Morphology Control of Electrospun Brominated Butyl Rubber Microfibrous Membrane
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization and Measurement
2.3. Electrospinning
3. Results and Discussions
3.1. Property of BIIR
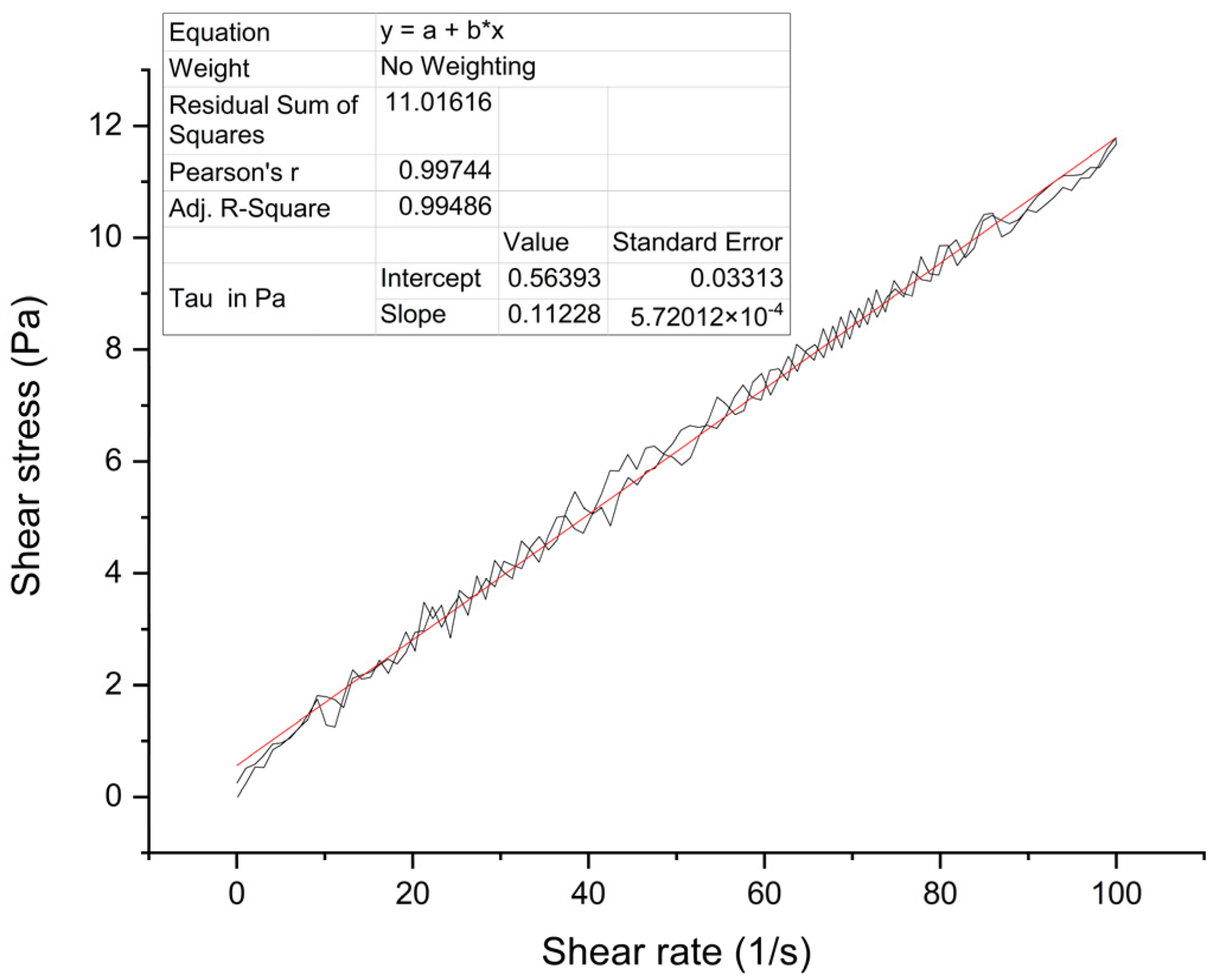
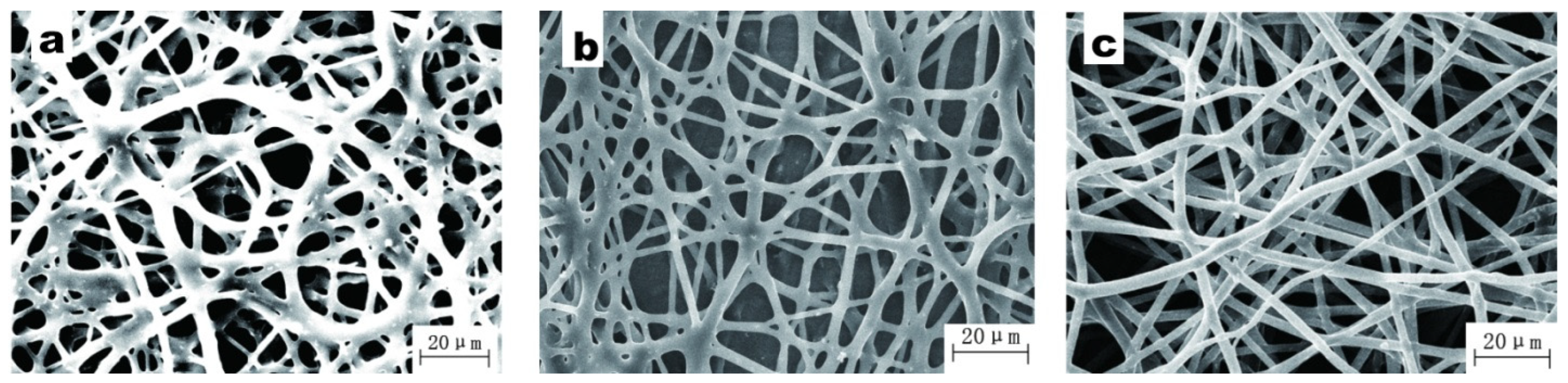
3.2. Rheological Property of BIIR Electrospinning Solution
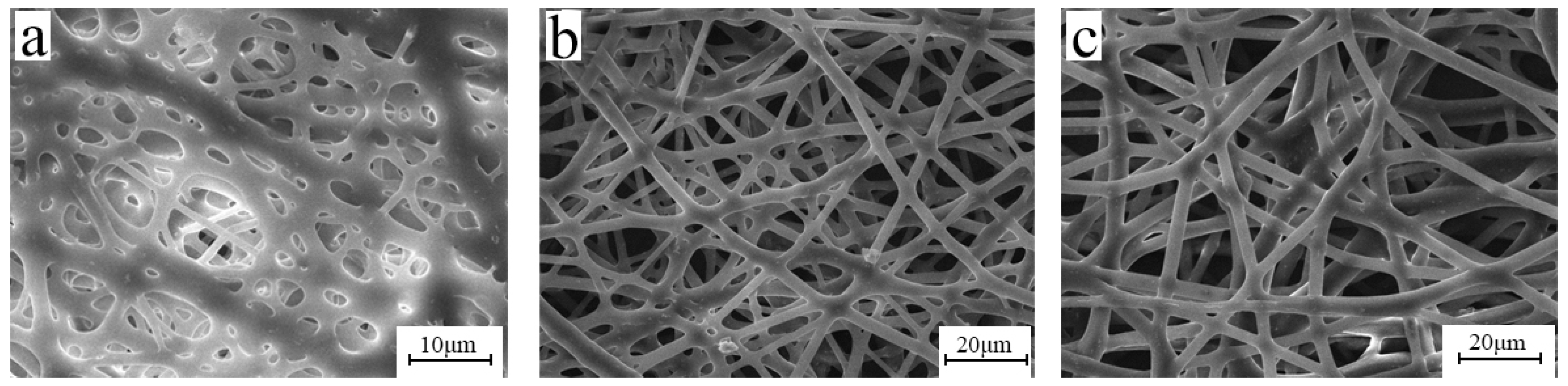
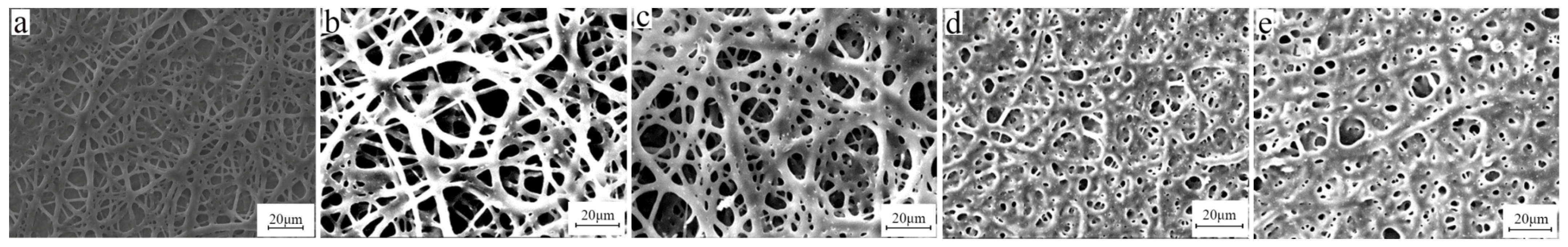
3.3. Solvent
3.4. Concentration of BIIR
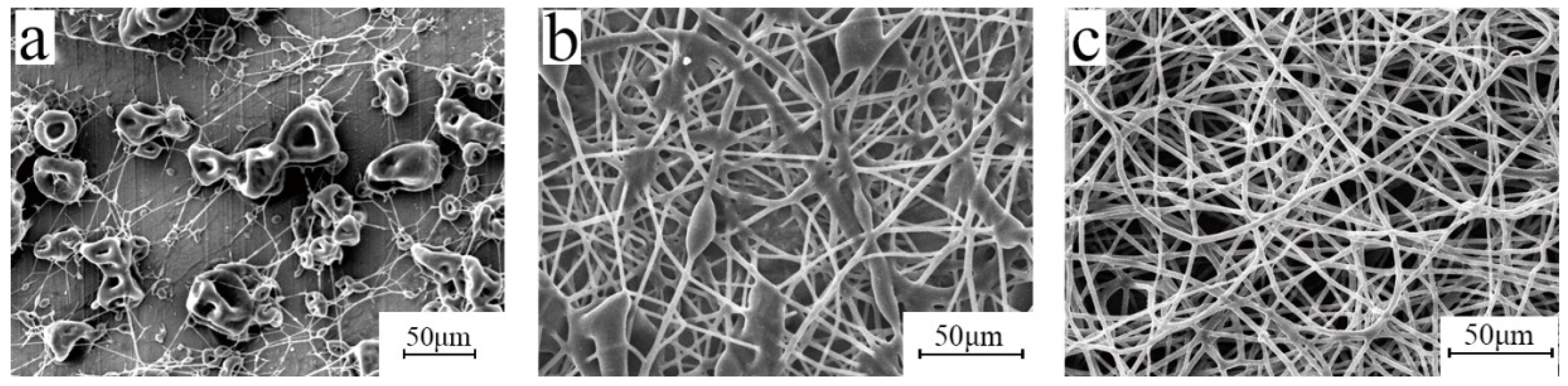
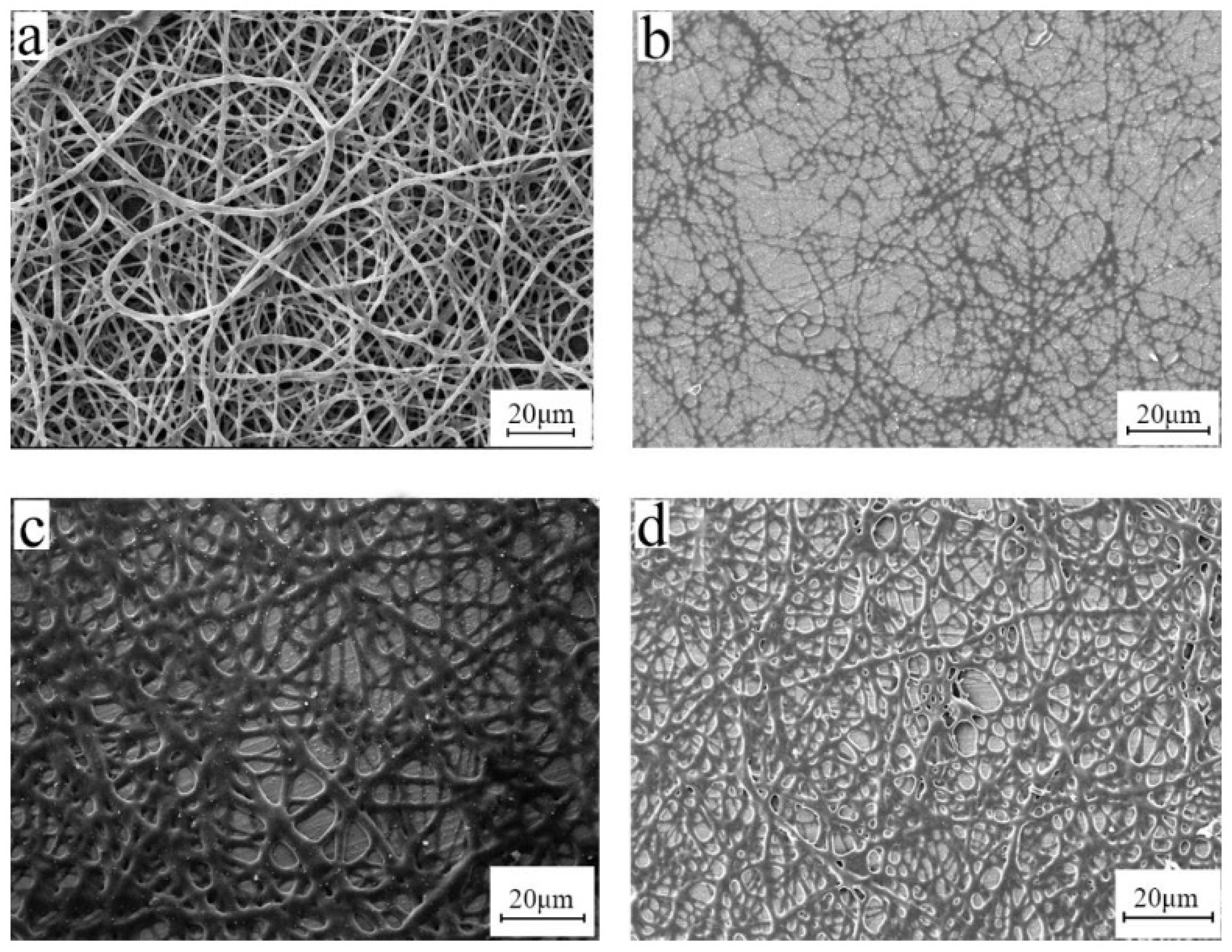
3.5. Electrospinning Process
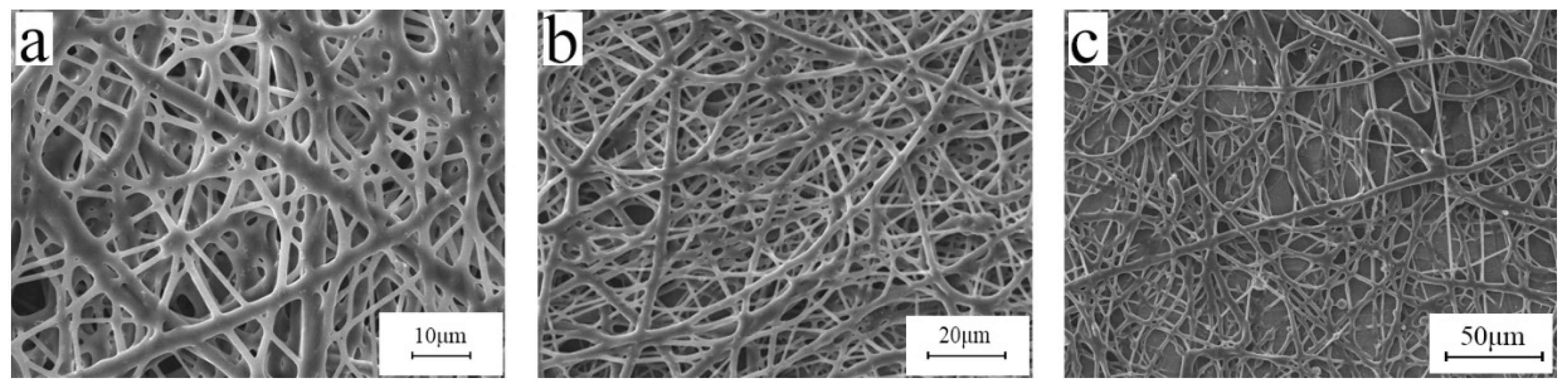
3.6. Effect of Post-Treatment Process
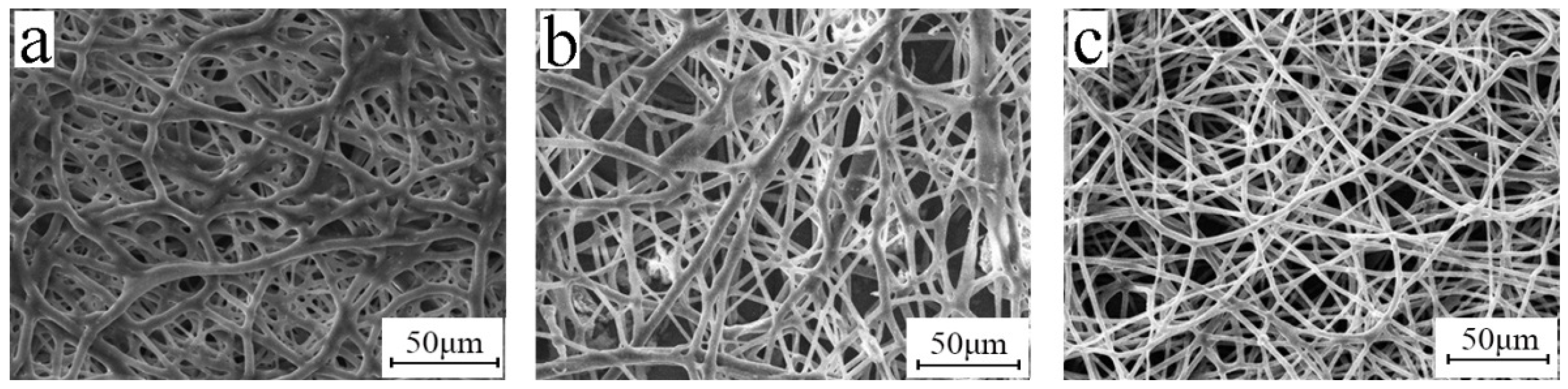
3.7. Release Agent
3.8. Antimicrobial Property
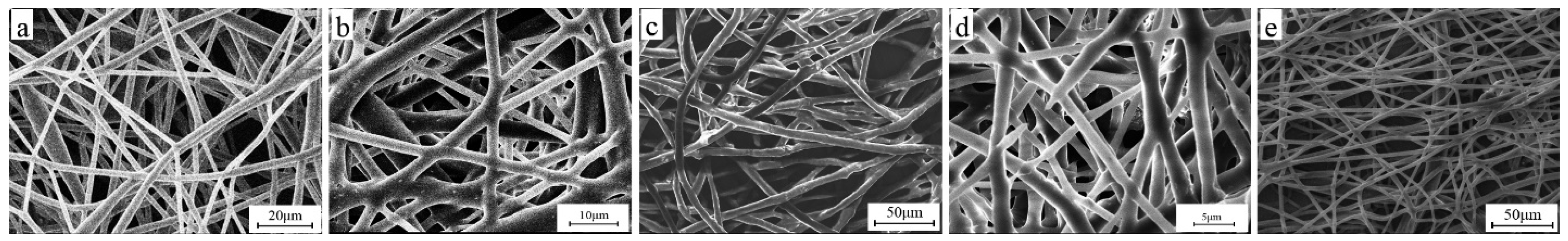
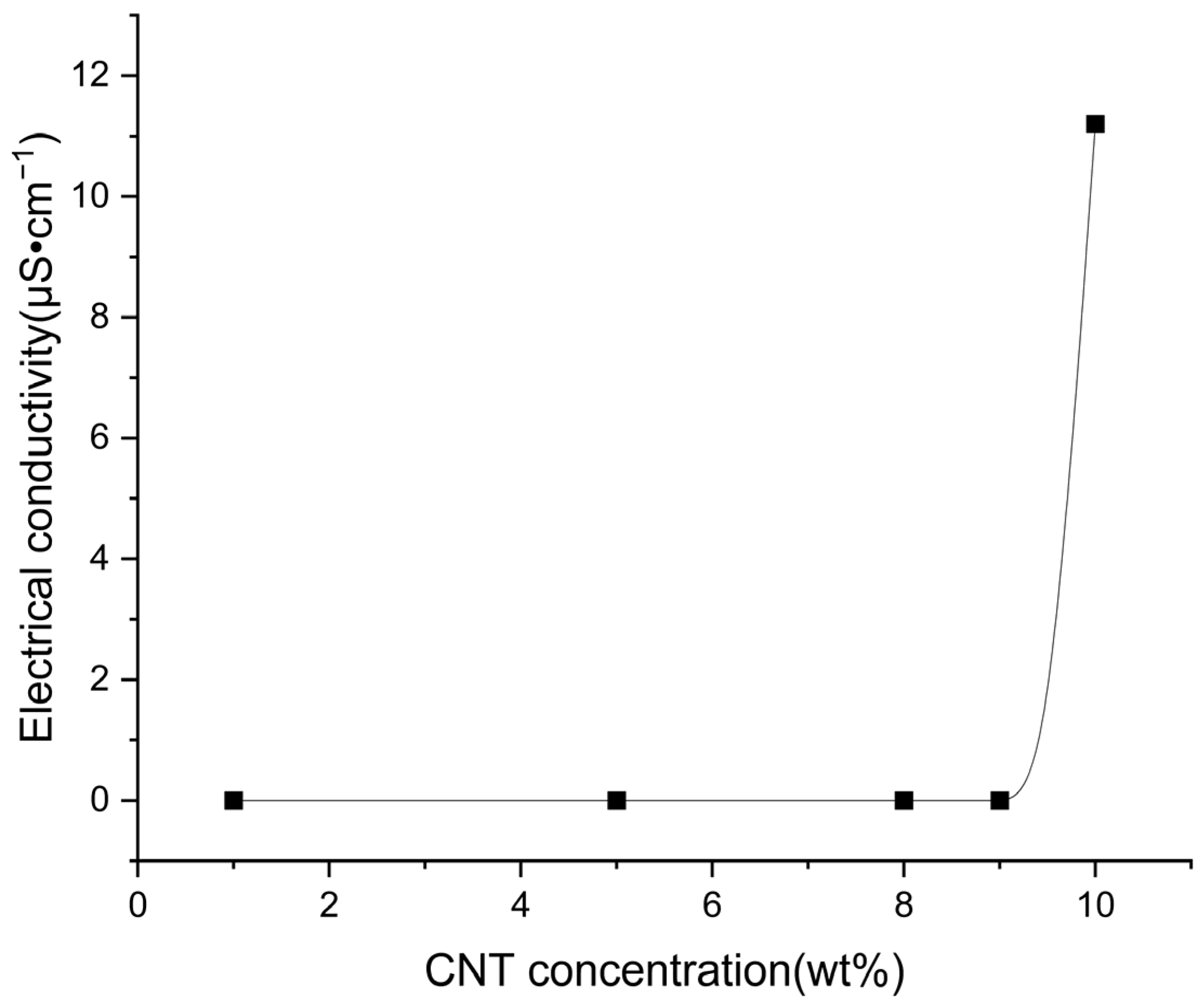
3.9. Electrical Properties
3.10. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Behera, P.K.; Kumar, A.; Mohanty, S.; Gupta, V.K. Overview on Post-Polymerization Functionalization of Butyl Rubber and Properties. Ind. Eng. Chem. Res. 2022, 61, 16910–16923. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, Y.; Liao, D.; He, Y.; Li, S.; Zhou, L.; Yu, C.; Chen, Y.; Liu, Y.; Liu, H. Fabrication of Cellulose Nanofiber/Reduced Graphene Oxide/Nitrile Rubber Flexible Films Using Pickering Emulsion Technology for Electromagnetic Interference Shielding and Piezoresistive Sensor. Macromol. Mater. Eng. 2021, 306, 2100070. [Google Scholar] [CrossRef]
- Cao, R.; Zhao, X.; Zhao, X.; Wu, X.; Li, X.; Zhang, L. Bromination Modification of Butyl Rubber and Its Structure, Properties, and Application. Ind. Eng. Chem. Res. 2019, 58, 16645–16653. [Google Scholar] [CrossRef]
- Le Floch, P.; Yao, X.; Liu, Q.; Wang, Z.; Nian, G.; Sun, Y.; Jia, L.; Suo, Z. Wearable and washable conductors for active textiles. ACS Appl. Mater. Interfaces 2017, 9, 25542–25552. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, G.; Jiang, Y.; Zhang, Y. Effects of boron nitride and carbon nanotube on damping properties, thermal conductivity and compression stress relaxation behavior of BIIR. Polym. Compos. 2022, 43, 1128–1135. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Zhang, X.; Xu, T. Treatment of simulated brominated butyl rubber wastewater by bipolar membrane electrodialysis. Sep. Purif. Technol. 2011, 80, 196–201. [Google Scholar] [CrossRef]
