Preparation and Performance of H-PDMS/PMHS/OTS Hybrid Nanosilica Hydrophobic and Self-Cleaning Coatings on Phosphogypsum Surface
Abstract
:1. Introduction
2. Materials and Experimental Detail
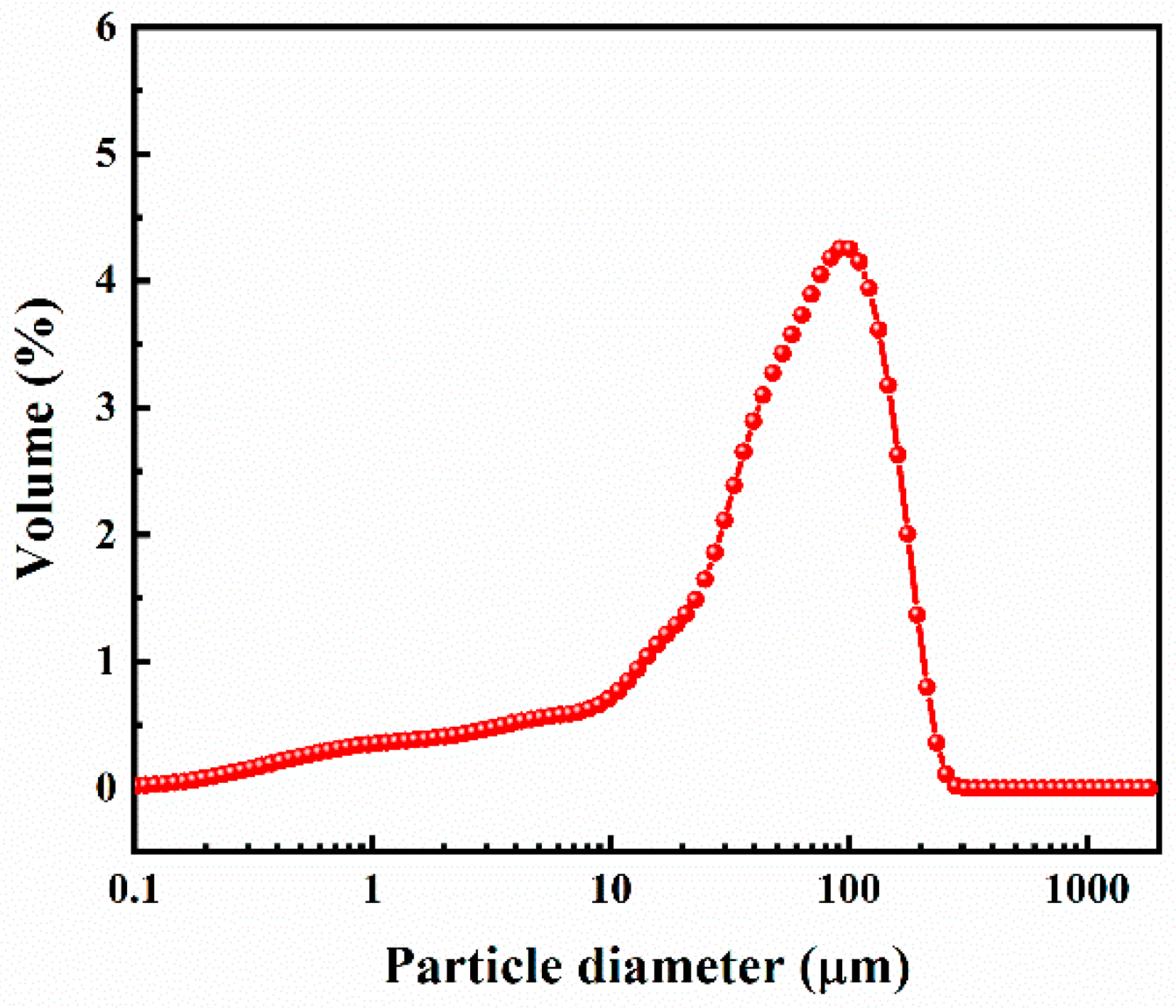
2.1. Materials
2.2. Experimental Methods
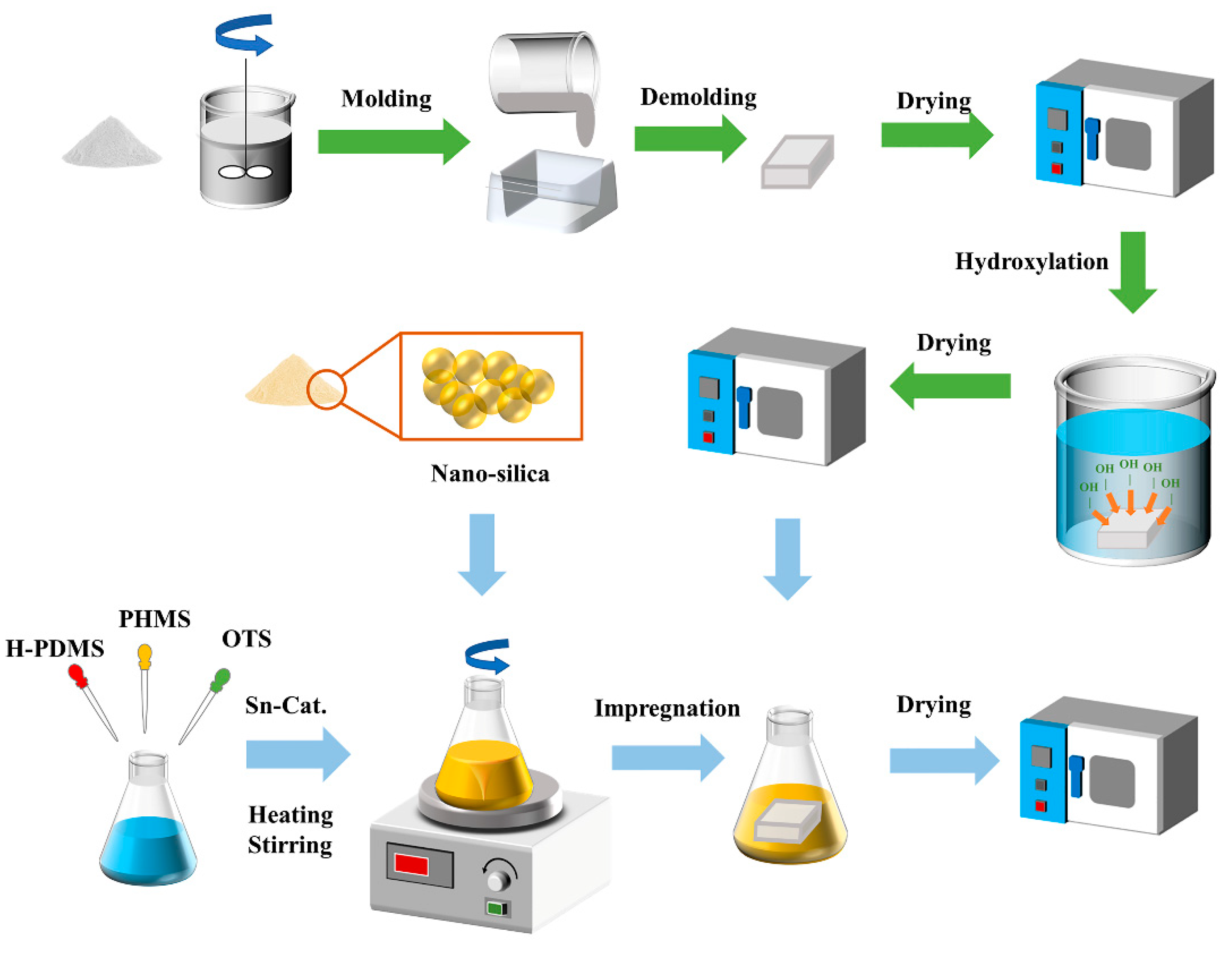
2.2.1. Preparation of PG Samples
