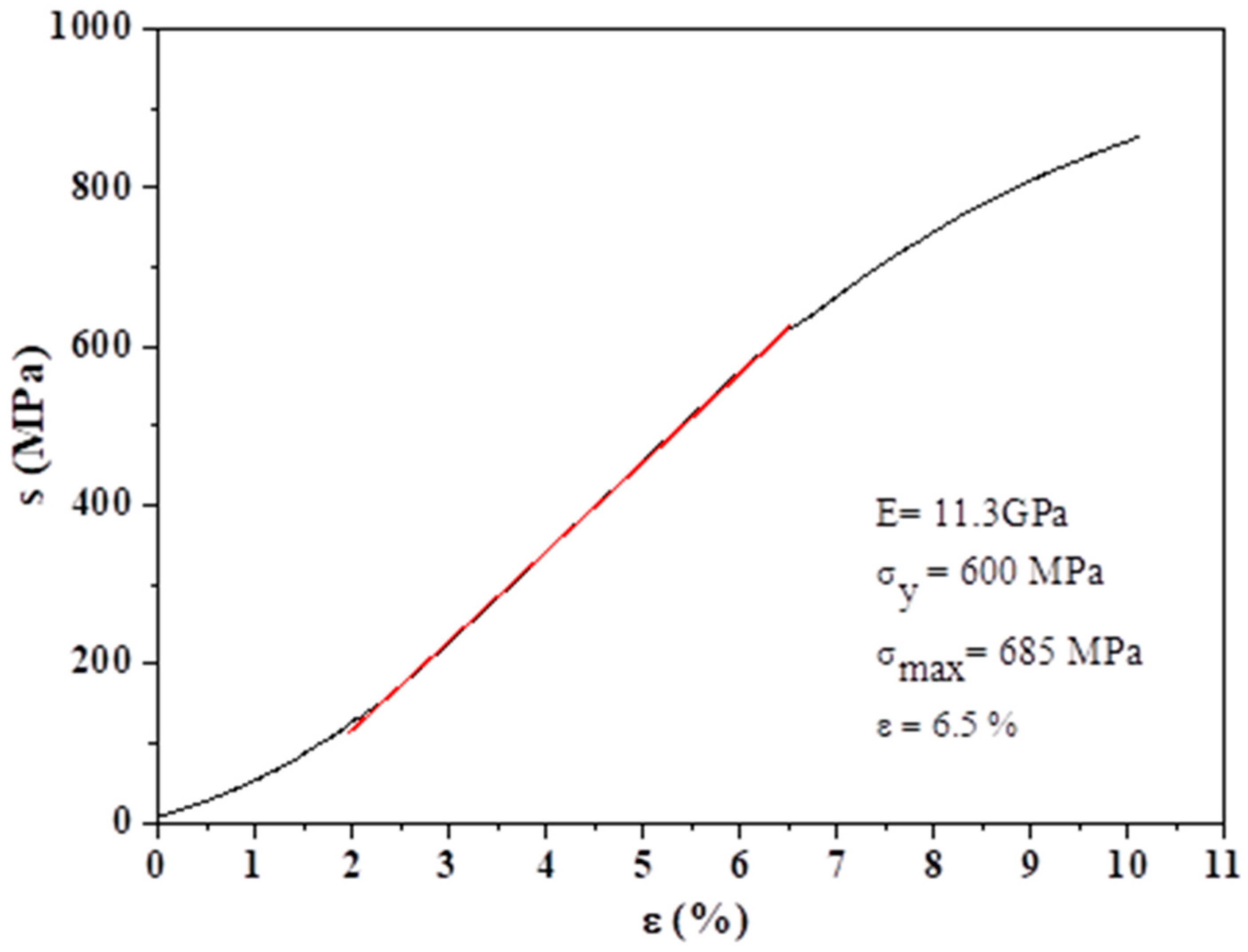
3.2. Fabrication and Characterization of the Titanium Foams
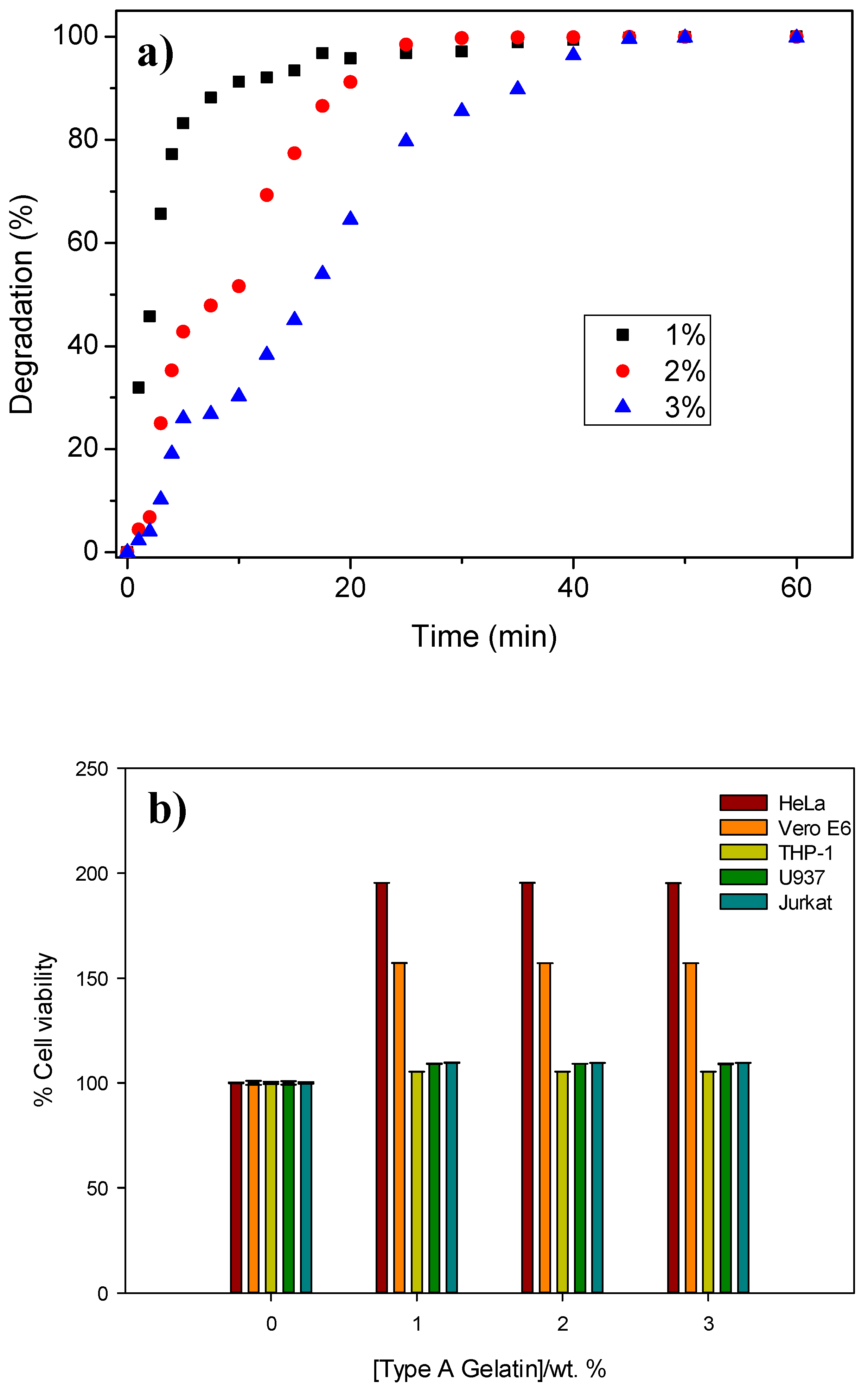
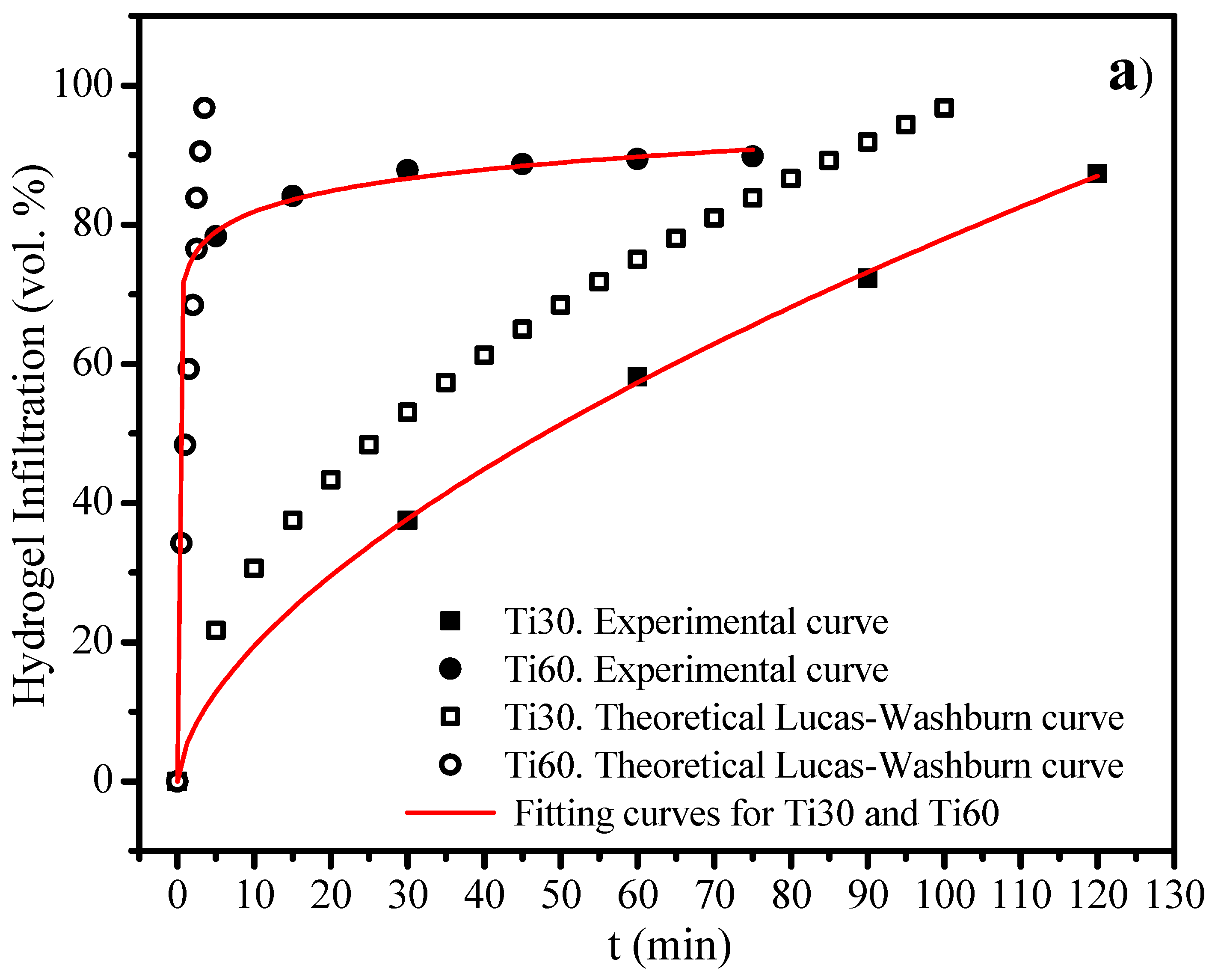
3.3. Infiltration-Degradation for the Gelatin-Based Hydrogels into the Titanium Foams
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chicardi, E.; Gutiérrez-González, C.F.; Sayagués, M.J.; García-Garrido, C. Development of a novel TiNbTa material potentially suitable for bone replacement implants. Mater. Des. 2018, 145, 88–96. [Google Scholar] [CrossRef]
- Giner, M.; Chicardi, E.; de Costa, A.F.; Santana, L.; Vázquez-Gámez, M.Á.; García-Garrido, C.; Colmenero, M.A.; Olmo-Montes, F.J.; Torres, Y.; Montoya-García, M.J. Biocompatibility and Cellular Behavior of TiNbTa Alloy with Adapted Rigidity for the Replacement of Bone Tissue. Metals 2021, 11, 130. [Google Scholar] [CrossRef]
- Parithimarkalaignan, S.; Padmanabhan, T.V. Osseointegration: An Update. J. Indian Prosthodont. Soc. 2013, 13, 2. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Wong, J.Y.; Bronzino, J.D. Biomaterials; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Russo, T.; De Santis, R.; Gloria, A.; Barbaro, K.; Altigeri, A.; Fadeeva, I.; Rau, J. Modification of PMMA Cements for Cranioplasty with Bioactive Glass and Copper Doped Tricalcium Phosphate Particles. Polymers 2020, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, S.; Tsoi, J.K.H.; Matinlinna, J.P. Binary titanium alloys as dental implant materials—A review. Regen. Biomater. 2017, 4, 315. [Google Scholar] [CrossRef] [PubMed]
