Dynamic Surface Properties of α-Lactalbumin Fibril Dispersions
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Sample Preparation
2.3. Methods
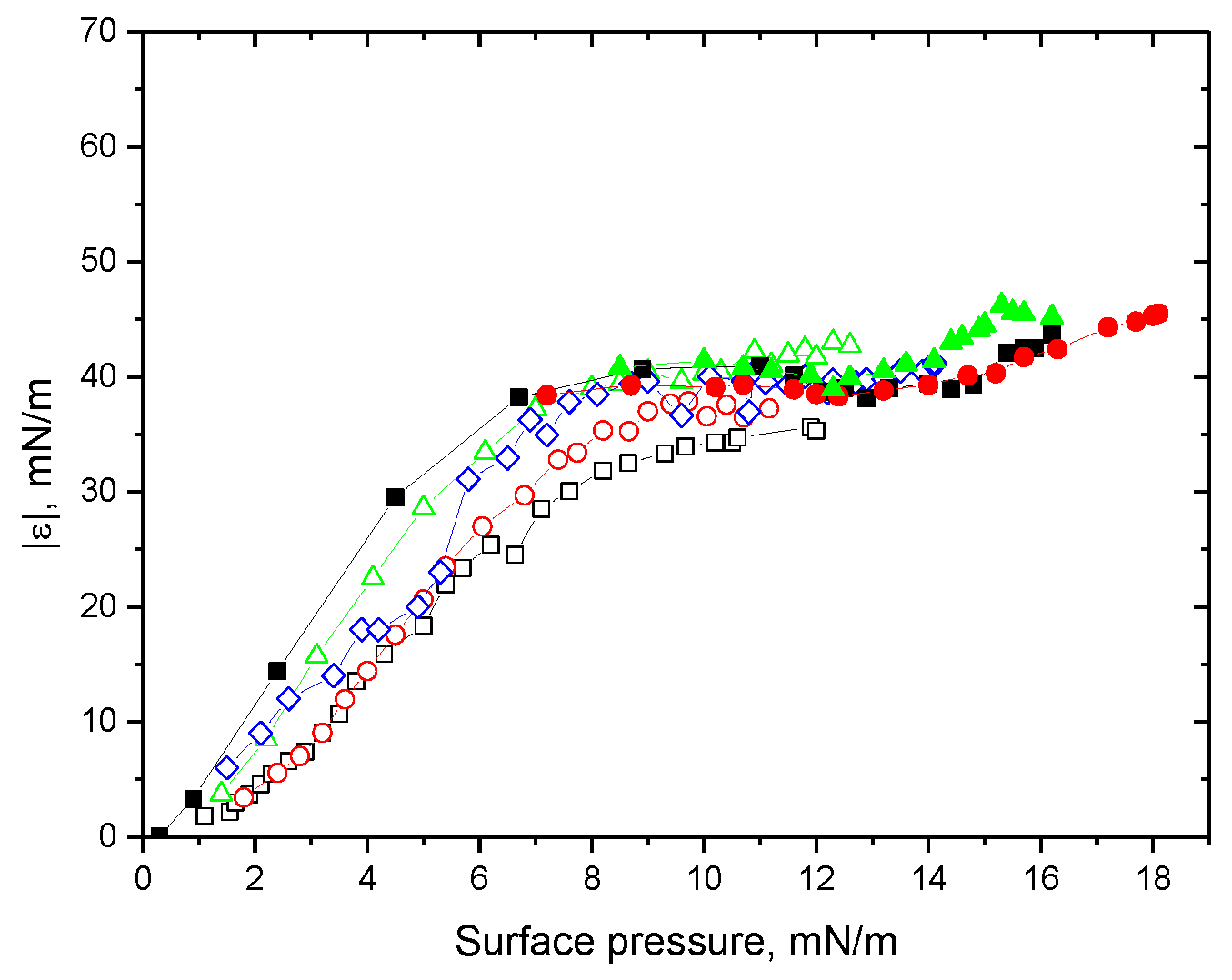
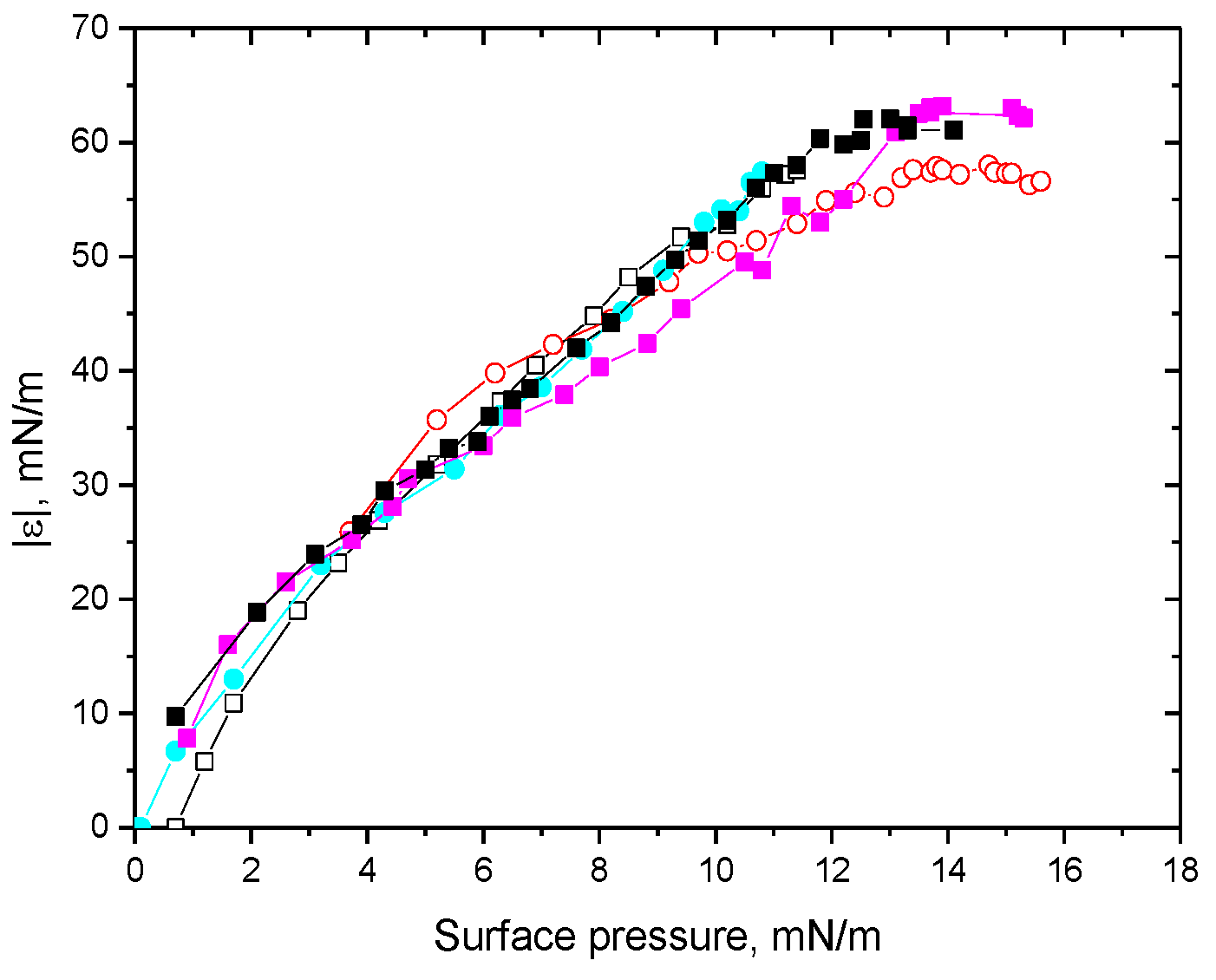
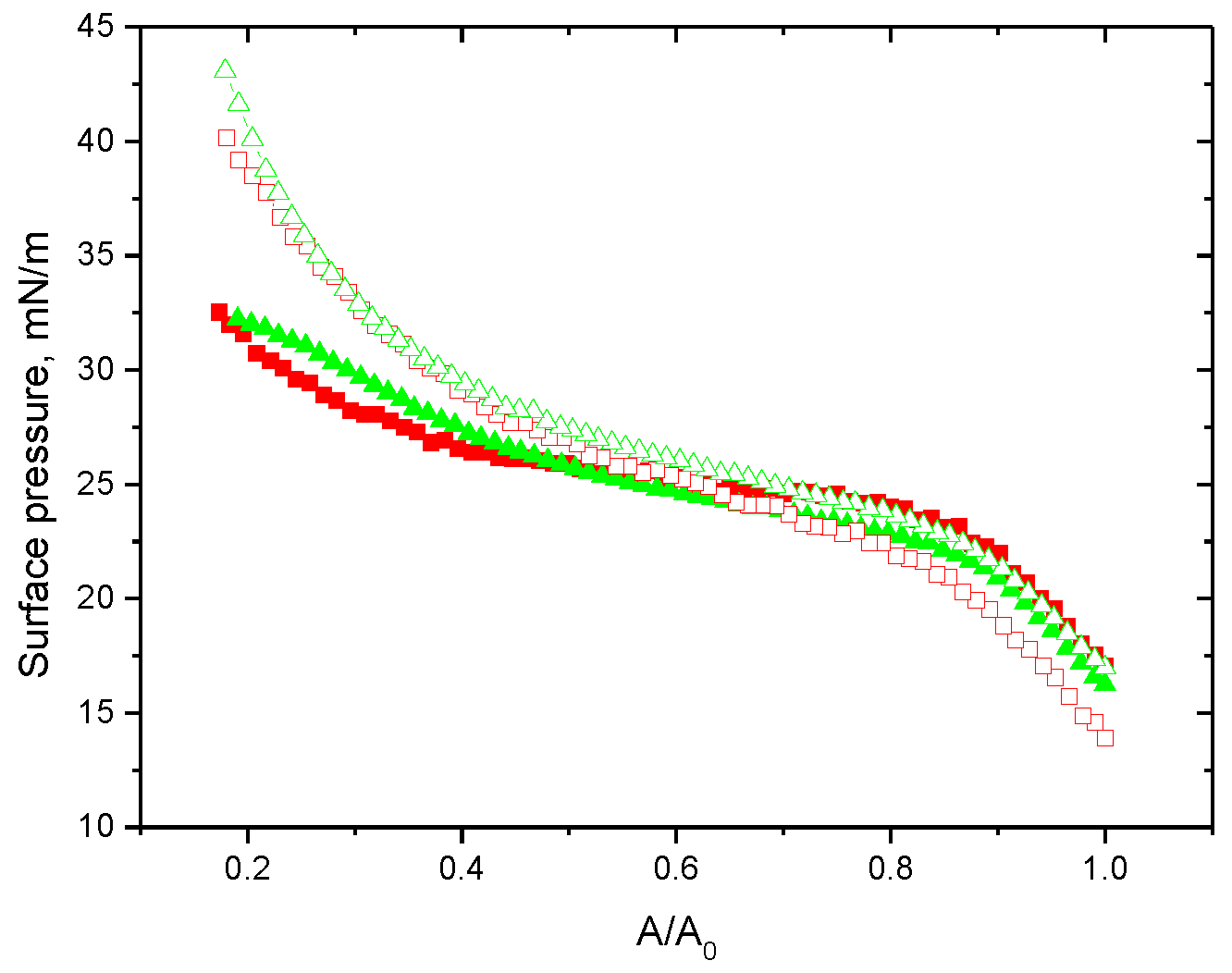
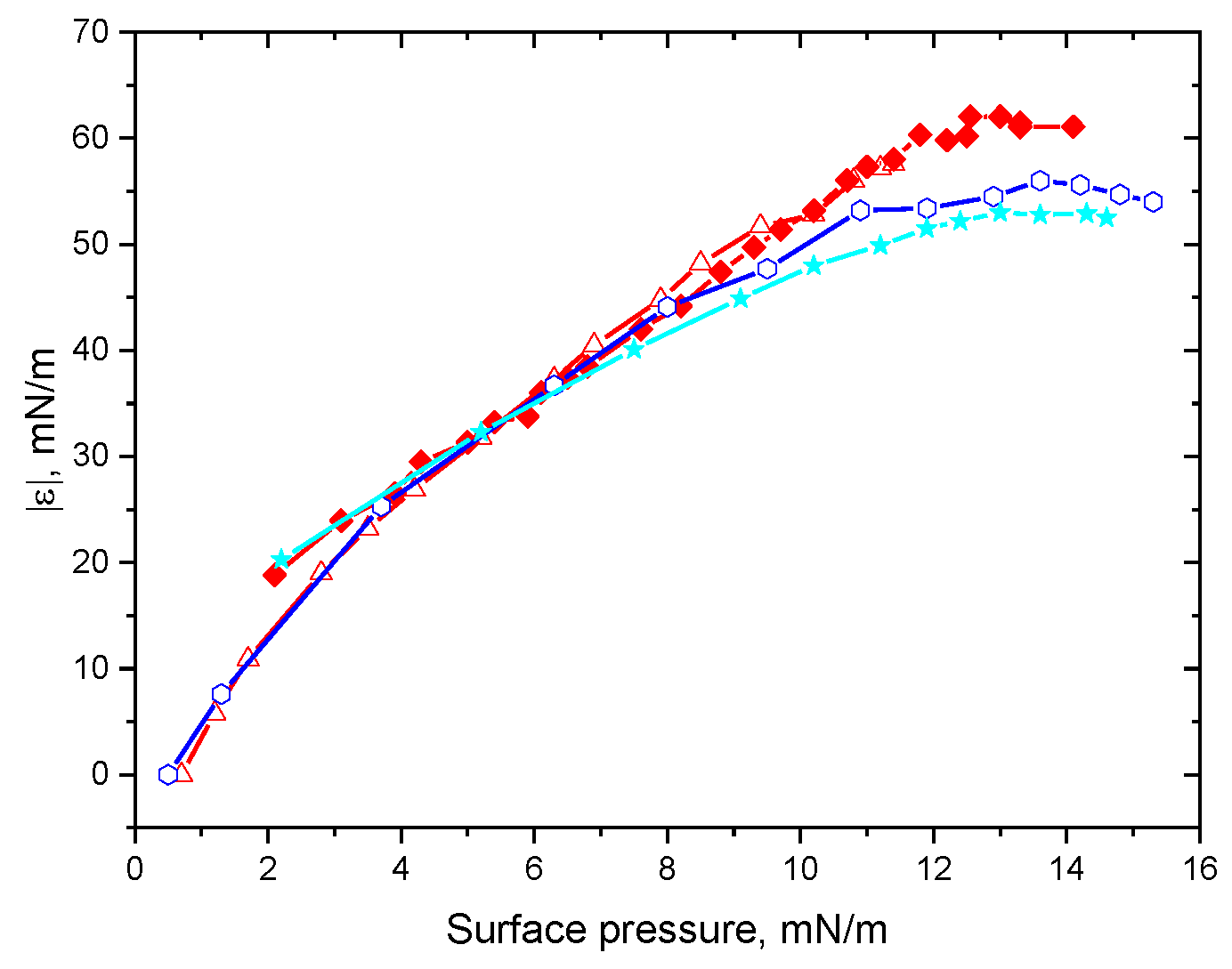
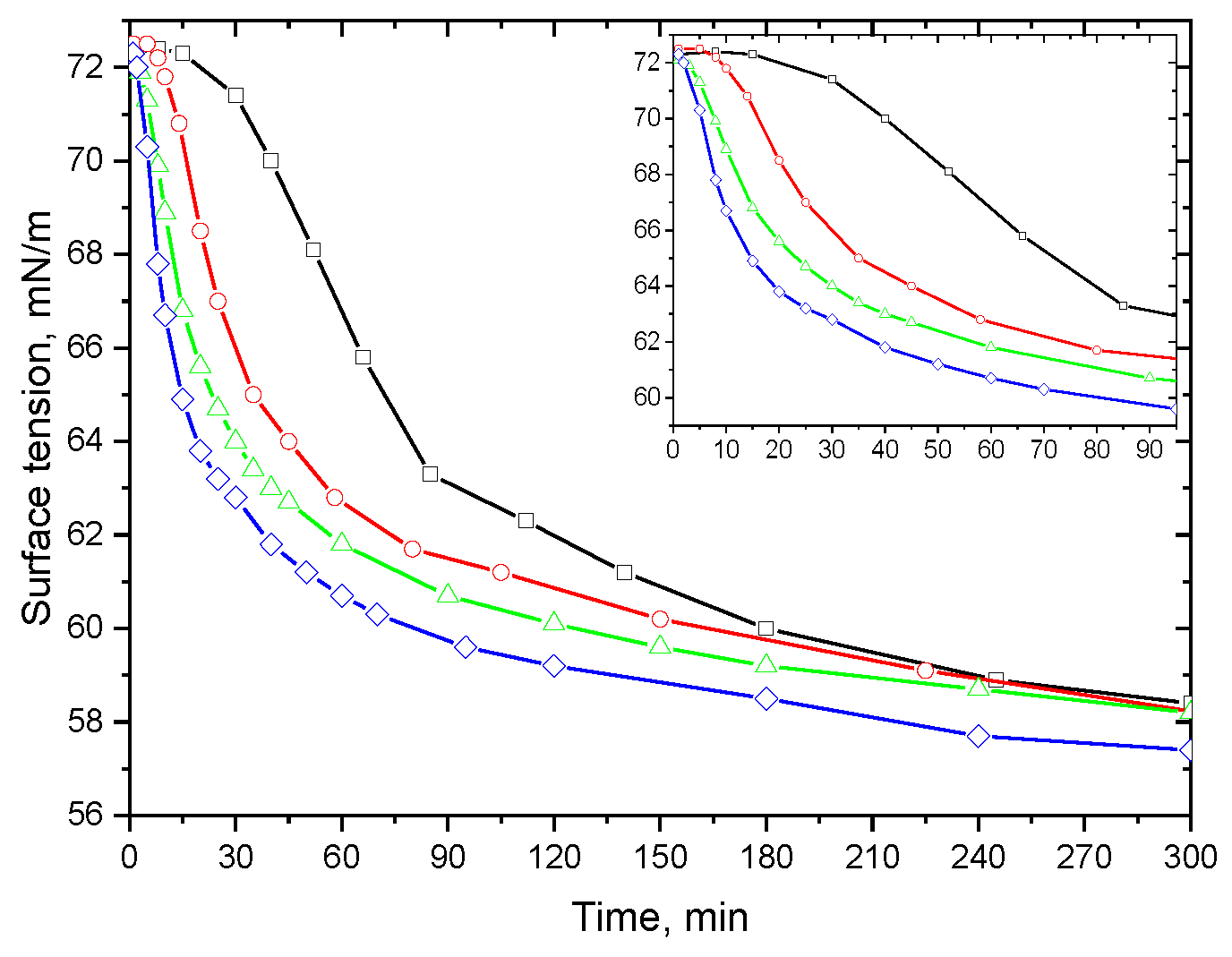
3. Results
3.1. Native Protein
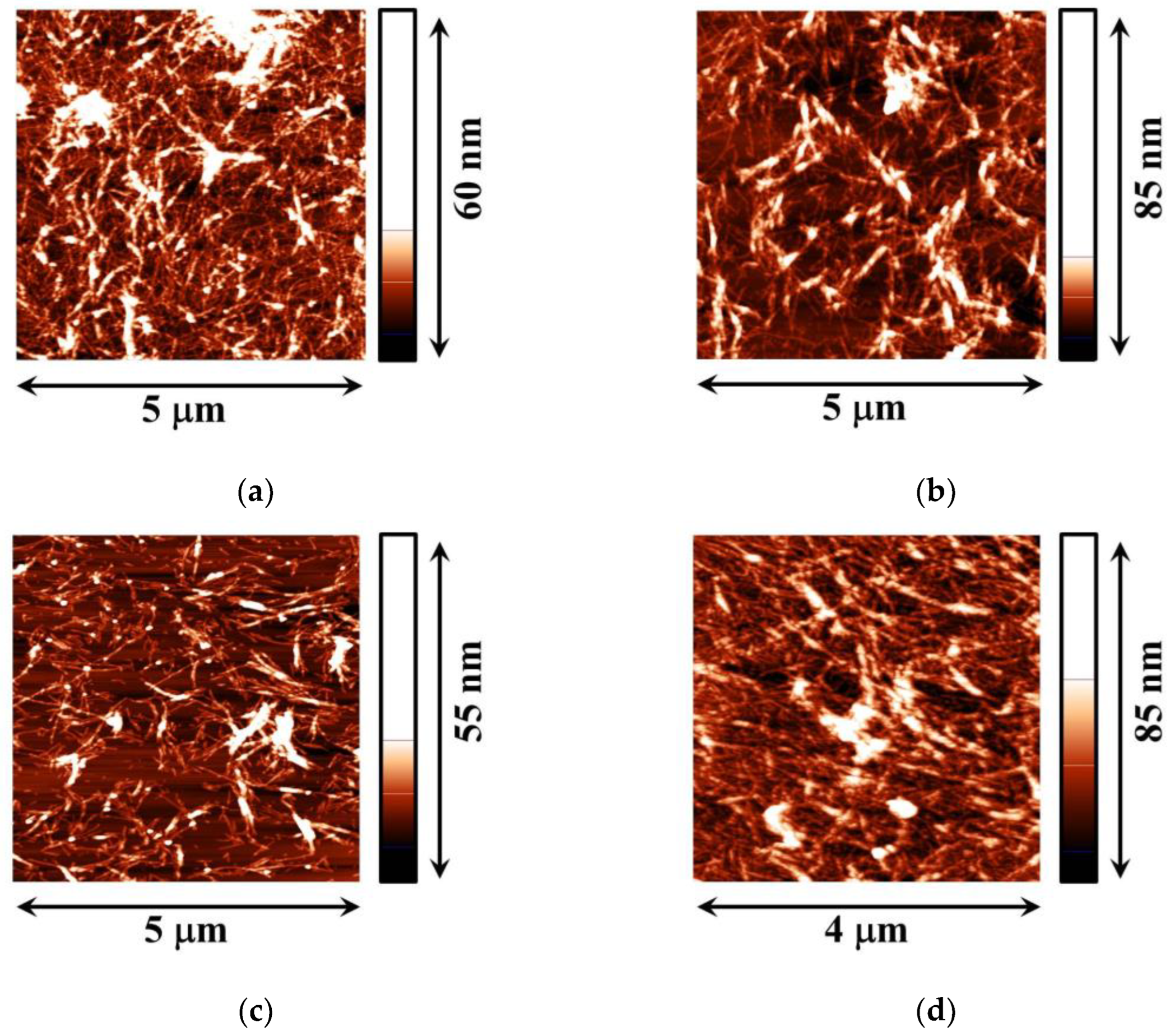
3.2. Fibrils of Proteins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knowles, T.P.J.; Mezzenga, R. Amyloid Fibrils as Building Blocks for Natural and Artificial Functional Materials. Adv. Mater. 2016, 28, 6546–6561. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qin, R.; Liu, R.; Miao, S.; Yang, P. Functional Amyloid Materials at Surfaces/Interfaces. Biomater. Sci. 2018, 6, 462–472. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Food Protein Amyloid Fibrils: Origin, Structure, Formation, Characterization, Applications and Health Implications. Adv. Colloid Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef]
- Ke, P.C.; Zhou, R.; Serpell, L.C.; Riek, R.; Knowles, T.P.J.; Lashuel, H.A.; Gazit, E.; Hamley, I.W.; Davis, T.P.; Fändrich, M.; et al. Half a Century of Amyloids: Past, Present and Future. Chem. Soc. Rev. 2020, 49, 5473–5509. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Wei, Z.; Xue, C. Protein Fibrils from Different Food Sources: A Review of Fibrillation Conditions, Properties, Applications and Research Trends. Trends Food Sci. Technol. 2022, 121, 59–75. [Google Scholar] [CrossRef]