2.2.2. Fabrication of Hydrophobic PG Sample
2.3. Sample Characterization
2.4. Stability and Durability Tests
3. Results and Discussion
3.1. The Effect of Surface Hydroxylation on the Properties of PG
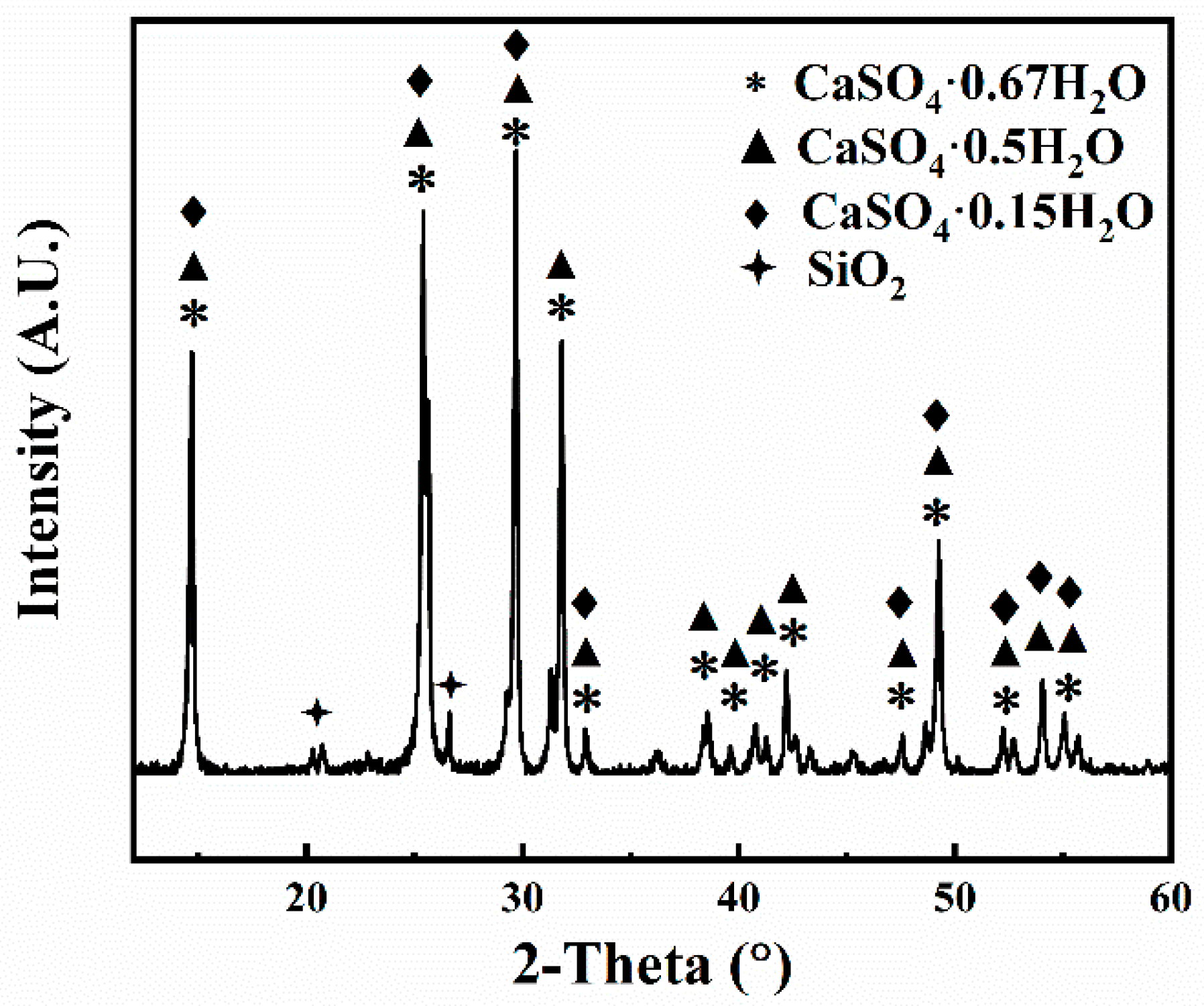
XRD and SEM Analysis
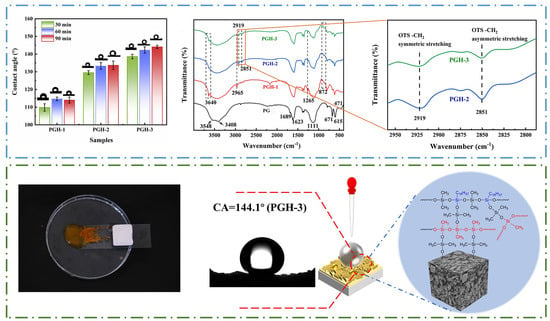
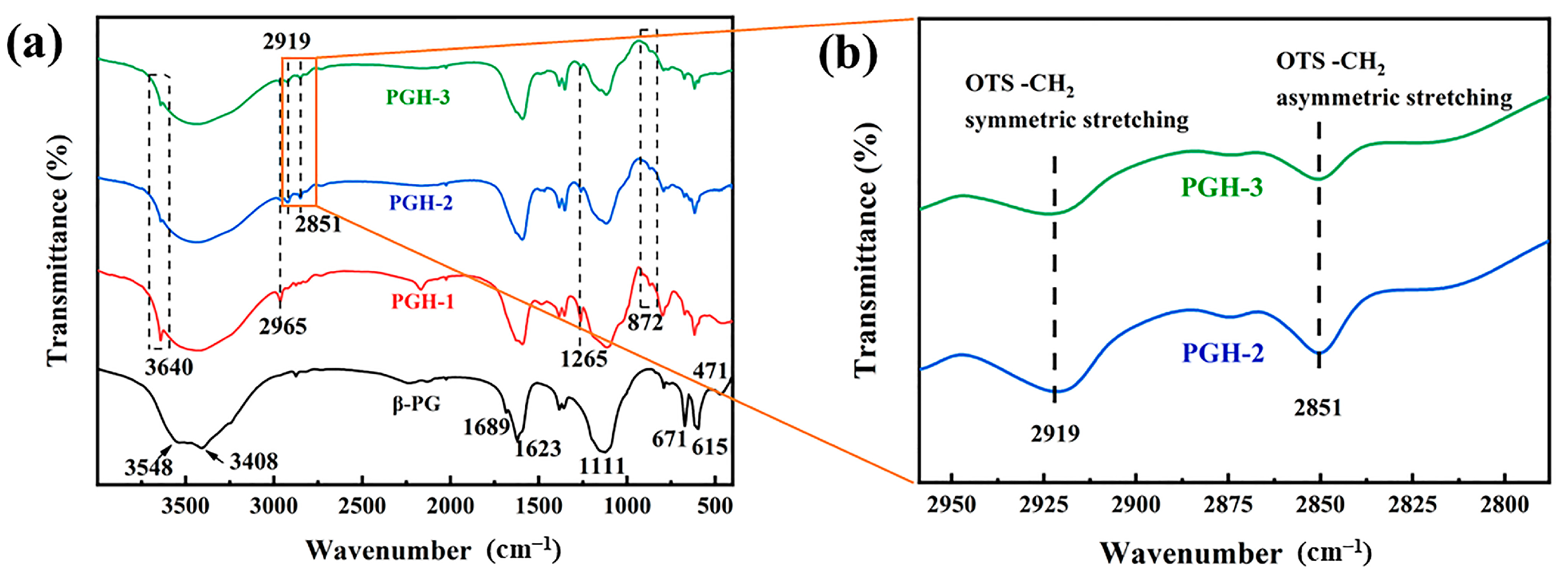
3.2. FTIR Analysis
