Thin-Film Composite Membrane with Porous Interlayer Composed of Dendritic Mesoporous Silica Nanoparticles for Enhanced Nanofiltration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of DMSNs with Different Sizes
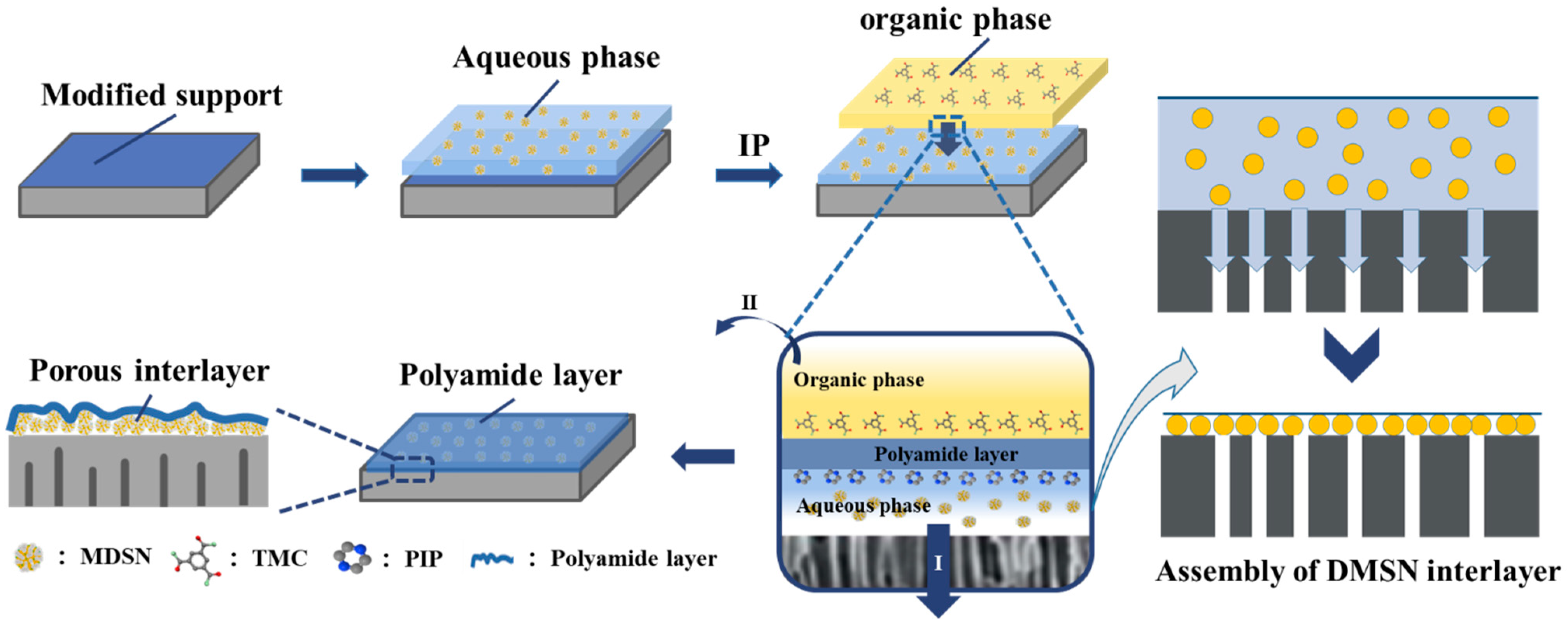
2.3. Fabrication of NF Membrane with DMSN Interlayer
2.4. Characterization of DMSNs
2.5. Characterization of DMSN-Interlayered NF Membranes
2.6. NF Membrane Performance
3. Results and Discussion
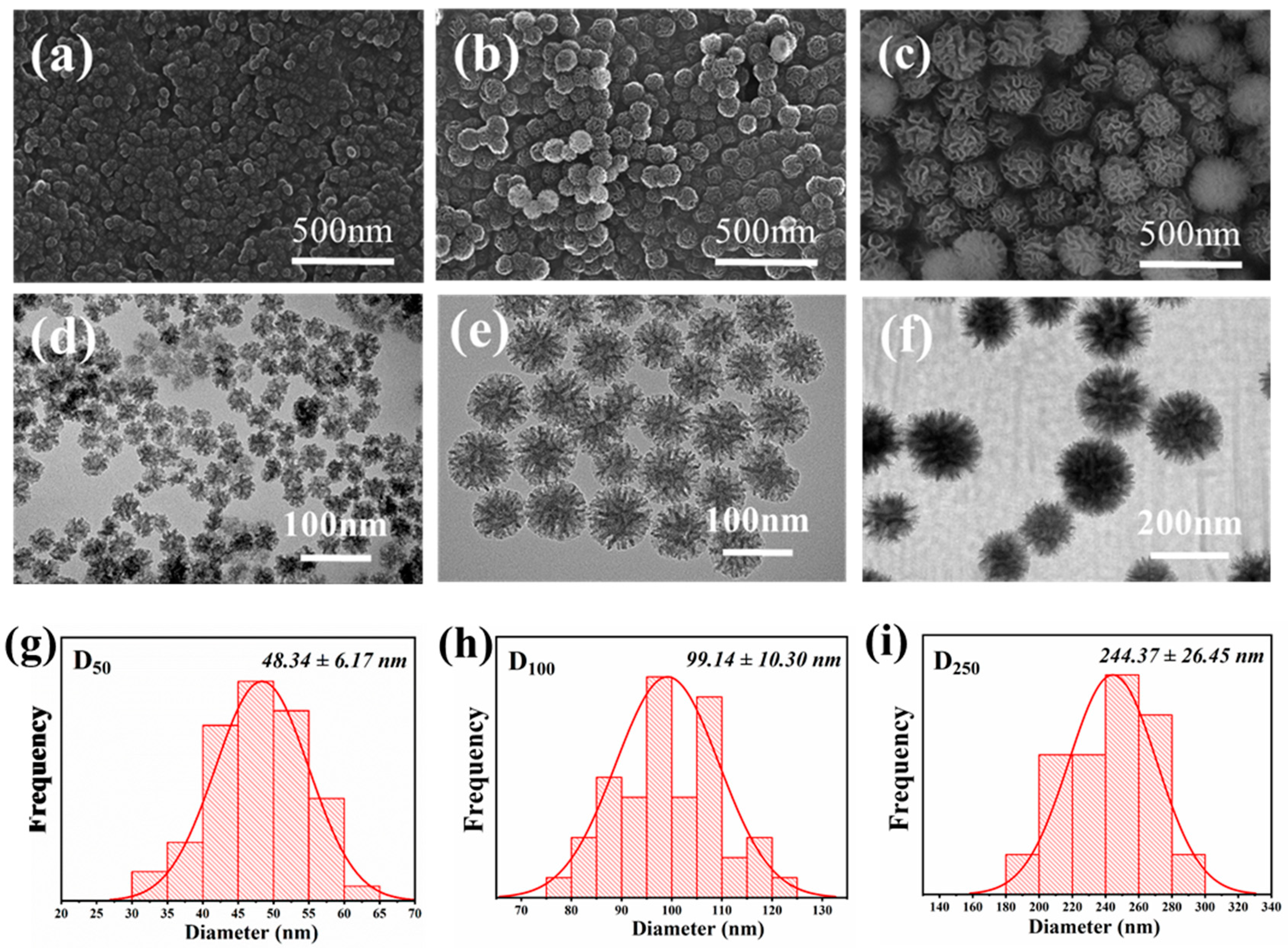
3.1. Characterization of the Synthesized DMSNs
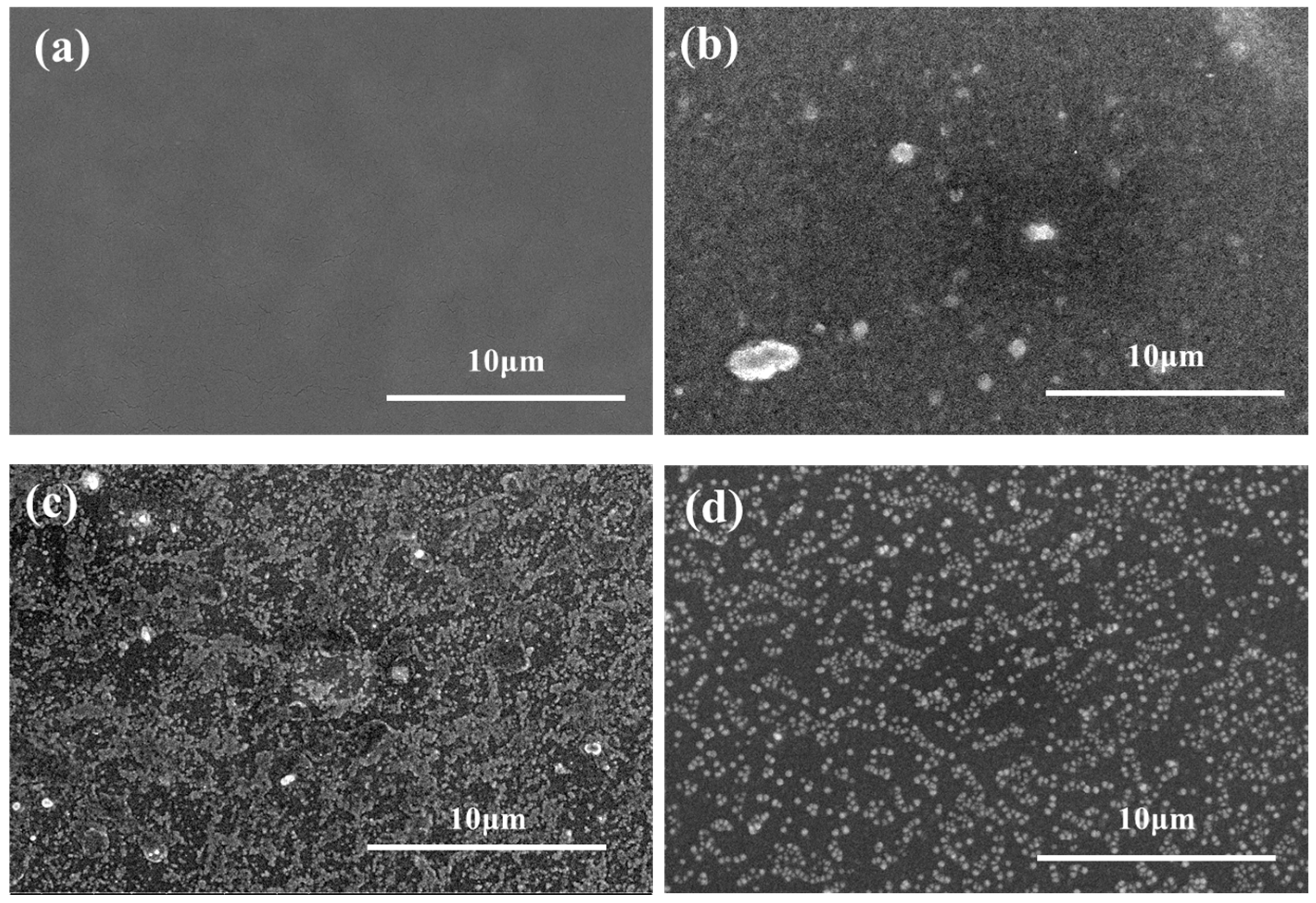
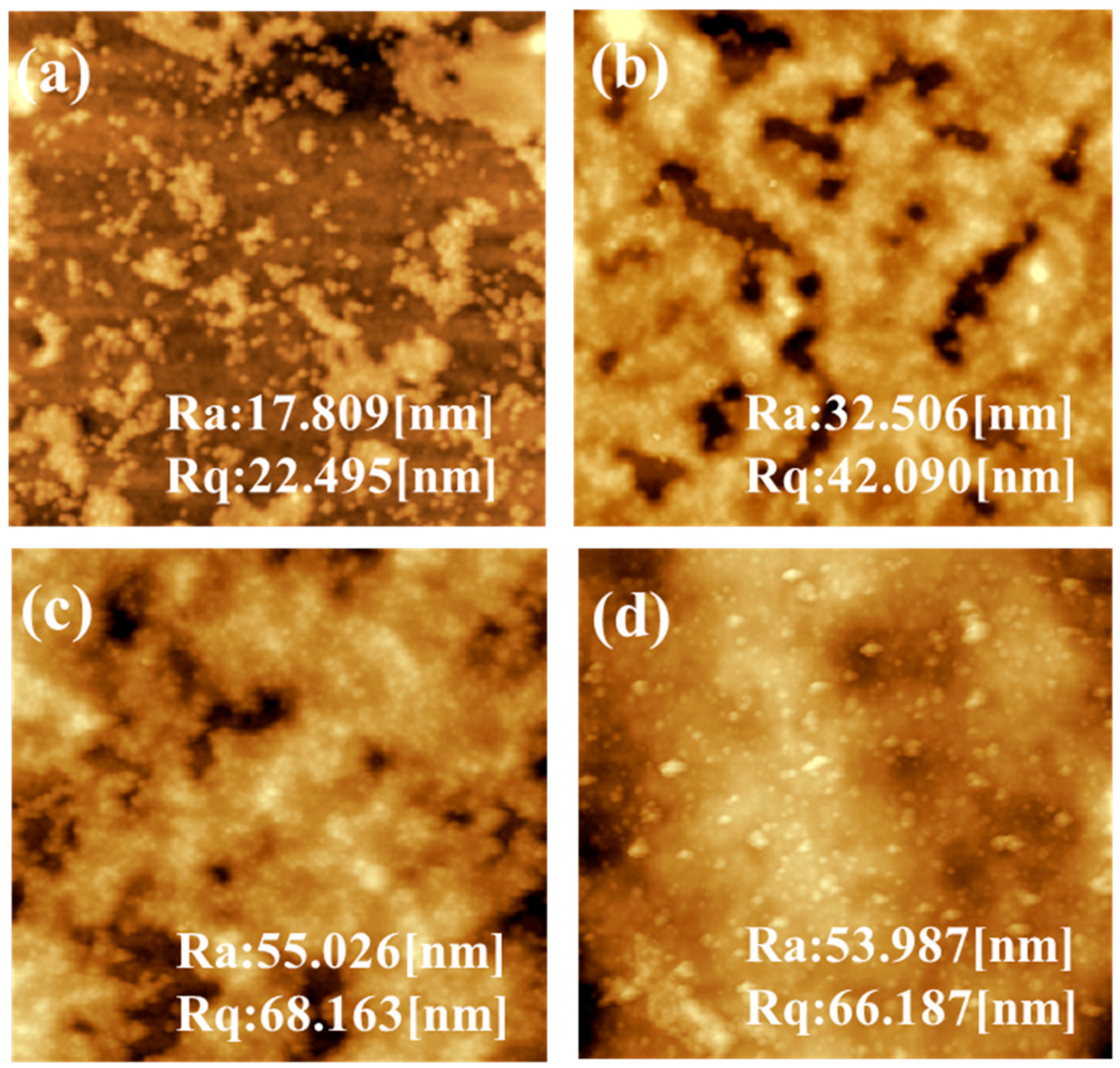
3.2. Physicochemical Properties of the Fabricated NF Membranes
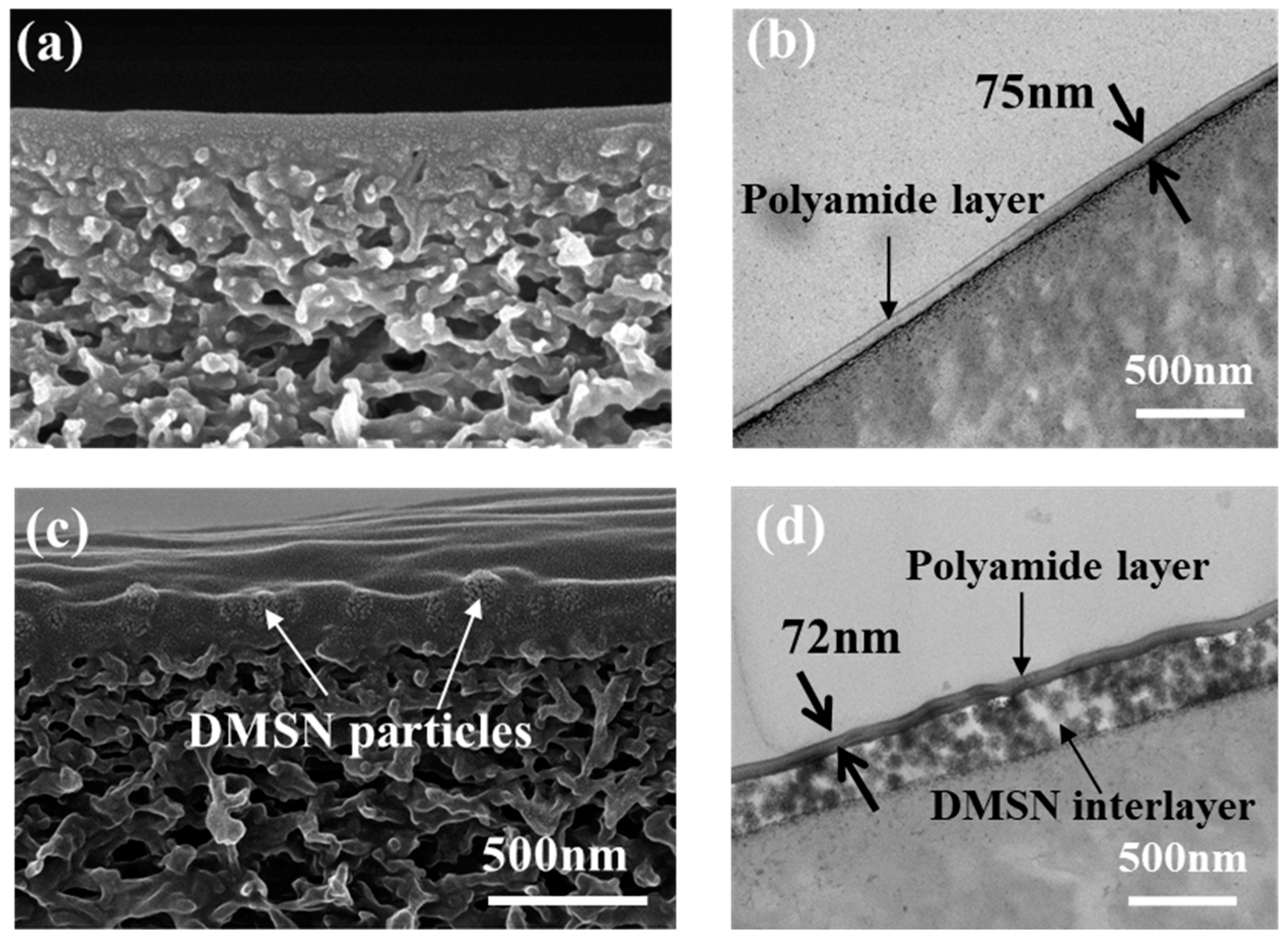
3.3. Structural Properties of the Fabricated NF Membranes
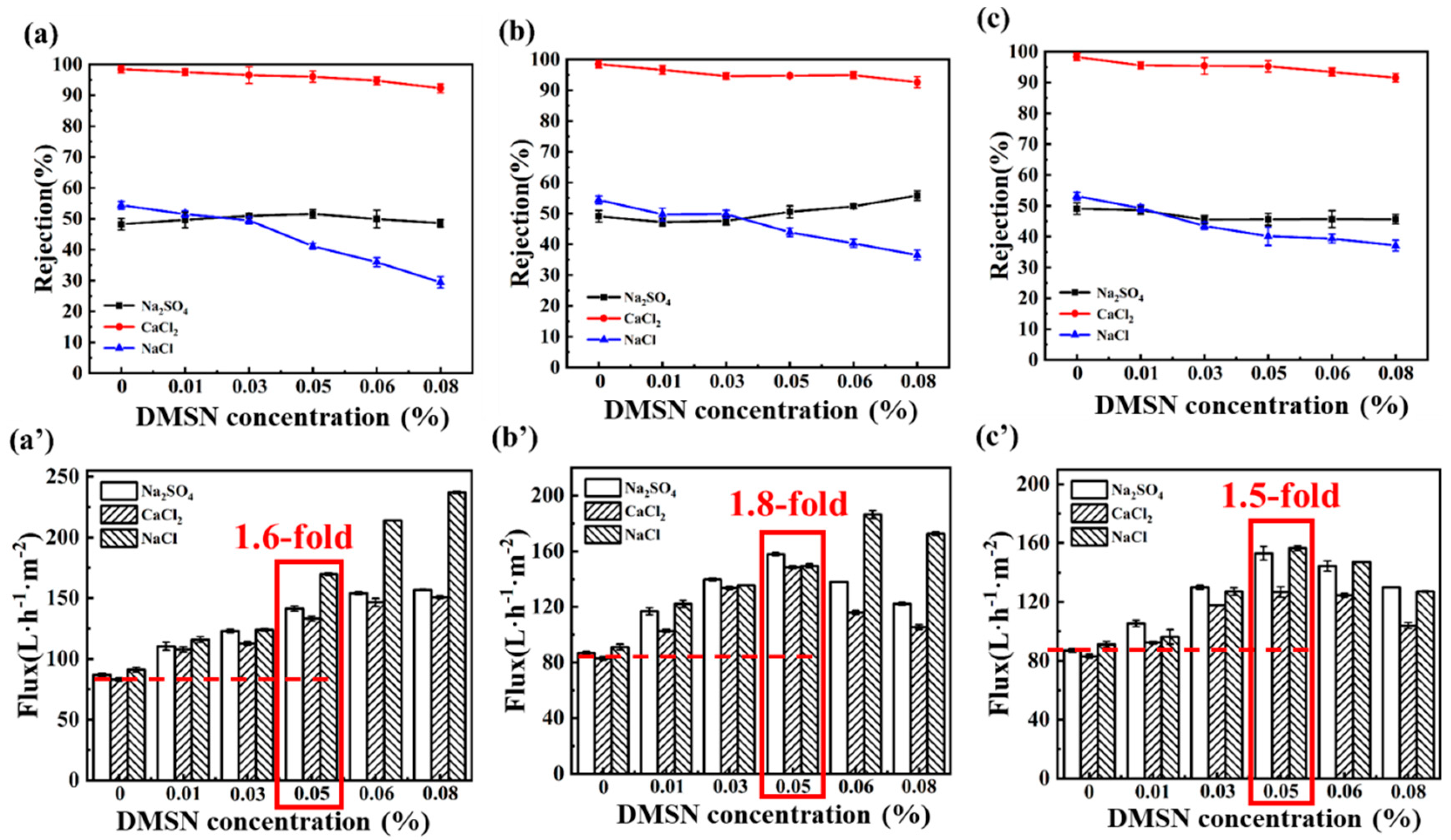
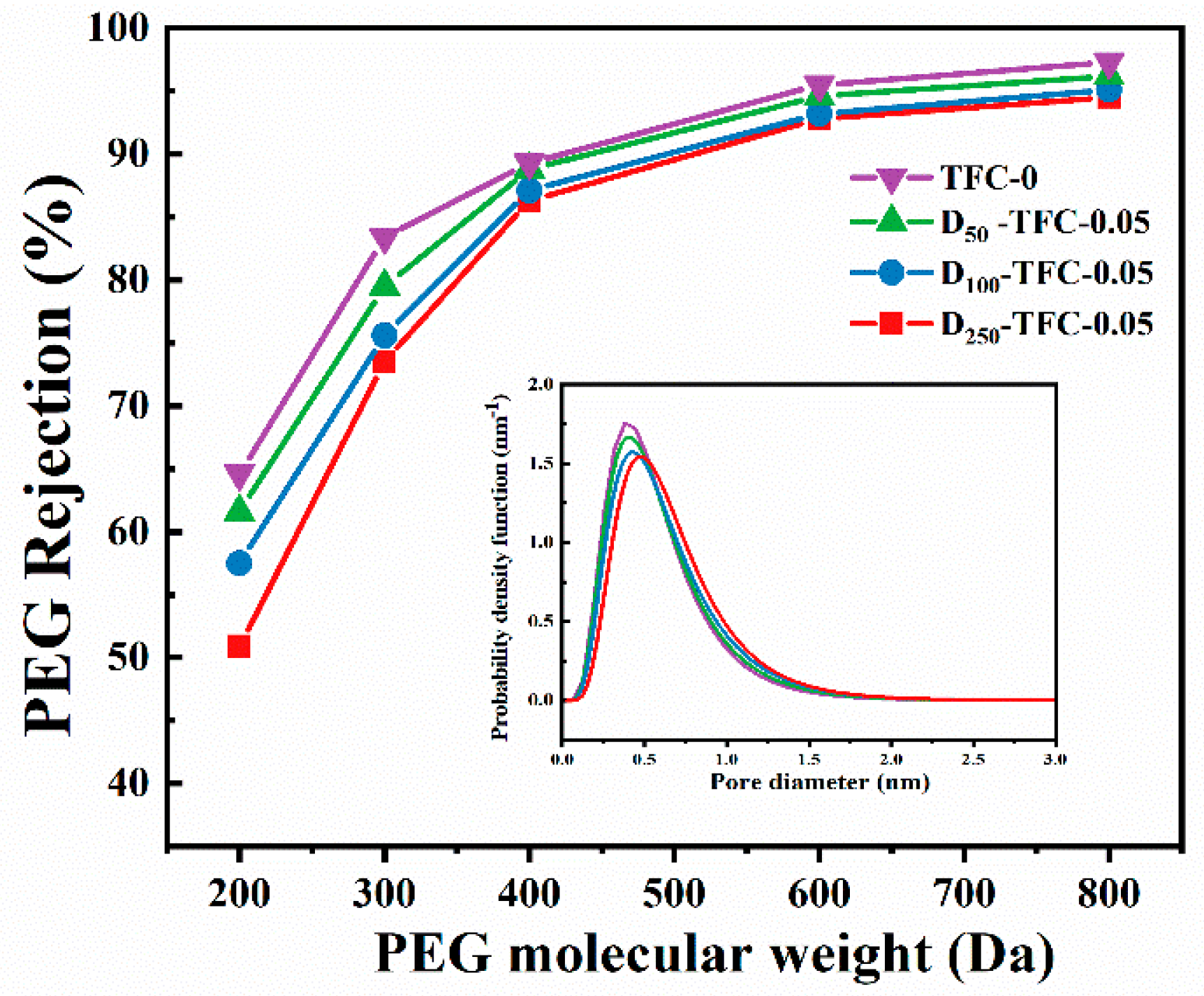
3.4. NF Membrane Performance
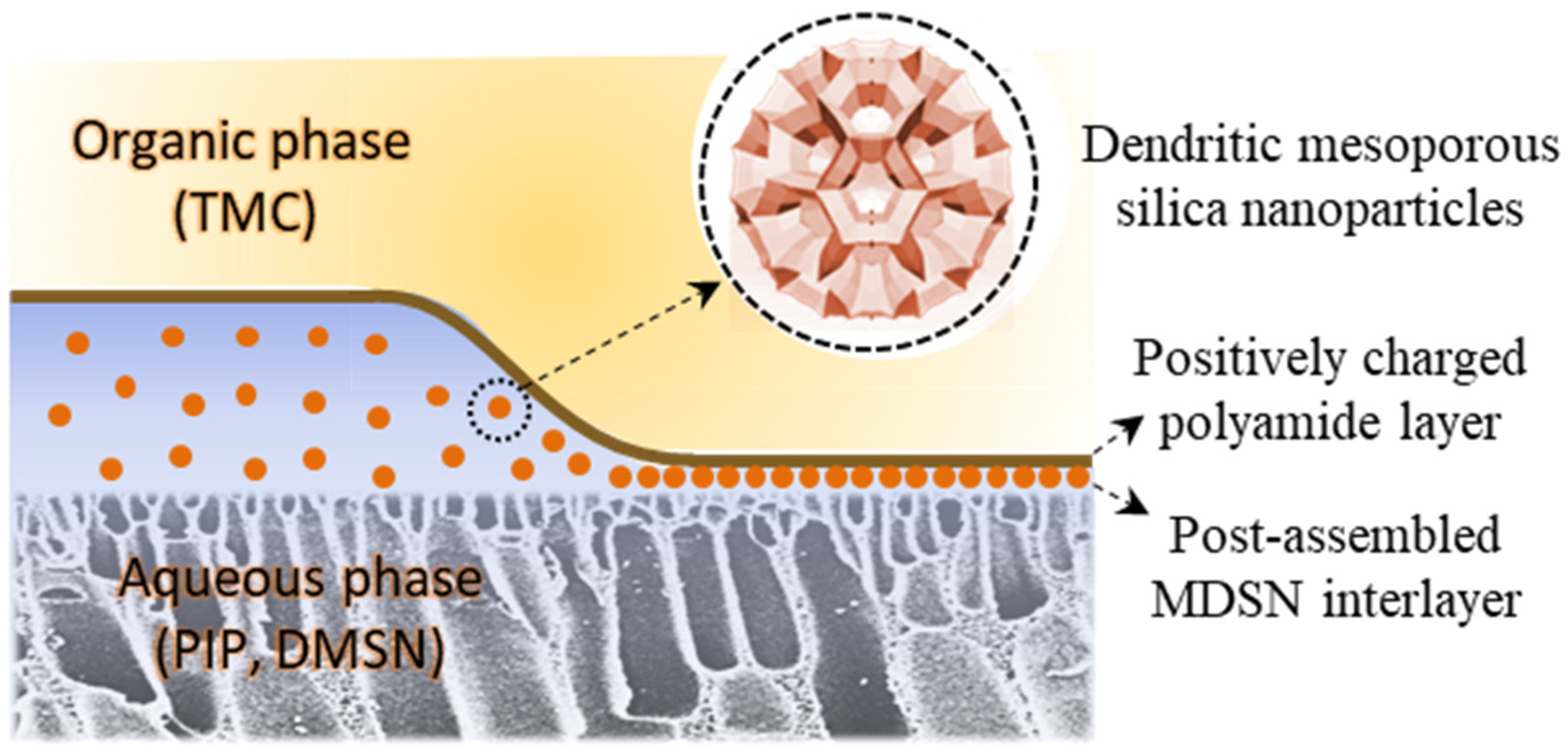
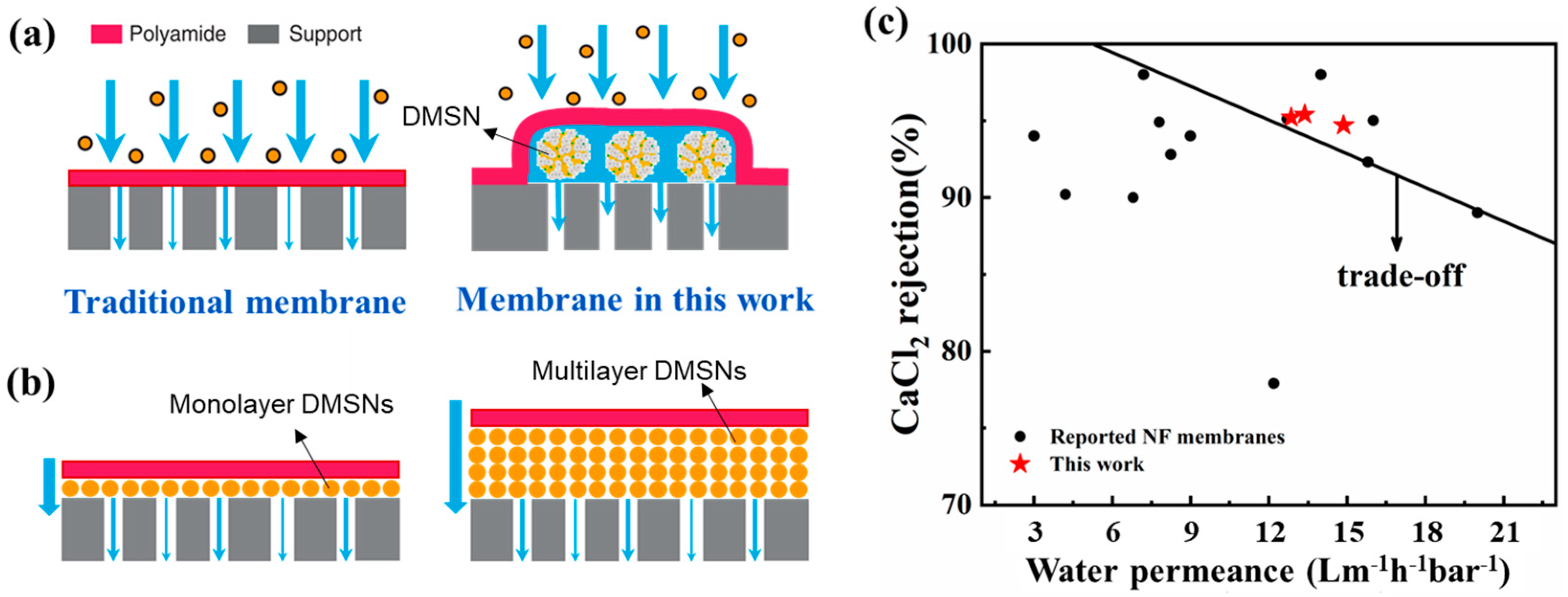