- Li, T.; Zhou, J.; Peydayesh, M.; Yao, Y.; Bagnani, M.; Kutzli, I.; Chen, Z.; Wang, L.; Mezzenga, R. Plant Protein Amyloid Fibrils for Multifunctional Sustainable Materials. Adv. Sustain. Syst. 2023, 7, 2200414. [Google Scholar] [CrossRef]
- John, T.; Martin, L.L.; Abel, B. Peptide Self-Assembly into Amyloid Fibrils at Hard and Soft Interfaces—From Corona Formation to Membrane Activity. Macromol. Biosci. 2023, 23, 2200576. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Cao, Y.; Zhou, J.; Yao, Y.; Wu, X.; Bolisetty, S.; Diener, M.; Handschin, S.; Lu, C.; Mezzenga, R. Interfacial Electrostatic Self-Assembly of Amyloid Fibrils into Multifunctional Protein Films. Adv Sci. 2023, 10, 2206867. [Google Scholar] [CrossRef]
- Nicolai, T.; Britten, M.; Schmitt, C. β-Lactoglobulin and WPI Aggregates: Formation, Structure and Applications. Food Hydrocoll. 2011, 25, 1945–1962. [Google Scholar] [CrossRef]
- Oboroceanu, D.; Wang, L.; Magner, E.; Auty, M.A.E. Fibrillization of Whey Proteins Improves Foaming Capacity and Foam Stability at Low Protein Concentrations. J. Food Eng. 2014, 121, 102–111. [Google Scholar] [CrossRef]
- Peng, J.; Simon, J.R.; Venema, P.; Van Der Linden, E. Protein Fibrils Induce Emulsion Stabilization. Langmuir 2016, 32, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Yang, X.; Sagis, L.M.C. Nonlinear Surface Dilatational Rheology and Foaming Behavior of Protein and Protein Fibrillar Aggregates in the Presence of Natural Surfactant. Langmuir 2016, 32, 3679–3690. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Yang, X.; Sagis, L.M.C. Contribution of Long Fibrils and Peptides to Surface and Foaming Behavior of Soy Protein Fibril System. Langmuir 2016, 32, 8092–8101. [Google Scholar] [CrossRef] [PubMed]