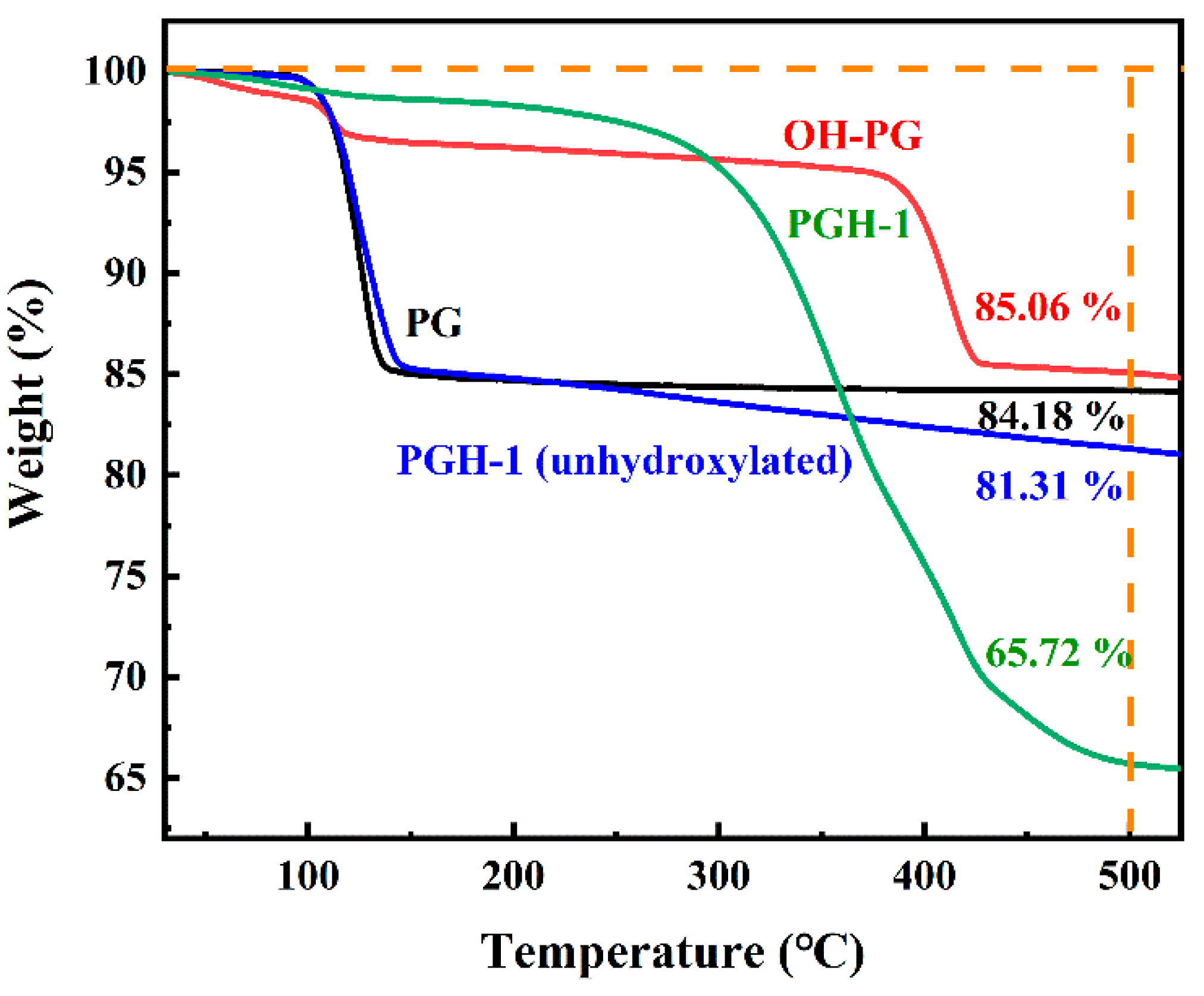
3.3. Thermogravimetric Analysis
3.4. Surface Wettability Analysis
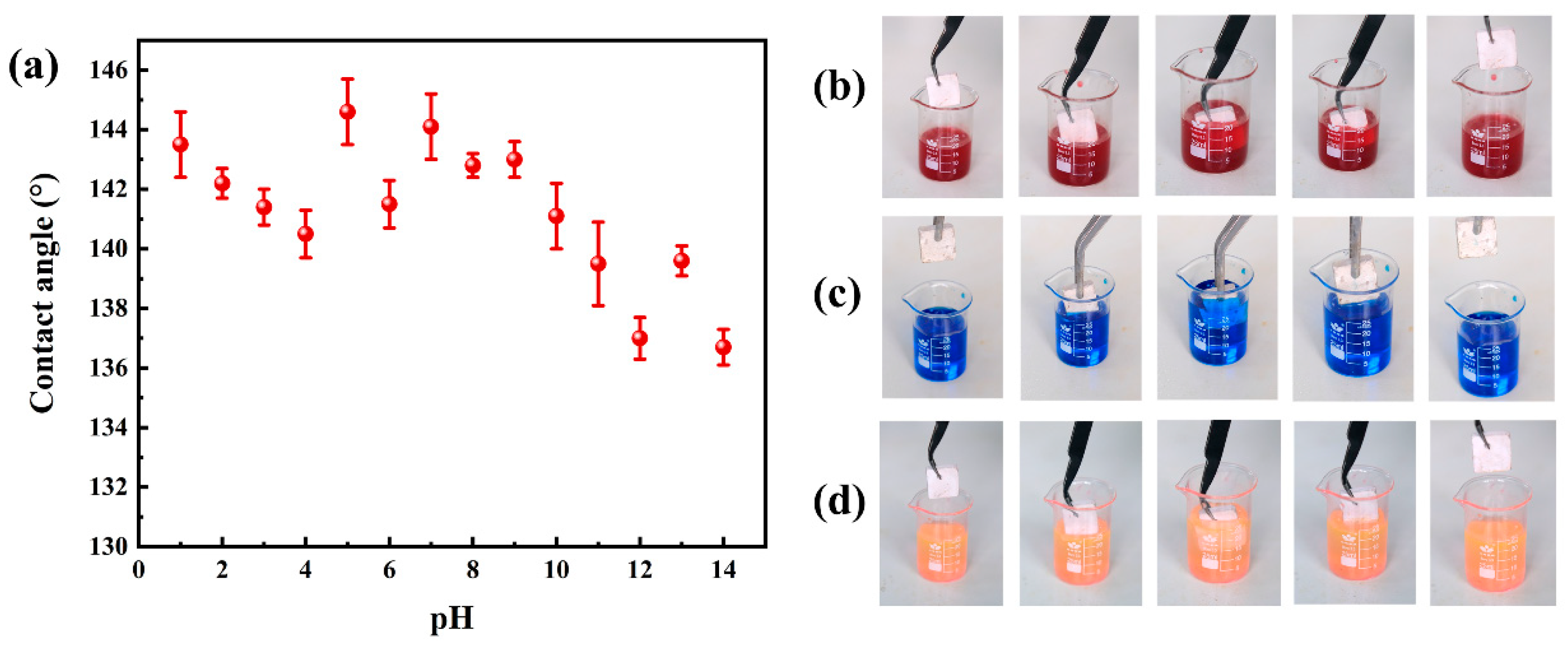
3.4.1. Contact Angle and Adhesion Analysis
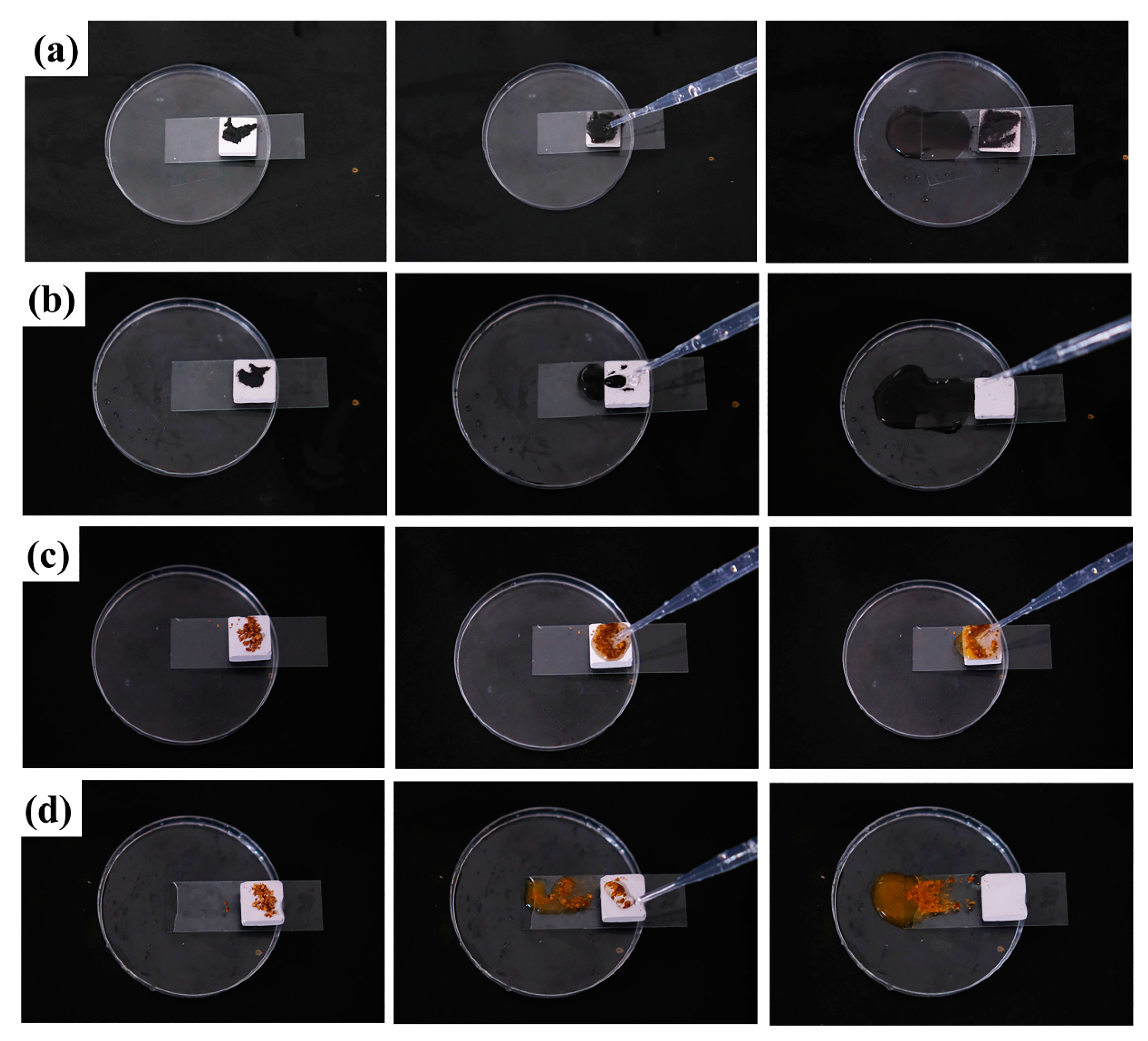
3.4.2. Self-Cleaning Properties
3.5. Surface Microstructure Analysis
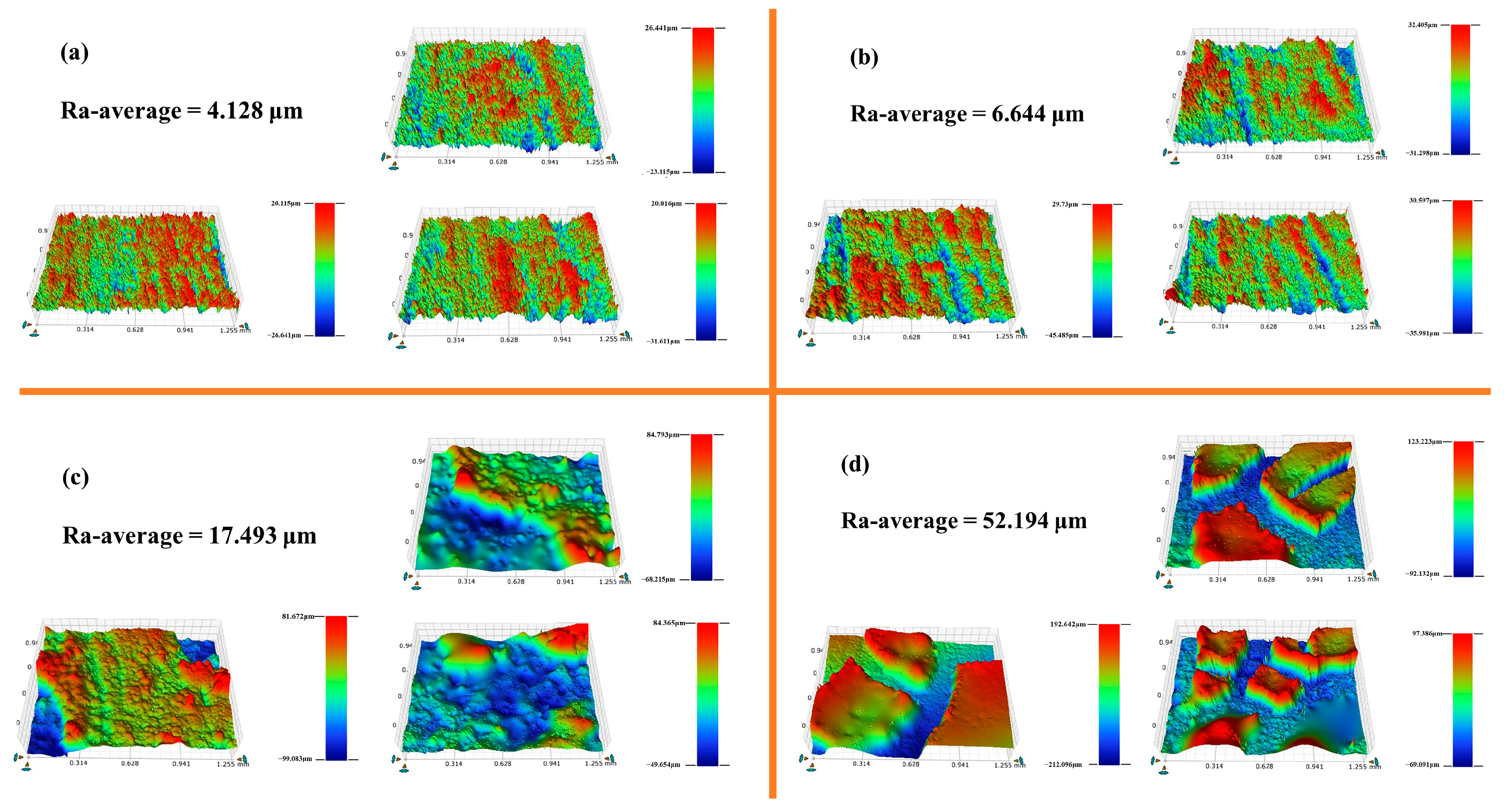
3.5.1. Surface 3D Morphology and Roughness Analysis