- Gottlow, J.; Dard, M.; Kjellson, F.; Obrecht, M.; Sennerby, L. Evaluation of a New Titanium-Zirconium Dental Implant: A Biomechanical and Histological Comparative Study in the Mini Pig. Clin. Implant Dent. Relat. Res. 2012, 14, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Aik Khor, K. Preparation and Properties of Coatings and Thin Films on Metal Implants. Encycl. Biomed. Eng. 2019, 1–3, 203–212. [Google Scholar]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases WJCC 2015, 3, 52. [Google Scholar] [CrossRef]
- Ellakany, P.; AlGhamdi, M.A.; Alshehri, T.; Abdelrahman, Z. Cytotoxicity of Commercially Pure Titanium (cpTi), Silver-Palladium (Ag-Pd), and Nickel-Chromium (Ni-Cr) Alloys Commonly Used in the Fabrication of Dental Prosthetic Restorations. Cureus 2022, 14, e31679. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Costa Pessoa, J. Vanadium ionic species from degradation of Ti-6Al-4V metallic implants: In vitro cytotoxicity and speciation evaluation. Mater. Sci. Eng. C 2019, 96, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; You, Y.; Li, B.; Song, Y.; Ma, A.; Chen, B.; Han, W.; Li, C. Improved Cell Adhesion and Osseointegration on Anodic Oxidation Modified Titanium Implant Surface. J. Hard Tissue Biol. 2019, 28, 13–20. [Google Scholar] [CrossRef]
- Sakka, S.; Coulthard, P. Implant failure: Etiology and complications. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e42–e44. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, Q.; Qian, Z.; Liu, D.; Liu, H. Surface Modification of Dental Titanium Implant by Layer-by-Layer Electrostatic Self-Assembly. Front. Physiol. 2017, 8, 574. [Google Scholar] [CrossRef]
- Matos, G.R.M. Surface Roughness of Dental Implant and Osseointegration. J. Maxillofac. Oral Surg. 2021, 20, 1–4. [Google Scholar] [CrossRef]