- Loveday, S.M.; Anema, S.G.; Singh, H. β-Lactoglobulin Nanofibrils: The Long and the Short of It. Int. Dairy J. 2017, 67, 35–45. [Google Scholar] [CrossRef]
- Peng, D.; Yang, J.; Li, J.; Tang, C.; Li, B. Foams Stabilized by β-Lactoglobulin Amyloid Fibrils: Effect of PH. J. Agric. Food Chem. 2017, 65, 10658–10665. [Google Scholar] [CrossRef]
- Mantovani, R.A.; de Figueiredo Furtado, G.; Netto, F.M.; Cunha, R.L. Assessing the Potential of Whey Protein Fibril as Emulsifier. J. Food Eng. 2018, 223, 99–108. [Google Scholar] [CrossRef]
- Mohammadian, M.; Madadlou, A. Technological Functionality and Biological Properties of Food Protein Nanofibrils Formed by Heating at Acidic Condition. Trends Food Sci. Technol. 2018, 75, 115–128. [Google Scholar] [CrossRef]
- Hu, J.; Yang, J.; Xu, Y.; Zhang, K.; Nishinari, K.; Phillips, G.O.; Fang, Y. Comparative Study on Foaming and Emulsifying Properties of Different Beta-Lactoglobulin Aggregates. Food Funct. 2019, 10, 5922–5930. [Google Scholar] [CrossRef]
- Murray, B.S. Recent Developments in Food Foams. Curr. Opin. Colloid Interface Sci. 2020, 50, 101394. [Google Scholar] [CrossRef]
- Fan, Y.; Peng, G.; Pang, X.; Wen, Z.; Yi, J. Physicochemical, Emulsifying, and Interfacial Properties of Different Whey Protein Aggregates Obtained by Thermal Treatment. LWT 2021, 149, 111904. [Google Scholar] [CrossRef]