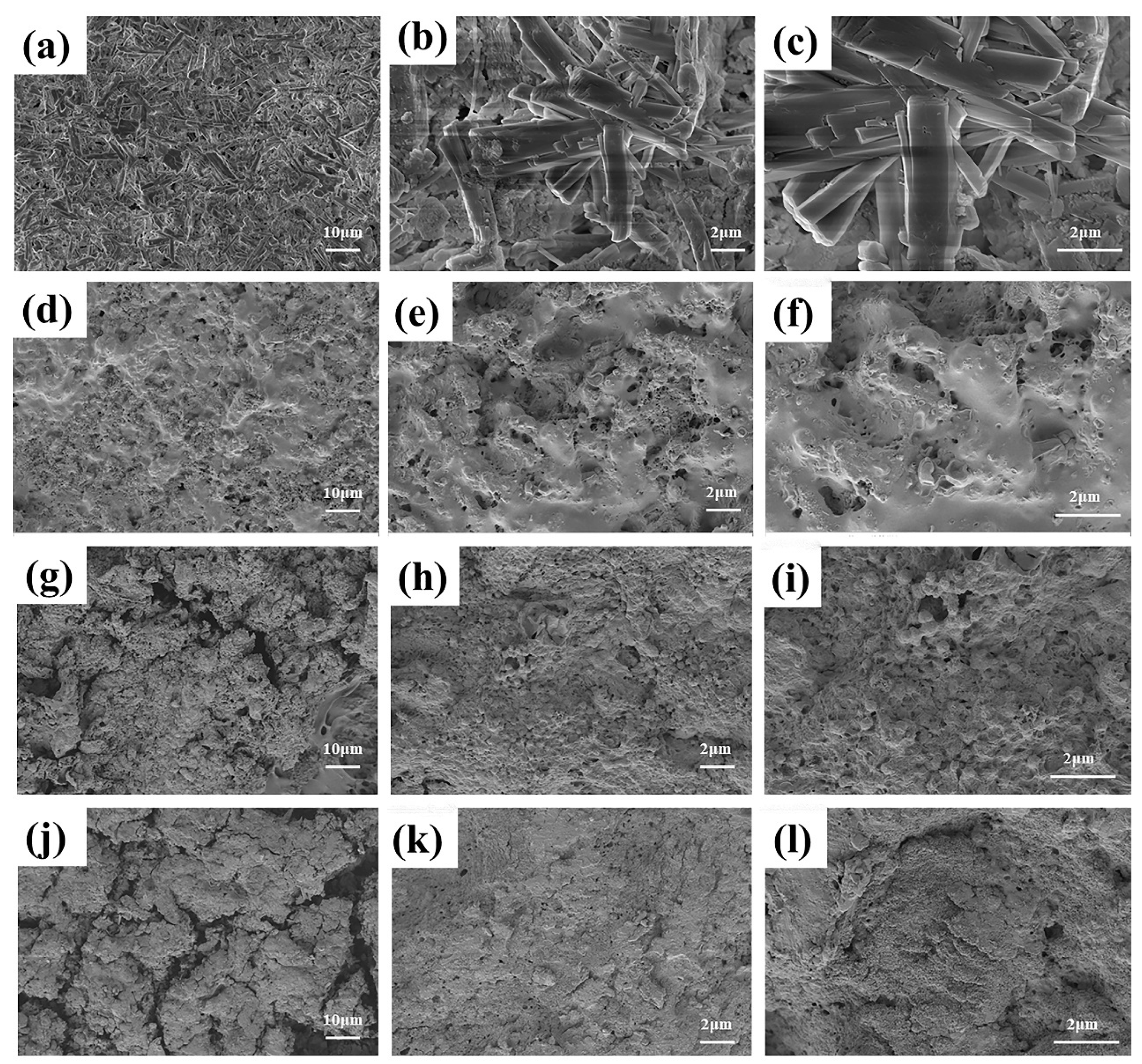
3.5.2. Surface Morphology Analysis
3.6. Surface Chemical Composition Analysis
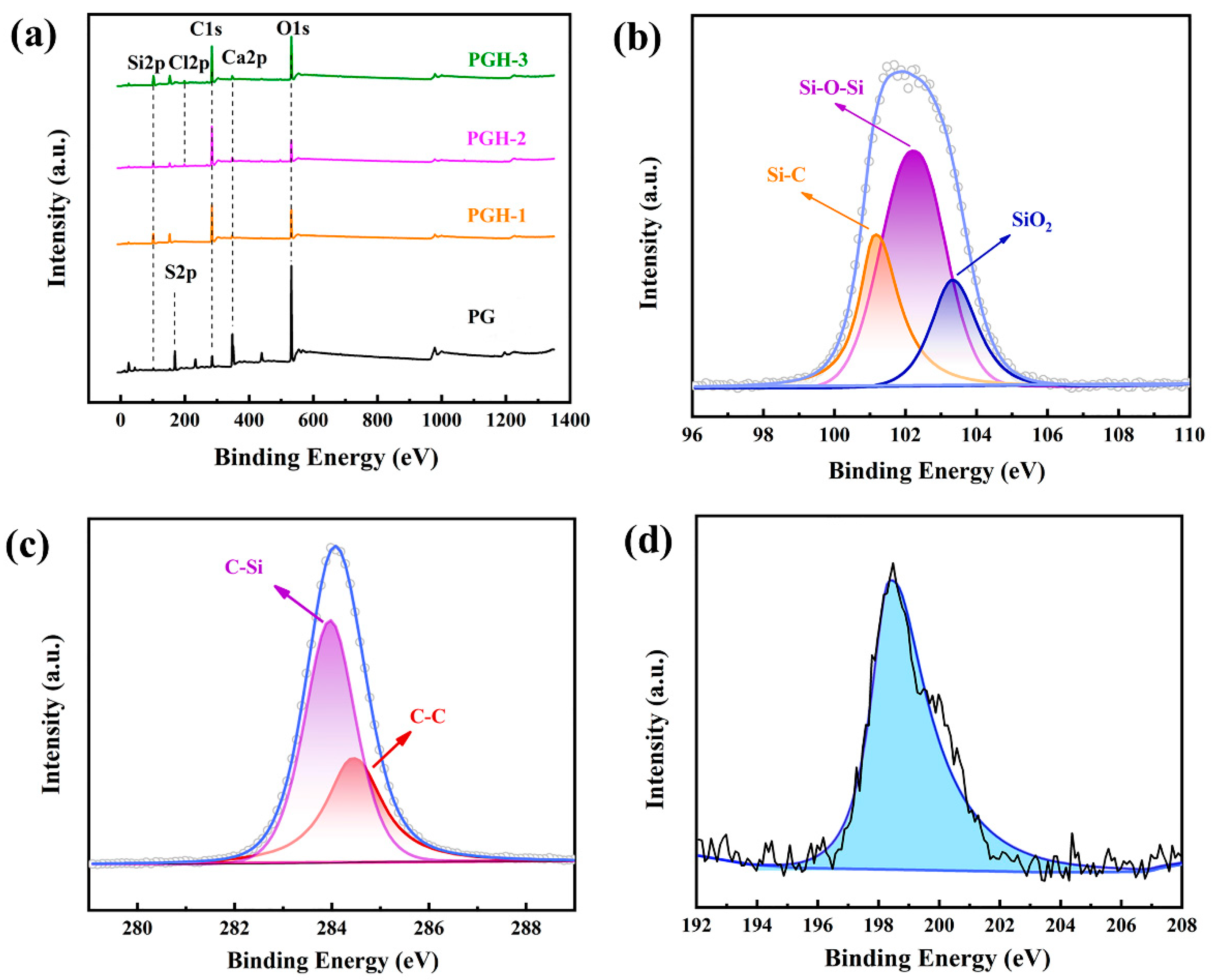
3.6.1. XPS Analysis
3.6.2. FTIR Analysis
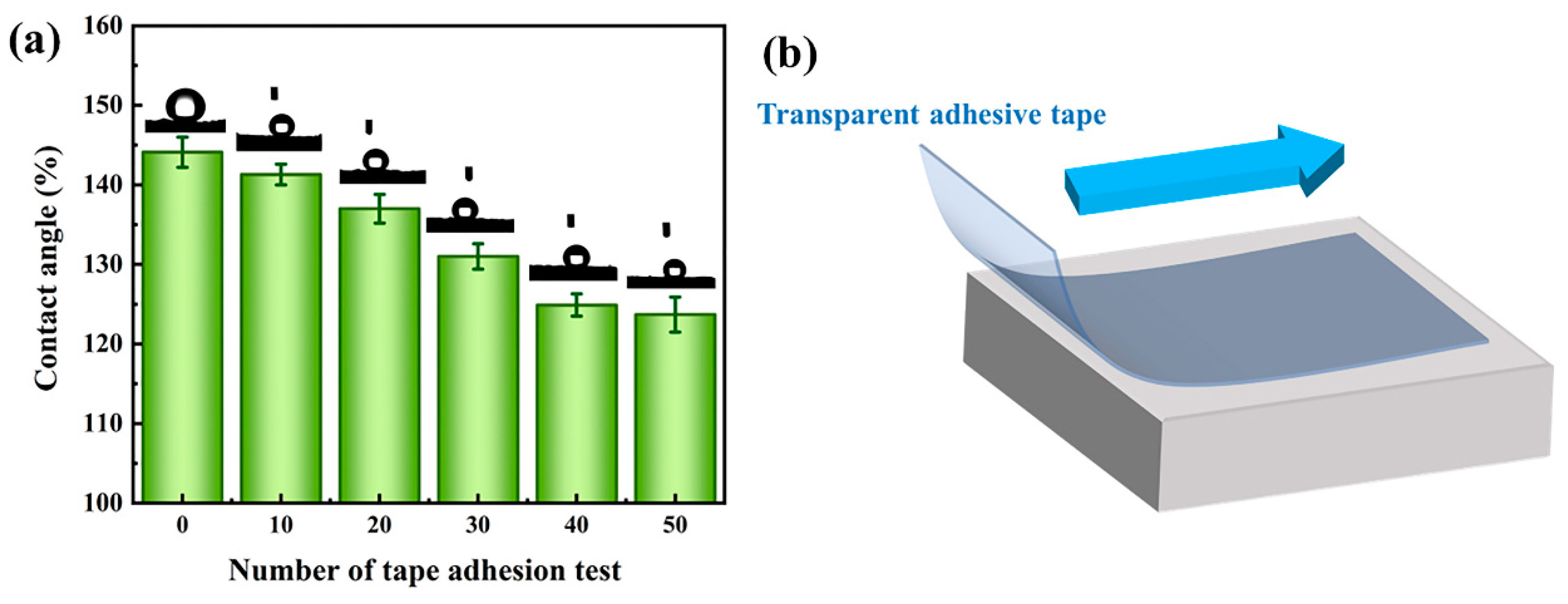
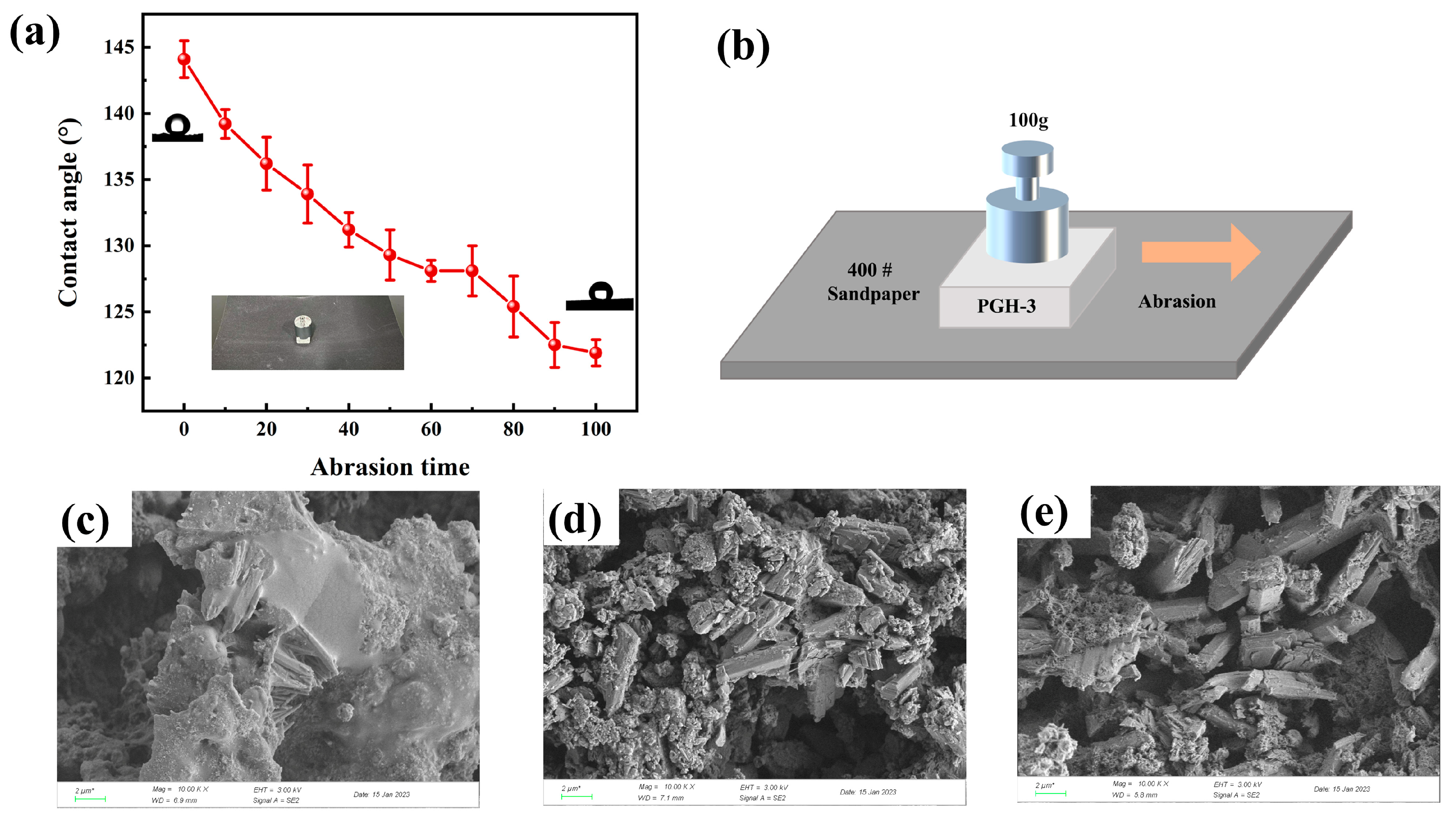
3.7. Stability and Durability Analysis
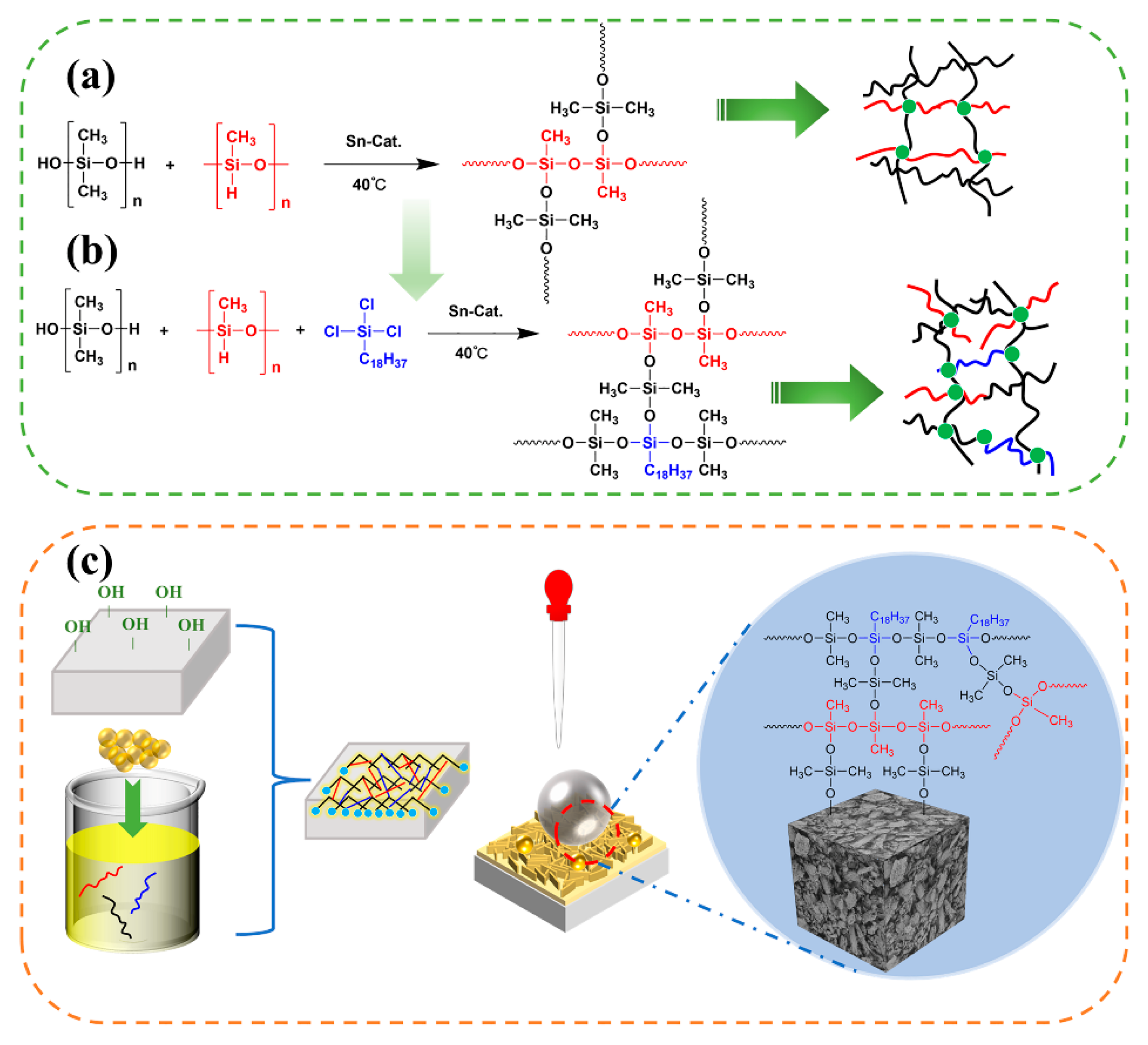
3.8. Mechanism Analysis
4. Conclusions
- The maximum contact angle of the PGH-3 surface was 144.1°, with low adhesion to water droplets and acceptable self-cleaning performance. From the surface 3D morphology and SEM images, it was observed that the surface micro- and nano-hydrophobic networks with a certain roughness formed a hydrophobic coating on the PG.
- XPS and FTIR tests demonstrated that the carbon and silicon elements on the PGH-3 surface increased to 48.82% and 17.91%, respectively, and the hydrophobic network of siloxane generated by the in situ reaction attached to the surface of the PG through hydrogen bonding such that the PG, which was originally completely hydrophilic, exhibited hydrophobicity.
