Antioxidant Activity of Biogenic Cinnamic Acid Derivatives in Polypropylene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
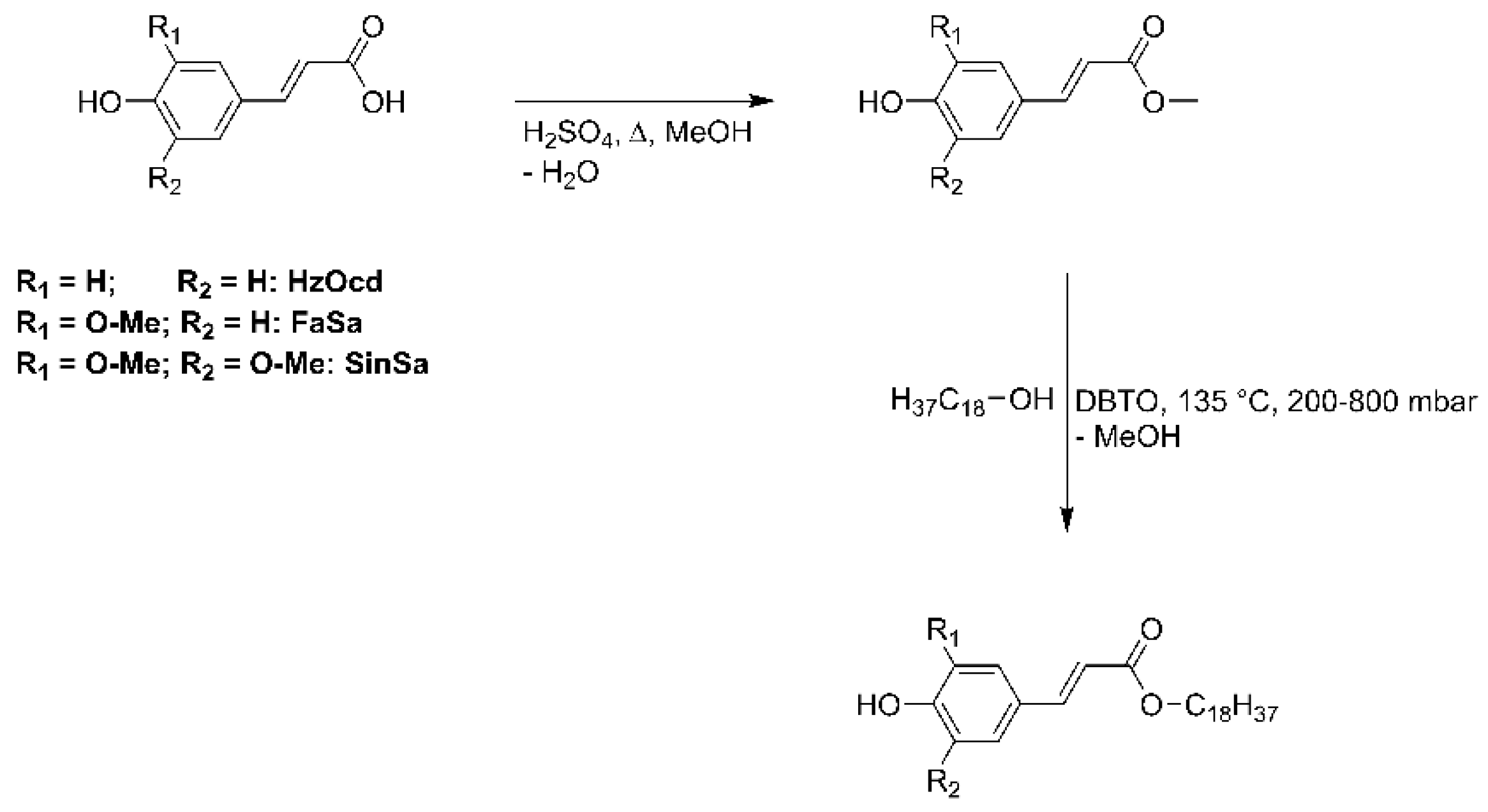
2.2. Synthesis Procedure
2.2.1. Synthesis of the Coumaric Acid Methyl Ester
2.2.2. Synthesis of the Ferulic Acid Methyl Ester
2.2.3. Synthesis of the Sinapic Acid Methyl Ester
2.2.4. Synthesis of the Cinnamic Acid Stearyl Esters
2.3. Methods and Characterization
2.3.1. Structural Characterization
2.3.2. DPPH Antioxidant Assay
2.3.3. Characterization of AOs
2.3.4. Microextruder Experiments: Determination of the Processing Stabilization Performance
2.3.5. Preparation of PP Compounds for OIT and Mechanical Properties
3. Results and Discussion
3.1. Characterization of the Hydroxycinnamic Acid Stearyl Esters
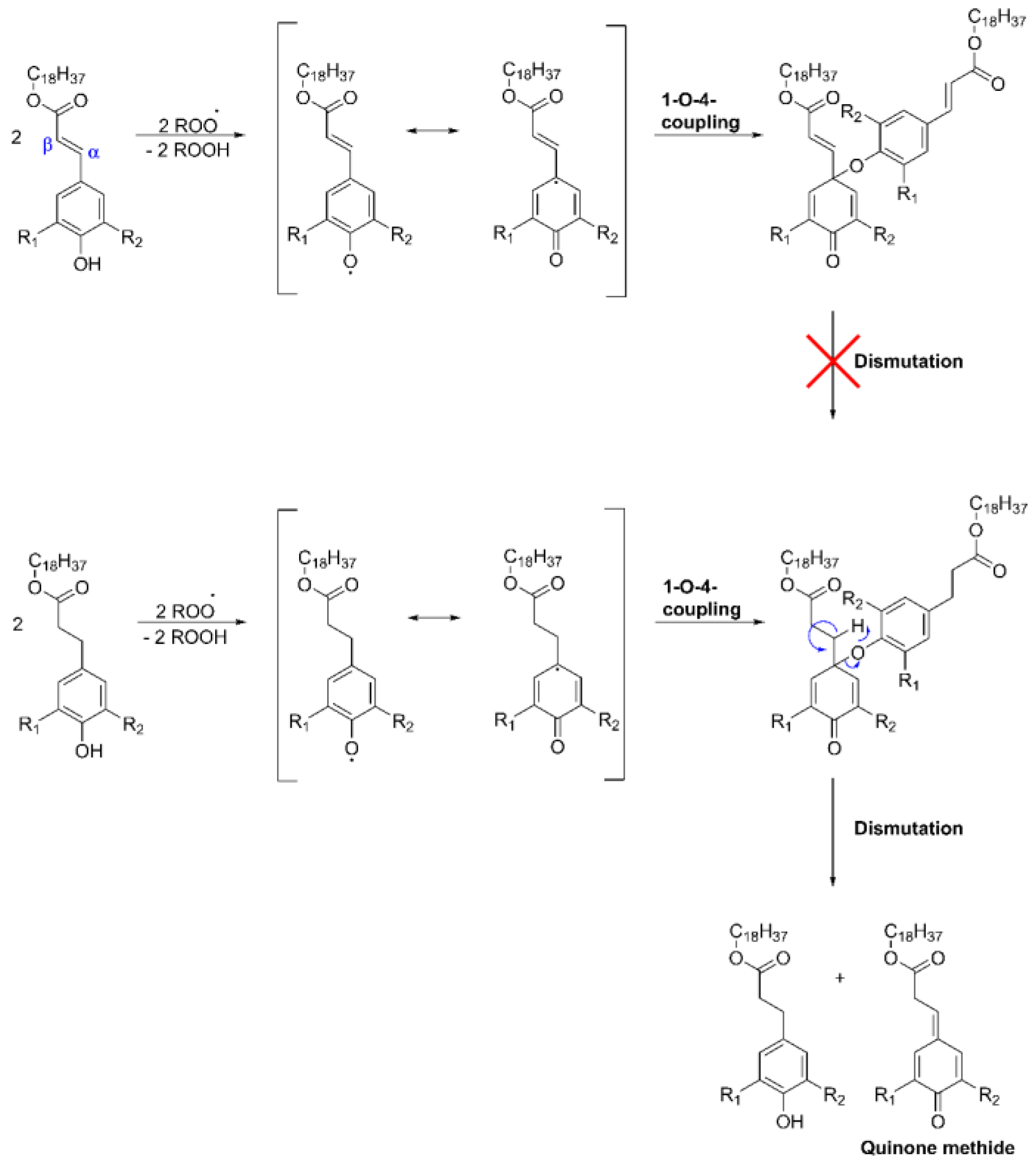
3.2. Thermal Stability of the Hydroxycinnamic Acid Stearyl Esters
3.3. Antioxidant Activity of the Hydroxycinnamic Acid Esters
3.4. Performance as Processing Stabilizer
3.5. OIT Measurements
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maddah, H.A. Polypropylene as a Promising Plastic: A Review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar]
- Bertoldo, M.; Ciardelli, F. Water extraction and degradation of a sterically hindered phenolic antioxidant in polypropylene films. Polymer 2004, 45, 8751–8759. [Google Scholar] [CrossRef]
- Simpson, D.M.; Vaughan, G.A. Ethylene polymers, LLDPE. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; pp. 441–482. [Google Scholar]
- Wegmann, A.; Le Gal, A.; Müller, D. Antioxidantien. In Handbuch Kunststoff-Additive, 4th ed.; Maier, R.-D., Schiller, M., Eds.; Hanser: Munich, Germany, 2016; pp. 1–153. [Google Scholar]
- Al-Malaika, S.; Axtell, F.; Rothon, R.; Gilbert, M. Additives for Plastics. In Brydson’s Plastic Materials, 8th ed.; Gilbert, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 127–168. [Google Scholar]
- Dexter, M.; Thomas, R.W.; King, R.E. Antioxidants. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; pp. 164–197. [Google Scholar]
- Al-Malaika, S. Mechanisms of antioxidant action and stabilisation technology—The Aston experience. Polym. Degrad. Stab. 1991, 34, 1–36. [Google Scholar] [CrossRef]
