Light-Driven Depolymerization of Cellulosic Biomass into Hydrocarbons
Abstract
:1. Introduction
2. Challenges in Cellulose Hydrolysis: Influence of Structure Function
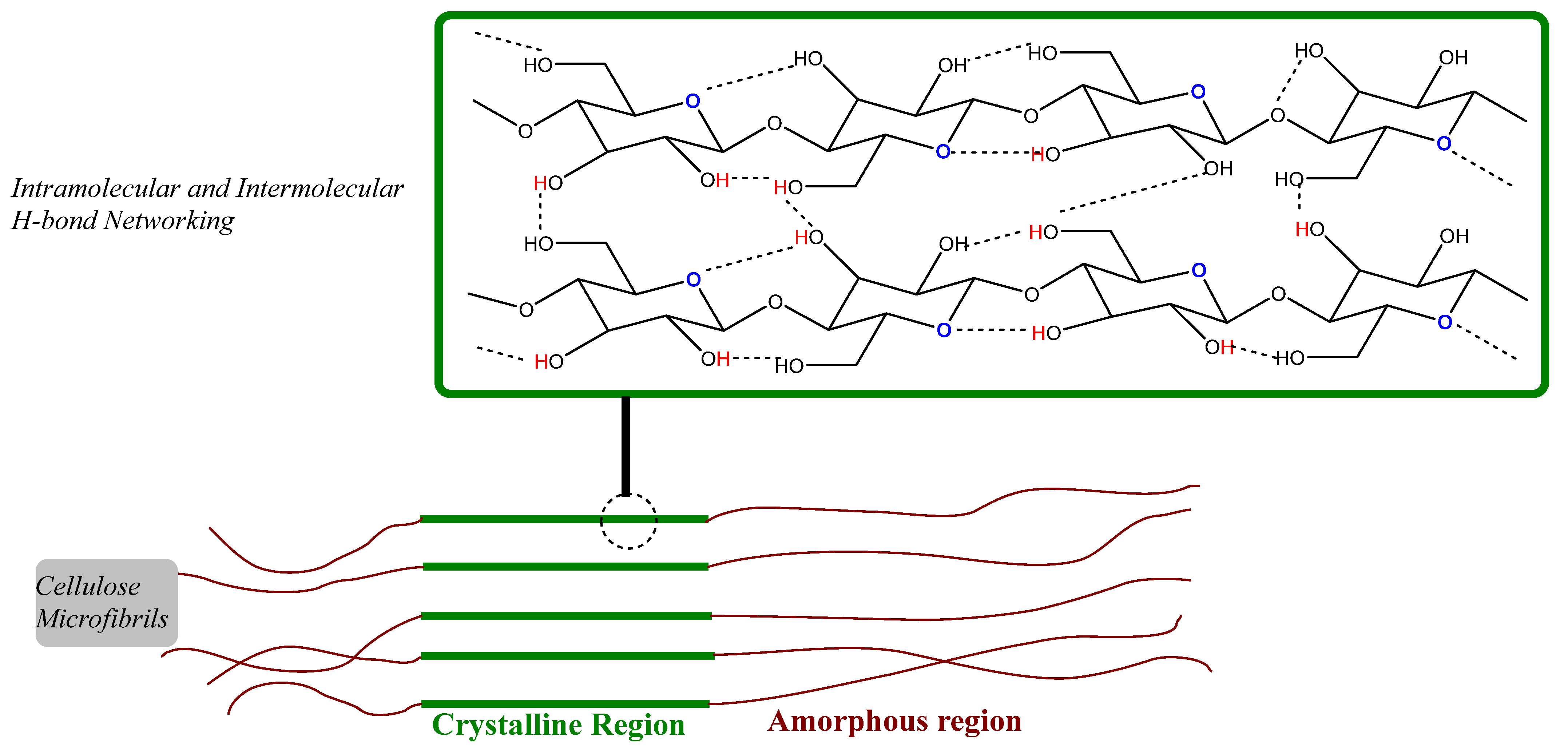
Structure of Cellulose
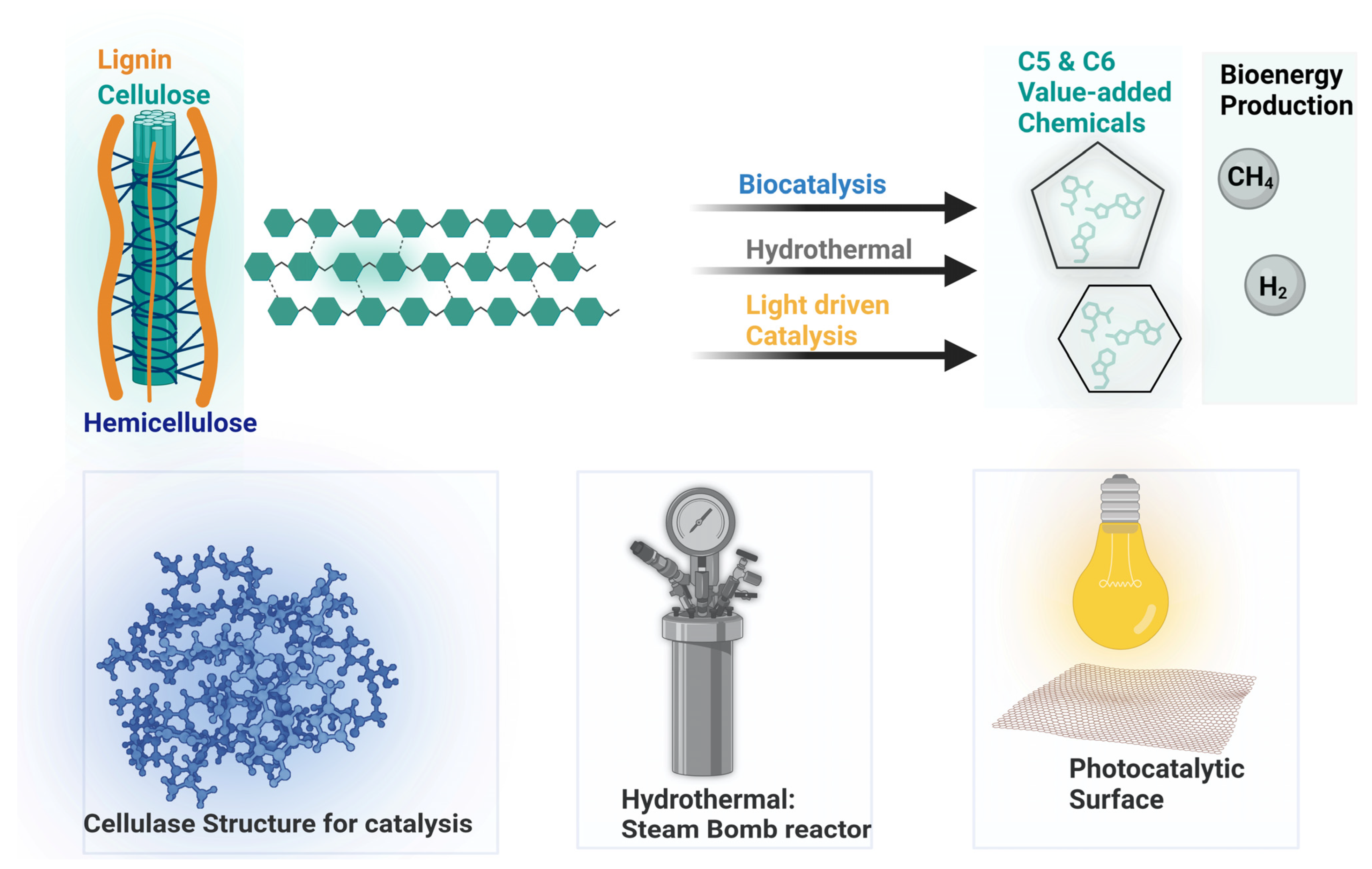
3. Methods of Cellulosic Biomass Conversion
3.1. Enzymatic Processes for Converting Cellulosic Biomass
3.2. Acidic Treatments for Converting Cellulosic Biomass
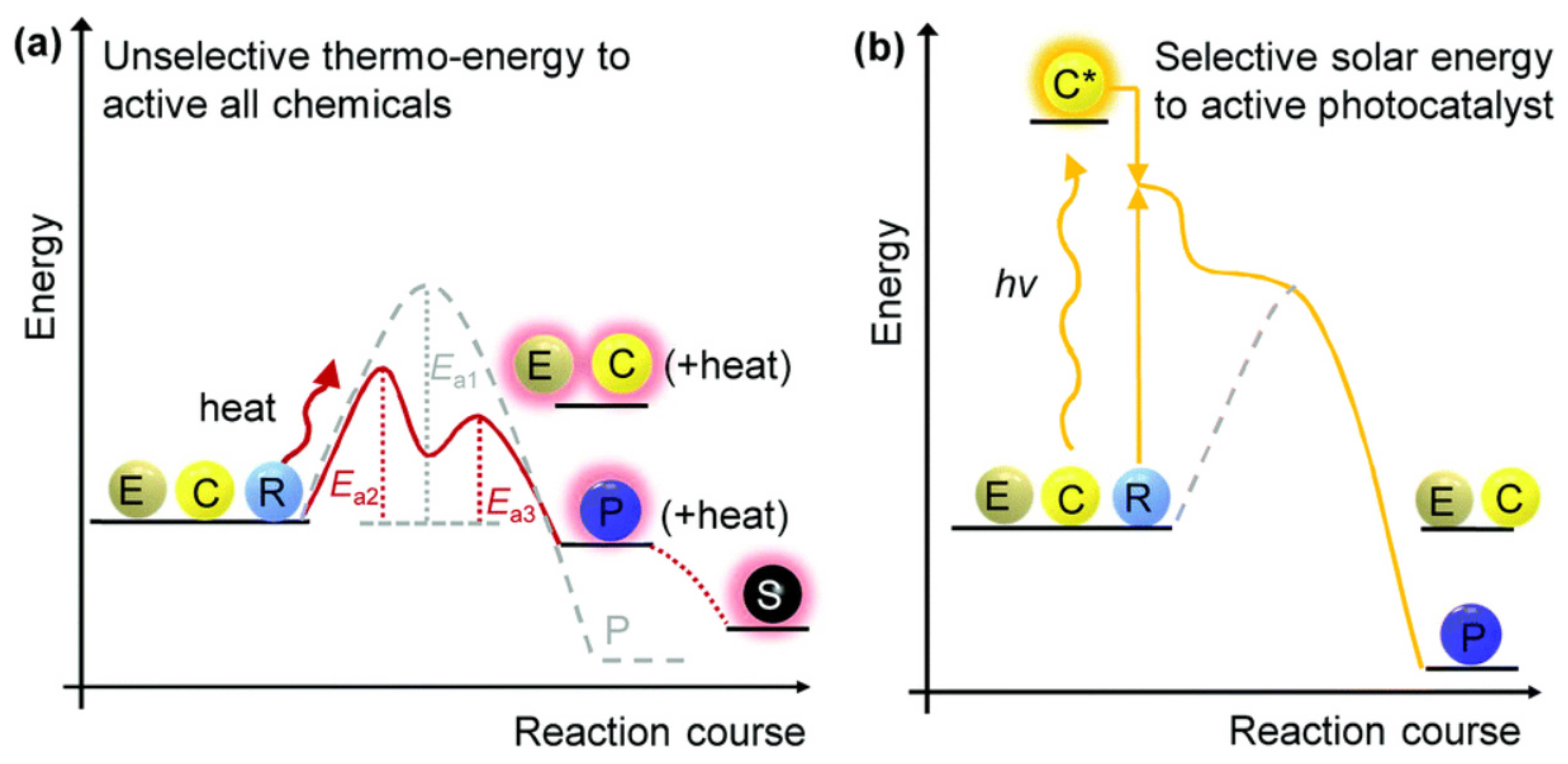
3.3. Photocatalytic Conversion of Cellulosic Biomass
3.4. Cellulosic-Biomass-Derived Hydrogen Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dewes, T.; Hünsche, E. Composition and microbial degradability in the soil of farmyard manure from ecologically-managed farms. Biol. Agric. Hortic. 1998, 16, 251–268. [Google Scholar] [CrossRef]
- Boopathy, R. Biological treatment of swine waste using anaerobic baffled reactors. Bioresour. Technol. 1998, 64, 1–6. [Google Scholar] [CrossRef]
- Cheung, S.W.; Anderson, B.C. Laboratory investigation of ethanol production from municipal primary wastewater solids. Bioresour. Technol. 1997, 59, 81–96. [Google Scholar] [CrossRef]
- Reshamwala, S.; Shawky, B.T.; Dale, B.E. Ethanol production from enzymatic hydrolysates of AFEX-treated coastal bermudagrass and switchgrass. Appl. Biochem. Biotechnol. 1995, 51, 43–55. [Google Scholar] [CrossRef]
- Nurdin, M.; Abimanyu, H.; Putriani, H.; Setiawan, L.I.; Maulidiyah, M.; Wibowo, D.; Ansharullah, A.; Natsir, M.; Salim, L.O.A.; Arham, Z. Optimization of OPEFB lignocellulose transformation process through ionic liquid [TEA][HSO4] based pretreatment. Sci. Rep. 2021, 11, 11338. [Google Scholar] [CrossRef]
- Ziaei-Rad, Z.; Pazouki, M.; Fooladi, J.; Azin, M.; Gummadi, S.N.; Allahverdi, A. Investigation of a robust pretreatment technique based on ultrasound-assisted, cost-effective ionic liquid for enhancing saccharification and bioethanol production from wheat straw. Sci. Rep. 2023, 13, 446. [Google Scholar] [CrossRef]
- Shrotri, A.; Kobayashi, H.; Fukuoka, A. Cellulose depolymerization over heterogeneous catalysts. Acc. Chem. Res. 2018, 51, 761–768. [Google Scholar] [CrossRef]
- Xiao, L.-P.; Sun, Z.-J.; Shi, Z.-J.; Xu, F.; Sun, R.-C. Impact of hot compressed water pretreatment on the structural changes of woody biomass for bioethanol production. BioResources 2011, 6, 1576–1598. [Google Scholar] [CrossRef]
- Orozco, R.; Redwood, M.; Leeke, G.; Bahari, A.; Santos, R.; Macaskie, L. Hydrothermal hydrolysis of starch with CO2 and detoxification of the hydrolysates with activated carbon for bio-hydrogen fermentation. Int. J. Hydrogen Energy 2012, 37, 6545–6553. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar]
- Dogaris, I.; Karapati, S.; Mamma, D.; Kalogeris, E.; Kekos, D. Hydrothermal processing and enzymatic hydrolysis of sorghum bagasse for fermentable carbohydrates production. Bioresour. Technol. 2009, 100, 6543–6549. [Google Scholar] [CrossRef]
- Rajan, K.; Carrier, D.J. Insights into exo-cellulase inhibition by the hot water hydrolyzates of rice straw. ACS Sustain. Chem. Eng. 2016, 4, 3627–3633. [Google Scholar] [CrossRef]
- Qi, W.; He, C.; Wang, Q.; Liu, S.; Yu, Q.; Wang, W.; Leksawasdi, N.; Wang, C.; Yuan, Z. Carbon-based solid acid pretreatment in corncob saccharification: Specific xylose production and efficient enzymatic hydrolysis. ACS Sustain. Chem. Eng. 2018, 6, 3640–3648. [Google Scholar] [CrossRef]
- Larnaudie, V.; Ferrari, M.D.; Lareo, C. Enzymatic hydrolysis of liquid hot water-pretreated switchgrass at high solid content. Energy Fuels 2019, 33, 4361–4368. [Google Scholar] [CrossRef]
- Xiao, X.; Bian, J.; Li, M.-F.; Xu, H.; Xiao, B.; Sun, R.-C. Enhanced enzymatic hydrolysis of bamboo (Dendrocalamus giganteus Munro) culm by hydrothermal pretreatment. Bioresour. Technol. 2014, 159, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Nitsos, C.K.; Choli-Papadopoulou, T.; Matis, K.A.; Triantafyllidis, K.S. Optimization of hydrothermal pretreatment of hardwood and softwood lignocellulosic residues for selective hemicellulose recovery and improved cellulose enzymatic hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 4529–4544. [Google Scholar] [CrossRef]