- Syam, S.; Wu, C.-J.; Lan, W.-C.; Ou, K.-L.; Huang, B.-H.; Lin, Y.-Y.; Saito, T.; Tsai, H.-Y.; Chuo, Y.-C.; Yen, M.-L.; et al. The Potential of a Surface-Modified Titanium Implant with Tetrapeptide for Osseointegration Enhancement. Appl. Sci. 2021, 11, 2616. [Google Scholar] [CrossRef]
- Elias, C.N.; Meirelles, L. Improving osseointegration of dental implants. Expert Rev. Med. Devices 2010, 7, 241–256. [Google Scholar] [CrossRef]
- Nuss, K.M.; Rechenberg, B. von Biocompatibility Issues with Modern Implants in Bone—A Review for Clinical Orthopedics. Open Orthop. J. 2008, 2, 66. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hady Gepreel, M.; Niinomi, M. Biocompatibility of Ti-alloys for long-term implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef]
- Wilk, A.; Szypulska-Koziarska, D.; Wiszniewska, B. The toxicity of vanadium on gastrointestinal, urinary and reproductive system, and its influence on fertility and fetuses malformations. Postepy Hig. Med. Dosw. 2017, 71, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.V.; Puleo, D.A. Infection, Inflammation, and Bone Regeneration: A Paradoxical Relationship. J. Dent. Res. 2011, 90, 1052. [Google Scholar] [CrossRef] [PubMed]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.-G.; Wang, L.; Li, X.R.; Williams, G. Energy-Saving Electrospinning with a Concentric Teflon-Core Rod Spinneret to Create Medicated Nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef]
- Sharma, S.; Tiwari, S. A review on biomacromolecular hydrogel classification and its applications. Int. J. Biol. Macromol. 2020, 162, 737–747. [Google Scholar] [CrossRef]
