Thermal, Morphological and Mechanical Properties of Multifunctional Composites Based on Biodegradable Polymers/Bentonite Clay: A Review
Abstract
:1. Introduction
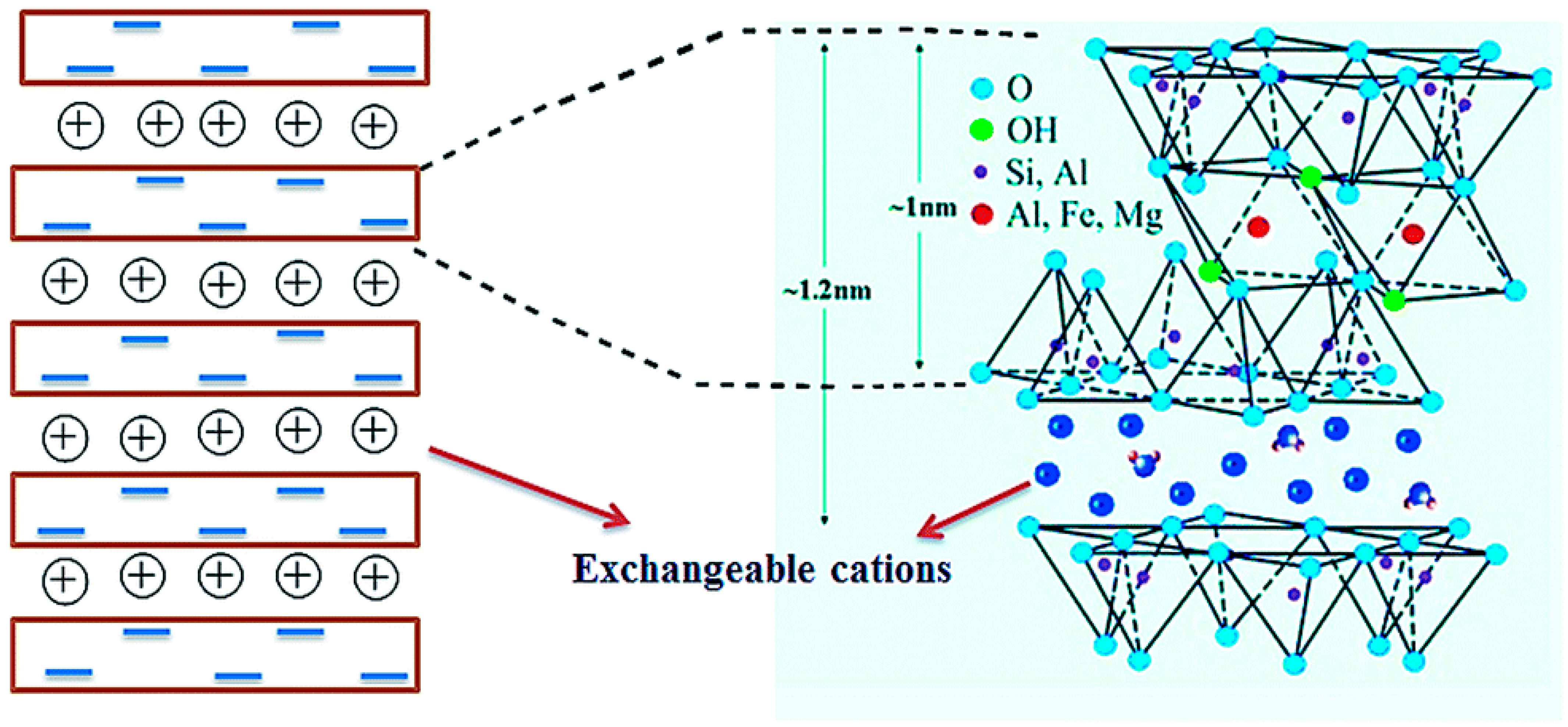
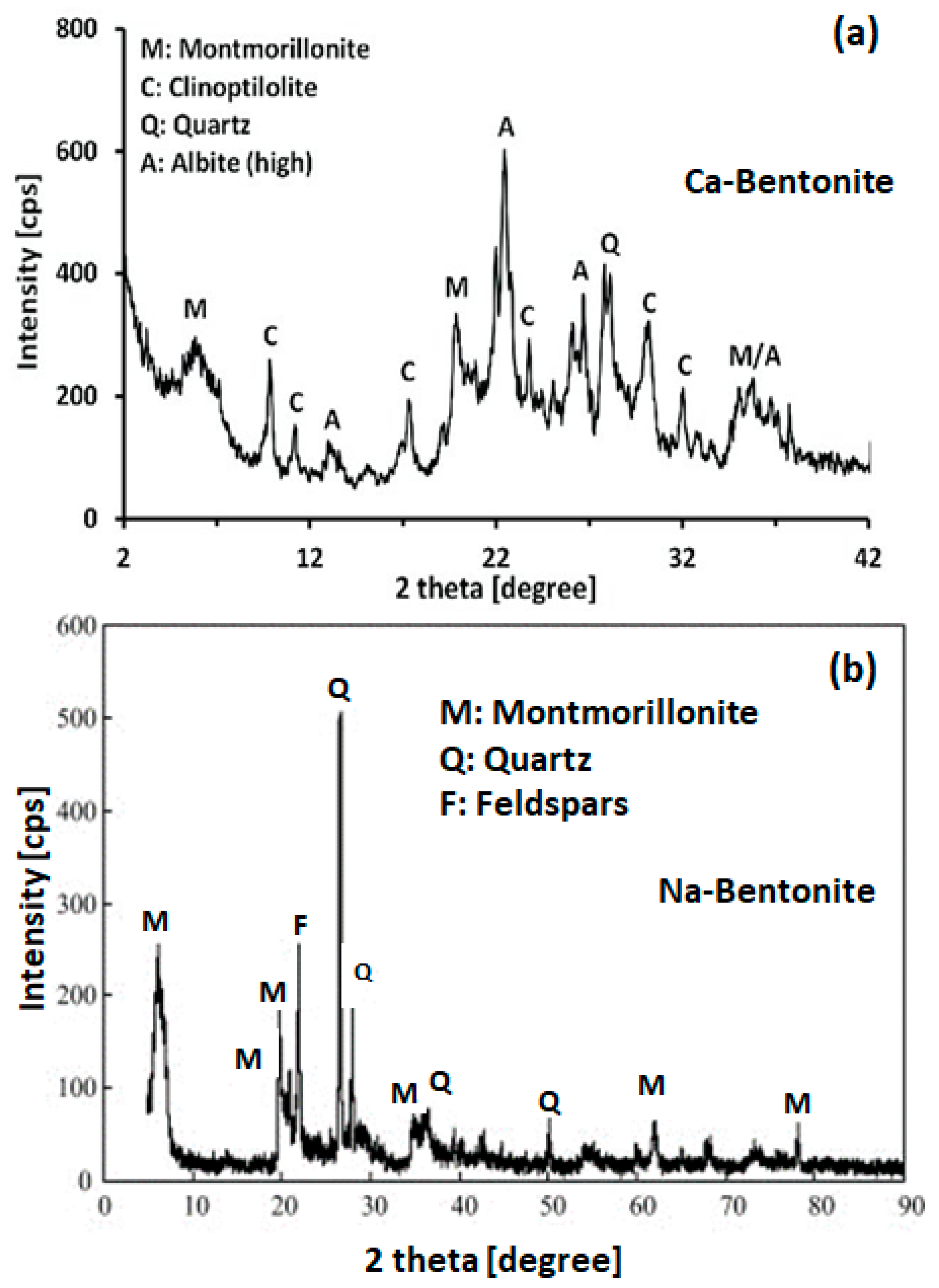
2. Bentonite Clay: Classification, Composition, and Structure
3. Thermal Stability of Bentonite Clay
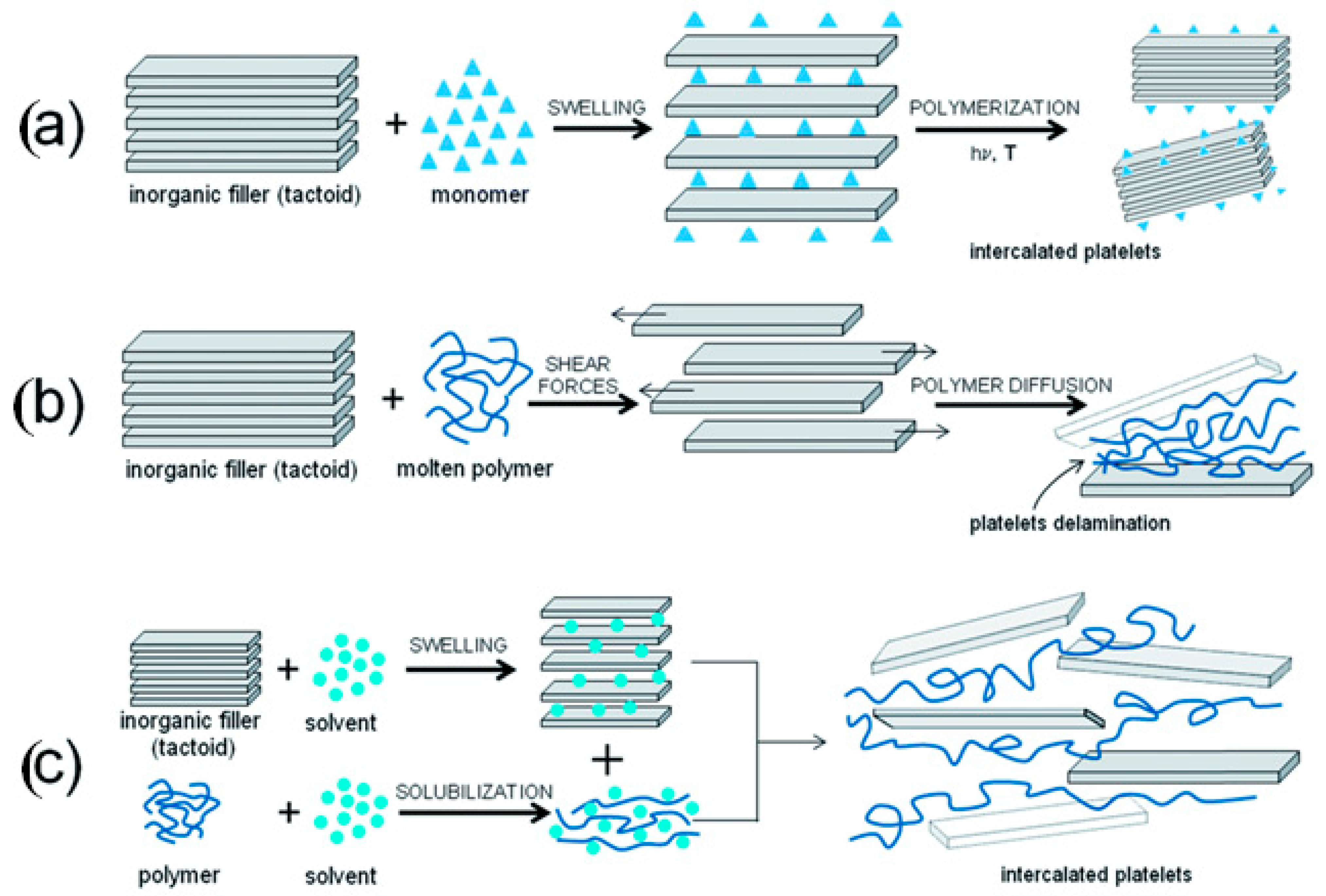
4. Preparation of Biodegradable Polymer/Bentonite Nanocomposites
4.1. Properties of Biodegradable Polymer-Based Bentonite Clay Composites
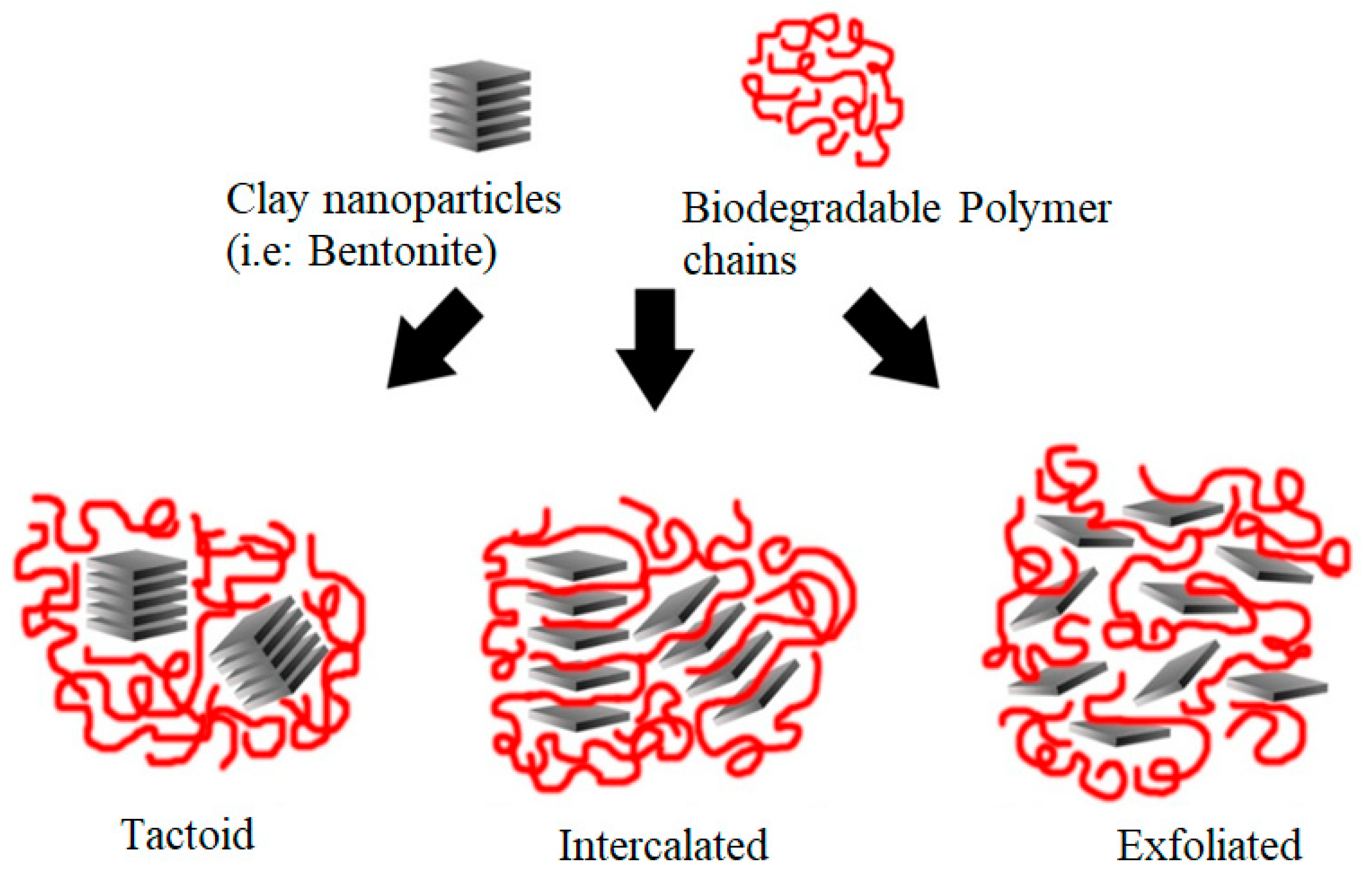
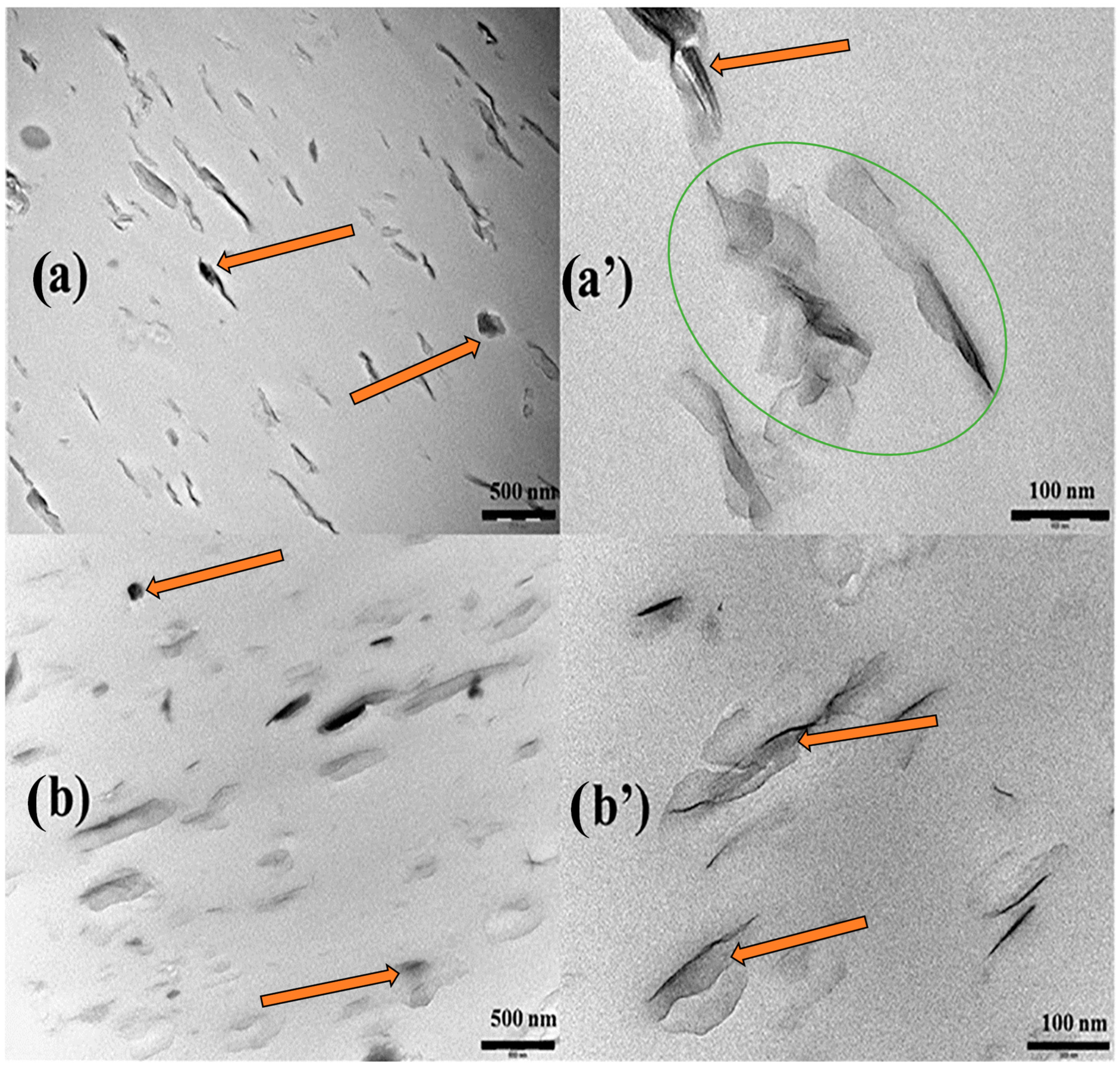
4.2. Microstructural Properties of Biodegradable Polymer-Based Bentonite Clay Materials
4.3. Mechanical Properties of Biodegradable Polymer-Based Bentonite Clay Materials
4.4. Thermal Stability of Biodegradable Polymer/Bentonite Clay Nanocomposites
4.5. Difficulties in Clay/Biodegradable Polymer Nanocomposite Development
4.6. Designing Biodegradable Polymer/Clay Nanocomposites: Theories and Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS | Acrylonitrile–butadiene–styrene |
| BNT | Bentonite |
| [P(AA-DEA)] | Poly(acrylic acid-N, N-diethyl acrylamide) |
| CaB | Calcium bentonites |
| CCP | Coal combustion product |
| kCCP | Hydraulic conductivity |
| ESO | Epoxidized soybean oil |
| MMT | Montmorillonite |
| MB | Modified bentonite |
| OMMT | Organo-montmorillonite |
| OBnt | Organo-modified bentonite |
| HS | Hui–Shia laminated model |
| HT | Halpin–Tsai laminated model |
| Empirical constant to control the stress transfer between the fillers and matrices | |
| Ec | Modulus of the composites |
| Em | Modulus of the matrices |
| Ep | Modulus of the fillers |
| ϕp | Volume fraction of the fillers in the composites |
| ηL | Stress-partitioning factor |
| ξ | Constant depending on the geometry and aspect ratios of the fillers in the composites |
| l | Length of dispersed fillers in the composites |
| t | Thickness/depth of dispersed fillers in the composites |
| σc | Tensile strength of the composites |
| σm | Tensile strength of the matrices |
| ψ | Area fraction in the cross section |
| a | Constant influenced by the particle shape and arrangement in the composites |
| b | Constant influenced by the particle shape and arrangement in the composites |
References
- De Oliveira, C.R.S.; da Silva Júnior, A.H.; Mulinari, J.; Ferreira, A.J.S.; da Silva, A. Fibrous microplastics released from textiles: Occurrence, fate, and remediation strategies. J. Contam. Hydrol. 2023, 256, 104169. [Google Scholar] [CrossRef] [PubMed]
- Miguel Silva, R., Jr.; de Oliveira, T.A.; Araque, L.M.; Alves, T.S.; de Carvalho, L.H.; Barbosa, R. Thermal behavior of biodegradable bionanocomposites: Influence of bentonite and vermiculite clays. J. Mater. Res. Technol. 2019, 8, 3234–3243. [Google Scholar]
- Ilsouk, M.; Raihane, M.; Castelvetro, V.; Lahcini, M.; Bronco, S.; Rhouta, B.; Bianchi, S.; Conzatti, L. Highly thermostable and crystalline poly (butylene adipate) bionanocomposites prepared by in situ polycondensation with organically modified Moroccan beidellite clay. Polym. Int. 2017, 66, 939–949. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y. Recent advances and perspectives on starch nanocomposites for packaging applications. J. Mater. Sci. 2018, 53, 15319–15339. [Google Scholar] [CrossRef]
- Mapossa, A.B.; López-Beceiro, J.; Díaz-Díaz, A.M.; Artiaga, R.; Moyo, D.S.; Mphateng, T.N.; Focke, W.W. Properties of mosquito repellent-plasticized poly (lactic acid) strands. Molecules 2021, 26, 5890. [Google Scholar] [CrossRef] [PubMed]
- Mphateng, T.N.; Mapossa, A.B.; Wesley-Smith, J.; Ramjee, S.; Focke, W.W. Cellulose acetate/organoclay nanocomposites as controlled release matrices for pest control applications. Cellulose 2022, 29, 3915–3933. [Google Scholar] [CrossRef]
- Tripathi, J.; Ambolikar, R.; Gupta, S.; Variyar, P.S. Preparation and characterization of methylated guar gum based nano-composite films. Food Hydrocoll. 2022, 124, 107312. [Google Scholar] [CrossRef]
- Ollier, R.P.; Casado, U.; Torres Nicolini, A.; Alvarez, V.A.; Pérez, C.J.; Ludueña, L.N. Improved creep performance of melt-extruded polycaprolactone/organo-bentonite nanocomposites. J. Appl. Polym. Sci. 2021, 138, 50961. [Google Scholar] [CrossRef]
- Ray, S.; Bhowmick, A.K.; Sarma, K.S.S.; Majali, A.B.; Tikku, V.K. Characterization of electron-beam-modified surface coated clay fillers and their influence on physical properties of rubbers. Radiat. Phys. Chem. 2002, 65, 627–640. [Google Scholar] [CrossRef]