- Yu, Q.; Zhuang, X.; Yuan, Z.; Wang, Q.; Qi, W.; Wang, W.; Zhang, Y.; Xu, J.; Xu, H. Two-step liquid hot water pretreatment of Eucalyptus grandis to enhance sugar recovery and enzymatic digestibility of cellulose. Bioresour. Technol. 2010, 101, 4895–4899. [Google Scholar] [CrossRef]
- Matsushita, Y.; Yamauchi, K.; Takabe, K.; Awano, T.; Yoshinaga, A.; Kato, M.; Kobayashi, T.; Asada, T.; Furujyo, A.; Fukushima, K. Enzymatic saccharification of Eucalyptus bark using hydrothermal pretreatment with carbon dioxide. Bioresour. Technol. 2010, 101, 4936–4939. [Google Scholar] [CrossRef]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.; Mitchinson, C.; Saddler, J.N. Comparative sugar recovery and fermentation data following pretreatment of poplar wood by leading technologies. Biotechnol. Prog. 2009, 25, 333–339. [Google Scholar] [CrossRef]
- Farzad, S.; Mandegari, M.A.; Guo, M.; Haigh, K.F.; Shah, N.; Görgens, J.F. Multi-product biorefineries from lignocelluloses: A pathway to revitalisation of the sugar industry? Biotechnol. Biofuels 2017, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Calderon, O.; Arantes, V. A review on commercial-scale high-value products that can be produced alongside cellulosic ethanol. Biotechnol. Biofuels 2019, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Wyman, C.E.; Balan, V.; Dale, B.E.; Elander, R.T.; Falls, M.; Hames, B.; Holtzapple, M.T.; Ladisch, M.R.; Lee, Y.; Mosier, N. Comparative data on effects of leading pretreatments and enzyme loadings and formulations on sugar yields from different switchgrass sources. Bioresour. Technol. 2011, 102, 11052–11062. [Google Scholar] [CrossRef]
- Wyman, C.E.; Dale, B.E.; Balan, V.; Elander, R.T.; Holtzapple, M.T.; Ramirez, R.S.; Ladisch, M.R.; Mosier, N.S.; Lee, Y.; Gupta, R. Comparative performance of leading pretreatment technologies for biological conversion of corn stover, poplar wood, and switchgrass to sugars. In Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals; Wiley: Hoboken, NJ, USA, 2013; pp. 239–259. [Google Scholar]
- Merino, S.T.; Cherry, J. Progress and challenges in enzyme development for biomass utilization. In Biofuels; Springer: Berlin/Heidelberg, Germany, 2007; pp. 95–120. [Google Scholar]
- Sun, Y.; Cheng, J. Enzymatic Hydrolysis of Rye Straw and Bermudagrass Using Cellulases Supplemented with Â-Glucosidase. Trans. ASAE 2004, 47, 343–349. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, M.; Hong, D.; Fang, Z.; Xiao, Y.; Fang, W.; Zhang, X. Improving the cellobiose hydrolysis activity of glucose-stimulating β-glucosidase Bgl2A. Enzym. Microb. Technol. 2023, 169, 110289. [Google Scholar] [CrossRef]
- Bezerra, R.M.; Dias, A.A. Enzymatic kinetic of cellulose hydrolysis: Inhibition by ethanol and cellobiose. Appl. Biochem. Biotechnol. 2005, 126, 49–59. [Google Scholar] [CrossRef]
- Padella, M.; O’Connell, A.; Prussi, M. What is still limiting the deployment of cellulosic ethanol? Analysis of the current status of the sector. Appl. Sci. 2019, 9, 4523. [Google Scholar]
- Lynd, L.R.; Liang, X.; Biddy, M.J.; Allee, A.; Cai, H.; Foust, T.; Himmel, M.E.; Laser, M.S.; Wang, M.; Wyman, C.E. Cellulosic ethanol: Status and innovation. Curr. Opin. Biotechnol. 2017, 45, 202–211. [Google Scholar] [CrossRef]
- Balan, V.; Chiaramonti, D.; Kumar, S. Review of US and EU initiatives toward development, demonstration, and commercialization of lignocellulosic biofuels. Biofuels Bioprod. Biorefin. 2013, 7, 732–759. [Google Scholar] [CrossRef]