- Ali, I.; Althakfi, S.H.; Suhail, M.; Locatelli, M.; Hsieh, M.-F.; Alsehli, M.; Hameed, A.M. Advances in Polymeric Colloids for Cancer Treatment. Polymers 2022, 14, 5445. [Google Scholar] [CrossRef] [PubMed]
- Albensi, B.C. What Is Nuclear Factor Kappa B (NF-κB) Doing in and to the Mitochondrion? Front. Cell Dev. Biol. 2019, 7, 154. [Google Scholar] [CrossRef]
- Hao, M.; Peng, X.; Sun, S.; Ding, C.; Liu, W. Chitosan/Sodium Alginate/Velvet Antler Blood Peptides Hydrogel Promoted Wound Healing by Regulating PI3K/AKT/mTOR and SIRT1/NF-κB Pathways. Front. Pharmacol. 2022, 13, 913408. [Google Scholar] [CrossRef]
- Hao, T.; Qian, M.; Zhang, Y.; Liu, Q.; Midgley, A.C.; Liu, Y.; Che, Y.; Hou, J.; Zhao, Q. An Injectable Dual-Function Hydrogel Protects Against Myocardial Ischemia/Reperfusion Injury by Modulating ROS/NO Disequilibrium. Adv. Sci. 2022, 9, 2105408. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, Z.; Zhou, Y.; Yu, Z.; Mai, H.; Xu, C.; Zhang, J.; Wang, J. The novel hyaluronic acid granular hydrogel attenuates osteoarthritis progression by inhibiting the TLR-2/ NF-κB signaling pathway through suppressing cellular senescence. Bioeng. Transl. Med. 2022. [Google Scholar] [CrossRef]
- Chafran, L.; Carfagno, A.; Altalhi, A.; Bishop, B. Green Hydrogel Synthesis: Emphasis on Proteomics and Polymer Particle-Protein Interaction. Polymers 2022, 14, 4755. [Google Scholar] [CrossRef]
- Sola, D.; Cases, R. High-Repetition-Rate Femtosecond Laser Processing of Acrylic Intra-Ocular Lenses. Polymers 2020, 12, 242. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate Hydrogels as Biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Wu, J.; Zhu, J.J.; Xiao, Z.C.; He, C.C.; Shi, H.X.; Li, X.K.; Yang, S.L.; Xiao, J. Research Advances in Tissue Engineering Materials for Sustained Release of Growth Factors. Biomed Res. Int. 2015, 2015, 808202. [Google Scholar] [CrossRef]