- Peng, D.; Jin, W.; Sagis, L.M.C.; Li, B. Adsorption of Microgel Aggregates Formed by Assembly of Gliadin Nanoparticles and a β-Lactoglobulin Fibril-Peptide Mixture at the Air/Water Interface: Surface Morphology and Foaming Behavior. Food Hydrocoll. 2022, 122, 107039. [Google Scholar] [CrossRef]
- Jiang, F.; Pan, Y.; Peng, D.; Huang, W.; Shen, W.; Jin, W.; Huang, Q. Tunable Self-Assemblies of Whey Protein Isolate Fibrils for Pickering Emulsions Structure Regulation. Food Hydrocoll. 2022, 124, 107264. [Google Scholar] [CrossRef]
- Han, Y.; Zhu, L.; Karrar, E.; Qi, X.; Zhang, H.; Wu, G. Pickering Foams Stabilized by Protein-Based Particles: A Review of Characterization, Stabilization, and Application. Trends Food Sci. Technol. 2023, 133, 148–159. [Google Scholar] [CrossRef]
- Jung, J.-M.; Gunes, D.Z.; Mezzenga, R. Interfacial Activity and Interfacial Shear Rheology of Native β-Lactoglobulin Monomers and Their Heat-Induced Fibers. Langmuir 2010, 26, 15366–15375. [Google Scholar] [CrossRef] [PubMed]
- Noskov, B.A.; Akentiev, A.V.; Bykov, A.G.; Loglio, G.; Miller, R.; Milyaeva, O.Y. Spread and Adsorbed Layers of Protein Fibrils at Water–Air Interface. Colloids Surf. B Biointerfaces 2022, 220, 112942. [Google Scholar] [CrossRef]
- Noskov, B.A.; Rafikova, A.R.; Milyaeva, O.Y. β-Lactoglobulin Microgel Layers at the Surface of Aqueous Solutions. J. Mol. Liq. 2022, 351, 118658. [Google Scholar] [CrossRef]
- Ruhs, P.A.; Scheuble, N.; Windhab, E.J.; Mezzenga, R.; Fischer, P. Simultaneous Control of PH and Ionic Strength during Interfacial Rheology of Beta-Lactoglobulin Fibrils Adsorbed at Liquid/Liquid Interfaces. Langmuir 2012, 28, 12536. [Google Scholar] [CrossRef]