- Horch, R.A.; Shahid, N.; Mistry, A.S.; Timmer, M.D.; Mikos, A.G.; Barron, A.R. Nanoreinforcement of poly (propylene fumarate)-based networks with surface modified alumoxane nanoparticles for bone tissue engineering. Biomacromolecules 2004, 5, 1990–1998. [Google Scholar] [CrossRef]
- Maiti, P.; Batt, C.A.; Giannelis, E.P. New biodegradable polyhydroxybutyrate/layered silicate nanocomposites. Biomacromolecules 2007, 8, 3393–3400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, G.; Abou-Hussein, R.; Allen, W.M.; Noda, I.; Mark, J.E. Biodegradable nanocomposites based on the polyester poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) and layered silicate or expanded graphite. J. Macromol. Sci. Part A 2008, 45, 431–439. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Q.; Mark, J.E.; Noda, I. A novel biodegradable nanocomposite based on poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) and silylated kaolinite/silica core–shell nanoparticles. Appl. Clay Sci. 2009, 46, 51–56. [Google Scholar] [CrossRef]
- LeBaron, P.C.; Wang, Z.; Pinnavaia, T.J. Polymer-layered silicate nanocomposites: An overview. Appl. Clay Sci. 1999, 15, 11–29. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, G.; Abou-Hussein, R.; Hassan, M.K.; Noda, I.; Mark, J.E. Some novel layered-silicate nanocomposites based on a biodegradable hydroxybutyrate copolymer. Eur. Polym. J. 2007, 43, 3128–3135. [Google Scholar] [CrossRef]
- Zini, E.; Focarete, M.L.; Noda, I.; Scandola, M. Bio-composite of bacterial poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) reinforced with vegetable fibers. Compos. Sci. Technol. 2007, 67, 2085–2094. [Google Scholar] [CrossRef]
- Ansarifar, A.; Azhar, A.; Ibrahim, N.; Shiah, S.F.; Lawton, J.M.D. The use of a silanised silica filler to reinforce and crosslink natural rubber. Int. J. Adhes. Adhes. 2005, 25, 77–86. [Google Scholar] [CrossRef]
- Tiwari, R.R.; Khilar, K.C.; Natarajan, U. Synthesis and characterization of novel organo-montmorillonites. Appl. Clay Sci. 2008, 38, 203–208. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Kausar, A.; Rizwan, M.; Sarwar, M.I. Probing the role of surface treated montmorillonite on the properties of semi-aromatic polyamide/clay nanocomposites. Appl. Surf. Sci. 2008, 255, 2080–2086. [Google Scholar] [CrossRef]
- Kotal, M.; Bhowmick, A.K. Polymer nanocomposites from modified clays: Recent advances and challenges. Prog. Polym. Sci. 2015, 51, 127–187. [Google Scholar] [CrossRef]
- Abdallah, W.; Yilmazer, U. Novel thermally stable organo-montmorillonites from phosphonium and imidazolium surfactants. Thermochim. Acta 2011, 525, 129–140. [Google Scholar] [CrossRef]
- Sanavada, K.; Shah, M.; Gandhi, D.; Unnarkat, A.; Vaghasiya, P. A Systematic and Comprehensive Study of Eco-friendly Bentonite Clay Application in Esterification and Wastewater Treatment. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100784. [Google Scholar] [CrossRef]
- Şahin, Ö.; Kaya, M.; Saka, C. Plasma-surface modification on bentonite clay to improve the performance of adsorption of methylene blue. Appl. Clay Sci. 2015, 116, 46–53. [Google Scholar] [CrossRef]
- Ollier, R.; Rodriguez, E.; Alvarez, V. Unsaturated polyester/bentonite nanocomposites: Influence of clay modification on final performance. Compos. A Appl. Sci. 2013, 48, 137–143. [Google Scholar] [CrossRef]
- Hedley, C.B.; Yuan, G.; Theng, B. Thermal analysis of montmorillonites modified with quaternary phosphonium and ammonium surfactants. Appl. Clay Sci. 2007, 35, 180–188. [Google Scholar] [CrossRef]
- Bala, P.; Samantaraya, B.K.; Srivastava, S.K. Synthesis and characterization of Na-montmorillonite-alkylammonium intercalation compounds. Mater. Res. Bull. 2000, 35, 1717–1724. [Google Scholar] [CrossRef]
- Xi, Y.; Frost, R.L.; He, H. Modification of the surfaces of Wyoming montmorillonite by the cationic surfactants alkyl trimethyl, dialkyl dimethyl, and trialkyl methyl ammonium bromides. J. Colloid Interface Sci. 2007, 305, 150–158. [Google Scholar] [CrossRef]
- Xie, W.; Gao, Z.; Pan, W.P.; Hunter, D.; Singh, A.; Vaia, R. Thermal degradation chemistry of alkyl quaternary ammonium montmorillonite. Chem. Mater. 2001, 13, 2979–2990. [Google Scholar] [CrossRef]
