Next-Generation Water Treatment: Exploring the Potential of Biopolymer-Based Nanocomposites in Adsorption and Membrane Filtration
Abstract
1. Introduction
| Sr. No. | Type Pollutants | Chemical Compositions/ Formula | Sources | Effects | Ref. |
|---|---|---|---|---|---|
| 1. | Kaolin clay | Al2Si2O5 (OH)4 | Industrial effluents from
| Water with kaolin clay causes
| [49,50] |
| 2. | Iron ore slimes | Fe2O3, Fe3O4, 2Fe2O. 3H2O, FeCO3 and FeS2 | Washing liquid from
| Water with iron slimes
| [51,52,53] |
| 3. | Coal | C, H, O, N, S |
| Coal water creates
| [54,55,56] |
| 4. | Silicon dioxide | SiO2 | Wastage from industries of
| Silica in drinking water causes:
| [57,58,59,60] |
| 5. | Copper | Cu | Wastewater from industries of
| Copper intoxication
| [61,62,63,64,65] |
| 6. | Nickel | Ni | Effluents from
| Nickel leads to effects such as
| [66,67] |
| 7. | Zinc | Zn | Industrial effluents from
| High concertation of zinc causes
| [68,69] |
| 8. | Lead | Pb | Wastewater from
| Lead causes mostly
| [70,71] |
| 9. | Mercury | Hg | Wastage from
| Mercury has dangerous effects on
| [72] |
| 10. | Chromium | Cr | Effluents from
| Cr (VI) is dangerous and creates
| [73,74] |
| 11. | Arsenic | As | Industrial wastewater from
| Long ingestion of arsenic (III) (>10 ppb) can lead to
| [75,76] |
| 12. | Malachite green (MG) | C23H25Cl2 | Industrial effluents from
| High concentration of MG
| [77] |
| 13. | Congo red (CR) | C32H22N6Na2O6S2 | Wastewater from
| High concentration of CR creates
| [78,79] |
| 14. | Methylene blue (MB) | C16H18ClN3S | Effluents from
| MB creates mostly
| [80,81] |
| 15. | Reactive Black 5 (RB5) | C26H21N5Na4O19S6 | Wastewater from
| Presence of RB5 in water bodies causes
| [82,83] |
| 16. | Reactive Blue 4 (RB4) | C23H14Cl2N6O8S2 | Effluent from
| Presence of RB4 in water bodies causes
| [84,85] |
| 17. | Reactive Blue 29 (RB29) | C29H15Cl2N5Na2O9S2 | Discharged wastewater from
| RB29 affects
| [86] |
| 18. | Reactive Yellow 145 (RY145) | C28H20ClN9Na4O16S5 | Wastewater from
| RY145 toxic towards
| [87,88] |
| 19. | Pharmaceuticals n: (Ibuprofen: Metformin: Simvastatin: Omeprazole: Fluoxetine: Ciprofloxacin: Amlodipine: Sertraline: Atorvastatin:) | C8H9NO2 C13H18O2 C4H11N5 C25H38O5 C17H19N3O3S C17H18F3NO C17H18FN3O3 C20H25ClN2O5 C17H17Cl2N C33H35FN2O5 |
|
| [89,90] |
| 20. | Personal Care (Products: Parabens: Triclosan: Benzophenone-3: Phthalates: Octinoxate: Formaldehyde: Sodium Lauryl Sulfate:) | C9H10O3 C12H7Cl3O2 C14H12O3 C8H4O4 C18H26O3 CH2O C12H25NaO4S |
|
| [91] |
| 21. | Microplastics (Polyethylene: Polypropylene: Polystyrene:) | (C2H4)n (C3H6)n (C8H8)n |
|
| [92,93] |
2. Effects of Pollutants on Human Health
3. Biopolymers


4. Biopolymer-Based Nanocomposite
4.1. Nano-Fillers for Water Treatment
4.2. Synthesis and Characterization
4.3. Applications in Water Treatment
4.3.1. Adsorption
| Sl. No | Adsorbent/Flocculent | Pollutants | Results (% or Qmax mg/g) | Ref. |
|---|---|---|---|---|
| 1. | GO-cl-potato starch bio-composite | MB | 500 mg/g (90%) | [157] |
| 2. | St-g-poly(AM-co-GO)/hydroxyapatite composite hydrogel | MG | 297 mg/g | [164] |
| 3. | AP-g-3D GO composites | tert-butyl hydroquinone o-nitrophenol p-aminophenol Hydroquinone p-nitrophenol Neutral red Alizarin red S Pb (II) Mn (II) Cr2O7−2 Cd (II) Cu (II) Nd (III) La (III) Y (III) Yb (III) Yr (III) | 22.17 mg/g 36.96 mg/g 116.4 mg/g 16.10 mg/g 36.96 mg/g 44.78 mg/g 39.92 mg/g 84.76 mg/g 7.92 mg/g 13.6 mg/g 17.64 mg/g 30.56 mg/g 25.25 mg/g 12.48 mg/g 16.96 mg/g 23.32 mg/g 30.32 mg/g | [161] |
| 4. | 3D GO-sodium alginate | MB | 833.3 mg/g, at 303K | [165] |
| 5. | GO-calcium/alginate | MB | 163.93 mg/g, at 298 K 140.85 mg/g, at 328 K | [166] |
| 6. | Dextrin-g-poly(m-phenylenediamine)-GO | Pb (II) MB | 80 mg/g 76.33 mg/g | [167] |
| 7. | St-g-3DGO | Pb (II) Cu (II) Cd (II) Yb (III) Nd (III) | 108.68 mg/g 32.12 mg/g 46.28 mg/g 41.76 mg/g 38.168 mg/g | [168] |
| 8. | Alginate–chitosan hybrid adsorbent | Pb (II) | 96.8%, pH 5.0 | [169] |
| 9. | PAA–chitosan and biochar-composite | Cu (II), Zn (II), Ni (II), Pb (II), Cd (II), Mn (II), Co (II), and Cr (VI) | 80%, pH 2–7 | [170] |
| 10. | Chitosan–GO–Hap composite | CR, acid red 1, and reactive red 2 | 43.06, 41.32, and 40.03 mg/g, pH 2 | [171] |
| 11. | Chitosan–GO composites | Reactive black 5 dye | 277 mg/g at 25 °C | [172] |
| 12. | GO–Chitosan composite | Cu (II), Pb (II), Cd (II) | 60.7, 48.7, 32.3 mg/g, pH 1 | [175] |
| 13. | Sodium alginate-CMC-GO-Gd3O3 | Pb (II), Cr (III) and As (V) | 29.16, 158.73, and 36.77 mg/g | [173] |
| 14. | Chitosan-activated carbon composite | Cr (VI) | 98.7% | [174] |
| 15. | Chitosan–graphene-oxide-dip-coated electrospun nanofiber membrane | MB CR | 201 mg/g 152 mg/g | [176] |
4.3.2. Magnetic Adsorbents
5. Membrane Filtration
6. Limitation, Challenges, and Opportunities
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Coccia, M.; Bontempi, E. New trajectories of technologies for the removal of pollutants and emerging contaminants in the environment. Environ. Res. 2023, 229, 115938. [Google Scholar] [CrossRef] [PubMed]
- Kolya, H.; Tripathy, T. Grafted polysaccharides based on acrylamide and N,N-dimethylacrylamide: Preparation and investigation of their flocculation performances. J. Appl. Polym. Sci. 2013, 127, 2786–2795. [Google Scholar] [CrossRef]
- Pal, S.; Mal, D.; Singh, R.P. Cationic starch: An effective flocculating agent. Carbohydr. Polym. 2005, 59, 417–423. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Jin, J.; Tian, Z.; Yang, W.; Graham, N.J.D.; Yang, Z. Enhanced removal of trace pesticides and alleviation of membrane fouling using hydrophobic-modified inorganic-organic hybrid flocculants in the flocculation-sedimentation-ultrafiltration process for surface water treatment. Water Res. 2023, 229, 119447. [Google Scholar] [CrossRef] [PubMed]
- Raeesh, M.; Devi, T.T.; Hirom, K. Recent Developments on Application of Different Turbulence and Multiphase Models in Sedimentation Tank Modeling—A Review. Water Air Soil Pollut. 2022, 234, 5. [Google Scholar] [CrossRef]
- Schelling, M.; Patil, K.; Boving, T.B. Sustainable Water Treatment with Induced Bank Filtration. Water 2023, 15, 361. [Google Scholar] [CrossRef]
- Qian, Y.; Shi, Y.; Guo, J.; Chen, Y.; Hanigan, D.; An, D. Molecular characterization of disinfection byproduct precursors in filter backwash water from 10 drinking water treatment plants. Sci. Total Environ. 2023, 856, 159027. [Google Scholar] [CrossRef]
- Ghorai, S.; Sarkar, A.; Raoufi, M.; Panda, A.B.; Schönherr, H.; Pal, S. Enhanced Removal of Methylene Blue and Methyl Violet Dyes from Aqueous Solution Using a Nanocomposite of Hydrolyzed Polyacrylamide Grafted Xanthan Gum and Incorporated Nanosilica. ACS Appl. Mater. Interfaces 2014, 6, 4766–4777. [Google Scholar] [CrossRef]
- Maraddi, A.; Halakarni, M.; Manohara Halanur, M.; Nataraj, S.K. Fe-MOF induced biopolymer-based sustainable self-cleaning membranes for effective selective separation and wastewater treatment. Sustain. Mater. Technol. 2023, 35, e00537. [Google Scholar] [CrossRef]
- Lee, Y.; Park, Y.-J.; Lee, J.; Bae, T.-H. Recent advances and emerging applications of membrane contactors. Chem. Eng. J. 2023, 461, 141948. [Google Scholar] [CrossRef]
- Mirza, A.; King, A.; Troakes, C.; Exley, C. Aluminium in brain tissue in familial Alzheimer’s disease. J. Trace Elem. Med. Biol. 2017, 40, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Tiehm, A.; Herwig, V.; Neis, U. Particle size analysis for improved sedimentation and filtration in waste water treatment. Water Sci. Technol. 1999, 39, 99–106. [Google Scholar] [CrossRef]
- Verma, S.; Daverey, A.; Sharma, A. Slow sand filtration for water and wastewater treatment—A review. Environ. Technol. Rev. 2017, 6, 47–58. [Google Scholar] [CrossRef]
- Lin, H.; Ye, C.; Chen, S.; Zhang, S.; Yu, X. Viable but non-culturable E. coli induced by low level chlorination have higher persistence to antibiotics than their culturable counterparts. Environ. Pollut. 2017, 230, 242–249. [Google Scholar] [CrossRef]
- Munter, R. Advanced oxidation processes–current status and prospects. Proc. Est. Acad. Sci. Chem. 2001, 50, 59–80. [Google Scholar] [CrossRef]
- Nabi, M.; Liang, H.; Cheng, L.; Yang, W.; Gao, D. A comprehensive review on the use of conductive materials to improve anaerobic digestion: Focusing on landfill leachate treatment. J. Environ. Manag. 2022, 309, 114540. [Google Scholar] [CrossRef]
