Modeling and Simulation of a Multizone Circulating Reactor for Polyethylene Production with Internal Cooling
Abstract
:1. Introduction
2. Model Development
2.1. Material Balance
2.2. Fluid Flow
2.3. Heat Transfer
3. Results and Discussion
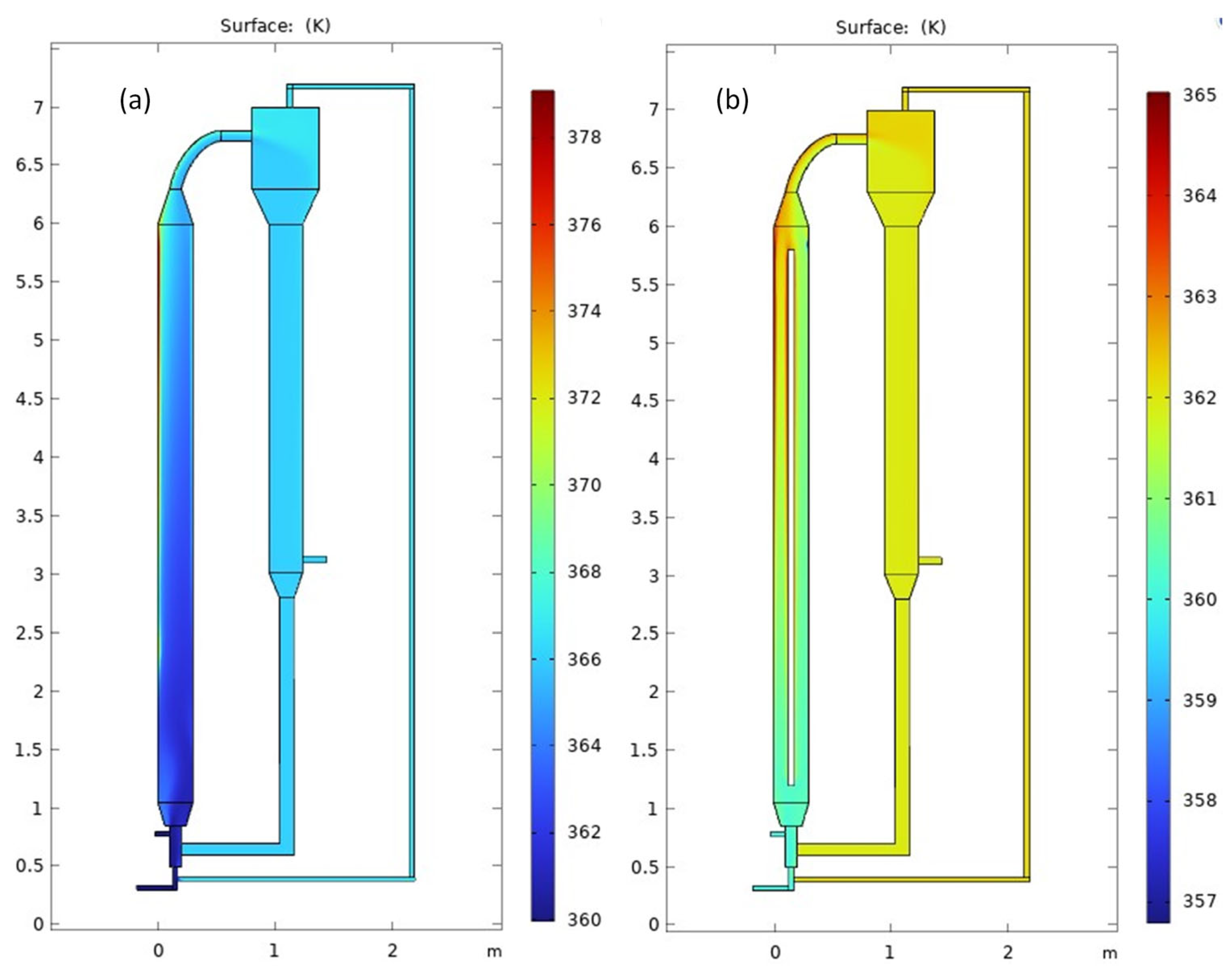
3.1. Temperature Distribution
3.2. Velocity Distribution Profile
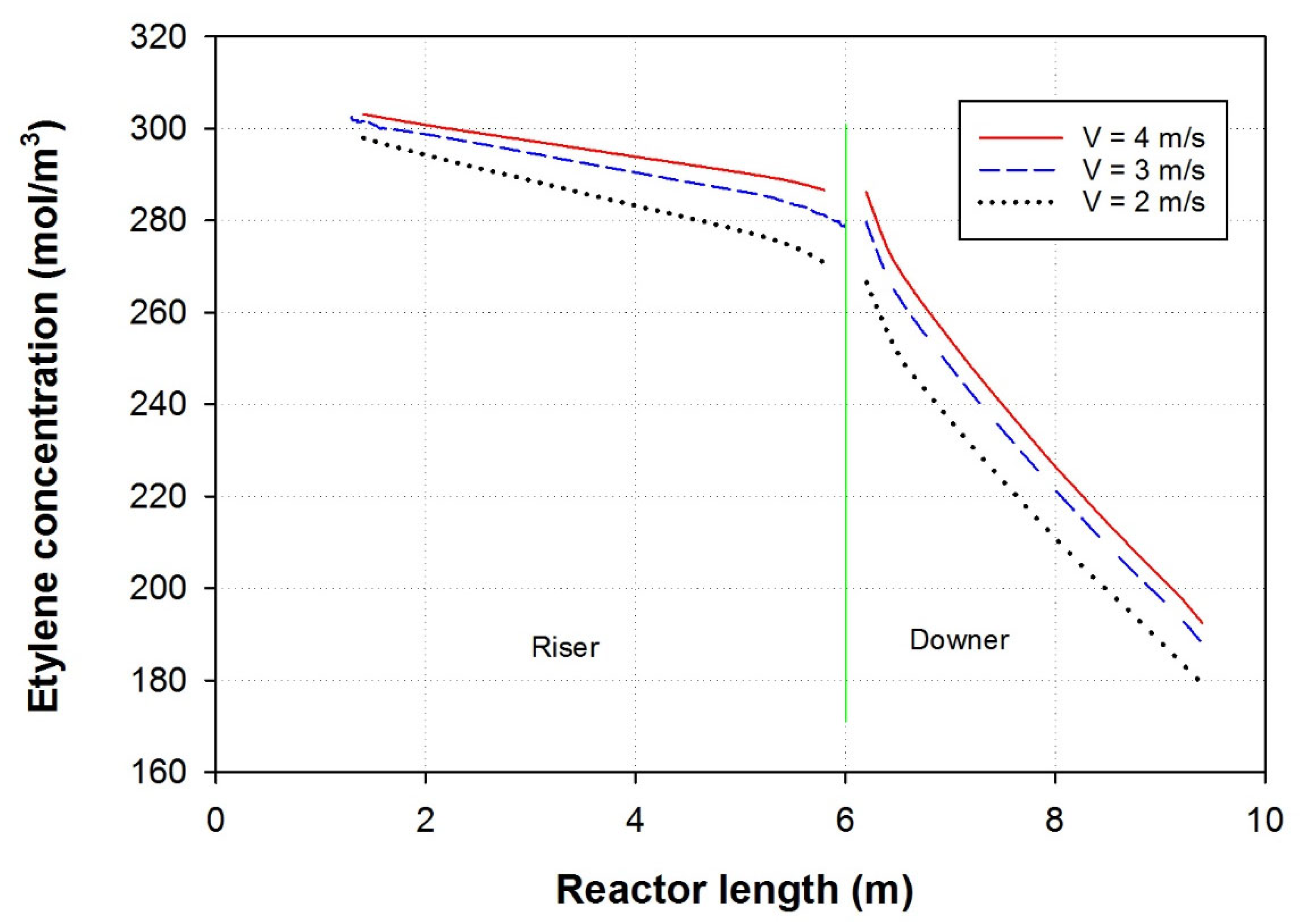
3.3. Effect of Feed Velocity
3.4. Influence of Cooler Insertion
3.5. Effect of Porosity
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Guarino, V.; Marrese, M.; Ambrosio, L. Chemical and Physical Properties of Polymers for Biomedical Use; Springer: Berlin/Heidelberg, Germany, 2014; pp. 67–90. ISBN 978-3-319-12478-0. [Google Scholar]
- Yang, Y.-N.; Liu, H.; Zhu, L.-T.; Jin, J.; Zhang, X.-B.; Luo, Z.-H. Simulation of Molecular and Particle Properties of Polypropylene Produced in Multizone Circulating Reactors. Ind. Eng. Chem. Res. 2023, 62, 6130–6141. [Google Scholar] [CrossRef]
- Hutley, T.J.; Ouederni, M. Polyolefins—The History and Economic Impact BT—Polyolefin Compounds and Materials: Fundamentals and Industrial Applications; Al-Ali AlMa’adeed, M., Krupa, I., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 13–50. ISBN 978-3-319-25982-6. [Google Scholar]
- Ghasem, N. CO2 removal from natural gas. In Advances in Carbon Capture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 479–501. [Google Scholar]
- Ghasem, N. Modeling and Simulation of CO2 Absorption Enhancement in Hollow-Fiber Membrane Contactors using CNT–Water-Based Nanofluids. J. Membr. Sci. Res. 2019, 5, 295–302. [Google Scholar] [CrossRef]
- Sui, Y.; Qiu, Z.; Liu, Y.; Li, J.; Cui, Y.; Wei, P.; Cong, C.; Meng, X.; Zhou, Q. Ultra-high molecular weight polyethylene (UHMWPE)/high-density polyethylene (HDPE) blends with outstanding mechanical properties, wear resistance, and processability. J. Polym. Res. 2023, 30, 222. [Google Scholar] [CrossRef]
- Covezzi, M.; Mei, G. The multizone circulating reactor technology. Chem. Eng. Sci. 2001, 56, 4059–4067. [Google Scholar] [CrossRef]
- Fernandas, F.A.N.; Lona, L.M.F. Multizone circulating reactor modeling for gas-phase polymerization. II. Reactor operating with gas barrier in the downer section. J. Appl. Polym. Sci. 2004, 93, 1053–1059. [Google Scholar] [CrossRef]
- Fernandas, F.A.N.; Lona, L.M.F. Multizone circulating reactor modeling for gas-phase polymerization. I. Reactor modeling. J. Appl. Polym. Sci. 2004, 93, 1042–1052. [Google Scholar] [CrossRef]