- Dhawan, A.; Kumar, V.; Parmar, V.S.; Cholli, A.L. Novel Polymeric Antioxidants for Materials. In Antioxidant Polymers: Synthesis, Properties, and Applications; Cirilo, G., Lemma, F., Eds.; Wiley: Hoboken, NJ, USA, 2012; pp. 385–425. [Google Scholar]
- Marturano, V.; Cerruti, P.; Ambrogi, V. Polymer additives. Phys. Sci. Rev. 2017, 2, 20160130. [Google Scholar] [CrossRef]
- Fiege, H.; Voges, H.-W.; Hamamoto, T.; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H.-J.; Garbe, D.; Paulus, W. Phenol Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000; pp. 521–582. [Google Scholar]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.-R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.-A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Reano, A.F.; Chérubin, J.; Peru, A.M.M.; Wang, Q.; Clément, T.; Domenek, S.; Allais, F. Structure–Activity Relationships and Structural Design Optimization of a Series of p-Hydroxycinnamic Acids-Based Bis- and Trisphenols as Novel Sustainable Antiradical/Antioxidant Additives. ACS Sustain. Chem. Eng. 2015, 3, 3486–3496. [Google Scholar] [CrossRef]
- Haider, N.; Karlsson, S. Kinetics of migration of antioxidants from polyolefins in natural environments as a basis for bioconversion studies. Biomacromolecules 2000, 1, 481–487. [Google Scholar] [CrossRef]
- Unice, K.M.; Bare, J.L.; Kreider, M.L.; Panko, J.M. Experimental methodology for assessing the environmental fate of organic chemicals in polymer matrices using column leaching studies and OECD 308 water/sediment systems: Application to tire and road wear particles. Sci. Total Environ. 2015, 533, 476–487. [Google Scholar] [CrossRef]
- Brocca, D.; Arvin, E.; Mosbæk, H. Identification of organic compounds migrating from polyethylene pipelines into drinking water. Water Res. 2002, 36, 3675–3680. [Google Scholar] [CrossRef]
- Laermer, S.F.; Zambetti, P.F. Alpha-Tocopherol (Vitamin E)—The Natural Antioxidant for Polyolefins. J. Plast. Film Sheeting 1992, 8, 228–248. [Google Scholar] [CrossRef]
- Al-Malaika, S.; Ashley, H.; Issenhuth, S. The antioxidant role of α-tocopherol in polymers. I. The nature of transformation products of α-tocopherol formed during melt processing of LDPE. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 3099–3113. [Google Scholar] [CrossRef]