- Ping, Q.; Cohen, B.; Dosoretz, C.; He, Z. Long-term investigation of fouling of cation and anion exchange membranes in microbial desalination cells. Desalination 2013, 325, 48–55. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Dharupaneedi, S.P.; Nataraj, S.K.; Nadagouda, M.; Reddy, K.R.; Shukla, S.S.; Aminabhavi, T.M. Membrane-based separation of potential emerging pollutants. Sep. Purif. Technol. 2019, 210, 850–866. [Google Scholar] [CrossRef]
- Thurakkal, L.; Porel, M. Removal of cationic and anionic dyes from waste water in five seconds by a facile synthesis of a covalent organic polymer. Eur. Polym. J. 2023, 194, 112169. [Google Scholar] [CrossRef]
- Procopio, E.Q.; Fukushima, T.; Barea, E.; Navarro, J.A.R.; Horike, S.; Kitagawa, S. A Soft Copper(II) Porous Coordination Polymer with Unprecedented Aqua Bridge and Selective Adsorption Properties. Chem. A Eur. J. 2012, 18, 13117–13125. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.-X.; Jin, Y.-L.; Sun, Z.-F.; Ren, F.; Pei, L.; Ren, P.-G. Facile synthesis of chitosan-based acid-resistant composite films for efficient selective adsorption properties towards anionic dyes. Carbohydr. Polym. 2021, 254, 117473. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, C.; Konwar, A.; Bora, P.; Rajguru, P.; Hazarika, S. Cellulose nanofiber-poly(ethylene terephthalate) nanocomposite membrane from waste materials for treatment of petroleum industry wastewater. J. Hazard. Mater. 2023, 442, 129955. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.-W. Bio-Based Polymeric Flocculants and Adsorbents for Wastewater Treatment. Sustainability 2023, 15, 9844. [Google Scholar] [CrossRef]
- Stephen, D.P.; Palanisamy, S.B. Advances in biopolymer composites and biomaterials for the removal of emerging contaminants. Phys. Sci. Rev. 2021, 000010151520210056. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Sheikh, M.F.; Zolfagharian, A.; Bodaghi, M. Biopolymeric sustainable materials and their emerging applications. J. Environ. Chem. Eng. 2022, 10, 108159. [Google Scholar] [CrossRef]
- Joshi, P.; Mehta, S.; Pandey, A.; Khatri, O.P. Inorganic Analogues of Graphene and Their Nanocomposites for Wastewater Treatment BT—Two-Dimensional Materials for Environmental Applications; Kumar, N., Gusain, R., Sinha Ray, S., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 75–102. ISBN 978-3-031-28756-5. [Google Scholar]
- Subash, A.; Naebe, M.; Wang, X.; Kandasubramanian, B. Biopolymer—A sustainable and efficacious material system for effluent removal. J. Hazard. Mater. 2023, 443, 130168. [Google Scholar] [CrossRef]
- Nawaz, S.; Tabassum, A.; Muslim, S.; Nasreen, T.; Baradoke, A.; Kim, T.H.; Boczkaj, G.; Jesionowski, T.; Bilal, M. Effective assessment of biopolymer-based multifunctional sorbents for the remediation of environmentally hazardous contaminants from aqueous solutions. Chemosphere 2023, 329, 138552. [Google Scholar] [CrossRef]
- Saha, A.; Bardhan, S.; Roy, S.; Dutta, S.; Das, S. Biopolymeric Hydrogels: A New Era in Combating Heavy Metal Pollution in Industrial Wastewater BT—Membranes for Water Treatment and Remediation; Nadda, A.K., Banerjee, P., Sharma, S., Nguyen-Tri, P., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 209–226. ISBN 978-981-19-9176-9. [Google Scholar]
- Haasler, S.; Christensen, M.L.; Reitzel, K. Synthetic and biopolymers for lake restoration—An evaluation of flocculation mechanism and dewatering performance. J. Environ. Manag. 2023, 331, 117199. [Google Scholar] [CrossRef]
- Rocha-Pimienta, J.; Navajas-Preciado, B.; Barraso-Gil, C.; Martillanes, S.; Delgado-Adámez, J. Optimization of the Extraction of Chitosan and Fish Gelatin from Fishery Waste and Their Antimicrobial Potential as Active Biopolymers. Gels 2023, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Romero-Montero, A.; Valencia-Bermúdez, J.L.; Rosas-Meléndez, S.A.; Núñez-Tapia, I.; Piña-Barba, M.C.; Leyva-Gómez, G.; Del Prado-Audelo, M.L. Biopolymeric Fibrous Aerogels: The Sustainable Alternative for Water Remediation. Polymers 2023, 15, 262. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Sharma, S.S. Biopolymer-Based Nanocomposites BT. In Handbook of Biopolymers; Thomas, S., Ajitha, A.R., Jose Chirayil, C., Thomas, B., Eds.; Springer Nature: Singapore, 2022; pp. 1–28. ISBN 978-981-16-6603-2. [Google Scholar]
- Meera, K.; Ramesan, M.T. Tailoring the performance of boehmite nanoparticles reinforced carboxymethyl chitosan/cashew gum blend nanocomposites via green synthesis. Polymer 2023, 268, 125706. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Nooshkam, M.; Zargar, M.; Garavand, F.; Ghosh, S.; Hadidi, M.; Forough, M. Green synthesis of nanomaterials for smart biopolymer packaging: Challenges and outlooks. J. Nanostruct. Chem. 2023, 1–24. [Google Scholar] [CrossRef]
- Khan, I.; Khan, I.; Saeed, K.; Ali, N.; Zada, N.; Khan, A.; Ali, F.; Bilal, M.; Akhter, M.S. 7—Polymer nanocomposites: An overview. In Micro and Nano Technologies; Ali, N., Bilal, M., Khan, A., Nguyen, T.A., Gupta, R.K.B.T.-S.P.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 167–184. ISBN 978-0-323-91611-0. [Google Scholar]
- Kolya, H.; Kuila, T.; Kim, N.H.; Lee, J.H. 10—Polymer Nanocomposites for Energy-Related Applications; Subramani, N.K., Siddaramaiah, H., Lee, J.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 215–248. ISBN 978-0-12-818484-4. [Google Scholar]
- Kolya, H.; Mondal, S.; Kang, C.-W.; Nah, C. The use of polymer-graphene composites in catalysis. In Polymer Nanocomposites Containing Graphene; Woodhead Publishing Series in Composites Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 537–556. [Google Scholar]
- Baigorria, E.; Souza dos Santos, S.; de Moura, M.R.; Fraceto, L.F. Nanocomposite hydrogels 3D printed for application in water remediation. Mater. Today Chem. 2023, 30, 101559. [Google Scholar] [CrossRef]
- Rani Sethy, T.; Biswal, T.; Kumar Sahoo, P. An indigenous tool for the adsorption of rare earth metal ions from the spent magnet e-waste: An eco-friendly chitosan biopolymer nanocomposite hydrogel. Sep. Purif. Technol. 2023, 309, 122935. [Google Scholar] [CrossRef]
- Nandanwar, P.; Jugade, R.; Gomase, V.; Shekhawat, A.; Bambal, A.; Saravanan, D.; Pandey, S. Chitosan-Biopolymer-Entrapped Activated Charcoal for Adsorption of Reactive Orange Dye from Aqueous Phase and CO2 from Gaseous Phase. J. Compos. Sci. 2023, 7, 103. [Google Scholar] [CrossRef]
- Gupta, P.; Rana, N.; Dash, S.; Gupta, N.; Singh, M.; Kaushik, S. Bionanocomposite Membranes and Adsorbents for Water and Wastewater Treatment. In Bionanotechnology Towards Sustainable Management of Environmental Pollution; CRC Press: Boca Raton, FL, USA, 2023; pp. 141–166. ISBN 1003270956. [Google Scholar]
- Das, A.; Ringu, T.; Ghosh, S.; Pramanik, N. A comprehensive review on recent advances in preparation, physicochemical characterization, and bioengineering applications of biopolymers. Polym. Bull. 2023, 80, 7247–7312. [Google Scholar] [CrossRef] [PubMed]
- Muhammed Shameem, M.; Sasikanth, S.M.; Annamalai, R.; Ganapathi Raman, R. A brief review on polymer nanocomposites and its applications. Mater. Today Proc. 2021, 45, 2536–2539. [Google Scholar] [CrossRef]
- Goyal, R.K. Nanomaterials and Nanocomposites: Synthesis, Properties, Characterization Techniques, and Applications; CRC Press: Boca Raton, FL, USA, 2017; ISBN 1498761674. [Google Scholar]
- Mittal, V. Characterization Techniques for Polymer Nanocomposites; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 3527654526. [Google Scholar]
- Hu, P.; Yang, H. Insight into the physicochemical aspects of kaolins with different morphologies. Appl. Clay Sci. 2013, 74, 58–65. [Google Scholar] [CrossRef]
- Elmore, A.R. Cosmetic Ingredient Review Expert Panel: Final report on the safety assessment of aluminum silicate, calcium silicate, magnesium aluminum silicate, magnesium silicate, magnesium trisilicate, sodium magnesium silicate, zirconium silicate, attapulgite, bentonite, Fuller’s earth, hectorite, kaolin. Int. J. Toxicol. 2003, 22 (Suppl. 1), 37–102. [Google Scholar] [PubMed]
- Muwanguzi, A.J.B.; Karasev, A.V.; Byaruhanga, J.K.; Jönsson, P.G. Characterization of Chemical Composition and Microstructure of Natural Iron Ore from Muko Deposits. ISRN Mater. Sci. 2012, 2012, 174803. [Google Scholar] [CrossRef]
- Das, S.K.; Kumar, S.; Ramachandrarao, P. Exploitation of iron ore tailing for the development of ceramic tiles. Waste Manag. 2000, 20, 725–729. [Google Scholar] [CrossRef]
- Gilmour, P.S.; Brown, D.M.; Lindsay, T.G.; Beswick, P.H.; MacNee, W.; Donaldson, K. Adverse health effects of PM10 particles: Involvement of iron in generation of hydroxyl radical. Occup. Environ. Med. 1996, 53, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, S.V.; Menendez, R.; Diaz-Somoano, M.; Martinez-Tarazona, M.R. Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilization. 2. Characterization of ceramic cenosphere and salt concentrates. Fuel 2004, 83, 585–603. [Google Scholar] [CrossRef]