- Ghasem, N.M. Effect of polymer particle size and inlet gas temperature on industrial fluidized bed polyethylene reactors. Chem. Eng. Technol. 1999, 22, 777–783. [Google Scholar] [CrossRef]
- Ghasem, N.M. Design of a fuzzy logic controller for regulating the temperature in industrial polyethylene fluidized bed reactor. Chem. Eng. Res. Des. 2006, 84, 97–106. [Google Scholar] [CrossRef]
- Whitfield, R.; Truong, N.P.; Messmer, D.; Parkatzidis, K.; Rolland, M.; Anastasaki, A. Tailoring polymer dispersity and shape of molecular weight distributions: Methods and applications. Chem. Sci. 2019, 10, 8724–8734. [Google Scholar] [CrossRef]
- Kulajanpeng, K.; Sheibat-Othman, N.; Tanthapanichakoon, W.; McKenna, T.F.L. Multiscale modelling of multizone gas phase propylene (co)polymerization reactors—A comprehensive review. Can. J. Chem. Eng. 2022, 100, 2505–2545. [Google Scholar] [CrossRef]
- Ge, S.; Lou, Z.; Yang, Y.; Huang, Z.; Sun, J.; Wang, J.; Yang, Y. Electrostatic effects on hydrodynamics in the riser of the circulating fluidized bed for polypropylene. AIChE J. 2020, 66, e16916. [Google Scholar] [CrossRef]
- Che, Y.; Tian, Z.; Liu, Z.; Zhang, R.; Gao, Y.; Zou, E.; Wang, S.; Liu, B. A CFD-PBM Model Considering Ethylene Polymerization for the Flow Behaviors and Particle Size Distribution of Polyethylene in a Pilot-Plant Fluidized Bed Reactor. Powder Technol. 2015, 286, 107–123. [Google Scholar] [CrossRef]
- Yan, W.-C.; Li, J.; Luo, Z.-H. A CFD-PBM coupled model with polymerization kinetics for multizone circulating polymerization reactors. Powder Technol. 2012, 231, 77–87. [Google Scholar] [CrossRef]
- Yan, W.-C.; Chen, G.-Q.; Luo, Z.-H. A CFD modeling approach to design a new gas barrier in a multizone circulating polymerization reactor. Ind. Eng. Chem. Res. 2012, 51, 15132–15144. [Google Scholar] [CrossRef]
- Upadhyay, M.; Kim, A.; Kim, H.; Lim, D.; Lim, H. An Assessment of Drag Models in Eulerian–Eulerian CFD Simulation of Gas–Solid Flow Hydrodynamics in Circulating Fluidized Bed Riser. ChemEngineering 2020, 4, 37. [Google Scholar] [CrossRef]
- Ngo, S.I.; Lim, Y.-I. Multiscale Eulerian CFD of Chemical Processes: A Review. ChemEngineering 2020, 4, 23. [Google Scholar] [CrossRef]