- Lv, H.; Guo, S.; Zhang, G.; He, W.; Wu, Y.; Yu, D.-G. Electrospun Structural Hybrids of Acyclovir-Polyacrylonitrile at Acyclovir for Modifying Drug Release. Polymers 2021, 13, 4286. [Google Scholar] [CrossRef] [PubMed]
- Yaszemski, M.J.; Payne, R.G.; Hayes, W.C.; Langer, R.S.; Aufdemorte, T.B.; Mikos, A.G. The Ingrowth of New Bone Tissue and Initial Mechanical Properties of a Degrading Polymeric Composite Scaffold. Tissue Eng. 2007, 1, 41–52. Available online: https://home.liebertpub.com/ten (accessed on 18 September 2021). [CrossRef] [PubMed]
- Ahmady, A.; Abu Samah, N.H. A review: Gelatine as a bioadhesive material for medical and pharmaceutical applications. Int. J. Pharm. 2021, 608, 121037. [Google Scholar] [CrossRef] [PubMed]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Fabrication and characterization of hydrogels based on gelatinised collagen with potential application in tissue engineering. Polymers 2020, 12, 1146. [Google Scholar] [CrossRef]
- Essawy, H.A.; Ghazy, M.B.M.; El-Hai, F.A.; Mohamed, M.F. Superabsorbent hydrogels via graft polymerization of acrylic acid from chitosan-cellulose hybrid and their potential in controlled release of soil nutrients. Int. J. Biol. Macromol. 2016, 89, 144–151. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Pranantyo, D.; Kang, E.