Dye-Doped Polymeric Microplastics: Light Tools for Bioimaging in Test Organisms
Abstract
:1. Introduction
2. Materials and Methods
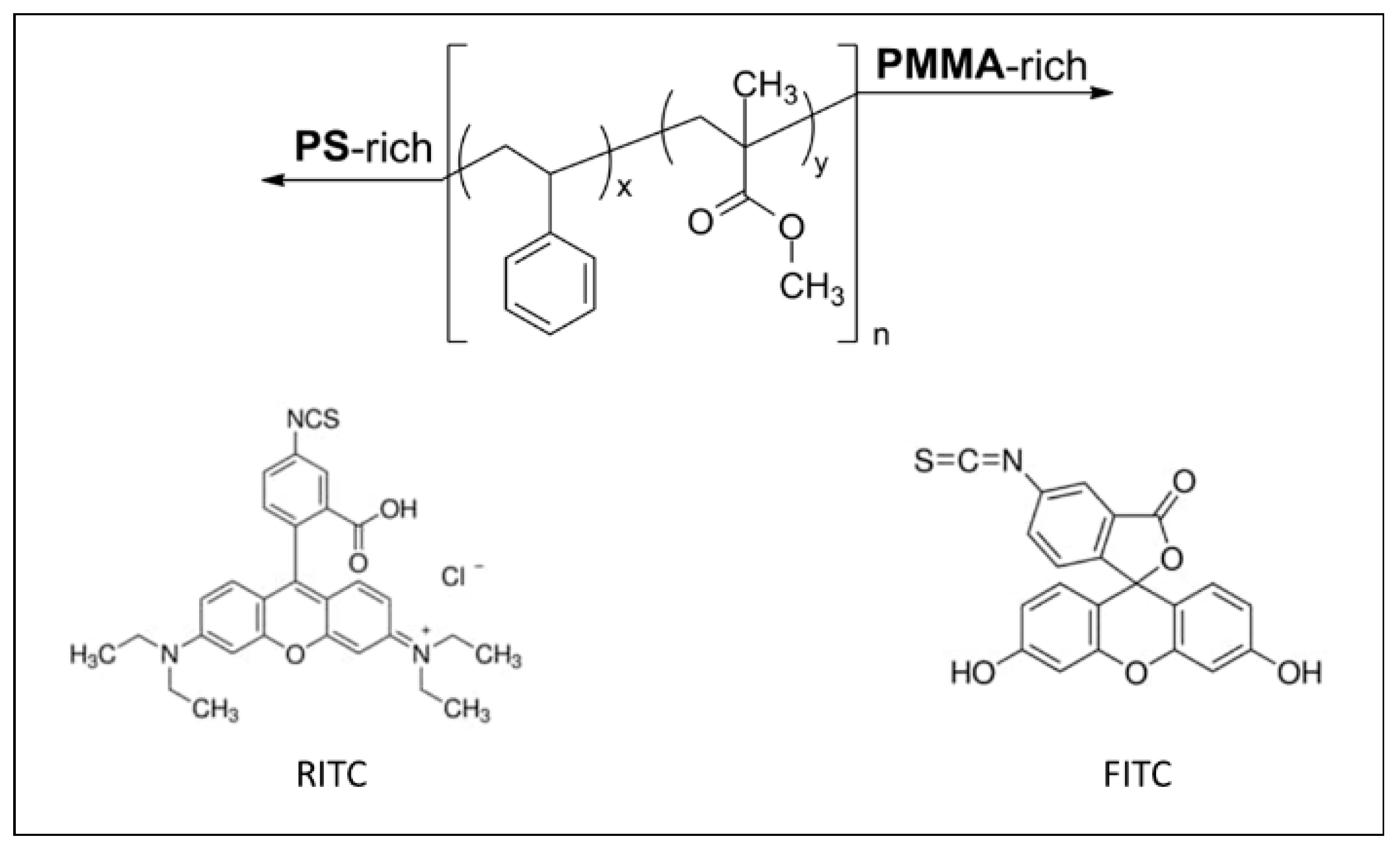
2.1. Materials
2.2. Dye-Doped Polymeric Microplastic Particles Preparation
2.3. Dye-Doped Polymeric Microplastic Particles’ Characterizations
2.4. Bioimaging in Test Organism
3. Results and Discussion
3.1. Dye-Doped MPs: Preparation and SEM Characteriztion
3.2. Dye-Doped MPs: Structural Characterizations
3.3. Bioimaging in Test Organism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Bukusoglu, E.; Miller, D.S.; Bedolla Pantoja, M.A.; Xiang, J.; Lavrentovich, O.D.; Abbott, N.L. Synthesis of Optically Complex, Porous, and Anisometric Polymeric Microparticles by Templating from Liquid Crystalline Droplets. Adv. Funct. Mater. 2016, 26, 7343–7351. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, M.-J.; Chu, L.-Y. Functional Polymeric Microparticles Engineered from Controllable Microfluidic Emulsions. Acc. Chem. Res. 2014, 47, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Venditti, I.; Fratoddi, I.; Palazzesi, C.; Prosposito, P.; Casalboni, M.; Cametti, C.; Battocchio, C.; Polzonetti, G.; Russo, M.V. Self-assembled nanoparticles of functional copolymers for photonic applications. J. Colloids Interface Sci. 2010, 348, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, L.; Xu, J.; Wang, K.; Wang, X.; He, X.; Dong, H.; Lin, S.; Zhu, J. Photoguided Shape Deformation of Azobenzene-Containing Polymer Microparticles. Langmuir ACS J. Surf. Colloids 2015, 31, 13094–13100. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.R.; Ding, Y.B.; Li, X.G.; Liu, Y.; Xi, K.; Gao, C.L.; Kumar, R.V. Synthesis of semiconducting polymer microparticles as solid ionophore with abundant complexing sites for long-life Pb(II) sensors. ACS Appl. Mater. Interfaces 2014, 6, 22096–22107. [Google Scholar] [CrossRef]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef]
- Shan, D.; Gerhard, E.; Zhang, C.; Tierney, J.W.; Xie, D.; Liu, Z.; Yang, J. Polymeric biomaterials for biophotonic applications. Bioact. Mater. 2018, 3, 434–445. [Google Scholar] [CrossRef]
- del Prado Garrido, M.; Borreguero, A.M.; Redondo, F.J.; Padilla, D.; Carmona, M.; Ramos, M.J.; Rodriguez, J.F. Functionalization of Poly(styrene-co-methyl methacrylate) Particles for Selective Removal of Bilirubin. Materials 2022, 15, 5989. [Google Scholar] [CrossRef]
- Takagai, Y.; Nojiri, Y.; Takase, T.; Hinze, W.L.; Butsugan, M.; Igarashi, S. “Turn-on” fluorescent polymeric microparticle sensors for the determination of ammonia and amines in the vapor state. Analyst 2010, 135, 1417–1425. [Google Scholar] [CrossRef]