- Drown, E.K.; Mohanty, A.K.; Parulekar, Y.; Hasija, D.; Harte, B.R.; Misra, M.; Kurian, J.V. The surface characteristics of organoclays and their effect on the properties of poly (trimethylene terephthalate) nanocomposites. Compos. Sci. Technol. 2007, 67, 3168–3175. [Google Scholar] [CrossRef]
- Franco-Urquiza, E.A. Clay-Based Polymer Nanocomposites: Essential Work of Fracture. Polymers 2021, 13, 2399. [Google Scholar] [CrossRef]
- Hebbar, R.S.; Isloor, A.M.; Ismail, A.F. Preparation and evaluation of heavy metal rejection properties of polyetherimide/porous activated bentonite clay nanocomposite membrane. RSC Adv. 2014, 4, 47240–47248. [Google Scholar] [CrossRef]
- Muhammad, N.; Siddiqua, S. Calcium bentonite vs sodium bentonite: The potential of calcium bentonite for soil foundation. Mater. Today Proc. 2022, 48, 822–827. [Google Scholar] [CrossRef]
- Fernández, A.M.; Marco, J.F.; Nieto, P.; León, F.J.; Robredo, L.M.; Clavero, M.Á.; Cardona, A.I.; Fernández, S.; Svensson, D.; Sellin, P. Characterization of Bentonites from the In Situ ABM5 Heater Experiment at Äspö Hard Rock Laboratory, Sweden. Minerals 2022, 12, 471. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Y.; Zhang, M.; Zhang, C.; Zhang, J.; Wang, C.; Zhang, N. The enhancement effect of Ca-bentonite on the working performance of red mud-slag based geopolymeric grout. Mater. Chem. Phys. 2022, 276, 125311. [Google Scholar] [CrossRef]
- Kumar, A.; Lingfa, P. Sodium bentonite and kaolin clays: Comparative study on their FT-IR, XRF, and XRD. Mater. Today Proc. 2020, 22, 737–742. [Google Scholar] [CrossRef]
- Choo, H.; Choi, Y.; Lee, W.; Lee, C. Effect of pH variations on the yield stress of calcium bentonite slurry treated with pH-responsive polymer. Materials 2020, 13, 2525. [Google Scholar] [CrossRef]
- Zhirong, L.; Uddin, M.A.; Zhanxue, S. FT-IR and XRD analysis of natural Na-bentonite and Cu (II)-loaded Na-bentonite. Spectrochim. Acta A 2011, 79, 1013–1016. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, H.K. Characterization of organobentonite used for polymer nanocomposites. Mater. Chem. Phys. 2004, 85, 410–415. [Google Scholar] [CrossRef]
- Massinga, P.H., Jr.; Focke, W.W.; De Vaal, P.L.; Atanasova, M. Alkyl ammonium intercalation of Mozambican bentonite. Appl. Clay Sci. 2010, 49, 142–148. [Google Scholar] [CrossRef]
- Zilg, C.; Mülhaupt, R.; Finter, J. Morphology and toughness/stiffness balance of nanocomposites based upon anhydride-cured epoxy resins and layered silicates. Macromol. Chem. Phys. 1999, 200, 661–670. [Google Scholar] [CrossRef]
- Ramos Filho, F.G.; Mélo, T.J.A.; Rabello, M.S.; Silva, S.M. Thermal stability of nanocomposites based on polypropylene and bentonite. Polym. Degrad. Stab. 2005, 89, 383–392. [Google Scholar] [CrossRef]
- Unalan, I.U.; Cerri, G.; Marcuzzo, E.; Cozzolino, C.A.; Farris, S. Nanocomposite films and coatings using inorganic nanobuilding blocks (NBB): Current applications and future opportunities in the food packaging sector. RSC Adv. 2014, 4, 29393–29428. [Google Scholar] [CrossRef]
- Gou, J.; Zhuge, J.; Liang, F. Processing of polymer nanocomposites. In Manufacturing Techniques for Polymer Matrix Composites (PMCs); Elsevier: Amsterdam, The Netherlands, 2012; pp. 95–119. [Google Scholar]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar]
- Devasenapathi, V.; Monish, P.; Balasivanandha Prabu, S. Experimental investigation of tensile creep behavior of polymer nanocomposites. Int. J. Adv. Manuf. Technol. 2009, 44, 412–418. [Google Scholar] [CrossRef]
- Soares, B.G.; Celestino, M.L.; Magioli, M.; Moreira, V.X.; Khastgir, D. Synthesis of conductive adhesives based on epoxy resin and polyaniline. DBSA using the in situ polymerization and physical mixing procedures. Synth. Met. 2010, 160, 1981–1986. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Pinto, J.C.; Cabral-Albuquerque, E.C.D.M.; Fialho, R.L.L. Coating of urea granules by in situ polymerization in fluidized bed reactors. Polímeros 2019, 29. [Google Scholar] [CrossRef]
- Nivasu, V.M.; Reddy, T.T.; Tammishetti, S. In situ polymerizable polyethyleneglycol containing polyesterpolyol acrylates for tissue sealant applications. Biomaterials 2004, 25, 3283–3291. [Google Scholar] [CrossRef]
- Chafran, L.; Carfagno, A.; Altalhi, A.; Bishop, B. Green Hydrogel Synthesis: Emphasis on Proteomics and Polymer Particle-Protein Interaction. Polymers 2022, 14, 4755. [Google Scholar] [CrossRef]