- Koppram, R.; Tomás-Pejó, E.; Xiros, C.; Olsson, L. Lignocellulosic ethanol production at high-gravity: Challenges and perspectives. Trends Biotechnol. 2014, 32, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Humbird, D.; Mohagheghi, A.; Dowe, N.; Schell, D.J. Economic impact of total solids loading on enzymatic hydrolysis of dilute acid pre-treated corn stover. Biotechnol. Prog. 2010, 26, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-H.; Wang, Z.; Dien, B.S.; Slininger, P.J.; Singh, V. Economic analysis of cellulosic ethanol production from sugarcane bagasse using a sequential deacetylation, hot water and disk-refining pretreatment. Processes 2019, 7, 642. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S.E. Enzymatic hydrolysis of biomass at high-solids loadings—A review. Biomass Bioenergy 2013, 56, 526–544. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Chen, H.-Z. Biomass–water interaction and its correlations with enzymatic hydrolysis of steam-exploded corn stover. ACS Sustain. Chem. Eng. 2016, 4, 1274–1285. [Google Scholar] [CrossRef]
- Wingren, A.; Galbe, M.; Zacchi, G. Energy considerations for a SSF-based softwood ethanol plant. Bioresour. Technol. 2008, 99, 2121–2131. [Google Scholar] [CrossRef]
- Unrean, P. Techno-economic assessment of bioethanol production from major lignocellulosic residues under different process configurations. In Sustainable Biotechnology-Enzymatic Resources of Renewable Energy; Springer: Berlin/Heidelberg, Germany, 2018; pp. 177–204. [Google Scholar]
- Zacchi, G.; Axelsson, A. Economic evaluation of preconcentration in production of ethanol from dilute sugar solutions. Biotechnol. Bioeng. 1989, 34, 223–233. [Google Scholar] [CrossRef]
- Varga, E.; Klinke, H.B.; Réczey, K.; Thomsen, A.B. High solid simultaneous saccharification and fermentation of wet oxidized corn stover to ethanol. Biotechnol. Bioeng. 2004, 88, 567–574. [Google Scholar] [CrossRef]
- Jørgensen, H.; Vibe-Pedersen, J.; Larsen, J.; Felby, C. Liquefaction of lignocellulose at high-solids concentrations. Biotechnol. Bioeng. 2007, 96, 862–870. [Google Scholar] [CrossRef]
- Kristensen, J.B.; Felby, C.; Jørgensen, H. Yield-determining factors in high-solids enzymatic hydrolysis of lignocellulose. Biotechnol. Biofuels 2009, 2, 11. [Google Scholar] [CrossRef]
- Liu, W.-J.; Li, W.-W.; Jiang, H.; Yu, H.-Q. Fates of chemical elements in biomass during its pyrolysis. Chem. Rev. 2017, 117, 6367–6398. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.P.; Ischay, M.A.; Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2010, 2, 527–532. [Google Scholar] [CrossRef]
- Schultz, D.M.; Yoon, T.P. Solar synthesis: Prospects in visible light photocatalysis. Science 2014, 343, 1239176. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Luo, N.; Xie, S.; Zhang, H.; Zhang, Q.; Wang, F.; Wang, Y. Photocatalytic transformations of lignocellulosic biomass into chemicals. Chem. Soc. Rev. 2020, 49, 6198–6223. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Zhang, L.; Xue, S.; Doherty, W.O.; O’Hara, I.M.; Ke, X. Sustainable conversion of cellulosic biomass to chemicals under visible-light irradiation. RSC Adv. 2015, 5, 85242–85247. [Google Scholar] [CrossRef]
- Zhang, X.; Ke, X.; Du, A.; Zhu, H. Plasmonic nanostructures to enhance catalytic performance of zeolites under visible light. Sci. Rep. 2014, 4, 3805. [Google Scholar] [CrossRef]
- Zhang, X.; Du, A.; Zhu, H.; Jia, J.; Wang, J.; Ke, X. Surface plasmon-enhanced zeolite catalysis under light irradiation and its correlation with molecular polarity of reactants. Chem. Commun. 2014, 50, 13893–13895. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Guo, L.; Chen, Z.; Li, C. Photothermally promoted cleavage of β-1, 4-glycosidic bonds of cellulosic biomass on Ir/HY catalyst under mild conditions. Appl. Catal. B Environ. 2018, 237, 660–664. [Google Scholar] [CrossRef]
- Wakerley, D.W.; Kuehnel, M.F.; Orchard, K.L.; Ly, K.H.; Rosser, T.E.; Reisner, E. Solar-driven reforming of lignocellulose to H2 with a CdS/CdOx photocatalyst. Nat. Energy 2017, 2, 17021. [Google Scholar] [CrossRef]
- Zhang, G.; Ni, C.; Huang, X.; Welgamage, A.; Lawton, L.A.; Robertson, P.K.; Irvine, J.T. Simultaneous cellulose conversion and hydrogen production assisted by cellulose decomposition under UV-light photocatalysis. Chem. Commun. 2016, 52, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Zhang, G.; Xu, X. One-pot photoreforming of cellulosic biomass waste to hydrogen by merging photocatalysis with acid hydrolysis. Appl. Catal. A Gen. 2018, 563, 73–79. [Google Scholar] [CrossRef]
