A Practical Primer: Raman Spectroscopy for Monitoring of Photopolymerization Systems
Abstract
:1. Introduction
2. Raman Measurements in Photopolymerization Systems
2.1. Conversion Measurements
2.2. Other Kinetic Data
3. Raman Measurements in Photopolymer Systems
3.1. Raman Microscopy
3.2. Physical Property Correlations
4. Practical Tips
4.1. Experimental Considerations
4.2. Confocal Raman Microscopy
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salim, M.S. Overview of UV Curable Coatings. In Radiation Curing of Polymers II; Randell, D.R., Ed.; The Royal Society of Chemistry: Cambridge, UK, 1991; pp. 3–21. [Google Scholar]
- Fouassier, J.P. Photoinitiation, Photopolymerization, and Photocuring: Fundamentals and Applications; Hanser Publishers: New York, NY, USA, 1995. [Google Scholar]
- Ingle, J.D., Jr.; Crouch, S.R. Spectrochemical Analysis; Prentice Hall: Hoboken, NJ, USA, 1988. [Google Scholar]
- Edwards, H.G.M.; Johnson, A.F.; Lewis, I.R. Applications of Raman spectroscopy to the study of polymers and polymerization processes. J. Raman Spectrosc. 1993, 24, 475–483. [Google Scholar] [CrossRef]
- Xue, G. Laser Raman Spectroscopy of Polymeric Materials. Prog. Polym. Sci. 1994, 19, 317–388. [Google Scholar] [CrossRef]
- Nelson, E.W.; Scranton, A.B. Kinetics of cationic photopolymerizations of divinyl ethers characterized using in situ Raman spectroscopy. J. Polym. Sci. Pt. A Polym. Chem. 1996, 34, 403–411. [Google Scholar] [CrossRef]
- Williams, K.P.J.; Wilcock, I.C. Raman Spectroscopy of Polymers. Polym. Polym. Compos. 1997, 5, 443–449. [Google Scholar] [CrossRef]
- Nelson, E.W.; Jacobs, J.L.; Scranton, A.B.; Anseth, K.S.; Bowman, C.N. Photo-differential scanning calorimetry studies of cationic polymerizations of divinyl ethers. Polymer 1995, 36, 4651–4656. [Google Scholar] [CrossRef]
- Falk, B.; Vallinas, S.M.; Crivello, J.V. Monitoring photopolymerization reactions with optical pyrometry. J. Polym. Sci. Pt. A Polym. Chem. 2003, 41, 579–596. [Google Scholar] [CrossRef]
- Roper, T.M.; Lee, T.Y.; Guymon, C.A.; Hoyle, C.E. In situ characterization of photopolymerizable systems using a thin-film calorimeter. Macromolecules 2005, 38, 10109–10116. [Google Scholar] [CrossRef]
- Udagawa, A.; Sakurai, F.; Takahashi, T. In situ study of photopolymerization by Fourier transform infrared spectroscopy. J. Appl. Polym. Sci. 1991, 42, 1861–1867. [Google Scholar] [CrossRef]
- Jager, W.F.; Volkers, A.A.; Neckers, D.C. Solvatochromic fluorescent probes for monitoring the photopolymerization of dimethacrylates. Macromolecules 1995, 28, 8153–8158. [Google Scholar] [CrossRef]
- Cai, Y.; Jessop, J.L.P. Decreased oxygen inhibition in photopolymerized acrylate/epoxide hybrid polymer coatings as demonstrated by Raman spectroscopy. Polymer 2006, 47, 6560–6566. [Google Scholar] [CrossRef]
- Schissel, S.M.; Jessop, J.L.P. Transferable shadow cure: A new approach to achieving cationic photopolymerization in light-restricted areas. J. Appl. Polym. Sci. 2002, 139, e52491. [Google Scholar] [CrossRef]
- Zou, Y.; Armstrong, S.R.; Jessop, J.L.P. Quantitative analysis of adhesive resin in the hybrid layer using Raman spectroscopy. J. Biomed. Mater. Res. Pt. A 2010, 94, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Khalid, H.; Sarfraz, Z.; Khan, M.; Iqbal, J.; Muhammad, N.; Fareed, M.A.; Rehman, I.U. Vibrational spectroscopy of selective dental restorative materials. Appl. Spectrosc. Rev. 2017, 52, 507–540. [Google Scholar] [CrossRef]
- Scherzer, T. Depth profiling of the conversion during the photopolymerization of acrylates using real-time FTIR-ATR spectroscopy. Vib. Spectrosc. 2002, 29, 139–145. [Google Scholar] [CrossRef]