- Lee, S.; Kim, C.H.; Park, J.K. Improvement of processability of clay/polylactide nanocomposites by a combinational method: In situ polymerization of l-lactide and melt compounding of polylactide. J. Appl. Polym. Sci. 2006, 101, 1664–1669. [Google Scholar] [CrossRef]
- Nabgui, A.; El Assimi, T.; El Meziane, A.; Luinstra, G.A.; Raihane, M.; Gouhier, G.; Lahcini, M. Synthesis and antibacterial behavior of bio-composite materials-based on poly (ε-caprolactone)/bentonite. Eur. Polym. J. 2021, 156, 110602. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Jiang, L.; Tong, S.; Li, Y.-C.; Pu, H.; Zhao, Q. Straightforward preparation of Ca-bentonite/polymer nanocomposite by confining salt-resistant copolymers into montmorillonite interlayers. Polymer 2022, 263, 125519. [Google Scholar] [CrossRef]
- Naz, A.; Chowdhury, A. Pollutant extraction from water and soil using Montmorillonite clay-polymer composite: A rapid review. Mater. Today Proc. 2022, 60, 1–7. [Google Scholar] [CrossRef]
- Omanović-Mikličanin, E.; Badnjević, A.; Kazlagić, A.; Hajlovac, M. Nanocomposites: A brief review. Health Technol. 2020, 10, 51–59. [Google Scholar] [CrossRef]
- Ray, J.R.; Shabtai, I.A.; Teixidó, M.; Mishael, Y.G.; Sedlak, D.L. Polymer-clay composite geomedia for sorptive removal of trace organic compounds and metals in urban stormwater. Water Res. 2019, 157, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Manna, S.; Behera, A.K.; Shee, M.; Basak, P.; Sharma, A.K. Current synthesis and characterization techniques for clay-based polymer nano-composites and its biomedical applications: A review. Environ. Res. 2022, 212, 113534. [Google Scholar] [CrossRef] [PubMed]
- Paramitha, T.; Wonoputri, V.; Sitompul, D.S.D.; Lee, H.W.; Sitompul, J.P. Properties of clays reinforced PLA nanocomposites by melt extrusion technique. Malays. J. Fundam. Appl. Sci. 2020, 16, 453–457. [Google Scholar] [CrossRef]
- Gardi, I.; Mishael, Y.-G.; Lindahl, M.; Muro-Pastor, A.M.; Undabeytia, T. Coagulation-flocculation of Microcystis aeruginosa by polymer-clay based composites. J. Clean. Prod. 2023, 394, 136356. [Google Scholar] [CrossRef]
- Zvulunov, Y.; Ben-Barak-Zelas, Z.; Fishman, A.; Radian, A. A self-regenerating clay-polymer-bacteria composite for formaldehyde removal from water. Chem. Eng. J. 2019, 374, 1275–1285. [Google Scholar] [CrossRef]
- Yang, J.; Huang, B.; Lin, M. Adsorption of hexavalent chromium from aqueous solution by a chitosan/bentonite composite: Isotherm, kinetics, and thermodynamics studies. J. Chem. Eng. Data 2020, 65, 2751–2763. [Google Scholar] [CrossRef]
- Dotto, G.L.; Rodrigues, F.K.; Tanabe, E.H.; Fröhlich, R.; Bertuol, D.A.; Martins, T.R.; Foletto, E.L. Development of chitosan/bentonite hybrid composite to remove hazardous anionic and cationic dyes from colored effluents. J. Environ. Chem. Eng. 2016, 4, 3230–3239. [Google Scholar] [CrossRef]
- Hristodor, C.M.; Vrinceanu, N.; Pui, A.; Novac, O.; Copcia, V.E.; Popovici, E. Textural and morphological characterization of chitosan/bentonite nanocomposite. Environ. Eng. Manag. J. 2012, 11, 573–578. [Google Scholar] [CrossRef]
- Devi, N.; Dutta, J. Preparation and characterization of chitosan-bentonite nanocomposite films for wound healing application. Int. J. Biol. Macromol. 2017, 104, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.L.; Rosa, P.D.T.V.; Realinho, V.; Antunes, M.; Velasco, J.I.; Morales, A.R. Influence of chemical composition of Brazilian organoclays on the morphological, structural and thermal properties of PLA-organoclay nanocomposites. Appl. Clay Sci. 2019, 180, 105186. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. NPJ Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Solarski, S.; Ferreira, M.; Devaux, E.; Fontaine, G.; Bachelet, P.; Bourbigot, S.; Dubois, P. Designing polylactide/clay nanocomposites for textile applications: Effect of processing conditions, spinning, and characterization. J. Appl. Polym. Sci. 2008, 109, 841–851. [Google Scholar] [CrossRef]
- Shanmathy, M.; Mohanta, M.; Thirugnanam, A. Development of biodegradable bioplastic films from Taro starch reinforced with bentonite. Carbohydr. Polym. 2021, 2, 100173. [Google Scholar] [CrossRef]
- Tan, S.X.; Andriyana, A.; Ong, H.C.; Lim, S.; Pang, Y.L.; Ngoh, G.C. A comprehensive review on the emerging roles of nanofillers and plasticizers towards sustainable starch-based bioplastic fabrication. Polymers 2022, 14, 664. [Google Scholar] [CrossRef]