-T.; Chan-Park, M.B. Smart nanomicelles with bacterial infection-responsive disassembly for selective antimicrobial applications. Biomater. Sci. 2021, 9, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A.; Punset, M.; Calero, J.A.; Gil, F.J.; Ruperez, E.; Manero, J.M. Powder metallurgy with space holder for porous titanium implants: A review. J. Mater. Sci. Technol. 2021, 76, 129–149. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Alonso-González, M.; Romero, A.; Perez-Puyana, V. Applied Rheology as Tool for the Assessment of Chitosan Hydrogels for Regenerative Medicine. Polymers 2021, 13, 2189. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Wellard, R.M.; Woodruff, M.; Klein, T. Tailoring Hydrogel Viscoelasticity with Physical and Chemical Crosslinking. Polymers 2015, 7, 2650–2669. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Rheological and Microstructural Evaluation of Collagen-Based Scaffolds Crosslinked with Fructose. Polymers 2021, 13, 632. [Google Scholar] [CrossRef]
- George, B.; Bhatia, N.; Kumar, A.; SK, S.; Vadakkadath Meethal, K.; TM, S.; TV, S. Bioinspired gelatin based sticky hydrogel for diverse surfaces in burn wound care. Sci. Rep. 2022, 12, 13735. [Google Scholar] [CrossRef]
- Ahmadian, E.; Eftekhari, A.; Kavetskyy, T.; Khosroushahi, A.Y.; Turksoy, V.A.; Khalilov, R. Effects of quercetin loaded nanostructured lipid carriers on the paraquat-induced toxicity in human lymphocytes. Pestic. Biochem. Physiol. 2020, 167, 104586. [Google Scholar] [CrossRef]
- Kanokpanont, S.; Damrongsakkul, S.; Ratanavaraporn, J.; Aramwit, P. An innovative bi-layered wound dressing made of silk and gelatin for accelerated wound healing. Int. J. Pharm. 2012, 436, 141–153. [Google Scholar] [CrossRef]