3.5. Mechanism and Performance Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elimelech, M.; Phillip, W.A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Yang, Z.; Guo, H.; Wen, J.J.; Nghiem, L.D.; Cornelissen, E. Potable Water Reuse through Advanced Membrane Technology. Environ. Sci. Technol. 2018, 52, 10215–10223. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Jons, S.D. Chemistry and fabrication of polymeric nanofiltration membranes: A review. Polymer 2016, 103, 417–456. [Google Scholar] [CrossRef]
- Zuo, K.C.; Wang, K.P.; DuChanois, R.M.; Fang, Q.Y.; Deemer, E.M.; Huang, X.C.; Xin, R.K.; Said, I.A.; He, Z.; Feng, Y.R.; et al. Selective membranes in water and wastewater treatment: Role of advanced materials. Mater. Today 2021, 50, 516–532. [Google Scholar] [CrossRef]
- Li, X.H.; Mo, Y.H.; Qing, W.H.; Shao, S.L.; Tang, C.Y.Y.; Li, J.X. Membrane-based technologies for lithium recovery from water lithium resources: A review. J. Membr. Sci. 2019, 591, 117317. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Oatley, D.L.; Williams, P.M.; Wright, C.J. Positively charged nanofiltration membranes: Review of current fabrication methods and introduction of a novel approach. Adv. Colloid Interface Sci. 2011, 164, 12–20. [Google Scholar] [CrossRef]