- Venditti, I.; Barbero, N.; Vittoria Russo, M.; Di Carlo, A.; Decker, F.; Fratoddi, I.; Barolo, C.; Dini, D. Electrodeposited ZnO with squaraine sentisizers as photoactive anode of DSCs. Mater. Res. Express 2014, 1, 015040. [Google Scholar] [CrossRef]
- Duarte, F.; Cuerva, C.; Fernández-Lodeiro, C.; Fernández-Lodeiro, J.; Jiménez, R.; Cano, M.; Lodeiro, C. Polymer Micro and Nanoparticles Containing B(III) Compounds as Emissive Soft Materials for Cargo Encapsulation and Temperature-Dependent Applications. Nanomaterials 2021, 11, 3437. [Google Scholar] [CrossRef] [PubMed]
- Venditti, I.; Cartoni, A.; Fontana, L.; Testa, G.; Scaramuzzo, F.A.; Faccini, R.; Terracciano, C.M.; Camillocci, E.S.; Morganti, S.; Giordano, A.; et al. Y3+ embedded in polymeric nanoparticles: Morphology, dimension and stability of composite colloidal system. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 125–131. [Google Scholar] [CrossRef]
- Balakrishnan, N.K.; Siebert, S.; Richter, C.; Groten, R.; Seide, G. Effect of Colorants and Process Parameters on the Properties of Dope-Dyed Polylactic Acid Multifilament Yarns. Polymers 2022, 14, 5021. [Google Scholar] [CrossRef] [PubMed]
- Perret, E.; Jakubowski, K.; Heuberger, M.; Hufenus, R. Effects of Nanoscale Morphology on Optical Properties of Photoluminescent Polymer Optical Fibers. Polymers 2022, 14, 3262. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ortiz, D.; Salameh, C.; Bechelany, M.; Miele, P. Nanostructured boron nitride–based materials: Synthesis and applications. Mater. Today Adv. 2020, 8, 100107. [Google Scholar] [CrossRef]
- Shtansky, D.V.; Matveev, A.T.; Permyakova, E.S.; Leybo, D.V.; Konopatsky, A.S.; Sorokin, P.B. Recent Progress in Fabrication and Application of BN Nanostructures and BN-Based Nanohybrids. Nanomaterials 2022, 12, 2810. [Google Scholar] [CrossRef]
- Cinan, Z.M.; Erol, B.; Baskan, T.; Mutlu, S.; Ortaç, B.; Savaskan Yilmaz, S.; Yilmaz, A.H. Radiation Shielding Tests of Crosslinked Polystyrene-b-Polyethyleneglycol Block Copolymers Blended with Nanostructured Sele-nium Dioxide and Boron Nitride Particles. Nanomaterials 2022, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- Fruncillo, S.; Su, X.; Liu, H.; Wong, L.S. Lithographic Processes for the Scalable Fabrication of Micro- and Nanostructures for Biochips and Biosensors. ACS Sens. 2021, 6, 2002–2024. [Google Scholar] [CrossRef]
- Singh, A.; Shi, A.; Claridge, S.A. Nanometer-scale patterning of hard and soft interfaces: From photolithography to molecular-scale design. Chem. Commun. 2022, 58, 13059–13070. [Google Scholar] [CrossRef]
