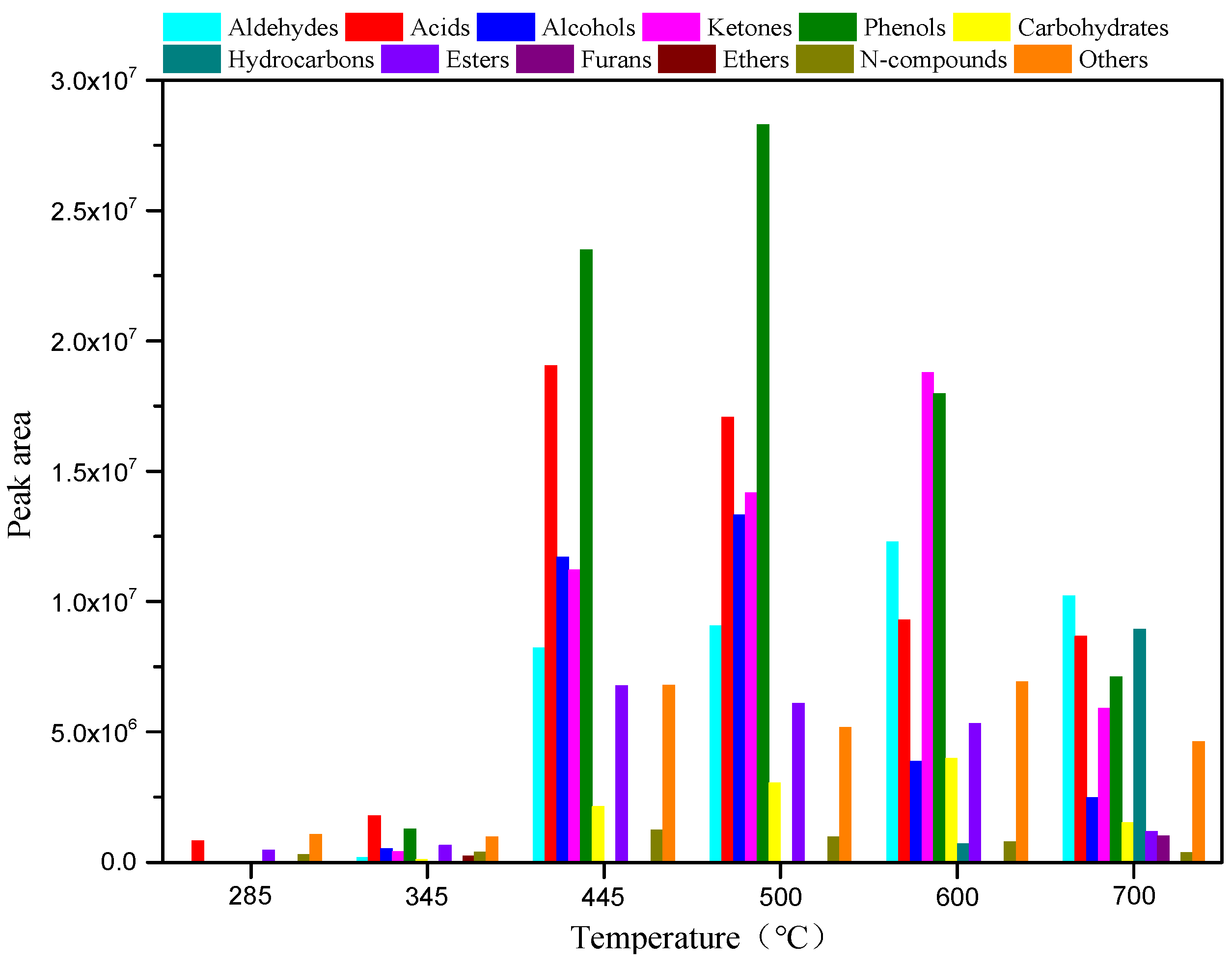
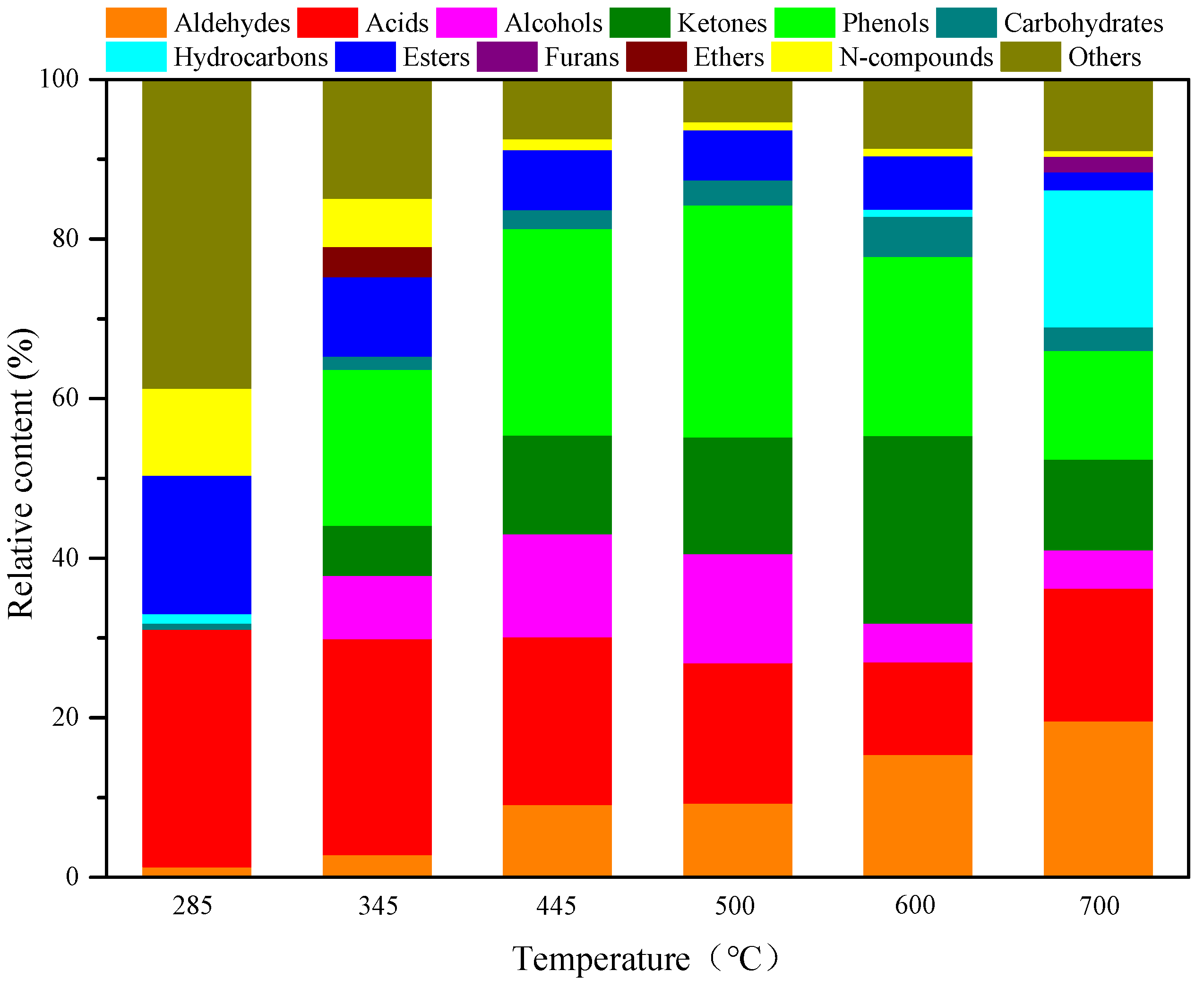
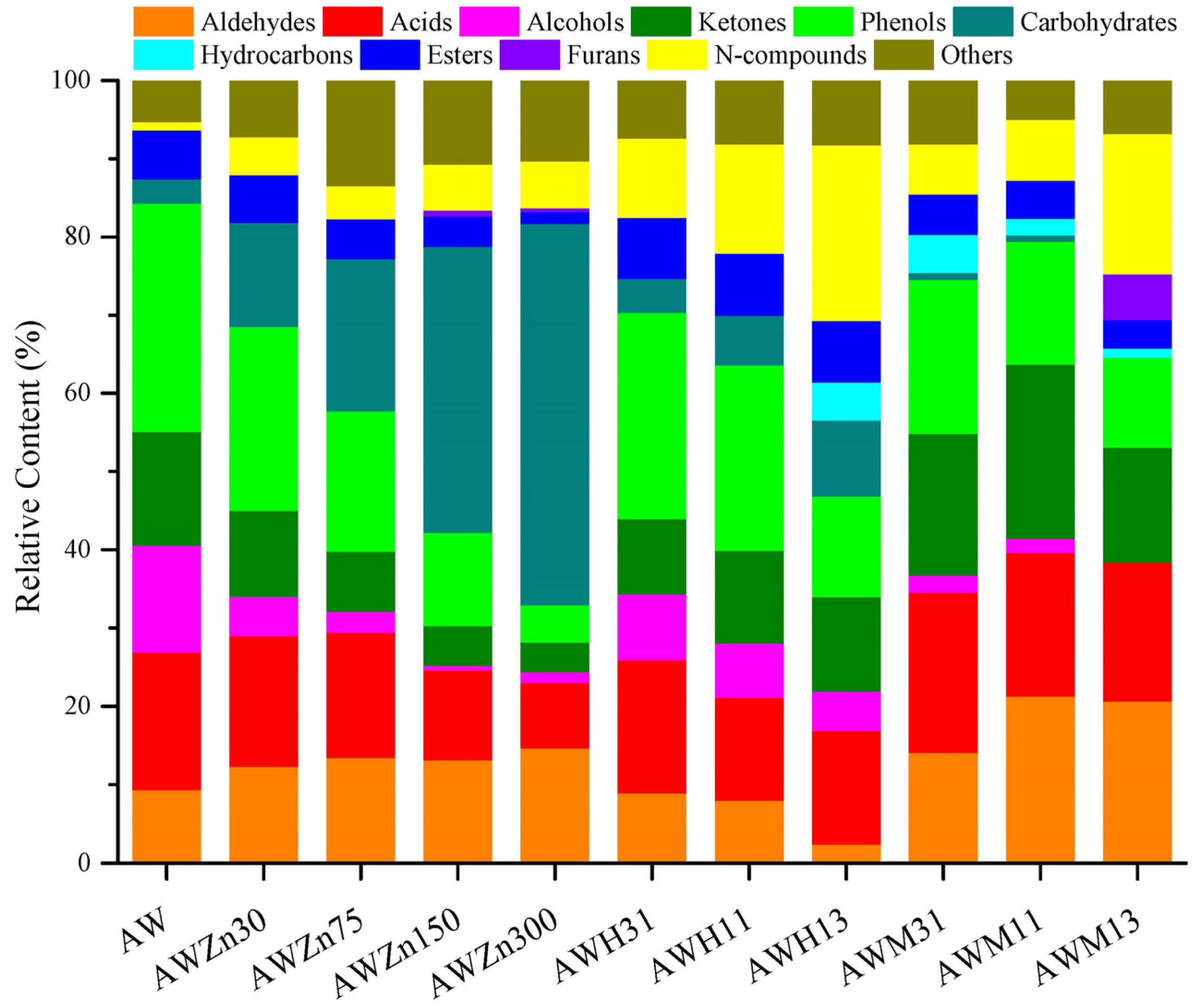
3.2. Influence of the Reaction Temperature on the Product Distribution from AW and RH Pyrolysis
To reveal the relationship between the structure and pyrolysis products of AW, AW was pyrolyzed for 10 s at different reaction temperatures with no catalysts. The pyrolysis product distribution is illustrated in
Figure 1 and
Figure 2. As shown in
Figure 1, AW pyrolysis produces diverse types of compounds, which can be classified into aldehydes, acids, alcohols, ketones, phenols, carbohydrates, hydrocarbons, esters, furans, ethers, and N-compounds. In addition, the total ion chromatographs of AW pyrolysis products contained hundreds of peaks, some of which were not identifiable. These were classified as “others”. The total peak areas at each temperature in the range of 285–700 °C were determined to be 2.77 × 10
6, 6.61 × 10
6, 9.07 × 10
7, 9.73 × 10
7, 8.00 × 10
7, and 5.21 × 10
7 by adding the peak areas of various compounds.