- Alexy, P.; Košíková, B.; Podstránska, G. The effect of blending lignin with polyethylene and polypropylene on physical properties. Polymer 2000, 41, 4901–4908. [Google Scholar] [CrossRef]
- Gregorová, A.; Cibulková, Z.; Košíková, B.; Šimon, P. Stabilization effect of lignin in polypropylene and recycled polypropylene. Polym. Degrad. Stab. 2005, 89, 553–558. [Google Scholar] [CrossRef]
- Gregorova, A.; Košíková, B.; Staško, A. Radical scavenging capacity of lignin and its effect on processing stabilization of virgin and recycled polypropylene. J. Appl. Polym. Sci. 2007, 106, 1626–1631. [Google Scholar] [CrossRef]
- Kirschweng, B.; Tilinger, D.M.; Hégely, B.; Samu, G.; Tátraaljai, D.; Földes, E.; Pukánszky, B. Melt stabilization of PE with natural antioxidants: Comparison of rutin and quercetin. Eur. Polym. J. 2018, 103, 228–237. [Google Scholar] [CrossRef]
- Tátraaljai, D.; Földes, E.; Pukánszky, B. Efficient melt stabilization of polyethylene with quercetin, a flavonoid type natural antioxidant. Polym. Degrad. Stab. 2014, 102, 41–48. [Google Scholar] [CrossRef]
- Kirschweng, B.; Bencze, K.; Sárközi, M.; Hégely, B.; Samu, G.; Hári, J.; Tátraaljai, D.; Földes, E.; Kállay, M.; Pukánszky, B. Melt stabilization of polyethylene with dihydromyricetin, a natural antioxidant. Polym. Degrad. Stab. 2016, 133, 192–200. [Google Scholar] [CrossRef]
- Xin, M.; Ma, Y.; Lin, W.; Xu, K.; Chen, M. Use of dihydromyricetin as antioxidant for polypropylene stabilization. J. Therm. Anal. Calorim. 2015, 120, 1741–1747. [Google Scholar] [CrossRef]
- Tátraaljai, D.; Kirschweng, B.; Kovács, J.; Földes, E.; Pukánszky, B. Processing stabilisation of PE with a natural antioxidant, curcumin. Eur. Polym. J. 2013, 49, 1196–1203. [Google Scholar] [CrossRef]
- Kirschweng, B.; Tátraaljai, D.; Földes, E.; Pukánszky, B. Efficiency of curcumin, a natural antioxidant, in the processing stabilization of PE: Concentration effects. Polym. Degrad. Stab. 2015, 118, 17–23. [Google Scholar] [CrossRef]
- Kirschweng, B.; Tátraaljai, D.; Földes, E.; Pukánszky, B. Natural antioxidants as stabilizers for polymers. Polym. Degrad. Stab. 2017, 145, 25–40. [Google Scholar] [CrossRef]