- Haibin, L.; Zhenling, L. Recycling utilization patterns of coal mining waste in China. Resour. Conserv. Recycl. 2010, 54, 1331–1340. [Google Scholar] [CrossRef]
- DeGraeve, G.M.; Overcast, R.L.; Bergman, H.L. Toxicity of underground coal gasification condenser water and selected constituents to aquatic biota. Arch. Environ. Contam. Toxicol. 1980, 9, 543–555. [Google Scholar] [CrossRef]
- Chen, A.; Lo, R. Semiconductor Packaging; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-1-4398-6205-6. [Google Scholar]
- Jäger-Waldau, A. Chapter IC-4—Progress in Chalcopyrite Compound Semiconductor Research for Photovoltaic Applications and Transfer of Results into Actual Solar Cell Production; McEvoy, A., Markvart, T., Castañer, L., Eds.; Academic Press: Boston, MA, USA, 2012; pp. 373–395. ISBN 978-0-12-385934-1. [Google Scholar]
- Humborg, C.; Conley, D.J.; Rahm, L.; Wulff, F.; Cociasu, A.; Ittekkot, V. Silicon Retention in River Basins: Far-reaching Effects on Biogeochemistry and Aquatic Food Webs in Coastal Marine Environments. AMBIO A J. Hum. Environ. 2000, 29, 45–50. [Google Scholar] [CrossRef]
- Rondeau, V.; Jacqmin-Gadda, H.; Commenges, D.; Helmer, C.; Dartigues, J.-F. Aluminum and Silica in Drinking Water and the Risk of Alzheimer’s Disease or Cognitive Decline: Findings From 15-Year Follow-up of the PAQUID Cohort. Am. J. Epidemiol. 2008, 169, 489–496. [Google Scholar] [CrossRef]
- Santore, R.C.; Di Toro, D.M.; Paquin, P.R.; Allen, H.E.; Meyer, J.S. Biotic ligand model of the acute toxicity of metals. 2. Application to acute copper toxicity in freshwater fish and Daphnia. Environ. Toxicol. Chem. 2001, 20, 2397–2402. [Google Scholar] [CrossRef]
- Erickson, R.J.; Benoit, D.A.; Mattson, V.R.; Leonard, E.N.; Nelson, H.P., Jr. The effects of water chemistry on the toxicity of copper to fathead minnows. Environ. Toxicol. Chem. 1996, 15, 181–193. [Google Scholar] [CrossRef]
- Sunda, W. The Relationship between Cupric Ion Activity and the Toxicity of Copper to Phytoplankton. Ph.D. Thesis, Massachusetts Institute of Technology and Woods Hole Oceanographic Institution, Cambridge, MA, USA, 1976; pp. 511–529. [Google Scholar]
- Brewer, G.J. Copper toxicity in Alzheimer’s disease: Cognitive loss from ingestion of inorganic copper. J. Trace Elem. Med. Biol. 2012, 26, 89–92. [Google Scholar] [CrossRef]
- Desai, V.; Kaler, S.G. Role of copper in human neurological disorders. Am. J. Clin. Nutr. 2008, 88, 855S–858S. [Google Scholar] [CrossRef] [PubMed]
- Das, K.K.; Das, S.N. Nickel, its adverse health effects & oxidative stress—ProQuest. Indian J. Med. Res. 2008, 128, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.C.T.-H. Nickel toxicity to human term placenta: In vitro study on lipid peroxidation. J. Toxicol. Environ. Heal. Part A 1998, 54, 37–47. [Google Scholar] [CrossRef]
- Fosmire, G.J. Zinc toxicity. Am. J. Clin. Nutr. 1990, 51, 225–227. [Google Scholar] [CrossRef]
- Lock, K.; Janssen, C.R. Modeling zinc toxicity for terrestrial invertebrates. Environ. Toxicol. Chem. 2001, 20, 1901–1908. [Google Scholar] [CrossRef]
- Gidlow, D.A. Lead toxicity. Occup. Med. 2004, 54, 76–81. [Google Scholar] [CrossRef]
- Senthil Kumar, P. Adsorption of lead(II) ions from simulated wastewater using natural waste: A kinetic, thermodynamic and equilibrium study. Environ. Prog. Sustain. Energy 2014, 33, 55–64. [Google Scholar] [CrossRef]
- Sigel, A.; Sigel, H. Metal Ions in Biological Systems: Volume 34: Mercury and Its Effects on Environment and Biology; CRC Press: Boca Raton, FL, USA, 1997; ISBN 0824798287. [Google Scholar]
- Losi, M.E.; Amrhein, C.; Frankenberger, W.T. Environmental Biochemistry of Chromium BT—Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: New York, NY, USA, 1994; pp. 91–121. ISBN 978-1-4612-2656-7. [Google Scholar]
- Costa, M.; Klein, C.B. Toxicity and Carcinogenicity of Chromium Compounds in Humans. Crit. Rev. Toxicol. 2006, 36, 155–163. [Google Scholar] [CrossRef]
- Lee, J.-J.; Kim, Y.-K.; Cho, S.-H.; Park, K.-S.; Chung, I.-J.; Cho, D.; Ryang, D.-W.; Kim, H.-J. Hemolytic Anemia as a Sequela of Arsenic Intoxication Following Long-Term Ingestion of Traditional Chinese Medicine. J. Korean Med. Sci. 2004, 19, 127–129. [Google Scholar] [CrossRef]
- Smith, R.; Knight, R.; Fendorf, S. Overpumping leads to California groundwater arsenic threat. Nat. Commun. 2018, 9, 2089. [Google Scholar] [CrossRef] [PubMed]
- Cleinmensen, S.; Jensen, J.C.; Jensen, N.J.; Meyer, O.; Olsen, P.; Würtzen, G. Toxicological studies on malachite green: A triphenylmethane dye. Arch. Toxicol. 1984, 56, 43–45. [Google Scholar] [CrossRef]
- Lorenzo, A.; Yankner, B.A. Beta-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc. Natl. Acad. Sci. USA 1994, 91, 12243–12247. [Google Scholar] [CrossRef]
- Afkhami, A.; Moosavi, R. Adsorptive removal of Congo red, a carcinogenic textile dye, from aqueous solutions by maghemite nanoparticles. J. Hazard. Mater. 2010, 174, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Top, W.M.; Gillman, P.K.; de Langen, C.J.; Kooy, A. Fatal methylene blue associated serotonin toxicity. Neth. J. Med. 2014, 72, 179–181. [Google Scholar] [PubMed]
- Littlefield, N.A.; Blackwell, B.-N.; Hewitt, C.C.; Gaylor, D.W. Chronic Toxicity and Carcinogenicity Studies of Gentian Violet in Mice. Toxicol. Sci. 1985, 5, 902–912. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Zhang, J.; Zhao, H.; Zhang, Y.; Quan, X.; Jin, Y. Characterisation of acute toxicity, genotoxicity and oxidative stress posed by textile effluent on zebrafish. J. Environ. Sci. 2012, 24, 2019–2027. [Google Scholar] [CrossRef]
- Rehman, K.; Shahzad, T.; Sahar, A.; Hussain, S.; Mahmood, F.; Siddique, M.H.; Siddique, M.A.; Rashid, M.I. Effect of Reactive Black 5 azo dye on soil processes related to C and N cycling. PeerJ 2018, 6, e4802. [Google Scholar] [CrossRef]
- Epolito, W.J.; Lee, Y.H.; Bottomley, L.A.; Pavlostathis, S.G. Characterization of the textile anthraquinone dye Reactive Blue 4. Dye. Pigment. 2005, 67, 35–46. [Google Scholar] [CrossRef]
- Carneiro, P.A.; Osugi, M.E.; Fugivara, C.S.; Boralle, N.; Furlan, M.; Zanoni, M.V.B. Evaluation of different electrochemical methods on the oxidation and degradation of Reactive Blue 4 in aqueous solution. Chemosphere 2005, 59, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.H.; Naghizadeh, A.; Rashidi, A.; Derakhshani, E. Adsorption of reactive blue 29 dye from aqueous solution by multiwall carbon nanotubes. Desalin. Water Treat. 2013, 51, 7655–7662. [Google Scholar] [CrossRef]
- Shiva Shankar, Y.; Ankur, K.; Bhushan, P.; Mohan, D. Utilization of Water Treatment Plant (WTP) Sludge for Pretreatment of Dye Wastewater Using Coagulation/Flocculation BT—Advances in Waste Management; Kalamdhad, A.S., Singh, J., Dhamodharan, K., Eds.; Springer: Singapore, 2019; pp. 107–121. [Google Scholar]
- Kazi, S.A.; Iqbal, H.H.; Shahid, N.; Shah, G.M.; Jameel, N. Removal of reactive dye yellow 145 by adsorption using white quartz. Bull. Environ. Stud 2016, 1, 43. [Google Scholar]
- Nassiri Koopaei, N.; Abdollahi, M. Health risks associated with the pharmaceuticals in wastewater. DARU J. Pharm. Sci. 2017, 25, 9. [Google Scholar] [CrossRef]
- Wasi, S.; Tabrez, S.; Ahmad, M. Toxicological effects of major environmental pollutants: An overview. Environ. Monit. Assess. 2013, 185, 2585–2593. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, L.; Zhang, X. Emerging pollutants–part I: Occurrence, fate and transport. Water Environ. Res. 2018, 90, 1301–1322. [Google Scholar] [CrossRef]
- Rochman, C.M.; Browne, M.A.; Underwood, A.J.; van Franeker, J.A.; Thompson, R.C.; Amaral-Zettler, L.A. The ecological impacts of marine debris: Unraveling the demonstrated evidence from what is perceived. Ecology 2016, 97, 302–312. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.-W. Synthesis of starch-based smart hydrogel derived from rice-cooked wastewater for agricultural use. Int. J. Biol. Macromol. 2023, 226, 1477–1489. [Google Scholar] [CrossRef]
- Kolya, H.; Jana, D.; Sasmal, D.; Jana, S.; Tripathy, T. Sulfated katira gum-graft-poly(N-vinyl imidazole): A useful scavenger of mercury(II) ions. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Kumari, S.; Kishor, R. Chapter 1—Chitin and Chitosan: Origin, Properties, and Applications; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–33. ISBN 978-0-12-817970-3. [Google Scholar]
