The Performance and Synthesis of Alkynyl−Functionalized Benzoxazine Containing Phthalide Side Groups and Cyano Groups with Different Molecular Weights
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Synthesis of BOZ-1N Series Resin
2.3. Preparation of T700/BOZ−1N Composite Laminate
2.4. Representation
3. Results
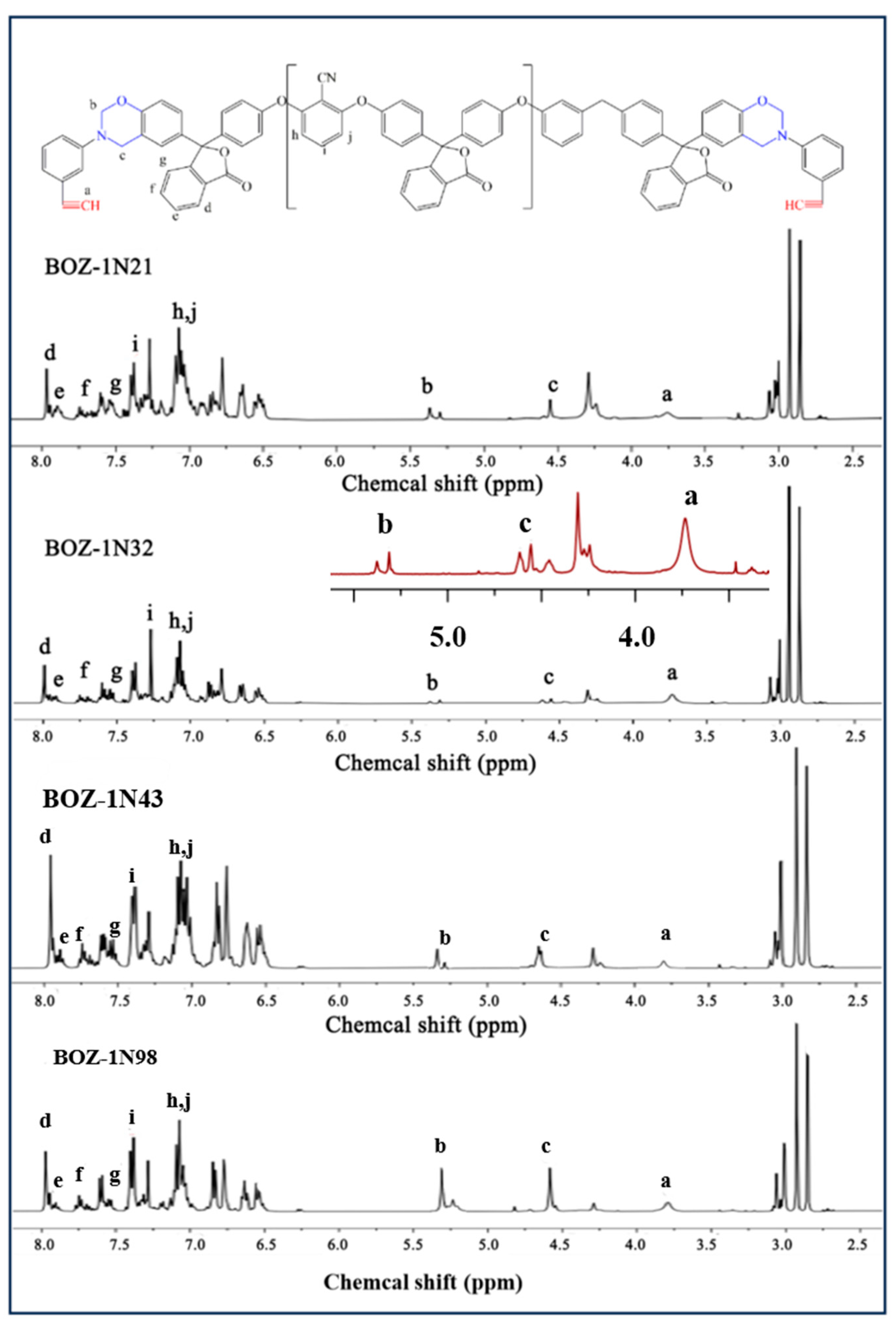
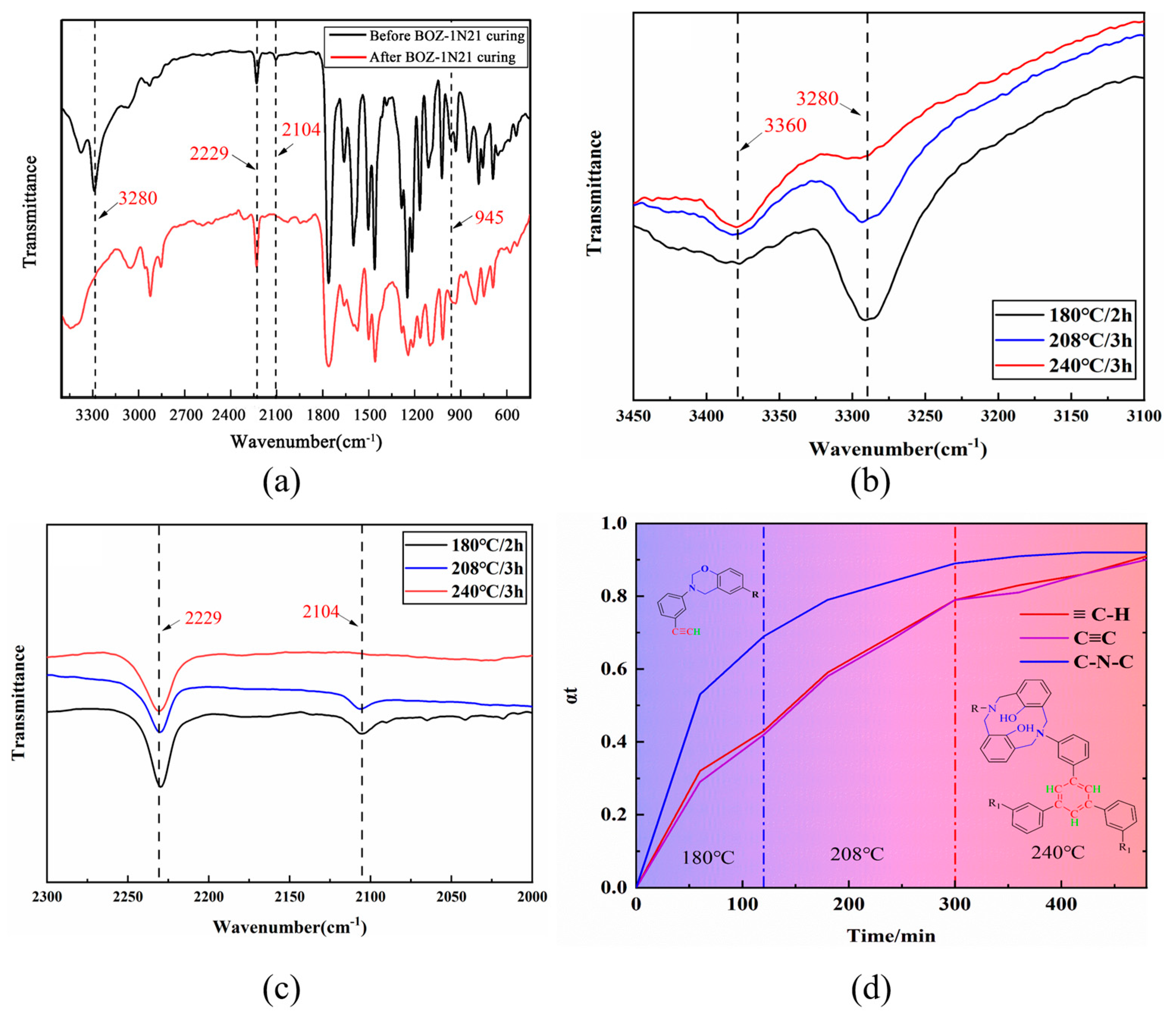
3.1. Chemical Structural Characterization of BOZ Series Resins
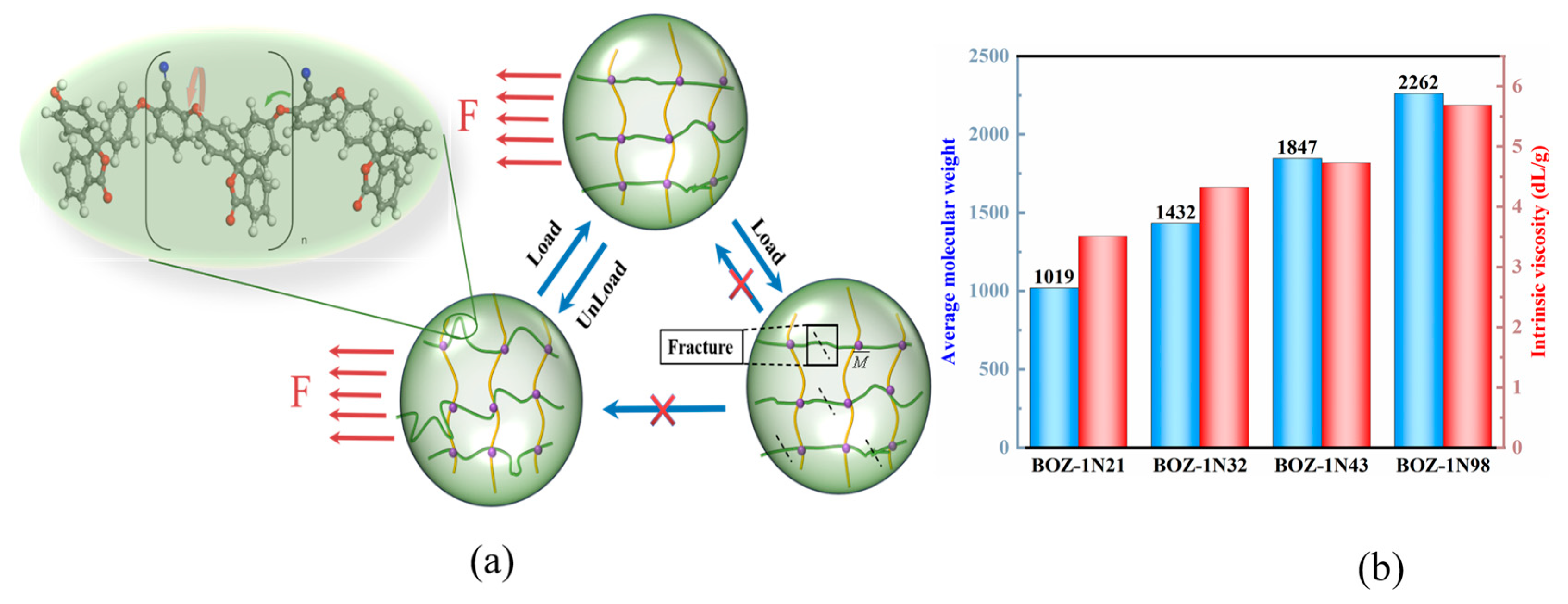
3.2. Intrinsic Viscosity and Average Molecular Weight of BOZ-1N Benzoxazine Resin
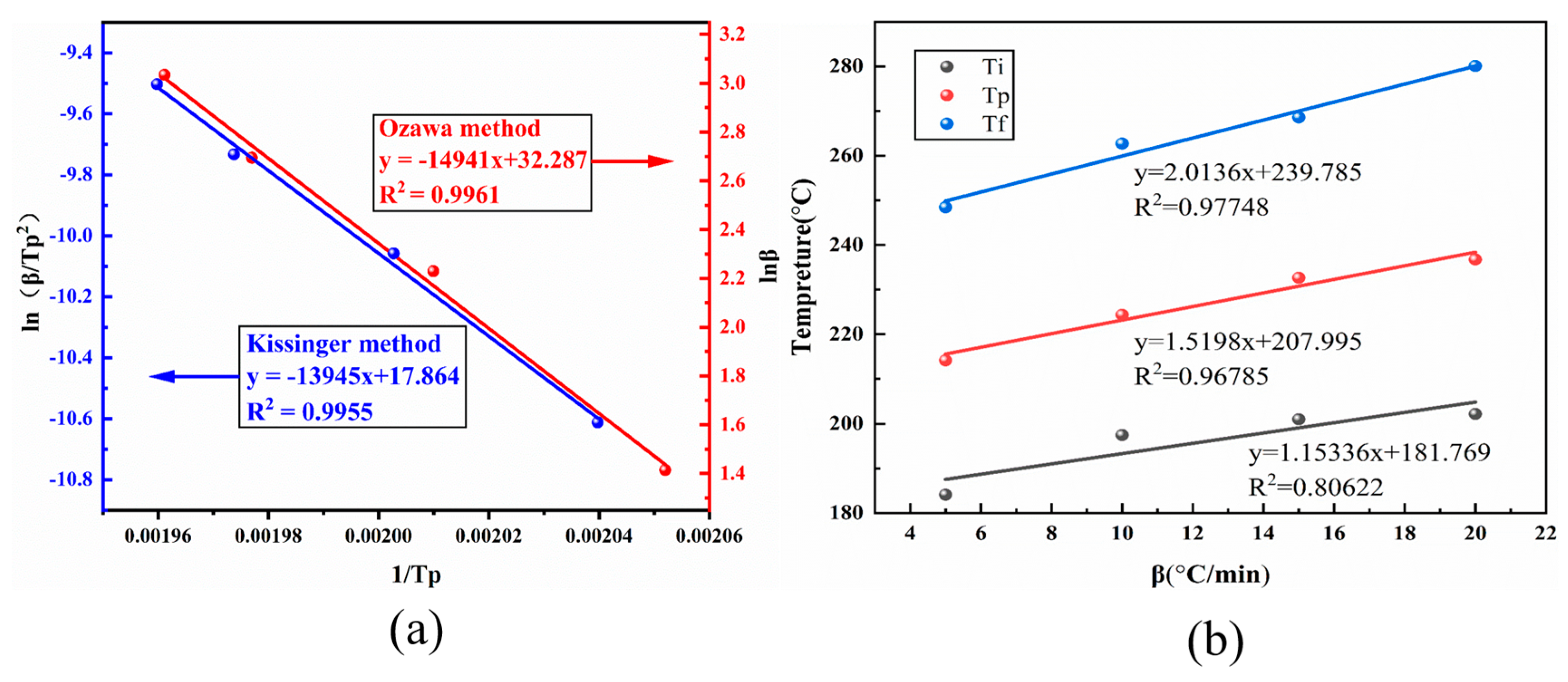
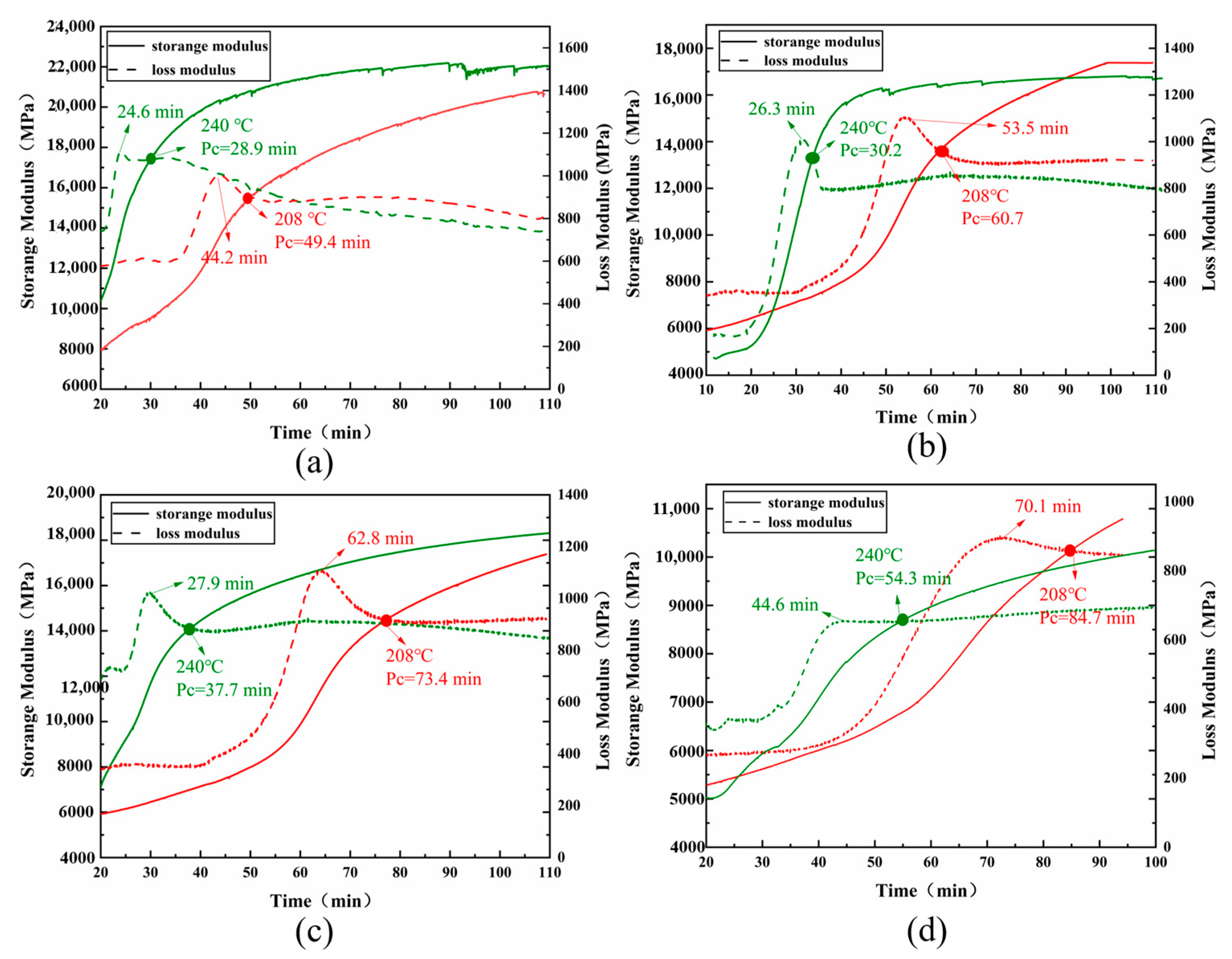
3.3. Curing Behavior and Gelation Point Analysis of BOZ-1N21 Resins
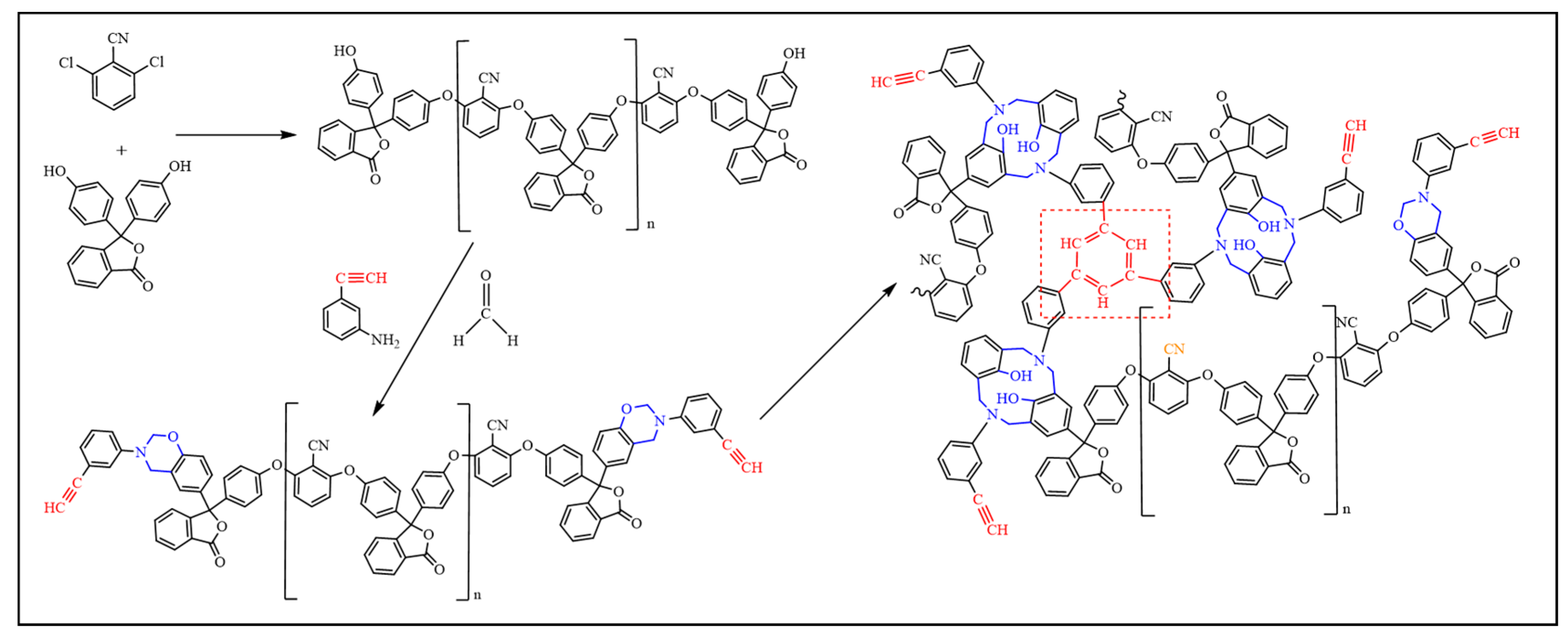
3.4. Curing Mechanism of BOZ-1N Resin
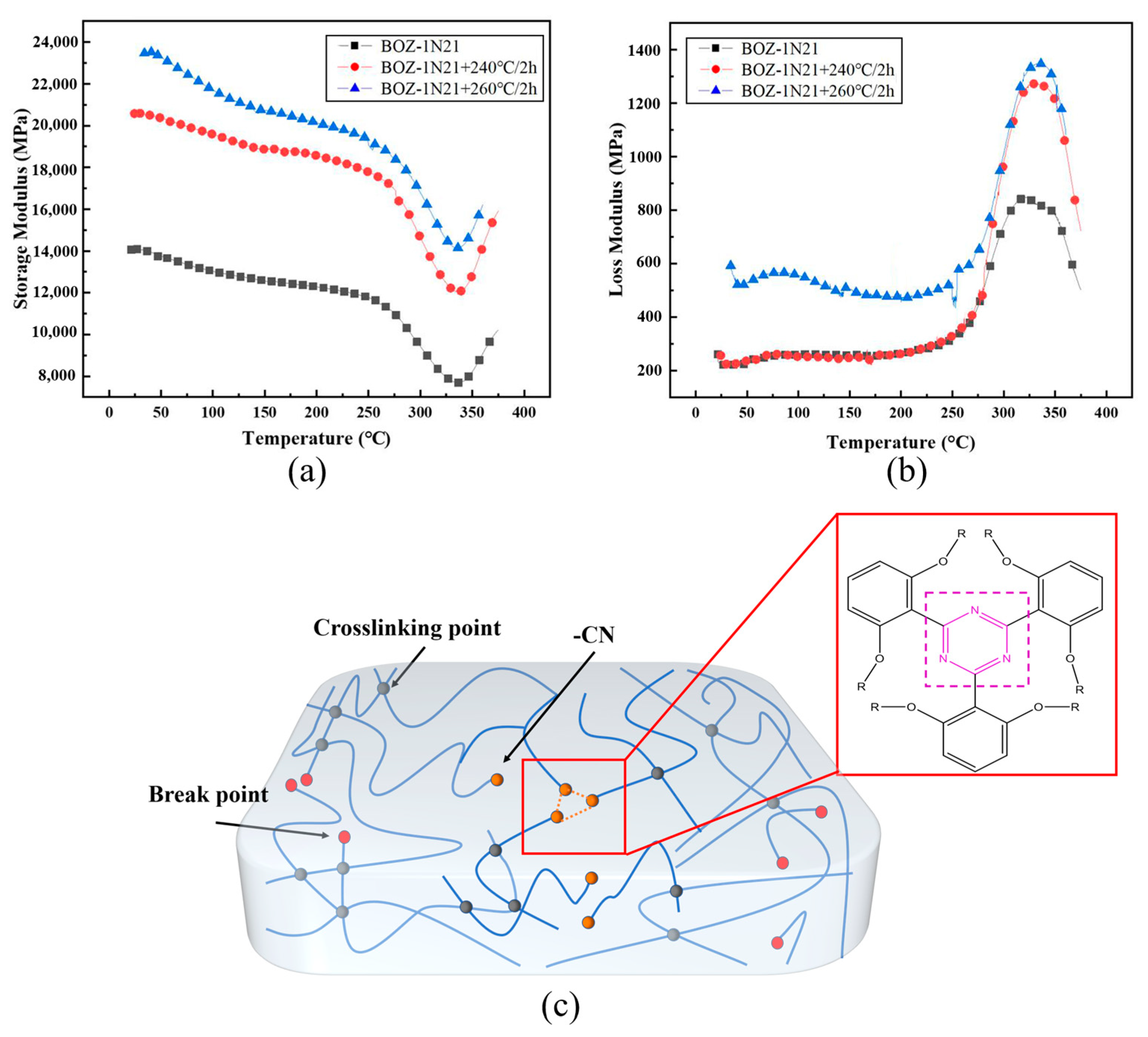
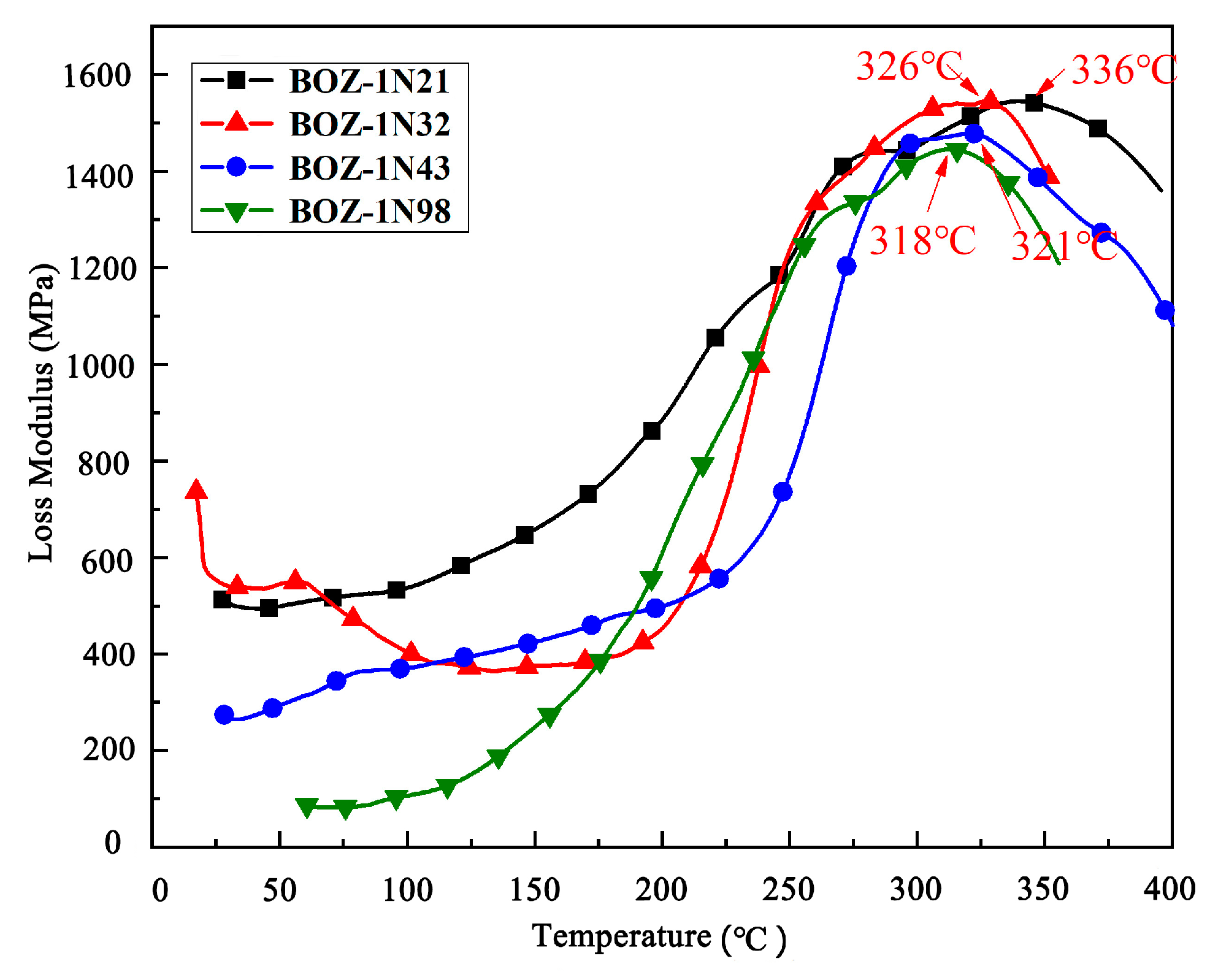
3.5. Dynamic Mechanical Analysis of BOZ-1N Composite Material
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dumas, L.; Bonnaud, L.; Olivier, M.; Poorteman, M.; Dubois, P. Chavicol benzoxazine: Ultrahigh Tg biobased thermoset with tunable extended network. Eur. Polym. J. 2016, 81, 337–346. [Google Scholar] [CrossRef]