- Pfaendner, R.; Melz, T. Biogene Kunststoff-Additive. In Biologische Transformation; Neugebauer, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 165–182. [Google Scholar]
- Elhamirad, A.H.; Zamanipoor, M.H. Thermal stability of some flavonoids and phenolic acids in sheep tallow olein. Eur. J. Lipid Sci. Technol. 2012, 114, 602–606. [Google Scholar] [CrossRef]
- Pouteau, C.; Dole, P.; Cathala, B.; Averous, L.; Boquillon, N. Antioxidant properties of lignin in polypropylene. Polym. Degrad. Stab. 2003, 81, 9–18. [Google Scholar] [CrossRef]
- Pouteau, C.; Baumberger, S.; Cathala, B.; Dole, P. Lignin-polymer blends: Evaluation of compatibility by image analysis. Comptes Rendus Biol. 2004, 327, 935–943. [Google Scholar] [CrossRef]
- Zheng, K.; Tang, H.; Chen, Q.; Zhang, L.; Wu, Y.; Cui, Y. Enzymatic synthesis of a polymeric antioxidant for efficient stabilization of polypropylene. Polym. Degrad. Stab. 2015, 112, 27–34. [Google Scholar] [CrossRef]
- Grigsby, W.J.; Bridson, J.H.; Lomas, C.; Frey, H. Evaluating Modified Tannin Esters as Functional Additives in Polypropylene and Biodegradable Aliphatic Polyester. Macromol. Mater. Eng. 2014, 299, 1251–1258. [Google Scholar] [CrossRef]
- Doudin, K.; Al-Malaika, S.; Sheena, H.H.; Tverezovskiy, V.; Fowler, P. New genre of antioxidants from renewable natural resources: Synthesis and characterisation of rosemary plant-derived antioxidants and their performance in polyolefins. Polym. Degrad. Stab. 2016, 130, 126–134. [Google Scholar] [CrossRef]
- Reano, A.F.; Domenek, S.; Pernes, M.; Beaugrand, J.; Allais, F. Ferulic Acid-Based Bis/Trisphenols as Renewable Antioxidants for Polypropylene and Poly(butylene succinate). ACS Sustain. Chem. Eng. 2016, 4, 6562–6571. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Zago, E.; Lecomte, J.; Barouh, N.; Aouf, C.; Carré, P.; Fine, F.; Villeneuve, P. Influence of rapeseed meal treatments on its total phenolic content and composition in sinapine, sinapic acid and canolol. Ind. Crops Prod. 2015, 76, 1061–1070. [Google Scholar] [CrossRef]