- Ait Hamdan, Y.; El Amerany, F.; Desbrières, J.; Aghrinane, A.; Oudadesse, H.; Rhazi, M. The evolution of the global COVID-19 epidemic in Morocco and understanding the different therapeutic approaches of chitosan in the control of the pandemic. Polym. Bull. 2022, 1–27. [Google Scholar] [CrossRef]
- Gacesa, P. Alginates. Carbohydr. Polym. 1988, 8, 161–182. [Google Scholar] [CrossRef]
- Pereira, L.; Cotas, J. Introductory Chapter: Alginates—A General Overview; Pereira, L., Ed.; IntechOpen: Rijeka, Croatia, 2020; p. Ch. 1. ISBN 978-1-78985-642-2. [Google Scholar]
- Alavinia, S.; Ghorbani-Vaghei, R.; Asadabadi, S.; Atrian, A. Sodium alginate/diethyleneamine-triazine-sulfonamide nanocomposite for adsorptive removal of Pb(II) and methyl violet from aqueous solutions. Mater. Chem. Phys. 2023, 293, 126915. [Google Scholar] [CrossRef]
- Kolya, H.; Pal, S.; Pandey, A.; Tripathy, T. Preparation of gold nanoparticles by a novel biodegradable graft copolymer sodium alginate-g-poly (N,N-dimethylacrylamide-co-acrylic acid) with anti micro bacterial application. Eur. Polym. J. 2015, 66, 139–148. [Google Scholar] [CrossRef]
- Sen, G.; Singh, R.P.; Pal, S. Microwave-initiated synthesis of polyacrylamide grafted sodium alginate: Synthesis and characterization. J. Appl. Polym. Sci. 2010, 115, 63–71. [Google Scholar] [CrossRef]
- Dong, Y.-D.; Zhang, H.; Zhong, G.-J.; Yao, G.; Lai, B. Cellulose/carbon Composites and their Applications in Water Treatment—A Review. Chem. Eng. J. 2021, 405, 126980. [Google Scholar] [CrossRef]
- Das, R.; Panda, A.B.; Pal, S. Synthesis and characterization of a novel polymeric hydrogel based on hydroxypropyl methyl cellulose grafted with polyacrylamide. Cellulose 2012, 19, 933–945. [Google Scholar] [CrossRef]
- Falua, K.J.; Pokharel, A.; Babaei-Ghazvini, A.; Ai, Y.; Acharya, B. Valorization of Starch to Biobased Materials: A Review. Polymers 2022, 14, 2215. [Google Scholar] [CrossRef]
- Kolya, H.; Das, S.; Tripathy, T. Synthesis of Starch-g-Poly-(N-methylacrylamide-co-acrylic acid) and its application for the removal of mercury (II) from aqueous solution by adsorption. Eur. Polym. J. 2014, 58, 1–10. [Google Scholar] [CrossRef]
- Kolya, H.; Roy, A.; Tripathy, T. Starch-g-Poly-(N, N-dimethyl acrylamide-co-acrylic acid): An efficient Cr (VI) ion binder. Int. J. Biol. Macromol. 2015, 72, 560–568. [Google Scholar] [CrossRef]
- Kolya, H.; Tripathy, T. Preparation, investigation of metal ion removal and flocculation performances of grafted hydroxyethyl starch. Int. J. Biol. Macromol. 2013, 62, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Guleria, S.; Singh, H.; Sharma, V.; Bhardwaj, N.; Arya, S.K.; Puri, S.; Khatri, M. Polyhydroxyalkanoates production from domestic waste feedstock: A sustainable approach towards bio-economy. J. Clean. Prod. 2022, 340, 130661. [Google Scholar] [CrossRef]
- Che, L.; Jin, W.; Zhou, X.; Han, W.; Chen, Y.; Chen, C.; Jiang, G. Current status and future perspectives on the biological production of polyhydroxyalkanoates. Asia-Pacific J. Chem. Eng. 2023, 18, e2899. [Google Scholar] [CrossRef]
- Liu, B.; Wen, Q.; Huang, L.; Chen, Z.; Lin, X.; Liu, S. Insights into integration of polyhydroxyalkanoates (PHAs) production into wastewater treatment: Comparison of different electron acceptors on system function and PHA-producer enrichment. Chem. Eng. J. 2023, 451, 138631. [Google Scholar] [CrossRef]
- Palaniraj, A.; Jayaraman, V. Production, recovery and applications of xanthan gum by Xanthomonas campestris. J. Food Eng. 2011, 106, 1–12. [Google Scholar] [CrossRef]
- Shekarforoush, E.; Faralli, A.; Ndoni, S.; Mendes, A.C.; Chronakis, I.S. Electrospinning of Xanthan Polysaccharide. Macromol. Mater. Eng. 2017, 302, 1700067. [Google Scholar] [CrossRef]
- Maji, B.; Maiti, S. Chemical modification of xanthan gum through graft copolymerization: Tailored properties and potential applications in drug delivery and wastewater treatment. Carbohydr. Polym. 2021, 251, 117095. [Google Scholar] [CrossRef]
- Kolya, H.; Tripathy, T.; De, B.R. Flocculation Performance of Grafted Xanthangum: A Comparative Study. J. Phys. Sci. 2012, 16, 221–234. [Google Scholar]
- Brethauer, S.; Shahab, R.L.; Studer, M.H. Impacts of biofilms on the conversion of cellulose. Appl. Microbiol. Biotechnol. 2020, 104, 5201–5212. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, M.; Yu, X.; Niu, N.; Chen, L. Application of lignin adsorbent in wastewater Treatment: A review. Sep. Purif. Technol. 2022, 302, 122116. [Google Scholar] [CrossRef]
- Beck, R.J.; Zhao, Y.; Fong, H.; Menkhaus, T.J. Electrospun lignin carbon nanofiber membranes with large pores for highly efficient adsorptive water treatment applications. J. Water Process Eng. 2017, 16, 240–248. [Google Scholar] [CrossRef]
- del Orta, M.M.; Martín, J.; Santos, J.L.; Aparicio, I.; Medina-Carrasco, S.; Alonso, E. Biopolymer-clay nanocomposites as novel and ecofriendly adsorbents for environmental remediation. Appl. Clay Sci. 2020, 198, 105838. [Google Scholar] [CrossRef]
- Biswas, S.; Pal, A. Application of biopolymers as a new age sustainable material for surfactant adsorption: A brief review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100145. [Google Scholar] [CrossRef]
- Paul, A.; Sharma, S.S. Handbook of Biopolymers; Thomas, S., Ajitha, A.R., Jose Chirayil, C., Thomas, B., Eds.; Springer Nature: Singapore, 2023; pp. 523–550. ISBN 978-981-19-0710-4. [Google Scholar]
- Thakur, K.; Kandasubramanian, B. Graphene and graphene oxide-based composites for removal of organic pollutants: A review. J. Chem. Eng. Data 2019, 64, 833–867. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene Oxide, Highly Reduced Graphene Oxide, and Graphene: Versatile Building Blocks for Carbon-Based Materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Fahim, I.S. Graphene Reinforced Biopolymer Nanocomposites for Water Filtration Applications BT—Graphene Based Biopolymer Nanocomposites; Sharma, B., Jain, P., Eds.; Springer: Singapore, 2021; pp. 221–232. ISBN 978-981-15-9180-8. [Google Scholar]
- Dimapilis, E.A.S.; Hsu, C.-S.; Mendoza, R.M.O.; Lu, M.-C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M.; Aragaw, B.A. Iron-based nanoparticles in wastewater treatment: A review on synthesis methods, applications, and removal mechanisms. J. Saudi Chem. Soc. 2021, 25, 101280. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.-W. Biogenic Synthesis of Silver-Iron Oxide Nanoparticles Using Kulekhara Leaves Extract for Removing Crystal Violet and Malachite Green Dyes from Water. Sustainability 2022, 14, 15800. [Google Scholar] [CrossRef]
- García, A.; Rodríguez, B.; Giraldo, H.; Quintero, Y.; Quezada, R.; Hassan, N.; Estay, H. Copper-Modified Polymeric Membranes for Water Treatment: A Comprehensive Review. Membranes 2021, 11, 93. [Google Scholar] [CrossRef]
- Kolya, H.; Kuila, T.; Kim, N.H.; Lee, J.H. Bioinspired silver nanoparticles/reduced graphene oxide nanocomposites for catalytic reduction of 4-nitrophenol, organic dyes and act as energy storage electrode material. Compos. Part B Eng. 2019, 173, 106924. [Google Scholar] [CrossRef]
- Safat, S.; Buazar, F.; Albukhaty, S.; Matroodi, S. Enhanced sunlight photocatalytic activity and biosafety of marine-driven synthesized cerium oxide nanoparticles. Sci. Rep. 2021, 11, 14734. [Google Scholar] [CrossRef] [PubMed]
- Fatima, H.; Azhar, M.R.; Zhong, Y.; Arafat, Y.; Khiadani, M.; Shao, Z. Rational design of ZnO-zeolite imidazole hybrid nanoparticles with reduced charge recombination for enhanced photocatalysis. J. Colloid Interface Sci. 2022, 614, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Celik Karakaya, M.; Karakaya, N.; Aydin, M.E. Effective removal of selected pharmaceuticals from sewerage treatment plant effluent using natural clay (Na-montmorillonite). Appl. Water Sci. 2023, 13, 129. [Google Scholar] [CrossRef]
- Makaremi, M.; De Silva, R.T.; Pasbakhsh, P. Electrospun Nanofibrous Membranes of Polyacrylonitrile/Halloysite with Superior Water Filtration Ability. J. Phys. Chem. C 2015, 119, 7949–7958. [Google Scholar] [CrossRef]
- Zhu, J.; Tian, M.; Zhang, Y.; Zhang, H.; Liu, J. Fabrication of a novel “loose” nanofiltration membrane by facile blending with Chitosan–Montmorillonite nanosheets for dyes purification. Chem. Eng. J. 2015, 265, 184–193. [Google Scholar] [CrossRef]
- Pasbakhsh, P.; Churchman, G.J.; Keeling, J.L. Characterisation of properties of various halloysites relevant to their use as nanotubes and microfibre fillers. Appl. Clay Sci. 2013, 74, 47–57. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, J.; Liu, M. Interphase in Polymer Nanocomposites. JACS Au 2022, 2, 280–291. [Google Scholar] [CrossRef]