Figure 1 demonstrates that a pyrolysis temperature of 500 °C is conducive to increasing the content of bio-oils and the yields of all compounds vary significantly with changes in the pyrolysis temperature. As the temperature is increased from 285 to 600 °C, the yields of aldehydes and carbohydrates show gradual increases and then begin to decline as the temperature is increased from 600 to 700 °C. In the experimental temperature range, the yields of acids, esters, and N-compounds increase at first, and then decrease as the pyrolysis temperature rises, and they all reach their maxima at 445 °C. Alcohols and phenols are not produced at a low temperature of 285 °C, while their yields tend to increase at first, and then decline as the temperature rises in the range of 345–700 °C, reaching their maximum at 500 °C. Similar to alcohols and phenols, no ketones are detected at 285 °C, while their yield increases at first, and then decreases when the temperature rises from 345 to 700 °C, and it reaches the maximum at 600 °C. Hydrocarbons are only produced at 285 °C, 600 °C, and 700 °C. At the low temperature of 285 °C, what is produced is a low content of alkanes; traces of olefins are generated at 600 °C. The yield of hydrocarbons is the highest at 700 °C; these mainly include olefins and aromatic hydrocarbons. Small amounts of ethers are produced only at 345 °C, while furans are only generated at 700 °C. As displayed in
Figure 2, many other substances are produced at the pyrolysis temperature of 285 °C, the content of which reaches 38.74%. Apart from this, acids are the most important pyrolysis products at 285 °C, with a relative content of 29.74%; esters and N-compounds also account for large proportions (17.31% and 10.87%). As the temperature rises to 345 °C, acids and phenols become the main pyrolysis products at the temperature and their contents, respectively, reach 27.09% and 19.52%. With a further temperature rise to 445–500 °C, phenols become the predominant products and their relative contents reach 25.92% and 29.08%, while the content of acids remains relatively high, being 21.03% and 17.59%. At a pyrolysis temperature of 600 °C, the proportions of ketones and aldehydes are enlarged while that of acids are reduced, so that ketones, phenols, and aldehydes become the most important pyrolysis products, with contents, respectively, of 23.50%, 22.48%, and 15.37%. At 700 °C, the proportion of phenols declines and the deoxygenation of oxygen compounds at high temperatures promotes the production of a greater number of hydrocarbons, the proportion of which reaches 17.17%. Aldehydes predominate (19.59%) at that temperature, while acids are also present in significant amounts (16.66%).
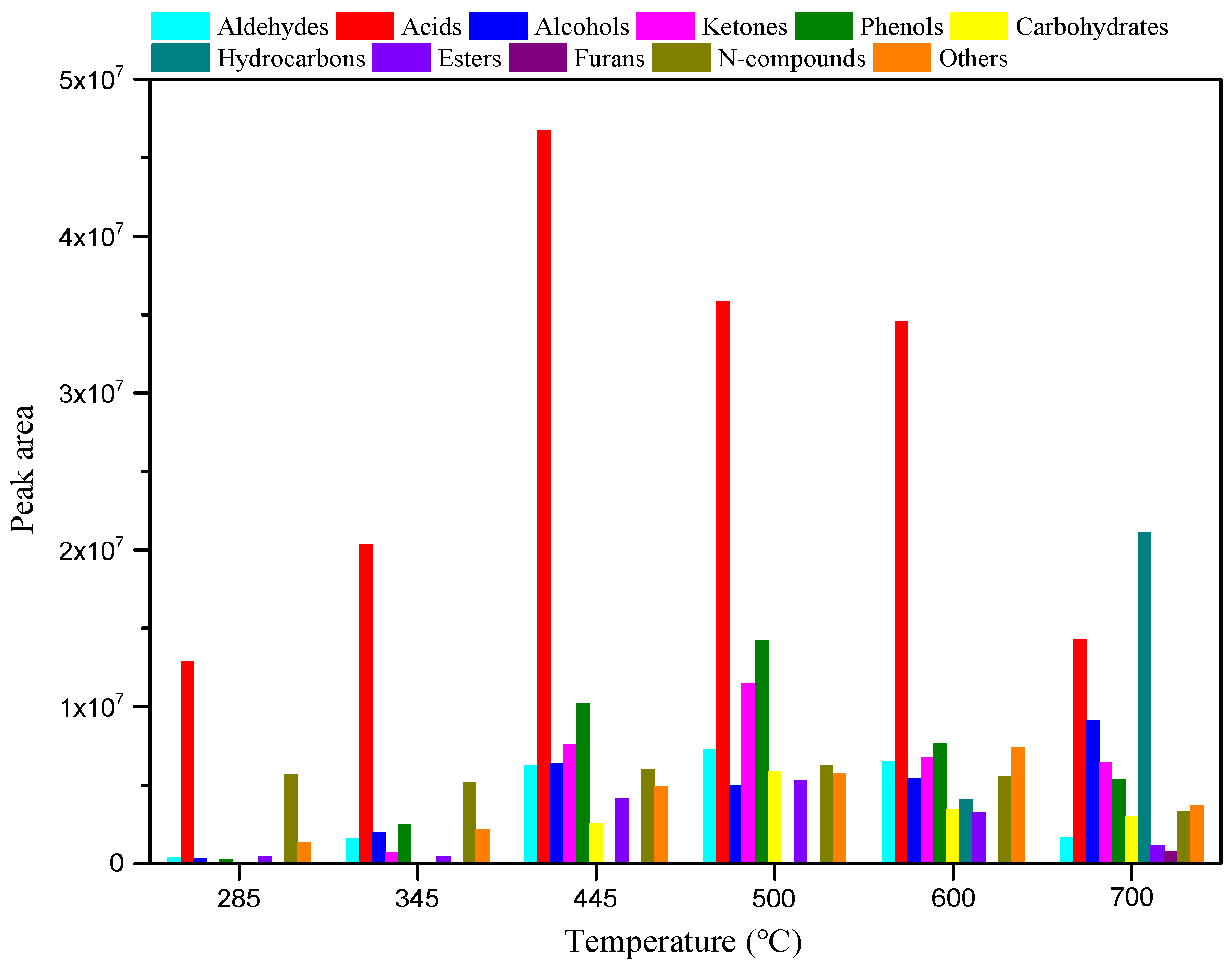
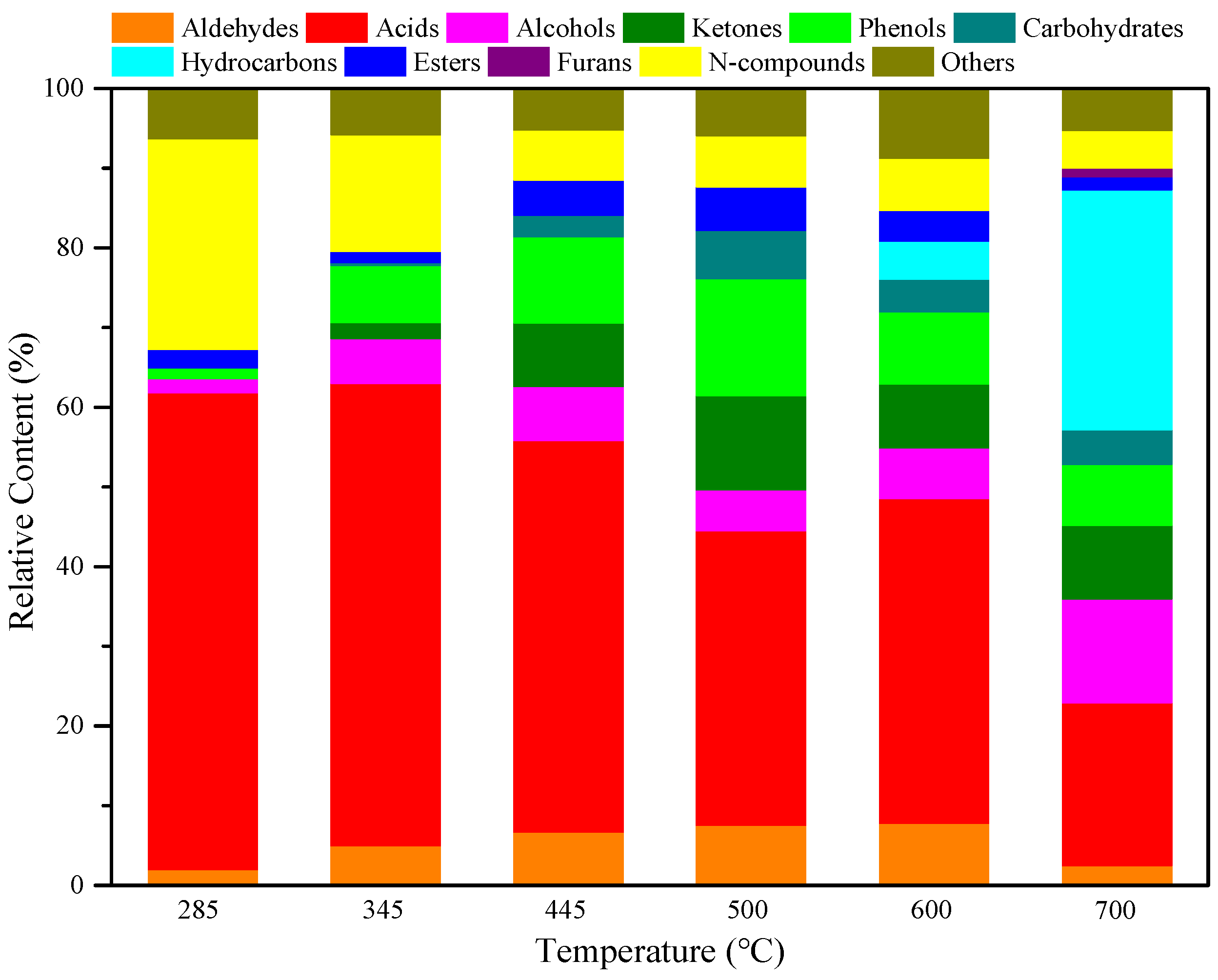