- Zou, Y.; Armstrong, S.R.; Jessop, J.L.P. Apparent conversion of adhesive resin in the hybrid layer, Part 1: Identification of an internal reference for Raman spectroscopy and the effects of water storage. J. Biomed. Mater. Res. Pt. A 2008, 86, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Nyquist, R. Interpreting Infrared, Raman, and Nuclear Magnetic Resonance Spectra; Academic Press: San Diego, CA, USA, 2001; Volumes 1 and 2. [Google Scholar]
- Kaalberg, S.M.; Schissel, S.M.; Soumounthong, M.; Jessop, J.L.P. Elucidation of network structure in cationic photopolymerization of cyclic ether comonomers. Polym. Chem. 2021, 12, 5999–6008. [Google Scholar] [CrossRef]
- Schissel, S.M.; Lapin, S.C.; Jessop, J.L.P. Internal reference validation for EB-cured polymer conversion measured via Raman spectroscopy. RadTech Rep. 2014, 46–50. [Google Scholar]
- Eom, H.S.; Jessop, J.L.P.; Scranton, A.B. Photoinitiated cationic copolymerizations: Effects of the oligomer structure and composition. Polymer 2013, 54, 4134–4142. [Google Scholar] [CrossRef]
- Kaalberg, S.; Jessop, J.L.P. Enhancing cationic ring-opening photopolymerization of cycloaliphatic epoxides via dark cure and oxetanes. J. Polym. Sci. Pt. A Polym. Chem. 2018, 56, 1436–1445. [Google Scholar] [CrossRef]
- De Santis, A. Photo-polymerisation effects on the carbonyl CO bands of composite resins measured by micro-Raman spectroscopy. Polymer 2005, 46, 5001–5004. [Google Scholar] [CrossRef]
- Schissel, S.M.; Jessop, J.L.P. Enhancing epoxide kinetics and tuning polymer properties using hydroxyl-containing (meth) acrylates in hybrid photopolymerizations. Polymer 2019, 161, 78–91. [Google Scholar] [CrossRef]
- Talungchit, S. Enhancing Resin-Dentin Bond Effectiveness and Durability: The Role of Ethanol-Wet Bonding Technique, MMP-inhibition (Chlorhexidine), and Photoinitiator Systems. Ph.D. Thesis, University of Iowa, Iowa City, IA, USA, 2012. [Google Scholar]
- Cai, Y.; Jessop, J.L.P. Effect of water concentration on photopolymerized acrylate/epoxide hybrid polymer coatings as demonstrated by Raman spectroscopy. Polymer 2009, 50, 5406–5413. [Google Scholar] [CrossRef]
- Hasa, E.; Scholte, J.P.; Jessop, J.L.P.; Stansbury, J.W.; Guymon, C.A. Kinetically controlled photoinduced phase separation for hybrid radical/cationic systems. Macromolecules 2019, 52, 2975–2986. [Google Scholar] [CrossRef]
- Knapp, E.; Kaalberg, S.; Jessop, J.L.P. Head-to-head comparison of cationic photoinitiator effectiveness. In Proceedings of the RadTech 2022 UV/EB Technology Conference, Orlando, FL, USA, 9–12 May 2022. [Google Scholar]
- Dillman, B.; Jessop, J.L.P. Chain transfer agents in cationic photopolymerization of a bis-cycloaliphatic epoxide monomer: Kinetic and physical property effects. J. Polym. Sci. Pt. A Polym. Chem. 2013, 51, 2058–2067. [Google Scholar] [CrossRef]
- Barnes, S.E.; Cygan, Z.T.; Yates, J.K.; Beers, K.L.; Amis, E.J. Raman spectroscopic monitoring of droplet polymerization in a microfluidic device. Analyst 2006, 131, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.T.H.; Jia, Z.; Kanaev, A.; Museur, L. Photopolymerization of TiO2-based hybrid materials: Effect of nanoparticles loading and photosensitive 1D microstructures fabrication. J. Mater. Sci. 2023, 58, 1127–1138. [Google Scholar] [CrossRef]
- Kaalberg, S.M. Improving Photopolymerization Outcomes Through Control of Cationic Ring-Opening Systems. Ph.D. Thesis, University of Iowa, Iowa City IA, USA, 2018. [Google Scholar]
- Cai, Y. Characterization of Hybrid Resin Systems Based on Epoxide/Acrylate Functionalities. Ph.D. Thesis, University of Iowa, Iowa City, IA, USA, 2006. [Google Scholar]
- Sipani, V.; Scranton, A.B. Kinetic studies of cationic photopolymerizations of phenyl glycidyl ether: Termination/trapping rate constants for iodonium photoinitiators. J. Photochem. Photobiol. A Chem. 2003, 159, 189–195. [Google Scholar] [CrossRef]