- Cao, G.P.; Chen, W.J.; Liu, X.B. Synthesis and thermal properties of the thermosetting resin based on cyano functionalized benzoxazine. Polym. Degrad. Stab. 2008, 93, 739–744. [Google Scholar] [CrossRef]
- Xiang, H.; Ling, H.; Wang, J.; Song, L.; Gu, Y. A novel high performance RTM resin based on benzoxazine. Polym. Compos. 2005, 26, 563–571. [Google Scholar] [CrossRef]
- Yu, L.; Yan, C.; Zhang, X.; Li, H.; Zhu, Y.; Liu, J.; Liao, D. Research on toughening of polybenzoxazine resin by epoxy methylphenyl silicone oil. Mater. Guide 2012, 26, 71–75. [Google Scholar]
- Holly, F.W.; Cope, A.C. Condensation products of aldehydes and ketones with o-aminobenzyl alcohol and o-hydroxybenzylamine. J. Am. Chem. Soc. 1944, 66, 1875–1879. [Google Scholar] [CrossRef]
- Riess, G.; Schwob, J.M.; Guth, G.; Roche, M.; Laude, B. Ring opening polymerization of benzoxazines—A new route to phenolic resins. In Advances in Polymer Synthesis; Springer: Boston, MA, USA, 1985; pp. 27–49. [Google Scholar]
- Nakamura, M.; Hatsuo, I. Synthesis and properties of new crosslinkable telechelics with benzoxazine moiety at the chain end. Polymer 2009, 50, 2688–2695. [Google Scholar] [CrossRef]
- Brunovska, Z.; Lyon, R.; Ishida, H. Thermal properties of phthalocyano functional polybenzoxazines. Thermochim. Acta 2000, 357, 195–203. [Google Scholar] [CrossRef]