- Kozlowska, H.; Naczk, M.; Shahidi, F.; Zadernowski, R. Phenolic acids and tannins in rapeseed and canola. In Canola and Rapeseed-Production, Chemistry, Nutrition, and Processing Technology; Shahidi, F., Ed.; Springer: Boston, MA, USA, 1990; pp. 193–210. [Google Scholar]
- Tilay, A.; Bule, M.; Kishenkumar, J.; Annapure, U. Preparation of ferulic acid from agricultural wastes: Its improved extraction and purification. J. Agric. Food Chem. 2008, 56, 7644–7648. [Google Scholar] [CrossRef]
- Percec, V.; Peterca, M.; Sienkowska, M.J.; Ilies, M.A.; Aqad, E.; Smidrkal, J.; Heiney, P.A. Synthesis and retrostructural analysis of libraries of AB3 and constitutional isomeric AB2 phenylpropyl ether-based supramolecular dendrimers. J. Am. Chem. Soc. 2006, 128, 3324–3334. [Google Scholar] [CrossRef]
- Masuda, T.; Yamada, K.; Maekawa, T.; Takeda, Y.; Yamaguchi, H. Antioxidant Mechanism Studies on Ferulic Acid: Isolation and Structure Identification of the Main Antioxidation Product from Methyl Ferulate. Food Sci. Technol. Res. 2006, 12, 173–177. [Google Scholar] [CrossRef]
- Fujita, M.; Yamada, M.; Nakajima, S.; Kawai, K.; Nagai, M. O-Methylation effect on the carbon-13 nuclear magnetic resonance signals of ortho-disubstituted phenols and its application to structure determination of new phthalides from Aspergillus silvaticus. Chem. Pharm. Bull. 1984, 32, 2622–2627. [Google Scholar] [CrossRef]
- Fischer, J.; Metzsch-Zilligen, E.; Zou, M.; Pfaendner, R. A novel class of high molecular weight multifunctional antioxidants for polymers based on thiol-ene click reaction. Polym. Degrad. Stab. 2020, 173, 109099. [Google Scholar] [CrossRef]
- Zhan, K.; Ejima, H.; Yoshie, N. Antioxidant and Adsorption Properties of Bioinspired Phenolic Polymers: A Comparative Study of Catechol and Gallol. ACS Sustain. Chem. Eng. 2016, 4, 3857–3863. [Google Scholar] [CrossRef]
- DIN EN ISO 11357-6:2013; Plastics—Differential Scanning Calorimetry (DSC)—Part 6: Determination of OXIDATION induction Time (isothermal OIT) and Oxidation Induction Temperature (dynamic OIT). German Institute for Standardisation: Berlin, Germany, 2013.
- ISO 1133-1:2022; Plastics—Determination of the Melt Mass-Flow Rate (MFR) and Melt Volume-Flow Rate (MVR) of Thermoplastics—Part 1: Standard Method. International Organization for Standardization: Geneva, Switzerland, 2022.