- Fan, H.; Li, G.; Yang, F.; Yang, L.; Zhang, S. Photodegradation of cellulose under UV light catalysed by TiO2. J. Chem. Technol. Biotechnol. 2011, 86, 1107–1112. [Google Scholar] [CrossRef]
- Li, S.; Deng, W.; Wang, S.; Wang, P.; An, D.; Li, Y.; Zhang, Q.; Wang, Y. Catalytic transformation of cellulose and its derivatives into functionalized organic acids. ChemSusChem 2018, 11, 1995–2028. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Lu, C.; Wang, J.; Chen, Y.; Xu, Z.; Varma, R.S. Selectivity enhancement in heterogeneous photocatalytic transformations. Chem. Rev. 2017, 117, 1445–1514. [Google Scholar] [CrossRef] [PubMed]
- Colmenares, J.C.; Magdziarz, A.; Bielejewska, A. High-value chemicals obtained from selective photo-oxidation of glucose in the presence of nanostructured titanium photocatalysts. Bioresour. Technol. 2011, 102, 11254–11257. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Magdziarz, A. Room temperature versatile conversion of biomass-derived compounds by means of supported TiO2 photocatalysts. J. Mol. Catal. A Chem. 2013, 366, 156–162. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Magdziarz, A.; Kurzydlowski, K.; Grzonka, J.; Chernyayeva, O.; Lisovytskiy, D. Low-temperature ultrasound-promoted synthesis of Cr–TiO2-supported photocatalysts for valorization of glucose and phenol degradation from liquid phase. Appl. Catal. B Environ. 2013, 134, 136–144. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Magdziarz, A.; Chernyayeva, O.; Lisovytskiy, D.; Kurzydłowski, K.; Grzonka, J. Sonication-Assisted Low-Temperature Routes for the Synthesis of Supported Fe–TiO2 Econanomaterials: Partial Photooxidation of Glucose and Phenol Aqueous Degradation. ChemCatChem 2013, 5, 2270–2277. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Luque, R. Heterogeneous photocatalytic nanomaterials: Prospects and challenges in selective transformations of biomass-derived compounds. Chem. Soc. Rev. 2014, 43, 765–778. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, Q.; Yang, C.; Zhang, B.; Deng, K. Photocatalytic oxidation of glucose in water to value-added chemicals by zinc oxide-supported cobalt thioporphyrazine. Catal. Sci. Technol. 2019, 9, 6909–6919. [Google Scholar] [CrossRef]
- Zhang, Q.; Ge, Y.; Yang, C.; Zhang, B.; Deng, K. Enhanced photocatalytic performance for oxidation of glucose to value-added organic acids in water using iron thioporphyrazine modified SnO2. Green Chem. 2019, 21, 5019–5029. [Google Scholar] [CrossRef]
- Chen, R.; Yang, C.; Zhang, Q.; Zhang, B.; Deng, K. Visible-light-driven selective oxidation of glucose in water with H-ZSM-5 zeolite supported biomimetic photocatalyst. J. Catal. 2019, 374, 297–305. [Google Scholar] [CrossRef]
- Zhou, B.; Song, J.; Zhang, Z.; Jiang, Z.; Zhang, P.; Han, B. Highly selective photocatalytic oxidation of biomass-derived chemicals to carboxyl compounds over Au/TiO. Green Chem. 2017, 19, 1075–1081. [Google Scholar] [CrossRef]
- Omri, M.; Sauvage, F.D.R.; Busby, Y.; Becuwe, M.; Pourceau, G.; Wadouachi, A. Gold catalysis and photoactivation: A fast and selective procedure for the oxidation of free sugars. ACS Catal. 2018, 8, 1635–1639. [Google Scholar] [CrossRef]
- Phan, T.-D.N.; Pham, H.-D.; Cuong, T.V.; Kim, E.J.; Kim, S.; Shin, E.W. A simple hydrothermal preparation of TiO2 nanomaterials using concentrated hydrochloric acid. J. Cryst. Growth 2009, 312, 79–85. [Google Scholar] [CrossRef]
- Tertzakian, P. A Thousand Barrels a Second: The Coming Oil Break Point and the Challenges Facing an Energy Dependent World; Mcgraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Murray, J.; King, D. Oil’s tipping point has passed. Nature 2012, 481, 433–435. [Google Scholar] [CrossRef]
- Stocker, T. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Li, X.; Raorane, C.J.; Xia, C.; Wu, Y.; Tran, T.K.N.; Khademi, T. Latest approaches on green hydrogen as a potential source of renewable energy towards sustainable energy: Spotlighting of recent innovations, challenges, and future insights. Fuel 2023, 334, 126684. [Google Scholar] [CrossRef]