- Tjong, S.C. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R Rep. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Saha, P.; Adhikari, J.; Deshmukh, K.; Ahamed, M.B.; Cabibihan, J.J. Recent advances in mechanical properties of biopolymer composites: A review. Polym. Compos. 2020, 41, 32–59. [Google Scholar] [CrossRef]
- Paspali, A.; Bao, Y.; Gawne, D.T.; Piestert, F.; Reinelt, S. The influence of nanostructure on the mechanical properties of 3D printed polylactide/nanoclay composites. Compos. B Eng. 2018, 152, 160–168. [Google Scholar] [CrossRef]
- Lee, D.C.; Jang, L.W. Preparation and characterization of PMMA–clay hybrid composite by emulsion polymerization. J. Appl. Polym. Sci. 1996, 61, 1117–1122. [Google Scholar] [CrossRef]
- Hasegawa, N.; Kawasumi, M.; Kato, M.; Usuki, A.; Okada, A. Preparation and mechanical properties of polypropylene-clay hybrids using a maleic anhydride-modified polypropylene oligomer. J. Appl. Polym. Sci. 1998, 67, 87–92. [Google Scholar] [CrossRef]
- Petersson, L.; Oksman, K. Biopolymer based nanocomposites: Comparing layered silicates and microcrystalline cellulose as nanoreinforcement. Compos. Sci. Technol. 2006, 66, 2187–2196. [Google Scholar] [CrossRef]
- Behera, L.; Mohanta, M.; Thirugnanam, A. Intensification of yam-starch based biodegradable bioplastic film with bentonite for food packaging application. Environ. Technol. Innov. 2022, 25, 102180. [Google Scholar] [CrossRef]
- Luduena, L.N.; Alvarez, V.A.; Vazquez, A. Processing and microstructure of PCL/clay nanocomposites. Mater. Sci. Eng. A 2007, 460, 121–129. [Google Scholar] [CrossRef]
- Petersson, L.; Mathew, A.P.; Oksman, K. Dispersion and properties of cellulose nanowhiskers and layered silicates in cellulose acetate butyrate nanocomposites. J. Appl. Polym. Sci. 2009, 112, 2001–2009. [Google Scholar] [CrossRef]
- Petersson, L.; Oksman, K.; Mathew, A.P. Using maleic anhydride grafted poly (lactic acid) as a compatibilizer in poly (lactic acid)/layered-silicate nanocomposites. J. Appl. Polym. Sci. 2006, 102, 1852–1862. [Google Scholar] [CrossRef]
- Ray, S.S.; Bousmina, M. Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Prog. Mater. Sci. 2005, 50, 962–1079. [Google Scholar]
- Paul, M.A.; Alexandre, M.; Degée, P.; Henrist, C.; Rulmont, A.; Dubois, P. New nanocomposite materials based on plasticized poly (L-lactide) and organo-modified montmorillonites: Thermal and morphological study. Polymer 2003, 44, 443–450. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Armentano, I.; Puglia, D.; Luzi, F.; Arciola, C.R.; Morena, F.; Martino, S.; Torre, L. Nanocomposites based on biodegradable polymers. Materials 2018, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, D. Can nanoparticles really enhance thermal stability of polymers? Part II: An overview on thermal decomposition of polycondensation polymers. Thermochim. Acta 2011, 523, 25–45. [Google Scholar] [CrossRef]
- El Assimi, T.; Blažic, R.; El Kadib, A.; Raihane, M.; Beniazza, R.; Luinstra, G.A.; Lahcini, M. Synthesis of poly (ε-caprolactone)-grafted guar gum by surface-initiated ring-opening polymerization. Carbohydr. Polym. 2019, 220, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Lahcini, M.; Elhakioui, S.; Szopinski, D.; Neuer, B.; El Kadib, A.; Scheliga, F.; Luinstra, G.A. Harnessing synergies in tin-clay catalyst for the preparation of poly (ε-caprolactone)/halloysite nanocomposites. Eur. Polym. J. 2016, 81, 1–11. [Google Scholar] [CrossRef]
- Bari, S.S.; Chatterjee, A.; Mishra, S. Biodegradable polymer nanocomposites: An overview. Polym. Rev. 2016, 56, 287–328. [Google Scholar] [CrossRef]
- Moja, T.N.; Bunekar, N.; Mishra, S.B.; Tsai, T.Y.; Hwang, S.S.; Mishra, A.K. Melt processing of polypropylene-grafted-maleic anhydride/Chitosan polymer blend functionalized with montmorillonite for the removal of lead ions from aqueous solutions. Sci. Rep. 2020, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, H.; Ramazani, A.S.A.; Nikkhah, S.J. Investigation of in situ prepared polypropylene/clay nanocomposites properties and comparing to melt blending method. Mater. Des. 2010, 31, 76–84. [Google Scholar] [CrossRef]
- Castillo-Pérez, R.; Hernández-Vargas, M.L.; Flores-Cedillo, O.; Campillo-Illanes, B.F. Effect on thermo-mechanical properties by in-situ emulsion polymerization of polymer/clay nanocomposites. Polym. Compos. 2019, 40, 263–276. [Google Scholar] [CrossRef]