- The PGH-3 sample exhibited excellent chemical stability, and the contact angle could be maintained greater than 135° under strongly acidic or strongly alkaline conditions. After 50 tape-bonding tests, the contact angle remained at 123.7°. After 100 wear cycles, the contact angle remained at 121.9°.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Attallah, M.; Metwally, S.; Moussa, S.; Soliman, M.A. Environmental impact assessment of phosphate fertilizers and phosphogypsum waste: Elemental and radiological effects. Microchem. J. 2019, 146, 789–797. [Google Scholar] [CrossRef]
- Chernysh, Y.; Yakhnenko, O.; Chubur, V.; Roubík, H. Phosphogypsum recycling: A review of environmental issues, current trends, and prospects. Appl. Sci. 2021, 11, 1575. [Google Scholar] [CrossRef]
- Xu, D.; Zhong, B.; Wang, X.; Li, X.; Zhong, Y.; Yan, Z.; Yang, J.; Li, X.; Wang, Y.; Zhou, X. The development road of ammonium phosphate fertilizer in China. Chin. J. Chem. Eng. 2022, 41, 170–175. [Google Scholar] [CrossRef]
- Haneklaus, N.; Bertau, M.; Fröhlich, P.; Pavón, S.; Barbossa, S.; Basallote, M.; Nieto, J.; Cánovas, C.; Bilal, E.; Chajduk, E. Closing the upcoming EU gypsum gap with phosphogypsum. Resour. Conserv. Recycl. 2022, 182, 106328. [Google Scholar] [CrossRef]
- Ding, W.; Chen, Q.; Sun, H.; Peng, T. Modified mineral carbonation of phosphogypsum for CO2 sequestration. J. CO2 Util. 2019, 34, 507–515. [Google Scholar] [CrossRef]
- Wu, F.; Ren, Y.; Qu, G.; Liu, S.; Chen, B.; Liu, X.; Zhao, C.; Li, J. Utilization path of bulk industrial solid waste: A review on the multi-directional resource utilization path of phosphogypsum. J. Environ. Manag. 2022, 313, 114957. [Google Scholar] [CrossRef]
- Bilal, E.; Bellefqih, H.; Bourgier, V.; Mazouz, H.; Dumitraş, D.-G.; Bard, F.; Laborde, M.; Caspar, J.P.; Guilhot, B.; Iatan, L. Phosphogypsum circular economy considerations: A critical review from more than 65 storage sites worldwide. J. Clean. Prod. 2023, 14, 137561. [Google Scholar] [CrossRef]
- Campos, M.; Costa, L.; Nisti, M.; Mazzilli, B. Phosphogypsum recycling in the building materials industry: Assessment of the radon exhalation rate. J. Environ. Radioact. 2017, 172, 232–236. [Google Scholar] [CrossRef]
- Ségalen, C.; Dieudé-Fauvel, E.; Baudez, J. Electrical and rheological properties of sewage sludge—Impact of the solid content. Water Res. 2015, 82, 25–36. [Google Scholar] [CrossRef]
- Singh, M. Treating waste phosphogypsum for cement and plaster manufacture. Cem. Concr. Res. 2002, 32, 1033–1038. [Google Scholar] [CrossRef]
- Cao, Y.; Cui, Y.; Yu, X.; Li, T.; Chang, I.-S.; Wu, J. Bibliometric analysis of phosphogypsum research from 1990 to 2020 based on literatures and patents. Environ. Sci. Pollut. Res. 2021, 28, 66845–66857. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bao, W.; Xiu, C.; Zhang, Y.; Xu, H. Energy conservation and circular economy in China’s process industries. Energy 2010, 35, 4273–4281. [Google Scholar] [CrossRef]
- Xiao, Y.D.; Jin, H.X.; Wang, M.L.; Guo, Y.L. Collaborative Utilization Status of Red Mud and Phosphogypsum: A Review. J. Sustain. Metall. 2022, 8, 1422–1434. [Google Scholar] [CrossRef]
- Ma, P.; Li, S.; Cheng, B.; Yu, B.; Gao, Y. Effect of cement and fluidized bed combustion ashes on properties of phosphogypsum composite cementitious materials. IOP Conf. Ser. Earth Environ. Sci. 2021, 636, 012035. [Google Scholar] [CrossRef]
- Singh, M. Role of phosphogypsum impurities on strength and microstructure of selenite plaster. Constr. Build. Mater. 2005, 19, 480–486. [Google Scholar] [CrossRef]
- Zhao, F.-Q.; Liu, H.-J.; Hao, L.-X.; Li, Q. Water resistant block from desulfurization gypsum. Constr. Build. Mater. 2012, 27, 531–533. [Google Scholar] [CrossRef]
- Cao, J.-Y.; Li, J.-P.; Jiang, Y.-M.; Wang, S.-L.; Ding, Y.; Oh, W.-C. Improvement in water resistance of desulfurized gypsum by novel modification of silicone oil paraffin composite emulsion-based waterproofing agent. J. Korean Ceram. Soc. 2019, 56, 558–565. [Google Scholar] [CrossRef]
- Li, J.; Cao, J.; Ren, Q.; Ding, Y.; Zhu, H.; Xiong, C.; Chen, R. Effect of nano-silica and silicone oil paraffin emulsion composite waterproofing agent on the water resistance of flue gas desulfurization gypsum. Constr. Build. Mater. 2021, 287, 123055. [Google Scholar] [CrossRef]
- Fornés, I.V.; Vaičiukynienė, D.; Nizevičienė, D.; Doroševas, V. The improvement of the water-resistance of the phosphogypsum by adding waste metallurgical sludge. J. Build. Eng. 2021, 43, 102861. [Google Scholar] [CrossRef]
- Jin, Z.; Ma, B.; Su, Y.; Lu, W.; Qi, H.; Hu, P. Effect of calcium sulphoaluminate cement on mechanical strength and waterproof properties of beta-hemihydrate phosphogypsum. Constr. Build. Mater. 2020, 242, 118198. [Google Scholar] [CrossRef]
- Ran, J.; Chen, J.; Su, X. Hydrophobic modification of building gypsum by peat soil: An in-depth analysis of characterization, mechanism, and mechanical properties. Constr. Build. Mater. 2022, 338, 127526. [Google Scholar] [CrossRef]
- Bai, X.; Yang, S.; Tan, C.; Jia, T.; Guo, L.; Song, W.; Jian, M.; Zhang, X.; Zhang, Z.; Wu, L. Synthesis of TiO2 based superhydrophobic coatings for efficient anti-corrosion and self-cleaning on stone building surface. J. Clean. Prod. 2022, 380, 134975. [Google Scholar] [CrossRef]
- Carrascosa, L.A.; Zarzuela, R.; Botana-Galvín, M.; Botana, F.J.; Mosquera, M.J. Achieving superhydrophobic surfaces with tunable roughness on building materials via nanosecond laser texturing of silane/siloxane coatings. J. Build. Eng. 2022, 58, 104979. [Google Scholar] [CrossRef]
- Konar, M.; Roy, B.; Govindaraju, T. Molecular Architectonics-Guided Fabrication of Superhydrophobic and Self-Cleaning Materials. Adv. Mater. Interfaces 2020, 7, 2000246. [Google Scholar] [CrossRef]
- Chen, J.; Wei, C.; Ran, J.; Su, X.; Wang, W.; Zhang, J. Functional hydrophobic coating for phosphogypsum via stoichiometric silanization, hydrophobic characterization, microstructure analysis, and durability evaluation. Constr. Build. Mater. 2022, 347, 128560. [Google Scholar] [CrossRef]
- Jiang, Q.; Huang, J.; Ma, B.; Yang, Z.; Zhang, T. SiO2/silicone hybrid superhydrophobic coating on gypsum-based materials with self-cleaning and moisture resistance. J. Sol-Gel Sci. Technol. 2020, 96, 207–218. [Google Scholar] [CrossRef]
- Jumrus, N.; Suttanon, N.; Sroila, W.; Tippo, P.; Panthawan, A.; Thongpan, W.; Kumpika, T.; Sroila, W.; Rianyoi, R.; Singjai, P. Durability and photocatalytic activity of superhydrophobic gypsum boards coated with PDMS/MTCS-modified SiO2-TiO2 NPs. Mater. Lett. 2023, 330, 133342. [Google Scholar] [CrossRef]
- Cao, Y.; Salvini, A.; Camaiti, M. One-step fabrication of robust and durable superamphiphobic, self-cleaning surface for outdoor and in situ application on building substrates. J. Colloid Interface Sci. 2021, 591, 239–252. [Google Scholar] [CrossRef]
- Jadav, G.L.; Aswal, V.K.; Singh, P.S. Preparation of bifunctional poly (dimethylsiloxane) membrane by dual X-linking. J. Mater. Chem. A 2013, 1, 4893–4903. [Google Scholar] [CrossRef]