- Das, R.; Ali, M.E.; Hamid, S.B.A.; Ramakrishna, S.; Chowdhury, Z.Z. Carbon nanotube membranes for water purification: A bright future in water desalination. Desalination 2014, 336, 97–109. [Google Scholar] [CrossRef]
- Aiswarya, R.; Kalaivani, T. Chemically Modified Carbon Nanotubes in Membrane Separation. In Chemically Modified Carbon Nanotubes for Commercial Applications; John Wiley & Sons: Newark, NJ, USA, 2023; pp. 169–195. [Google Scholar]
- Noamani, S.; Niroomand, S.; Rastgar, M.; Sadrzadeh, M. Carbon-based polymer nanocomposite membranes for oily wastewater treatment. npj Clean Water 2019, 2, 20. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; Mckay, G.; Manawi, Y.; Malaibari, Z.; Hussien, M.A. Outstanding adsorption performance of high aspect ratio and super-hydrophobic carbon nanotubes for oil removal. Chemosphere 2016, 164, 142–155. [Google Scholar] [CrossRef]
- Mazrouaa, A.M.; Mousa, A.A.; Mohamed, M.G. Chemically Modified Carbon Nanotubes for Water Purification System. In Chemically Modified Carbon Nanotubes for Commercial Applications; John Wiley & Sons: Newark, NJ, USA, 2023; pp. 197–214. [Google Scholar]
- Akpotu, S.O.; Diagboya, P.N.; Lawal, I.A.; Sanni, S.O.; Pholosi, A.; Peleyeju, M.G.; Mtunzi, F.M.; Ofomaja, A.E. Designer composite of montmorillonite-reduced graphene oxide-PEG polymer for water treatment: Enrofloxacin sequestration and cost analysis. Chem. Eng. J. 2023, 453, 139771. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Wang, X.; Ma, Y.; Yang, Y.; Zhuang, L.; Zhang, S.; Jehan, R.; Chen, J.; Wang, X. Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: A review. Environ. Pollut. 2019, 252, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M. Polymer Nanocomposites—A Comparison between Carbon Nanotubes, Graphene, and Clay as Nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Klopocinska, A.; Horvat, K.; Abdel Hamid, Z. Graphene-Based Nanocomposites: Synthesis, Mechanical Properties, and Characterizations. Polymers 2021, 13, 2869. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Singha, N.R. Polymeric Nanocomposite Membranes for Next Generation Pervaporation Process: Strategies, Challenges and Future Prospects. Membranes 2017, 7, 53. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.-H. Advances in Functional Biopolymer-Based Nanocomposites for Active Food Packaging Applications. Polymers 2021, 13, 4198. [Google Scholar] [CrossRef]
- Gangarapu, S.; Sunku, K.; Babu, P.S.; Sudarsanam, P. Fabrication of Polymer-Graphene Nanocomposites BT—Handbook of Polymer and Ceramic Nanotechnology; Hussain, C.M., Thomas, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–15. ISBN 978-3-030-10614-0. [Google Scholar]
- Poshina, D.; Otsuka, I. Electrospun Polysaccharidic Textiles for Biomedical Applications. Textiles 2021, 1, 152–169. [Google Scholar] [CrossRef]
- Ghorbani, M.; Hassan Vakili, M.; Ameri, E. Fabrication and evaluation of a biopolymer-based nanocomposite membrane for oily wastewater treatment. Mater. Today Commun. 2021, 28, 102560. [Google Scholar] [CrossRef]
- Valandro, S.R.; Lombardo, P.C.; Poli, A.L.; Horn, M.A., Jr.; Neumann, M.G.; Cavalheiro, C.C.S. Thermal properties of poly (methyl methacrylate)/organomodified montmorillonite nanocomposites obtained by in situ photopolymerization. Mater. Res. 2014, 17, 265–270. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.W. Polyvinyl acetate/reduced graphene oxide-poly (diallyl dimethylammonium chloride) composite coated wood surface reveals improved hydrophobicity. Prog. Org. Coat. 2021, 156, 106253. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. Application of adsorption process for effective removal of emerging contaminants from water and wastewater. Environ. Pollut. 2021, 280, 116995. [Google Scholar] [CrossRef]
- Yu, C.; Han, X. Adsorbent material used in water treatment-a review. In Proceedings of the 2015 2nd International Workshop on Materials Engineering and Computer Sciences, Jinan, China, 10–11 October 2015; Atlantis Press: Amsterdam, The Netherlands, 2015; pp. 286–289. [Google Scholar]
- Saleem, J.; Bin Shahid, U.; Hijab, M.; Mackey, H.; McKay, G. Production and applications of activated carbons as adsorbents from olive stones. Biomass Convers. Biorefinery 2019, 9, 775–802. [Google Scholar] [CrossRef]
- Biswas, S.; Fatema, J.; Debnath, T.; Rashid, T.U. Chitosan–Clay Composites for Wastewater Treatment: A State-of-the-Art Review. ACS EST Water 2021, 1, 1055–1085. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Banerjee, B.; Ghorai, S.; Rana, D.; Roy, I.; Sarkar, G.; Saha, N.R.; De, S.; Ghosh, T.K.; Sadhukhan, S.; et al. Development of an auto-phase separable and reusable graphene oxide-potato starch based cross-linked bio-composite adsorbent for removal of methylene blue dye. Int. J. Biol. Macromol. 2018, 116, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yang, M.; Xu, W.; He, S.; Men, Y. Natural polysaccharides-modified graphene oxide for adsorption of organic dyes from aqueous solutions. J. Colloid Interface Sci. 2017, 486, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Politaeva, N.; Yakovlev, A.; Yakovleva, E.; Chelysheva, V.; Tarantseva, K.; Efremova, S.; Ilyashenko, S. Graphene Oxide-Chitosan Composites for Water Treatment from Copper. Water 2022, 14, 1430. [Google Scholar] [CrossRef]
- Trang, T.T.C.; Takaomi, K. Chitosan and its biomass composites in application for water treatment. Curr. Opin. Green Sustain. Chem. 2021, 29, 100429. [Google Scholar] [CrossRef]
- Zhao, X.-R.; Xu, X.; Teng, J.; Zhou, N.; Zhou, Z.; Jiang, X.-Y.; Jiao, F.-P.; Yu, J.-G. Three-dimensional porous graphene oxide-maize amylopectin composites with controllable pore-sizes and good adsorption-desorption properties: Facile fabrication and reutilization, and the adsorption mechanism. Ecotoxicol. Environ. Saf. 2019, 176, 11–19. [Google Scholar] [CrossRef]
- Vo, T.S.; Vo, T.T.B.C. Organic dye removal and recycling performances of graphene oxide-coated biopolymer sponge. Prog. Nat. Sci. Mater. Int. 2022, 32, 634–642. [Google Scholar] [CrossRef]
- Bernard, K.N.M.; Prakash, O.; Hippargi, G.; Sylvere, N.K.; Joseph, K.G.; Pal, S. Exploring the applicability of a geopolymer and a biopolymer as an environmentally benign treatment option for heavy metals contaminated water. J. Taiwan Inst. Chem. Eng. 2022, 135, 104392. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Ramin, S. Fabrication of starch-graft-poly(acrylamide)/graphene oxide/hydroxyapatite nanocomposite hydrogel adsorbent for removal of malachite green dye from aqueous solution. Int. J. Biol. Macromol. 2018, 106, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Chang, P.R.; Zheng, P.; Zhao, F.; Ma, X. Fabrication of ultra-light graphene-based gels and their adsorption of methylene blue. Chem. Eng. J. 2014, 240, 595–600. [Google Scholar] [CrossRef]
- Li, Y.; Du, Q.; Liu, T.; Peng, X.; Wang, J.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y.; et al. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem. Eng. Res. Des. 2013, 91, 361–368. [Google Scholar] [CrossRef]
- Zare, E.N.; Lakouraj, M.M.; Kasirian, N. Development of effective nano-biosorbent based on poly m-phenylenediamine grafted dextrin for removal of Pb (II) and methylene blue from water. Carbohydr. Polym. 2018, 201, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Zeng, X.; Xu, X.; Yu, J.-G. Assembly of a novel porous 3D graphene oxide-starch architecture by a facile hydrothermal method and its adsorption properties toward metal ions. Mater. Lett. 2018, 214, 31–33. [Google Scholar] [CrossRef]
- Kumari, N.; Mishra, S. Synthesis, characterization and flocculation efficiency of grafted Moringa gum based derivatives. Carbohydr. Polym. 2022, 281, 119079. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, S.; He, F.; Liu, Y.; Mao, W.; Guan, Y. Highly efficient and selective capture of heavy metals by poly(acrylic acid) grafted chitosan and biochar composite for wastewater treatment. Chem. Eng. J. 2019, 378, 122215. [Google Scholar] [CrossRef]
- Sirajudheen, P.; Karthikeyan, P.; Ramkumar, K.; Meenakshi, S. Effective removal of organic pollutants by adsorption onto chitosan supported graphene oxide-hydroxyapatite composite: A novel reusable adsorbent. J. Mol. Liq. 2020, 318, 114200. [Google Scholar] [CrossRef]
- Travlou, N.A.; Kyzas, G.Z.; Lazaridis, N.K.; Deliyanni, E.A. Graphite oxide/chitosan composite for reactive dye removal. Chem. Eng. J. 2013, 217, 256–265. [Google Scholar] [CrossRef]