- Li, W.; Shi, C.; Zhou, A.Y.; He, X.; Sun, Y.W.; Zhang, J.L. A positively charged composite nanofiltration membrane modified by EDTA for LiCl/MgCl2 separation. Sep. Purif. Technol. 2017, 186, 233–242. [Google Scholar] [CrossRef]
- Li, X.H.; Zhang, C.J.; Zhang, S.N.; Li, J.X.; He, B.Q.; Cui, Z.Y. Preparation and characterization of positively charged polyamide composite nanofiltration hollow fiber membrane for lithium and magnesium separation. Desalination 2015, 369, 26–36. [Google Scholar] [CrossRef]
- Lin, C.E.; Fang, L.F.; Du, S.Y.; Yao, Z.K.; Zhu, B.K. A novel positively charged nanofiltration membrane formed via simultaneous cross-linking/quaternization of poly(m-phenylene 5 isophthalamide)/polyethyleneimine blend membrane. Sep. Purif. Technol. 2019, 212, 101–109. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Xu, Z.L.; Ding, H.; Tang, Y.J. Positively charged capillary nanofiltration membrane with high rejection for Mg2+ and Ca2+ and good separation for Mg2+ and Li+. Desalination 2017, 420, 158–166. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Sun, W.; Hu, Y.H.; Tang, H.H. Membrane technologies for Li+/Mg2+ separation from salt-lake brines and seawater: A comprehensive review. J. Ind. Eng. Chem. 2020, 81, 7–23. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Wang, Z.X.; Jiang, X.; Li, T.X.; Lau, C.H.; Guo, Z.H.; Ma, J.; Shao, L. Towards sustainable ultrafast molecular-separation membranes: From conventional polymers to emerging materials. Prog. Mater. Sci. 2018, 92, 258–283. [Google Scholar] [CrossRef]
- Hao, Y.F.; Li, Q.; He, B.Q.; Liao, B.; Li, X.H.; Hu, M.Y.; Ji, Y.H.; Cui, Z.Y.; Younas, M.; Li, J.X. An ultrahighly permeable-selective nanofiltration membrane mediated by an in situ formed interlayer. J. Mater. Chem. A 2020, 8, 5275–5283. [Google Scholar] [CrossRef]
- CJi, H.; Zhai, Z.; Jiang, C.; Hu, P.; Zhao, S.Z.; Xue, S.M.; Yang, Z.; He, T.; Niu, Q.J. Recent advances in high-performance TFC membranes: A review of the functional interlayers. Desalination 2021, 500, 114869. [Google Scholar]
- Yang, Z.; Sun, P.F.; Li, X.H.; Gan, B.W.; Wang, L.; Song, X.X.; Park, H.D.; Tang, C.Y. A Critical Review on Thin-Film Nanocomposite Membranes with Interlayered Structure: Mechanisms, Recent Developments, and Environmental Applications. Environ. Sci. Technol. 2020, 54, 15563–15583. [Google Scholar] [CrossRef]