- Emami, M.; Parvazinia, M.; Abedini, H. Gas-phase polymerization of propylene at low reaction rates: A precise look at catalyst fragmentation. Iran. Polym. J. 2017, 26, 871–883. [Google Scholar] [CrossRef]
- Wu, G.-L.; Tian, Z.; Wang, J.-J.; Gu, X.-P.; Feng, L.-F. Simulation of multizone circulating reactor for propylene gas-phase polymerization. Huaxue Gongcheng/Chem. Eng. 2011, 39, 79–83+97. [Google Scholar]
- Adli, H.; Mostoufi, N.; Ghafelebashi, S.M. Modeling of a multizone gas-phase polyethylene reactor with a cluster-based approach. J. Appl. Polym. Sci. 2011, 122, 393–405. [Google Scholar] [CrossRef]
- Wei, L.-H.; Yan, W.-C.; Luo, Z.-H. A preliminary CFD study of the gas-solid flow fields in multizone circulating polymerization reactors. Powder Technol. 2011, 214, 143–154. [Google Scholar] [CrossRef]
- Mei, G.; Beccarini, E.; Caputo, T.; Fritze, C.; Massari, P.; Agnoletto, D.; Pitteri, S. Recent technical advances in polypropylene. J. Plast. Film Sheeting 2009, 25, 95–113. [Google Scholar] [CrossRef]
- Ghasem, N.M.; Ang, W.L.; Hussain, M.A. Dynamics and stability of ethylene polymerization in multizone circulating reactors. Korean J. Chem. Eng. 2009, 26, 603–611. [Google Scholar] [CrossRef]
- Ghasem, N.M.; Ang, W.-L.; Hussain, M.A. Dynamic model for polyethylene production in a multizone circulating reactor. Chem. Prod. Process Model. 2008, 3. [Google Scholar] [CrossRef]
- Santos, J.L.; Asua, J.M.; De La Cal, J.C. Modeling of olefin gas-phase polymerization in a multizone circulating reactor. Ind. Eng. Chem. Res. 2006, 45, 3081–3094. [Google Scholar] [CrossRef]
- Wu, C.; Chen, W. Recent advances in technology of impact PP prepared in-situ in reactor. Hecheng Shuzhi Ji Suliao/China Synth. Resin Plast. 2006, 23, 71–74. [Google Scholar]
- Marandi, R.; Kamyabi, M.; Mostoufi, N. Hydrodynamic design of multi-zone circulating reactors using CFD. Can. J. Chem. Eng. 2018, 96, 670–678. [Google Scholar] [CrossRef]
- Dompazis, G.; Kanellopoulos, V.; Touloupides, V.; Kiparissides, C. Development of a multi-scale, multi-phase, multi-zone dynamic model for the prediction of particle segregation in catalytic olefin polymerization FBRs. Chem. Eng. Sci. 2008, 63, 4735–4753. [Google Scholar] [CrossRef]
- Dadebo, S.A.; Bell, M.L.; McLellan, P.J.; McAuley, K.B. Temperature control of industrial gas phase polyethylene reactors. J. Process Control 1997, 7, 83–95. [Google Scholar] [CrossRef]