- Liu, J.; Agag, T.; Ishida, H. Main-chain benzoxazine oligomers: A new approach for resin transfer moldable neat benzoxazines for high performance applications. Polymer 2010, 51, 5688–5694. [Google Scholar] [CrossRef]
- Agag, T.; Takeichi, T. Synthesis and characterization of novel benzoxazine monomers containing allyl groupss and their high performance thermosets. Macromolecules 2003, 36, 6010–6017. [Google Scholar] [CrossRef]
- Agag, T.; Takeichi, T. Novel benzoxazine monomers containing p-phenyl propargyl ether: Polymerization of monomers and properties of polybenzoxazines. Macromolecules 2001, 34, 7257–7263. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Y. Polybenzoxazine/Bismaleimide Alloys. In Advanced and Emerging Polybenzoxazine Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 301–318. [Google Scholar]
- Jubsilp, C.; Damrongsakkul, S.; Takeichi, T.; Rimdusit, S. Curing kinetics of arylamine-based polyfunctional benzoxazine resins by dynamic differential scanning calorimetry. Thermochim. Acta 2006, 447, 131–140. [Google Scholar] [CrossRef]
- Kim, H.J.; Brunovska, Z.; Ishida, H. Dynamic mechanical analysis on highly thermally stable polybenzoxazines with an acetylene functional groups. J. Appl. Polym. Sci. 1999, 73, 857–862. [Google Scholar] [CrossRef]
- Zhong, Z.X.; Geng, L.Y.; Zhu, J.J.; Yu, Z.M.; Cheng, S.; Liu, L.; Huang, Y. Progress of thermostable resin containing alkynyl and cyanogen. Chem. Bond. 2018, 40, 357–362. [Google Scholar]