- Courtecuisse, F.; Karasu, F.; Allonas, X.; Croutxé-Barghorn, C.; van der Ven, L. Confocal Raman microscopy study of several factors known to influence the oxygen inhibition of acrylate photopolymerization under LED. Prog. Org. Coat. 2016, 92, 1–7. [Google Scholar] [CrossRef]
- Thiher, N.L.K.; Schissel, S.M.; Jessop, J.L.P. Quantifying UV/EB dual cure for successful mitigation of oxygen inhibition and light attenuation. Prog. Org. Coat. 2020, 138, 105378. [Google Scholar] [CrossRef]
- Navarra, C.O.; Cadenaro, M.; Armstrong, S.R.; Jessop, J.L.P.; Antoniolli, F.; Sergo, V.; Di Lenarda, R.; Breschi, L. Degree of conversion of Filtek Silorane Adhesive System and Clearfil SE Bond within the hybrid and adhesive layer: An in situ Raman analysis. Dent. Mater. 2009, 25, 1178–1185. [Google Scholar] [CrossRef]
- Zou, Y.; Jessop, J.L.P.; Armstrong, S.R. Apparent conversion of adhesive resin in the hybrid layer, Part II: In situ studies of the resin-dentin bond. J. Biomed. Mater. Res. Pt. A 2008, 89, 355–362. [Google Scholar] [CrossRef]
- Biria, S.; Malley, P.P.A.; Kahan, T.F.; Hosein, I.D. Tunable nonlinear optical pattern formation and microstructure in cross-linking acrylate systems during free-radical polymerization. J. Phys. Chem. C 2016, 120, 4517–4528. [Google Scholar] [CrossRef]
- Chikama, K.; Mastubara, K.; Oyama, S.; Tomita, Y. Three-dimensional confocal Raman imaging of volume holograms formed in ZrO2 nanoparticle-photopolymer composite materials. J. Appl. Phys. 2008, 103, 113108. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, W.; Wang, Y.; Fan, Y.; Qiu, L.; Zhu, K. Improving spatial resolution of confocal Raman microscopy by super-resolution image restoration. Opt. Express 2016, 24, 10767–10776. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xu, D.; Li, A.; Su, Y.; Zhao, W.; Qiu, L.; Cui, H. High spatial resolution of topographic imaging and Raman mapping by differential correlation-confocal Raman microscopy. Opt. Express 2022, 30, 41447–41458. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, U.; Hild, S.; Ibach, W.; Hollricher, O. Characterization of thin polymer films on the nanometer scale with confocal Raman AFM. Macromol. Symp. 2005, 230, 133–143. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, W. Hybrid AFM for nanoscale physicochemical characterization: Recent development and emerging applications. Small 2017, 13, 1603525. [Google Scholar] [CrossRef]
- Chevrel, M.C.; Brun, N.; Hoppe, S.; Meimaroglou, D.; Falk, L.; Chapron, D.; Bourson, P.; Durand, A. In situ monitoring of acrylic acid polymerization in aqueous solution using rheo-Raman technique. Experimental investigation and theoretical modelling. Chem. Eng. Sci. 2014, 106, 242–252. [Google Scholar] [CrossRef]
- Brown, T.E.; Malavé, V.; Higgins, C.I.; Kotula, A.P.; Caplins, B.W.; Garboczi, E.J.; Killgore, J.P. Voxel-scale conversion mapping informs intrinsic resolution in stereolithographic additive manufacturing. ACS Appl. Polym. Mater. 2020, 3, 290–298. [Google Scholar] [CrossRef]
- Chen, Y.C.; Ferracane, J.L.; Prahl, S.A. Quantum yield of conversion of the photoinitiator camphorquinone. Dent. Mater. 2007, 23, 655–664. [Google Scholar] [CrossRef]
- Navarra, C.O.; Alcantar, J.; Armstrong, S.R.; Jessop, J.L.P. 514 nm Raman Laser Contributes to Conversion of Methacrylates Used in Dental Resins; University of Iowa: Iowa City, IA, USA, 2006; unpublished work. [Google Scholar]
- Osipov, A.A. High-temperature Raman spectroscopy. Pure Appl. Chem. 2019, 91, 1749–1756. [Google Scholar] [CrossRef]
- Technical Note: Sample Damage in Raman Spectroscopy. Available online: https://www.stellarnet.us/technical-note-sample-damage-in-raman-spectroscopy/ (accessed on 22 June 2023).