- Bitinis, N.; Hernández, M.; Verdejo, R.; Kenny, J.M.; Lopez-Manchado, M.A. Recent advances in clay/polymer nanocomposites. Adv. Mater. 2011, 23, 5229–5236. [Google Scholar] [CrossRef] [PubMed]
- Piekarska, K.; Sowinski, P.; Piorkowska, E.; Haque, M.M.U.; Pracella, M. Structure and properties of hybrid PLA nanocomposites with inorganic nanofillers and cellulose fibers. Compos. Part A Appl. Sci. 2016, 82, 34–41. [Google Scholar] [CrossRef]
- Sessini, V.; Arrieta, M.P.; Raquez, J.M.; Dubois, P.; Kenny, J.M.; Peponi, L. Thermal and composting degradation of EVA/Thermoplastic starch blends and their nanocomposites. Polym. Degrad. Stab. 2019, 159, 184–198. [Google Scholar] [CrossRef]
- Shabtai, I.A.; Mishael, Y.G. Catalytic polymer-clay composite for enhanced removal and degradation of diazinon. J. Hazard. Mater. 2017, 335, 135–142. [Google Scholar] [CrossRef]
- Lvov, Y.; Abdullayev, E. Functional polymer–clay nanotube composites with sustained release of chemical agents. Prog. Polym. Sci. 2013, 38, 1690–1719. [Google Scholar] [CrossRef]
- Salam, H.; Dong, Y. Theoretical Modelling Analysis on Tensile Properties of Bioepoxy/Clay Nanocomposites Using Epoxidised Soybean Oils. J. Nanomater. 2019, 2019, 4074869. [Google Scholar] [CrossRef]
- Djebara, Y.; El Moumen, A.; Kanit, T.; Madani, S.; Imad, A. Modeling of the effect of particles size, particles distribution and particles number on mechanical properties of polymer-clay nano-composites: Numerical homogenization versus experimental results. Compos. Part B Eng. 2016, 86, 135–142. [Google Scholar] [CrossRef]
- Mahadeva Raju, G.K.; Dakshayini, B.S.; Madhu, G.M.; Ameen Khan, M.; Dinesh Sankar Reddy, P. Characterizing and Modeling of Mechanical Properties of Epoxy Polymer Composites Reinforced with Bentonite Clay. Mater. Today Proc. 2018, 5, 28098–28107. [Google Scholar] [CrossRef]
- Hirsch, T.J. Modulus of Elasticity iof Concrete Affected by Elastic Moduli of Cement Paste Matrix and Aggregate. ACI J. Proc. 1962, 59, 427–452. [Google Scholar]
- Affdl, J.C.H.; Kardos, J.L. The Halpin-Tsai equations: A review. Polym. Eng. Sci. 1976, 16, 344–352. [Google Scholar] [CrossRef]
- Danusso, F.; Tieghi, G. Strength versus composition of rigid matrix particulate composites. Polymer 1986, 27, 1385–1390. [Google Scholar] [CrossRef]
- Nicolais, L.; Narkis, M. Stress-strain behavior of styrene-acrylonitrile/glass bead composites in the glassy region. Polym. Eng. Sci. 1971, 11, 194–199. [Google Scholar] [CrossRef]
- Seidi, A.; Benhamou, M.; Khalil, D. Exact scaling of adsorption isotherms of clay-polymer composites: Comparison to experiment. Comput. Mater. Sci. 2023, 219, 111994. [Google Scholar] [CrossRef]
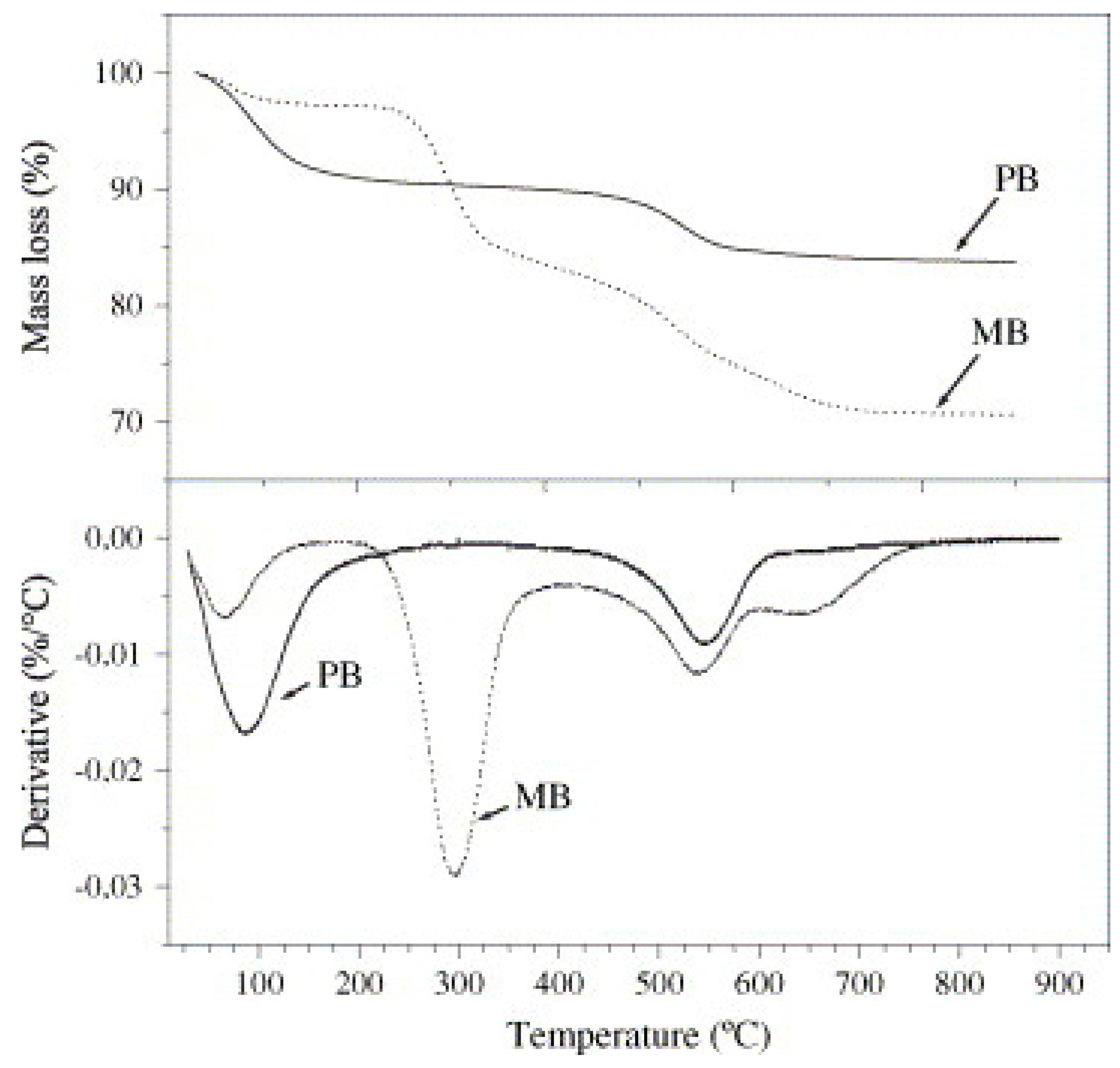
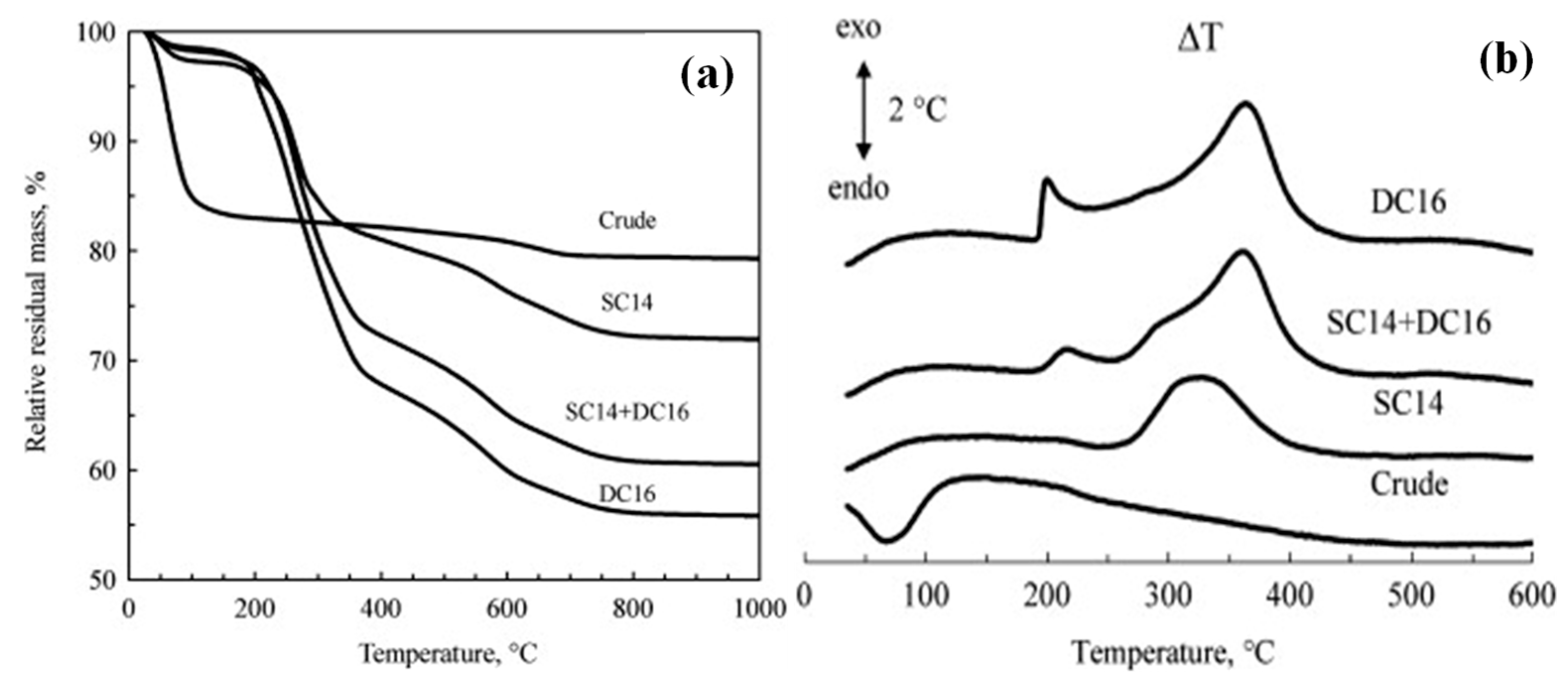
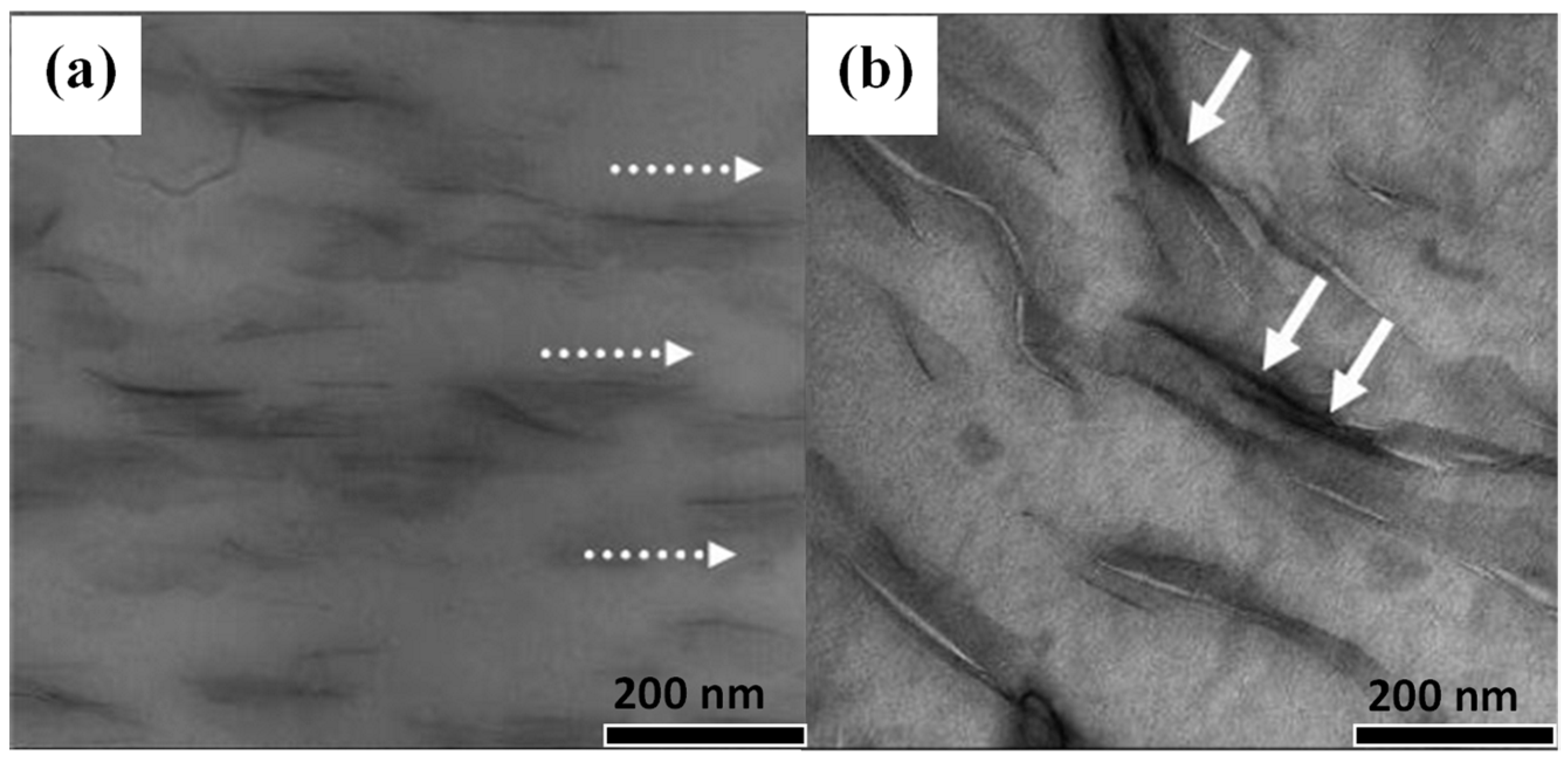
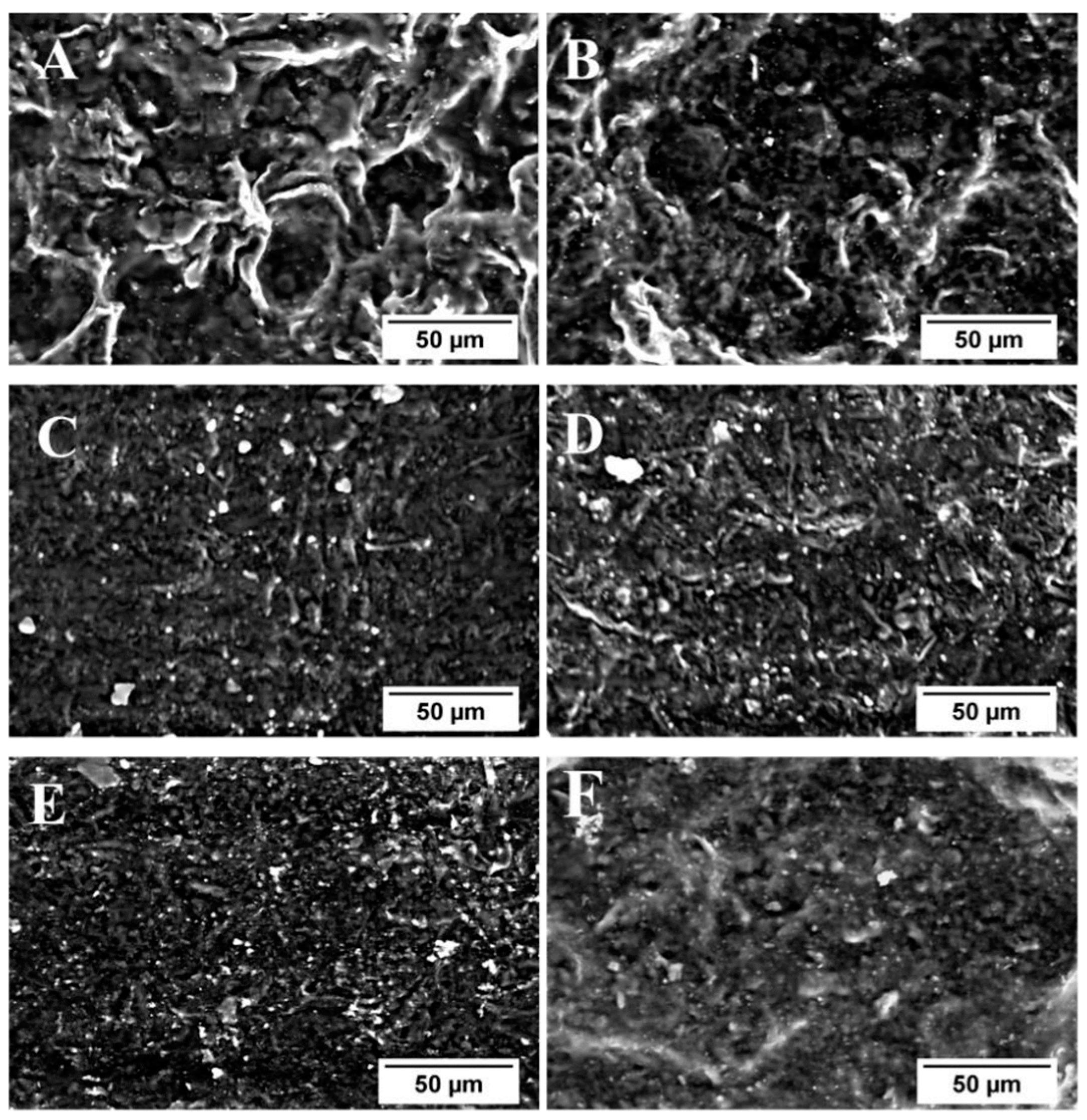
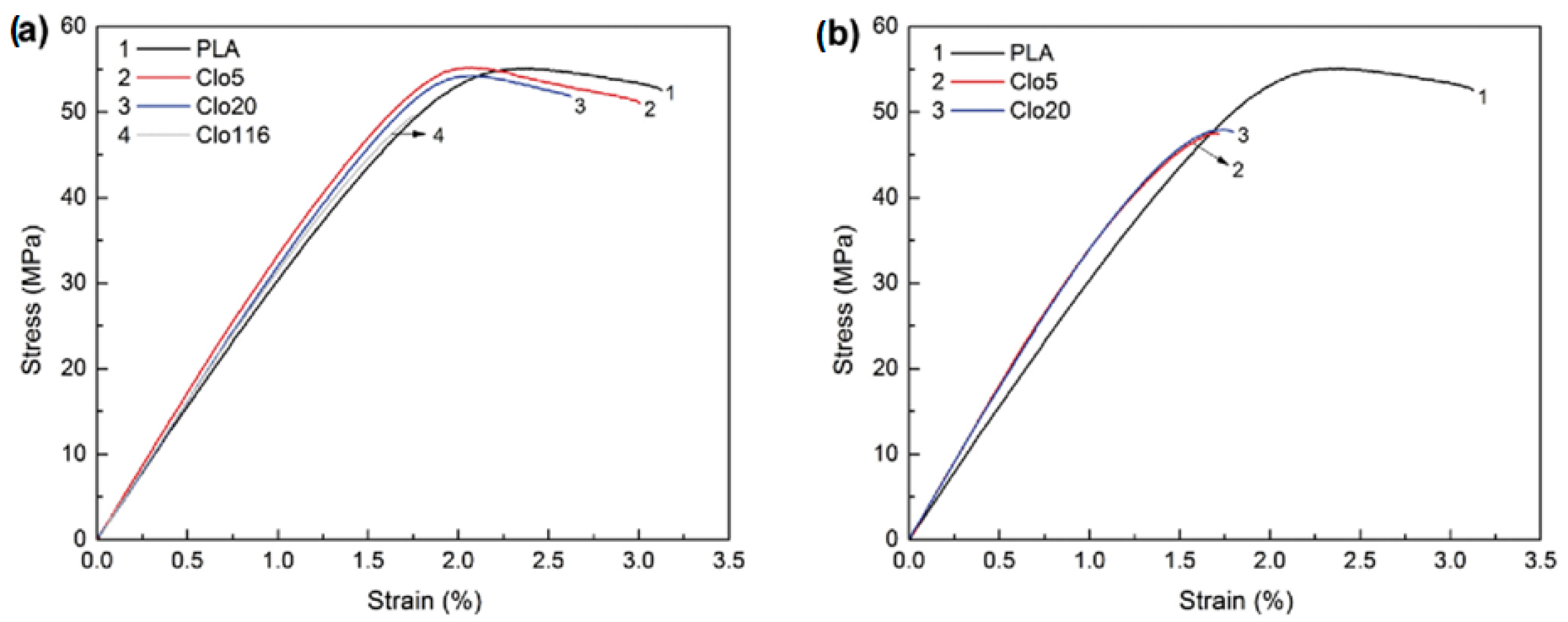
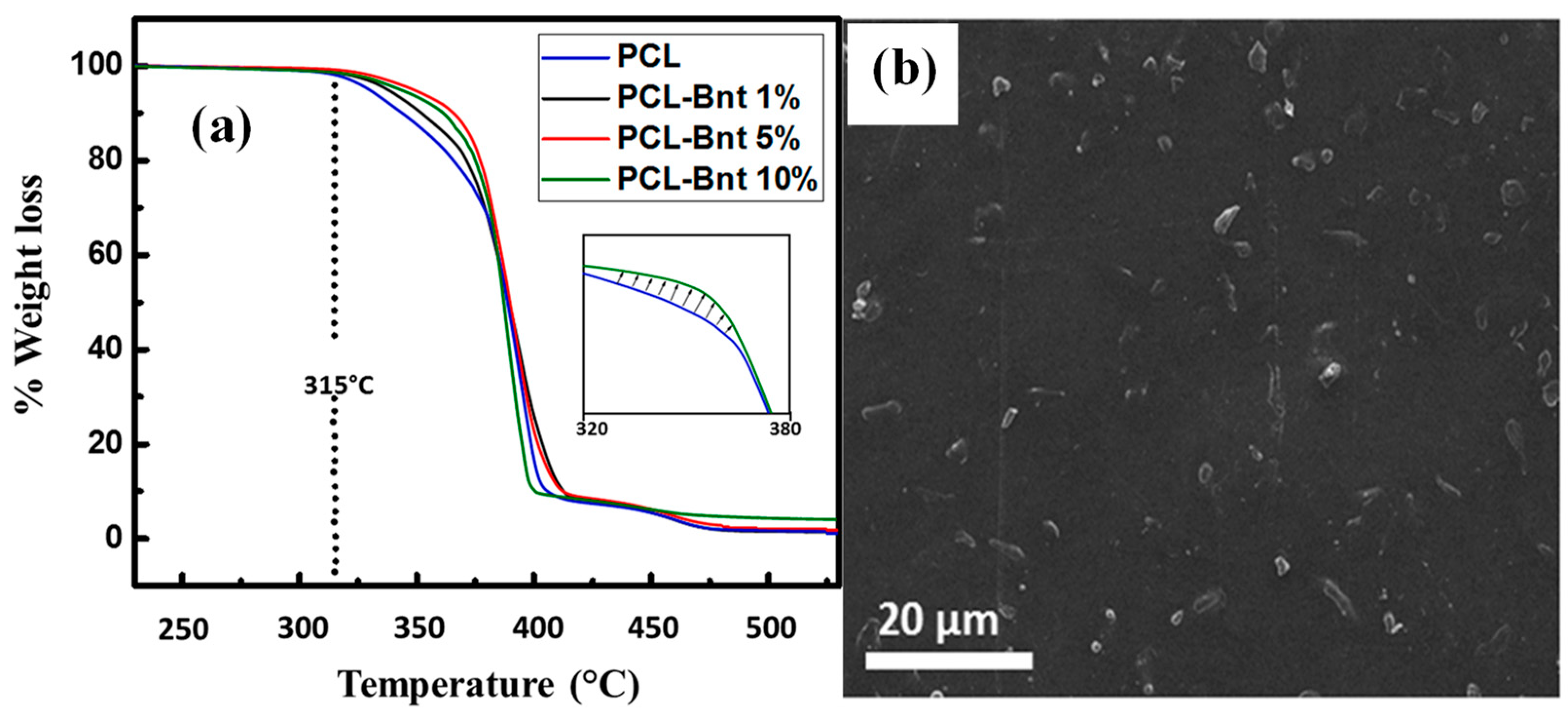
| Sample | Residual Mass (%), 150 °C | Residual Mass (%), 1000 °C | Theory Organic Content (%) | Experimental Organic Content (%) |
|---|---|---|---|---|
| Crude bentonite | 83.4 | 79.3 | - | - |
| SC14 | 97.1 | 71.9 | 19.0 | 20.9 |
| SC14 + DC16 | 97.9 | 60.5 | 28.1 | 34.0 |
| DC16 | 98.1 | 55.8 | 35.3 | 39.3 |
| Sample | Young’s Modulus (MPa) | Tensile Strength (MPa) | Reference |
|---|---|---|---|
| Neat PCL | 114.8 ± 2.0 | 12.4 ± 0.2 | [8] |
| PCL–1.5 wt.-% Bent | 194.7 ± 12 | 13.9 ± 0.2 | [8] |
| PCL–3.0 wt.-% Bent | 180.8 ± 22.1 | 13.6 ± 0.5 | [8] |
| PCL–1.5 wt.-% bCBK | 203.2 ± 20.9 | 19.3 ± 0.8 | [8] |
| PCL–3.0 wt.-% bCBK | 257.3 ± 37.0 | 19.8 ± 3.1 | [8] |
| Neat PLA | 28.5 ± 3.8 | 1.7 ± 0.2 | [75] |
| PLA/Bentonite | 42.0 ± 4.3 | 2.6 ± 0.3 | [75] |
| Starch films | 40.9 ± 6.04 | 2.56 ± 0.13 | [76] |
| Starch/Bentonite (0.5 wt.-%) | 45.35 ± 3.03 | 2.66 ± 0.72 | [76] |
| Starch/Bentonite (1 wt.-%) | 54.38 ± 7.89 | 3.053 ± 0.361 | [76] |
| Starch/Bentonite (1.5 wt.-%) | 72.65 ± 11.42 | 4.063 ± 0.12 | [76] |
| Neat PLA | 452 ± 66 | 37.0 ± 6.0 | [77] |
| PCL/Bentonite (5 wt.-%) | 767 ± 61 | 42.3 ± 2.5 | [77] |
| Biodegradable Polymer/Bentonite Clay Nanocomposites | Remarks | Reference |
|---|---|---|
| Poly(hydroxybutyrate)/polyethylene glycol blend (PHB/PEG) with organobentonite (1 wt.-% and 3 wt.-%) | It was observed that the initial temperature for the degradation of bionanocomposites showed different behavior for organobentonite (1 wt.-% and 3 wt.-%). The thermal stability of bionanocomposites increased with the organobentonite content. Furthermore, it was also verified that clay addition to most systems led to an increase in crystallinity compared to the PHB matrix, which was attributed to the clay nucleating effect. | [2] |