- Smith, C.; Bucke, C.; van der Horst, D. Green hydrogen powering sustainable festivals: Public perceptions of generators, production and ownership. Int. J. Hydrogen Energy 2023, 48, 8370–8385. [Google Scholar] [CrossRef]
- Yusaf, T.; Mahamude, A.S.F.; Kadirgama, K.; Ramasamy, D.; Farhana, K.; Dhahad, H.A.; Talib, A.R.A. Sustainable hydrogen energy in aviation—A narrative review. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Tiseira, A.; Novella, R.; Garcia-Cuevas, L.; Lopez-Juarez, M. Concept design and energy balance optimization of a hydrogen fuel cell helicopter for unmanned aerial vehicle and aerotaxi applications. Energy Convers. Manag. 2023, 288, 117101. [Google Scholar] [CrossRef]
- Hai, T.; Ali, M.A.; Zeki, F.M.; Chauhan, B.S.; Metwally, A.S.M.; Ullah, M. Optimal design of inter-state hydrogen fuel cell vehicle fueling station with on-site hydrogen production. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Novella, R.; Pastor, J.; Gomez-Soriano, J.; Sánchez-Bayona, J. Numerical study on the use of ammonia/hydrogen fuel blends for automotive spark-ignition engines. Fuel 2023, 351, 128945. [Google Scholar] [CrossRef]
- Wu, C.; Xue, S.; Qin, Z.; Nazari, M.; Yang, G.; Yue, S.; Tong, T.; Ghasemi, H.; Hernandez, F.C.R.; Xue, S. Making g-C3N4 ultra-thin nanosheets active for photocatalytic overall water splitting. Appl. Catal. B Environ. 2021, 282, 119557. [Google Scholar] [CrossRef]
- Zhen, W.; Ning, X.; Yang, B.; Wu, Y.; Li, Z.; Lu, G. The enhancement of CdS photocatalytic activity for water splitting via anti-photocorrosion by coating Ni2P shell and removing nascent formed oxygen with artificial gill. Appl. Catal. B Environ. 2018, 221, 243–257. [Google Scholar] [CrossRef]
- Semelsberger, T.A.; Brooks, K.P. Chemical hydrogen storage material property guidelines for automotive applications. J. Power Sources 2015, 279, 593–609. [Google Scholar] [CrossRef]
- Squadrito, G.; Maggio, G.; Nicita, A. The green hydrogen revolution. Renew. Energy 2023, 216, 119041. [Google Scholar] [CrossRef]
- Foorginezhad, S.; Mohseni-Dargah, M.; Falahati, Z.; Abbassi, R.; Razmjou, A.; Asadnia, M. Sensing advancement towards safety assessment of hydrogen fuel cell vehicles. J. Power Sources 2021, 489, 229450. [Google Scholar] [CrossRef]
- Iulianelli, A.; Liguori, S.; Wilcox, J.; Basile, A. Advances on methane steam reforming to produce hydrogen through membrane reactors technology: A review. Catal. Rev. 2016, 58, 1–35. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Zinoviev, S.; Müller-Langer, F.; Das, P.; Bertero, N.; Fornasiero, P.; Kaltschmitt, M.; Centi, G.; Miertus, S. Next-generation biofuels: Survey of emerging technologies and sustainability issues. ChemSusChem 2010, 3, 1106–1133. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Choi, S.; Lee, I.; Nguyen, T.V.T.; Bae, S.; Kim, Y.H.; Ryu, J.; Park, S.; Ryu, J. Solar Biomass Reforming and Hydrogen Production with Earth-Abundant Si-Based Photocatalysts. Adv. Mater. 2023, 35, 2301576. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Kang, F.; Zhu, Y.; Teng, M.; Shi, J.; Qi, H.; Huang, Z.; Si, C.; Jiang, F.; Hu, J. Photoreforming lignocellulosic biomass for hydrogen production: Optimized design of photocatalyst and photocatalytic system. Chem. Eng. J. 2023, 452, 138980. [Google Scholar] [CrossRef]
- Xu, X.; Shi, L.; Zhang, S.; Ao, Z.; Zhang, J.; Wang, S.; Sun, H. Photocatalytic reforming of lignocellulose: A review. Chem. Eng. J. 2023, 469, 143972. [Google Scholar] [CrossRef]
- Singh, T.I.; Li, S.; Leem, G.; Lee, S. Photocatalytic Hydrogen Production by Biomass Reforming. In Photocatalytic Hydrogen Production for Sustainable Energy; Wiley: Hoboken, NJ, USA, 2023; pp. 191–217. [Google Scholar]
- Puga, A.V. Photocatalytic production of hydrogen from biomass-derived feedstocks. Coord. Chem. Rev. 2016, 315, 1–66. [Google Scholar] [CrossRef]