To clarify the relationship between the structure and pyrolysis products of RH, RH was pyrolyzed for 10 s at different temperatures without adding catalysts. The pyrolysis product distribution is illustrated in
Figure 3 and
Figure 4. As shown in
Figure 3, diverse compounds produced from RH pyrolysis can be divided into aldehydes, acids, alcohols, ketones, phenols, carbohydrates, hydrocarbons, esters, furans, and N-compounds. After the summation of peak areas of various compounds in
Figure 3, the total peak areas at each temperature in the range of 285–700 °C are obtained as 2.15 × 10
7, 3.51 × 10
7, 9.50 × 10
7, 9.71 × 10
7, 8.48 × 10
7, and 7.01 × 10
7.
Figure 3 also shows that the pyrolysis temperature in the range of 445–500 °C is conducive to improving the yield of bio-oils, and the yields of all compounds vary significantly as the pyrolysis temperature changes. As the temperature is increased from 285 to 500 °C, the yields of aldehydes, phenols, and esters increase, while they begin to decline as the temperature is increased from 500 to 700 °C. The yield of acids increases at first and then reduces with the rise of the pyrolysis temperature in the experimental range, and it reaches the maximum at 445 °C. The maximum yields of alcohols and N-compounds are obtained separately at 700 and 500 °C. Ketones and carbohydrates are not detected at 285 °C and their yields increase, and then decrease as the temperature rises from 345 to 700 °C, reaching the maximum at 500 °C. Hydrocarbons are only produced at 600–700 °C and their yield increases with increasing temperature, and they always include olefins, alkyne, and aromatic hydrocarbons, whereas, furans are only generated at a high temperature of 700 °C. As illustrated in
Figure 4, acids are the main pyrolysis products at 285 °C, with a relative content of 59.79%, and N-compounds also account for a large proportion at 26.42%. As the temperature increases to 345 °C, acids and N-compounds are still the most important pyrolysis products and their contents are, respectively, 58.00% and 14.67%. With the rise of pyrolysis temperature to 445 °C, the content of N-compounds reduces while that of phenols increases; acids and phenols are major pyrolysis products under this condition, separately accounting for 49.16% and 10.80%. At the pyrolysis temperature of 500 °C, the content of acids declines to some extent; acids (36.95%), phenols (14.67%), and ketones (11.84%) represent three predominant compounds. Akin to the case at 285–500 °C, acids are also the compounds of the highest content at 600 °C and their content can reach 40.79%. With a further rise of the pyrolysis temperature to 700 °C, deoxygenation of oxygen compounds at high temperatures facilitates the generation of a large number of hydrocarbons, so hydrocarbons are compounds of the largest content (30.12%) under this condition; acids and alcohols also have high contents of 20.41% and 13.04%, respectively.