- Sha, X.L.; Yuan, L.; Liang, G.; Gu, A. Heat-resistant and robust biobased benzoxazine resins developed with a green synthesis strategy. Polym. Chem. 2021, 12, 432–438. [Google Scholar] [CrossRef]
- Li, P.; Dai, J.; Xu, Y.; Ran, Q.; Gu, Y. A conjugated alkyne functional bicyclic polybenzoxazine with superior heat resistance. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1587–1592. [Google Scholar] [CrossRef]
- Yagci, Y.; Baris, K.; Narendra, N.G. Recent advancement on polybenzoxazine—A newly developed high performance thermoset. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5565–5576. [Google Scholar] [CrossRef]
- Wang, Y.; You, S.; Hu, J.; Zhang, K. Synthesis and properties of benzoxazine monomers bearing both 3-methyltetrahydrophtalimide and cyano groupss: Para-para vs. ortho-ortho. Macromol. Res. 2020, 28, 74–81. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, K. Studies on the isomeric effect of cyano functionality on the polymerization and thermal properties of ortho-norbornene-based benzoxazine resins. J. Polym. Res. 2020, 27, 130. [Google Scholar] [CrossRef]
- Chaisuwan, T.; Ishida, H. High-performance maleimide and cyano-functionalized benzoxazines with good processibility for advanced composites applications. J. Appl. Polym. Sci. 2006, 101, 548–558. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Han, L.; Wang, J.; Ishida, H. Synthesis and thermally induced structural transformation of phthalimide and cyano-functionalized benzoxazine: Toward smart ortho-benzoxazine chemistry for low thermosets. RSC Adv. 2019, 9, 1526–1535. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Ye, J.; Sun, H.; Liu, X. Probing the copolymerization of alkynyl and cyano groupss using monocyclic benzoxazine as model compound. Polymer 2022, 252, 124932. [Google Scholar] [CrossRef]
- Ghetiya, R.M.; Kundariya, D.S.; Parsania, P.H.; Patel, V.A. Synthesis and characterization of cardo bisbenzoxazines and their thermal polymerization. Polym.-Plast. Technol. Eng. 2008, 47, 836–841. [Google Scholar] [CrossRef]
- Laskoski, M.; Schear, M.B.; Neal, A.; Dominguez, D.D.; Ricks-Laskoski, H.L.; Hervey, J.; Keller, T.M. Improved synthesis and properties of aryl ether-based oligomeric phthalocyano resins and polymers. Polymer 2015, 67, 185–191. [Google Scholar] [CrossRef]
- Zhang, K.; Froimowicz, P.; Han, L.; Ishida, H. Hydrogen-bonding characteristics and unique ring-opening polymerization behavior of Ortho-methylol functional benzoxazine. J. Polym. Sci. Part A: Polym. Chem. 2016, 54, 3635–3642. [Google Scholar] [CrossRef]