- Conrad, B.; Hayashi, C.; Yang, F. Gelatin-Based Microribbon Hydrogels Guided Mesenchymal Stem Cells to Undergo Endochondral Ossification In Vivo with Bone-Mimicking Mechanical Strength. Regen. Eng. Transl. Med. 2021, 7, 301–311. [Google Scholar] [CrossRef]
- Cao, C.; Yang, N.; Zhao, Y.; Yang, D.; Hu, Y.; Yang, D.; Song, X.; Wang, W.; Dong, X. Biodegradable hydrogel with thermo-response and hemostatic effect for photothermal enhanced anti-infective therapy. Nano Today 2021, 39, 101165. [Google Scholar] [CrossRef]
- Chang, W.-C.; Tai, A.-Z.; Tsai, N.-Y.; Li, Y.-C.E. An Injectable Hybrid Gelatin Methacryloyl (GelMA)/Phenyl Isothiocyanate-Modified Gelatin (Gel-Phe) Bioadhesive for Oral/Dental Hemostasis Applications. Polymers 2021, 13, 2386. [Google Scholar] [CrossRef]
- Torres-Sanchez, C.; Norrito, M.; Almushref, F.R.; Conway, P.P. The impact of multimodal pore size considered independently from porosity on mechanical performance and osteogenic behaviour of titanium scaffolds. Mater. Sci. Eng. C 2021, 124, 112026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.C.; Chen, L.Y. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef]
- Lloreda-Jurado, P.J.; Chicardi, E.; Paúl, A.; Sepúlveda, R. Effect of processing parameters on the properties of freeze-cast Ni wick with gradient porosity. Mater. Des. 2021, 206, 109795. [Google Scholar] [CrossRef]
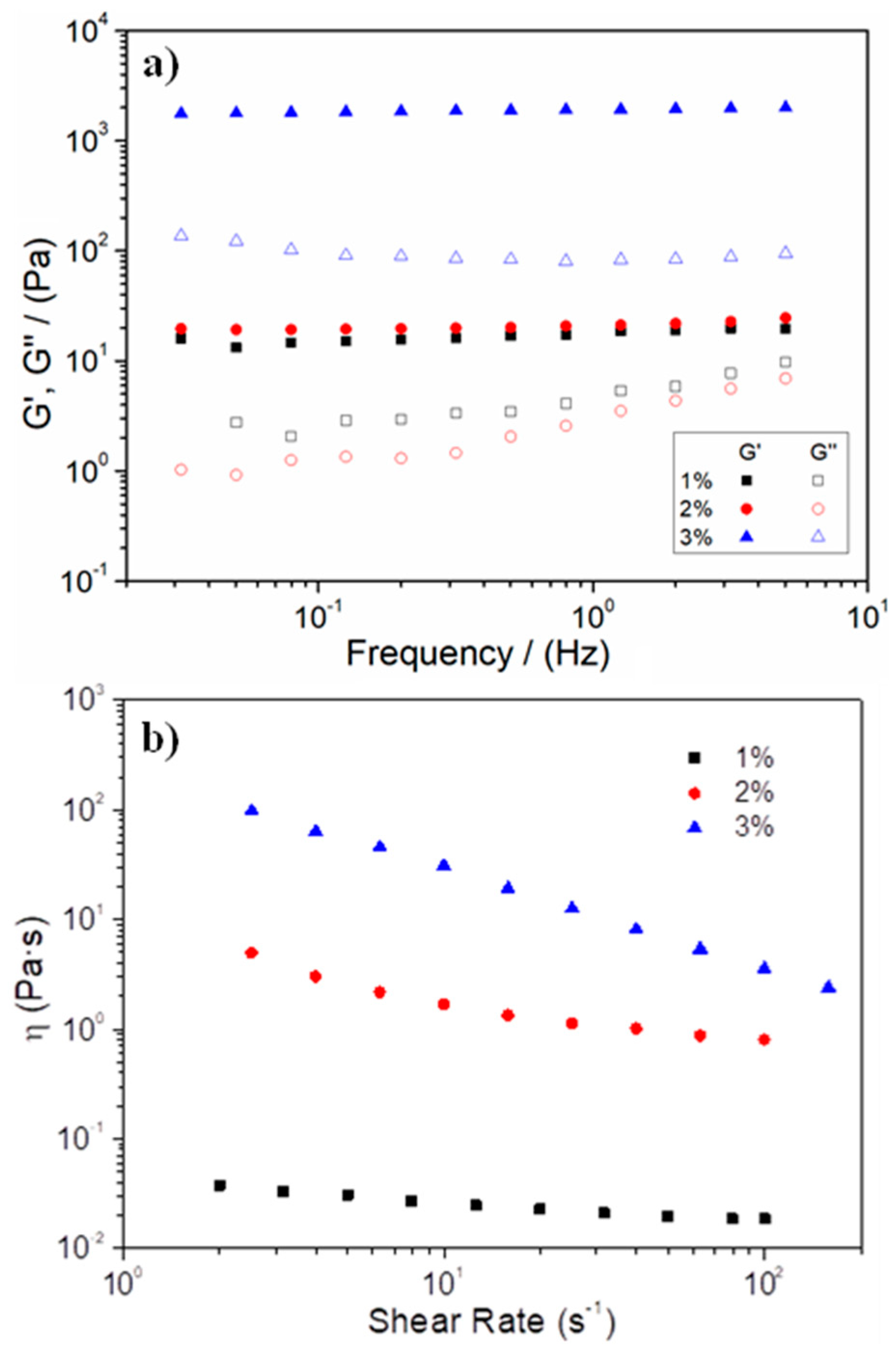

| Systems | Critical Strain (%) | G′1 (Pa) | tan δ1 (-) | η100 (Pa·s) | k (min−n) | n |
|---|---|---|---|---|---|---|
| 1% hydrogel | 0.5 ± 0.2 | 17.9 ± 4.1 | 0.26 ± 0.03 | 0.06 ± 0.05 | 51.9 | 0.19 |
| 2% hydrogel | 20.6 ± 6.6 | 18.3 ± 3.7 | 0.19 ± 0.02 | 0.63 ± 0.04 | 17.3 | 0.51 |
| 3% hydrogel | 14.8 ± 0.3 | 1983 ± 75.5 | 0.04 ± 0.01 | 3.69 ± 0.21 | 8.6 | 0.64 |