The above results show that the types and yields of compounds generated by the non-catalytic pyrolysis of AW and RH were influenced significantly by temperature. In addition, the compositional and structural differences between these two types of biomasses led to marked differences in product distributions in the pyrolysis process. As provided in the
Supplementary Data (Tables S1 and S2), the types of pyrolytic compounds of AW and RH increased and the molecular structures of those tended to be simpler with increasing temperature from 285 °C to 700 °C. After a sequence of reactions including depolymerization, cracking, dehydration, deoxygenation, decarbonylation, and decarboxylation under high temperatures, the macromolecular oxygenates and N-compounds were converted into small molecular compounds. Moreover, higher temperatures can remove certain oxygen-containing functional groups to obtain hydrocarbons or promote the dehydration, oligomerization, and aromatization of small molecules to generate aromatics [
4].
3.3. Effects of Various Catalysts and Their Concentration on Product Distribution from AW Pyrolysis
Three different catalysts (i.e., ZnCl
2, HZSM-5, and MCM-41) were used to perform catalytic rapid pyrolysis experiments on AW, and the results are shown in
Figure 5 and
Figure 6. Data in
Figure 5 indicate that adding ZnCl
2 promotes the generation of aldehydes, the content of which increases with increasing amounts of catalyst. When using ZnCl
2 as the catalyst, the contents of most oxygen compounds decrease, including acids, alcohols, ketones, phenols, and esters. The addition of a high content of ZnCl
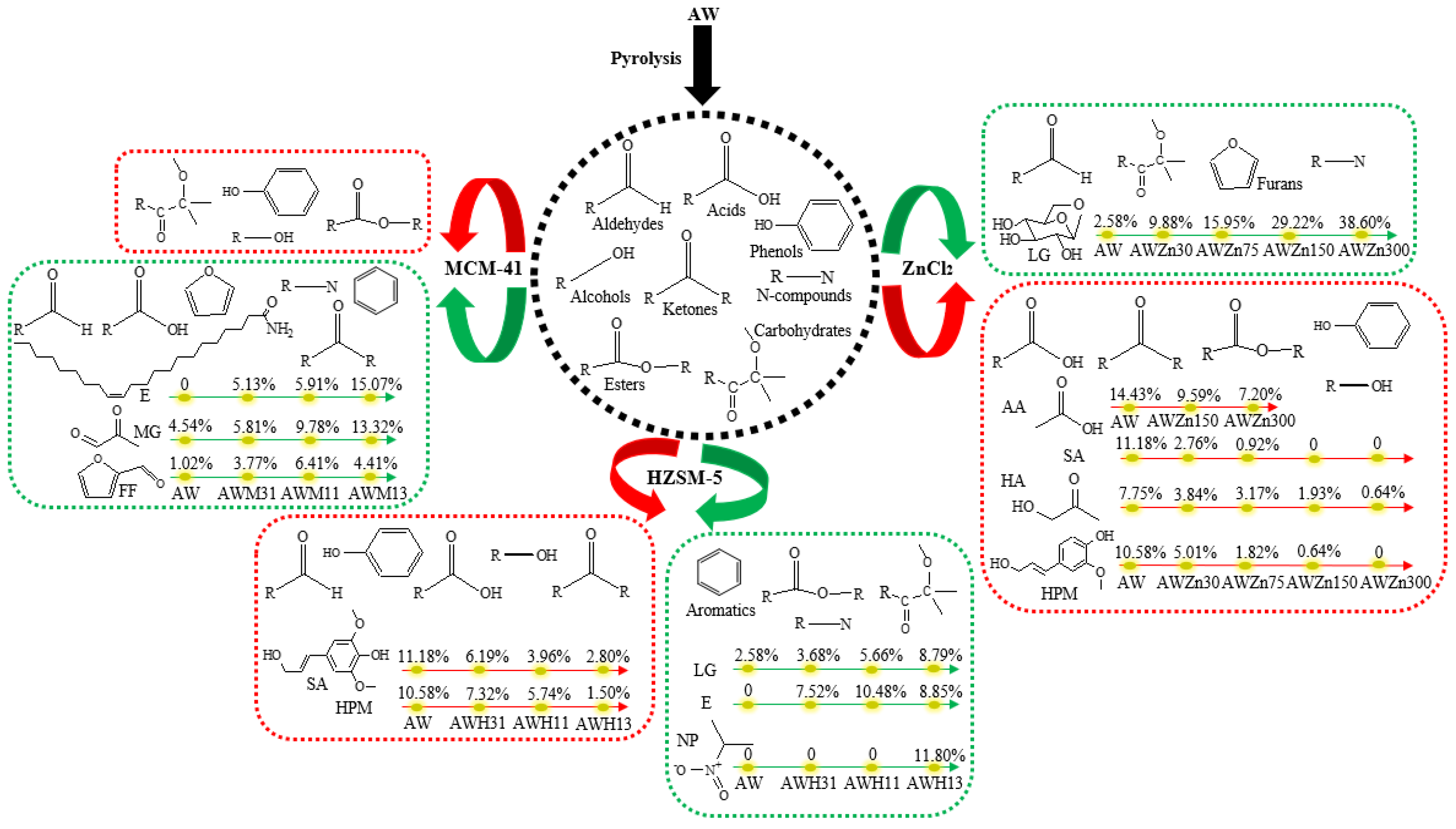
2 significantly inhibits the production of acids; within the experimental range, the acid content can be reduced by up to 9.24%. Variation in the content of acids is mainly shown as the reduction of the AA content, which was reduced by 7.23% (
Figure 6). This is consistent with previous studies, wherein it was found that ZnCl
2 supplementation can reduce the AA content of corn stalk pyrolysis products by up to 6.19% [
6]. These show that ZnCl
2 can inhibit the formation of AA in biomass pyrolysis products. In addition, ZnCl
2 can significantly reduce the alcohol content, up to 13.08% (
Figure 5). Changes in the content of alcohols are mainly reflected by the decreased content of
trans-sinapyl alcohol (SA), the content of which can be reduced from 11.18% to 0 (
Figure 6). As displayed in
Figure 5, when adding ZnCl
2, the content of ketones and phenols also reduces by up to 10.78% and 24.35%, respectively (
Figure 5). In detail, the reduction of the content of ketones is manifested in a decreased content of HA from 7.75% (AW) to 0.64% (AWZn300) (
Figure 6). The decrease in the content of phenols is mainly reflected by the reduced content of 4-((1
E)-3-hydroxy-1-propenyl)-2-methoxyphenyl (HPM) from 10.58% (AW) to 0 (AWZn300) (
Figure 6). The contents of alcohols, ketones, and phenols also decrease significantly when the added amount of ZnCl
2 is high, which indicates that ZnCl
2 may change the main path of pyrolysis-product generation for AW. As shown in
Figure 5, ZnCl
2 can significantly increase the content of carbohydrates from 3.14% without adding the catalyst to 48.75% (AWZn300). Variation of the content of carbohydrates is mainly mirrored by the increased content of LG from 2.58% (AW) to 38.60% (AWZn300) (
Figure 6). This shows that ZnCl
2 can make the pyrolysis reaction of AW produce more carbohydrates (especially LG), which is consistent with previous studies; ZnCl
2 supplementation can improve the LG content of corn stalk pyrolysis products by up to 18.16% [
6]. ZnCl
2 does not promote the generation of hydrocarbons from AW pyrolysis, so the yield of hydrocarbons is always zero. If adding high contents of ZnCl
2 (AWZn150 and AWZn300), it promotes the production of trace amounts of furans. Moreover, ZnCl
2 can also suppress the generation of esters and facilitate the production of N-compounds. Such inhibiting or promoting effects become increasingly obvious with increasing amounts of catalyst. In all catalysis experiments, the lowest contents of acids (8.35%), ketones (3.79%), phenols (4.73%), and esters (1.50%) and the highest content of carbohydrates (48.75%) are always determined in the case of AWZn300.