- Dou, F.; Peng, C.; Zou, M.; Zhang, X. Direct Imprinting of Large-Area Metallic Photonic Lattices for Infrared Po-larization Filters with Broadband Tunability. Nanomaterials 2023, 13, 1022. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Akagi, T.; Kaneko, T.; Kida, T.; Akashi, M. Preparation and characterization of biodegradable nanoparticles based on poly(g-glutamic acid) with l-phenylalanine as a protein carrier. J. Control. Release 2005, 108, 226–236. [Google Scholar] [CrossRef]
- Jeong, Y.-I.; Cho, C.-S.; Kim, S.-H.; Ko, K.-S.; Kim, S.-I.; Shim, Y.-H.; Nah, J.-W. Preparation of poly(DL-lactide-co-glycolide) nanoparticles without surfactant. J. Appl. Polym. Sci. 2001, 80, 2228–2236. [Google Scholar] [CrossRef]
- Lee, M.S.; Yee, D.W.; Ye, M.; Macfarlane, R.J. Nanoparticle Assembly as a Materials Development Tool. J. Am. Chem. Soc. 2022, 144, 3330–3346. [Google Scholar] [CrossRef]
- Fang, J.; Li, X.; Xie, W.; Sun, K. A Novel Fabrication of Single Electron Transistor from Patterned Gold Nanoparticle Array Template-Prepared by Polystyrene Nanospheres. Nanomaterials 2022, 12, 3102. [Google Scholar] [CrossRef] [PubMed]
- Escudero, A.; Carrillo-Carrión, C.; Romero-Ben, E.; Franco, A.; Rosales-Barrios, C.; Castillejos, M.; Khiar, N. Molecular Bottom-Up Approaches for the Synthesis of Inorganic and Hybrid Nanostructures. Inorganics 2021, 9, 58. [Google Scholar] [CrossRef]
- Matusinovic, Z.; Rogosic, M.; Sipusic, J. Synthesis and characterization of poly(styrene-co-methyl methacrylate)/layered double hydroxide nanocomposites via in situ polymerization. Polym. Degrad. Stab. 2009, 94, 95–101. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.; Chen, X.; He, Y.; Bolan, N.; Rinklebe, J.; Lam, S.S.; Peng, W.; Sonne, C. A discussion of microplastics in soil and risks for ecosystems and food chains. Chemosphere 2023, 313, 137637. [Google Scholar] [CrossRef] [PubMed]
- Corsi, I.; Venditti, I.; Trotta, F.; Punta, C. Environmental safety of nanotechnologies: The eco-design of manufactured nanomaterials for environmental remediation. Sci. Total Environ. 2023, 864, 161181. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Matei Brazdis, R.I.; Manaila-Maximean, D. Natural and Natural-Based Polymers: Recent Developments in Management of Emerging Pollutants. Polymers 2023, 15, 2063. [Google Scholar] [CrossRef]
- Karakolis, E.G.; Nguyen, B.; You, J.B.; Rochman, C.M.; Sinton, D. Fluorescent Dyes for Visualizing Microplastic Particles and Fibers in Laboratory-Based Studies. Environ. Sci. Technol. Lett. 2018, 5, 62–67. [Google Scholar] [CrossRef]