- Zhai, Z.; Jiang, C.; Zhao, N.; Dong, W.J.; Lan, H.L.; Wang, M.; Niu, Q.J. Fabrication of advanced nanofiltration membranes with nanostrand hybrid morphology mediated by ultrafast Noria-polyethyleneimine codeposition. J. Mater. Chem. A 2018, 6, 21207–21215. [Google Scholar] [CrossRef]
- Argyo, C.; Weiss, V.; Brauchle, C.; Bein, T. Multifunctional Mesoporous Silica Nanoparticles as a Universal Platform for Drug Delivery. Chem. Mater. 2014, 26, 435–451. [Google Scholar] [CrossRef]
- Slowing, I.I.; Trewyn, B.G.; Giri, S.; Lin, V.S.Y. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv. Funct. Mater. 2007, 17, 1225–1236. [Google Scholar] [CrossRef]
- Huang, R.; Shen, Y.W.; Guan, Y.Y.; Jiang, Y.X.; Wu, Y.; Rahman, K.; Zhang, L.J.; Liu, H.J.; Luan, X. Mesoporous silica nanoparticles: Facile surface functionalization and versatile biomedical applications in oncology. Acta Biomater. 2020, 116, 1–15. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Park, D.S.; Yun, D.; Choi, Y.; Kim, T.Y.; Oh, S.; Cho, J.H.; Yi, J. Effect of 3D open-pores on the dehydration of n-butanol to di-n-butyl ether (DNBE) over a supported heteropolyacid catalyst. Chem. Eng. J. 2013, 228, 889–895. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, L.P.; Li, P.; Sun, H.X.; Hou, Y.F.; Niu, Q.J. Ultrathin Film Composite Membranes Fabricated by Novel In Situ Free Interfacial Polymerization for Desalination. ACS Appl. Mater Interfaces 2020, 12, 25304–25315. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Liu, Z.Y.; Jiang, L.; Fei, Z.H.; Hou, Y.F. Rapid transport of water and monovalent ions through ultrathin polyamide nanofilms for highly efficient mono/bivalent ions separation. Appl. Surf. Sci. 2023, 608, 155025. [Google Scholar] [CrossRef]
- Xing, Y.; Pan, Q.; Du, X.; Xu, T.L.; He, Y.; Zhang, X.J. Dendritic Janus Nanomotors with Precisely Modulated Coverages and Their Effects on Propulsion. ACS Appl. Mater. Interfaces 2019, 11, 10426–10433. [Google Scholar] [CrossRef] [PubMed]
- Karan, S.; Jiang, Z.W.; Livingston, A.G. Sub-10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 2015, 348, 1347–1351. [Google Scholar] [CrossRef]