- Luo, G.; Pang, B.; Luo, X.; Zeng, X.; Wang, Y.; Zhao, L. Self-healing and enhanced anticorrosion coatings based on graphene-reinforced brominated butyl rubber ionomer. Prog. Org. Coat. 2023, 174, 107245. [Google Scholar] [CrossRef]
- Yang, S.; Wu, H.; Li, C.; Xiong, Y.; Guo, S. Constructing oriented two-dimensional large-sized modified graphene oxide barrier walls in brominated butyl rubber to achieve excellent gas barrier properties. ACS Appl. Mater. Interfaces 2019, 12, 3976–3983. [Google Scholar] [CrossRef]
- Parent, J.S.; White, G.D.; Thom, D.J.; Whitney, R.A.; Hopkins, W. Sulfuration and reversion reactions of brominated poly (isobutylene-co-isoprene). J. Polym. Sci. Part A Polym. Chem. 2003, 41, 1915–1926. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89. [Google Scholar] [CrossRef]
- Shabafrooz, V.; Mozafari, M.; Vashaee, D.; Tayebi, L. Electrospun nanofibers: From filtration membranes to highly specialized tissue engineering scaffolds. J. Nanosci. Nanotechnol. 2014, 14, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun nanofibers: New generation materials for advanced applications. Mater. Sci. Eng. B 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Qureshi, U.A.; Khatri, Z.; Ahmed, F.; Ibupoto, A.S.; Khatri, M.; Mahar, F.K.; Brohi, R.Z.; Kim, I.S. Highly efficient and robust electrospun nanofibers for selective removal of acid dye. J. Mol. Liq. 2017, 244, 478–488. [Google Scholar] [CrossRef]
- Muslumova, S.; Yetiskin, B.; Okay, O. Highly stretchable and rapid self-recoverable cryogels based on butyl rubber as reusable sorbent. Gels 2019, 5, 1. [Google Scholar] [CrossRef]
- JIS Z 2801: 2012; Test for Antimicrobial Activity of Plastics. Microchem Laboratory: Round Rock, TX, USA, 2012.
- GB/T 1040-2006/ISO 527:1993; Plastics-Determination of Tensile Properties. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, National Standardization Administration of China: Beijing, China, 2006.
- Liu, Z.; Tang, Y.; Zhao, K.; Zhang, Q. Superhydrophobic SiO2 micro/nanofibrous membranes with porous surface prepared by freeze electrospinning for oil adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 356–361. [Google Scholar] [CrossRef]