- Cook, S.; Chan, H.L.; Abolfathi, S.; Bending, G.D.; Schäfer, H.; Pearson, J.M. Longitudinal dispersion of microplastics in aquatic flows using fluorometric techniques. Water Res. 2020, 170, 115337. [Google Scholar] [CrossRef]
- Tong, H.; Jiang, Q.; Zhong, X.; Hu, X. Rhodamine B dye staining for visualizing microplastics in laborato-ry-based studies. Environ. Sci. Pollut. Res. Int. 2021, 28, 4209–4215. [Google Scholar] [CrossRef]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef] [Green Version]
- Wang, W. Bioimaging of metals in environmental toxicological studies: Linking localization and functionality. Crit. Rev. Environ. Sci. Technol. 2021, 52, 3384–3414. [Google Scholar] [CrossRef]
- Modlitbová, P.; Střítežská, S.; Hlaváček, A.; Prochazka, D.; Pořízka, P.; Kaiser, J. Laser-induced breakdown spec-troscopy as a straightforward bioimaging tool for plant biologists; the case study for assessment of pho-ton-upconversion nanoparticles in Brassica oleracea L. plant. Ecotoxicol. Environ. Saf. 2021, 214, 112113. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Fratoddi, I.; Palocci, C.; Venditti, I.; Russo, M.V. Osmosis based method drives the self-assembly of polymeric chains into micro- and nanostructures. Langmuir ACS J. Surf. Colloids 2009, 25, 11940–11946. [Google Scholar] [CrossRef]
- Ceschin, S.; Mariani, F.; Di Lernia, D.; Venditti, I.; Pelella, E.; Iannelli, M.A. Effects of Microplastic Contamination on the Aquatic Plant Lemna minuta (Least Duckweed). Plants 2023, 12, 207. [Google Scholar] [CrossRef]
- Mariani, F.; Di Lernia, D.; Venditti, I.; Pelella, E.; Muzzi, M.; Di Giulio, A.; Ceschin, S. Trophic transfer of micro-plastics from producer (Lemna minuta) to primary consumer (Cataclysta lemnata) in a freshwater food chain. Sci. Total Environ. 2023, 891, 164459. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Pulingam, T.; Foroozandeh, P.; Chuah, J.A.; Sudesh, K. Exploring Various Techniques for the Chemical and Biological Synthesis of Polymeric Nanoparticles. Nanomaterials 2022, 12, 576. [Google Scholar] [CrossRef]
- Visaveliya, N.R.; Köhler, J.M. Softness Meets with Brightness: Dye-Doped Multifunctional Fluorescent Polymer Particles via Microfluidics for Labeling. Adv. Opt. Mater. 2021, 9, 2002219. [Google Scholar] [CrossRef]
- Moniz, T.; Nunes, A.; Silva, A.M.; Queirós, C.; Ivanova, G.; Gomes, M.S.; Rangel, M. Rhodamine labeling of 3-hydroxy-4-pyridinone iron chelators is an important contribution to target Mycobacterium avium infection. J. Inorg. Biochem. 