- Schissel, S.M.; Scranton, A.B.; Jessop, J.L.P. Characterization of Dark Cure and Shadow Cure in Cationic Photopolymerizations: Annual Report for the Fundamentals and Applications of Photopolymerizations Industry; University Cooperative Research Center, University of Iowa: Iowa City, IA, USA, 2013; unpublished work. [Google Scholar]
- Everall, N.J. Modeling and measuring the effect of refraction on the depth resolution of confocal Raman microscopy. Appl. Spectrosc. 2000, 54, 773–782. [Google Scholar] [CrossRef]
- Everall, N.J. Confocal Raman microscopy: Why the depth resolution and spatial accuracy can be much worse than you think. Appl. Spectrosc. 2000, 54, 1515–1520. [Google Scholar] [CrossRef]
- Baia, L.; Gigant, K.; Posset, U.; Schottner, G.; Kiefer, W.; Popp, J. Confocal micro-Raman spectroscopy: Theory and application to a hybrid polymer coating. Appl. Spectrosc. 2002, 56, 536–540. [Google Scholar] [CrossRef]
- Schissel, S.M. Developing Property and Kinetic Control Strategies for Radiation Polymerization. Ph.D. Thesis, University of Iowa, Iowa City, IA, USA, 2016. [Google Scholar]
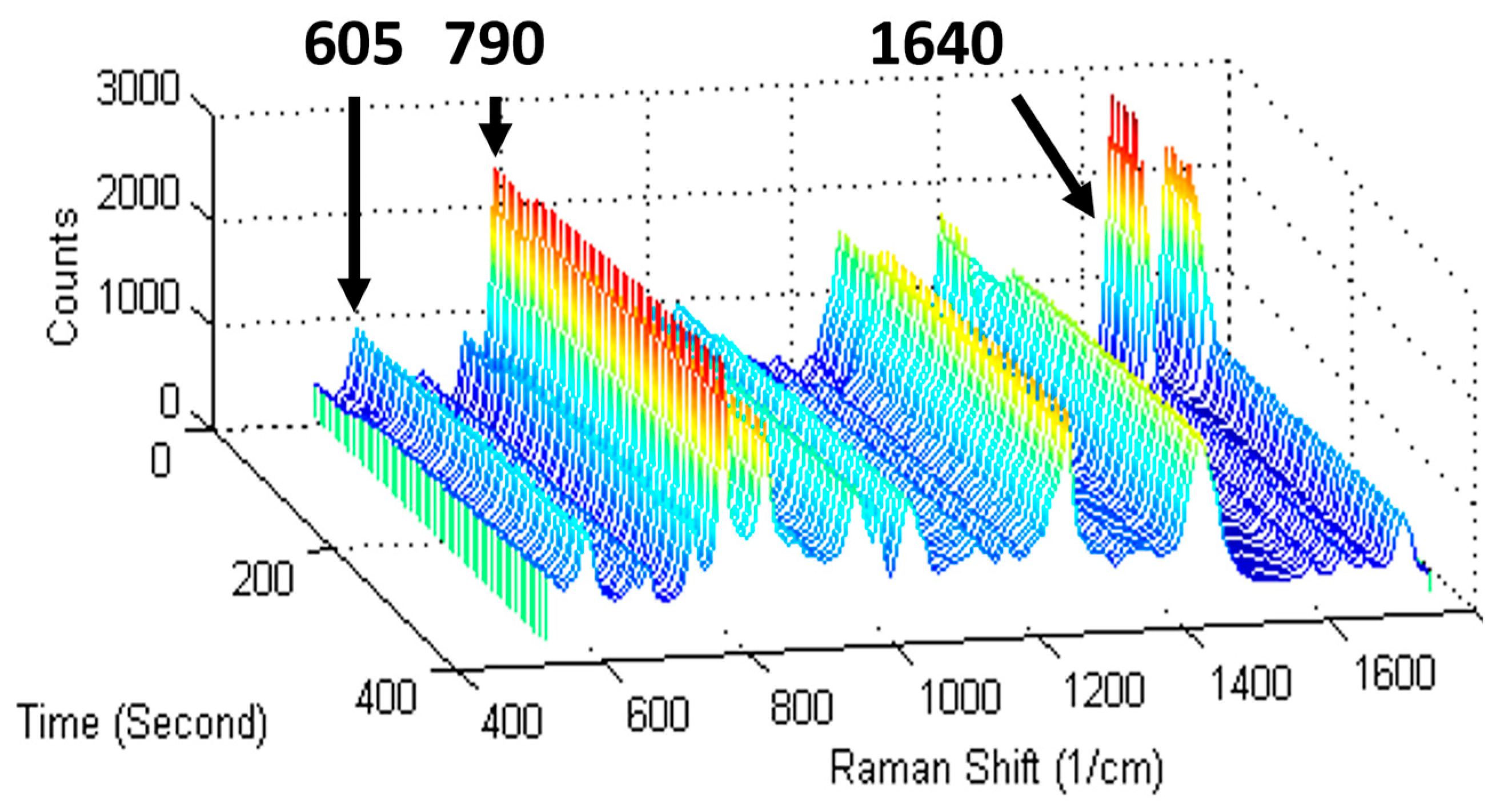


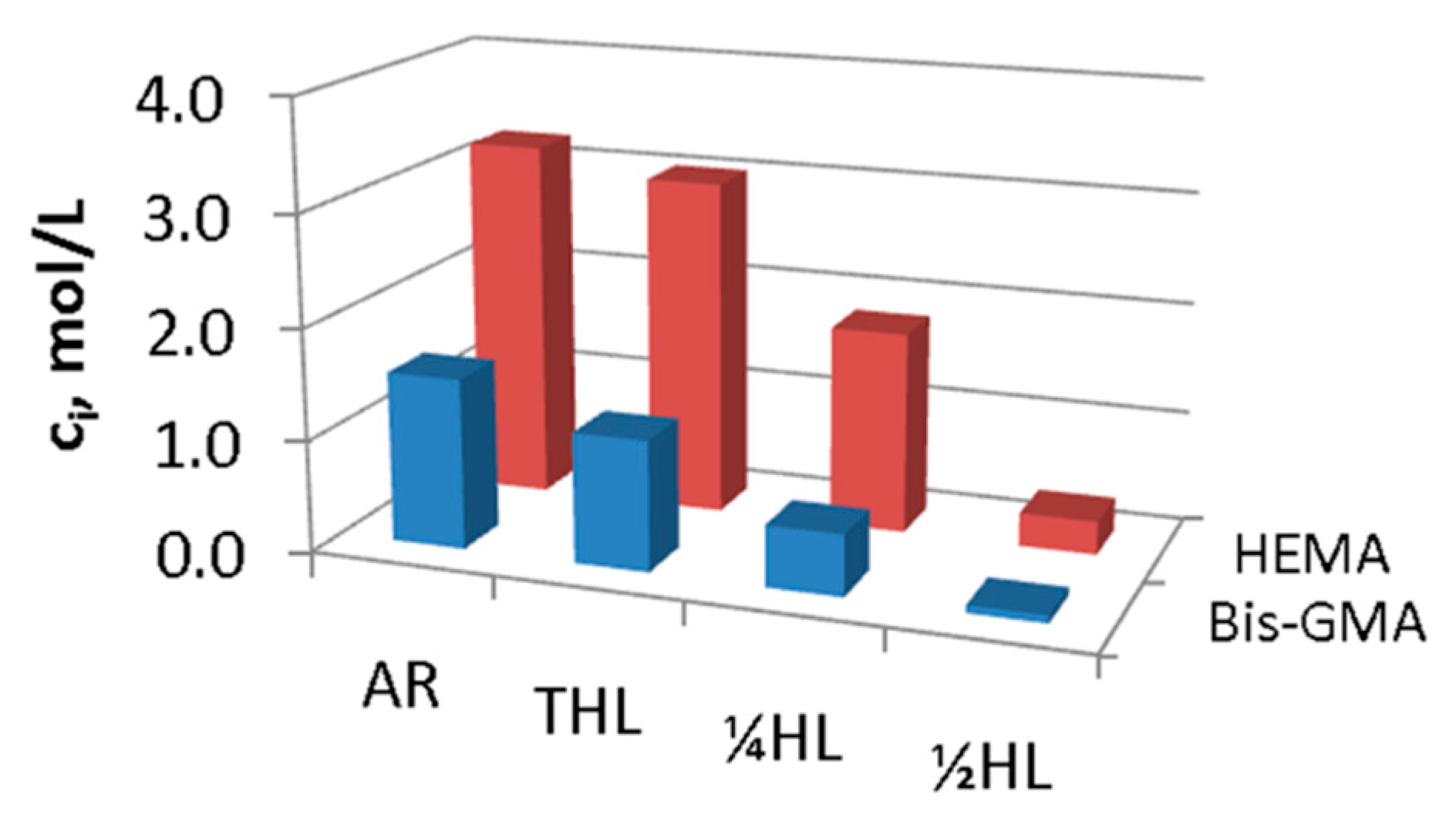
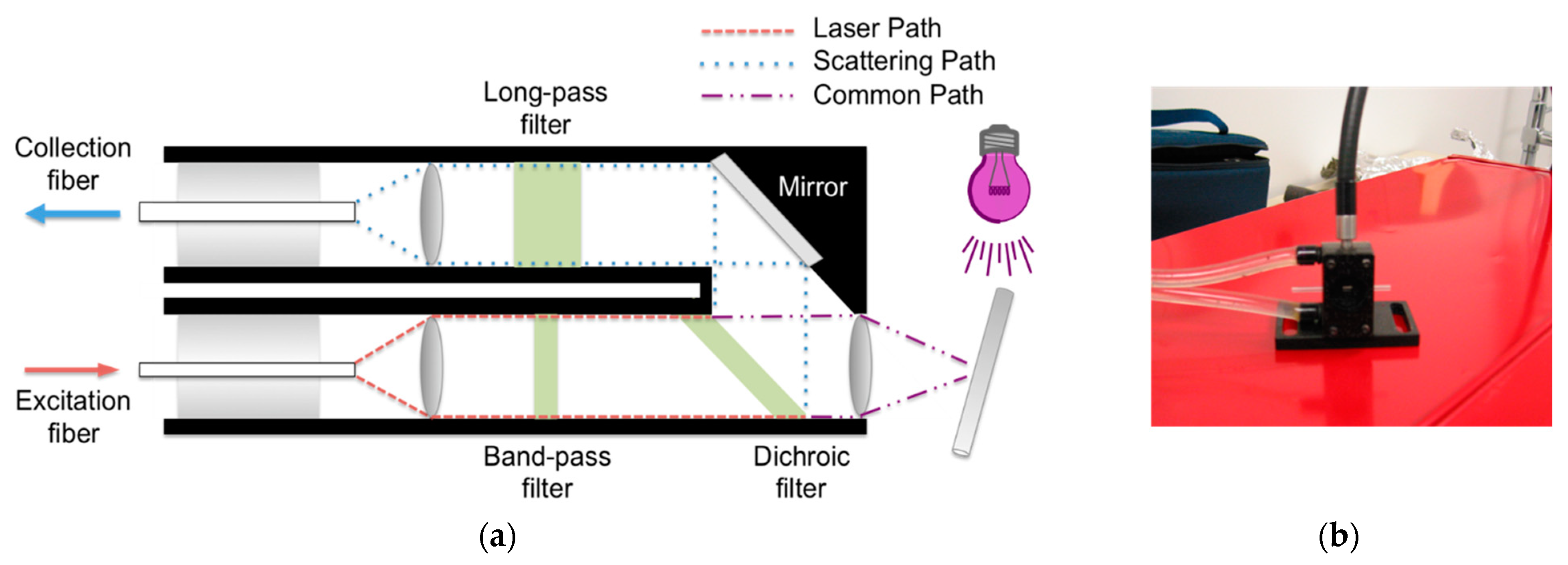
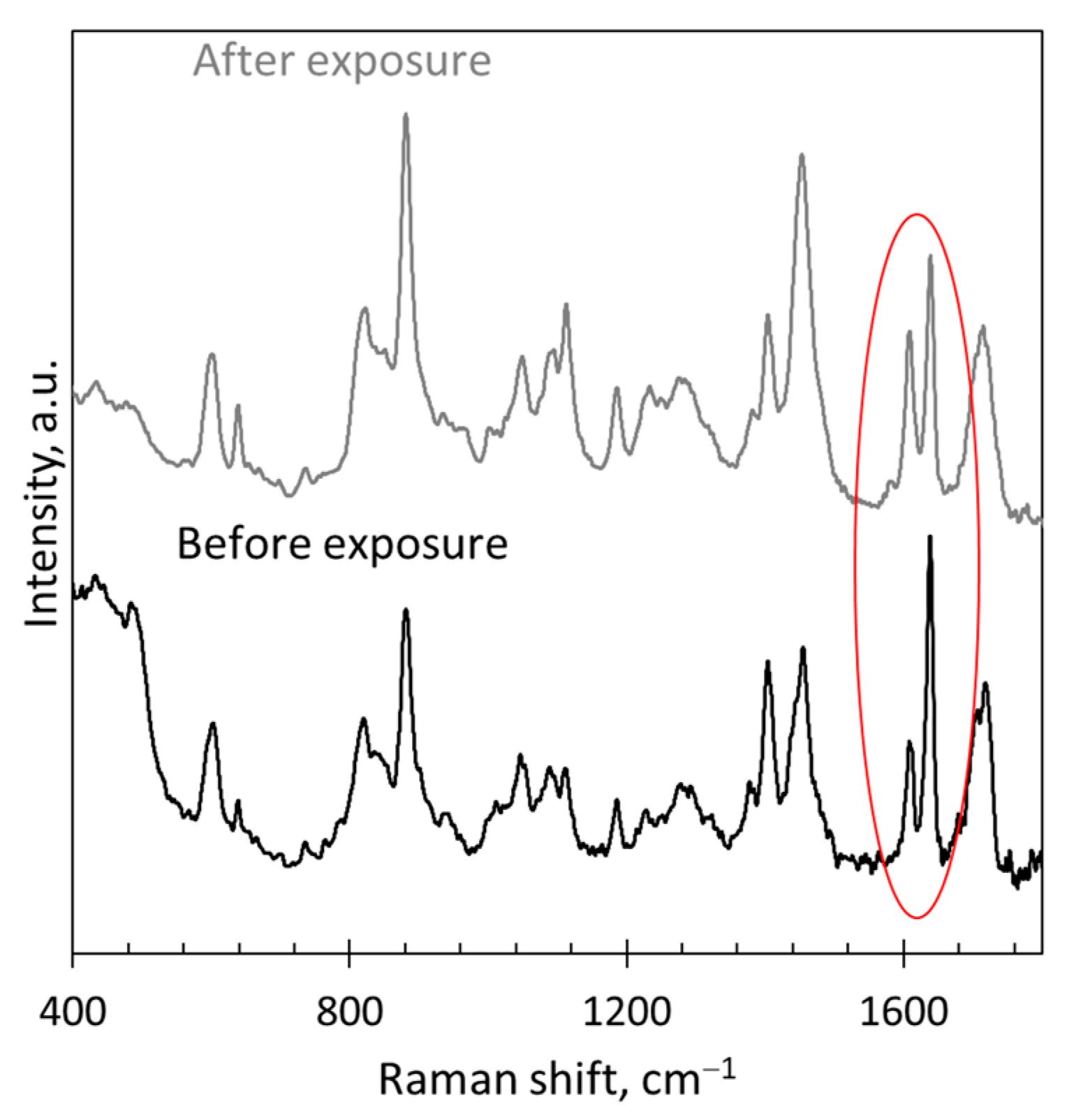