- Asadi-Eydivand, M.; Solati-Hashjin, M.; Shafiei, S.S.; Mohammadi, S.; Hafezi, M.; Abu Osman, N.A. Structure, properties, and in vitro behavior of heat-treated calcium sulfate scaffolds fabricated by 3D printing. PLoS ONE 2016, 11, e0151216. [Google Scholar] [CrossRef]
- Anjum, A.S.; Sun, K.C.; Ali, M.; Riaz, R.; Jeong, S.H. Fabrication of coral-reef structured nano silica for self-cleaning and super-hydrophobic textile applications. Chem. Eng. J. 2020, 401, 125859. [Google Scholar] [CrossRef]
- Wu, Y.; Jia, S.; Qing, Y.; Luo, S.; Liu, M. A versatile and efficient method to fabricate durable superhydrophobic surfaces on wood, lignocellulosic fiber, glass, and metal substrates. J. Mater. Chem. A 2016, 4, 14111–14121. [Google Scholar] [CrossRef]
- Playà, E.; Recio, C.; Mitchell, J. Extraction of gypsum hydration water for oxygen isotopic analysis by the guanidine hydrochloride reaction method. Chem. Geol. 2005, 217, 89–96. [Google Scholar] [CrossRef]
- Sievert, T.; Wolter, A.; Singh, N. Hydration of anhydrite of gypsum (CaSO4.II) in a ball mill. Cem. Concr. Res. 2005, 35, 623–630. [Google Scholar] [CrossRef]
- Guo, K.; Sharma, A.; Toh, R.J.; Alvárez de Eulate, E.; Gengenbach, T.R.; Cetó, X.; Voelcker, N.H.; Prieto-Simón, B. Porous silicon nanostructures as effective faradaic electrochemical sensing platforms. Adv. Funct. Mater. 2019, 29, 1809206. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Wang, S.; Shao, W.; Qin, W.; Zhao, X.; Kong, F. Facile fabrication and structure control of SiO2/carbon via in situ doping from liquefied bio-based sawdust for supercapacitor applications. Ind. Crops Prod. 2020, 151, 112490. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Wang, S.; Shao, W.; Wu, Q.; Zhao, X.; Kong, F. A new lamellar larch-based carbon material: Fabrication, electrochemical characterization and supercapacitor applications. Ind. Crops Prod. 2020, 148, 112306. [Google Scholar] [CrossRef]
- Javidparvar, A.; Ramezanzadeh, B.; Ghasemi, E. Effect of various spinel ferrite nanopigments modified by amino propyl trimethoxy silane on the corrosion inhibition properties of the epoxy nanocomposites. Corrosion 2016, 72, 761–774. [Google Scholar] [CrossRef]
- Yu, W.; Wang, J.; Huang, W.; Cui, L.; Wang, L. Improving high temperature tribological performances of Si doped diamond-like carbon by using W interlayer. Tribol. Int. 2020, 146, 106241. [Google Scholar] [CrossRef]
- Lin, B.; Zheng, C.; Li, X.; Zhu, Q.; Xie, F.; Liu, G. Characterization and application of superhydrophobic and superoleophilic OTS-LDH/melamine sponge. J. Therm. Anal. Calorim. 2021, 147, 1031–1040. [Google Scholar] [CrossRef]
- Angst, D.L.; Simmons, G.W. Moisture absorption characteristics of organosiloxane self-assembled monolayers. Langmuir 1991, 7, 2236–2242. [Google Scholar] [CrossRef]
- Ke, Q.; Fu, W.; Wang, S.; Tang, T.; Zhang, J. Facile preparation of superhydrophobic biomimetic surface based on octadecyltrichlorosilane and silica nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 2393–2398. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Wang, Y.; Wang, J.; Liu, S.; Li, Z.; Yang, S. Self-assembly of octadecyltrichlorosilane on graphene oxide and the tribological performances of the resultant film. J. Phys. Chem. C 2011, 115, 10080–10086. [Google Scholar] [CrossRef]
- Ren, S.; Yang, S.; Zhao, Y.; Zhou, J.; Xu, T.; Liu, W. Friction and wear studies of octadecyltrichlorosilane SAM on silicon. Tribol. Lett. 2002, 13, 233–239. [Google Scholar] [CrossRef]
- Iler, K.R. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties and Biochemistry of Silica; John Wiley and Sons: New York, NY, USA, 1979. [Google Scholar]
- Jephcott, C.; Johnston, J. Solubility of Silica and Alumina. Arch. Indust. Hyg. Occup. Med. 1950, 1, 323–340. [Google Scholar]
- Nechyporchuk, O.; Ulmefors, H.; Teleman, A. Silica-rich regenerated cellulose fibers enabled by delayed dissolution of silica nanoparticles in strong alkali using zinc oxide. Carbohydr. Polym. 2021, 264, 118032. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, P.; Pang, C.; Cui, K.; Yu, C.; Huang, L. A superhydrophobic surface with a synergistic abrasion-corrosion resistance effect prepared by femtosecond laser treatment on an FeMnSiCrNiNb shape memory alloy coating. New J. Chem. 2022, 46, 19188–19197. [Google Scholar] [CrossRef]
- Xu, L.; Wan, J.; Yuan, X.; Pan, H.; Wang, L.; Shen, Y.; Sheng, Y. Preparation of durable superamphiphobic cotton fabrics with self-cleaning and liquid repellency. J. Adhes. Sci. Technol. 2022, 36, 1–20. [Google Scholar] [CrossRef]
- Gjersing, E.; Chinn, S.; Giuliani, J.R.; Herberg, J.; Maxwell, R.S.; Eastwood, E.; Bowen, D.; Stephens, T. Investigation of network heterogeneities in filled, trimodal, highly functional PDMS networks by 1H multiple quantum NMR. Macromolecules 2007, 40, 4953–4962. [Google Scholar] [CrossRef]



| Composition | Content/wt % | Composition | Content/wt % |
|---|---|---|---|
| SO3 | 58.620 | TiO2 | 0.075 |
| CaO | 36.180 | SrO | 0.057 |
| SiO2 | 2.340 | BaO | 0.046 |
| P2O5 | 0.999 | Y2O3 | 0.007 |
| Al2O3 | 0.748 | ZrO2 | 0.006 |
| Fe2O3 | 0.532 | Ar | 0.004 |
| MgO | 0.157 | Lu2O3 | 0.002 |
| Na2O | 0.130 | CuO | 0.002 |
| K2O | 0.096 | NiO | 0.001 |
| Demand of Standards | Test Results | |
|---|---|---|
| Heavy metals | Pb ≤ 90 mg/Kg | Pb = 3.52 mg/Kg |
| Cd ≤ 90 mg/Kg | Cd = 1.05 mg/Kg | |
| Cr ≤ 90 mg/Kg | Cr = 11.54 mg/Kg | |
| Hg ≤ 90 mg/Kg | Hg = 5.64 mg/Kg |
| Scheme 1 | Ra-1/μm | Ra-2/μm | Ra-3/μm | Ra-Average/μm |
|---|---|---|---|---|
| PG | 4.523 | 4.218 | 3.642 | 4.128 |
| PGH-1 | 6.105 | 6.413 | 7.414 | 6.644 |
| PGH-2 | 14.754 | 21.568 | 16.156 | 17.493 |
| PGH-3 | 29.077 | 43.524 | 83.981 | 52.194 |
| Samples | C 1s/% | Ca 2p/% | O 1s/% | S 2p/% | Si 2p/% | Cl 2p/% |
|---|---|---|---|---|---|---|
| PG | 16.63 | 11.79 | 54.28 | 13.34 | 3.24 | 0.72 |
| PGH-1 | 53.39 | 1.07 | 25.05 | 1.7 | 18.42 | 0.37 |
| PGH-2 | 65.96 | 2.2 | 18.84 | 1.72 | 9.19 | 2.09 |
| PGH-3 | 48.82 | 1.61 | 28.41 | 1.54 | 17.91 | 1.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Chen, Z.; Lv, C.; Deng, L.; Luo, X.; Li, Y.; He, S.; Liu, Q. Preparation and Performance of H-PDMS/PMHS/OTS Hybrid Nanosilica Hydrophobic and Self-Cleaning Coatings on Phosphogypsum Surface. Polymers 2023, 15, 3574. https://doi.org/10.3390/polym15173574
Yang G, Chen Z, Lv C, Deng L, Luo X, Li Y, He S, Liu Q. Preparation and Performance of H-PDMS/PMHS/OTS Hybrid Nanosilica Hydrophobic and Self-Cleaning Coatings on Phosphogypsum Surface. Polymers. 2023; 15(17):3574. https://doi.org/10.3390/polym15173574
Chicago/Turabian StyleYang, Guang, Zhonghua Chen, Changwei Lv, Lei Deng, Xiaofeng Luo, Yi Li, Songtao He, and Qibin Liu. 2023. "Preparation and Performance of H-PDMS/PMHS/OTS Hybrid Nanosilica Hydrophobic and Self-Cleaning Coatings on Phosphogypsum Surface" Polymers 15, no. 17: 3574. https://doi.org/10.3390/polym15173574
APA StyleYang, G., Chen, Z., Lv, C., Deng, L., Luo, X., Li, Y., He, S., & Liu, Q. (2023). Preparation and Performance of H-PDMS/PMHS/OTS Hybrid Nanosilica Hydrophobic and Self-Cleaning Coatings on Phosphogypsum Surface. Polymers, 15(17), 3574. https://doi.org/10.3390/polym15173574