- Rühs, P.A.; Affolter, C.; Windhab, E.J.; Fischer, P. Shear and Dilatational Linear and Nonlinear Subphase Controlled Interfacial Rheology of β-Lactoglobulin Fibrils and Their Derivatives. J. Rheol. 2013, 57, 1003–1022. [Google Scholar] [CrossRef]
- Rühs, P.A.; Scheuble, N.; Windhab, E.J.; Fischer, P. Protein Adsorption and Interfacial Rheology Interfering in Dilatational Experiment. Eur. Phys. J. Spec. Top. 2013, 222, 47–60. [Google Scholar] [CrossRef]
- Humblet-Hua, N.-P.K.; van der Linden, E.; Sagis, L.M.C. Surface Rheological Properties of Liquid–Liquid Interfaces Stabilized by Protein Fibrillar Aggregates and Protein–Polysaccharide Complexes. Soft Matter 2013, 9, 2154–2165. [Google Scholar] [CrossRef]
- Sagis, L.M.C.; Humblet-Hua, K.N.P.; Van Kempen, S.E.H.J. Nonlinear Stress Deformation Behavior of Interfaces Stabilized by Food-Based Ingredients. J. Phys. Condens. Matter 2014, 26, 464105. [Google Scholar] [CrossRef] [PubMed]
- Jordens, S.; Rühs, P.A.; Sieber, C.; Isa, L.; Fischer, P.; Mezzenga, R. Bridging the Gap between the Nanostructural Organization and Macroscopic Interfacial Rheology of Amyloid Fibrils at Liquid Interfaces. Langmuir 2014, 30, 10090–10097. [Google Scholar] [CrossRef] [PubMed]
- Jordens, S.; Riley, E.E.; Usov, I.; Isa, L.; Olmsted, P.D.; Mezzenga, R. Adsorption at Liquid Interfaces Induces Amyloid Fibril Bending and Ring Formation. ACS Nano 2014, 8, 11071–11079. [Google Scholar] [CrossRef] [PubMed]
- Permyakov, E.A. α-Lactalbumin, Amazing Calcium-Binding Protein. Biomolecules 2020, 10, 1210. [Google Scholar] [CrossRef]