- Maier, R.D.; Kristiansen, P.M. Nukleierungsmittel und Transparenzverstärker. In Handbuch Kunststoff-Additive, 4th ed.; Maier, R.-D., Schiller, M., Eds.; Hanser: Munich, Germany, 2016; pp. 679–736. [Google Scholar]
- Foti, M.C. Use and Abuse of the DPPH• Radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef]
- Xie, J.; Schaich, K.M. Re-evaluation of the 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Sekher Pannala, A.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C.A. Flavonoid B-ring chemistry and antioxidant activity: Fast reaction kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Olszowy, M. On practical problems in estimation of antioxidant activity of compounds by DPPH method (Problems in estimation of antioxidant activity). Food Chem. 2012, 131, 1037–1043. [Google Scholar] [CrossRef]
- Von Gadow, A.; Joubert, E.; Hansmann, C.F. Comparison of the Antioxidant Activity of Aspalathin with That of Other Plant Phenols of Rooibos Tea (Aspalathus linearis), α-Tocopherol, BHT, and BHA. J. Agric. Food Chem. 1997, 45, 632–638. [Google Scholar] [CrossRef]
- Al-Malaika, S.; Goodwin, C.; Issenhuth, S.; Burdick, D. The antioxidant role of α-tocopherol in polymers II. Melt stabilising effect in polypropylene. Polym. Degrad. Stab. 1999, 64, 145–156. [Google Scholar] [CrossRef]
- Bergenudd, H.; Eriksson, P.; DeArmitt, C.; Stenberg, B.; Malmström Jonsson, E. Synthesis and evaluation of hyperbranched phenolic antioxidants of three different generations. Polym. Degrad. Stab. 2002, 76, 503–509. [Google Scholar] [CrossRef]
- Pauquet, J.-R.; Todesco, R.V.; Drake, W.O. Limitations and Applications of Oxidative Induction Time (OIT) to Quality Control of Polyolefins. In Proceedings of the 42nd International Wire & Cable Symposium, St. Louis, MO, USA, 15–18 November 1993. [Google Scholar]
- Schmid, M.; Affolter, S. Interlaboratory tests on polymers by differential scanning calorimetry (DSC): Determination and comparison of oxidation induction time (OIT) and oxidation induction temperature (OIT*). Polym. Test. 2003, 22, 419–428. [Google Scholar] [CrossRef]
- Pospîsil, J.; Nešpůrek, S.; Zweifel, H. The role of quinone methides in thermostabilization of hydrocarbon polymers -I. Formation and reactivity of quinone methides. Polym. Degrad. Stab. 1996, 54, 7–14. [Google Scholar] [CrossRef]
- Pospîsil, J.; Nešpůrek, S.; Zweifel, H. The role of quinone methides in thermostabilization of hydrocarbon polymers-II. Properties and activity mechanisms. Polym. Degrad. Stab. 1996, 54, 15–21. [Google Scholar] [CrossRef]
- Beer, S.; Teasdale, I.; Brueggemann, O. Immobilization of antioxidants via ADMET polymerization for enhanced long-term stabilization of polyolefins. Eur. Polym. J. 2013, 49, 4257–4264. [Google Scholar] [CrossRef]
- Al-Malaika, S.; Issenhuth, S. The antioxidant role of α-tocopherol in polymers III. Nature of transformation products during polyolefins extrusion. Polym. Degrad. Stab. 1999, 65, 143–151. [Google Scholar] [CrossRef]
- Ehrenstein, G.W.; Pongratz, S. Resistance and Stability of Polymers; Hanser Publishers: Munich, Germany, 2013; pp. 139–271. [Google Scholar]