| Poly(butylene adipate) (PBA)/organoclay nanocomposites | The organophilic modification was observed to promote efficient clay delamination, resulting in nanocomposites with a predominantly exfoliated morphology when the appropriate organoclay (i.e., with the optimal organomodifier/clay ratio) in the appropriate concentration range of 2–3 wt.-% was used. The increased crystallinity of PBA suggests that the nanoclay acts as a heterophase-nucleating agent. The enhanced thermal stability of the PBA matrix confirmed the good quality of the delamination and dispersion of the clay lamellae in the polymer matrix. | [3] |
| Polycaprolactone/organobentonite nanocomposites | The morphology characterization of the nanocomposites showed that the organo-modification of the clay greatly improved its dispersion in the polymer matrix. As a consequence, it is demonstrated that the reinforcement of PCL with 3 wt.-% loading of organoclay produces the strongest improvement in creep resistance. The instantaneous creep strain and experimental creep rate decrease by more than 9% and 27%, respectively. | [8] |
| Polyester resin (Dolplast)/bentonite nanocomposites | The results clearly showed that the chemical modifications of the bentonite clay caused a desired effect on its final properties (thermal, barrier (water absorption), mechanical (flexural), and dynamic mechanical properties), improving the performance of the nanocomposites. The enhancements were directly related to the dispersion of the clay inside the matrix viewed via transmission electron microscopy. | [24] |
| Poly(ε-caprolactone)/bentonite nanocomposites | The prepared poly(ε-caprolactone)/bentonite presented better thermal stability regardless of the content of bentonite as a reinforcing filler. In fact, the melting point and poly(ε-caprolactone)/bentonite degree of crystallinity determined via TGA and DSC analyses, increased by increasing the amount of the filler. Furthermore, the fractured surfaces of the neat polymer (PCL) and poly(ε-caprolactone)/bentonite were compared using SEM, whereby the adhesion between clay particles and the polymer was clearly exposed, as well as the dispersion of the bentonite filler in the polymeric matrix. Finally, the bio-composites with 5 wt.-% of bentonite in the poly(ε-caprolactone) matrix displayed the highest resistance under stress with 66 MPa instead to 46 MPa only for the neat poly(ε-caprolactone). | [51] |
| PLA/bentonites nanocomposites | Nanocomposites were prepared at low clay compositions of 0.5, 1, 3, and 5 wt.-% of bentonite. From the XRD spectra, the partial exfoliation of the nanoclay layers occurred during the melting extrusion. This resulted in improvement of mechanical properties, such as Young’s modulus, tensile strength, and elongation at break. The highest tensile strength was obtained with the addition of 0.5 wt.-% commercial bentonite, increasing about 23.25% compared to the neat PLA. The increasing composition of the clays revealed a decrease in mechanical properties due to filler–filler interactions. Furthermore, the water absorption of nanocomposites up to 1 wt.-% of clays was better than that of the neat PLA. Biodegradability was enhanced in the presence of a higher clay composition due to the high hydrophilicity of clay, high water uptake, and high interactions. | [57] |
| Chitosan/bentonite nanocomposites | The characterization of the chitosan/bentonite composite via FT-IR and XRD indicates evidence of the interaction between the functional groups of chitosan with bentonite, which is beneficial for the adsorption of hexavalent chromium. SEM micrographs of the chitosan/bentonite composite revealed its slightly smooth surface with cotton-like accumulation of irregular shapes. After adsorption, the surface morphology of the chitosan/bentonite composite remained practically unchanged. | [60] |
| Chitosan/bentonite nanocomposites | The good mechanical structure was confirmed through the tensile strength and elongation values. The chitosan/bentonite composite presented a tensile strength of 40.5 ± 1.6 MPa and elongation of 60.0 ± 0.5%. These mechanical properties were better in relation to the chitosan film (pure chitosan), confirming that the insertion of bentonite was favorable. SEM micrographs demonstrated the presence of bentonite particles in the chitosan/bentonite composite. Additionally, the results showed that the chitosan/bentonite composite presented a heterogeneous structure. | [61] |