- Wen, Y.; Kok, M.D.; Tafoya, J.P.V.; Sobrido, A.B.J.; Bell, E.; Gostick, J.T.; Herou, S.; Schlee, P.; Titirici, M.-M.; Brett, D.J. Electrospinning as a route to advanced carbon fibre materials for selected low-temperature electrochemical devices: A review. J. Energy Chem. 2021, 59, 492–529. [Google Scholar] [CrossRef]
- Ning, N.; Hu, L.; Yao, P.; Wu, H.; Han, J.; Zhang, L.; Tian, H.; Tian, M. Study on the microstructure and properties of bromobutyl rubber (BIIR)/polyamide-12 (PA12) thermoplastic vulcanizates (TPVs). J. Appl. Polym. Sci. 2016, 133, 43043. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, J.; Wang, C.; Gao, S.; Guo, S.; Zhang, Y. Influence of 1,2-polybutadiene on properties of dicumyl peroxide cured brominated butyl rubber. J. Appl. Polym. Sci. 2016, 133, 43280. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Duan, B.; Yuan, X.; Zhu, Y.; Zhang, Y.; Li, X.; Zhang, Y.; Yao, K. A nanofibrous composite membrane of PLGA–chitosan/PVA prepared by electrospinning. Eur. Polym. J. 2006, 42, 2013–2022. [Google Scholar] [CrossRef]