- Xiong, C.; Bai, L.; Li, H.; Guo, Y.; Yu, Y.; Lin, G. Experimental study on a R134a loop heat pipe with high heat transfer capacity. Heat Mass Transf. 2022, 58, 903–916. [Google Scholar] [CrossRef]
- Wu, D.; Wu, W. Battery Powered Portable Thermal Cycler for Continuous-Flow Polymerase Chain Reaction Diagnosis by Single Thermostatic Thermoelectric Cooler and Open-Loop Controller. Sensors 2019, 19, 1609. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Y.; Shi, X.; Wang, C.; Gao, J.; Lan, X. 3D CPFD Simulation of Circulating Fluidized Bed Downer and Riser: Comparisons of Flow Structure and Solids Back-Mixing Behavior. Processes 2020, 8, 161. [Google Scholar] [CrossRef]
- Chalermsinsuwan, B.; Gidaspow, D.; Piumsomboon, P. Comparisons of particle cluster diameter and concentration in circulating fluidized bed riser and downer using computational fluid dynamics simulation. Korean J. Chem. Eng. 2013, 30, 963–975. [Google Scholar] [CrossRef]
- Kissin, Y.V.; Mink, R.I.; Nowlin, T.E.; Brandolini, A.J. Kinetics and Mechanism of Ethylene Polymerization and Copolymerization Reactions with Heterogeneous Titanium-Based Ziegler-Natta Catalysts BT—Metalorganic Catalysts for Synthesis and Polymerization; Kaminsky, W., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 60–75. [Google Scholar]
- Thakur, A.K.; Gupta, S.K.; Chaudhari, P. Modeling and simulation of an industrial slurry phase ethylene polymerization reactor: Effect of reactor operating variables. Iran. Polym. J. 2020, 29, 811–825. [Google Scholar] [CrossRef]
- Deng, B.; Jiang, Y.; Gao, L.; Zhao, B. CFD modeling of ethylene degradation in gas-phase photocatalytic reactors. Environ. Sci. Pollut. Res. 2023, 30, 24132–24142. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ni, L.; Chen, Q.; Jiang, J.; Zhou, K. Temperature Control of Exothermic Reactions Using n-Octadecane@MF Resin microPCMs Based on Esterification Reactions. Processes 2022, 10, 239. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, B.; He, F.; Fu, Z.; Xu, J.; Fan, Z. Comparative Study on Kinetics of Ethylene and Propylene Polymerizations with Supported Ziegler–Natta Catalyst: Catalyst Fragmentation Promoted by Polymer Crystalline Lamellae. Polymers 2019, 11, 358. [Google Scholar] [CrossRef]
- Ahsan Bashir, M.; McKenna, T.F.L. Reaction Engineering of Polyolefins: The Role of Catalyst Supports in Ethylene Polymerization on Metallocene Catalysts BT—Polymer Reaction Engineering of Dispersed Systems: Volume I; Pauer, W., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 19–63. ISBN 978-3-319-73479-8. [Google Scholar]