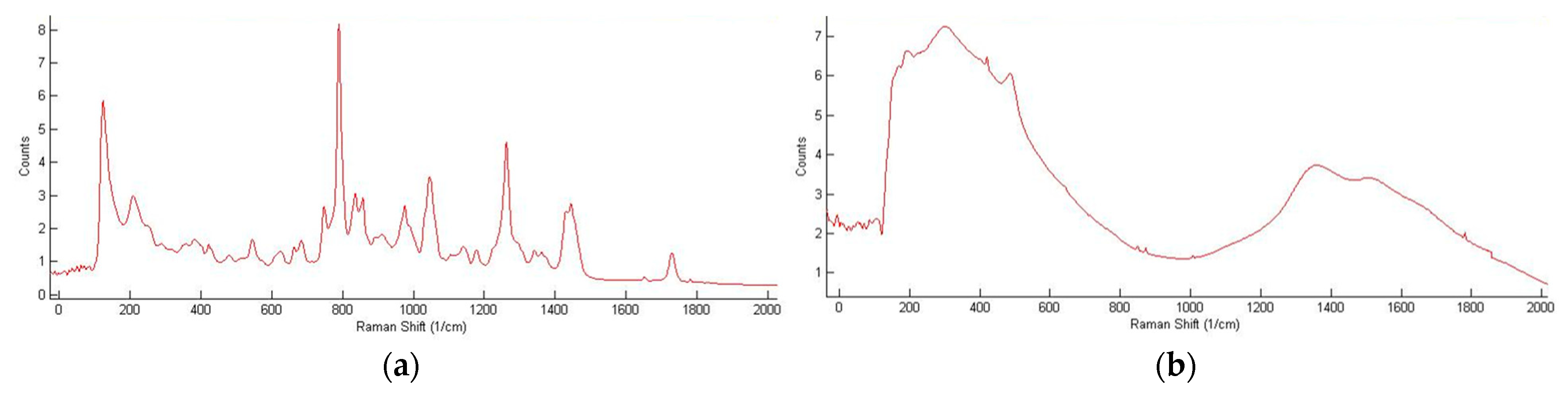
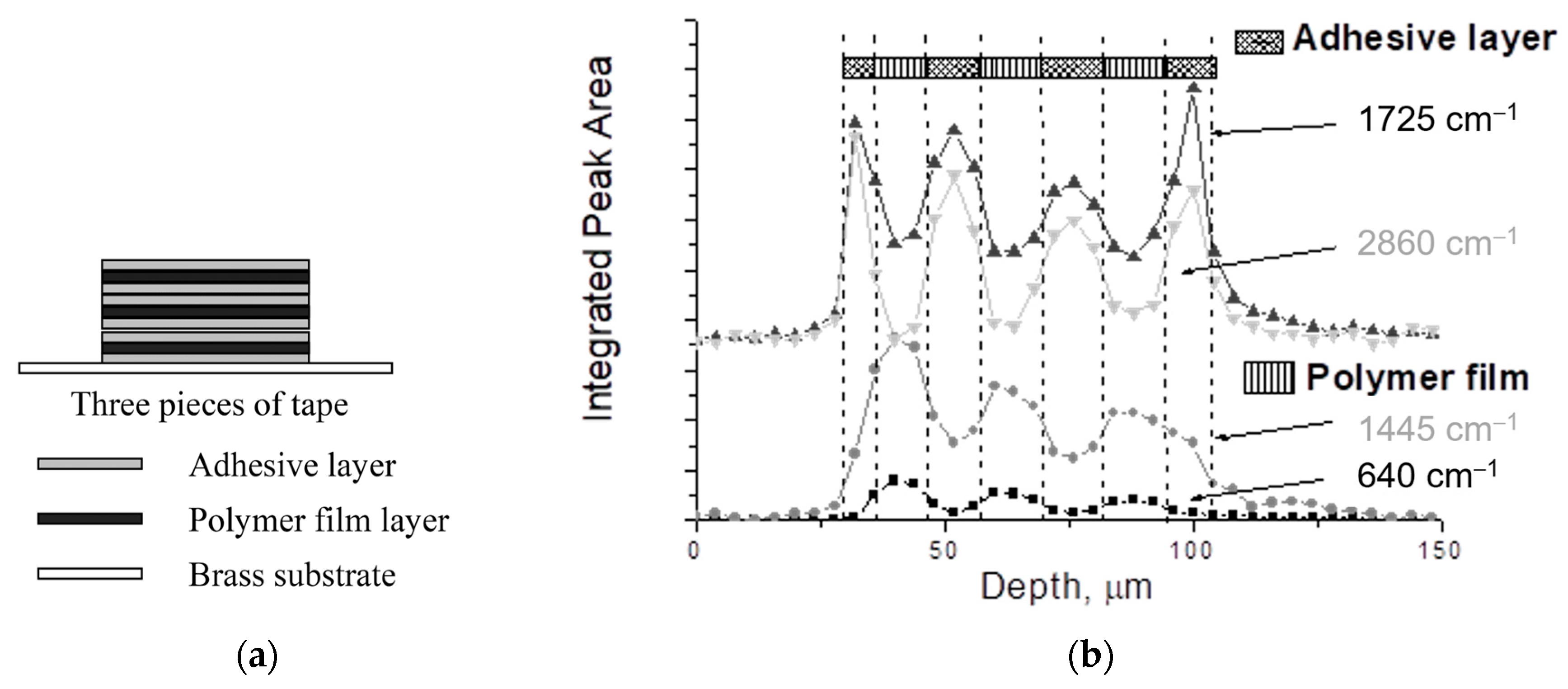
| Reference Peak | Reaction Peak | Representative Reference | |||
|---|---|---|---|---|---|
| Peak (cm−1) | Moiety | Peak (cm−1) | Moiety | ||
| trimethylolpropane triacrylate (TMPTA, acrylate) | 1070 | aliphatic groups | 1636 | vinyl C=C group | [22] |
| hydroxyethyl methacrylate (HEMA, methacrylate) | 605 | carbonyl (C–C–O) stretch | 1640 | vinyl C=C group | [18] |
| 3,6,9,12-tetraoxatetradeca-1,13-diene (DVE-3, vinyl ether) | 1458 | wagging and bending of ethyl-ether hydrogens | 1322 | C–H bending of hydrogen attached to a vinyl carbon | [6] |