- Xu, P.; Gonzales, R.R.; Hong, J.; Guan, K.; Chiao, Y.H.; Mai, Z.; Li, Z.; Rajabzadeh, S.; Matsuyama, H. Fabrication of highly positively charged nanofiltration membranes by novel interfacial polymerization: Accelerating Mg2+ removal and Li+ enrichment. J. Membr. Sci. 2023, 668, 121251. [Google Scholar] [CrossRef]
- Li, P.; Lan, H.; Chen, K.; Ma, X.; Wei, B.; Wang, M.; Li, P.; Hou, Y.; Niu, Q.J. Novel high-flux positively charged aliphatic polyamide nanofiltration membrane for selective removal of heavy metals. Sep. Purif. Technol. 2022, 280, 119949. [Google Scholar] [CrossRef]
- Lv, Y.; Du, Y.; Qiu, W.Z.; Xu, Z.K. Nanocomposite Membranes via the Codeposition of Polydopamine/Polyethylenimine with Silica Nanoparticles for Enhanced Mechanical Strength and High Water Permeability. ACS Appl. Mater. Interfaces 2017, 9, 2966–2972. [Google Scholar] [CrossRef]
- Lv, Y.; Du, Y.; Chen, Z.X.; Qiu, W.Z.; Xu, Z.K. Nanocomposite membranes of polydopamine/electropositive nanoparticles/polyethyleneimine for nanofiltration. J. Membr. Sci. 2018, 545, 99–106. [Google Scholar] [CrossRef]