As shown in
Figure 5, taking HZSM-5 as the catalyst can apparently decrease the contents of aldehydes, acids, and alcohols, which reduce more remarkably with increasing amounts of catalyst added. Therein, changes in the content of alcohols are manifested as the content of SA declines from 11.18% (AW) to 6.19% (AWH31), 3.96% (AWH11), and 2.80% (AWH13) (
Figure 6). The content of ketones is also inhibited by HZSM-5, which is more obvious when the added amount of catalyst is low. HZSM-5 can significantly reduce the content of phenols from 29.08% without adding the catalyst to 26.33% (AWH31), 23.67% (AWH11), and 12.88% (AWH13) (
Figure 5). Variation of the content of phenols is mainly reflected by the reduction of the HPM content from 10.58% (AW) to 7.32% (AWH31), 5.74% (AWH11), and 1.50% (AWH13) (
Figure 6). High amounts of HZSM-5 (AWH13) may promote the generation of aromatic hydrocarbons, increasing their content from 0 to 4.81% (
Figure 5). The production of aromatic hydrocarbons is probably because HZSM-5 can facilitate deoxygenation and promote the transformation of phenolic substances to aromatic hydrocarbons in the pyrolysis. HZSM-5 can promote the generation of carbohydrates, and such an effect becomes increasingly significant with increasing amounts of catalyst. The increased content of carbohydrates is manifested in the increase in the LG content from 2.58% (AW) to 3.68% (AWH31), 5.66% (AWH11), and 8.79% (AWH13) (
Figure 5 and
Figure 6). It can be seen from
Figure 5 that HZSM-5 can slightly increase the content of esters, which is not affected to any significant extent by the amount of catalyst added. HZSM-5 does not promote the generation of furans during AW pyrolysis. Additionally, taking HZSM-5 as the catalyst can significantly augment the content of N-compounds from 1.01%, without adding the catalyst, to 10.14% (AWH31), 13.93% (AWH11), and 22.43% (AWH13). The increased content of N-compounds is mainly reflected by the increased contents of erucamide (E) and 2-nitropropane (NP). The contents of E and NP separately grow from 0 (AW) to 7.52% (AWH31), 10.48% (AWH11), and 8.85% (AWH13), and from 0 (AW) to 11.80% (AWH13) (
Figure 6). In all catalysis experiments, the lowest content of aldehydes (2.41%) and the highest content of N-compounds (22.43%) were both obtained in the case of AWH13, while the highest content of esters (7.97%) was produced under AWH11.
As shown in
Figure 5, the content of aldehydes rises significantly from 9.32%, without adding the catalyst, to 14.13% (AWM31), 21.29% (AWM11), and 20.64% (AWM13) when adding MCM-41 as the catalyst. Changes in the content of aldehydes are manifested as variations in methylglyoxal (MG) and FF contents: the content of MG grows from 4.54% (AW) to 5.81% (AWM31), 9.78% (AWM11), and 13.32% (AWM13); that of FF increases from 1.02% (AW) to 3.77% (AWM31), 6.41% (AWM11), and 4.41% (AWM13) (
Figure 6). MCM-41 can promote the generation of acids, while the promoting effect becomes increasingly attenuated with increasing amounts of catalyst. Addition of MCM-41 results in a significant reduction of the content of alcohols from 13.68%, without adding the catalyst, to 2.15% (AWM31), 1.78% (AWM11), and 0 (AWM13). Changes in the content of alcohols are manifested in the reduction of the SA content from 11.18% (AW) to 0 (AWM31, AWM11, and AWM13).
Figure 5 also shows that adding low amounts of MCM-41 (AWM31 and AWM11) can promote the production of ketones. In addition, MCM-41 reduces the content of phenols remarkably from 29.08% (AW) to 19.66% (AWM31), 15.68% (AWM11), and 11.50% (AWM13). Variation of the content of phenols is mainly reflected by the reduction of the HPM content from 10.58% (AW) to 0 (AWM31, AWM11, and AWM13). As illustrated in
Figure 5, MCM-41 decreases the generation of carbohydrates and esters, which becomes increasingly apparent as the added amount of the catalyst increases. The presence of MCM-41 promotes the generation of hydrocarbons from AW pyrolysis, while the content of hydrocarbons tends to decrease with increasing addition of the catalyst. Furans are only produced under conditions of a high amount of catalyst (AWM13) and their content can reach 5.88%. Taking MCM-41 as the catalyst can significantly increase the content of N-compounds. In particular, when adding a large amount of the catalyst, the content of N-compounds grows from 1.01% (AW) to 17.89% (AWM13). Changes in the content of N-compounds are mainly reflected by the increasing content of E from 0 (AW) to 5.13% (AWM31), 5.91% (AWM11), and 15.07% (AWM13) (
Figure 6). In all catalysis experiments, AWM13 leads to the highest contents of aldehydes (20.64%) and furans (5.88%), with the lowest contents of alcohols (0) and carbohydrates (0). The highest content of ketones (22.30%) is obtained under AWM11, while those of acids (20.46%) and hydrocarbons (4.85%) are attained in the case of AWM31.