- Kuehnel, M.F.; Reisner, E. Solar hydrogen generation from lignocellulose. Angew. Chem. Int. Ed. 2018, 57, 3290–3296. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, W.; Qiao, F.; Sun, J.; Fan, Y.; Li, Z. Hydrothermal synthesis of MoS2-NiS/CdS with enhanced photocatalytic hydrogen production activity and stability. J. Solid State Chem. 2019, 270, 531–538. [Google Scholar] [CrossRef]
- Imizcoz, M.; Puga, A.V. Assessment of photocatalytic hydrogen production from biomass or wastewaters depending on the metal co-catalyst and its deposition method on TiO2. Catalysts 2019, 9, 584. [Google Scholar] [CrossRef]
- Xia, Q.; Chen, Z.; Shao, Y.; Gong, X.; Wang, H.; Liu, X.; Parker, S.F.; Han, X.; Yang, S.; Wang, Y. Direct hydrodeoxygenation of raw woody biomass into liquid alkanes. Nat. Commun. 2016, 7, 11162. [Google Scholar] [CrossRef]
- Li, C.; Zheng, M.; Wang, A.; Zhang, T. One-pot catalytic hydrocracking of raw woody biomass into chemicals over supported carbide catalysts: Simultaneous conversion of cellulose, hemicellulose and lignin. Energy Environ. Sci. 2012, 5, 6383–6390. [Google Scholar] [CrossRef]
- Speltini, A.; Sturini, M.; Dondi, D.; Annovazzi, E.; Maraschi, F.; Caratto, V.; Profumo, A.; Buttafava, A. Sunlight-promoted photocatalytic hydrogen gas evolution from water-suspended cellulose: A systematic study. Photochem. Photobiol. Sci. 2014, 13, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Sakata, T. Conversion of carbohydrate into hydrogen fuel by a photocatalytic process. Nature 1980, 286, 474–476. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y.; Yang, Y.; Song, Y.; Deng, P.; Li, J.; Liu, W.; Shen, Y.; Tian, X. Recent advances in cadmium sulfide-based photocatalysts for photocatalytic hydrogen evolution. Renewables 2023, 1, 39–56. [Google Scholar] [CrossRef]
- Cao, S.; Chen, Y.; Wang, C.-J.; Lv, X.-J.; Fu, W.-F. Spectacular photocatalytic hydrogen evolution using metal-phosphide/CdS hybrid catalysts under sunlight irradiation. Chem. Commun. 2015, 51, 8708–8711. [Google Scholar] [CrossRef] [PubMed]
- Lakhera, S.K.; Rajan, A.; Rugma, T.; Bernaurdshaw, N. A review on particulate photocatalytic hydrogen production system: Progress made in achieving high energy conversion efficiency and key challenges ahead. Renew. Sustain. Energy Rev. 2021, 152, 111694. [Google Scholar] [CrossRef]
- Toe, C.Y.; Tsounis, C.; Zhang, J.; Masood, H.; Gunawan, D.; Scott, J.; Amal, R. Advancing photoreforming of organics: Highlights on photocatalyst and system designs for selective oxidation reactions. Energy Environ. Sci. 2021, 14, 1140–1175. [Google Scholar] [CrossRef]
- Estahbanati, M.K.; Feilizadeh, M.; Attar, F.; Iliuta, M.C. Current developments and future trends in photocatalytic glycerol valorization: Process analysis. React. Chem. Eng. 2021, 6, 197–219. [Google Scholar] [CrossRef]
- Nwosu, U.; Wang, A.; Palma, B.; Zhao, H.; Khan, M.A.; Kibria, M.; Hu, J. Selective biomass photoreforming for valuable chemicals and fuels: A critical review. Renew. Sustain. Energy Rev. 2021, 148, 111266. [Google Scholar] [CrossRef]
- Qi, M.-Y.; Conte, M.; Anpo, M.; Tang, Z.-R.; Xu, Y.-J. Cooperative coupling of oxidative organic synthesis and hydrogen production over semiconductor-based photocatalysts. Chem. Rev. 2021, 121, 13051–13085. [Google Scholar] [CrossRef]
- Wu, X.; Fan, X.; Xie, S.; Lin, J.; Cheng, J.; Zhang, Q.; Chen, L.; Wang, Y. Solar energy-driven lignin-first approach to full utilization of lignocellulosic biomass under mild conditions. Nat. Catal. 2018, 1, 772–780. [Google Scholar] [CrossRef]



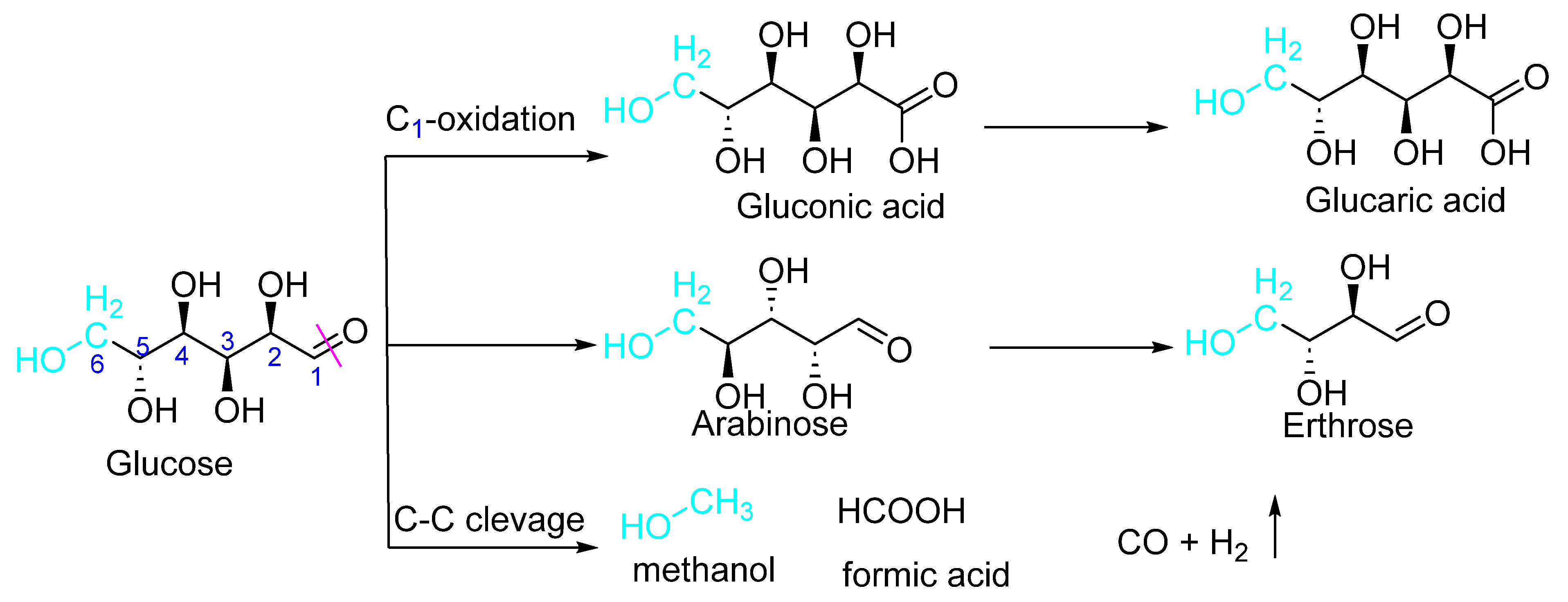
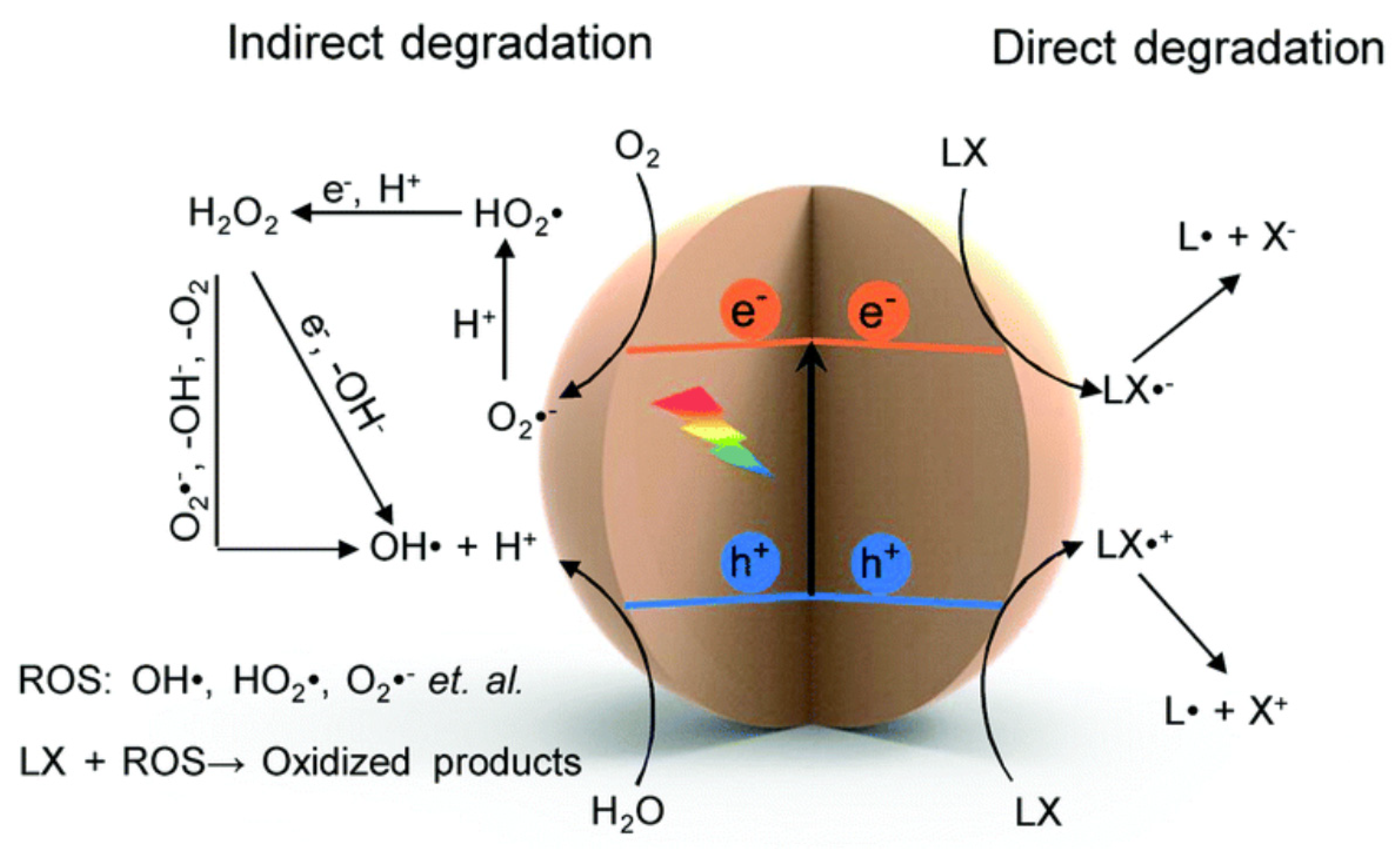
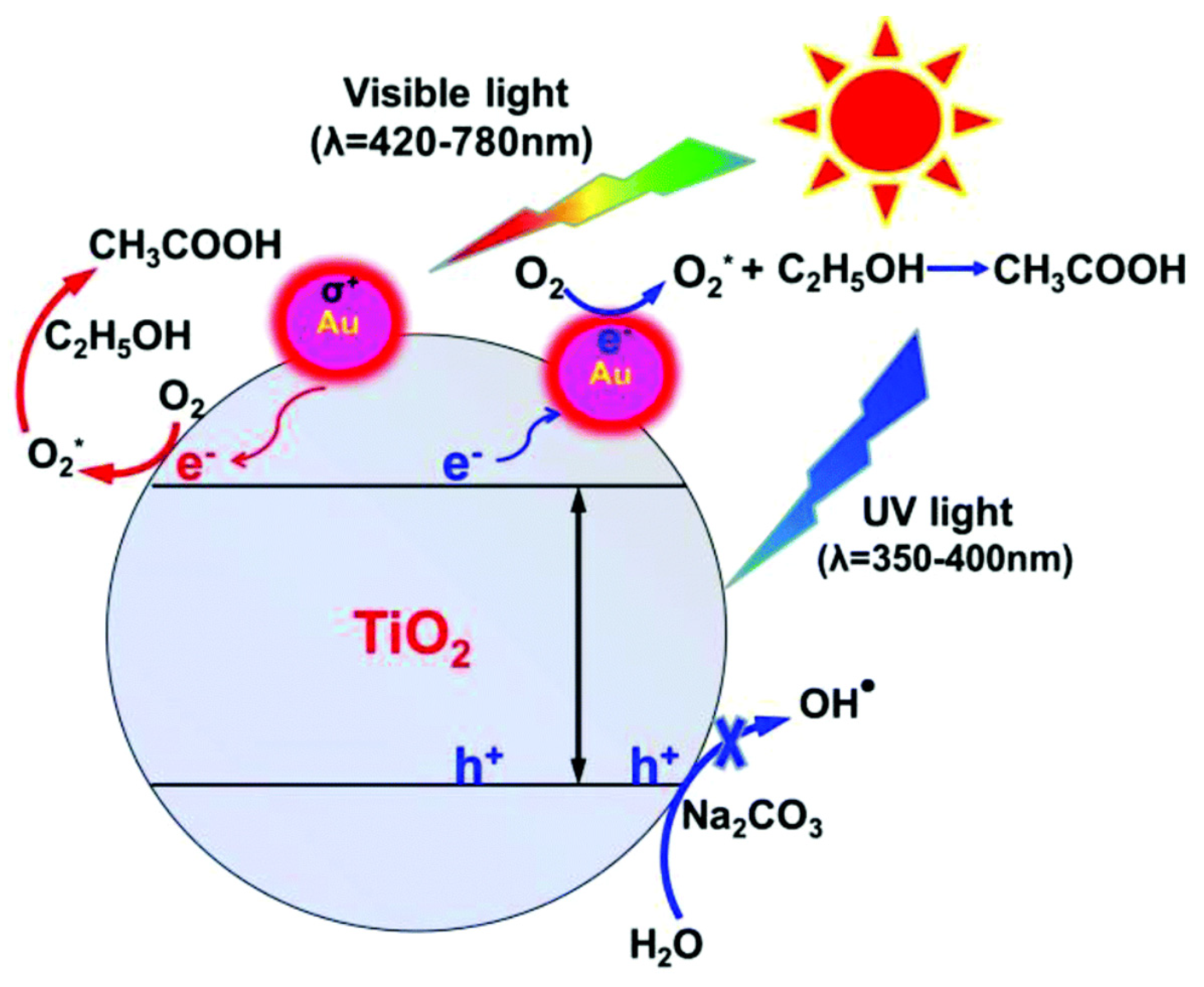
| Lignocellulosic Materials | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|
| Hardwoods stem | 40–55 | 24–40 | 18–25 |
| Softwoods stem | 45–50 | 25–35 | 25–35 |
| Nutshells | 25–30 | 25–30 | 30–40 |
| Corn cobs | 45 | 35 | 15 |
| Grasses | 25–40 | 35–50 | 10–30 |
| Paper | 85–99 | 0 | 0–15 |
| Oil palm empty fruit bunches a | NA | 24–35 | 18–35 |
| Wheat straw b | 33–38 | 26–32 | 17–18 |