2013, 121, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Dong, B.; Chen, B.; Wang, J.; Xu, L.; Zhang, S.; Song, H. Multifunctional Au@mSiO2/rhodamine B isothiocyanate nanocomposites: Cell imaging, photocontrolled drug release, and photothermal therapy for can-cer cells. Small 2013, 9, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xia, H.; Tang, H.; Yang, L.; Sun, G. Tissue distribution of Lycium barbarum polysaccharides in rat tis-sue by fluorescein isothiocyanate labeling. Food Sci. Hum. Wellness 2022, 11, 837–844. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X. Spectrometric Identification of orgenic Compounds, 6th ed.; Wiley ED: Hoboken, NJ, USA, 1998. [Google Scholar]
- Fratoddi, I.; Battocchio, C.; Iucci, G.; Catone, D.; Cartoni, A.; Paladini, A.; O’Keeffe, P.; Nappini, S.; Cerra, S.; Venditti, I. Silver Nanoparticles Functionalized by Fluorescein Isothiocyanate or Rhodamine B Isothiocyanate: Fluorescent and Plasmonic Materials. Appl. Sci. 2021, 11, 2472. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database, Version 4.1 (National Institute of Standards and Technology, Gaithersburg, 2012). Available online: http://srdata.nist.gov/xps/ (accessed on 20 July 2023).




| Solvent | P(S-co-MMA) (g) | Dw (µm) | Dn (µm) | PI = Dw/Dn |
|---|---|---|---|---|
| Acetone (10 mL) | 0.0530 | 1.95 | 1.98 | 0.98 |
| Acetone (10 mL) | 0.3015 | 2.32 | 2.36 | 0.98 |
| DMF (10 mL) | 0.0530 | 0.70 | 0.72 | 0.98 |
| DMF (10 mL) | 0.3015 | 0.93 | 0.93 | 1.00 |
| Acetone (100 mL) | 0.3015 + RITC * | 2.04 | 2.08 | 0.98 |
| Acetone (10 mL) | 0.3015 + FITC * | 9.17 | 9.19 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertelà, F.; Battocchio, C.; Iucci, G.; Ceschin, S.; Di Lernia, D.; Mariani, F.; Di Giulio, A.; Muzzi, M.; Venditti, I. Dye-Doped Polymeric Microplastics: Light Tools for Bioimaging in Test Organisms. Polymers 2023, 15, 3245. https://doi.org/10.3390/polym15153245
Bertelà F, Battocchio C, Iucci G, Ceschin S, Di Lernia D, Mariani F, Di Giulio A, Muzzi M, Venditti I. Dye-Doped Polymeric Microplastics: Light Tools for Bioimaging in Test Organisms. Polymers. 2023; 15(15):3245. https://doi.org/10.3390/polym15153245
Chicago/Turabian StyleBertelà, Federica, Chiara Battocchio, Giovanna Iucci, Simona Ceschin, Dario Di Lernia, Flaminia Mariani, Andrea Di Giulio, Maurizio Muzzi, and Iole Venditti. 2023. "Dye-Doped Polymeric Microplastics: Light Tools for Bioimaging in Test Organisms" Polymers 15, no. 15: 3245. https://doi.org/10.3390/polym15153245
APA StyleBertelà, F., Battocchio, C., Iucci, G., Ceschin, S., Di Lernia, D., Mariani, F., Di Giulio, A., Muzzi, M., & Venditti, I. (2023). Dye-Doped Polymeric Microplastics: Light Tools for Bioimaging in Test Organisms. Polymers, 15(15), 3245. https://doi.org/10.3390/polym15153245