- Kurouski, D.; Lauro, W.; Lednev, I.K. Amyloid Fibrils Are “Alive”: Spontaneous Refolding from One Polymorph to Another. Chem. Commun. 2010, 46, 4249–4251. [Google Scholar] [CrossRef]
- Rahamtullah, R.M. Nicking and fragmentation are responsible for α-lactalbumin amyloid fibril formation at acidic pH and elevated temperature. Protein Sci. 2021, 30, 1919–1934. [Google Scholar] [CrossRef]
- Noskov, B.A.; Akentiev, A.V.; Bilibin, A.Y.; Zorin, I.M.; Miller, R. Dilational Surface Viscoelasticity of Polymer Solutions. Adv. Colloid Interface Sci. 2003, 104, 245–271. [Google Scholar] [CrossRef]
- Noskov, B.A.; Loglio, G.; Miller, R. Dilational Surface Visco-Elasticity of Polyelectrolyte/Surfactant Solutions: Formation of Heterogeneous Adsorption Layers. Adv. Colloid Interface Sci. 2011, 168, 179–197. [Google Scholar] [CrossRef]
- Langmuir, I.; Schaefer, J. Properties and Structure Protein Monolayers. Chem. Rev. 1938, 24, 181–202. [Google Scholar] [CrossRef]
- Graham, D.E.; Phillips, M.C. Proteins at Liquid Interfaces. IV. Dilatational Properties. J. Colloid Interface Sci. 1980, 76, 227–239. [Google Scholar] [CrossRef]
- Lu, J.R.; Su, T.J.; Thomas, R.K. Structural Conformation of Bovine Serum Albumin Layers at the Air-Water Interface Studied by Neutron Reflection. J. Colloid Interface Sci. 1999, 213, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.R.; Su, T.J.; Howlin, B.J. The Effect of Solution PH on the Structural Conformation of Lysozyme Layers Adsorbed on the Surface of Water. J. Phys. Chem. B 1999, 103, 5903–5909. [Google Scholar] [CrossRef]
- Taylor, D.J.F.; Thomas, R.K.; Penfold, J. Polymer/Surfactant Interactions at the Air/Water Interface. Adv. Colloid Interface Sci. 2007, 132, 69–110. [Google Scholar] [CrossRef]