- Qi, H.; Ren, H.; Pan, G.; Zhuang, Y.; Huang, F.; Du, L. Synthesis and characteristic of polybenzoxazine with phenylnitrile functional group. Polym. Adv. Technol. 2009, 20, 268–272. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.; Wu, H.; Mei, L.; Chen, J.; Qiao, J.; Xi, M.; Wu, W. Preparation and analysis of alkynyl benzoxazines. FRP Compos. Mater. 2009, 5, 69–71. [Google Scholar]
- Li, J.; Ran, Q.; Gu, Y. Synthesis study of 3-acetylene aniline/phenol type benzoxazine. Thermosetting Resin 2017, 32, 13–18. [Google Scholar]
- Catalani, A.; Bonicelli, M.G. Kinetics of the curing reaction of a diglycidyl ether of bisphenol A with a modified polyamine. Thermochim. Acta 2005, 438, 126–129. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Crane, L.W.; Dynes, P.J.; Kaelble, D.H. Analysis of curing kinetics in polymer composites. J. Polym. Sci. Polym. Lett. Ed. 1973, 11, 533–540. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, P.; Zhang, S.; Li, Y.; Gu, Y. Curing kinetics of phenolphthalein–aniline-based benzoxazine investigated by non-isothermal differential scanning calorimetry. J. Therm. Anal. Calorim. 2015, 120, 1755–1764. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Chen, Z.; Duan, X.; Yu, Y.; Sun, L. Curing behavior, thermal, and mechanical properties of N,N′-(4,4′-diphenylmethane)bismaleimide/2,2′-diallylbisphenol A/3-allyl-5,5-dimethylhydantoin resin system. High Perform. Polym. 2020, 32, 631–644. [Google Scholar] [CrossRef]