| Sorted refuse | 60 | 20 | 20 |
| Leaves | 15–20 | 80–85 | 0 |
| Cotton seed fibers | 80–95 | 5–20 | 0 |
| Newspaper waste | 40–55 | 25–40 | 18–30 |
| Waste papers from chemical pulps | 60–70 | 10–20 | 5–10 |
| Primary wastewater solids | 8–15 | NA | 24–29 |
| Swine waste | 6.0 | 28 | NA |
| Solid cattle manure | 1.6–4.7 | 1.4–3.3 | 2.7–5.7 |
| Coastal Bermuda grass | 25 | 35.7 | 6.4 |
| Switchgrass | 45 | 31.4 | 12.0 |
| Biomass Source | Hydrothermal (HT) Pretreatments | Biocatalytic Hydrolysis Phase | Outcome | Ref. |
|---|---|---|---|---|
| Tamarix ramosissima bark (Salt cedar) | Hot compressed water (HCW) pretreatment: Deionized H2O (10% w/v of dry solid mass) at 100–200 °C in a batch-type reactor | Cellulase loading 35 FPU/g | Enzymatic saccharification of HCW pre-treated cellulose-rich biomass yield 88% hydrolysis, 3–4 times higher than untreated method | [1,9] |
| Starch | Hot compressed water (HCW) pretreat-mint in the presence of CO2 a at 180–235 °C for 15 min | Fermentation: Escherichia coli HD701 cultured (inoculate 10 μL of sample equiv. to 0.001% inoculum). cell concentration measured by optical density using a conversion factor; OD6001 = 0.482 g dry weight. L−1. 0.5–1 mL of cell suspension added to make up the 1 g L−1 dry weight concentration | Addition of CO2 into HCW method increases sugars yields (~1.5-fold compared to a N2 control at 250 °C for 15 min) from cellulose-enriched biomass and decreases yields of organic acids (also act as fermentation inhibitors, such as 5-hydroxymethylfurfural and furfural) in lignocellulose hydrolysates. Activated carbon-treated hydrolysates exhibited higher hydrogen production than untreated, exhibiting the efficiency of activated carbon treatments in removing organic acids (which act as inhibitors) for Escherichia coli HD701. | [10] |
| Sorghum bagasse | Microwave digestion equipment with IR sensor: microwave power (700 W), liquor to solid ratio (6.7 g g−1) at 160–210 °C (3–30 min) | Enzymes derived from wild-type strain F3 of Fusarium oxysporum and Neurospora crassa DSM 1129 | Cellulases from Fusarium oxysporum demonstrated an extended depolymerization of cellulose (Yield of glucose ~60%) higher than cellulases from Neurospora crassa (could be due to relatively higher cellobiohydrolase activity of enzymes in Fusarium oxysporum) | [11,12] |
| Rice straw | H2O, 220 °C, 52 min and pH 7.0, in a 1 L Parr 4525 reactor, at 10% solid loading (wet weight) | Cellulase activity quantified by determining the required enzyme to release one µM of 4-methylumbelliferone/min. The enzyme loading (41.7 U/mL) was used. | Fractionation using centrifugal partition chromatography of hot water hydrolysates (from rice straw) exhibits a more efficient separation method than inhibiting different chemical classes using exo-cellulase (derived from the prehydrolysate). Phenolics derived from rice straw subjected to potent inhibition to cellulase enzymes followed by furans and acetic acid. | [13] |
| Corncob | Carbon-based solid (C-SO3H) acid catalyst/H2O at 120–160 °C for 4–6 h | Cellulase loadings of 20 and 40 FPU/g for 1–96 h | Pretreatment resulted in direct xylose released (78.1%) from corncob. Enzymatic hydrolysis of prehydrolysate yielded up to 91.6% in 48 h. | [14] |
| Switchgrass (Energy crop, Panicum virgatum) | Liquid hot water: 15% w/w solid content at 200 °C for 5 min | Cellulase in a ratio of 50 mgprotein/gglucan (20 FPU/gglucan), at the pH (4, 5, 6, and 7 for solid contents, 4.8 for 15%, and 5.5 for 20%). | Achieving high glucose yields and hydrolysis efficiencies recorded for high solid content (with high enzyme dosage) | [15] |
| Bamboo(Dendrocalamus giganteus Munro) | Non-isothermally pre-treated with hot H2O at 140–200 °C for different times (10–120 min) | Cellulase loading at 14.5 FPU/g | Maximum glucose yield was achieved (75.7%). | [16] |
| Residual poplar branches (Hardwood, 20 years old Populus deltoides trees) | Deionized H2O (liquid/solid ratio = 15) heating rate (~7 °C/min) to temperatures (170–220 °C), with reaction times (15–180 min) | Cellulase (60 FPU/g) | Prolong HT pretreatment at 170 °C (90 min) to 220 °C (15 min) showed improvement in glucose recovery (31.2 wt% versus 43.6 wt%) | [17] |