- Lee, S.; Lingamdinne, L.P.; Yang, J.-K.; Koduru, J.R.; Chang, Y.-Y.; Naushad, M. Biopolymer mixture-entrapped modified graphene oxide for sustainable treatment of heavy metal contaminated real surface water. J. Water Process Eng. 2022, 46, 102631. [Google Scholar] [CrossRef]
- Bahador, F.; Foroutan, R.; Esmaeili, H.; Ramavandi, B. Enhancement of the chromium removal behavior of Moringa oleifera activated carbon by chitosan and iron oxide nanoparticles from water. Carbohydr. Polym. 2021, 251, 117085. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, L.; Ma, J.; Tian, Y. Preparation of graphene oxide/chitosan complex and its adsorption properties for heavy metal ions. Green Process. Synth. 2020, 9, 294–303. [Google Scholar] [CrossRef]
- Pathirana, M.A.; Dissanayake, N.S.L.; Wanasekara, N.D.; Mahltig, B.; Nandasiri, G.K. Chitosan-Graphene Oxide Dip-Coated Polyacrylonitrile-Ethylenediamine Electrospun Nanofiber Membrane for Removal of the Dye Stuffs Methylene Blue and Congo Red. Nanomaterials 2023, 13, 498. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tian, J.; Li, Y.; Sun, N.; Mi, S.; Xie, Y.; Chen, Z. Enhanced dyes adsorption from wastewater via Fe3O4 nanoparticles functionalized activated carbon. J. Hazard. Mater. 2019, 373, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Yang, C.-H.; Huang, K.-S.; Yeh, C.-S.; Wang, A.H.-J.; Chen, C.-H. Electrostatic droplets assisted in situ synthesis of superparamagnetic chitosan microparticles for magnetic-responsive controlled drug release and copper ion removal. J. Mater. Chem. B 2013, 1, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, X.; Liu, Y.; Zhang, T. Review on preparation and adsorption properties of chitosan and chitosan composites. Polym. Bull. 2021, 79, 2633–2665. [Google Scholar] [CrossRef]
- Mallik, A.K.; Kabir, S.M.F.; Bin Abdur Rahman, F.; Sakib, M.N.; Efty, S.S.; Rahman, M.M. Cu(II) removal from wastewater using chitosan-based adsorbents: A review. J. Environ. Chem. Eng. 2022, 10, 108048. [Google Scholar] [CrossRef]
- Wang, M.; You, X. Critical review of magnetic polysaccharide-based adsorbents for water treatment: Synthesis, application and regeneration. J. Clean. Prod. 2021, 323, 129118. [Google Scholar] [CrossRef]
- Van Hoa, N.; Khong, T.T.; Thi Hoang Quyen, T.; Si Trung, T. One-step facile synthesis of mesoporous graphene/Fe3O4/chitosan nanocomposite and its adsorption capacity for a textile dye. J. Water Process Eng. 2016, 9, 170–178. [Google Scholar] [CrossRef]
- Jazzar, A.; Alamri, H.; Malajati, Y.; Mahfouz, R.; Bouhrara, M.; Fihri, A. Recent advances in the synthesis and applications of magnetic polymer nanocomposites. J. Ind. Eng. Chem. 2021, 99, 1–18. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Bediako, J.K.; Choi, J.-W.; Yun, Y.-S. Functionalized magnetic biopolymeric graphene oxide with outstanding performance in water purification. NPG Asia Mater. 2019, 11, 4. [Google Scholar] [CrossRef]
- Saya, L.; Malik, V.; Singh, A.; Singh, S.; Gambhir, G.; Singh, W.R.; Chandra, R.; Hooda, S. Guar gum based nanocomposites: Role in water purification through efficient removal of dyes and metal ions. Carbohydr. Polym. 2021, 261, 117851. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Abedin-Moghanaki, A.; Tavakoli, A. Efficient removal of cationic dyes using a new magnetic nanocomposite based on starch-g-poly(vinylalcohol) and functionalized with sulfate groups. RSC Adv. 2016, 6, 38042–38051. [Google Scholar] [CrossRef]
- Stan, M.; Lung, I.; Soran, M.-L.; Opris, O.; Leostean, C.; Popa, A.; Copaciu, F.; Lazar, M.D.; Kacso, I.; Silipas, T.-D.; et al. Starch-coated green synthesized magnetite nanoparticles for removal of textile dye Optilan Blue from aqueous media. J. Taiwan Inst. Chem. Eng. 2019, 100, 65–75. [Google Scholar] [CrossRef]
- Xie, G.; Xi, P.; Liu, H.; Chen, F.; Huang, L.; Shi, Y.; Hou, F.; Zeng, Z.; Shao, C.; Wang, J. A facile chemical method to produce superparamagnetic graphene oxide–Fe3O4 hybrid composite and its application in the removal of dyes from aqueous solution. J. Mater. Chem. 2012, 22, 1033–1039. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Saber-Samandari, S.; Nezafati, N.; Yahya, K. Efficient removal of lead (II) ions and methylene blue from aqueous solution using chitosan/Fe-hydroxyapatite nanocomposite beads. J. Environ. Manag. 2014, 146, 481–490. [Google Scholar] [CrossRef]
- Wang, D.; Liu, L.; Jiang, X.; Yu, J.; Chen, X. Adsorption and removal of malachite green from aqueous solution using magnetic β-cyclodextrin-graphene oxide nanocomposites as adsorbents. Colloids Surf. A Physicochem. Eng. Asp. 2015, 466, 166–173. [Google Scholar] [CrossRef]
- Karimi, F.; Ayati, A.; Tanhaei, B.; Sanati, A.L.; Afshar, S.; Kardan, A.; Dabirifar, Z.; Karaman, C. Removal of metal ions using a new magnetic chitosan nano-bio-adsorbent; A powerful approach in water treatment. Environ. Res. 2022, 203, 111753. [Google Scholar] [CrossRef]
- He, H.; Meng, X.; Yue, Q.; Yin, W.; Gao, Y.; Fang, P.; Shen, L. Thiol-ene click chemistry synthesis of a novel magnetic mesoporous silica/chitosan composite for selective Hg(II) capture and high catalytic activity of spent Hg(II) adsorbent. Chem. Eng. J. 2021, 405, 126743. [Google Scholar] [CrossRef]
- Zheng, C.; Zheng, H.; Hu, C.; Wang, Y.; Wang, Y.; Zhao, C.; Ding, W.; Sun, Q. Structural design of magnetic biosorbents for the removal of ciprofloxacin from water. Bioresour. Technol. 2020, 296, 122288. [Google Scholar] [CrossRef]
- Zheng, X.; Zheng, H.; Xiong, Z.; Zhao, R.; Liu, Y.; Zhao, C.; Zheng, C. Novel anionic polyacrylamide-modify-chitosan magnetic composite nanoparticles with excellent adsorption capacity for cationic dyes and pH-independent adsorption capability for metal ions. Chem. Eng. J. 2020, 392, 123706. [Google Scholar] [CrossRef]
- Perez, T.; Pasquini, D.; de Faria Lima, A.; Rosa, E.V.; Sousa, M.H.; Cerqueira, D.A.; de Morais, L.C. Efficient removal of lead ions from water by magnetic nanosorbents based on manganese ferrite nanoparticles capped with thin layers of modified biopolymers. J. Environ. Chem. Eng. 2019, 7, 102892. [Google Scholar] [CrossRef]
- Wu, S.; Guo, J.; Wang, Y.; Huang, C.; Hu, Y. Facile preparation of magnetic sodium alginate/carboxymethyl cellulose composite hydrogel for removal of heavy metal ions from aqueous solution. J. Mater. Sci. 2021, 56, 13096–13107. [Google Scholar] [CrossRef]
- Lin, S.; Zou, C.; Liang, H.; Peng, H.; Liao, Y. The effective removal of nickel ions from aqueous solution onto magnetic multi-walled carbon nanotubes modified by β-cyclodextrin. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126544. [Google Scholar] [CrossRef]
- Santhosh, C.; Daneshvar, E.; Tripathi, K.M.; Baltrėnas, P.; Kim, T.; Baltrėnaitė, E.; Bhatnagar, A. Synthesis and characterization of magnetic biochar adsorbents for the removal of Cr(VI) and Acid orange 7 dye from aqueous solution. Environ. Sci. Pollut. Res. 2020, 27, 32874–32887. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Wang, Z.; Zhu, Y. Facile preparation and highly efficient sorption of magnetic composite graphene oxide/Fe3O4/GC for uranium removal. Sci. Rep. 2021, 11, 8440. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Y.; Fu, Y.; Yang, C.; Jiang, J.; Yan, G.; Hu, J. Rapid and high selective removal of Hg(II) ions using tannic acid cross-linking cellulose/polyethyleneimine functionalized magnetic composite. Int. J. Biol. Macromol. 2021, 182, 1120–1129. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Li, X.; Lu, F.; Qiu, H.; Sun, M. Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J. Hazard. Mater. 2012, 215–216, 272–279. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, Y.; Qin, G.; Song, H.; Shu, C.; Zheng, Y.; Ji, S. Designing degradable lignin-grafted magnetic nano-composite materials for cost-effectively sustainable removal of fluoroquinolone antibiotics from environmental water. J. Clean. Prod. 2022, 360, 132215. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, L.; Niu, N. Enhanced adsorption for malachite green by functionalized lignin magnetic composites: Optimization, performance and adsorption mechanism. J. Mol. Struct. 2022, 1260, 132842. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Li, H.; Lu, M.; Xie, J.; Guan, S.; Wang, X.; Liu, X.; Lu, J. Preparation and adsorbability of magnetic composites based on cellulose nanofiber/graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128373. [Google Scholar] [CrossRef]
- Ling, C.; Yimin, D.; Qi, L.; Chengqian, F.; Zhiheng, W.; Yaqi, L.; Ling, C.; Bo, L.; Yue-Fei, Z.; Yan, L.; et al. Novel High-efficiency adsorbent consisting of magnetic Cellulose-based ionic liquid for removal of anionic dyes. J. Mol. Liq. 2022, 353, 118723. [Google Scholar] [CrossRef]