- Babayevsky, P.G.; Gillham, J.K. Epoxy thermosetting systems: Dynamic mechanical analysis of the reactions of aromatic diamines with the diglycidyl ether of bisphenol A. J. Appl. Polym. Sci. 1973, 17, 2067–2088. [Google Scholar] [CrossRef]
- Wolff, F.; Kugler, C.; Münstedt, H. Time-and temperature-dependent crosslinking behaviour of a silicone resin. Rheol. Acta 2012, 51, 71–80. [Google Scholar] [CrossRef]
- Tung, C.Y.M.; Dynes, P.J. Relationship between viscoelastic properties and gelationation in thermosetting systems. J. Appl. Polym. Sci. 1982, 27, 569–574. [Google Scholar] [CrossRef]
- Xiong, X.; Ren, R.; Cui, X.; Chen, P. Alkynyl-functionalized benzoxazine containing phthalide side groups: Synthesis, characterization and curing mechanism. Polym. Test. 2018, 72, 232–237. [Google Scholar] [CrossRef]
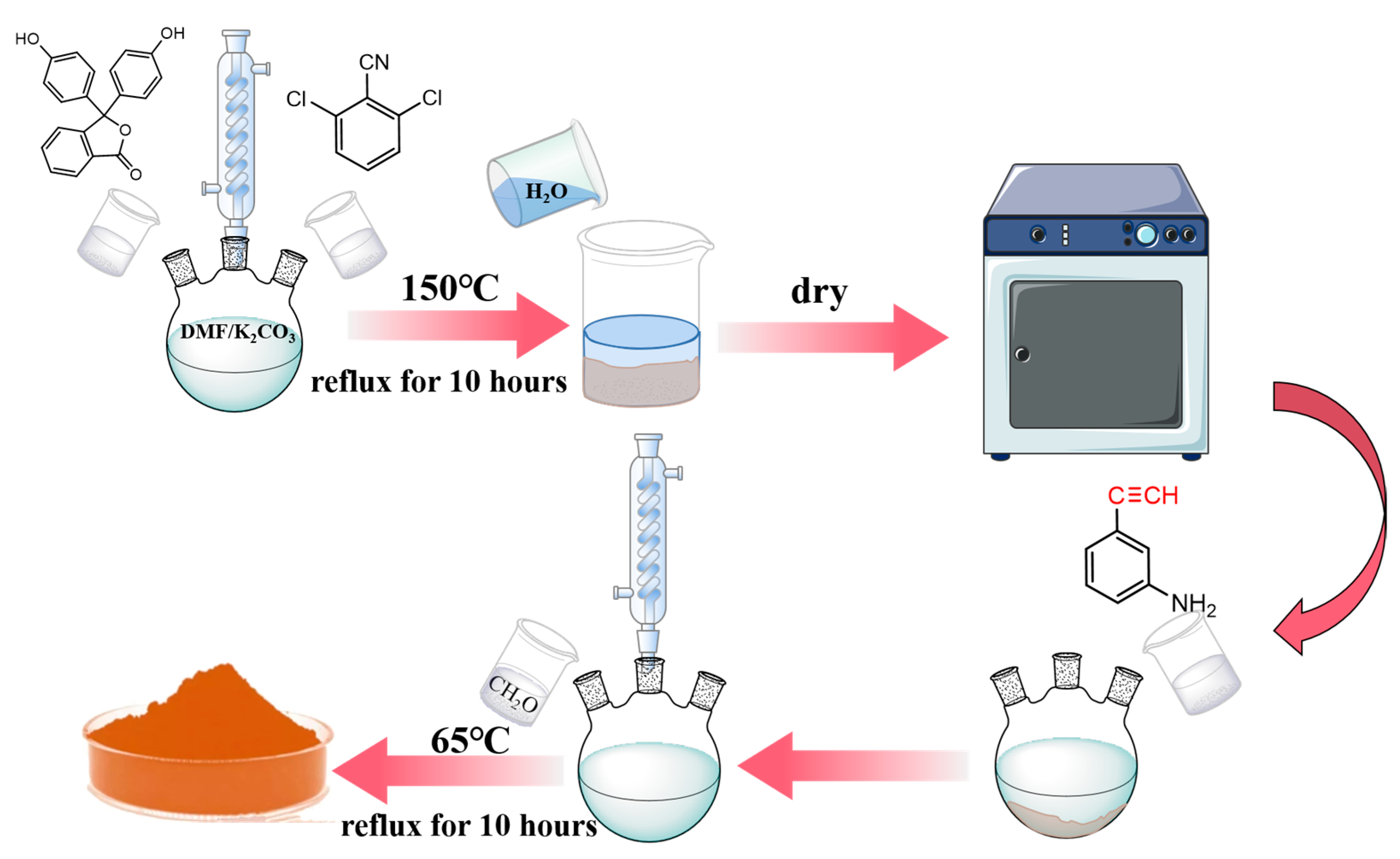



| PP:2,6-Dichlorobenzonitrile | PP (g) | 2,6-Dichlorobenzonitrile (g) | K2CO3 (g) | |
|---|---|---|---|---|
| 1 | 2:1 | 63.6 | 17.2 | 27.6 g |
| 2 | 3:2 | 95.4 | 34.4 | 41.4 g |
| 3 | 4:3 | 63.6 | 25.8 | 27.6 g |
| 4 | 9:8 | 57.24 | 27.52 | 24.84 g |
| Heating Rate (℃/min) | Ti (°C) | Tp (°C) | Tf (°C) |
|---|---|---|---|