- Sans, V.; Karbass, N.; Burguete, M.I.; García-Verdugo, E.; Luis, S. V Residence time distribution, a simple tool to understand the behaviour of polymeric mini-flow reactors. RSC Adv. 2012, 2, 8721–8728. [Google Scholar] [CrossRef]
- Lovreglio, P.; Buist, K.A.; Kuipers, J.A.M.; Peters, E.A.J.F. Analysis of Particle-Resolved CFD Results for Dispersion in Packed Beds. Fluids 2022, 7, 199. [Google Scholar] [CrossRef]
- Johnson, M.D.; May, S.A.; Groh, J.M.C.; Webster, L.P.; Shankarraman, V.; Spencer, R.D.; Luciani, C.V.; Polster, C.S.; Braden, T. Understanding Residence Time, Residence Time Distribution, and Impact of Surge Vessels BT—Continuous Pharmaceutical Processing; Nagy, Z.K., El Hagrasy, A., Litster, J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 51–85. ISBN 978-3-030-41524-2. [Google Scholar]






| Parameter | Value |
|---|---|
| Pressure (atm) | 30 |
| Gas density (kg/m3) | 29 |
| Gas viscosity (μPa.s) | 11.6 |
| Polymer density (kg/m3) | 950 |
| Polymer heat capacity (J/g K) | 1.839 |
| Ethylene heat capacity (J/mol K) | 46 |
| Nitrogen heat capacity (J/mol K) | 28 |
| Gas thermal conductivity (W/mol K) | 0.03015 |
| Polymer thermal conductivity (W/mol K) | 0.34 |
| The heat of reaction (kJ/mol) | 94.5 |
| Parameter | Values |
|---|---|
| Riser length (m) | 5 |
| Downer length (m) | 3 |
| Riser diameter (m) | 0.3 |
| Downer diameter (m) | 0.3 |
| Riser void fraction | 0.9 |
| Downer void fraction | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasem, N. Modeling and Simulation of a Multizone Circulating Reactor for Polyethylene Production with Internal Cooling. Polymers 2023, 15, 3741. https://doi.org/10.3390/polym15183741
Ghasem N. Modeling and Simulation of a Multizone Circulating Reactor for Polyethylene Production with Internal Cooling. Polymers. 2023; 15(18):3741. https://doi.org/10.3390/polym15183741
Chicago/Turabian StyleGhasem, Nayef. 2023. "Modeling and Simulation of a Multizone Circulating Reactor for Polyethylene Production with Internal Cooling" Polymers 15, no. 18: 3741. https://doi.org/10.3390/polym15183741
APA StyleGhasem, N. (2023). Modeling and Simulation of a Multizone Circulating Reactor for Polyethylene Production with Internal Cooling. Polymers, 15(18), 3741. https://doi.org/10.3390/polym15183741