- He, D.; Hu, B.; Yao, Q.-F.; Wang, K.; Yu, S.-H. Large-scale synthesis of flexible free-standing SERS substrates with high sensitivity: Electrospun PVA nanofibers embedded with controlled alignment of silver nanoparticles. ACS Nano 2009, 3, 3993–4002. [Google Scholar] [CrossRef] [PubMed]
- Viriyabanthorn, N.; Stacer, R.G.; Sung, C.; Mead, J.L. Effect of Carbon Black Loading on Electrospun Butyl Rubber Nonwoven Mats; ACS Publications: Washington, DC, USA, 2003. [Google Scholar]
- Zhang, W.; Zhang, Y.; Yang, G.; Hao, X.; Lv, X.; Wu, F.; Liu, J.; Zhang, Y. Wearable and self-powered sensors made by triboelectric nanogenerators assembled from antibacterial bromobutyl rubber. Nano Energy 2021, 82, 105769. [Google Scholar] [CrossRef]
- Hu, A.; Long, J.; Shu, C.; Liang, R.; Li, J. Three-dimensional interconnected network architecture with homogeneously dispersed carbon nanotubes and layered MoS2 as a highly efficient cathode catalyst for lithium–oxygen battery. ACS Appl. Mater. Interfaces 2018, 10, 34077–34086. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, C.; Satyam, M. Studies on electrical conductivity of insulator-conductor composites. J. Appl. Phys. 1978, 49, 5536–5542. [Google Scholar] [CrossRef]