| Chitosan/bentonite nanocomposites | The incorporation of an appropriate amount of bentonite in the solution mixture with respect to chitosan in order to fabricate nanocomposite films dramatically improved almost all the properties required for an ideal wound dressing material. FTIR spectra confirmed the H-bonding interactions between the hydroxyl group of bentonites with the hydroxyl and amino groups of chitosan. Further, the SEM images showed a rough surface of the nanocomposite film that might be due to the entrapment of undissolved bentonite particles in the nanocomposite film. | [63] |
| PLA/organoclay nanocomposites | The results showed that the hybrid organo-modified montmorillonites with di(alkyl ester) dimethyl ammonium chloride (EA) and ethoxylated tallow amine (ETA) (both containing polar groups) conferred compatibility between the organoclays and PLA, while the phosphonium compound unexpectedly failed to promote the dispersion of the clay layers in PLA nanocomposites, related to its poor compatibility with PLA. However, these systems may be useful when combined with other polymers. In addition, the use of hybrid organo-modified montmorillonites (OMt) may be a good approach to obtain polymer clay nanocomposites with final desirable properties, depending on the type, amount, and surfactant combinations in the hybrid system, being a field that still has to further be explored. DSC showed dependence on the nanocomposite thermal behavior with the dispersion level of the organoclays. In general, the presence of organoclays reduced the glass transition temperature of PLA and its cold crystallization temperature, and increased its crystallinity, which was related to an effective heterogeneous crystal nucleation promoted by the organoclay. | [64] |
| Polylactide/bentonite nanocomposites | Because of good compatibility with the PLA matrix, the dispersion of bentonite (B104) occurred under different conditions without difficulty. The results obtained showed that at low temperatures of mixing, the shear stress exerted on the polymer had a key role in the extent of intercalation and delamination. Bentonite could be added up to 4 wt.-% into PLA without detrimentally sacrificing the tensile strength of melt-spun filaments, especially at a high draw ratio. Interestingly, the composites-based multifilament was knitted, and the flammability studied using cone calorimeter at 35 kW/m2. A strong decrease, up to 46%, in the heat release rate was observed. | [67] |
| Taro starch/bentonite nanocomposites | SEM and FTIR results showed that the films formed were homogeneous and confirmed the functional groups of Taro starch. With the increase in bentonite concentration, the tensile strength of the bioplastic film was found to increase. The bentonite films exhibited more resistance to salt and acid, were susceptible to alkali, and showed minor swelling. | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mapossa, A.B.; da Silva Júnior, A.H.; de Oliveira, C.R.S.; Mhike, W. Thermal, Morphological and Mechanical Properties of Multifunctional Composites Based on Biodegradable Polymers/Bentonite Clay: A Review. Polymers 2023, 15, 3443. https://doi.org/10.3390/polym15163443
Mapossa AB, da Silva Júnior AH, de Oliveira CRS, Mhike W. Thermal, Morphological and Mechanical Properties of Multifunctional Composites Based on Biodegradable Polymers/Bentonite Clay: A Review. Polymers. 2023; 15(16):3443. https://doi.org/10.3390/polym15163443
Chicago/Turabian StyleMapossa, António Benjamim, Afonso Henrique da Silva Júnior, Carlos Rafael Silva de Oliveira, and Washington Mhike. 2023. "Thermal, Morphological and Mechanical Properties of Multifunctional Composites Based on Biodegradable Polymers/Bentonite Clay: A Review" Polymers 15, no. 16: 3443. https://doi.org/10.3390/polym15163443
APA StyleMapossa, A. B., da Silva Júnior, A. H., de Oliveira, C. R. S., & Mhike, W. (2023). Thermal, Morphological and Mechanical Properties of Multifunctional Composites Based on Biodegradable Polymers/Bentonite Clay: A Review. Polymers, 15(16), 3443. https://doi.org/10.3390/polym15163443