- Meinders, M.B.J.; De Jongh, H.H.J. Limited Conformational Change of β-Lactoglobulin When Adsorbed at the Air-Water Interface. Biopolym.-Biospectrosc. Sect. 2002, 67, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.H.; Meinders, M.B.J.; Bos, M.A.; Cohen Stuart, M.A.; van Vliet, T. Conformational Aspects of Proteins at the Air/Water Interface Studied by Infrared Reflection−Absorption Spectroscopy. Langmuir 2003, 19, 2922–2928. [Google Scholar] [CrossRef]
- Noskov, B.A.; Mikhailovskaya, A.A.A. Adsorption Kinetics of Globular Proteins and Protein/Surfactant Complexes at the Liquid–Gas Interface. Soft Matter 2013, 9, 9392–9402. [Google Scholar] [CrossRef]
- Noskov, B.A. Protein Conformational Transitions at the Liquid–Gas Interface as Studied by Dilational Surface Rheology. Adv. Colloid Interface Sci. 2014, 206, 222–238. [Google Scholar] [CrossRef]
- Cornec, M.; Kim, D.A.; Narsimhan, G. Adsorption Dynamics and Interfacial Properties of α-Lactalbumin in Native and Molten Globule State Conformation at Air–Water Interface. Food Hydrocoll. 2001, 15, 303–313. [Google Scholar] [CrossRef]
- Gurkov, T.D.; Russev, S.C.; Danov, K.D.; Ivanov, I.B.; Campbell, B. Monolayers of Globular Proteins on the Air/Water Inter-face: Applicability of the Volmer Equation of State. Langmuir 2003, 19, 7362–7369. [Google Scholar] [CrossRef]
- Tihonov, M.M.; Milyaeva, O.Y.; Noskov, B.A. Dynamic surface properties of lysozyme solutions. Impact of urea and guanidine hydrochloride. Colloids Surf. B Biointerfaces 2015, 129, 114–120. [Google Scholar] [CrossRef]