- Chen, B.; Long, F.; Chen, S.; Cao, Y.; Pan, X. Magnetic chitosan biopolymer as a versatile adsorbent for simultaneous and synergistic removal of different sorts of dyestuffs from simulated wastewater. Chem. Eng. J. 2020, 385, 123926. [Google Scholar] [CrossRef]
- Bui, N.T.; Nguyen, V.H.; Le, D.T.; van Tran, T.T.; Bui, T.H. Superparamagnetic cobalt ferric nanoparticles incorporated biopolymers extracted from dragon fruit (hylocereus undatus) peels for nickel(II) removal. Environ. Technol. Innov. 2021, 23, 101773. [Google Scholar] [CrossRef]
- Amini Herab, A.; Salari, D.; Ostadrahimi, A.; Olad, A. Preparation of magnetic inulin nanocomposite and its application in the removal of methylene blue and heavy metals from aqueous solution. Mater. Chem. Phys. 2022, 291, 126580. [Google Scholar] [CrossRef]
- Jiang, R.; Shen, T.-T.; Zhu, H.-Y.; Fu, Y.-Q.; Jiang, S.-T.; Li, J.-B.; Wang, J.-L. Magnetic Fe3O4 embedded chitosan–crosslinked-polyacrylamide composites with enhanced removal of food dye: Characterization, adsorption and mechanism. Int. J. Biol. Macromol. 2023, 227, 1234–1244. [Google Scholar] [CrossRef]
- Cevallos-Mendoza, J.; Amorim, C.G.; Rodríguez-Díaz, J.M.; Montenegro, M.d.C.B.S.M. Removal of Contaminants from Water by Membrane Filtration: A Review. Membranes 2022, 12, 570. [Google Scholar] [CrossRef]
- Wu, J.; Wang, N.; Wang, L.; Dong, H.; Zhao, Y.; Jiang, L. Unidirectional water-penetration composite fibrous film via electrospinning. Soft Matter 2012, 8, 5996–5999. [Google Scholar] [CrossRef]
- Lee, M.; Wu, Z.; Li, K. 2—Advances in ceramic membranes for water treatment. In Woodhead Publishing Series in Energy; Basile, A., Cassano, A., Rastogi, N.K., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 43–82. ISBN 978-1-78242-121-4. [Google Scholar]
- Matsuyama, H.; Teramoto, M.; Uesaka, T. Membrane formation and structure development by dry-cast process. J. Memb. Sci. 1997, 135, 271–288. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, X.-Y.; Xiao, K.; Liu, Q.; Xie, G.; Li, P.; Ma, J.; Tian, Y.; Wen, L.; Jiang, L. Engineered Asymmetric Heterogeneous Membrane: A Concentration-Gradient-Driven Energy Harvesting Device. J. Am. Chem. Soc. 2015, 137, 14765–14772. [Google Scholar] [CrossRef] [PubMed]
- Schacher, F.H.; Rupar, P.A.; Manners, I. Functional Block Copolymers: Nanostructured Materials with Emerging Applications. Angew. Chemie Int. Ed. 2012, 51, 7898–7921. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Hamilton, A.L.; Delaney, K.T.; Fredrickson, G.H.; Kramer, E.J.; Ntaras, C.; Avgeropoulos, A.; Lynd, N.A. Creating Extremely Asymmetric Lamellar Structures via Fluctuation-Assisted Unbinding of Miktoarm Star Block Copolymer Alloys. J. Am. Chem. Soc. 2015, 137, 6160–6163. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.R.; Roy, U.; Das, P.; Mukhopadhayay, A. Chapter 6—Membrane processes for removal of polyaromatic hydrocarbons from wastewater. In Advances in Green and Sustainable Chemistry; Sharma, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 189–207. ISBN 978-0-12-817742-6. [Google Scholar]
- Kamide, K.; Manabe, S. Mechanism of Permselectivity of Porous Polymeric Membranes in Ultrafiltration Process. Polym. J. 1981, 13, 459–479. [Google Scholar] [CrossRef][Green Version]
- Kolya, H.; Singh, V.K.; Kang, C.-W. Polymeric Membranes and Hybrid Techniques for Water Purification Applications BT—Polymer-Based Advanced Functional Materials for Energy and Environmental Applications; Subramani, N.K., Nataraj, S.K., Patel, C., Shivanna, S., Eds.; Springer: Singapore, 2022; pp. 75–91. ISBN 978-981-16-8755-6. [Google Scholar]
- Baig, N.; Matin, A.; Faizan, M.; Anand, D.; Ahmad, I.; Khan, S.A. Antifouling low-pressure highly permeable single step produced loose nanofiltration polysulfone membrane for efficient Erichrome Black T/divalent salts fractionation. J. Environ. Chem. Eng. 2022, 10, 108166. [Google Scholar] [CrossRef]
- Guo, S.; Wan, Y.; Chen, X.; Luo, J. Loose nanofiltration membrane custom-tailored for resource recovery. Chem. Eng. J. 2021, 409, 127376. [Google Scholar] [CrossRef]
- Feng, X.; Peng, D.; Zhu, J.; Wang, Y.; Zhang, Y. Recent advances of loose nanofiltration membranes for dye/salt separation. Sep. Purif. Technol. 2022, 285, 120228. [Google Scholar] [CrossRef]
- Hakami, M.W.; Alkhudhiri, A.; Al-Batty, S.; Zacharof, M.-P.; Maddy, J.; Hilal, N. Ceramic Microfiltration Membranes in Wastewater Treatment: Filtration Behavior, Fouling and Prevention. Membranes 2020, 10, 248. [Google Scholar] [CrossRef]
- Kotobuki, M.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-Polymer Composite Membranes for Water and Wastewater Treatment: Bridging the Big Gap between Ceramics and Polymers. Molecules 2021, 26, 3331. [Google Scholar] [CrossRef]
- Barhoum, A.; Deshmukh, K.; García-Betancourt, M.-L.; Alibakhshi, S.; Mousavi, S.M.; Meftahi, A.; Sabery, M.S.K.; Samyn, P. Nanocelluloses as sustainable membrane materials for separation and filtration technologies: Principles, opportunities, and challenges. Carbohydr. Polym. 2023, 317, 121057. [Google Scholar] [CrossRef]
- Garcia-Ivars, J.; Alcaina-Miranda, M.-I.; Iborra-Clar, M.-I.; Mendoza-Roca, J.-A.; Pastor-Alcañiz, L. Enhancement in hydrophilicity of different polymer phase-inversion ultrafiltration membranes by introducing PEG/Al2O3 nanoparticles. Sep. Purif. Technol. 2014, 128, 45–57. [Google Scholar] [CrossRef]
- Cai, C.; Sun, W.; He, S.; Zhang, Y.; Wang, X. Ceramic membrane fouling mechanisms and control for water treatment. Front. Environ. Sci. Eng. 2023, 17, 126. [Google Scholar] [CrossRef]
- Mpala, T.J.; Etale, A.; Richards, H.; Nthunya, L.N. Biofouling phenomena in membrane distillation: Mechanisms and mitigation strategies. Environ. Sci. Adv. 2023, 2, 39–54. [Google Scholar] [CrossRef]
- Manjarrez Nevárez, L.; Ballinas Casarrubias, L.; Canto, O.S.; Celzard, A.; Fierro, V.; Ibarra Gómez, R.; González Sánchez, G. Biopolymers-based nanocomposites: Membranes from propionated lignin and cellulose for water purification. Carbohydr. Polym. 2011, 86, 732–741. [Google Scholar] [CrossRef]
- Thakur, S. Gum Based Green Nanocomposites and Their Applications BT—Green-Based Nanocomposite Materials and Applications; Avalos Belmontes, F., González, F.J., López-Manchado, M.Á., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 295–315. ISBN 978-3-031-18428-4. [Google Scholar]
- Mukherjee, M.; De, S. Antibacterial polymeric membranes: A short review. Environ. Sci. Water Res. Technol. 2018, 4, 1078–1104. [Google Scholar] [CrossRef]
- Cheng, M.; Huang, L.; Wang, Y.; Zhao, Y.; Tang, J.; Wang, Y.; Zhang, Y.; Hedayati, M.; Kipper, M.J.; Wickramasinghe, S.R. Synthesis of graphene oxide/polyacrylamide composite membranes for organic dyes/water separation in water purification. J. Mater. Sci. 2019, 54, 252–264. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, J.; Lu, T.; Tang, G.; Wu, S.; Ma, W.; Huang, C. Robust, functionalized reduced graphene-based nanofibrous membrane for contaminated water purification. Chem. Eng. J. 2021, 404, 126347. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Jafari, S.M. Bio-nanocomposites of graphene with biopolymers; fabrication, properties, and applications. Adv. Colloid Interface Sci. 2021, 292, 102416. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Ullah, A. Chapter 14—Biopolymers in Environmental Applications: Industrial Wastewater Treatment; Thomas, S., Gopi, S., Amalraj, A.B.T.-B., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 331–349. ISBN 978-0-12-819240-5. [Google Scholar]
- Suyambulingam, I.; Gangadhar, L.; Sana, S.S.; Divakaran, D.; Siengchin, S.; Kurup, L.A.; Iyyadurai, J.; Albert Bernad Noble, K.E. Chitosan Biopolymer and Its Nanocomposites: Emerging Material as Adsorbent in Wastewater Treatment. Adv. Mater. Sci. Eng. 2023, 2023, 9387016. [Google Scholar] [CrossRef]
- Rouf, T.B.; Kokini, J.L. Biodegradable biopolymer–graphene nanocomposites. J. Mater. Sci. 2016, 51, 9915–9945. [Google Scholar] [CrossRef]
- Leudjo Taka, A.; Klink, M.; Yangkou Mbianda, X.; Mtunzi, F.; Bobby Naidoo, E. Carbon Nanotubes Reinforced Polymeric Hybrid Materials for Water Purification BT—Nanohybrid Materials for Water Purification; Swain, S.K., Ed.; Springer Nature: Singapore, 2022; pp. 197–223. ISBN 978-981-19-2332-6. [Google Scholar]