| Grapevine pruning (Hardwood, Vitis vinifera) | Deionized H2O (liquid/solid ratio = 15) heating rate (~7 °C/min) to temperatures (170–220 °C), with reaction times (15–180 min) | Cellulase (60 FPU/g) | Prolong HT pretreatment at 170 °C (90 min) to 220 °C (15 min) showed modest improvement in glucose recovery (54.0 wt% versus 63.0 wt%) | [17] |
| Pine tree sawdust (Softwood, Pinus sylvestris)) | Deionized H2O (liquid/solid ratio = 15) heating rate (~7 °C/min) to temperatures (170–220 °C), with reaction times (15–180 min) | Cellulase (60 FPU/g) | Prolong HT pretreatment at 170 °C (90 min) to 220 °C (15 min) showed modest improvement in glucose recovery (21.6 wt% versus 22.6 wt%) | [17] |
| Eucalyptus grandis | Two-step liquid hot water pretreatment: (a) 5% w/v cellulosic enriched biomass in H2O at 180–200 °C for 10–60 min (b) Addition of H2O (5% w/v of dry solids) to the collected hydrolysate, heated (180–240 °C) | Cellulase -loading amount 40 FPU/g dry solid | Pretreatment of cellulose is highly dependent on reaction temperature rather than time. “72 h digestibility of samples treated at 180 °C for 20 min was 72.8% (increased to 97.2% after the IInd pretreatment at 240 °C). The slightest improvement in enzymatic digestibility from 81.2% to 86.6% was recorded when IInd pretreatment reaction time was prolonged from 20 to 60 min at 200 °C.” | [18] |
| Eucalyptus bark (Cryptomeria japonica) | CO2 a/H2O at 175–200 °C for 4 h | Enzyme Mixture: Cellulases from Trichoderma reesei, pectinases from Aspergillus niger, and α-amylase from Aspergillus oryzae. Dosage of each enzyme = 1.25 U/mg | Glucose yield achieved about 80% (as an α-cellulose) during HT treatment. | [19] |
| Poplar wood | SO2 steam explosion | Cellulase Loading of 15 FPU/g Glucan | Glucose yields (74.3%) achieved. Total sugar yield (Glucose + Xylose = 95.9%) | [20] |
| Pretreatments | Xylose Yields | Glucose Yields | Total Sugars | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ↓/Stages | I | II | Total | I | II | Total | I | II | Total |
| Untreated | NA | 1.9 | 1.9 | NA | 8.4 | 8.4 | NA | 10.3 | 10.3 |
| Maximum | 39.4 | 60.6 | 100 | ||||||
| Dil. H2SO4 | 29.3 | 3.4 | 32.6 | 4.3 | 42.2 | 46.5 | 33.6 | 45.6 | 79.2 |
| Sulfur dioxide steam explosion | 28.7 | 3.2 | 31.9 | 3.0 | 48.3 | 51.4 | 31.7 | 51.5 | 83.2 |
| Liquid hot water | 25.9 | 5.3 | 31.3 | 4.1 | 47.3 | 51.4 | 30 | 52.6 | 82.6 |
| Lime | 13.6 | 22.4 | 36 | 0.9 | 54 | 54.9 | 14.5 | 76.4 | 90.9 |
| Soaking in aqueous ammonia | 9.5 | 17.8 | 27.3 | 0.2 | 39.8 | 40 | 9.7 | 57.6 | 69.2 |
| Ammonia fiber expansion | 11.1 | 25.6 | 36.7 | 0.8 | 47.1 | 47.9 | 11.9 | 72.7 | 84.6 |
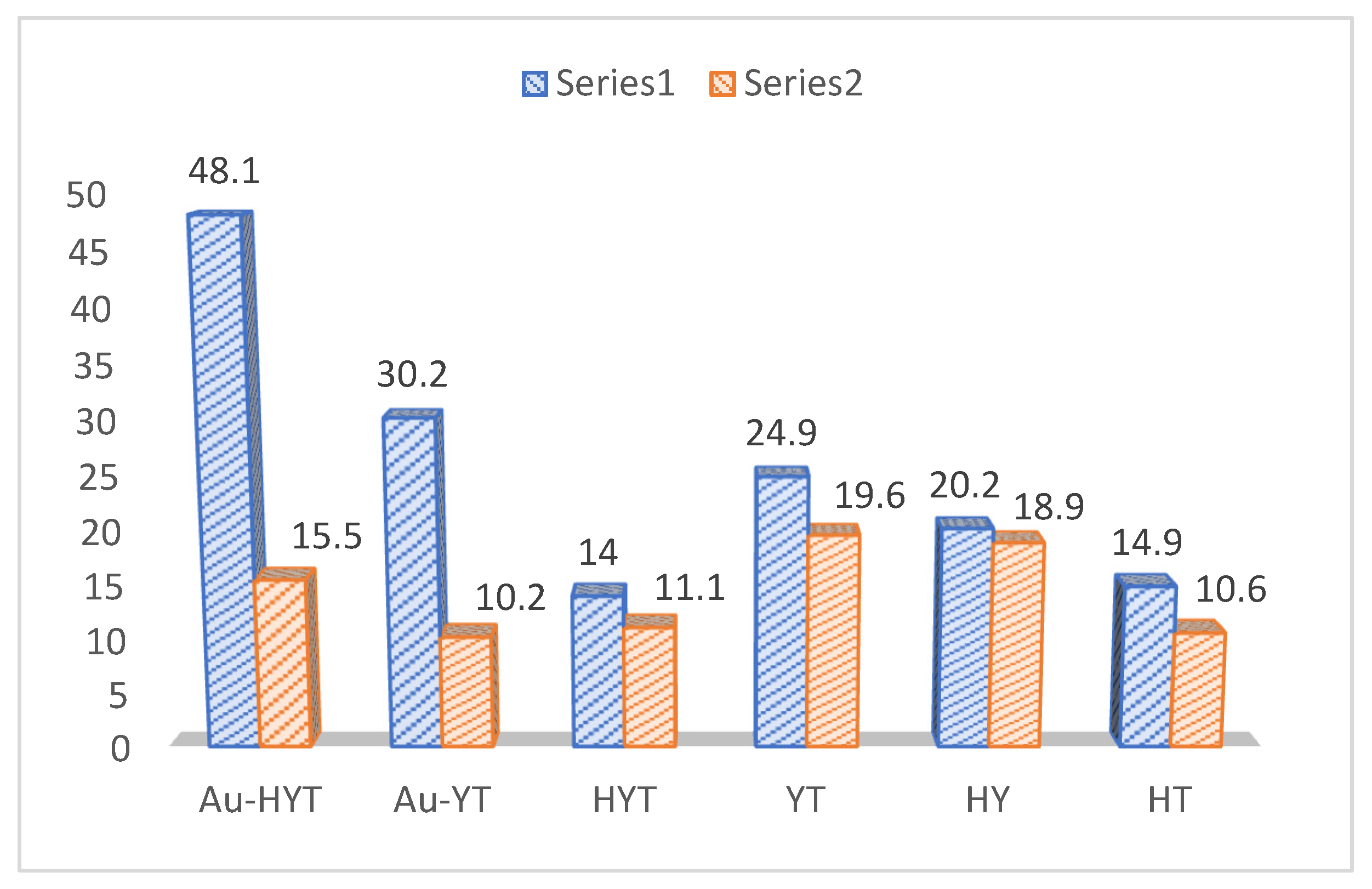
| Light-On | Light-Off | |||||
|---|---|---|---|---|---|---|
| Catalysts | Glucose | HMF | Total Conversion | Glucose | HMF | Total Conversion |
| Au-HYT | 48.1 | 10.6 | 58.7 | 15.5 | 0 | 15.5 |
| Au-YT | 30.2 | 2.6 | 32.8 | 10.2 | 0 | 10.2 |
| HYT | 14 | 10.1 | 24.1 | 11.1 | 7.5 | 18.6 |
| YT | 24.9 | 5.7 | 30.6 | 19.6 | 4.6 | 24.2 |
| HY | 20.2 | 19.1 | 39.3 | 18.9 | 17.1 | 36 |
| HT | 14.9 | 5.7 | 20.6 | 10.6 | 4.9 | 15.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negi, A.; Kesari, K.K. Light-Driven Depolymerization of Cellulosic Biomass into Hydrocarbons. Polymers 2023, 15, 3671. https://doi.org/10.3390/polym15183671
Negi A, Kesari KK. Light-Driven Depolymerization of Cellulosic Biomass into Hydrocarbons. Polymers. 2023; 15(18):3671. https://doi.org/10.3390/polym15183671
Chicago/Turabian StyleNegi, Arvind, and Kavindra Kumar Kesari. 2023. "Light-Driven Depolymerization of Cellulosic Biomass into Hydrocarbons" Polymers 15, no. 18: 3671. https://doi.org/10.3390/polym15183671
APA StyleNegi, A., & Kesari, K. K. (2023). Light-Driven Depolymerization of Cellulosic Biomass into Hydrocarbons. Polymers, 15(18), 3671. https://doi.org/10.3390/polym15183671