- Noskov, B.A.; Bykov, A.G.; Gochev, G.; Lin, S.Y.; Loglio, G.; Miller, R.; Milyaeva, O.Y. Adsorption Layer Formation in Dis-persions of Protein Aggregates. Adv. Colloid Interface Sci. 2020, 276, 102086. [Google Scholar] [CrossRef] [PubMed]
- Noskov, B.A. Dynamic Surface Elasticity of Polymer Solutions. Colloid Polym. Sci. 1995, 273, 263–270. [Google Scholar] [CrossRef]
- Kurouski, D.; Lu, X.; Popova, L.; Wan, W.; Shanmugasundaram, M.; Stubbs, G.; Dukor, R.K.; Lednev, I.K.; Nafie, L.A. Is Su-pramolecular Filament Chirality the Underlying Cause of Major Morphology Differences in Amyloid Fibrils? J. Am. Chem. Soc. 2014, 136, 2302–2312. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noskov, B.; Loglio, G.; Miller, R.; Milyaeva, O.; Panaeva, M.; Bykov, A. Dynamic Surface Properties of α-Lactalbumin Fibril Dispersions. Polymers 2023, 15, 3970. https://doi.org/10.3390/polym15193970
Noskov B, Loglio G, Miller R, Milyaeva O, Panaeva M, Bykov A. Dynamic Surface Properties of α-Lactalbumin Fibril Dispersions. Polymers. 2023; 15(19):3970. https://doi.org/10.3390/polym15193970
Chicago/Turabian StyleNoskov, Boris, Giuseppe Loglio, Reinhard Miller, Olga Milyaeva, Maria Panaeva, and Alexey Bykov. 2023. "Dynamic Surface Properties of α-Lactalbumin Fibril Dispersions" Polymers 15, no. 19: 3970. https://doi.org/10.3390/polym15193970
APA StyleNoskov, B., Loglio, G., Miller, R., Milyaeva, O., Panaeva, M., & Bykov, A. (2023). Dynamic Surface Properties of α-Lactalbumin Fibril Dispersions. Polymers, 15(19), 3970. https://doi.org/10.3390/polym15193970