3.4. Effects of Various Catalysts and Their Concentration on Product Distribution from RH Pyrolysis
Catalytic rapid pyrolysis experiments of RH were conducted using three different catalysts including ZnCl
2, HZSM-5, and MCM-41, and the results are illustrated in
Figure 7 and
Figure 8. Data in
Figure 7 indicate that the addition of ZnCl
2 can promote the generation of aldehydes while inhibiting the production of alcohols and N-compounds, whereas, the contents of the three compounds do not change in any particular way with the increased amount of added catalyst. Adding ZnCl
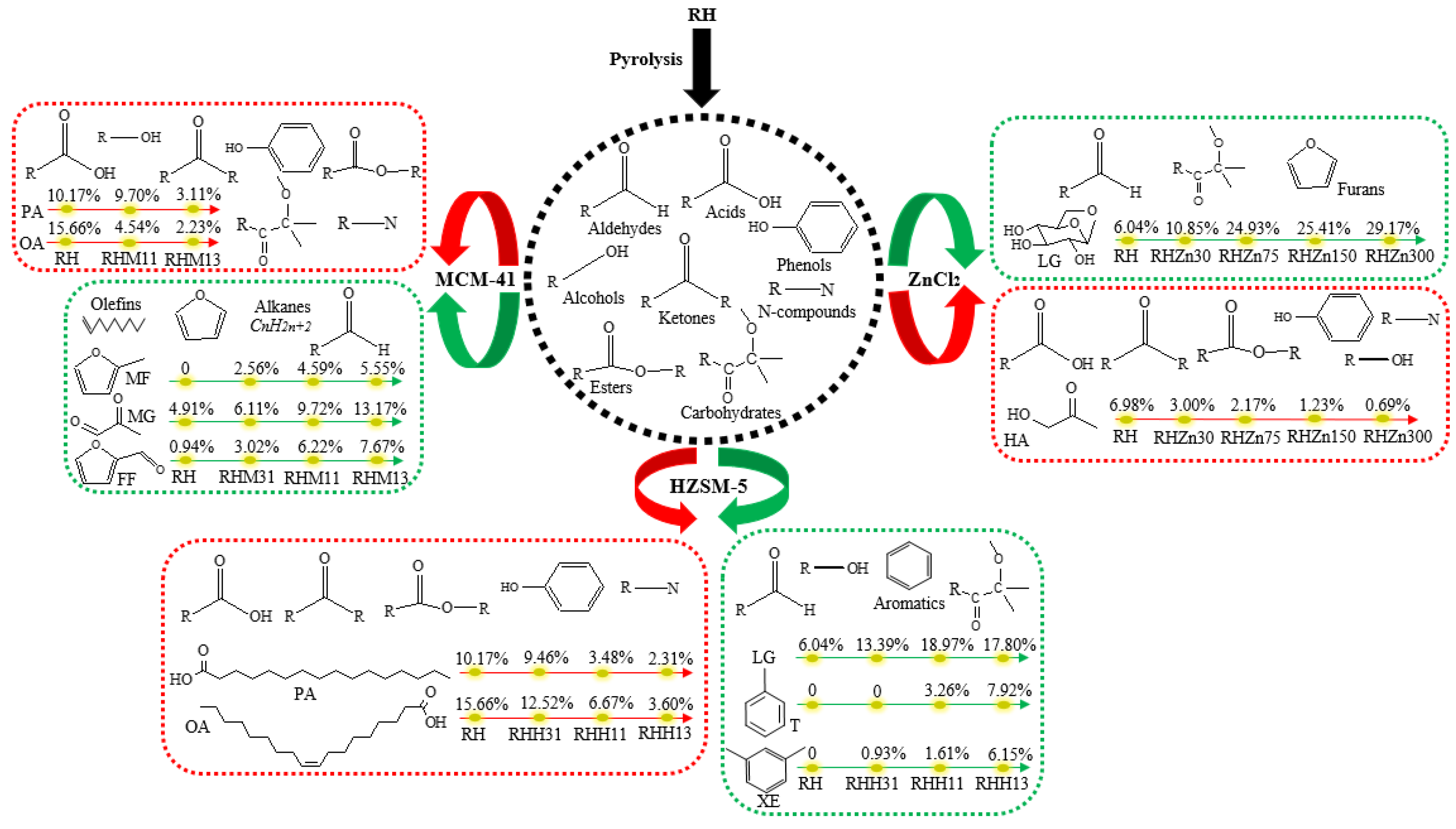
2 can apparently reduce the generation of acids from 36.95% without adding the catalyst to 35.50% (RHZn30), 23.93% (RHZn75), 27.27% (RHZn150), and 27.10% (RHZn300). Changes in the content of acids are mainly shown in the reduction of the content of multiple acids. In addition, taking ZnCl
2 as the catalyst can also decrease the contents of ketones, phenols, and esters, and their contents reduce more apparently as the amount of catalyst added increases. Variation of the content of ketones is mainly reflected by changes in the HA content, which decreases from 6.98% (RH) to 3.00% (RHZn30), 2.17% (RHZn75), 1.23% (RHZn150), and 0.69% (RHZn300) (
Figure 8). Variation of the content of phenols is manifested in the reduction of the contents of multiple phenols. The presence of ZnCl
2 can promote the generation of carbohydrates from 6.04%, without adding the catalyst, to 14.80% (RHZn30), 31.88% (RHZn75), 32.09% (RHZn150), and 39.16% (RHZn300) (
Figure 7). Variation in the content of carbohydrates is mainly reflected by the increment of the LG content from 6.04% (RH) to 10.85% (RHZn30), 24.93% (RHZn75), 25.41% (RHZn150), and 29.17% (RHZn300) (
Figure 8). As shown in
Figure 7, ZnCl
2 does not promote the generation of hydrocarbons during RH pyrolysis, so the content of hydrocarbons always remains 0. The addition of high amounts of ZnCl
2 (RHZn75, RHZn150, and RHZn300) facilitates the generation of slight furans and variation in the added amount of catalyst does not influence the furan content to any significant extent. In all catalysis experiments, the lowest contents of ketones (3.89%), phenols (5.20%), and esters (1.13%) and the highest content of carbohydrates (39.16%) are obtained under RHZn300. Separately, RHZn75 and RHZn30 result in the lowest contents of alcohols (2.34%) and N-compounds (3.09%).