- Li, C.; Sun, P.; Guo, S.; Zhang, Z.; Wang, J. Relationship between bridged groups and antioxidant activity for aliphatic diamine bridged hindered phenol in polyolefins. J. Appl. Polym. Sci. 2017, 134, 45095. [Google Scholar] [CrossRef]
- Strandberg, C.; Albertsson, A.-C. Process efficiency and long-term performance of α-tocopherol in film-blown linear low-density polyethylene. J. Appl. Polym. Sci. 2005, 98, 2427–2439. [Google Scholar] [CrossRef]
- Mallégol, J.; Carlsson, D.; Deschênes, L. A comparison of phenolic antioxidant performance in HDPE at 32–80 °C. Polym. Degrad. Stab. 2001, 73, 259–267. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Hao, Y.; Liu, Y. Identification of the DPPH radical scavenging reaction adducts of ferulic acid and sinapic acid and their structure-antioxidant activity relationship. LWT 2021, 146, 111411. [Google Scholar] [CrossRef]
- Al-Malaika, S. Oxidative degradation and stabilisation of polymers. Int. Mater. Rev. 2003, 48, 165–185. [Google Scholar] [CrossRef]




| Abbreviation | Chemical Structure |
|---|---|
| HzOcd |  |
| FaSa | 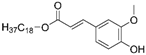 |
| SinSa | 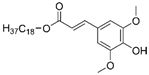 |
| AO-1076 | 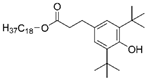 |
| α-Tocopherol | 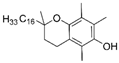 |
| Sample | PP | HzOcd | FaSa | SinSa | AO-1076 | α-Tocopherol |
|---|---|---|---|---|---|---|
| [wt.%] | ||||||
| PP0 | 100 | |||||
| PP1 | 99.5 | 0.5 | ||||
| PP2 | 99.5 | 0.5 | ||||
| PP3 | 99.5 | 0.5 | ||||
| PP-C1 | 99.5 | 0.5 | ||||
| PP-C2 | 99.5 | 0.5 |
| Sample | Temperatures of Mass Loss | Temperatures of Maximum Mass Loss | ||||
|---|---|---|---|---|---|---|
| T5% [°C] | T10% [°C] | Tmax [°C] | ||||
| Air | N2 | Air | N2 | Air | N2 | |
| HzOcd | 297 | 308 | 318 | 327 | 362 | 378 |
| FaSa | 289 | 302 | 312 | 317 | 361 | 364 |
| SinSa | 299 | 321 | 318 | 337 | 364 | 378 |
| Sample | Force Retention [%] | ||
|---|---|---|---|
| 10 min | 20 min | 30 min | |
| PP0 | 53.9 ± 0.9 | 27.8 ± 0.1 | 13.9 ± 0.5 |
| PP1 | 65.0 ± 0.8 | 49.0 ± 0.5 | 36.8 ± 0.2 |
| PP2 | 74.6 ± 0.6 | 59.9 ± 1.6 | 49.5 ± 2.0 |
| PP3 | 80.5 ± 0.1 | 67.8 ± 1.6 | 57.8 ± 2.3 |
| PP-C1 | 79.9 ± 0.0 | 69.4 ± 1.9 | 59.0 ± 2.1 |
| PP-C2 | 89.5 ± 0.2 | 84.8 ± 0.1 | 79.9 ± 0.1 |
| Sample | OIT [min] TM = 220 °C |
|---|---|
| PP0 | 2.5 ± 0.1 |
| PP1 | 9.1 ± 1.7 |
| PP2 | 17.6 ± 3.0 |
| PP3 | 26.8 ± 2.1 |
| PP-C1 | 100.6 ± 12.1 |
| PP-C2 | 103.6 ± 9.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayer, J.; Steinbrecher, R.; Metzsch-Zilligen, E.; Pfaendner, R. Antioxidant Activity of Biogenic Cinnamic Acid Derivatives in Polypropylene. Polymers 2023, 15, 3621. https://doi.org/10.3390/polym15173621
Mayer J, Steinbrecher R, Metzsch-Zilligen E, Pfaendner R. Antioxidant Activity of Biogenic Cinnamic Acid Derivatives in Polypropylene. Polymers. 2023; 15(17):3621. https://doi.org/10.3390/polym15173621
Chicago/Turabian StyleMayer, Jannik, René Steinbrecher, Elke Metzsch-Zilligen, and Rudolf Pfaendner. 2023. "Antioxidant Activity of Biogenic Cinnamic Acid Derivatives in Polypropylene" Polymers 15, no. 17: 3621. https://doi.org/10.3390/polym15173621
APA StyleMayer, J., Steinbrecher, R., Metzsch-Zilligen, E., & Pfaendner, R. (2023). Antioxidant Activity of Biogenic Cinnamic Acid Derivatives in Polypropylene. Polymers, 15(17), 3621. https://doi.org/10.3390/polym15173621