- Singh, S.; Khulbe, K.C.; Matsuura, T.; Ramamurthy, P. Membrane characterization by solute transport and atomic force microscopy. J. Membr. Sci. 1998, 142, 111–127. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Zhang, M.; Hou, Y. Thin-Film Composite Membrane with Porous Interlayer Composed of Dendritic Mesoporous Silica Nanoparticles for Enhanced Nanofiltration. Polymers 2023, 15, 3912. https://doi.org/10.3390/polym15193912
Jiang C, Zhang M, Hou Y. Thin-Film Composite Membrane with Porous Interlayer Composed of Dendritic Mesoporous Silica Nanoparticles for Enhanced Nanofiltration. Polymers. 2023; 15(19):3912. https://doi.org/10.3390/polym15193912
Chicago/Turabian StyleJiang, Chi, Mengmeng Zhang, and Yingfei Hou. 2023. "Thin-Film Composite Membrane with Porous Interlayer Composed of Dendritic Mesoporous Silica Nanoparticles for Enhanced Nanofiltration" Polymers 15, no. 19: 3912. https://doi.org/10.3390/polym15193912
APA StyleJiang, C., Zhang, M., & Hou, Y. (2023). Thin-Film Composite Membrane with Porous Interlayer Composed of Dendritic Mesoporous Silica Nanoparticles for Enhanced Nanofiltration. Polymers, 15(19), 3912. https://doi.org/10.3390/polym15193912