- Mostafavi, A.H.; Mishra, A.K.; Gallucci, F.; Kim, J.H.; Ulbricht, M.; Coclite, A.M.; Hosseini, S.S. Advances in surface modification and functionalization for tailoring the characteristics of thin films and membranes via chemical vapor deposition techniques. J. Appl. Polym. Sci. 2023, 140, e53720. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, J.; Li, R.; Shi, S.; Gong, Y.-K. Mussel adhesion and cell membrane antifouling mimetic strategies for durable fouling-resistant coating. Prog. Org. Coat. 2023, 182, 107636. [Google Scholar] [CrossRef]
- Dongre, R.S.; Sadasivuni, K.K.; Deshmukh, K.; Mehta, A.; Basu, S.; Meshram, J.S.; Al-Maadeed, M.A.A.; Karim, A. Natural polymer based composite membranes for water purification: A review. Polym. Technol. Mater. 2019, 58, 1295–1310. [Google Scholar] [CrossRef]
- Bessbousse, H.; Verchère, J.-F.; Lebrun, L. Characterisation of metal-complexing membranes prepared by the semi-interpenetrating polymer networks technique. Application to the removal of heavy metal ions from aqueous solutions. Chem. Eng. J. 2012, 187, 16–28. [Google Scholar] [CrossRef]
- Dutta, K.; De, S. Aromatic conjugated polymers for removal of heavy metal ions from wastewater: A short review. Environ. Sci. Water Res. Technol. 2017, 3, 793–805. [Google Scholar] [CrossRef]
- Bessbousse, H.; Rhlalou, T.; Verchère, J.-F.; Lebrun, L. Mercury removal from wastewater using a poly(vinylalcohol)/poly(vinylimidazole) complexing membrane. Chem. Eng. J. 2010, 164, 37–48. [Google Scholar] [CrossRef]
- Refaat Alawady, A.; Ali Alshahrani, A.; Ali Aouak, T.; Mohamed Alandis, N. Polysulfone membranes with CNTs/Chitosan biopolymer nanocomposite as selective layer for remarkable heavy metal ions rejection capacity. Chem. Eng. J. 2020, 388, 124267. [Google Scholar] [CrossRef]
- Saucedo-Rivalcoba, V.; Martínez-Hernández, A.L.; Martínez-Barrera, G.; Velasco-Santos, C.; Rivera-Armenta, J.L.; Castaño, V.M. Removal of Hexavalent Chromium from Water by Polyurethane–Keratin Hybrid Membranes. Water Air Soil Pollut. 2011, 218, 557–571. [Google Scholar] [CrossRef]
- Wu, S.; Li, F.; Wang, H.; Fu, L.; Zhang, B.; Li, G. Effects of poly (vinyl alcohol) (PVA) content on preparation of novel thiol-functionalized mesoporous PVA/SiO2 composite nanofiber membranes and their application for adsorption of heavy metal ions from aqueous solution. Polymer 2010, 51, 6203–6211. [Google Scholar] [CrossRef]
- Terbish, N.; Popuri, S.R.; Lee, C.-H. Improved performance of organic–inorganic nanocomposite membrane for bioelectricity generation and wastewater treatment in microbial fuel cells. Fuel 2023, 332, 126167. [Google Scholar] [CrossRef]
- Barman, S.R.; Banerjee, P.; Mukhopadhayay, A.; Das, P. Biopolymer linked activated carbon-nano-bentonite composite membrane for efficient elimination of PAH mixture from aqueous solutions. Biomass Convers. Biorefinery 2022, 1–15. [Google Scholar] [CrossRef]
- Gajera, R.; Patel, R.V.; Yadav, A.; Labhasetwar, P.K. Adsorption of cationic and anionic dyes on photocatalytic flyash/TiO2 modified chitosan biopolymer composite. J. Water Process Eng. 2022, 49, 102993. [Google Scholar] [CrossRef]
- Kour, G.; Majhi, P.K.; Bharti, A.; Kothari, R.; Jain, A.; Singh, A.; Tyagi, V.V.; Pathania, D. Biopolymer-Based Nanocomposites and Water Treatment: A Global Outlook. In Biorenewable Nanocomposite Materials, Vol. 2: Desalination and Wastewater Remediation; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2022; Volume 1411, pp. 2–25. ISBN 9780841297807. [Google Scholar]
- Osman, A.I.; El-Monaem, E.M.A.; Elgarahy, A.M.; Aniagor, C.O.; Hosny, M.; Farghali, M.; Rashad, E.; Ejimofor, M.I.; López-Maldonado, E.A.; Ihara, I.; et al. Methods to prepare biosorbents and magnetic sorbents for water treatment: A review. Environ. Chem. Lett. 2023, 21, 2337–2398. [Google Scholar] [CrossRef]
- Nure, J.F.; Nkambule, T.T.I. The recent advances in adsorption and membrane separation and their hybrid technologies for micropollutants removal from wastewater. J. Ind. Eng. Chem. 2023, 126, 92–114. [Google Scholar] [CrossRef]
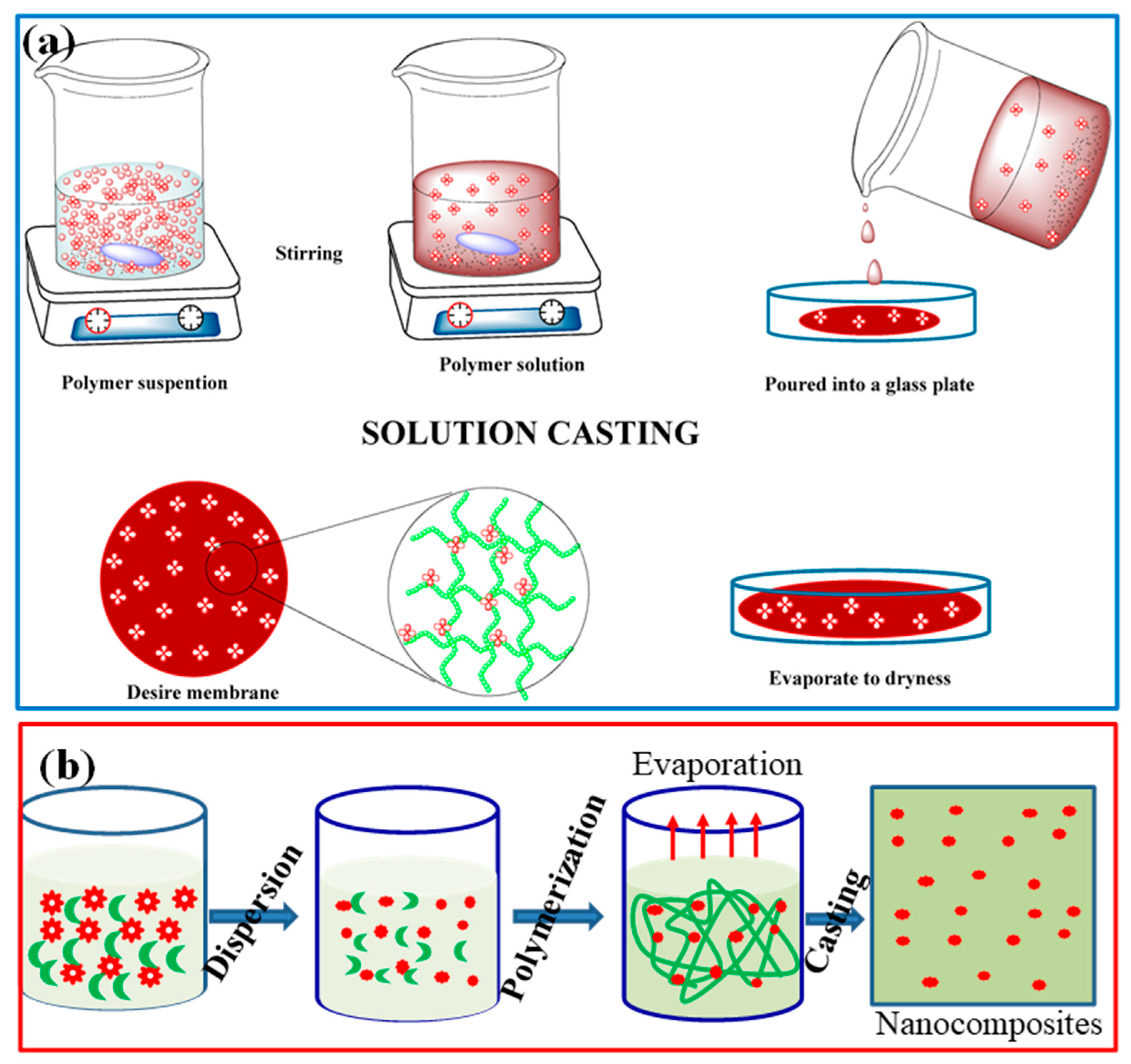
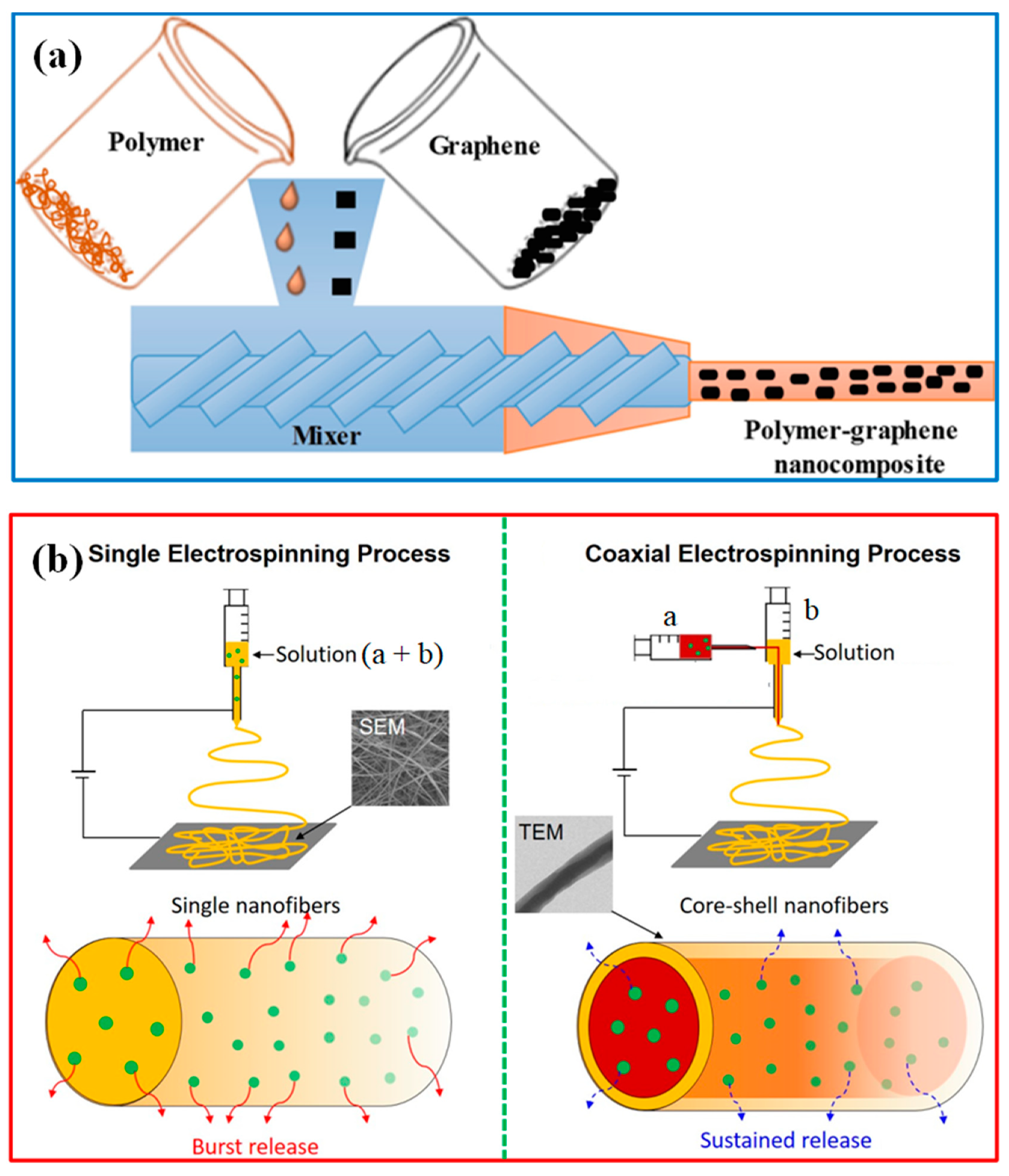
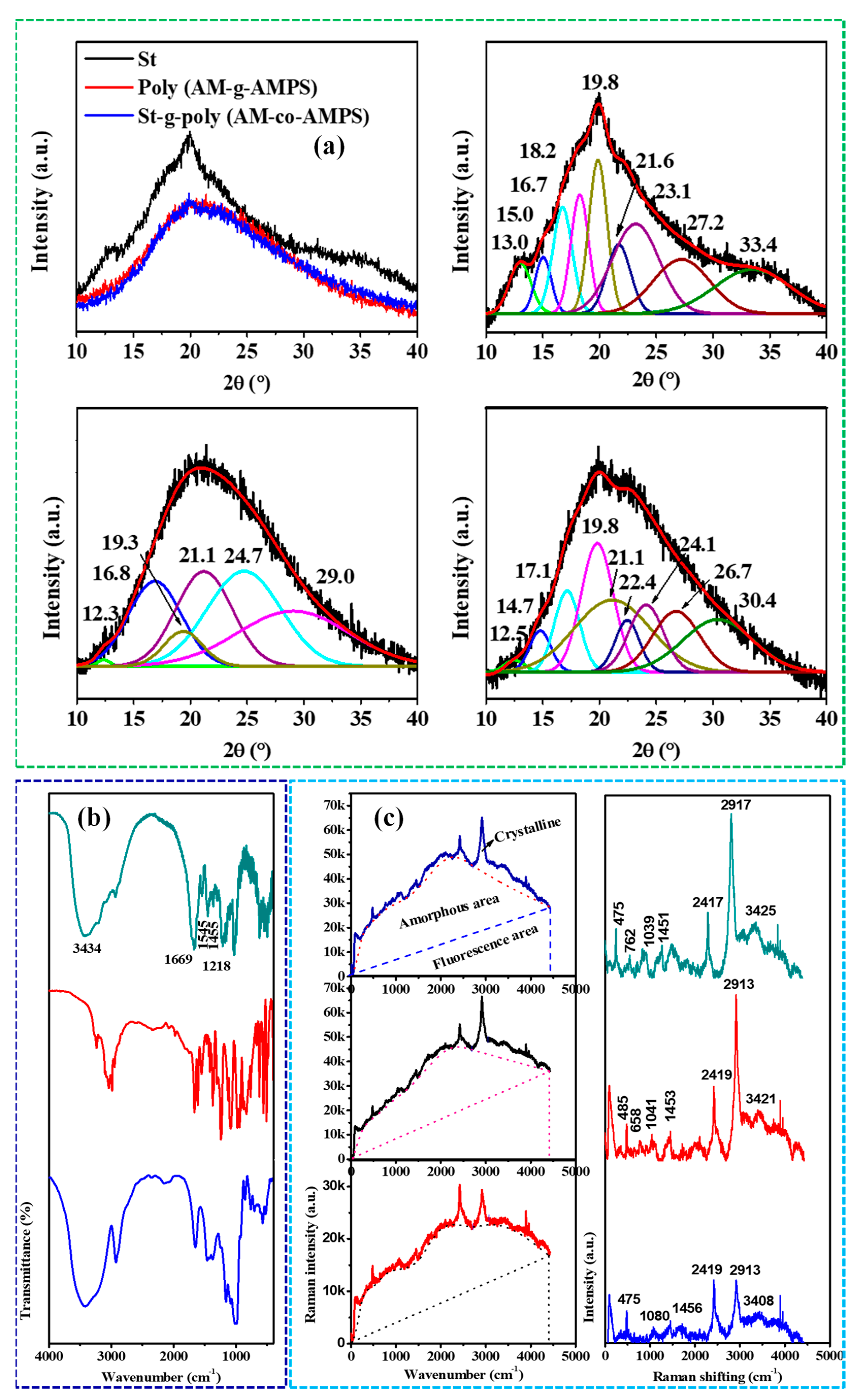
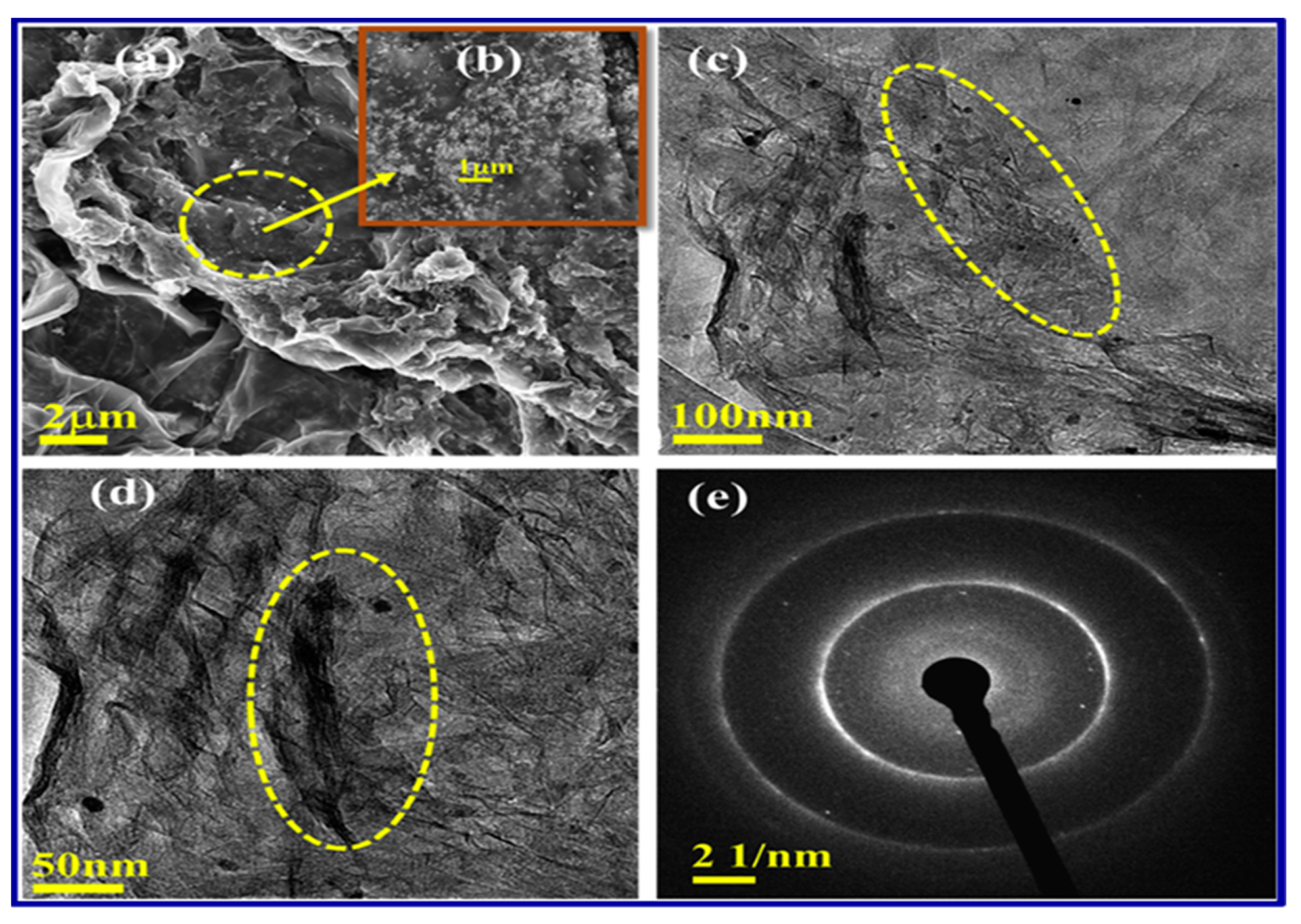
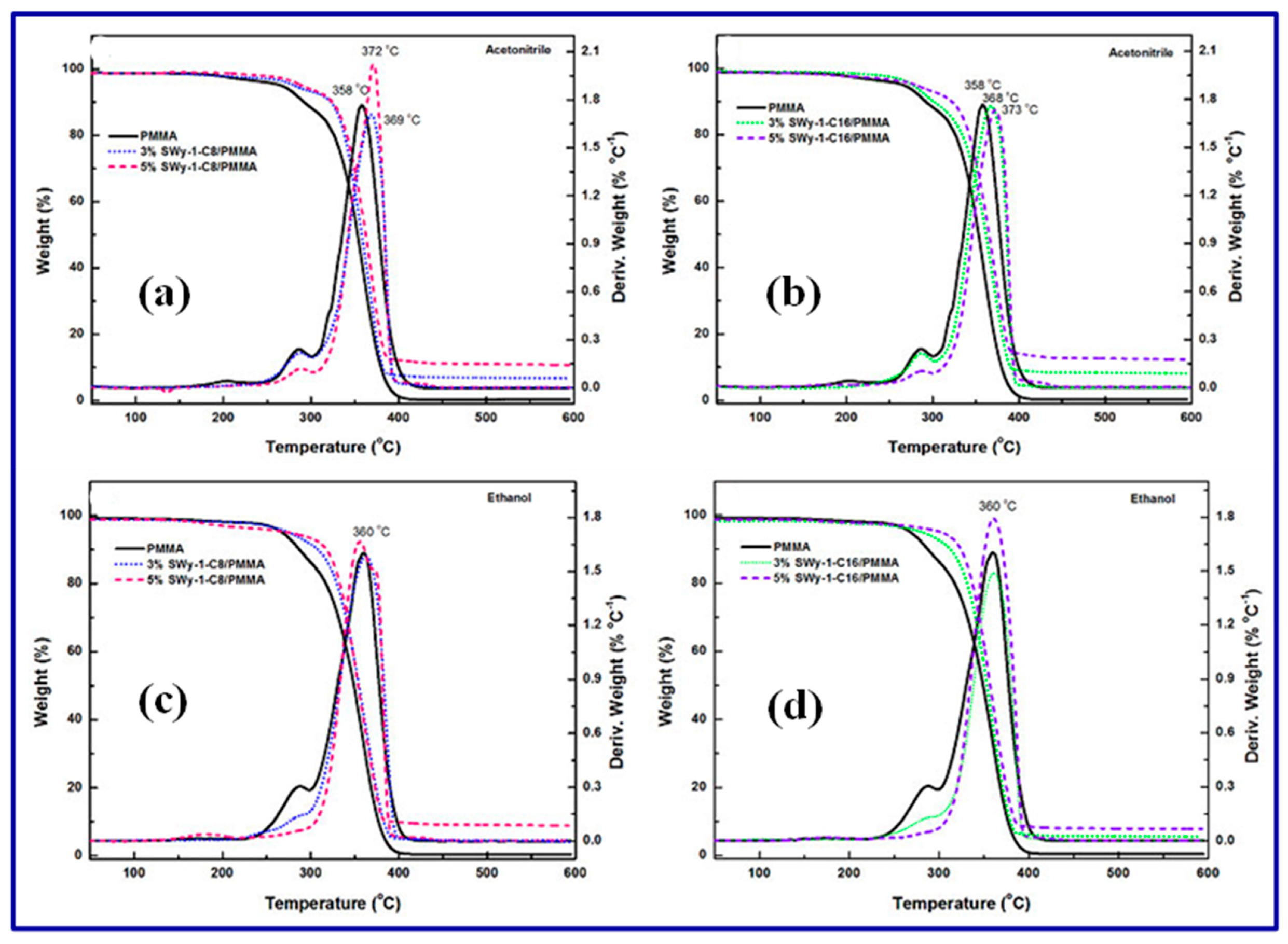
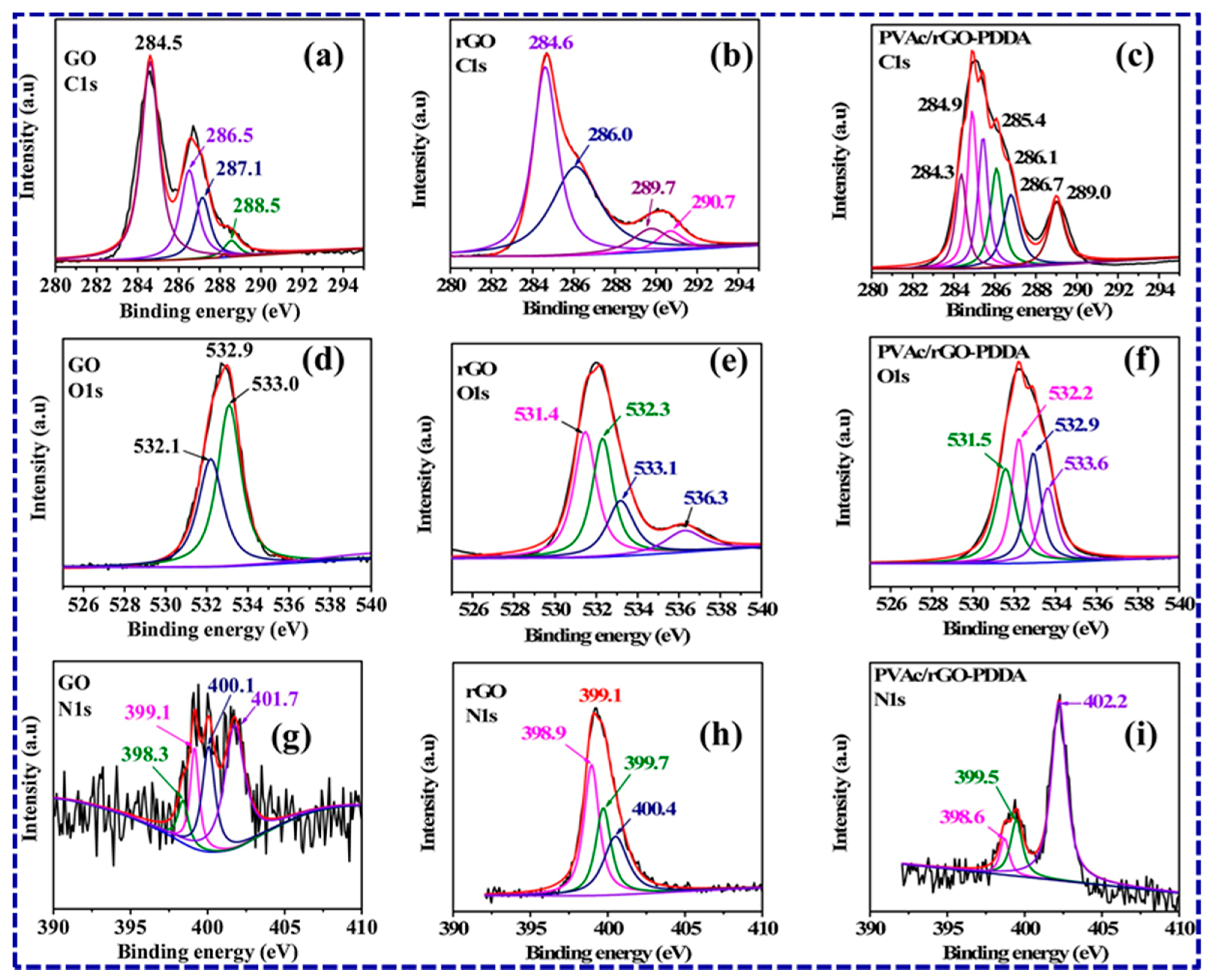
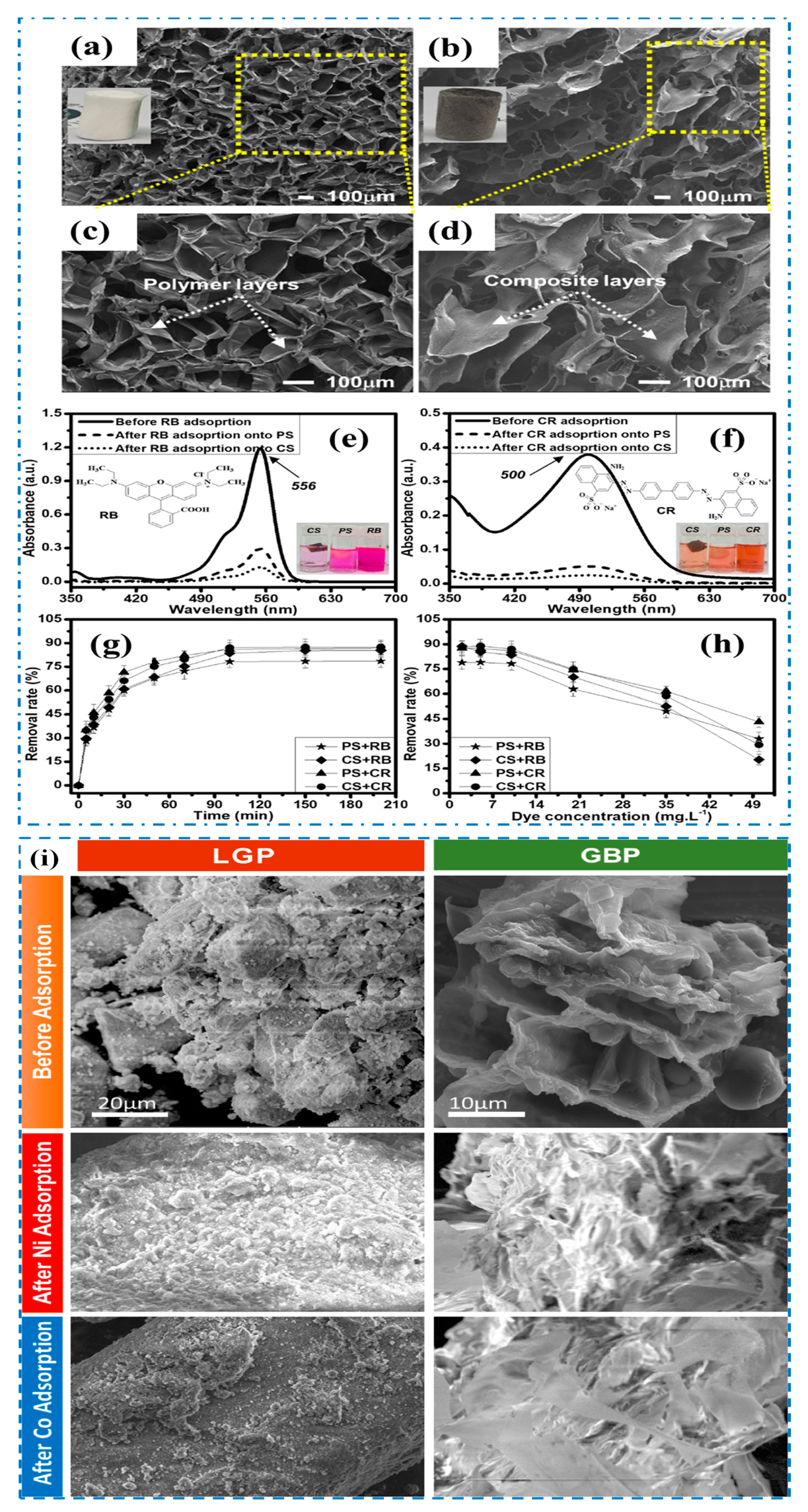

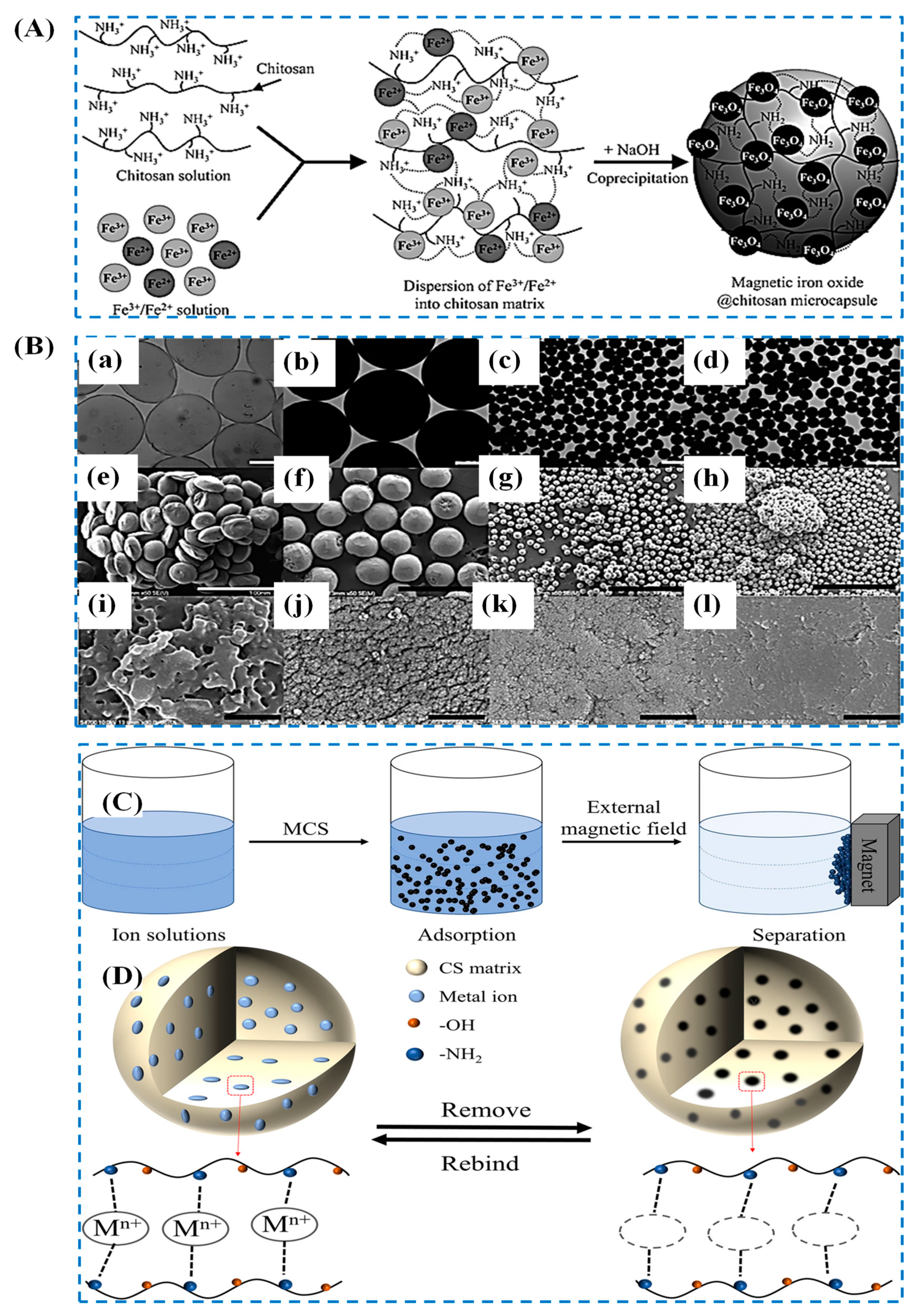


| Sl. No | Adsorbent/Flocculent | Pollutant | Results (% or Qmax mg/g) | Ref. |
|---|---|---|---|---|
| 1. | Fe3O4-Starch-g-PVA | MB MG | 621 mg/g 567 mg/g | [187] |
| 2. | Starch-coated Fe3O4 Nanoparticles | Textile dye Optilan Blue | 74.05 mg/g | [188] |
| 3. | GO–Fe3O4 hybrid composite | MB Natural red | 167.2 mg/g 171.3 mg/g | [189] |
| 4. | Chitosan/Fe–hydroxyapatite beads | MB | 1324 mg/g | [190] |
| 5. | Fe3O4/β-cyclodextrin/GO | MG | 740.7 mg/g | [191] |
| 6. | Magnetic chitosan nano-adsorbent | Cu Cd Zn | 99.9% 95.0% 81.7% | [192] |
| 7. | Magnetic mesoporous silica–chitosan composite | Hg (II) | 437.8 mg/g | [193] |
| 8. | Carboxymethyl-chitosan-based magnetic | Ciprofloxacin | 527.9 mg/g | [194] |
| 9. | Anionic-polyacrylamide-modified chitosan magnetic composite | MB | 1044.1 | [195] |
| 10. | Manganese ferrite nanoparticles covered with carboxymethyl starch | Pb (II) | 34–64 mg/g | [196] |
| 11. | Sodium alginate/CMC/Fe3O4 | Mn (II), Pb (II), Ni (II) | 71.83, 89.49, 105.93 mg/g | [197] |
| 12. | β-cyclodextrin-Fe3O4/MWCNT | Ni (II) | 103 mg/g | [198] |
| 13. | Fe3O4/wood biochar | Acid orange and Cr (VI) | 110.27, 80.96 mg/g | [199] |
| 14. | GO/Fe3O4/glucose | U (VI) | 390.70 mg/g | [200] |
| 15. | Cellulose/polyethyleneimine-Fe3O4 | Hg (II) | 247.51 mg/g | [201] |
| 16. | Chitosan activated carbon-Fe3O4 composites | Cr (VI) | 99.8% | [174] |
| 17. | Magnetic chitosan−GO nanocomposites | MB | 95.31 mg/g, pH 5.3, 303K | [202] |
| 18. | GO-Fe3O4-chitosan composites | MB | 98.0% | [183] |
| 19. | Chitosan/poly (methacrylic acid)/Fe3O4 -GO composite | MB | 2478 mg/g | [185] |
| 20. | Lignin-grafted magnetic nanocomposite | Fluoroquinolone | 108.2 mg/g | [203] |
| 21. | Lignin magnetic composites | Malachite green | 456.62 mg/g | [204] |
| 22. | Cellulose nanofiber–GO magnetic composite | Methylene blue | 83.53% | [205] |
| 23. | Magnetic-cellulose-based ionic liquid | Congo red and methyl blue | 1299.3 and 1068.1 mg/g | [206] |
| 24. | Fe3O4–chitosan composite | Methyl orange | 638.6 mg/g | [207] |
| 25. | Dragon fruit biopolymer–CoFe2O4 | Ni (II) | 88% | [208] |
| 26. | Inulin–Fe3O4 nanocomposite | Co (II), Cu (II), Hg (II) | 152.5, 167.7, 198.0 mg/g | [209] |
| 27. | Fe3O4–chitosan–PAMm composite | Food dye | 359.71 mg/g | [210] |
| Sl No | Membrane | Pollutants | Optimum Rejection (%) | Pressure (bar) | pH | Ref. |
|---|---|---|---|---|---|---|
| 1. | CNTs/chitosan biopolymer nanocomposite | Cu(II), Ni(II), Pb (II), Cd(II), and Co(II) ions. | 71–92.2 | 22 | 3.0 | [246] |
| 2. | Lignin–cellulose citrate | Anions Cations | 12–42 27–54 | - | >7.0 | [230] |
| 3. | Polylactic acid-polybutylene succinate-polypropylene carbonate-polyhydroxybutyrate. | Oil–grease TDS | 98.6 89.15 | 1 4 | - | [150] |
| 4. | Poly (urethane)/keratin biofiber | Cr (VI) | 38.0 11.0 | 0.7 | >8–<4 | [247] |
| 5. | PVA and poly (vinylimidazole) | Hg (II) | 99.4 | 3.0 | 2.5 | [245] |
| 6. | Polyvinyl alcohol/SiO2 composites | Cu (II) | 93.1 | - | 5–6 | [248] |
| 7. | Nanoclay montmorillonite-Chitosan | COD | 65.7 | - | - | [249] |
| 8. | Chitosan-linked activated carbon–nano-bentonite | Polyaromatic hydrocarbons | 99.3 | - | 6.0 | [250] |
| 9. | TiO2 loaded fly ash chitosan composite | Congo red Methylene blue | 98.0 55.7 | - | 1.8 | [251] |
| 10. | Polydopamine-functionalized polysulfone membrane | Eriochrome Black T | 99.9 | 1–3 | - | [221] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolya, H.; Kang, C.-W. Next-Generation Water Treatment: Exploring the Potential of Biopolymer-Based Nanocomposites in Adsorption and Membrane Filtration. Polymers 2023, 15, 3421. https://doi.org/10.3390/polym15163421
Kolya H, Kang C-W. Next-Generation Water Treatment: Exploring the Potential of Biopolymer-Based Nanocomposites in Adsorption and Membrane Filtration. Polymers. 2023; 15(16):3421. https://doi.org/10.3390/polym15163421
Chicago/Turabian StyleKolya, Haradhan, and Chun-Won Kang. 2023. "Next-Generation Water Treatment: Exploring the Potential of Biopolymer-Based Nanocomposites in Adsorption and Membrane Filtration" Polymers 15, no. 16: 3421. https://doi.org/10.3390/polym15163421
APA StyleKolya, H., & Kang, C.-W. (2023). Next-Generation Water Treatment: Exploring the Potential of Biopolymer-Based Nanocomposites in Adsorption and Membrane Filtration. Polymers, 15(16), 3421. https://doi.org/10.3390/polym15163421