- Zhang, J.N.; Wang, C.Y.; Guo, Z.; Zhou, Y.G.; Wu, H.H. Prediction on electrical resistivity of thin-ply unidirectional composites considering electric tunnel effect. Polym. Compos. 2020, 41, 4318–4328. [Google Scholar] [CrossRef]




| BIIR No. | Bromine Content (wt%) | Mooney Viscosity (ML (1 + 8) of 125 °C) (MU) | Volatile Component (wt%) | Antioxygen Content (wt%) |
|---|---|---|---|---|
| 2301 | 2.1 ± 0.2 | 32 ± 5 | ≤2 ± 5 | ≥2 ± 5 |
| 2302 | 1.9 ± 0.2 | 32 ± 5 | ≤2 ± 5 | ≥2 ± 5 |
| 2501 | 1.9 ± 0.2 | 46 ± 5 | ≤6 ± 5 | ≥6 ± 5 |
| Antibactrial Rate (%) | Escherichia coli | Staphylococcus aureus | |
|---|---|---|---|
| Sample | |||
| Standard PE samples | -- | -- | |
| blank | >99 | >99 | |
| Nano silver 1‰ | >99 | >99 | |
| Nano silver 3‰ | >99 | >99 | |
| Triclosan 3‰ | >99 | >99 | |
| Triclosan 5‰ | >99 | >99 | |
| Triclosan 7‰ | >99 | >99 | |
| Tensile Strength (kPa) | Elastic Modulus (kPa) | Elongation at Break (%) | |
|---|---|---|---|
| blank | 2.7 ± 0.01 | 430 ± 2.15 | 10.85 ± 0.05 |
| 1‰ nano-silver | 20 ± 0.10 | 570 ± 2.85 | 37.64 ± 0.19 |
| 3‰ nano-silver | 11 ± 0.06 | 480 ± 2.40 | 33.00 ± 0.17 |
| 3‰ triclosan | 10 ± 0.05 | 180 ± 0.90 | 56.72 ± 0.26 |