As shown in
Figure 7, adding a high amount of HZSM-5 (RHH13) can promote the production of aldehydes, while the content of acids declines significantly in the presence of HZSM-5 from 36.95%, without adding the catalyst, to 33.82% (RHH31), 20.89% (RHH11), and 13.21% (RHH13). Variation in the content of acids is manifested in the reduction in contents of palmitic acid (PA) and oleic acid (OA). The former reduces from 10.17% (RH) to 9.46% (RHH31), 3.48% (RHH11), and 2.31% (RHH13), while the latter declines from 15.66% (RH) to 12.52% (RHH31), 6.67% (RHH11), and 3.60% (RHH13) (
Figure 8). Data in
Figure 7 also show that adding a low amount of HZSM-5 (RHH31) can augment the content of alcohols (albeit slightly), whereas, HZSM-5 suppresses the generation of ketones and phenols, and the inhibitory effect becomes increasingly prominent with the increasing amounts of catalyst. Using HZSM-5 as the catalyst can promote the generation of carbohydrates from 6.04% (RH) to 15.09% (RHH31), 22.85% (RHH11), and 21.84% (RHH13). Changes in the content of carbohydrates are mainly reflected by the variation of the LG content, which grows from 6.04% (RH) to 13.39% (RHH31), 18.97% (RHH11), and 17.80% (RHH13) (
Figure 8). As displayed in
Figure 7, the addition of HZSM-5 significantly enlarges the content of hydrocarbons (aromatic hydrocarbons) from 0 (RH) to 0.93% (RHH31), 5.96% (RHH11), and 18.28% (RHH13). Variation in the content of hydrocarbons is mainly shown in the increased contents of toluene (T) and m-xylene (XE). The former grows from 0 (RH) to 3.26% (RHH11) and 7.92% (RHH13), while the latter rises from 0 (RH) to 0.93% (RHH31), 1.61% (RHH11), and 6.15% (RHH13) (
Figure 8). HZSM-5 also plays a role in reducing the production of esters, while the effect is not significant. Moreover, HZSM-5 does not promote the generation of furans during RH pyrolysis, so the furan content is always zero. In addition, the presence of HZSM-5 decreases the content of N-compounds, while changes in the amount of added catalyst do not apparently affect the content thereof (
Figure 7). In all catalysis experiments, alcohols reach their highest concentration (6.66%) under RHH31, while RHH13 leads to the lowest content of acids (13.21%) and the highest content of hydrocarbons (18.28%).
As illustrated in
Figure 7, the content of aldehydes can be increased from 7.50% (RH) to 13.18% (RHM31), 20.33% (RHM11), and 25.33% (RHM13) when using MCM-41 as the catalyst. Changes in the content of aldehydes are mainly shown as increments of the MG and FF contents. The former grows from 4.91% (RH) to 6.11% (RHM31), 9.72% (RHM11), and 13.17% (RHM13), and the latter increases from 0.94% (RH) to 3.02% (RHM31), 6.22% (RHM11), and 7.67% (RHM13) (
Figure 8). MCM-41 with a high adding amount can significantly reduce the content of acids from 36.95% without adding the catalyst to 25.40% (RHM11) and 15.38% (RHM13) (
Figure 7). Variation in the content of acids is manifested in the reduction of the PA and OA contents. The former decreases from 10.17% (RH) to 9.70% (RHM11) and 3.11% (RHM13), and the latter decreases from 15.66% (RH) to 4.54% (RHM11) and 2.23% (RHM13) (
Figure 8). As displayed in
Figure 7, MCM-41 can inhibit the generation of alcohols while changes in the amount added do not significantly affect the content of alcohols. The presence of MCM-41 slightly decreases the content of ketones; at the same time, it suppresses the production of phenols and carbohydrates, and the contents of the two reduce more significantly with the increasing addition of the catalyst. Hydrocarbons are only produced when adding a larger amount of the catalyst (RHM13) and their content rises from 0 (RH) to 6.38% (RHM13); moreover, MCM-41 also reduces the generation of esters and N-compounds, the contents of which, however, are unaffected by the amount of the catalyst added. The presence of MCM-41 promotes the generation of 2-methylfuran (MF), so that the MF content increases from 0 (RH) to 2.56% (RHM31), 4.59% (RHM11), and 5.55% (RHM13) (
Figure 8). In all catalysis experiments, the highest contents of aldehydes (25.33%) and furans (5.55%), while the lowest content of carbohydrates (1.42%) are all attained in the case of RHM13.
According to the aforementioned studies on catalytic pyrolysis of two types of biomasses, the compositional and structural differences between AW and RH led to marked differences in product distributions in the pyrolysis process. In addition, the difference in catalyst performance also had different effects on the distribution of the pyrolysis products of biomass. The above results show that ZnCl
2 has no effect on the formation of hydrocarbons during AW and RH pyrolysis but can significantly change the distribution of oxygen-containing compounds. ZnCl
2 can increase the contents of aldehydes, carbohydrates, and furans while reducing the contents of acids, alcohols, ketones, phenols, and esters. Changes in the relative amount of carbohydrates are mainly manifested in the change in the proportion of LG. These catalytic effects are more obvious at higher doses. This is consistent with previous research [
6].
The addition of HZSM-5 is conducive to the formation of carbohydrates and aromatic hydrocarbons during the pyrolysis of AW and RH, while suppressing the production of oxygen-containing compounds such as acids, ketones, and phenols. This may be because the acidity of HZSM-5 promoted deoxygenation and facilitated the conversion of phenols to aromatic hydrocarbons during pyrolysis. The formation of aromatic hydrocarbons may be related to the polymerization of alkenes or alkynes, or to the condensation of compounds containing C=O [
26].
MCM-41 can improve the selectivity of aldehydes, furans, and hydrocarbons, while also decreasing the generation of alcohols, phenols, carbohydrates, and esters during the pyrolysis of AW and RH. Changes in the relative amounts of aldehydes, furans, and carbohydrates were mainly manifested in changes in the proportions of MG, FF, MF, and LG, respectively. MG, FF, and MF are valuable products that can be converted into other useful chemicals. They are typically derived from hemicellulose or produced by the dehydration of carbohydrates, mainly LG, which are derived from cellulose and hemicellulose pyrolysis [
4]. Compared with HZSM-5, MCM-41 had a larger specific surface area, pore volume, and average pore diameter, which were conducive to the provision of sufficient reaction sites and selectivity. Therefore, MCM-41 can also promote several deoxygenation reactions that convert oxygenates into olefins and aromatics.