| 3,4-epoxycyclohexylmethyl-3′,4′-epoxycyclohexanecarboxylate (EEC, cycloaliphatic epoxide) | 1727 | skeletal bending of the non-reactive CC=O group | 789 | asymmetric cycloaliphatic epoxide ring deformation | [23] |
| epoxidized polybutadiene oligomer (EPOH, aliphatic epoxide) | 1640 | 1,2-vinyl C=C stretching vibrations | 1271 | in-phase breathing vibrations of oxirane ring | [23] |
| 3-ethyl-3- [(2-ethylhexyloxy)methyl] oxetane (EHOX, oxetane) | 1450 | aliphatic groups | 1150 | oxetane ring | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jessop, J.L.P. A Practical Primer: Raman Spectroscopy for Monitoring of Photopolymerization Systems. Polymers 2023, 15, 3835. https://doi.org/10.3390/polym15183835
Jessop JLP. A Practical Primer: Raman Spectroscopy for Monitoring of Photopolymerization Systems. Polymers. 2023; 15(18):3835. https://doi.org/10.3390/polym15183835
Chicago/Turabian StyleJessop, Julie L. P. 2023. "A Practical Primer: Raman Spectroscopy for Monitoring of Photopolymerization Systems" Polymers 15, no. 18: 3835. https://doi.org/10.3390/polym15183835
APA StyleJessop, J. L. P. (2023). A Practical Primer: Raman Spectroscopy for Monitoring of Photopolymerization Systems. Polymers, 15(18), 3835. https://doi.org/10.3390/polym15183835