| 5 | 184.11 | 214.18 | 248.46 |
| 10 | 197.47 | 224.38 | 262.71 |
| 15 | 201.05 | 232.66 | 268.59 |
| 20 | 202.16 | 236.75 | 280.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, N.; Yang, S.; Xiong, X.; Han, A.; Ren, R.; Wang, J. The Performance and Synthesis of Alkynyl−Functionalized Benzoxazine Containing Phthalide Side Groups and Cyano Groups with Different Molecular Weights. Polymers 2023, 15, 3478. https://doi.org/10.3390/polym15163478
Kang N, Yang S, Xiong X, Han A, Ren R, Wang J. The Performance and Synthesis of Alkynyl−Functionalized Benzoxazine Containing Phthalide Side Groups and Cyano Groups with Different Molecular Weights. Polymers. 2023; 15(16):3478. https://doi.org/10.3390/polym15163478
Chicago/Turabian StyleKang, Nianjun, Shuai Yang, Xuhai Xiong, Anchang Han, Rong Ren, and Jing Wang. 2023. "The Performance and Synthesis of Alkynyl−Functionalized Benzoxazine Containing Phthalide Side Groups and Cyano Groups with Different Molecular Weights" Polymers 15, no. 16: 3478. https://doi.org/10.3390/polym15163478
APA StyleKang, N., Yang, S., Xiong, X., Han, A., Ren, R., & Wang, J. (2023). The Performance and Synthesis of Alkynyl−Functionalized Benzoxazine Containing Phthalide Side Groups and Cyano Groups with Different Molecular Weights. Polymers, 15(16), 3478. https://doi.org/10.3390/polym15163478