| Specimen | Absolute Density (g·cm−3) | Relative Densification (%) | Total Porosity (%) | Open Porosity (%) | Closed Porosity (%) |
|---|---|---|---|---|---|
| Ti30 | 2.8 ± 0.2 | 61.0 ± 2.7 | 39.0 ± 2.7 | 34.3 ± 2.1 | 4.7 ± 2.5 |
| Ti60 | 1.8 ± 0.1 | 39.6 ± 0.1 | 60.4 ± 0.1 | 43.8 ± 3.3 | 16.6 ± 6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehdi-Sefiani, H.; Perez-Puyana, V.; Ostos, F.J.; Sepúlveda, R.; Romero, A.; Rafii-El-Idrissi Benhnia, M.; Chicardi, E. Type-A Gelatin-Based Hydrogel Infiltration and Degradation in Titanium Foams as a Potential Method for Localised Drug Delivery. Polymers 2023, 15, 275. https://doi.org/10.3390/polym15020275
Mehdi-Sefiani H, Perez-Puyana V, Ostos FJ, Sepúlveda R, Romero A, Rafii-El-Idrissi Benhnia M, Chicardi E. Type-A Gelatin-Based Hydrogel Infiltration and Degradation in Titanium Foams as a Potential Method for Localised Drug Delivery. Polymers. 2023; 15(2):275. https://doi.org/10.3390/polym15020275
Chicago/Turabian StyleMehdi-Sefiani, Hanaa, Víctor Perez-Puyana, Francisco José Ostos, Ranier Sepúlveda, Alberto Romero, Mohammed Rafii-El-Idrissi Benhnia, and Ernesto Chicardi. 2023. "Type-A Gelatin-Based Hydrogel Infiltration and Degradation in Titanium Foams as a Potential Method for Localised Drug Delivery" Polymers 15, no. 2: 275. https://doi.org/10.3390/polym15020275
APA StyleMehdi-Sefiani, H., Perez-Puyana, V., Ostos, F. J., Sepúlveda, R., Romero, A., Rafii-El-Idrissi Benhnia, M., & Chicardi, E. (2023). Type-A Gelatin-Based Hydrogel Infiltration and Degradation in Titanium Foams as a Potential Method for Localised Drug Delivery. Polymers, 15(2), 275. https://doi.org/10.3390/polym15020275