| 5‰ triclosan | 18 ± 0.09 | 170 ± 0.85 | 70.99 ± 0.36 |
| 7‰ triclosan | 34 ± 0.17 | 350 ± 1.75 | 63.31 ± 0.32 |
| 10% CNT | 70 ± 0.35 | 270 ± 1.35 | 90.24 ± 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, T.; Tian, R.; Wu, L.; Zhang, D.; Chen, L.; Zhang, X.; Hao, X.; Hu, P. Morphology Control of Electrospun Brominated Butyl Rubber Microfibrous Membrane. Polymers 2023, 15, 3909. https://doi.org/10.3390/polym15193909
Zhu T, Tian R, Wu L, Zhang D, Chen L, Zhang X, Hao X, Hu P. Morphology Control of Electrospun Brominated Butyl Rubber Microfibrous Membrane. Polymers. 2023; 15(19):3909. https://doi.org/10.3390/polym15193909
Chicago/Turabian StyleZhu, Tianxiao, Ruizhi Tian, Liang Wu, Dingyi Zhang, Leying Chen, Xianmei Zhang, Xiangyang Hao, and Ping Hu. 2023. "Morphology Control of Electrospun Brominated Butyl Rubber Microfibrous Membrane" Polymers 15, no. 19: 3909. https://doi.org/10.3390/polym15193909
APA StyleZhu, T., Tian, R., Wu, L., Zhang, D., Chen, L., Zhang, X., Hao, X., & Hu, P. (2023). Morphology Control of Electrospun Brominated Butyl Rubber Microfibrous Membrane. Polymers, 15(19), 3909. https://doi.org/10.3390/polym15193909





